Abstract
Background:
Following an attack of acute diverticulitis (AD), many patients continue to suffer from a complex of symptoms, titled ‘symptomatic uncomplicated diverticular disease (SUDD)’. To date, there is no validated clinical score for standardized assessment of patients with SUDD, thereby hampering the interpretation of observational studies and the conductance of clinical trials.
We aimed to develop a validated SUDD clinical score.
Methods:
Data from previous prospective study of patients after AD was used to devise the score’s first version. Validation was first performed using a focus group of patients after AD SUDD who underwent a structured cognitive personal interview. Thereafter, the diverticular clinical score (DICS) was applied for a second validation cohort. DICS scores of validation cohort were compared with physicians’ global assessment for disease severity and inflammatory markers.
Results:
In DICS second validation using 48 patients prospectively recruited after AD SUDD, a correlation matrix demonstrated strong correlation between total questionnaire’s score and the presence of elevated inflammatory markers (ρ = 0.84). Mean score in patients with elevated inflammatory markers compared with those without inflammation was 17.8 versus 6.2, respectively, p < 0.001. Cronbach’s α for measuring internal consistency was 0.91. DICS discriminated accurately between patients with/without active disease, as gauged by the physicians global assessment (area under the curve receiver operating characteristic = 0.989).
Conclusions:
Patients suffering from post-AD SUDD exhibit a wide range of symptoms. The newly developed DICS accurately and reproducibly quantitates SUDD-related symptom severity. The DICS may prove useful for monitoring SUDD in clinical practice and in research settings, as well as facilitating patient stratification and therapeutic decisions.
Keywords: acute diverticulitis, clinical score, diverticular disease, symptomatic uncomplicated diverticular disease
Introduction
Diverticulosis of the colon is a common condition in western societies; by the age of 85 years two-thirds of the population in western countries will have developed colonic diverticula.1,2 While most patients remain asymptomatic, a minor portion will suffer from diverticular disease, most commonly acute diverticulitis (AD) occurring in 10–25% of the patients2–6 or even less (up to 4% according to recent literature).7 In most patients, the disease course is mild, and does not recur.8,9
However, a substantial group of patients that experienced AD continue to suffer from recurrent abdominal pain, change of bowel habits and bloating in the absence of overt inflammation. This specific patient subgroup is defined as suffering from symptomatic uncomplicated diverticular disease (SUDD) after AD.10,11 This entity was recently defined by the International Consensus on Diverticulosis and Diverticular Disease11 as chronic inflammatory disease with prolonged chronic symptoms, high levels of systemic serum inflammatory markers, high levels of tissue inflammatory cytokine and chronic inflammatory infiltrates in the affected colonic tissue. Recent data suggest a prolonged subclinical inflammatory process as the underlying mechanism for post-AD SUDD. Patients with SUDD were found to have increased levels of fecal calprotectin,12 elevated expression of the proinflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β, and increased numbers of lymphocytes and lymphocyte aggregates in the affected mucosa.13,14 Moreover, several clinical studies demonstrated clinical improvement using anti-inflammatory therapies, such as 5 aminosalicylic acid or treatments modulating bacterial–mucosal crosstalk, such as rifaximin and probiotics.15–20
However, despite its often-prolonged relapsing–remitting course, a major gap in the study of SUDD is the absence of a validated clinical symptom score. Recent guidance from regulatory bodies such as the United States Food and Drug Administration (FDA) underscores the importance of patient reported outcomes (PROs) in assessment of disease status and response to therapy.21 The absence of validated standardized score of patient-reported clinical symptoms undermines the interpretation of different observational studies in the field of diverticular disease and hampers the conductance of clinical trials.
The present study aimed to develop a validated score for clinical symptoms associated with post-AD SUDD that will enable physicians to evaluate disease severity and its impact on patients’ quality of life (QoL) and ultimately contribute to future clinical research in the field of diverticula-associated disease.
Methods
A three-stage approach was implemented for score development. First, data from a previous long-term prospective study of 261 patients assessing their symptoms post-AD were retrieved.22 Data were reviewed and patients’ reported clinical symptoms were recorded and processed in order to preliminarily develop the post-AD SUDD clinical score items, designated to be included in the diverticular clinical score (DICS). Thereafter, a simplified questionnaire was designed based on the most frequent clinical symptoms reported. The questionnaire was developed coupled with a relevant validated questionnaire for pain assessment, the visual analog scale (VAS),23 and with reference to a model score, that is, inflammatory bowel disease activity (‘IBD control’).24 In order to validate the questionnaire, a pilot focus group of 20 patients afflicted by SUDD after a documented attack of AD was recruited and asked to fill out a questionnaire. Subsequently, a personal interview with these patients was conducted. All interviews were conducted by the same senior gastroenterologist (AL), and lasted 30–45 min. Structured feedback regarding proposed items was obtained from the patients by conducting two rounds of individual interviews. Interviews were executed based on the guidance issued by the National Institutes of Health Patient Reported Outcomes Measurement Information System (PROMIS) consortium to evaluate respondent perceptions about language, comprehensibility, ambiguity, and relevance of each draft item.25 We utilized a standard set of queries as published by the PROMIS network for structured cognitive interview questions, assessing five main domains of the survey:
Directions (e.g. How would you make the directions more clear/easy to understand?)
