Abstract
Background:
Although regulatory T cells (Tregs) play crucial roles in the maintenance of immune hemostasis, the numbers of peripheral Tregs in patients with psoriatic arthritis (PsA) remain unclear. We measured these numbers and the efficacy and safety of low-dose interleukin-2 (IL-2) therapy.
Methods:
We recruited 95 PsA patients, of whom 22 received subcutaneous low-dose IL-2 [0.5 million international units (MIU) per day for 5 days] combined with conventional therapies. The absolute numbers of cells in peripheral CD4+ T cell subsets were measured via modified flow cytometry. Clinical and laboratory indicators were compared before and after treatment.
Results:
PsA patients had lower peripheral Treg numbers than healthy controls (p < 0.01), correlating significantly and negatively with the levels of disease indicators (p < 0.05). Although low-dose IL-2 significantly increased the Th17 and Treg numbers in PsA patients compared with the baseline values, the Treg numbers rose much more rapidly than those of Th17 cells, re-balancing the Th17 and Treg proportions. Low-dose IL-2 combination therapy rapidly reduced PsA disease activities as indicated by the DAS28 instrument, thus the number of tender joints, visual analog scale pain, physician global assessment, the dermatology life quality index score, and the health assessment questionnaire score (all p < 0.05).
Conclusion:
PsA patients exhibited low Treg numbers. Low-dose IL-2 combination treatment increased these numbers and relieved disease activity without any apparent side effects. Additional studies are required to explore the long-term immunoregulatory utility of IL-2 treatment.
Keywords: immunoregulation, low-dose IL-2, psoriatic arthritis, regulatory T cells
Introduction
Psoriatic arthritis (PsA) is a chronic, immune system-mediated, disabling inflammatory disease with a long and repetitive course; the multiple manifestations include peripheral arthritis, enthesitis, dactylitis, and psoriasis.1 Skin and joint treatments remain suboptimal; PsA is heterogeneous and the underlying causes are obscure. It is accepted that activated CD4+ T helper (Th) subsets (Th1, Th2, and Th17 cells) play indispensable roles in autoimmune diseases,2–5 and are the targets of conventional immunosuppressive therapies. Also, various biological agents targeting inflammatory cytokines such as interleukin (IL)-17, IL-23, and tumor necrosis factor (TNF)-α have been used to alleviate the joint and skin symptoms of PsA patients.6 However, the clinical effects remain unsatisfactory.7 New therapies are essential. Recently, immunosuppressive regulatory T cells (Tregs) have been shown to maintain immune system hemostasis and peripheral tolerance to self- and non-self-antigens.8 CD4+CD25+ T lymphocytes constitute a well-studied subgroup of Tregs; the forkhead box transcription factor FOXP3 mediates the immunosuppressive phenotypes.7 Disruption of the crucial balance between Th17 and CD4+CD25+FOXP3+ Treg numbers, caused by changes in the numbers and/or functions of either cell type, have been described in patients with various inflammatory and autoimmune diseases, including primary Sjogren’s syndrome (pSS), rheumatoid arthritis (RA), ankylosing spondylitis (AS), multiple sclerosis, systemic lupus erythematosus (SLE), psoriasis, and PsA.9–12 Notably, Tregs isolated from both peripheral blood (PB) and psoriatic skin do not effectively suppress effector T cell actions in patients with psoriasis.13,14 However, the absolute numbers of Treg and Th17 cells in the PB of PsA patients remain controversial.
IL-2 plays stimulatory and inhibitory roles in the immune system by binding to IL-2 receptors (IL-2Rs), either high-affinity trimeric receptors [IL-2Rα (CD25), IL-2Rβ (CD122), and IL-2Rγ (CD132)] or intermediate-affinity dimeric IL-2Rs (with β and γ chains).15,16 Low-dose IL-2 is required for the optimal development, survival, and function of Tregs that express high levels of IL-2Rα (CD25).17 Exogenous low-dose IL-2 therapy effectively and safely treated autoimmune disorders primarily by restoring Treg numbers and functions, and rebalancing the Th17/Treg ratios.9,18 Here, we measured the numbers of CD4+ T lymphocytes of various subsets, including Th17 cells and Tregs, and calculated Th17/Treg ratios in the PB of PsA patients. We also evaluated the immunoregulatory utility and clinical safety of low-dose IL-2 combination therapy.
Methods
Patients and healthy controls
We consecutively enrolled 95 patients with PsA (50 females and 45 males), including 21 new-onset and 74 previously treated patients. We also enrolled 106 healthy controls (56 females and 50 males) who visited the rheumatology clinic or the physical examination center of the Second Hospital of Shanxi Medical University from January 2016 to August 2018. The patients ranged from 23 to 70 years of age and met the 2006 PsA (CASPAR) criteria.19 The exclusion criteria included an IL-2 allergy or intolerance; any other autoimmune disease; a malignant tumor; any heart, kidney, or liver dysfunction; and/or a recent serious infection. The study was performed in accordance with all relevant tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University. Informed consent was obtained from each patient at the time of study entry.
