Abstract
Background
Fibroblast growth factor receptors (FGFRs) play vital roles in the development and progression of human cancers. This study aimed to comprehensively understand the prognostic performances of FGFR1–4 expression in breast cancer (BC) by mining databases.
Material/Methods
The levels of FGFR1–4 expression in BC were analyzed by online databases, GEPIA (Gene Expression Profiling Interactive Analysis) and UALCAN. Survival analysis of FGFR1–4 was carried out by Kaplan-Meier plotter. GSE74146 was downloaded from Gene Expression Omnibus (GEO) and analyzed by GEO2R to screen the differentially expressed genes (DEGs) between FGFR2-silenced BC cells and control. Over-presentation for DEGs were done by Enrichr tool. Networks of DEGs were obtained by using Search Tool for the Retrieval of Interacting Genes (STRING) and Cytoscape software. Hub genes were identified by cytoHubba Cytoscape plugin.
Results
The online databases showed that FGFR1 was significantly downregulated whereas FGFR3 was upregulated in BC. Kaplan-Meier plotter demonstrated the upregulation of both FGFR1 and FGFR3 indicated favorable relapse free survival (RFS) whereas FGFR4 overexpression predicted unfavorable overall survival (OS) in BC patients. Importantly, our results showed FGFR2 overexpression robustly predicted favorable OS and RFS in BC. Further bioinformatics analysis of GSE74146 suggested FGFR2 mainly participated in regulating degradation and organization of the extracellular matrix and signaling of retinoic acid. Moreover, CXCL8, CD44, MMP9, and BMP7 were identified as crucial FGFR2-related hub genes.
Conclusions
Our study comprehensively analyzed the prognostic values of FGFR1–4 expression in BC and proposed FGFR2 might serve as a promising biomarker. However, the underlying mechanisms remain to be elucidated.
MeSH Keywords: Breast Neoplasms; Computational Biology; Receptor, Fibroblast Growth Factor, Type 2
Background
Breast cancer (BC) is one of the most common malignancies for women and is also responsible for enormous cancer-associated deaths among females worldwide [1]. In China, its incidence increased approximately 30%, and the related mortality has doubled over the past 30 years [2,3]. Notably, breast carcinoma exhibits significant heterogeneity. According to its histopathological features, BC has currently been classified into 4 distinct intrinsic subtypes including luminal A, luminal B, human epidermal growth factor receptor 2 (HER2) enriched and basal-like. Each BC subtype can be distinguished from the others based on gene cluster expression patterns [4]. Although the specific biomarkers are used to predict response to therapy and prognoses, the clinically useful prognostic parameters are still insufficient, and the underlying mechanism in the BC development is largely unknown. Thus, it is important to develop and broaden additional prognostic predictors.
Fibroblast growth factor receptors (FGFRs) belong to a subfamily of highly conserved receptor tyrosine kinases that mainly comprises 4 cell membrane bound receptor (FGFR1–4). FGFRs bind extracellular ligands to initiate a complex intracellular signaling cascades. FGFR signaling has been observed to participate in a wide spectrum of physiologic processes and pathological conditions in cell proliferation, apoptosis, migration, invasion, angiogenesis, and metastasis [5,6]. Genetic aberration of FGFRs genes occurred in various human malignancies including urothelial, breast, endometrial, and lung cancer [7]. Furthermore, the alterations of FGFRs genes have observed to be significantly correlated with different types of tumor. Take FGFR1 gene for example, the alteration of FGFR1 gene has been linked to head and neck carcinoma and lung cancer [8,9], whereas the aberration of FGFR3 have been observed to be correlated with bladder cancers [10]. Together, the aberrations of FGFRs have implicated in the development of different human tumors.
Based on the significance of FGFRs in various human malignancies, the association of aberrant FGFRs with breast carcinoma is not surprising. In BC, FGFRs have been shown to be frequently deregulated. Among the 4 FGFR family members, FGFR1 amplification has been demonstrated to be the most frequent genomic aberration and its amplification was found to occur in nearly 14% of BC patients with the hormone receptor-positive luminal B tumor [7,11,12]. Moreover, FGFR1 gene amplification has been demonstrated to be strongly correlated with shorter overall survival (OS) in ER-positive BC and the resistance to endocrine-based therapies [13–15]. In contrast to FGFR1 aberration, the amplification of other FGFRs family members (FGFR2–4) is less relevant [5]. Amplification of the FGFR3 and FGFR4 genes has been found in less than 1% and around 2.3% of BC patients, respectively [7]. As for FGFR2, its amplification has not been reported. Although these findings have exhibited the importance of FGFRs amplification in BC patients, the prognostic performance of FGFR1–4 expression have not been investigated in BC so far.
The rise of high throughput technologies as well as numerous public databases has recently allowed observational studies hold promise in enhancing our comprehensive understanding of tumors. In the field of oncology, great advance has been witnessed in the publication of observational analyses using the large public databases, which provide new tools for investigators to probe into questions. As aforementioned, the performances of FGFR1–4 expression in BC prognosis are still elusive, although the distinctive roles of FGFR1–4 in the development of various human cancers. Here, we systemically explored the prognostic values of FGFR1–4 expression in BC by mining public databases. Furthermore, we investigated the potential mechanism of key FGFRs to provide a comprehensive analysis of FGFR1–4 in BC. To our knowledge, this is the first study to report the prognostic values of FGFR1–4 in BC patients.
Material and Methods
Analysis of FGFR1–4 expression in BC within public databases
To analyze the levels of the FGFR1–4 messenger RNA (mRNA) in BC, the GEPIA (Gene Expression Profiling Interactive Analysis) and UALCAN databases were analyzed. GEPIA (http://gepia.cancer-pku.cn/) is a comprehensive and interactive web resource for analyzing cancer data, which includes 9736 tumors and 8587 normal samples from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) [16]. In our study, the levels of FGFR1–4 in BC were explored by using GEPIA database. Additionally, the UALCAN database was further used to compare the expression patterns of FGFR1–4 between various BC sub-groups. UALCAN is a comprehensive and interactive web resource to analyze gene expression and clinical data from TCGA database (http://ualcan.path.uab.edu) [17]. Finally, the FGFR1–4 expression at the protein level was mined by using the Human Protein Atlas (HPA) (https://www.proteinatlas.org/) [18]. In this study, immunohistochemistry (IHC) data for FGFRs were downloaded from the HPA in both normal breast and BC tissues. The levels of FGFRs protein were compared in normal breast tissues and BC tissues within the HPA database.
Kaplan-Meier analysis of FGFR1–4 expression in BC patients
To explore the associations between the levels of FGFR1–4 genes and patients’ clinical outcomes, we performed prognostic analysis within the Kaplan-Meier database (www.kmplot.com). Kaplan-Meier is an online public database to draw survival plots, which can be used to study the effects of gene expression on the clinical outcome in BC patients [19]. We used the database to assess the relationship between the levels of FGFR1–4 mRNA and patients’ survival. The log-rank P-value with <0.05 was considered as statistical significance.
Bioinformatics analysis of GSE74146
Based on the robust relativity between the FGFR2 transcriptional level and the patients’ survivals in BC, we analyzed the Gene Expression Omnibus (GEO) database to explore the potential mechanism of FGFR2 in the BC development. GEO database was search according to the following criteria, (FGFR2 [Description] AND breast cancer [Description]) AND “Homo sapiens”[porgn: __txid9606]). The GSE74146 dataset was obtained. GSE74146 is an expression dataset of human BC cell line MCF-7 upon silencing of FGFR2 with small interfering RNA (siRNA). The dataset was based on the GPL10558 Illumina Human HT-12V4.0 expression bead chip platform. The dataset consisted of 24 microarray samples from MCF-7 treated under different conditions at a time point of 6 hours. The raw data were download and analyzed by GEO2R tool to distinguish differentially expressed genes (DEGs) between FGFR2-silenced cells and control cell. An adjusted P-value <0.05 and |log2FC| >1.0 were used as the cutoff for DEGs [20]. A volcano plot was used to visualize the distribution of DEGs in GSE74146.
