Abstract
Aims:
Insulin resistance (IR) adversely impacts memory and executive functioning in non-Hispanic whites without diabetes. Less is known in Hispanics/Latinos, despite the fact that Hispanics/Latinos have higher rates of insulin resistance than non-Hispanic whites. We investigated the association between IR and cognition and its variation by age.
Methods:
Data from 5,987 participants 45–74 years old without diabetes from the Hispanic Community Health Study/Study of Latinos. IR was considered continuously using homeostasis model assessment for insulin resistance (HOMA-IR) and also dichotomized based on clinically relevant thresholds for hyperinsulinemia (fasting insulin>84.73pmol/L or HOMA-IR>2.6) and sample-based norms (75th percentile of fasting insulin or HOMA-IR). Cognitive testing included the Brief Spanish English Verbal Learning Test (B-SEVLT), Verbal Fluency, and Digit Symbol Substitution.
Results:
There was 90% overlap in participant categorization comparing clinically relevant and sample-based thresholds. In separate fully-adjusted linear regression models, age modified the association between HOMA-IR and Digit Symbol Substitution (p=0.02); advancing age combined with higher HOMA-IR levels resulted in higher scores. Age also modified the association between clinically relevant hyperinsulinemia and B-SEVLT recall (p=0.03); with increasing age came worse performance for individuals with hyperinsulinemia.
Conclusion:
The relationship of IR with cognition in Hispanics/Latinos without diabetes may reflect an age- and test-dependent state.
Keywords: aging, cognition, epidemiology, Hispanics, insulin resistance, Latinos
1.0. Introduction
Diabetes is one of the most significant public health concerns with 30.3 million Americans affected by the disease [1]. Previous work conducted in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), the largest epidemiological study of cardiovascular disease risk factors in Hispanics and Latinos in the US to date, reported that 17% of community dwelling Hispanics/Latinos age 18 or older meet criteria for type 2 diabetes [2, 3]. The high prevalence of diabetes within the Hispanic/Latino community is a critical public health concern as this condition not only heightens the risk of cardiovascular disease, but also increases vulnerability for age-related cognitive decline and dementia [4, 5]. Preclinical changes in the ability to regulate glucose uptake and utilization, defined as insulin resistance (IR), are thought to be an early marker along the course of developing type 2 diabetes [6]. IR has been found to adversely impact memory and executive function in mid- to late-life (majority) non-Hispanic whites without diabetes.[7–10] Nearly half of individuals 65 years and older not diagnosed with diabetes (48.3%) have preclinical alterations in glucose uptake and utilization [1]. Furthermore, Hispanics/Latinos tend to have higher rates than non-Hispanic Whites [11]. Thus, more work is needed to investigate the relationship of IR with cognition in Hispanics/Latinos independent of diabetes.
Few studies have investigated the relationship of IR with cognition in Hispanics/Latinos independent of diabetes. Instead, most studies have reported on glucose dysregulation as an important etiological factor contributing to various cognitive functions in Hispanics/Latinos with diabetes. For example, poor glycemic control in Hispanic/Latino with diabetes has been associated with lower cognition, specifically lower executive functioning in adults 45 and older of the HCHS/SOL [12]. Additionally, in a US-based sample of adults 65 and older with and without diabetes (44% representation of Caribbean Hispanics/Latinos), declines in memory performance were found to be steeper in individuals with hyperinsulinemia compared to those without hyperinsulinemia at baseline [5]. However, no previous studies have expressly examined IR as it relates to cognition independent of diabetes in a diverse Hispanic/Latino population. Further, no prior research has investigated the role of age in the varying associations between IR and different cognitive domains. This is despite the fact that Hispanics/Latinos without diabetes (especially those from a Mexican background) have greater IR compared to non-Hispanic/Latino whites without diabetes, even after adjusting for other comorbid conditions including high body mass index (BMI) [11].