Study design and treatment
The absolute numbers of CD4+ T lymphocytes in blood from all subjects were derived via modified flow cytometry. All patients were on prednisone and/or other immunosuppressive medications to control disease activity. A total of 22 patients were randomly chosen (using a random number table) to receive subcutaneous human IL-2 (aldesleukin) at 0.50 million International Units (MIU) per day for 5 days. Laboratory and clinical data were collected before and after treatment for all patients, but only those receiving low-dose IL-2 underwent re-evaluation of CD4+ T cell subset counts within 2 days of treatment completion.
Outcomes
Efficacy
The primary efficacy outcomes were the clinical indicators, including the tender joint count (TJC), swollen joint count (SJC), visual analog (pain) score (VAS), dermatology life quality index (DLQI) score, physician global assessment (PHGA), health assessment questionnaire (HAQ) score, erythrocyte sedimentation rate (ESR), disease activity score (DAS) on the 28-ESR instrument, and C-reactive protein (CRP) level.
The indicators of immunological efficacy were the absolute numbers of CD4+ T lymphocytes, including Th1, Th2, and Th17 cells, and Tregs, and the Th17/Treg and Th1/Th2 ratios, derived via modified flow cytometry.9 We added the appropriate monoclonal antibodies and whole blood directly to BD Trucount tubes. The lyophilized antibody pellet dissolved in each tube, releasing a known number of fluorescent beads. The absolute numbers (cells/μL) of positive cells were determined by comparing cellular and bead events. The absolute numbers of cells in the various CD4+ T subsets were the percentages of each CD4+ T subset multiplied by the total number of CD4+ T cells. This single-platform method (the gold standard for absolute cell assessments) minimizes the risk of aberrant results.
Safety
The safety indicators included drug resistance, allergy, fever, fatigue, redness, and pain at the injection site. We assessed the effects of IL-2 on all vital organs and recorded all adverse events.
Statistical analysis
The demographic parameters of healthy controls and all patients were compared using the unpaired t-test for parametric data (age) or the independent-samples Mann–Whitney U test, Kruskal–Wallis test, or one-way analysis of variance (ANOVA) for non-parametric data (the TJC, SJC, and CD4+ T cell subset numbers; and routine blood, liver, and renal function data). We employed the χ2 test to compare proportions (sex). Clinical and immune system efficacy and safety were compared before and after IL-2 treatment using the Wilcoxon signed-rank test. We calculated Pearson correlations between CD4+ T subset numbers and disease activities. All p-values are two-tailed; p < 0.05 was considered to reflect statistical significance. We employed SPSS ver. 22.0 and GraphPad Prism ver. 6.0 software.
Results
Sex and mean age did not differ significantly between patients and controls (sex: χ2 = 0.001, p > 0.05; age: t = 0.344, p > 0.05) or among the 21 new-onset patients, the 74 treated patients, and the 106 healthy controls (sex: χ2 = 2.286, p > 0.05; age: F = 0.254, p > 0.05). A total of 22 patients received low-dose IL-2 combination treatment and completed the entire study. The baseline clinical characteristics of all patients, including age; sex; disease duration; DAS28-ESR index score; ESR; CRP level; VAS, PHGA, DLQI, and HAQ scores; and combination therapies are listed in Table 1 and Supplementary Table 1.
Table 1.
A summary of baseline demographics and disease characteristics of all enrolled patients.
| New-onset | Treated | All PsA patients | |
|---|---|---|---|
| n | 21 | 74 | 95 |
| Sex (female/male) | 8/13 | 42/32 | 50/45 |
| Age (years) | 46 ± 11.42 | 45 ± 11.76 | 44 ± 13.3 |
| Duration of disease (years), median (range) | 0.2 (0.10–11) | 3 (0.05–30) | 2 (0.05–30) |
| DAS28, median (range) | 4.5 (2.7–6.5) | 4.5 (2.2–6.5) | 4.5 (2.2–6.5) |
| TJC, median (range) | 8 (4–16) | 8 (2–12) | 8 (2–16) |
| SJC, median (range) | 2 (0–6) | 2 (0–4) | 2 (0–6) |
| ESR (mm/h), median (range) | 24 (3–100) | 24 (1–120) | 24 (1–120) |
| CRP (mg/l), median (range) | 4.21 (1.25–111) | 9.16 (1–165) | 8.49 (1–165) |
| VAS, median (range) | 7 (4–9) | 6 (3–9) | 7 (3–9) |
| PHGA, median (range) | 6 (4–8) | 6 (3–9) | 6 (3–9) |
| DLQI, median (range) | 12 (9–19) | 12 (6–18) | 12 (6–19) |
| HAQ, median (range) | 11 (7–17) | 10 (5–16) | 10 (5–17) |
| Use of concomitant agents (no. of patients) | |||
| Methotrexate | 6 | 24 | 30 |
| Leflunomide | 3 | 7 | 10 |
| Sulfasalazine | 1 | 4 | 5 |
| Cyclophosphamide | 0 | 8 | 8 |
| Thalidomide | 0 | 4 | 4 |
| Rapamycin | 3 | 14 | 17 |
| Tretinoin tablets | 14 | 20 | 34 |
| Biological agents | 12 | 56 | 68 |
| Total glucosides of paeony capsules | 1 | 21 | 22 |
CRP, C-reactive protein; DAS28, disease activity score 28S; DLQI, dermatology life quality index score; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire score; PHGA, physician’s global assessment; PsA, psoriatic arthritis; SJC, swollen joint count; TJC, tender joint count; VAS, visual analog (pain) score.