For the functional enrichment of FGFR2, DEGs were analyzed by Enrichr web tool (https://amp.pharm.mssm.edu/Enrichr) [21]. Gene Ontology (GO) enrichment terms included molecular functions (MF), biological processes (BP), and cellular components (CC) of genomic products. The enriched pathways included genomes (KEGG, Kyoto Encyclopedia of Genes and Genomes) pathways and REACTOME pathways. The enriched GO and pathways were visualized as a bar diagram. P<0.05 was considered significant difference.
The networks of DEGs was constructed by the Search Tool for the Retrieval of Interacting Genes (STRING) database was visualized by Cytoscape software (version 3.4.0). Additionally, the Cytoscape plugin cytoHubba were used to analyze the interaction of the DEGs and screen hub gene. The parameters of cytoHubba were set as follows: hubba nodes=top 10 nodes ranked by degree, display options=check the first-stage nodes, display the shortest path and display the expanded subnetwork.
Results
Differential expression of FGFR1–4 in BC
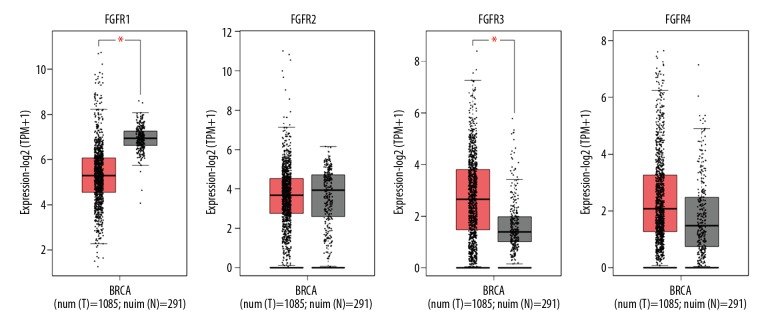
The 4 FGFR family members (FGFR1–4) were investigated in BC by the GEPIA online database. As shown in Figure 1, the level of FGFR1 mRNA was strongly lower in BC tissues (n=1085) than that in normal tissues (n=291). Conversely, FGFR3 was significantly upregulated in BC tissues. However, the other 2 members, FGFR2 and FGFR4, did not show differential expression between BC tissues and normal tissues (Figure 1).
Figure 1.
The expression profiles of FGFR1–4 genes in breast cancer within GEPIA database. Boxplot showed the FGFR1–4 expression in tumor tissues (T, red box, n=1085) and normal tissues (N, grey box, n=291). The asterisk (*) indicates statistical difference in comparison with normal tissues (P<0.01). FGFR1–4 – fibroblast growth factor receptors 1–4; GEPIA – Gene Expression Profiling Interactive Analysis.
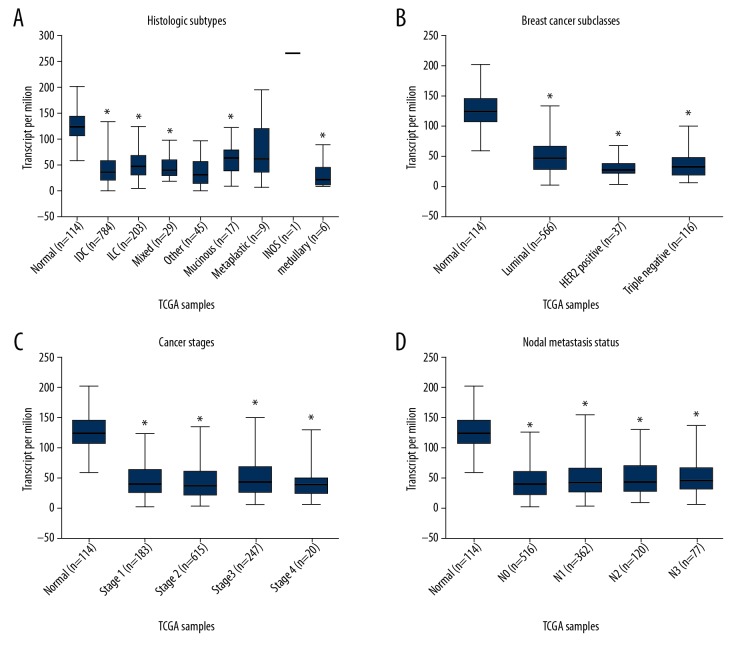
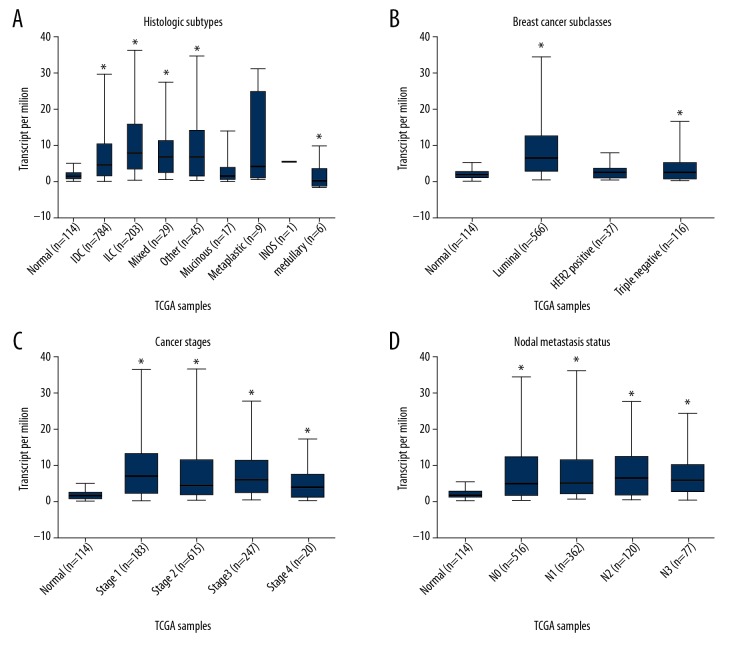
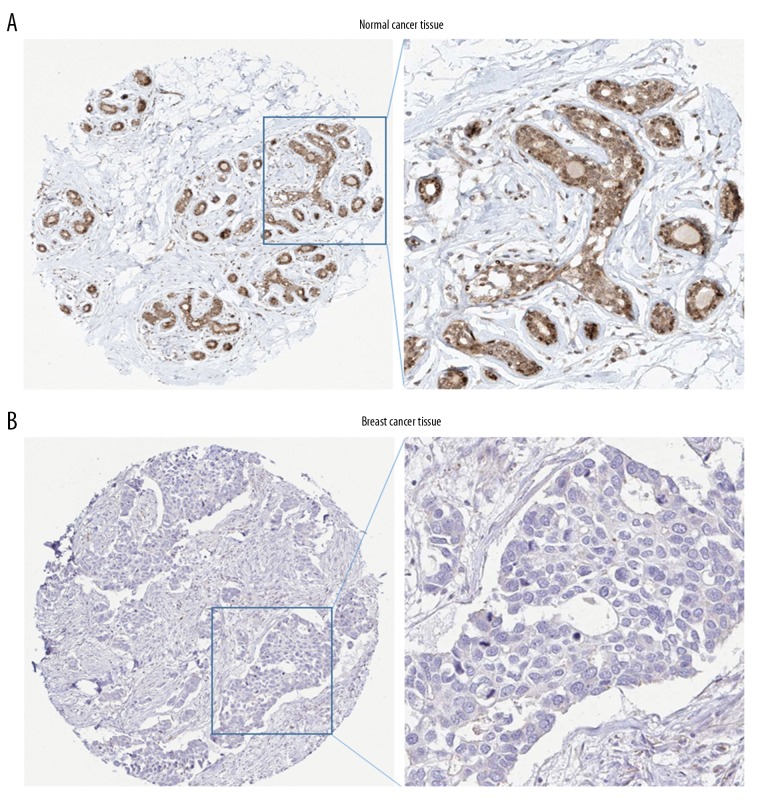
To further investigate the expression patterns of FGFR1 and FGFR3 in various BC subtypes, we analyzed the UALCAN online database. FGFR1 gene showed a relatively downregulated expression pattern nearly in all BC subtypes. When sorting the BC patients by histological subtypes, BC subclasses, cancer stages and nodal metastasis status, the levels of FGFR1 mRNA were still significantly decreased in all subgroups compared with normal control (Figure 2). Inversely, FGFR3 was found to exhibit almost an opposite expression pattern. FGFR3 was observed to be highly expressed in various BC subtypes including histological subtypes, BC subclasses, cancer stages and nodal metastasis status (Figure 3). To further verify the expression of FGFR1 and FGFR3 at the protein levels, we analyzed the immunostaining images from the HPA database. As shown in Figure 4, the level of FGFR1 protein was downregulated in BC tissues compared with normal breast tissues (Figure 4). Conversely, the protein expression of FGFR3 was obviously upregulated in BC tissues (Figure 5). Overall, given the data from multiple databases, FGFR1 could be downregulated in BC tissues compared with normal counterparts whereas FGFR3 could be upregulated in BC tissues. As for FGFR2 and FGFR4, the 2 FGFR members did not show significant differences between tumor tissues and normal controls.