The HCHS/SOL provides a unique opportunity to systematically examine this important public health topic as it includes individuals of varying Hispanic/Latino heritages living in the US. Thus, this study evaluated the association between IR and cognition in adults without diabetes from the HCHS/SOL. We hypothesized that IR would be associated with poorer executive function and memory performance. We further hypothesized that age would serve as a modifier of these results (i.e., associations would be stronger with advanced age) given that older Hispanics/Latinos are at increased risk of both IR as well as cognitive decline and dementia. In addition to examining IR as a continuous variable, we compared individuals with and without hyperinsulinemia. We hypothesized that individuals with hyperinsulinemia would show poorer executive function and memory performance when compared to individuals without hyperinsulinemia. Given that hyperinsulinemia cut points have not been validated in HCHS/SOL, and measures of IR are thought to vary based on population-specific characteristics [13], we examined IR as a dichotomized variable using clinically relevant criteria [9, 14] as well as HCHS/SOL study sample-based normative data.
2.0. Subjects, Materials and Methods
The HCHS/SOL is a population-based prospective cohort study of 16,415 Hispanics/Latinos aged 18–74 years from four U.S. cities (Chicago, IL; Miami, FL; Bronx, NY; San Diego, CA) that oversampled persons ages 45–74 to facilitate examination of target outcomes [15]. The baseline examination (2008 to 2011) [16] consisted of comprehensive biological, behavioral, and sociodemographic assessments. Cognitive testing was also conducted during this baseline examination, but only for individuals 45 years and older. The cohort includes participants who self-identified as being of Central American, Cuban, Dominican, Mexican, Puerto Rican, or South American backgrounds. The sample design and cohort selection have been described in detail elsewhere [15]. The HCHS/SOL was approved by the Institutional Review Boards at all sites and all participants provided written informed consent.
2.1. Participants
Men and women ages ≥45 years with data related to insulin resistance and cognition contributed to this analysis. From this sample of 9,060 participants, we excluded those who self-reported acute stroke (n=183) and/or substance abuse (n=358), were found to have psychotropic medication use based on medication review (n=217), or who were missing data on covariates (n=67). We further excluded 2,248 participants with the glycemic criteria set forth by the American Diabetes Association [17]. Diabetes was defined by at least one of the following glycemic values: random glucose of ≥11.1 mmol/L, fasting glucose≥6.99 mmol/L, hemoglobin A1C ≥48 mmol/mol (6.5%), or (if available) 2-hour post load glucose≥11.1 mmol/L during an oral glucose tolerance test (OGTT). Diabetes was also defined based on participant’s self-report of taking medication for diabetes that was verified by medication review at the study visit. These exclusions resulted in 5,987 participants for the current analyses.
2.2. Determination of Insulin Resistance
Blood was drawn following a minimum 8-hour fast. Plasma glucose was assessed using a hexokinase enzymatic method (Roche Diagnostics Corporation, Indianapolis, IN, USA) and hemoglobin A1C was measured in EDTA whole blood using a Tosoh G7 automated high-performance liquid chromatography analyzer (Tosoh Bioscience Inc., San Francisco, CA, USA). Insulin levels collected prior to October 29, 2009 were calibrated using the following regression equation: y = 1.00494x – 1.4504, where y = adjusted insulin value, x = original insulin value using Merdcodia assay. This was done to account for a change in assay from Merdcodia to Roche Elecsys analyzers which occurred at that time. All fasting insulin values, regardless of date of collection were converted to pmol/L. Additionally, participants received a 2-hour OGTT unless they had fasting plasma glucose levels >8.32 mmol/L. Post-OGTT blood levels were taken 2 hours after a 75g glucose load.
IR was considered both as a continuous variable as well as a categorical one. First, we quantified IR as a continuous variable using homeostasis model assessment of insulin resistance (HOMA-IR) to represent the interaction of glucose and insulin concentrations which may arise from both the presence and extent of IR being expressed [18]. This numeric variable was defined using the following calculation: (fasting glucose*fasting insulin)/405. We also created a categorical variable based on the presence or absence of hyperinsulinemia (a distinct condition from diabetes that may occur as a result of early stage diabetes or pre-diabetes. Thus, using clinically relevant cut-points outlined in several large-scale cohort studies [9, 14], hyperinsulinemia was defined by fasting insulin>84.73 pmol/L or HOMA-IR >2.6. Given that these thresholds have not been validated in the HCHS/SOL population, we also separately defined hyperinsulinemia based on the 75th percentile of fasting insulin or HOMA-IR within our HCHS/SOL study sample. This percentile cut-point was utilized and demonstrated to have relevance for cognition in the Atherosclerosis Risk in Communities (ARIC) cohort [9]. Thus, we determined clinically relevant hyperinsulinemia as well as HCHS/SOL-determined hyperinsulinemia (accounting for the sample design, including sampling weights, to allow appropriate generalization to other Hispanic/Latino cohort studies) as two separate methods to distinguish hyperinsulinemia status (yes/no) in our sample.