CD4+ T cell subset numbers
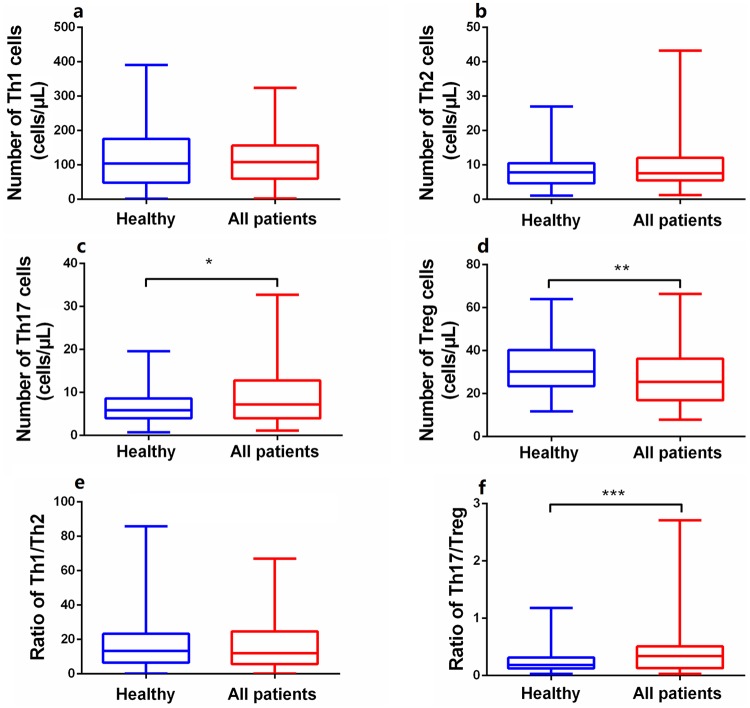
The absolute numbers and percentages of circulating CD4+ T subset cells in all subjects were derived via modified flow cytometry. PsA patients exhibited significant increases in the absolute number of Th17 cells (Z = 2.120, p < 0.05), significant reductions in Treg numbers (Z = 2.953, p < 0.05), and a higher Th17/Treg ratio (Z = 3.833, p < 0.001) (Figure 1) compared with normal controls. No significant difference in Th1 (Z = 0.237, p > 0.05) or Th2 (Z = 1.191, p > 0.05) cell numbers or the ratio (Z = 0.034, p > 0.05) was evident between patients and controls (Figure 1), indicating that Th17 cells and Tregs were more relevant to PsA pathogenesis than were Th1 or Th2 cells. In contrast, the proportions of other CD4+ T subsets did not differ significantly between patients and controls, unlike their absolute numbers (Supplementary Figure S1), indicating that the cell proportions did not completely parallel their absolute numbers. This suggested that reduced absolute numbers of Tregs affected PsA development.
Figure 1.
Comparison of the absolute numbers of peripheral CD4+T subsets between PsA patients (n = 95) and healthy controls (n = 106). (a, b, e) No statistically significant difference in the Th1or Th2 cell numbers or the ratio between the patients and healthy controls. (c, d, f) The levels of Th17 were significantly increased while that of Tregs reduced in PsA patients, resulting in a higher ratio of Th17/Treg, imbalance of pro- and anti-inflammation T cells. Data were calculated and compared with Mann–Whitney U test and are presented as median (range).
PsA, psoriatic arthritis; Tregs, regulatory T cells.
*p < 0.05, **p < 0.01, ***p < 0.001. Asymptotic significances (2-sided tests) were displayed. The significance level was p < 0.05.