Figure 2.
The FGFR1 expression based on clinical characteristics of breast cancer within UALCAN database. Boxplot showed the FGFR1 mRNA expression levels in tumor tissues with different clinical characteristics including histological subtypes (A), breast cancer subclasses (B), cancer stages (C) and nodal metastasis status (D). The asterisk (*) indicates statistical difference in comparison with normal tissues (P<0.01). FGFR1 – fibroblast growth factor receptors 1; mRNA – messenger RNA.
Figure 3.
The FGFR3 expression based on clinical characteristics of breast cancer within UALCAN database. Boxplot showed the FGFR3 mRNA expression levels in tumor tissues with different clinical characteristics including histological subtypes (A), breast cancer subclasses (B), cancer stages (C), and nodal metastasis status (D). The asterisk (*) indicates statistical difference in comparison with normal tissues (P<0.01). FGFR3 – fibroblast growth factor receptors 3; mRNA – messenger RNA.
Figure 4.
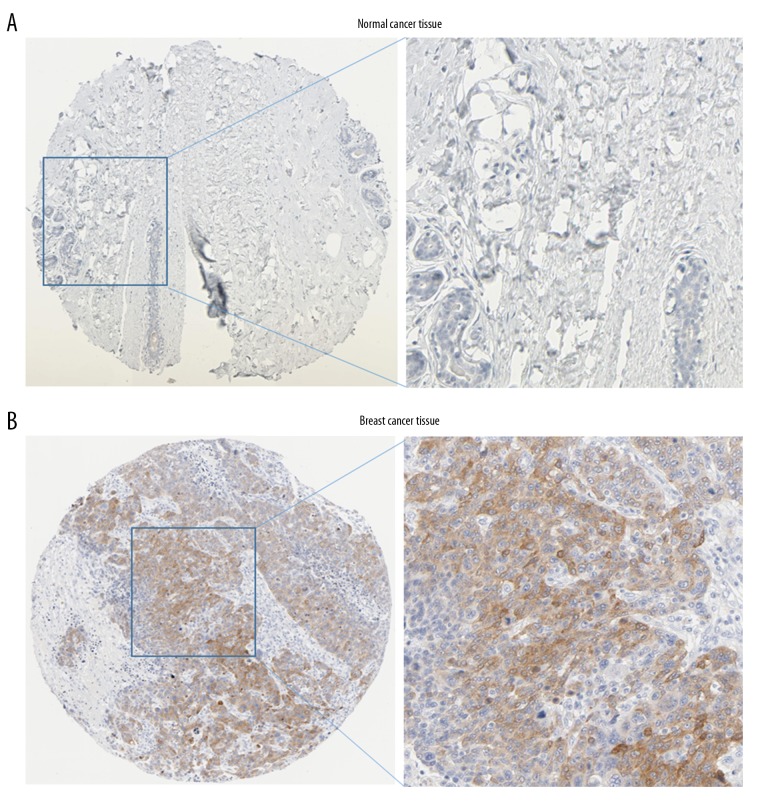
Immunohistochemistry analysis of FGFR1 expression in HPA database. Representative immunostaining images of FGFR1 in breast cancer: (A) normal breast tissue; (B) breast cancer tissues. FGFR1 – fibroblast growth factor receptors 1; HPA – Human Protein Atlas.
Figure 5.
Immunohistochemistry analysis of FGFR3 expression in HPA database. Representative immunostaining images of FGFR3 in breast cancer: (A) normal breast tissue; (B) breast cancer tissues. FGFR3 – fibroblast growth factor receptors 3; HPA – Human Protein Atlas.
The prognosis analysis of FGFR1–4
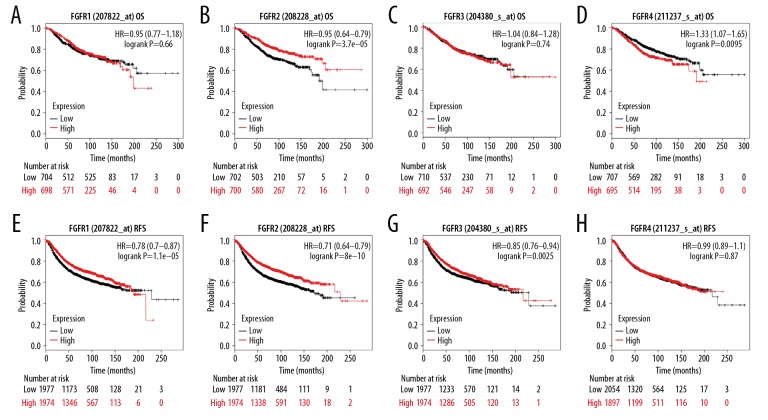
To date, it is still unknown about the possible prognostic value of FGFR1–4 expression in BC. Thus, survival analysis of FGFR1–4 was performed by online database Kaplan-Meier plotter. As for FGFR1, the levels of FGFR1 mRNA could not be considered a marker for OS in 1402 BC patients (hazard ratio [HR]=0.95; 95% confidence interval [CI]: 0.77–1.18, P-value=0.65, Figure 6A), but it could be regarded as an indicator for relapse-free survival (RFS) with a total of 3951 BC patients (HR=0.89; 95% CI: 0.8–0.99, P-value=0.034, Figure 6B). As for FGFR2, its median expression levels were observed to have a significant positive effect in the OS analysis (patients=1402, HR=0.66; 95% CI: 0.53–0.82, P-value=0.00012, Figure 6C). Moreover, median FGFR2 levels could be regarded as a significant biomarker for RFS with a total of 3951 BC patients (HR=0.78; 95% CI: 0.7–0.87, P-value=6.7e-06, Figure 6D). With regard to FGFR3, its median expression levels had no significant effect on the OS of patients (patients =1402, HR=1.04; 95% CI: 0.84–1.28, P-value=0.74, Figure 6E), whereas it exhibited an obvious positive effect on the RFS of patients (patients=3951, HR=0.85; 95% CI: 0.76–0.94, P-value=0.0025, Figure 6F). As for FGFR4, its median expression levels were observed to exhibit a significant negative effect on the OS of patients (patients=1402, HR=1.33; 95% CI: 1.07–1.65, P-value=0.0095, Figure 6G), whereas it had no effect on the RFS of patients (patients=3951, HR=0.99; 95% CI: 0.89–1.1, P-value=0.87, Figure 6H). In brief, only FGFR2 could be considered as a prognosis biomarker for BC compared with the other FGFR family members.