2.3. Cognitive Testing
A set of four test measures, outlined below, were administered in the participants’ preferred language during face-to-face interviews by study staff trained and supervised by doctorate-level, licensed, clinical psychologists. While only four tests of cognition were administered, they assessed important outcomes associated with aging including learning, memory, and attention/executive functioning. The Brief Spanish English Verbal Learning Test (B-SEVLT) [19] asked participants to recall items from a 15-item list presented for three consecutive ‘learning’ trials. This is followed by a 15-item distractor list and a delayed free recall trial immediately following the distractor trial [20, 21]. Variables of interest included total learning across all 3 trials (range=0–45) and recall post-interference (memory; range=0–15). Verbal Fluency required participants to generate as many words as possible within 60 seconds that began with a specific letter [22, 23]. In HCHS/SOL, two trials/letters were used: ‘F’ and ‘A’. The total number of correctly generated words was summed across both trials and represents the executive ability of establishing and maintaining mental set as well as word retrieval flexibility.
The Digit Symbol Subtest (DSST) of the Wechsler Adult Intelligence Scale-Revised measures executive functioning and information processing speed [24] by requiring the rapid copying and encoding of symbols to numbers within a 90 second period. The variable of interest is the total number of correctly transcribed symbols during the time allotted.
2.4. Covariables
In addition to age, sex, and education (i.e., less than high school, high school, greater than high school), we adjusted for potential confounding variables including BMI, total cholesterol, systolic blood pressure, and high sensitivity C-reactive protein (hsCRP) as continuous variables, and smoking status and language of test administration as binary variables as outlined below.
Measures of weight (kg) and height (m) were used to estimate BMI. Fasting total cholesterol and CRP levels were also collected. Blood pressure was measured on the right arm using an OMRON HEM-907 XL (Omron Healthcare, Inc., Lake Forest, IL, USA) automatic sphygmomanometer with the participant in a seated position and the arm resting. Three readings were obtained at 1-minute intervals following a 5-minute rest period with the average of the three systolic blood pressure readings used as a covariate. Self-reported current present or absent tobacco smoking was noted and information was obtained on language preference for testing (Spanish or English).
2.5. Statistical Analyses
All statistical analyses were conducted in STATA 15.1 and accounted for the HCHS/SOL sample design (including sampling weights) to allow appropriate generalization to the target population, cluster sampling, and stratification [25]. Descriptive statistics were compared for each hyperinsulinemia group (separately for clinically relevant and study-specific) with formal comparisons carried out via overall survey-adjusted Wald tests [26]. For continuous responses, all means and prevalence estimates were calculated using survey linear regression. Separate multivariable linear regressions were used to adjust for potential confounders. Statistical significance was defined as p<0.05 unless otherwise noted as 95% confidence intervals (CIs).
Cognitive outcomes were deemed normal based on Q-Q plots and Kolmogorov Smirnov testing [27]. For each cognitive outcome (B-SEVLT learning and memory, Verbal Fluency, and DSST performance), we fit a survey linear regression model using HOMA-IR as a continuous variable adjusting for age, sex, education, BMI, total cholesterol, systolic blood pressure, CRP, smoking status, and language of test administration. In order to determine whether age (as a continuous variable) was an effect modifier, we ran a second model adding the interaction term age*HOMA-IR. HOMA-IR was log 2 transformed, i.e., log2(HOMA-IR), to approximate symmetry of the distribution and improve model fit [28, 29], thus beta weights reflect the effect of doubling HOMA-IR on cognition. For each outcome in survey linear regressions using hyperinsulinemia group, we fit similar models as outlined above with hyperinsulinemia group (separately for clinically relevant and study-specific) as the independent variable and with the age*hyperinsulinemia group interaction term then added to determine whether age was an effect modifier. Significance for all analyses was set to p<0.05. To aid the interpretation of the interaction (where significant), we plotted cognitive test scores for participants with and without hyperinsulinemia.