Items (e.g. In your own words, what do you think this question is asking?)
Domains (e.g. In your own words, what do you think this group of questions is asking about?)
Response choices (e.g. What do you think about the response choices? How would you make the response choices clearer or easier to understand?)
Overall assessment (e.g. Are there things that we forgot to ask about that you think are important? Overall thoughts/opinions of the questionnaire? Anything you would change in the questionnaire as a whole?).26
Following interviews with the first group of 10 patients, questions were revised and processed, to be employed on the second 10 patients’ group.
In the third and final stage, a validation cohort consisting of 48 patients, similarly experiencing a SUDD post-AD episode, were prospectively recruited. Patients operated for complicated diverticular disease were excluded. All patients attended regularly a specialized diverticular disease (DD) clinic at the Chaim Sheba Medical Center, a tertiary referral center in Israel. Patients were asked to fill out the questionnaire during a routine follow-up visit and DICS scores were derived. Scores were compared with the physician global assessment (PGA) for disease severity obtained during the visit and inflammatory markers, such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) or fecal calprotectin, obtained within 1 month of the clinic visit.
Definitions
AD was defined as lower abdominal pain, usually on the left side, fever, and leukocytosis coupled with abdominal computed tomography (CT) contrast-enhanced findings characteristic for AD.27,28 Before inclusion, all CT scans were re-examined by senior abdominal radiologist, and AD diagnosis was confirmed. CT criteria for AD included the presence of colonic diverticulae with thickening of the colonic wall of at least 5mm at the site of the diverticulum and pericolonic fat infiltration.28 AD was considered complicated by radiographic criteria if an evident abscess or extraluminal air or extraluminal contrast was evident on CT study, according to the criteria of Ambrosetti and colleagues.27
In accordance with current literature, SUDD was defined as a syndrome characterized by recurrent abdominal symptoms, mainly abdominal pain (prolonged painful episodes), bloating and altered bowel habits according to definitions in the Italian consensus conference for colonic diverticulosis and DD and to recent studies.10,29,30 Active disease was defined by current active symptoms, mainly prolonged recurrent abdominal pain more than three times a week that lasted for more than 1 h, which may or may not be accompanied by a change in bowel habits and bloating. The finding of elevated inflammatory markers (CRP, ESR or fecal calprotectin) was considered as an additional factor supporting the presence of active disease, but was not deemed necessary.12,13
PGA was determined following complete symptom evaluation and physical examination performed by one experienced senior physician. Disease was defined as active or nonactive. All patients included in the study were more than 6 months after the last documented event of AD, without current symptoms of AD according to PGA. Patients after severe complicated AD per CT (mainly abscess) were only included in the study if subsequent imaging showed resolution of the complication.
Patients with previous history of chronic abdominal pain (mainly IBS and endometriosis) were excluded from the study.
Questionnaire
The questionnaire consists of nine basic questions, assessing frequency, duration and severity of abdominal pain, bloating, tenesmus, change in bowel habits, missed planned activities, mood disturbances and desire for treatment. The full questionnaire is attached in Appendix 1.
Questionnaires were written in English and used in a Hebrew version after being validated by translation and back translation.
Ethical considerations
This study has been approved by the local (Chaim Sheba Medical Center) ethics committee, approval number 4290-17-SMC. Informed consent was obtained from all study participants.
Statistical analysis
The sample size calculation (see below) was carried out using: PASS 14 Power Analysis and Sample Size Software, 2015 (NCSS, LLC, Kaysville, UT, USA; ncss.com/software/pass).
The reliability of a questionnaire was evaluated using Cronbach’s α (coefficient α) with threshold of ≥0.70 for the questionnaire reliability.
Data analysis was carried out using the R language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/).
Power calculation
A sample of 50 participants each responding to nine items achieves 90% power to detect the difference between the coefficient α under the null hypothesis of 0.5 and the coefficient α under the alternative hypothesis of 0.75 using a two-sided F test with a significance level of 0.05. Sample size graph is shown in Figure 1.
Figure 1.