Figure 6.
Prognostic values of FGFR1–4 in breast cancer within the database Kaplan-Meier plotter. (A–D) OS and (E–H) RFS of FGFR1–4 were evaluated by using Kaplan-Meier plotter database. FGFR1–4 – fibroblast growth factor receptors 1–4; OS – overall survival; RFS – relapse-free survival.
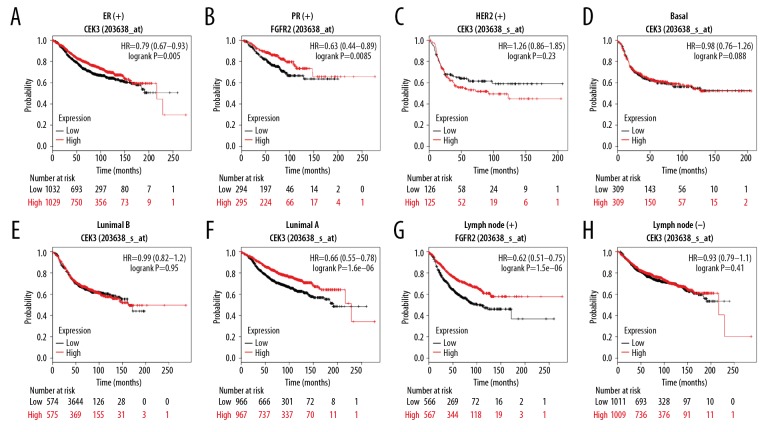
To further investigate the prognostic value of FGFR2 in various BC subgroups, we analyzed the RFS of BC patients with different clinical parameters. When BC patients were sorted by estrogen receptor (ER) status, progesterone receptor (PR) status and HER2 status, our results showed that FGFR2 expression revealed a significant positive effect on the RFS in ER-positive patients (patients=2061, HR=0.79; 95% CI: 0.67–0.93, P-value=0.005, Figure 7A), PR-positive patients (patients=589, HR=0.63; 95% CI: 0.44–0.89, P-value=0.0085, Figure 7B). But FGFR2 expression could not be considered as a biomarker in HER2-positive patients (patients=251, HR=1.26; 95% CI: 0.86–1.85, P-value=0.23, Figure 7C). As for intrinsic subtype, our results showed the levels of FGFR2 expression were not associated with RFS time in basal BC (patients=618, HR=0.98; 95% CI: 0.76–1.26, P-value=0.88, Figure 7D) and the luminal B subtype (patients=1149, HR=0.99; 95% CI: 0.82–1.2, P-value=0.95, Figure 7E). However, patients with high FGFR2 expression experienced a significantly longer RFS time in luminal A patients (patients=1403, HR=0.66; 95% CI: 0.55–0.78, P-value=1.6e-06, Figure 7F). Additionally, patients with high FGFR2 expression experienced a significantly longer RFS time in the lymph node-positive subtype (patients=1133, HR=0.62; 95% CI: 0.51–0.75, P-value=1.5e-06, Figure 7G), whereas the association did not occur in patients with negative lymph nodes (patients=2020, HR=0.93; 95% CI: 0.79–1.1, P-value=0.41, Figure 7H). Overall, the higher expression of FGFR2 predicted a better RFS time in BC patients with ER-positive, PR-positive, the luminal A or lymph node-positive subtype.
Figure 7.
Prognostic values of FGFR2 in breast cancer subgroups. Kaplan-Meier plot demonstrated the RFS of FGFR2 in patients with different clinical characteristics including (A) ER-positive breast cancer, (B) PR-positive breast cancer, (C) HER2-positive breast cancer, (D) basal subtype, (E) luminal B subtype, (F) luminal A subtype, (G) lymph node positive status, and (H) lymph node negative status. FGFR2 – fibroblast growth factor receptors 2; RFS – relapse-free survival; ER – estrogen receptor; PR – progesterone receptor; HER2 – human epidermal growth factor receptor 2.
Identification of DEGs in BC cells upon silencing of FGFR2
Based on the significant prognostic value of FGFR2 in BC patients, we explore the potential mechanism by mining GEO database. As described in the Method Section, we obtained the transcriptome of human BC cell lines upon silencing of FGFR2 (GSE74146). A total of 82 DEGs were identified in GSE74146 dataset. As shown in Figure 8, there were 10 upregulated DGEs and 72 downregulated DGEs in MCF-7 treated with FGFR2 siRNA (Figure 8). As shown in Tables 1 and 2, the top 10 upregulated and downregulated DEGs were listed, respectively.
Figure 8.
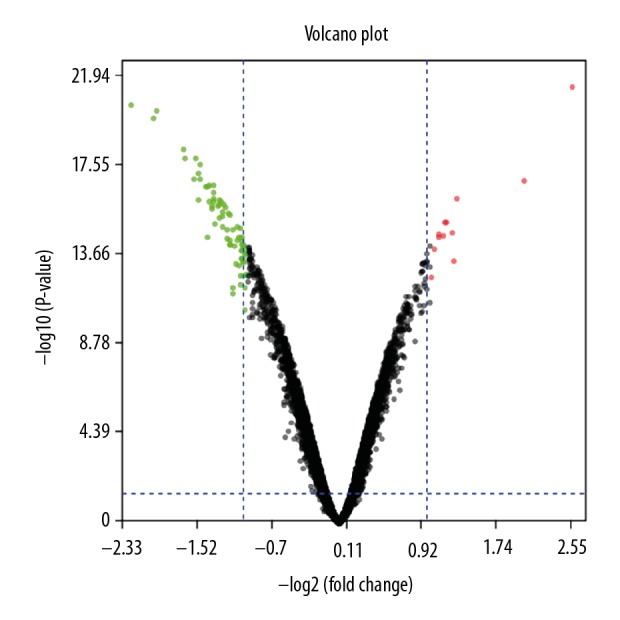
Identification of the DEGs between FGFR2-silenced BC cells and control. The volcano plot indicated the distributions of DEGs in GSE74146. The green and red dots represented the downregulated and upregulated DEGs, respectively. DEGs – differentially expressed genes; FGFR2 – fibroblast growth factor receptors 2; BC – breast cancer.
Table 1.
Top 10 downregulated genes in MCF-7 treated with FGFR2 siRNA.
| Gene title | Gene symbol | Adj. P value | Log2 FC |
|---|---|---|---|
| Mal, T-cell differentiation protein like | MALL | 5.46E-18 | −2.33 |
| Transglutaminase 2 | TGM2 | 4.35E-17 | −2.26 |
| Deiodinase, iodothyronine, type II | DIO2 | 1.21E-16 | −2.01 |
| Cathepsin V | CTSV | 6.51E-17 | −1.98 |
| Calpain 8 | CAPN8 | 3.38E-15 | −1.69 |
| STAM binding protein like 1 | STAMBPL1 | 6.88E-15 | −1.67 |
| Oxytocin receptor | OXTR | 4.47E-14 | −1.58 |
| Sprouty related EVH1 domain containing 1 | SPRED1 | 6.88E-15 | −1.55 |
| Transmembrane protein 189 | TMEM189 | 2.68E-14 | −1.52 |
| Zinc finger protein 365 | ZNF365 | 2.56E-13 | −1.52 |
Table 2.