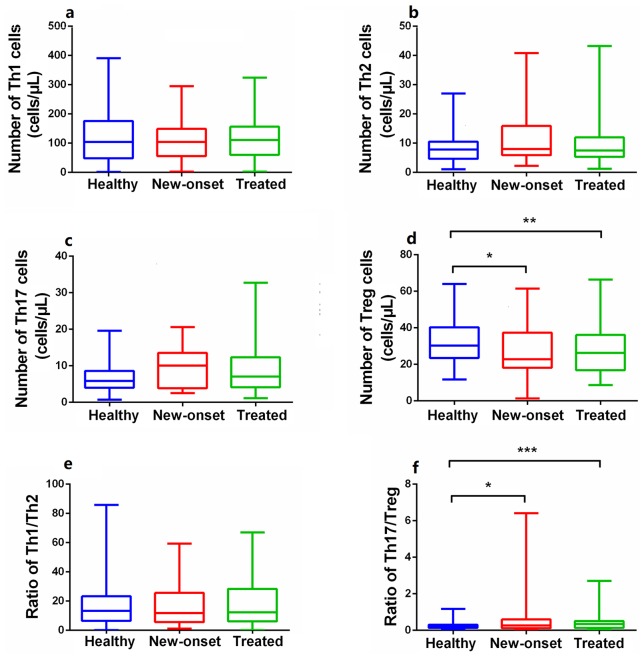
Peripheral CD4+ T cell subset numbers in new-onset and previously treated patients
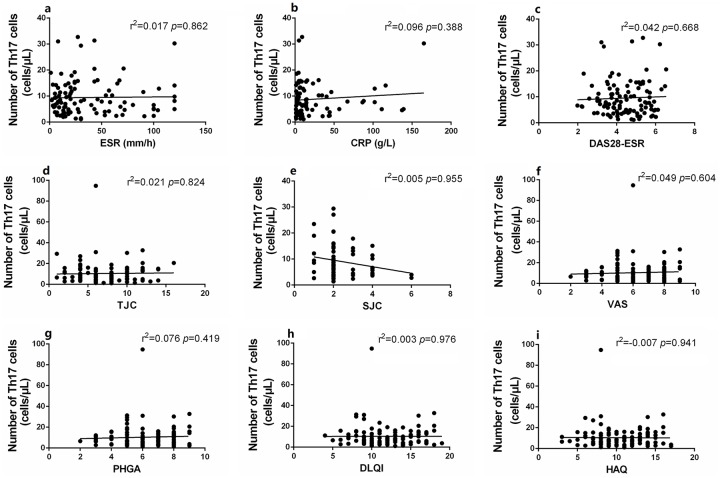
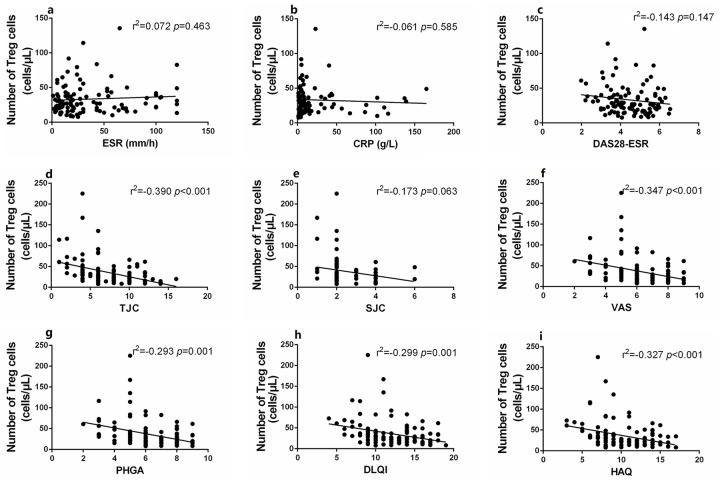
Compared with healthy controls, the absolute numbers of peripheral Tregs in new-onset PsA patients (Z = 2.174, p < 0.05) and patients on immunosuppressants (Z = 2.561, p < 0.05) were significantly reduced, but no significant difference in the level of Th1, Th2, or Th17 cells was apparent (all p > 0.05) (Figure 2). Notably, the levels (both percentages and absolute numbers) of CD4+ T subset cells did not differ significantly between new-onset and treated patients (all p > 0.05) (Figure 2, Supplementary Figure 2). The Treg numbers were significantly (negatively) correlated with many disease activity indicators, including the TJC and the VAS, PHGA, DLQI, and HAQ scores; the Th17 numbers were not so correlated (Figures 3 and 4). This suggested that a decrease in the absolute number of Tregs rather than Th17 cells played the more important role in PsA pathogenesis and disease activity.
Figure 2.
The differences of absolute numbers of CD4+T subgroups between PsA patients (including new-onset and treated) and healthy controls. (a, b, c, e) Absolute numbers (cells/µL) of Th1, Th2, and Th17 cells and the ratio of Th1/Th2 in PB were not significantly different among the healthy controls (n = 106), new-onset (n = 21), and previously treated patients (n = 74). (d, f) The absolute numbers of Tregs in PB were lower and the ratio of Th17/Treg was higher in PsA patients (including new and treated) than healthy controls, while there was no statistical difference between new-onset and treated patients in these indicators. Data are presented as median (range). Statistical analysis was performed using the Independent-Samples Kruskal–Wallis H test.
PsA, psoriatic arthritis; PB, peripheral blood; Tregs, regulatory T cells.
*p < 0.05, **p < 0.01, ***p < 0.001. Asymptotic significances (2-sided tests) were displayed. The significance level was p < 0.05.
Figure 3.
Correlation of the absolute numbers of Th17 with disease activities. (a–i) The absolute counts of Th17 were not significantly correlated with disease activity indicators including ESR (a), CRP (b), DAS28-ESR (c), TJC (d), SJC (e), VAS (f), PHGA (g), DLQI (h), HAQ (i). Statistical analysis was determined by Pearson coefficient.