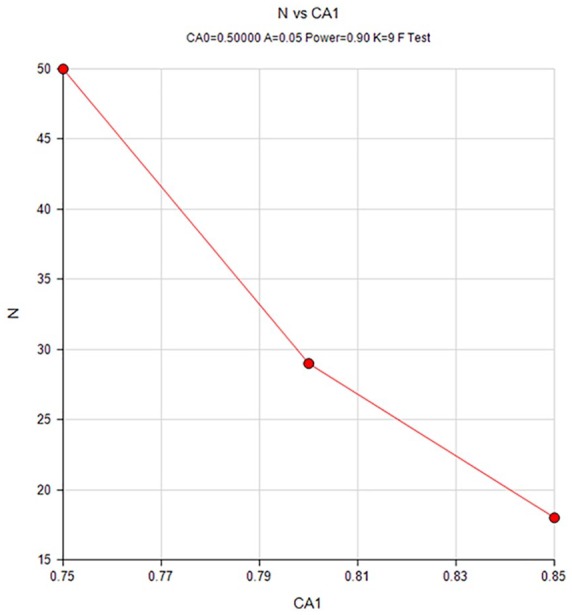
Sample size graph.
Reliability and construct validity calculation
The reliability of a questionnaire can be considered as the consistency of the survey results, as evaluated by its internal consistency, using Cronbach’s α. Cronbach’s α of at least 0.70 has been suggested to indicate adequate internal consistency.31 The construct validity of a questionnaire is evaluated by estimating its association with other variables, using a correlation matrix to examine the expected patterns of association between different measures of the same construct. It has been suggested that correlation coefficients of 0.1 should be considered as small, 0.3 as moderate, and 0.5 as large.32
Results
A review of a list of symptoms reported by 261 participants in a previous prospective post-AD study,20 yielded 15 possible clinical items. Of these items, the six most commonly reported items (abdominal pain, bloating, tenesmus, change of bowel habits, lack of energy, anxiety/depression) were chosen along with three severity items (nocturnal awakening, missed activities and ‘desire-for-therapy’). These were incorporated in the preliminary version of the DICS and question format was adapted after validation by personal interviews and scoring of a run-in preliminary cohort of 20 patients post-AD SUDD. Thereafter, as second validation, 48 consecutive patients post-AD SUDD filled out the questionnaire and received a DICS score. Patient baseline characteristics are shown in Table 1. Notably, 50% of patients had suffered from a complicated attack of AD.
Table 1.
Patient baseline characteristics.
| n | 48 |
| Sex (male) (%) | 20 (41.7) |
| Age at first AD attack [mean (SD)] | 55.17 (13.64) |
| (Range) | (29–82) |
| No. of past AD attacks [mean (SD)] | 3.08 (1.92) |
| (Range) | (1–10) |
| Complicated AD (%) | 24 (50%) |
| Time since last documented AD attack (months) (SD) | 23.75 (12.9) |
| (Range) | (60–6) |
| Comorbidities n (%) | |
| Autoimmune disease | 5 (10.4) |
| Cardiovascular disease | 16 (33) |
| Endocrine disorders | 7 (14.5) |
| Malignancy | 4 (8) |
| No comorbidities | 20 (41.7) |
| Concomitant medications n (%) | |
| Aspirin/NSAIDs | 10 (20.8) |
| Othera | 19 (39.5) |
| None | 18 (37.5) |
| Previous abdominal operationsb n (%) | 10 (20.8) |
Other medications (groups): thyroid replacement hormones, beta-blockers, statins, calcium channel blockers, proton pump inhibitors, oral diabetic treatment.
any laparotomy.
AD, acute diverticulitis; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation.
All patients included in this study that suffered from malignant disease in the past were more than 5 years after malignancy and considered cured. Their malignant diseases were: three patients with breast carcinoma after lumpectomy with no distant metastases; and one patient with carcinoma of the thyroid, after thyroidectomy with no distant metastases. Patients with past history of abdominal operation were included after at least 10 years after the procedure. Operations categories were: (n = 1) bariatric surgery (laparoscopic gastric banding), (n = 1) laparoscopic surgery for perforated duodenal ulcer, (n = 3) laparoscopic cholecystectomy, (n = 2) appendectomy, and (n = 3) Caesarian surgery.
Since four patients filled the survey twice on two different visits (one during disease exacerbation and one during disease remission), a total of 52 questionnaires were eligible and constituted the analysis dataset for this study. PGA based upon clinic visit history taking of clinical symptoms and physical examination was recorded and compared with questionnaire results. Patients were classified as either being in clinical remission or as experiencing a disease exacerbation at the time of the assessment, according to PGA. A total of 25 patients were on clinical remission, 24 suffered from active disease and 3 patients were classified as suffering from moderate disease activity according to PGA (based on a moderate severity of abdominal discomfort symptoms). A descriptive table of patient answers is shown in Table 2. Elevated inflammatory markers were measured in 22 patients (44%). The number of previous AD attacks was 2.65 ± 2.1 for patients in clinical remission compared with 3.05 ± 1.3 for patients in disease exacerbation (p value was nonsignificant).
Table 2.