Top 10 upregulated genes in MCF-7 treated with FGFR2 siRNA.
| Gene title | Gene symbol | Adj. P value | Log2 FC |
|---|---|---|---|
| BPI fold containing family A member 4, pseudogene | BPIFA4P | 2.57E-10 | 1.01 |
| Chloride intracellular channel 3 | CLIC3 | 2.09E-11 | 1.04 |
| Ral GEF with PH domain and SH3 binding motif 1 | RALGPS1 | 5.34E-12 | 1.09 |
| RWD domain containing 2A | RWDD2A | 7.52E-12 | 1.10 |
| Cytochrome P450 family 26 subfamily A member 1 | CYP26A1 | 6.41E-12 | 1.15 |
| Inhibitor of DNA binding 2, HLH protein | ID2 | 1.76E-12 | 1.16 |
| FYVE, RhoGEF and PH domain containing 3 | FGD3 | 1.69E-12 | 1.17 |
| RNA, 7SK small nuclear | RN7SK | 4.86E-12 | 1.24 |
| RNA, 7SK small nuclear | RN7SK | 6.18E-11 | 1.26 |
| Protein phosphatase 1 regulatory subunit 3C | PPP1R3C | 2.47E-13 | 1.29 |
Annotation for DEGs in BC cells upon silencing of FGFR2
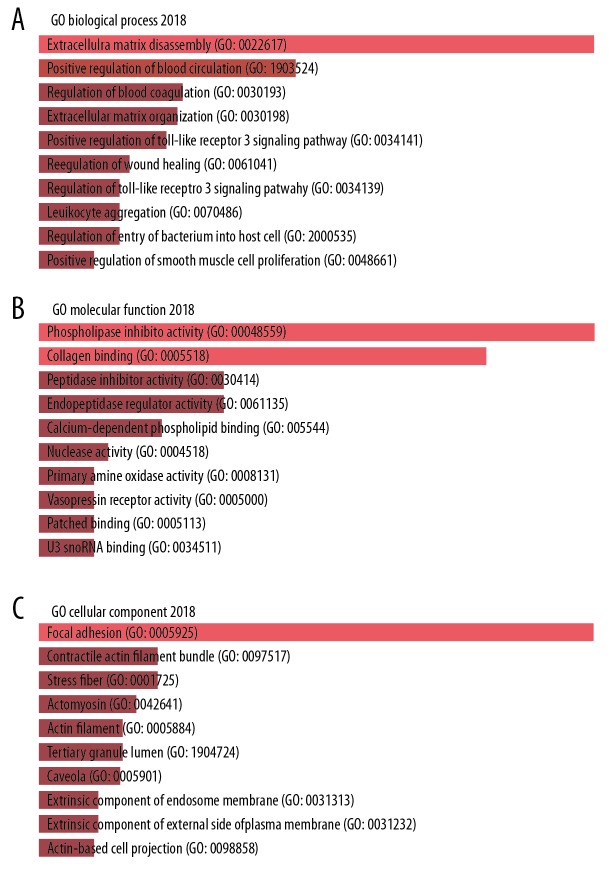
To explore the functions of the DEGs, all the 82 DEGs were imported to Enrichr tool for GO term enrichment analysis. For GO BP analysis, DEGs were mainly involved in extracellular matrix disassembly, positive regulation of blood circulation, regulation of blood coagulation, extracellular matrix organization, and positive regulation of Toll-like receptor 3 signaling pathway (Figure 9A). As for GO term MF, the top 5 significant MF GO terms included phospholipase inhibitor activity, collagen binding, peptidase inhibitor activity, endopeptidase regulator activity, and calcium-dependent phospholipid binding (Figure 9B). As for GO term CC, the DEGs was significantly enriched in the term of focal adhesion, contractile actin filament bundle, stress fiber, actomyosin, and actin filament (Figure 9C).
Figure 9.
GO enrichment analysis of FGFR2-correlated DEGs by the Enrichr web. Bar graph showed the top 10 terms: (A) biological process, (B) molecular function, and (C) cellular component. GO – Gene Ontology; DEGs – differentially expressed genes; FGFR2 – fibroblast growth factor receptors 2; BC – breast cancer.
Pathway enrichment analysis of DEGs in BC cells upon silencing of FGFR2
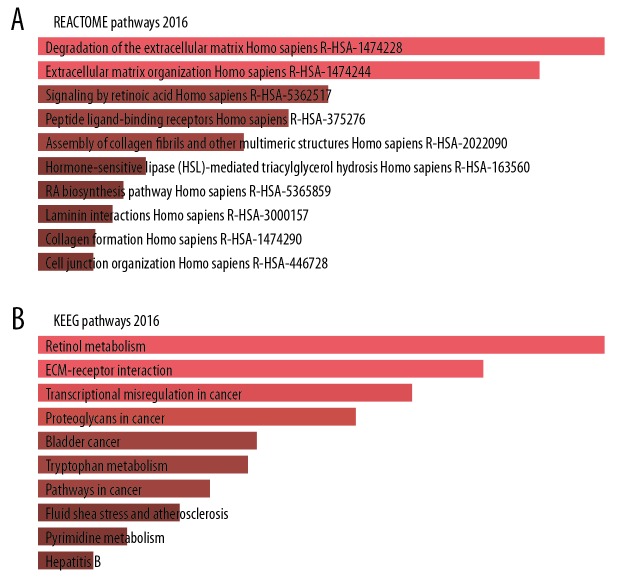
Following the GO enrichment analysis, we analyzed the pathway enrichment by using online Enrichr tool. The top 5 REACTOME pathways correlated with the DEGs were robustly associated with degradation of the extracellular matrix, extracellular matrix organization, signaling by retinoic acid, peptide ligand-binding receptors, and assembly of collagen fibrils and other multimeric structures (Figure 10A). Furthermore, the KEGG enrichment analysis showed the DEGs were mainly related to retinol metabolism, extracellular matrix-receptor interaction, and transcriptional misregulation in cancer and proteoglycans in cancer (Figure 10B).
Figure 10.
Pathway enrichment analysis of FGFR2-correlated DEGs by the Enrichr web. Bar graph showed FGFR2-correlated pathways analyzed by (A) REACTOME pathways 2016, and (B) KEGG pathways 2019. FGFR2 – fibroblast growth factor receptors 2; DEGs – differentially expressed genes; KEGG – Kyoto Encyclopedia of Genes and Genomes.