CRP, C-reactive protein; DAS28-ESR, disease activity score on the 28-ESR instrument; DLQI, dermatology life quality index score; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire score; PHGA, physician global assessment; SJC, swollen joint count; TJC, tender joint count; VAS, visual analog (pain) score.
Figure 4.
Correlation of the absolute numbers of Tregs with disease activities. (a–i) The absolute numbers of Tregs were significantly negatively correlated with disease activity indicators including TJC (d), VAS (f), PHGA (g), DLQI (h), HAQ (i), but not with ESR (a), CRP (b), DAS28-ESR (c), SJC (e). Statistical analysis was determined by Pearson coefficient.
CRP, C-reactive protein; DAS28-ESR, disease activity score on the 28-ESR instrument; DLQI, dermatology life quality index score; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire score; PHGA, physician global assessment; SJC, swollen joint count; TJC, tender joint count; Tregs, regulatory T cells; VAS, visual analog (pain) score.
Clinical outcomes of IL-2 treatment
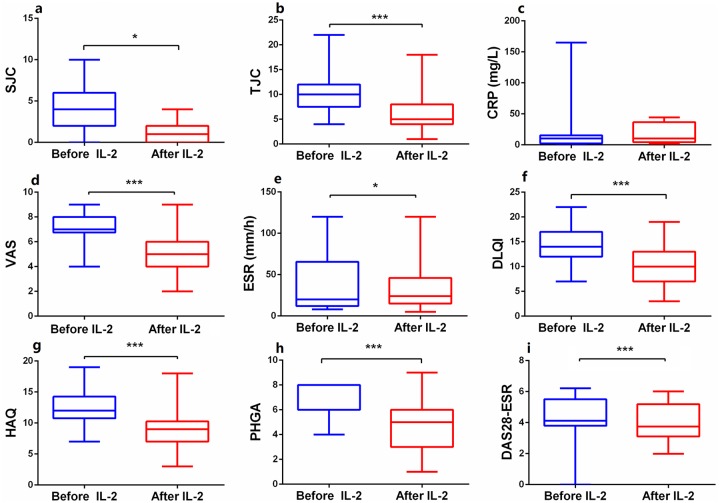
PsA patients who received IL-2 treatment for 5 days exhibited significant improvements in most disease activity measures: TJC (Z = 3.868, p < 0.05), SJC (Z = 2.295, p < 0.05), VAS (Z = 3.687, p < 0.05), ESR (Z = 2.511, p < 0.05), DAS28-ESR (Z = 3.360, p < 0.05), HAQ (Z = 3.612, p < 0.05), DLQI (Z = 3.750, p < 0.05), and PHGA (Z = 3.567, p < 0.05) (Figure 5).
Figure 5.
The changes of disease activities after low-dose IL-2 combination therapies. (a–i) The levels of disease activities, including SJC (a), TJC (b), VAS (d), ESR (e), DLQI (f), HAQ (g), PHGA (h), and DAS28-ESR (i) decreased significantly after IL-2 treatment, while CRP (c) showed no significant change. Within-group comparisons were made using the Wilcoxon test before (blue) and after (red) IL-2 therapy. *p < 0.05, ***p < 0.001. Asymptotic significances (2-sided tests) were displayed. The significance level was p < 0.05.
CRP, C-reactive protein; DAS28-ESR, disease activity score 28-ESR; DLQI, dermatology life quality index score; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire score; PHGA, physician’s GLobal assessment; SJC, swollen joint count; TJC, tender joint count; VAS, visual analog (pain) score.
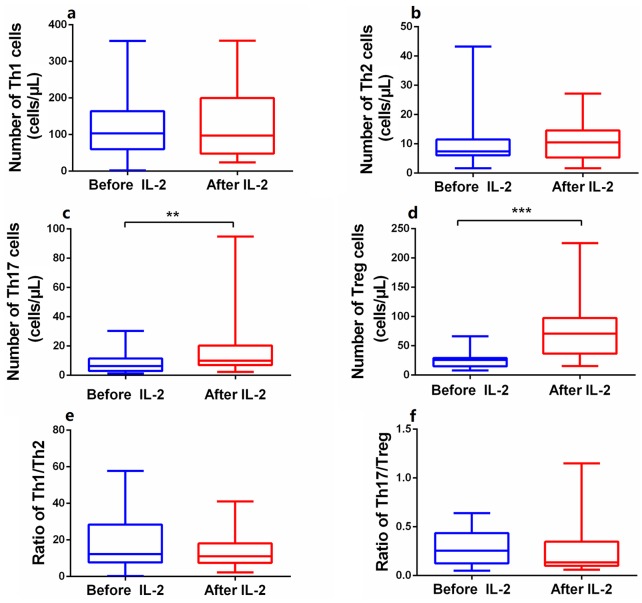
The effect of IL-2 treatment on CD4+ T lymphocyte numbers
The numbers of anti-inflammatory Tregs in the PB of patients treated with low-dose IL-2 rose dramatically from 25.5 ± 13.83 to 77.0 ± 51.26 cells/μL (Z = 3.95, p < 0.001) (Figure 6d, Supplementary Figure S3d). The absolute numbers of pro-inflammatory Th17 cells increased modestly from 7.7 ± 6.54 to 16.5 ± 19.39 cells/μL (Z = 2.81, p < 0.01) (Figure 6c, Supplementary Figure S3c). Thus, the Th17/Treg ratio tended to decrease, although statistical significance was not attained (Z = 0.82, p > 0.05) (Figure 6f). The levels of Th1 and Th2 cells, and the Th1/Th2 ratio, did not change significantly after IL-2 treatment (p > 0.05) (Figure 6a, b, and e; Supplementary Figure S3a and b).