Patient response to questionnaire.
| Demographic and clinical characteristics | Overall |
|---|---|
| n | 52 |
| Age [mean (SD)] | 59.02 (13.43) |
| Sex (male) (%) | 21 (40.4) |
| Disease activity according to PGA (%) | |
| remission | 25 (48.1) |
| active disease | 24 (46.2) |
| Moderate disease activity | 3 (5.8) |
| Abdominal pain, frequency (%) | |
| 1 Less than once a week | 18 (34.6) |
| 2 1–2 times a week | 9 (17.3) |
| 3 3–6 times per week | 7 (13.5) |
| 4 Daily | 18 (34.6) |
| Abdominal pain, duration (%) | |
| 1 <30 min | 17 (32.7) |
| 2 0.5 h to 1 h | 7 (13.5) |
| 3 1–6 h | 6 (11.5) |
| 4 >6 h | 22 (42.3) |
| Abdominal pain, severity (%) | |
| 0 No pain | 12 (23.1) |
| 1 Mild pain | 8 (15.4) |
| 2 Moderate pain | 7 (13.5) |
| 3 Severe pain | 4 (7.7) |
| 4 Very severe pain | 21 (40.4) |
| Number of additional symptoms (%) (choose any of the following: bloating, tenesmus, change in bowel habits) | |
| 0 | 11 (21.2) |
| 1 | 18 (34.6) |
| 2 | 6 (11.5) |
| 3 | 17 (32.6) |
| Missed activities during last 2 weeks (%) | 24 (46.2) |
| Woken up at night during last 2 weeks (%) | 23 (44.2) |
| Experienced lack of energy in last 2 weeks (%) | 32 (61.5) |
| Felt anxious or depressed in last 2 weeks (%) | 24 (46.2) |
| Felt the need to change treatment in last 2 weeks (%) | 23 (44.2) |
PGA, physician global assessment; SD, standard deviation.
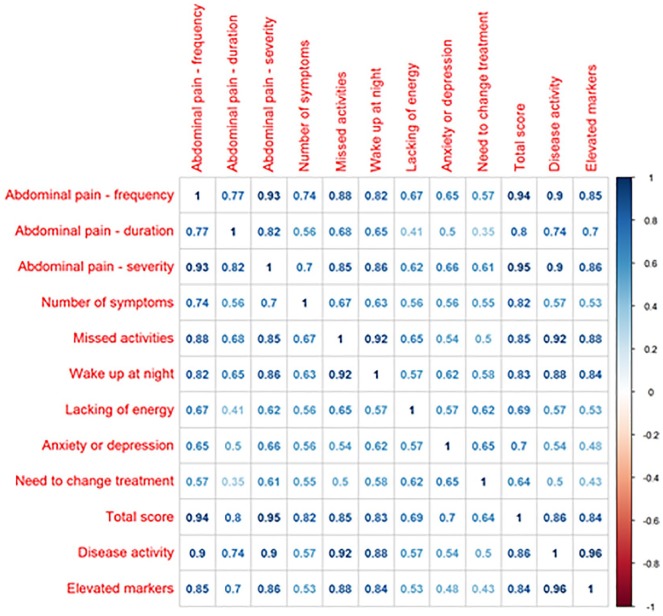
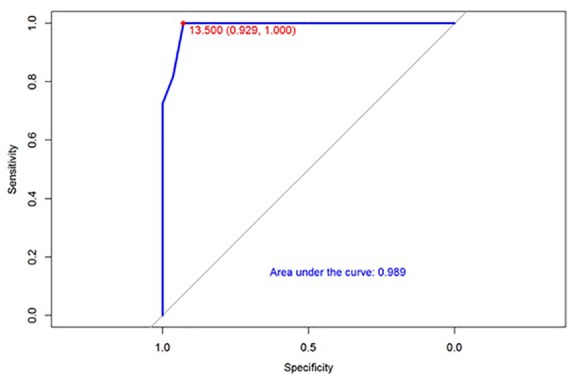
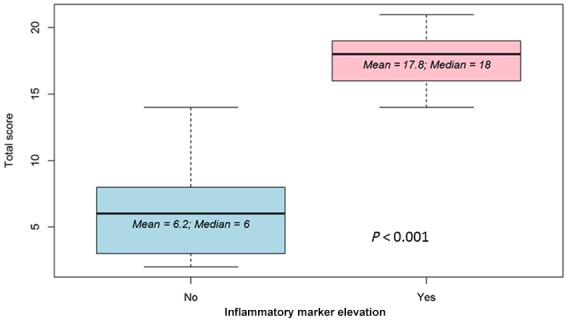
Initial questionnaire validation was performed by calculating the reliability and the validity of the results. The reliability was calculated using Cronbach α and yielded high internal consistency with a Cronbach’s α of 0.91. [(α) = 0.91 with mean score (standard deviation) = 1.27 (0.77)]. The construct validity was evaluated by the correlation between single questions and total score with disease severity and inflammatory markers (Figure 2). Notably, this correlation matrix demonstrated a very strong correlation between the total score and the elevation of inflammatory markers (ρ = 0.84). Correlation between total score and inflammatory markers is shown graphically in Figure 4. Next, the ability of the score scale to discriminate between individuals with or without active disease, as defined by PGA, was assessed by receiver operating characteristic analysis. This validation step showed an area under the curve of 0.989, that is, very accurate discriminatory accuracy to differentiate between disease activity states (Figure 3).