Analysis of protein-protein interactions (PPI) and hub genes
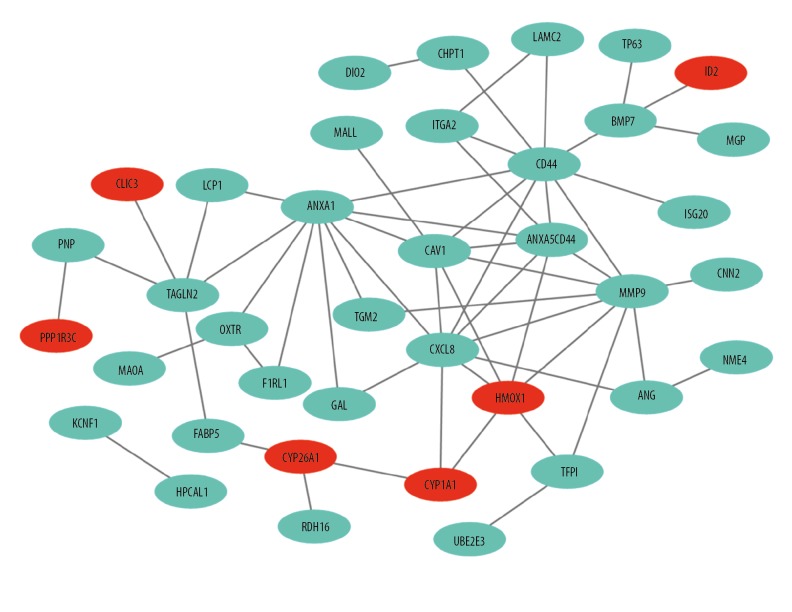
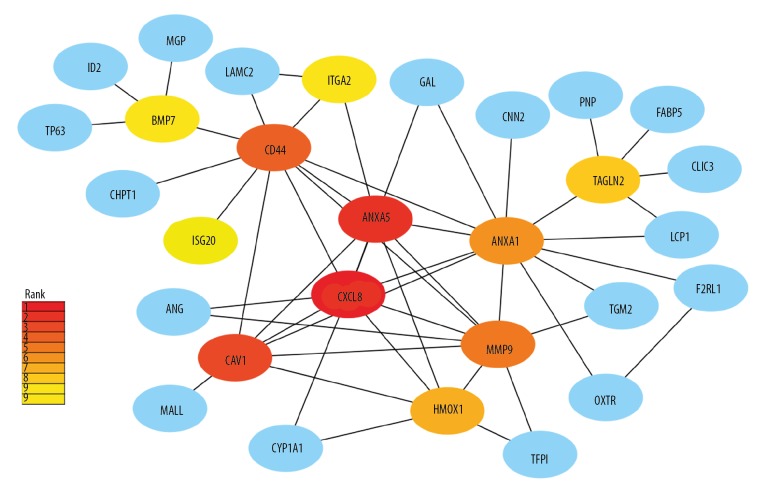
Despite all DEGs, we further performed the analysis of protein-protein interactions (PPI) network and hub genes were analyzed. The interactions among the 82 DEGs were analyzed by STRING with medium confidence and then visualized by Cytoscape software. The PPI network of the DEGs consisted of 69 nodes and 59 edges (Figure 11). CytoHubba analysis obtained top 10 hub genes, including CXCL8, ANXA5, CAV1, CD44, MMP9, ANXA1, HMOX1, TAGLN2, ITGA1, and BMP7 (Figure 12). Of the 10 hub genes, only HMOX1 was upregulated whereas the other 9 hub genes were downregulated.
Figure 11.
PPI network of FGFR2-correlated DEGs. The green and red dots represented the downregulated and upregulated DEGs, respectively. PPI – protein-protein interaction; FGFR2 – fibroblast growth factor receptors 2; DEGs – differentially expressed genes.
Figure 12.
Identification of hub genes by cytoHubba plugin. The rank of connection degree is represented by different degrees of color (from red to yellow).
Discussion
Breast carcinoma is one of the most common malignancies in the world. Despite the incredible progress in the field of BC, the useful prognostic signatures are still insufficient, and the underlying mechanism in the BC is still elusive. FGFRs have play vital roles in various human tumor [4–6]. Although genomic aberrations of the FGFRs have been found in BC, the expression profiles of the FGFR family members and its prognostic performance remain unknown. Here, we integrated the public databases to investigate the expression patterns and their prognostic performance of four FGFR family members genes (FGFR1–4). Our results showed that the high levels of FGFR2 mRNA were correlated with longer OS and RFS in all BC patients along with several specific pathological subtypes. Based on the significant prognostic value of FGFR2, we performed bioinformatics analysis of MCF-7 cells upon silencing of FGFR2 to decipher its potential mechanism. Together, we systemically deciphered the expression profiles and prognostic performance of each FGFR family member in BC, revealing that the FGFR1–4 has a distinct significance in the BC development.
FGFR1 amplifications have implied in various human tumors. In breast carcinoma, the amplification of FGFR1 gene was initially observed in a subset of metastatic lobular breast carcinoma [12]. Similar result was reported by Jiang and his colleagues, who studied the amplification FGFR1 gene in invasive BC and found the amplification of FGFR1 was an independent prognostic factor for poor disease-free survival for all BC patients [22]. Subsequently, FGFR1 expression was evaluated by immunochemistry and the level of FGFR1 was shown to be an independently prognostic of OS in triple-negative BC [23]. Similarly, Shi et al. used the immunochemistry approach to evaluated FGFR1 expression in a large cohort of breast carcinoma and observed that the expression of FGFR1 was shown to have an adverse impact on disease free survival in luminal A cancers [24]. In contrast, our results demonstrated the level of FGFR1 mRNA was downregulated in all BC tissues and its high level of FGFR1 was further demonstrated to be correlated with longer RFS in all BC patients. The discrepancy between our result and previous observations may be seemly attributed to the asynchrony between FGFR1 gene expression and copy number. In line with the speculation, the level of FGFR1 mRNA was elevated in primary lung tumors without FGFR1 amplification whereas FGFR1 mRNA was not upregulated in all FGFR1-amplified tumor tissues [25]. Thus, taking our and the aforementioned studies into account, the effect of FGFR1 on patients’ prognosis in BC appears to be highly dependent on the mRNA and protein levels.
In contrast to FGFR1 expression, our result demonstrated FGFR2 mRNA exhibited no differential expression between tumor tissues and normal control. However, the elevated levels of FGFR2 mRNA were shown to be correlated with longer OS and RFS in all BC patients and this association between FGFR2 level and RFS was further observed in specific pathological BC including ER-positive, PR-positive and the luminal A subtypes. In this respect with FGFR2 gene in BC, numerous previous studies have focused on the single nucleotide polymorphisms (SNPs) in FGFR2 and showed SNPs in FGFR2 were linked to increased BC susceptibility [5,26,27]. As for the prognostic value of FGFR2 expression in BC, previous studies revealed inconsistent results. Increased FGFR2 protein expression has been reported be associated with longer OS and RFS [28]. Conversely, high level of FGFR2 protein was found to be correlated with poor prognosis in 125 cases with invasive ductal carcinoma [29]. In other previous studies using a reverse transcriptase (RT) polymerase chain reaction (PCR) method, the level of FGFR2 mRNA was essentially unrelated to prognosis or clinical status [30,31]. Thus, our and the aforementioned studies suggested the FGFR2 expression might be a potential prognostic factor in BC. To our best knowledge, our study is the first study to investigate the prognostic performance of FGFR2 mRNA by integrating the databases.