Figure 6.
Efficacy of short-term and low-dose IL-2 treatment in regulating levels of CD4+T subsets. (a, b, e, f) Absolute numbers of Th1, Th2 and the ratio of Th1/Th2 and Th17/Treg in PB were no statistical difference after IL-2 treatment. (c, d) Absolute numbers of Th17 and Treg cells in PB, especially Tregs, were significantly increased in patients after IL-2 treatment. Within-group comparisons were made using the Wilcoxon test before (blue) and after (red) IL-2 therapy.
IL-2, interleukin-2; PB, peripheral blood; Tregs, regulatory T cells.
**p < 0.01, ***p < 0.001. Asymptotic significances (2-sided tests) were displayed. The significance level was p < 0.05.
The safety of low-dose IL-2 therapy
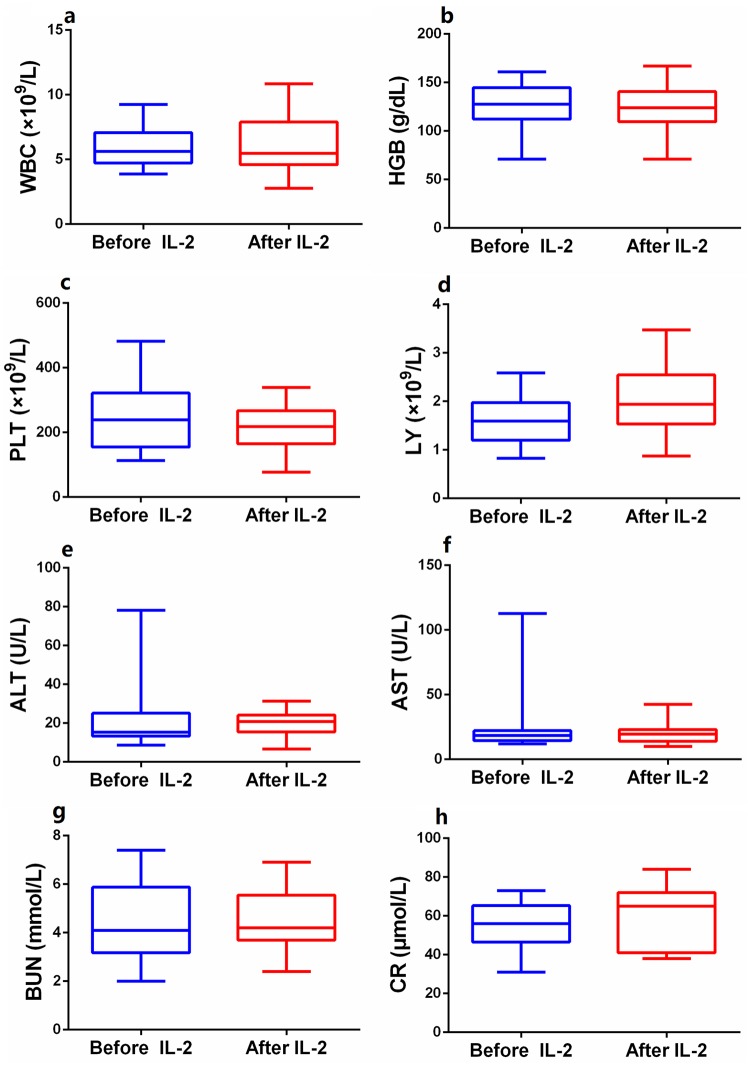
Of the 22 PsA patients who received low-dose IL-2 (0.5 MIU/day for 5 days), 1 developed a skin rash at the injection site on day 4. This healed spontaneously within 1 week. All blood, liver, and renal function data were within normal ranges (Figure 7).
Figure 7.
Safety outcomes of routine blood count, liver and kidney functions. The levels of white blood cell (a), hemoglobin (b), platelets (c), lymphocyte (d), alanine aminotransferase (e), aspartate aminotransferase (f), blood urea nitrogen (g), and serum creatinine (h) showed no significant difference before and after IL-2 treatment. Within-group comparisons were made using the paired-samples t test before (blue) and after (red) IL-2 therapy. Asymptotic significances (2-sided tests) were displayed. The significance level was p < 0.05.
IL-2, interleukin-2.