Figure 2.
Correlation between single questions and total score to disease severity and inflammatory markers.
Figure 4.
Total score predicting marker elevation.
Figure 3.
Total score by inflammatory marker elevation.
On a separate sensitivity analysis of the 52 cases, there was no difference in the DICS at the current assessment, and the original classification of the AD attack into either complicated AD (n = 24) or noncomplicated AD (n = 24, p value not significant).
Discussion
Following an episode of AD, a substantial subset of patients continue to suffer from SUDD manifesting as recurrent attacks of abdominal pain, change in bowel habits, bloating and diminished QoL in the absence of unequivocal symptoms of a full AD attack.10,33–37 Studies assessing variety of treatments for their potential efficacy in SUDD have addressed symptom improvement as a major therapeutic goal.38–47 A recently published prospective randomized trial comparing elective sigmoidectomy with conservative management in patients with recurrent symptoms following an attack of AD further support these findings.48 The study assessed patient symptoms and QoL at 5 years of follow up. Patient outcome was measured using a general QoL questionnaires [SF-36, VAS, EQ5D and Gastrointestinal Quality of Life Index (GIQLI)].23,49–53 Results showed a significantly increased QoL following elective sigmoidectomy due to symptomatic improvement. In this study, and in line with our previous prospective long-term observational study,22 the majority of patients had ongoing abdominal symptoms at inclusion, (59% and 68% in conservative versus surgical management groups, respectively).
However, to the best of our knowledge, none of the aforementioned studies used a validated symptom-based post-AD SUDD disease-specific questionnaire in order to determine disease activity (as has been customary for many years in other chronic intestinal disorders, such as Crohn’s disease and ulcerative colitis).54–56 Indeed, this gap was acknowledged by many of the expert authors of the previously cited studies, who emphasized the need for disease-specific clinical scores in order to perform validated and uniform data collection. Accordingly, disease-specific QoL questionnaire-based scoring was recently published.36 Another important progress in the field was a recently developed endoscopic score for grading mucosal inflammation associated with DD (the DICA classification). This endoscopic score assess endoscopic disease severity and was found to predict occurrence/recurrence of disease complications.57 However, the DICA is an exclusively endoscopic score without clinical components included. Therefore, the need for a simplified clinical score to be used during routine clinic follow-up visits as well as in the research arena still remains unmet.
Herein, we developed and validated a simplified disease-specific questionnaire-based clinical score for post-AD SUDD. DICS was developed in accordance with accepted standards recommended by the United States FDA and PROMIS,25,26,58 and proved to be accurate in disease assessment within an additional validation cohort of 48 patients. The DICS also differentiated with high accuracy between patients with active disease and patients with quiescent disease, as evident from the area under curve of 0.989 on the receiver operating characteristic analysis (Figure 2).
As shown in Table 1, patient population was diverse with regard to age, background comorbidities and concomitant medications. Disease-specific data were complete and available for all patients, and varied widely between patients. These features ensure representation of diverse SUDD subsets of patients, thereby increasing the generalizability of the DICS and lending further support to its clinical validity and relevance, notwithstanding that the number of patients in this cohort is only modest. Notably, 50% of our patients post-AD SUDD had suffered from complicated AD at the initial attack, a much higher proportion form the known complication rate (10–15%),59 further supporting the relation between severe initial inflammation to continuous symptoms and chronic inflammatory disease.
Moreover, the association between elevated inflammatory markers and disease activity strengthens our results and supports the chronic inflammatory nature of post-AD SUDD. Our results are in line with previous studies that confirmed the presence of elevated inflammatory markers in many patients with SUDD and support the inflammatory mechanism behind the symptoms.11–14,60–62 On the other hand, recent data suggest that DD per se is not associated with underlying inflammation.62,63 However, these studies assessed an asymptomatic screening colonoscopy population who were found to have incidental diverticulosis during colonoscopy, and did not assess symptomatic patients post-AD, that is, SUDD. Therefore, their findings do probably not apply to our patient population.
Our study had a few limitations. First, this was a modest-size study, although sample size calculation supports its sufficient power (see sample size graph, Figure 3). Nonetheless, the small number of patients with moderate disease activity precludes firm conclusions to be drawn. Another limitation was the study being single center and conducted in Hebrew. The score presented herein was developed in English, translated and back-translated as mandated by standard procedure for language validity verification. Nonetheless, as true for all questionnaires, corroboration of the findings by additional studies is warranted for other native-tongue populations. Another potential limitation is the effect of patients’ past medical history (specifically, past abdominal operations) on their current symptoms. In order to avoid this potential bias we only included patients who were more than a decade past their operation, and only patients after upper abdomen operations or localized lower abdominal operations (Caesarian section and appendectomy).