3.0. Results
Within our sample of 5,987 individuals, the majority of individuals were from either a Mexican (31.7%) or Cuban (27.3%) background. The mean and standard error for fasting insulin was 86.12±0.7 pmol/L while the mean and standard error for HOMA-IR was 3.0±0.0. Using this information for study-specific cut-points, the 75th percentile for fasting insulin was 102.8 pmol/L while the 75th percentile for HOMA-IR was 3.6. As previously stated, the clinically derived cut points for fasting insulin and HOMA-IR were 84.73 pmol/L and 2.6, respectively.
3.1. Participant Characteristics
Using clinically derived hyperinsulinemia criteria, there were 2,567 adults in the hyperinsulinemia group and 3,420 adults with normal insulin levels. These groups were equivalent in regards to age and education (Table 1) and did not differ in terms of language preference for testing (~85% of both groups preferred Spanish, p=0.35). The hyperinsulinemia group tended to have less women (Table 1) and had a significantly lower proportion of current smokers (17.3% versus 22.8%, p=0.002).
Table 1.
Entire sample characteristics and by clinically relevant hyperinsulinemia groups (hyperinsulinemia defined by fasting insulin>84.73 pmol/L or HOMA-IR>2.6).
| All N=5987 | Hyperinsulinem ia Group n=2567 | Non-Hyper insulinemia Comparison Group n=3420 | p-value | ||
|---|---|---|---|---|---|
| Age (mean, confidence interval) | 55.22 (54.89–55.55) |
55.38 (54.90– 55.88) |
55.06 (54.59–55.53) |
0.36 | |
| Sex (N, %) | |||||
| Male | 2205, 44.30% | 973, 46.33% | 1232, 42,74% | 0.05 | |
| Female | 3782, 55.70% | 1594, 53.67% | 2188, 57.26% | ||
| Education (N, %) | |||||
| Less than high school | 2369, 35.83% | 1033, 37.01% | 1336, 34.92% | 0.36 | |
| High school degree or equivalent | 1304, 21.59% | 576, 21.96% | 728, 21.31% | ||
| Some college, college graduate or above | 2314, 42.58% | 958, 41.03% | 1356, 43.78% | ||
| Glycemic Indicators (mean, confidence interval) | |||||
| HOMA-IR | 3.01 (2.95–3.07) |
4.46 (4.35–4.58) |
1.59 (1.56–1.61) |
<.0001 | |
| Glucose, mmol/L | 96.41 (96.08–96.73) |
99.42 (98.88–99.95) |
93.44 (93.10–93.79) |
<.0001 | |
| HbA1C, mmol/mol [%] | 37.87 (5.6) [37.71–38.03, (5.6–5.6)] |
38.53 (5.7) [38.30–38.75, 5.6–5.7)] |
37.23 (5.6) [37.01–37.44, (5.5–5.6)] |
<.0001 | |
| Insulin, pmol/L | 12.46 (12.24–12.69) |
18.14 (17.70–18.60) |
6.86 (6.75–6.98) |
<.0001 | |
| Post-OGTT Glucose, mmol/L | 125.24 (123.95–126.54) |
133.17 (131.33–135.01) |
117.42 (115.75–119.09) |
<.0001 | |
| Post-OGTT Insulin, pmol/L | 97.88 (95.20–100.55) |
135.74 (130.62–140.86) |
60.50 (58.53–62.47) |
<.0001 | |
| Cognition (mean, CI) | |||||
| B-SEVLT Total Learning | 22.96 (22.73–23.20) |
22.80 (22.45–23.14) |
23.13 (22.82–23.44) |
0.15 | |
| B-SEVLT Recall Post-interference (Memory) | 8.30 (8.19–8.40) |
8.23 (8.08–8.38) |
8.36 (8.22–8.50) |
0.22 | |
| Verbal Fluency | 18.90 (18.55–19.22) |
18.73 (18.21–19.25) |
19.04 (18.65–19.44) |
0.32 | |
| Digit Symbol Substitution Test | 35.37 (34.76–36.00) |
35.47 (34.65–36.30) |
35.27 (34.47–36.08) |
0.71 | |
NOTE: Data presented in this table are weighted to account for the HCHS/SOL sample design including sampling weights allowing for appropriate generalization of our findings to the target population, cluster sampling, and stratification.