Based on the significant prognostic performance of FGFR2 expression, we explored the molecular mechanism underlying targeting silence of FGFR2 in MCF-7 cells. Our GO enrichment result showed FGFR2 was mainly involved in the extracellular matrix disassembly and its closely related pathway was correlated with the degradation of the extracellular matrix. Consistent with our result, Sumbal et al. demonstrated that FGF/FGFR signaling regulated the production of various extracellular matrix proteins including collagens, fibronectin, osteopontin, and matrix metalloproteinases (MMPs). In his work, FGF/FGFR pathway in mammary fibroblasts have been shown to enhance fibroblast-induced branching and that FGFR2 knockdown in mammary fibroblasts reduces [32], suggesting the significance of FGFR2 in extracellular matrix and mammary epithelial morphogenesis. Additionally, our bioinformatics analysis also showed FGFR2 was significantly with the 10 hub genes including CXCL8, ANXA5, CAV1, CD44, MMP9, ANXA1, HMOX1, TAGLN2, ITGA1, and BMP7. Among the hub genes, most of the hub genes were enriched in the regulation of extracellular matrix (ITGA1 CD44, MMP9) and focal adhesion (ANXA5, ANXA1, CAV1), which was in line with our knowledge that FGF signaling pathway regulated a variety of cellular functions including cell proliferation, migration, and differentiation. Among the 10 hub genes, 4 genes (CXCL8, CD44, MMP9, and BMP7) were shown to have potential correlation with FGFR2. Next, we discuss the correlations between FGFR2 and the other 3 hub genes. Notably, our study showed CXCL8 was the top ranked hub gene in MCF-7 cells treated with FGFR2-siRNA, suggesting FGFR2 might be involved in CXCL8 expression. CXCL8 is a chemokine whose biological effects are mediated by its G-protein-coupled receptors CXCR1 and CXCR2. The CXCL8-CXCR1/2 axis has been reported to play multiple roles in cancer, such as increasing proliferation, angiogenesis, invasion, and metastases [33]. Unfortunately, few studies have reported the correlations between FGFRs signaling and CXCL8 expression. Only one recent study demonstrated fibroblasts derived FGF2 robustly induced CXCL8 expression in pancreatic tumor cells [34], which was similar with our result that interference of FGFR2 significantly retarded the CXCL8 expression. CD44 is a major adhesion molecule for extracellular matrix components and implicated in leukocyte homing and activation, wound healing, cell migration, and tumor metastasis [35]. Our study showed silencing of FGFR2 with siRNA suppressed the CD44 expression, indicating a possible cooperation between these molecules. Consistent with the result, Park et al. demonstrated the levels CD44 mRNA were reduced by inducible FGFR2 knockdown and the levels FGFR2 mRNA were also reduced by CD44 knockdown in gastric cancer [36]. In the Park et al. study, FGFR2 and CD44 positively regulated each other’s expression by c-Myc [36]. MMP9 has been shown to play vital roles in FGFs/FGFRs signaling. Intriguingly, different effects on MMP9 expression have been reported for FGFR2. For instance, MMP9 expression was demonstrated to be reduced in thyroid epithelial cancer cells with enforced FGFR2 expression [37], indicating negative effect of FGFR2 on MMP9 expression. Conversely, our result showed silencing of FGFR2 with siRNA resulted in the decreased expression of MMP9, suggesting a positive relation between 2 molecules. Additionally, MMP9 was found no significant difference after treatment with the FGFR2 inhibitors, which was performed in xenograft mouse model of cholangiocarcinoma [38]. BMP7 belongs to the BMP-subfamily within the transforming growth factor (TGF)-superfamily of cysteine-knot fold cytokine-growth factors. BMP7 has pivotal functions during branching morphogenesis. The branching morphogenesis regulator BMP7 was showed to be downregulated by partial loss of FGFR2 [39], revealing a positive regulation of BMP7 by FGFR2. In line with the result, our study showed silencing of FGFR2 with siRNA decreased the expression of BMP7. Altogether, our study revealed the potential correlation between FGFR2 and crucial hub genes.
In parallel, we also evaluated the expression pattern of FGFR3–4 and its prognostic performance in BC patients. Our result showed FGFR3 mRNA was upregulated in all tumor tissues regardless of pathological subtypes. Moreover, high level of FGFR3 was correlated with long RFS in all BC patients. In contrast with our result, Madden et al. integrated gene expression and survival data from 26 datasets and demonstrated the elevated expression of FGFR3 was associated with poor disease-free survival [40]. Similarly, the level of FGFR3 expression was also observed to be strongly associated with OS in a cohort of 50 invasive BC although FGFR3 was not shown to be correlated with specific clinicopathological parameters [41]. The discrepancy between our result and previous observations may be seemly attributed to the different detection methods and datasets. Intriguingly, the level of FGFR3 expression was dropped in pancreatic carcinoma tissues and FGFR3 was considered as a tumor suppressor in cancer cells of epithelial phenotype [42], which was in line with our result. Thus, given our result and previous observations, FGFR3 might exhibit a context-dependent functional role in the progress of BC. In contrast with FGFR3, our study found the elevated levels of FGFR4 mRNA were shown to be associated with shorter OS in all BC patients, whereas FGFR4 mRNA exhibited no differential expression between tumor tissues and normal control. Accordance with our result, Meijer et al. has studied the predictive value of FGFR4 for the duration of progression-free survival (PFS) in 285 patients with ER-positive breast carcinomas. In his work, high FGFR4 mRNA levels was demonstrated to be significantly correlated with short PFS as an independent predictive factor [31]. Similarly, recent observation showed FGFR4 activation increased dramatically in endocrine-treated distant metastases of invasive lobular carcinoma [43]. Collectively, our results, together with previous studies, suggest FGFR4 is an important mediator in breast carcinoma.
In spite of the aforementioned findings, there were some limitations in this study. First, we only deciphered the mRNA expression of FGFRs in BC by using the public databases. Further validation using PCR as well as western blotting should be performed in the near future. Secondly, the biological functions of FGFR2 in BC should be investigated. Based on the bioinformatics analysis of FGFR2, we will focus on the FGFR-mediated retinol metabolism in BC, a novel field of FGF/FGFR signaling. Although there were the aforementioned limitations in our study, we explored the prognostic values of FGFR1–4 expression in BC patients and explored the potential mechanism linked with FGFR2 in BC. When these outcomes are confirmed, the FGFRs, especially FGFR2 genes, might hold a substantial prognostic value in BC. Nevertheless, future verification with a larger study population is required to confirm that the FGFRs could be involved in diagnosis and prognostic monitoring of BC.
Conclusions
In brief, we systematically analyzed the FGFR1–4 expression patterns and prognostic performances of FGFR1–4 in BC patients by bioinformatics analysis based on several public online datasets. Our results demonstrated the FGFR2 overexpression predicted favorable prognosis in BC and indicated that FGFR2 might act as a promising biomarker for BC patients. One of our future works will concentrate on identification of the hub genes that is regulated by FGFR2. By study of the hub genes, we hope our study will broaden the understanding of diagnostic and therapy designs for BC patients.
Footnotes
Conflicts of interest
None.