In conclusion, we developed and validated a simplified SUDD-specific disease activity questionnaire-based patient-reported clinical score (DICS), which demonstrated high accuracy for discriminating between symptomatic and quiescent patients as well as between different grades of disease activity. If corroborated by additional studies, this validated disease-specific score may improve data collection and promote clinical research on SUDD, as well as facilitate treatment outcome assessment, thereby providing more tools for both patients and physicians during decision making throughout the SUDD disease course.
Supplemental Material
Supplemental material, Appendix_1_1 for Development and validation of a diverticular clinical score for symptomatic uncomplicated diverticular disease after acute diverticulitis in a prospective patient cohort by Adi Lahat, Herma H Fidder and Shomron Ben-Horin in Therapeutic Advances in Gastroenterology
Acknowledgments
AL conceived the study; AL and SBH designed the study and drafted the manuscript; HF was involved in analysis and interpretation of data. All authors also participated in critical revision of the manuscript for important intellectual property and all approved the final draft submitted.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Adi Lahat  https://orcid.org/0000-0003-1513-7280
https://orcid.org/0000-0003-1513-7280
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Adi Lahat, Department of Gastroenterology, Sheba Medical Center, Sackler School of Medicine, Tel-Aviv University, Tel Hashomer, Tel-Aviv 52621, Israel.
Herma H Fidder, Department of Gastroenterology and Hepatology, University Medical Center Utrecht, GA Utrecht, Netherlands.
Shomron Ben-Horin, Department of Gastroenterology, Sheba Medical Center, Sackler School of Medicine, Tel Hashomer, Tel Aviv University, Israel.
References
- 1. Hughes LE. Postmortem survey of diverticular disease of the colon. Gut 1969; 10: 336–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia G. Diverticulitis. In: Blaser MT, Smith DD, Ravdin JI, et al. (eds) Infections of the gastrointestinal tract. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2002, pp. 306–316. [Google Scholar]
- 3. Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol 1975; 4: 53–69. [PubMed] [Google Scholar]
- 4. Painter NS, Burkitt DP. Diverticular disease of the colon, a 20th century problem. Clin Gastroenterol 1975; 4: 3–21. [PubMed] [Google Scholar]
- 5. Farrell RJ, Farrell JJ, Morrin MM. Diverticular disease in the elderly. Gastroenterol Clic North Am 2001; 30: 475–496. [DOI] [PubMed] [Google Scholar]
- 6. Wong WD, Wexner SD, Lowry A, et al. Practice parameters for the treatment of sigmoid diverticulitis- supporting documentation. Dis Colon Rectum 2000; 43: 289–297. [DOI] [PubMed] [Google Scholar]
- 7. Shahedi K, Fuller G, Bolus R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol 2013; 11: 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaiser AM, Jiang JK, Lake JP, et al. The management of complicated diverticulitis and the role of computed tomography. Am J Gastroenterol 2005; 100: 910–917. [DOI] [PubMed] [Google Scholar]
- 9. Daniels L, Ünlü Ç, de Korte N, et al. ; Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg 2017; 104: 52–61. [DOI] [PubMed] [Google Scholar]
- 10. Cuomo R, Barbara G, Pace F, et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United European Gastroenterol J 2014; 2: 413–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tursi A, Brandimarte G, Di Mario F, et al. International consensus on diverticulosis and diverticular disease. Statements from the 3rd international symposium on diverticular disease. J Gastrointestin Liver Dis 2019; 28(Suppl. 4): 57–65. [DOI] [PubMed] [Google Scholar]
- 12. Tursi A, Brandimarte G, Elisei W, et al. Faecal calprotectin in colonic diverticular disease: a case-control study. Int J Colorectal Dis 2009; 24: 49–55. [DOI] [PubMed] [Google Scholar]
- 13. Tursi A, Elisei W, Brandimarte G, et al. Mucosal tumour necrosis factor α in diverticular disease of the colon is overexpressed with disease severity. Colorectal Dis 2012; 14: e258–e263. [DOI] [PubMed] [Google Scholar]
- 14. Lahat A, Necula D, Yavzori M, et al. Prolonged recurrent abdominal pain is associated with ongoing underlying mucosal inflammation in patients who had an episode of acute complicated diverticulitis. J Clin Gastroenterol. Epub ahead of print 19 January 2018. DOI: 10.1097/MCG.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 15. Bianchi M, Festa V, Moretti A, et al. Meta-analysis: long-term therapy with rifaximin in the management of uncomplicated diverticular disease. Aliment Pharmacol Ther 2011; 33: 902–910. [DOI] [PubMed] [Google Scholar]
- 16. Kruis W, Meier E, Schumacher M, et al. ; German SAG-20 Study Group. Randomised clinical trial: mesalazine (Salofalk granules) for uncomplicated diverticular disease of the colon – a placebo-controlled study. Aliment Pharmacol Ther 2013; 37: 680–690. [DOI] [PubMed] [Google Scholar]