When the hyperinsulinemia groups were defined using the HCHS/SOL sample (i.e. 75th percentile of fasting insulin or HOMA-IR), there were 1,570 adults with hyperinsulinemia and 4,417 adults without hyperinsulinemia. These groups did not differ in regards to age or education (Supplemental Table 1). Like the clinically derived groups, ~85% of both groups preferred Spanish. Additionally, the hyperinsulinemia group defined using the HCHS/SOL sample tended to have less women (p=0.07) when compared to the non-hyperinsulinemia comparison group but groups did not differ in terms of current smokers (18.4% and 21.2%, respectively).
Of the 5,987 participants in this study, 90% were categorized similarly, i.e., as either having (n=1,560) or not having (3,853) hyperinsulinemia regardless of the method used. Thus, only 10% of our sample were classified differently based on the different methods. All but 1 participant was categorized as having hyperinsulinemia based on the clinically relevant cut-points but categorized as not having hyperinsulinemia using HCHS/SOL-derived cut-points. Given the degree of categorization overlap between methods, and the utility of abiding by clinically relevant standards for hyperinsulinemia, we report results for the clinically relevant approach only.
3.2. Cognitive Test Performance
3.2.1. Insulin Resistance:
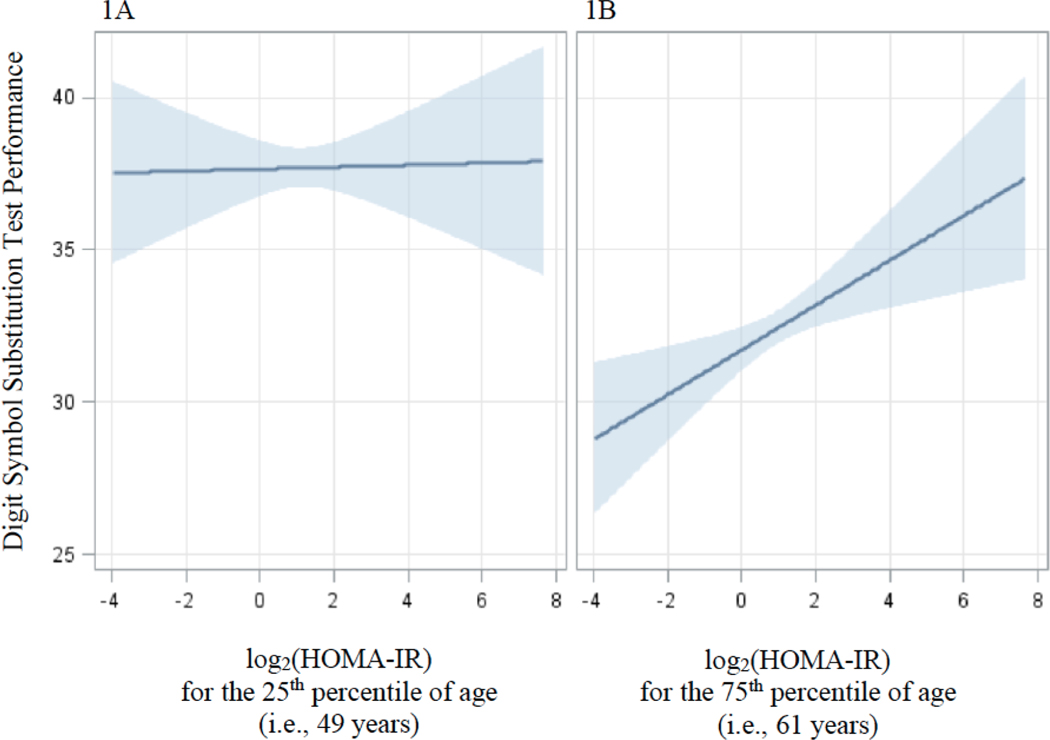
Table 2 displays results for the association of log2(HOMA-IR) as a continuous variable and all cognitive test variables. Age significantly modified the association between log2(HOMA-IR) and DSST performance [age*log2(HOMA-IR): β=0.05±0.02, p=0.02] after adjusting for covariates such that with increasing age and higher insulin resistance came higher performance. For ease of interpretation only, we plotted this cognitive test score for participants with and without hyperinsulinemia based on the magnitude of the difference for individuals at the 25th percentile of age (i.e., Figure 1A representing 350 participants at 49 years of age at testing) and the 75th percentile of age (i.e., Figure 1B representing 197 participants at 61 years of age at testing). Figure 1B shows that those at the 75th percentile of age showed an association of higher log2(HOMA-IR) levels with higher DSST scores while those at the 25th percentile of age (Figure 1A) did not. log2(HOMA-IR) was not significantly associated with any other cognitive test score.