Discussion
Previous studies have suggested that increases in Th17 cells and pro-inflammatory cytokines play major roles in PsA immunopathology, and that immunosuppressive therapies are required.6,20 However, in patients with various autoimmune diseases, including PsA, Tregs play vital roles in immune system homeostasis and the prevention of autoimmunity development in both mice and humans.4 Tregs (CD4+CD25+Foxp3+ T cells) not only control the immune response, but also promote tissue homeostasis by suppressing inflammation and aiding tissue repair.21 It is clear that decreases in the absolute numbers of Tregs, or dysfunction of such cells, underlie many illnesses, not only those of the immune system.9–12 However, evidence supporting the suggestion that low Treg numbers play a critical role in PsA development is lacking.
We found fewer Tregs and more Th17 cells in the PB of PsA patients than controls; also, the absolute number of Tregs was significantly (negatively) correlated with PsA disease activity. The CD4+ T subset numbers (including those of Tregs) did not differ significantly between new-onset and treated patients, indicating that the decrease was associated with the disease per se rather than the use of glucocorticoids or immunosuppressive agents.22 This reduction may be increased by conventional therapies, especially immunosuppressive agents, increasing the risks of malignancy and infection.23–25
A recent meta-analysis found that the proportion of Tregs in patients with active SLE was lower than that in patients in remission, suggesting that SLE activity is related to a decrease in Treg numbers.26 Similarly, Miao et al.9 showed that a decrease in the absolute number of circulating CD4+ Tregs, rather than an increase in either the absolute number or proportion of Th17 cells, crucially affected the immune disorders of pSS patients; pSS is also a chronic, autoantigen-mediated inflammatory disease. Tregs inhibit the pro-inflammatory activity of macrophages, which are the principal immune effector cells in patients with TNF-mediated psoriasis.27 Thus, increasing Treg numbers may be therapeutically effective in PsA patients.
Although the cause of the decrease in the absolute number of peripheral Tregs in PsA patients remains unclear, it is possible that a relative deficiency of IL-2 is in play.28 IL-2, a cell growth factor, critically regulates the immune system balance by enhancing the survival and proliferation of various types of T cells.29 The pleiotropic functions of IL-2 depend on the dose; high-dose IL-2 enhances anti-cancer immune responses by activating effector T cells. Low-dose IL-2 increases Treg numbers and activates the cells; the therapeutic potential is thus broad.29 IL-2R signaling is required to control T cell activation, to maintain homeostasis, and to prevent the development of autoimmunity.30 Tregs express high levels of CD25 (IL-2Rα) and are thus sensitive to low-dose IL-2; effector T cells are not.
Restoration of IL-2 levels in patients with autoimmune diseases, thus increasing Treg numbers, may be therapeutically valuable. Some recent studies found that low-dose IL-2 alleviated some autoimmune disease activities by regulating the balances of lymphocyte subsets,9,31,32 which would be expected to inhibit PsA development. We found that low-dose IL-2 not only alleviated disease activity (the TJC and SJC; and the VAS, DAS28-ESR, DLQI, HAQ, and PHGA scores) but also increased the absolute numbers of certain CD4+ T cell subsets, notably Tregs. Of interest, although Th17 cell numbers also increased slightly after IL-2 treatment, the disease activity indices decreased significantly, confirming that a lack of Tregs, rather than an increase in Th17 cell numbers, may play an important role in PsA pathogenesis. Low-dose IL-2 rapidly increases Treg numbers, controlling pSS, SLE, RA, and AS disease activities.9,32
However, we gave IL-2 for only 1 week; long-term evaluation of efficacy and safety is required. We focused principally on a role for low-dose IL-2 in terms of enhancing peripheral Treg proliferation and remitting arthritis. However, our skin and rash clinical data were incomplete; we could not accurately score the extent of skin involvement. The lack of skin rash data is an important limitation; we will follow this clinical index carefully in future. We are also testing other methods that may maintain immune system competence in the long term; we seek to induce and reconstitute immune tolerance in immunodeficient patients.
Conclusion
We found that a decrease in the absolute number of Tregs, rather than a percentage fall or a higher level of Th17 cells, played a crucial role in PsA disease progression. Low-dose IL-2 injections combined with conventional therapies may rapidly alleviate PsA symptoms. This would not constitute over-treatment. We recorded no side effects, but the long-term benefits afforded by such immunoregulatory therapy require further clarification.
Supplemental Material
Supplemental material, supplementary_figure for The numbers of peripheral regulatory T cells are reduced in patients with psoriatic arthritis and are restored by low-dose interleukin-2 by Jia Wang, Sheng-Xiao Zhang, Yu-Fei Hao, Meng-Ting Qiu, Jing Luo, Yu-Yao Li, Chong Gao and Xiao-Feng Li in Therapeutic Advances in Chronic Disease
Footnotes
Author contributions: Study design and manuscript writing: JW and SZ. Data extraction, quality assessment, analysis, and interpretation of data: YH and MQ. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Xiao-Feng Li had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval and consent to participate: This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (2016 KY-007).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jia Wang  https://orcid.org/0000-0003-2181-0750
https://orcid.org/0000-0003-2181-0750
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jia Wang, Department of Rheumatology, the Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Sheng-Xiao Zhang, Department of Rheumatology, the Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Yu-Fei Hao, Department of Rheumatology, the Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Meng-Ting Qiu, Department of Rheumatology, the Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Jing Luo, Department of Rheumatology, the Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China.