- 17. Tursi A, Brandimarte G, Elisei W, et al. Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease – a double-blind, randomised, placebo-controlled study. Aliment Pharmacol Ther 2013; 38: 741–751. [DOI] [PubMed] [Google Scholar]
- 18. Lahner E, Esposito G, Zullo A, et al. High-fiber diet and Lactobacillus paracasei B21060 in symptomatic uncomplicated diverticular disease. World J Gastroenterol 2012; 18: 5918–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colecchia A, Vestito A, Pasqui F, et al. Efficacy of long term cyclic administration of the poorly absorbed antibiotic rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol 2007; 13: 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campanini A1, De Conto U, Cavasin F, et al. A primary-care interventional model on the diverticular disease: searching for the optimal therapeutic schedule. J Clin Gastroenterol 2016; 50(Suppl. 1): S93–S96. [DOI] [PubMed] [Google Scholar]
- 21. US Department of Health and Human Services FDA Center for Drug Evaluation and Research; US Department of Health and Human Services FDA Center for Biologics Evaluation and Research and US Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006; 4: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lahat A, Avidan B, Sakhnini E, et al. Acute diverticulitis: a decade of prospective follow-up. J Clin Gastroenterol 2013; 47: 415–419. [DOI] [PubMed] [Google Scholar]
- 23. Huskisson EC. Measurement of pain. Lancet 1974; 2: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 24. Bodger K, Ormerod C, Shackcloth D, et al. ; IBD Control Collaborative. Development and validation of a rapid, generic measure of disease control from the patient’s perspective: the IBD-control questionnaire. Gut 2014; 63: 1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeWalt DA, Rothrock N, Yount S, et al. Evaluation of item candidates: the PROMIS qualitative item review. Med Care 2007; 45: S12–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irwin DE, Varni JW, Yeatts K, et al. Cognitive interviewing methodology in the development of a pediatric item bank: a patient reported outcomes measurement information system (PROMIS) study. Health Qual Life Outcomes 2009; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ambrosetti P, Becker C, Terrier F. Colonic diverticulitis: impact of imaging on surgical management—a prospective study of 542 patients. Eur Radiol 2002; 12: 1145–1149. [DOI] [PubMed] [Google Scholar]
- 28. Tursi A, Brandimarte G, Giorgetti G, et al. The clinical picture of uncomplicated versus complicated diverticulitis of the colon. Dig Dis Sci 2008; 53: 2474–2479. [DOI] [PubMed] [Google Scholar]
- 29. Annibale B, Lahner E, Maconi G, et al. Clinical features of symptomatic uncomplicated diverticular disease: a multicenter Italian survey. Int J Colorectal Dis 2012; 27: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 30. Salem TA, Molloy RG, O’Dwyer PJ. Prospective, five years follow-up study of patients with symptomatic uncomplicated diverticular disease. Dis Colon Rectum 2007; 50: 1460–1464. [DOI] [PubMed] [Google Scholar]
- 31. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951; 16: 297–334. [Google Scholar]
- 32. Bolarinwa OA. Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Niger Postgrad Med J 2015; 22: 195–201. [DOI] [PubMed] [Google Scholar]
- 33. Cuomo R, Barbara G, Andreozzi P, et al. Symptom patterns can distinguish diverticular disease from irritable bowel syndrome. Eur J Clin Invest 2013; 43: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 34. Bolster LT, Papagrigoriadis S. Diverticular disease has an impact on quality of life – results of a preliminary study. Colorectal Dis 2003; 5: 320–323. [DOI] [PubMed] [Google Scholar]
- 35. Comparato G, Fanigliulo L, Aragona G, et al. Quality of life in uncomplicated symptomatic diverticular disease: is it another good reason for treatment? Dig Dis 2007; 25: 252–259. [DOI] [PubMed] [Google Scholar]
- 36. Spiegel BM, Reid MW, Bolus R, et al. Development and validation of a disease-targeted quality of life instrument for chronic diverticular disease: the DV-QOL. Qual Life Res 2015; 24: 163–179. [DOI] [PubMed] [Google Scholar]
- 37. Van Dijk ST, Daniels L, de Korte N, et al. Quality of life and persistent symptoms after uncomplicated acute diverticulitis. Dis Colon Rectum 2019; 62: 608–614. [DOI] [PubMed] [Google Scholar]
- 38. Trepsi E, Colla C, Panizza P, et al. Therapeutic and prophylactic role of mesalazine (5-ASA) in symptomatic diverticular disease of the large intestine. 4 year follow-up results. Minerva Gastroenterol Dietol 1999; 45: 245–252. [PubMed] [Google Scholar]
- 39. Tursi A, Brandimarte G, Giorgetti GM, et al. Mesalazine and/or Lactobacillus casei in preventing recurrence of symptomatic uncomplicated diverticular disease of the colon: a prospective, randomized, open-label study. J Clin Gastroenterol 2006; 40: 312–316. [DOI] [PubMed] [Google Scholar]