Table 2.
The association of log2(HOMA-IR) with cognition
| Cognitive Function Beta±SE (p-value) | |||||
|---|---|---|---|---|---|
| Total Learning | Recall Post-Interference (Memory) | Fluency | Digit Symbol Substitution Test | ||
| Age | −0.13±0.01 (<0.001) | −0.06±0.05 (<0.001) | 0.005±0.02 (0.81) | −0.42±0.02 (<0.001) | |
| log2(HOMA-IR) | 0.02±0.11 (0.82) | 0.008±0.05 (0.88) | 0.18±0.16 (0.25) | 0.36±0.22 (0.10) | |
| Effect Modification of Age | |||||
| Age | −0.11±0.02 (<0.001) | −0.06±0.01 (<0.001) | 0.01±0.03 (0.61) | −0.50±0.04 (<0.001) | |
| log2(HOMA-IR) | 0.84±0.72 (0.24) | 0.38±0.37 (0.30) | 0.70±0.97 (0.47) | −2.83±1.50 (0.05) | |
| Age*log2(HOMA-IR) | −0.01±0.01 (0.24) | −0.006±0.006 (0.31) | −0.01±0.02 (0.60) | 0.05±0.02 (0.02) | |
Note: log2(HOMA-IR) was log 2 transformed to approximate normality and improve model fit; thus, beta weights reflect the effect of doubling HOMA-IR on cognition. Analyses adjusted for age, sex, education, body mass index, total cholesterol, systolic blood pressure, C-reactive protein, smoking status, and language of test administration. Age is listed in the table given its interaction with log2(HOMA-IR) was central to determining effect modification; however, only significant results with log2(HOMA-IR) and age* log2(HOMA-IR) are denoted with bold text given these were the outcomes of interest.
Figure 1.
Pictorial representation of the significant interaction of age*log2(HOMA-IR) for performance on the Digit Symbol Substitution Test based on Model 2 which adjusted for age, sex, education, body mass index, total cholesterol, systolic blood pressure, C-reactive protein, smoking status, and language of test administration. For ease of interpretation only, differences in test score for those with and without hyperinsulinemia are based on the magnitude of the difference for individuals at (A) the 25th percentile of age representing 350 participants at 49 years of age at testing, and (B) the 75th percentile of age representing 197 participants at 61 years of age at testing. Blue banding represents 95% Confidence Intervals.
3.2.2. Clinically Relevant Hyperinsulinemia:
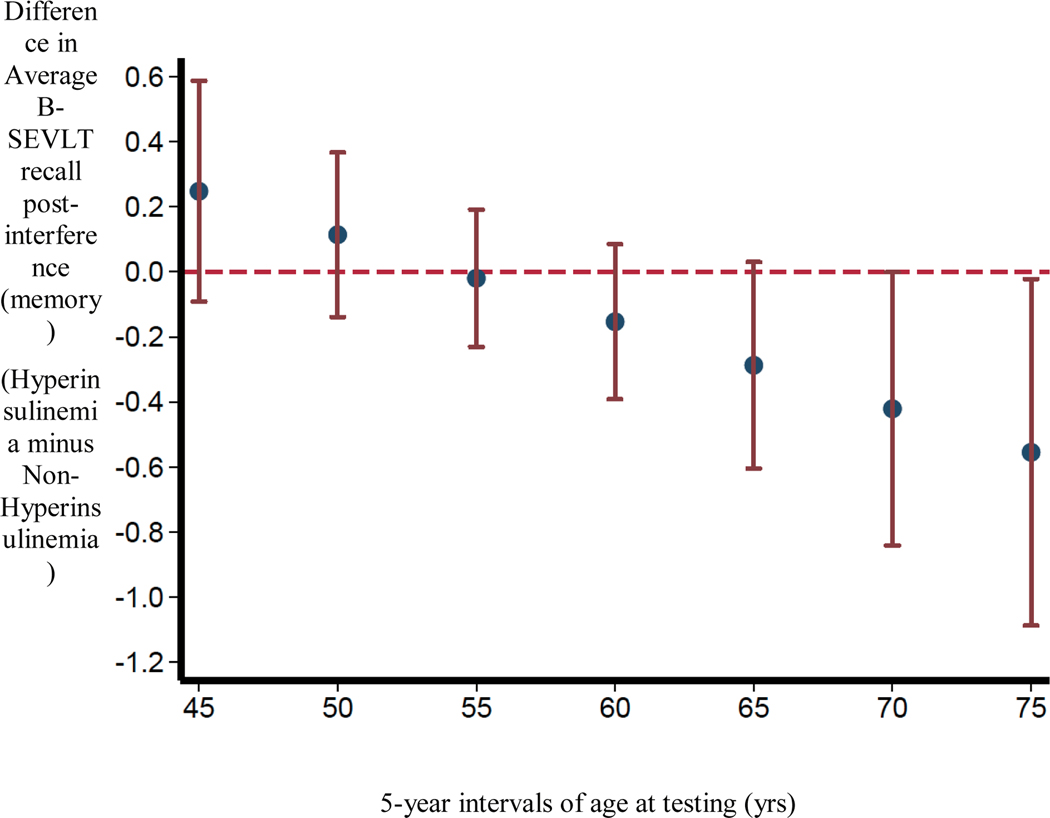
For hyperinsulinemia groups defined using standardized criteria (fasting insulin>84.73 pmol/L or HOMA-IR>2.6), there was a significant age*group interaction associated with B-SEVLT recall post-interference, i.e., memory (β=0.02±0.01, p=0.03) after controlling for covariates. Thus, age significantly modified the association between clinically relevant hyperinsulinemia status and memory such