Yu-Yao Li, Department of Rheumatology, Shanxi Li Xiaofeng Medical Groups, Taiyuan, Shanxi, China.
Chong Gao, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Xiao-Feng Li, Department of Rheumatology, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi 030001, China.
References
- 1. Zeng QY, Chen R, Darmawan J, et al. Rheumatic diseases in China. Arthritis Res Ther 2008; 10: R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chemin K, Gerstner C, Malmstrom V. Effector functions of CD4+ T cells at the site of local autoimmune inflammation-lessons from rheumatoid arthritis. Front Immunol 2019; 10: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerriets VA, Kishton RJ, Nichols AG, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 2015; 125: 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10: 490–500. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Chang C, Lu Q. Epigenetics of CD4+ T cells in autoimmune diseases. Curr Opin Rheumatol 2017; 29: 361–368. [DOI] [PubMed] [Google Scholar]
- 6. Ronholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Szentpetery A, Heffernan E, Gogarty M, et al. Abatacept reduces synovial regulatory T-cell expression in patients with psoriatic arthritis. Arthritis Res Ther 2017; 19: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell 2008; 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 9. Miao M, Hao Z, Guo Y, et al. Short-term and low-dose IL-2 therapy restores the Th17/Treg balance in the peripheral blood of patients with primary Sjogren’s syndrome. Ann Rheum Dis 2018; 77: 1838–1840. [DOI] [PubMed] [Google Scholar]
- 10. Waite JC, Skokos D. Th17 response and inflammatory autoimmune diseases. Int J Inflam 2012; 2012: 819467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beringer A, Noack M, Miossec P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol Med 2016; 22: 230–241. [DOI] [PubMed] [Google Scholar]
- 12. Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2010; 2: 52ra72. [DOI] [PubMed] [Google Scholar]
- 13. Sugiyama H, Gyulai R, Toichi E, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol 2005; 174: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 2010; 10: 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malek TR. The biology of interleukin-2. Annu Rev Immunol 2008; 26: 453–479. [DOI] [PubMed] [Google Scholar]
- 16. Minami Y, Kono T, Miyazaki T, et al. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol 1993; 11: 245–268. [DOI] [PubMed] [Google Scholar]
- 17. Asano T, Meguri Y, Yoshioka T, et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood 2017; 129: 2186–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012; 12: 180–190. [DOI] [PubMed] [Google Scholar]
- 19. Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 20. Benham H, Norris P, Goodall J, et al. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther 2013; 15: R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol 2013; 14: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitley NT, Day MJ. Immunomodulatory drugs and their application to the management of canine immune-mediated disease. J Small Anim Pract 2011; 52: 70–85. [DOI] [PubMed] [Google Scholar]
- 23. Pandya JM, Loell I, Hossain MS, et al. Effects of conventional immunosuppressive treatment on CD244+ (CD28null) and FOXP3+ T cells in the inflamed muscle of patients with polymyositis and dermatomyositis. Arthritis Res Ther 2016; 18: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hengstman GJ, van den Hoogen FH, van Engelen BG. Treatment of dermatomyositis and polymyositis with anti-tumor necrosis factor-alpha: long-term follow-up. Eur Neurol 2004; 52: 61–63. [DOI] [PubMed] [Google Scholar]
- 25. Zong M, Lundberg IE. Pathogenesis, classification and treatment of inflammatory myopathies. Nat Rev Rheumatol 2011; 7: 297–306. [DOI] [PubMed] [Google Scholar]
- 26. Zhang SX, Ma XW, Li YF, et al. The proportion of regulatory T cells in patients with systemic lupus erythematosus: a meta-analysis. J Immunol Res 2018; 2018: 7103219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leite Dantas R, Masemann D, Schied T, et al. Macrophage-mediated psoriasis can be suppressed by regulatory T lymphocytes. J Pathol 2016; 240: 366–377. [DOI] [PubMed] [Google Scholar]
- 28. Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J Biomed Biotechnol 2010; 2010: 740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 2015; 15: 283–294. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki H, Kundig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science 1995; 268: 1472–1476. [DOI] [PubMed] [Google Scholar]
- 31. Jeffery HC, Jeffery LE, Lutz P, et al. Low-dose interleukin-2 promotes STAT-5 phosphorylation, Treg survival and CTLA-4-dependent function in autoimmune liver diseases. Clin Exp Immunol 2017; 188: 394–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenzwajg M, Lorenzon R, Cacoub P, et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann Rheum Dis 2019; 78: 209–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplementary_figure for The numbers of peripheral regulatory T cells are reduced in patients with psoriatic arthritis and are restored by low-dose interleukin-2 by Jia Wang, Sheng-Xiao Zhang, Yu-Fei Hao, Meng-Ting Qiu, Jing Luo, Yu-Yao Li, Chong Gao and Xiao-Feng Li in Therapeutic Advances in Chronic Disease