Source of support: National Natural Science Foundation of China (No.81401616) and Natural Science Foundation of Shanghai (14ZR1405100)
References
- 1.Colozza M, Azambuja E, Cardoso F, et al. Proliferative markers as prognostic and predictive tools in early breast cancer: Where are we now? Ann Oncol. 2005;16:1723–39. doi: 10.1093/annonc/mdi352. [DOI] [PubMed] [Google Scholar]
- 2.Lin H, Huang JF, Qiu JR, et al. Significantly upregulated TACSTD2 and cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp Mol Pathol. 2013;94(1):73–78. doi: 10.1016/j.yexmp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Xu X, Tang X, Lu M, et al. Overexpression of MAGE-A9 predicts unfavorable outcome in breast cancer. Exp Mol Pathol. 2014;97(3):579–84. doi: 10.1016/j.yexmp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17(5):318–32. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Garcia J, Munoz-Couselo E, Soberino J, et al. Targeting FGFR pathway in breast cancer. Breast. 2018;37:126–33. doi: 10.1016/j.breast.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Kang X, Lin Z, Xu M, et al. Deciphering role of FGFR signalling pathway in pancreatic cancer. Cell Prolif. 2019;52(3):e12605. doi: 10.1111/cpr.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helsten T, Elkin S, Arthur E, et al. The FGFR landscape in cancer: Analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22(1):259–67. doi: 10.1158/1078-0432.CCR-14-3212. [DOI] [PubMed] [Google Scholar]
- 8.Koole K, Brunen D, van Kempen PM, et al. FGFR1 Is a potential prognostic biomarker and therapeutic target in head and neck squamous cell carcinoma. Clin Cancer Res. 2016;22(15):3884–93. doi: 10.1158/1078-0432.CCR-15-1874. [DOI] [PubMed] [Google Scholar]
- 9.Preusser M, Berghoff AS, Berger W, et al. High rate of FGFR1 amplifications in brain metastases of squamous and non-squamous lung cancer. Lung Cancer. 2014;83(1):83–89. doi: 10.1016/j.lungcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Gust KM, McConkey DJ, Awrey S, et al. Fibroblast growth factor receptor 3 is a rational therapeutic target in bladder cancer. Mol Cancer Ther. 2013;12:1245–54. doi: 10.1158/1535-7163.MCT-12-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letessier A, Sircoulomb F, Ginestier C, et al. Frequency, prognostic impact, and subtype association of 8p12, 8q24, 11q13, 12p13, 17q12, and 20q13 amplifications in breast cancers. BMC Cancer. 2006;6:245. doi: 10.1186/1471-2407-6-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunello E, Brunelli M, Bogina G, et al. FGFR-1 amplification in metastatic lymph-nodal and haematogenous lobular breast carcinoma. J Exp Clin Cancer Res. 2012;31:103. doi: 10.1186/1756-9966-31-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia MJ, Pole JC, Chin SF, et al. A 1 Mb minimal amplicon at 8p11-12 in breast cancer identifies new candidate oncogenes. Oncogene. 2005;24(3):5235–45. doi: 10.1038/sj.onc.1208741. [DOI] [PubMed] [Google Scholar]
- 14.Turner N, Pearson A, Sharpe R, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70(5):2085–94. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Formisano L, Lu Y, Servetto A, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10(1):1373. doi: 10.1038/s41467-019-09068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Z, Li C, Kang B, et al. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–58. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlen M, Zhang C, Lee S, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352) doi: 10.1126/science.aan2507. pii: eaan2507. [DOI] [PubMed] [Google Scholar]
- 19.Gyorffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–31. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 20.Tan A, Luo R, Liang H, et al. Bioinformatics approach reveals the key role of CXC motif chemokine receptor 2 in endometriosis development. Mol Med Rep. 2018;18(3):2841–49. doi: 10.3892/mmr.2018.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang M, Kim E, Choi Y, et al. FGFR1 is amplified during the progression of in situ to invasive breast carcinoma. Breast Cancer Res. 2012;14(4):R115. doi: 10.1186/bcr3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng CL, Thike AA, Tan SY, et al. Expression of FGFR1 is an independent prognostic factor in triple-negative breast cancer. Breast Cancer Res Treat. 2015;151(1):99–111. doi: 10.1007/s10549-015-3371-x. [DOI] [PubMed] [Google Scholar]
- 24.Shi YJ, Tsang JY, Ni YB, et al. FGFR1 is an adverse outcome indicator for luminal A breast cancers. Oncotarget. 2016;7(4):5063–73. doi: 10.18632/oncotarget.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rooney C, Geh C, Williams V, et al. Characterization of FGFR1 locus in sqNSCLC reveals a broad and heterogeneous amplicon. PLoS One. 2016;11(2):e0149628. doi: 10.1371/journal.pone.0149628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Luca A, Frezzetti D, Gallo M, et al. FGFR-targeted therapeutics for the treatment of breast cancer. Expert Opin Investig Drugs. 2017;26(3):303–11. doi: 10.1080/13543784.2017.1287173. [DOI] [PubMed] [Google Scholar]
- 27.Sobhani N, Ianza A, D’Angelo A, et al. Current status of fibroblast growth factor receptor-targeted therapies in breast cancer. Cells. 2018;7(7) doi: 10.3390/cells7070076. pii: E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanckaert VD, Hebbar M, Louchez MM, et al. Basic fibroblast growth factor receptors and their prognostic value in human breast cancer. Clin Cancer Res. 1998;4(12):2939–47. [PubMed] [Google Scholar]
- 29.Sun S, Jiang Y, Zhang G, et al. Increased expression of fibroblastic growth factor receptor 2 is correlated with poor prognosis in patients with breast cancer. J Surg Oncol. 2012;105(8):773–79. doi: 10.1002/jso.22120. [DOI] [PubMed] [Google Scholar]
- 30.Luqmani YA, Bansal GS, Mortimer C, et al. Expression of FGFR2 BEK and K-SAM mRNA variants in normal and malignant human breast. Eur J Cancer. 1996;32A(3):518–24. doi: 10.1016/0959-8049(95)00563-3. [DOI] [PubMed] [Google Scholar]
- 31.Meijer D, Sieuwerts AM, Look MP, et al. Fibroblast growth factor receptor 4 predicts failure on tamoxifen therapy in patients with recurrent breast cancer. Endocr Relat Cancer. 2008;15(1):101–11. doi: 10.1677/ERC-07-0080. [DOI] [PubMed] [Google Scholar]
- 32.Sumbal J, Koledova Z. FGF signaling in mammary gland fibroblasts regulates multiple fibroblast functions and mammary epithelial morphogenesis. Development,k. 2019;146(23) doi: 10.1242/dev.185306. pii: dev185306. [DOI] [PubMed] [Google Scholar]
- 33.Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7(6):1543–88. doi: 10.7150/thno.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awaji M, Futakuchi M, Heavican T, et al. Cancer-associated fibroblasts enhance survival and progression of the aggressive pancreatic tumor via FGF-2 and CXCL8. Cancer Microenviron. 2019;12(1):37–46. doi: 10.1007/s12307-019-00223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senbanjo LT, Chellaiah MA. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J, Kim HJ, Kim KM, et al. A reciprocal regulatory circuit between CD44 and FGFR2 via c-myc controls gastric cancer cell growth. Oncotarget. 2016;7(19):28670–83. doi: 10.18632/oncotarget.8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo M, Liu W, Serra SL, et al. FGFR2 isoforms support epithelial-stromal interactions in thyroid cancer progression. Cancer Res. 2012;72(8):2017–27. doi: 10.1158/0008-5472.CAN-11-3985. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Ding S, Wang CD, et al. Antitumor effect of FGFR inhibitors on a novel cholangiocarcinoma patient derived xenograft mouse model endogenously expressing an FGFR2-CCDC6 fusion protein. Cancer Lett. 2016;380(1):163–73. doi: 10.1016/j.canlet.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuslak SL, Thielen JL, Marker PC. The mouse seminal vesicle shape mutation is allelic with Fgfr2. Development. 2007;134(3):557–65. doi: 10.1242/dev.02741. [DOI] [PubMed] [Google Scholar]
- 40.Madden SF, Clarke C, Gaule P, et al. BreastMark: An integrated approach to mining publicly available transcriptomic datasets relating to breast cancer outcome. Breast Cancer Res. 2013;15(4):R52. doi: 10.1186/bcr3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuroso K, Imai Y, Kobayashi M, et al. Immunohistochemical detection of fibroblast growth factor receptor 3 in human breast cancer: Correlation with clinicopathological/molecular parameters and prognosis. Pathobiology. 2010;77(5):231–40. doi: 10.1159/000314346. [DOI] [PubMed] [Google Scholar]
- 42.Lafitte M, Moranvillier I, Garcia S, et al. FGFR3 has tumor suppressor properties in cells with epithelial phenotype. Mol Cancer. 2013;12:83. doi: 10.1186/1476-4598-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine KM, Priedigkeit N, Basudan A, et al. FGFR4 overexpression and hotspot mutations in metastatic ER+ breast cancer are enriched in the lobular subtype. NPJ Breast Cancer. 2019;5:19. doi: 10.1038/s41523-019-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]