- 40. Comparato G, Fanigliulo L, Aragona G, et al. Quality of life in uncomplicated symptomatic diverticular disease: is it another good reason for treatment? Dig Dis 2007; 25: 252–259. [DOI] [PubMed] [Google Scholar]
- 41. Comparato G, Fanigliulo L, Cavallaro LG, et al. Prevention of complications and symptomatic recurrences in diverticular disease with mesalazine: a 12-month follow-up. Dig Dis Sci 2007; 52: 2934–2941. [DOI] [PubMed] [Google Scholar]
- 42. Smith J, Humes D, Garsed K, et al. OC-119 Mechanistic randomized control trial of mesalazine in symptomatic diverticular disease. Gut 2012; 61: A51–A52. [Google Scholar]
- 43. Kruis W, Meier E, Schumacher M, et al. Randomised clinical trial: mesalazine (Salofalk granules) for uncomplicated diverticular disease of the colon—a placebo-controlled study. Aliment Pharmacol Ther 2013; 37: 680–690. [DOI] [PubMed] [Google Scholar]
- 44. Parente F, Bargiggia S, Prada A, et al. Intermittent treatment with mesalazine in the prevention of diverticulitis recurrence: a randomised multicentre pilot double-blind placebo-controlled study of 24-month duration. Int J Colorectal Dis 2013; 28: 1423–1431. [DOI] [PubMed] [Google Scholar]
- 45. Stollman N, Magowan S, Shanahan F, et al. A randomized controlled study of mesalamine afer acute diverticulitis: results of the DIVA trial. J Clin Gastroenterol 2013; 47: 621–629. [DOI] [PubMed] [Google Scholar]
- 46. Tursi A, Brandimarte G, Elisei W, et al. Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease - a double blind, randomised, placebo-controlled study. Aliment Pharmacol Ther 2013; 38: 741–751. [DOI] [PubMed] [Google Scholar]
- 47. Iannone A, Ruospo M, Wong G, et al. Mesalazine for people with diverticular disease: a systematic review of randomized controlled trials. Can J Gastroenterol Hepatol 2018; 2018: 5437135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bolkenstein HE, Consten ECJ, van der Palen J, et al. Long-term outcome of surgery versus conservative management for recurrent and ongoing complaints after an episode of diverticulitis. Ann Surg 2019; 269: 612–620. [DOI] [PubMed] [Google Scholar]
- 49. McHorney CA, Ware JE, Jr, Lu JF, et al. The MOS 36-item short-form health survey (SF-36). III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994; 32: 40–66. [DOI] [PubMed] [Google Scholar]
- 50. McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short form health survey (SF-36): psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31: 247–263. [DOI] [PubMed] [Google Scholar]
- 51. Ware JE. SF-36 health survey. Manual and interpretation guide. Boston: The Health Institute, New England Medical Center, 1993. [Google Scholar]
- 52. Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal quality of life index: development, validation and application of a new instrument. Br J Surg 1995; 82: 216–222. [DOI] [PubMed] [Google Scholar]
- 53. EuroQol Group. EuroQol: a new facility for the measurement of health related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 54. Harvey RF, Bradshaw JM. A simple index of Crohn’s disease activity. Lancet 1980; 315: 514. [DOI] [PubMed] [Google Scholar]
- 55. Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National cooperative Crohn’s disease study. Gastroenterology 1976; 70: 439–444. [PubMed] [Google Scholar]
- 56. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalcylic acid therapy for mildly to moderately active ulcerative colitis. N Eng J Med 1987; 317: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 57. Tursi A, Brandimarte G, Di Mario F, et al. Development and validation of an endoscopic classification of diverticular disease of the colon: the DICA classification. Dig Dis 2015; 33: 68–76. [DOI] [PubMed] [Google Scholar]
- 58. Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res 2009; 18: 115–123. [DOI] [PubMed] [Google Scholar]
- 59. Strate LL, Morris AM. Epidemiology, pathophysiology, and treatment of diverticulitis. Gastroenterology 2019; 156: 1282–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Simpson J, Scholefield JH, Spiller RC. Origin of symptoms in diverticular disease. Br J Surg 2003; 90: 899–908. [DOI] [PubMed] [Google Scholar]
- 61. Simpson J, Neal KR, Scholefield JH, et al. Patterns of pain in diverticular disease and the influence of acute diverticulitis. Eur J Gastroenterol Hepatol 2003; 15: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 62. Peery AF, Keku TO, Addamo C, et al. Colonic diverticula are not associated with mucosal inflammation or chronic gastrointestinal symptoms. Clin Gastroenterol Hepatol 2018; 16: 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Järbrink-Sehgal ME, Rassam L, Jasim A, et al. Diverticulosis, symptoms and colonic inflammation: a population-based colonoscopy study. Am J Gastroenterol 2019; 114: 500–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1_1 for Development and validation of a diverticular clinical score for symptomatic uncomplicated diverticular disease after acute diverticulitis in a prospective patient cohort by Adi Lahat, Herma H Fidder and Shomron Ben-Horin in Therapeutic Advances in Gastroenterology