that with increasing age came worse performance for individuals with hyperinsulinemia compared to individuals without hyperinsulinemia. For ease of interpretation only, differences in average test scores for those with and without hyperinsulinemia are plotted at 5-year intervals of age. Only when participants’ age moved into the sixth decade did those with hyperinsulinemia begin to show divergence on B-SEVLT recall post-interference (Figure 2). Clinically relevant hyperinsulinemia group status was not associated with any other cognitive test score (Table 3).
Figure 2.
Pictorial representation of the significant interaction of age*group for clinically-relevant hyperinsulinemia and B-SEVLT recall post-interference (memory) based on Model 2 which adjusted for age, sex, education, body mass index, total cholesterol, systolic blood pressure, C-reactive protein, smoking status, and language of test administration. For ease of interpretation only, differences in average test scores for those with and without hyperinsulinemia are plotted at 5-year intervals of age. Red bars represent 95% Confidence Intervals.
Table 3.
The association of clinically relevant hyperinsulinemia group with cognition.
| Cognitive Function Beta±SE (p-value) | |||||
|---|---|---|---|---|---|
| Total Learning | Recall Post-Interference (Memory) | Fluency | Digit Symbol Substitution Test | ||
| Age | −0.13+0.01 (<0.001) | −0.06+0.006 (<0.001) | 0.005+0.02 (0.80) | −0.42+0.02 (<0.001) | |
| Hyperinsulinemia Group | −0.09±0.23 (0.67) | −0.03±0.10 (0.75) | 0.25±0.34 (0.46) | 0.57±0.42 (0.17) | |
| Effect Modification of Age | |||||
| Age | −0.11+0.01 (<0.001) | −0.05+0.008 (<0.001) | 0.02+0.03 (0.45) | −0.43+0.03 (<0.001) | |
| Hyperinsulinemia Group | 2.07±1.49 (0.16) | 1.45±0.72 (0.04) | 2.60±2.03 (0.20) | −1.39±3.08 (0.65) | |
| Age*Hyperinsulinem ia Group | −0.04±0.02 (0.14) | −0.02±0.01 (0.03) | −0.04±0.03 (0.26) | 0.03±0.05 (0.52) | |
Note: Clinically relevant hyperinsulinemia was defined as fasting insulin>84.73 pmol/L or HOMA-IR >2.6. Analyses adjusted for age, sex, education, body mass index, total cholesterol, systolic blood pressure, C-reactive protein, smoking status, and language of test administration. Age is listed in the table given its interaction with log2(HOMA-IR) was central to determining effect modification; however, only significant results with log2(HOMA-IR) and age* log2(HOMA-IR) are denoted with bold text given these were the outcomes of interest.
4.0. Discussion
In this study, we investigated the relationship between insulin resistance and hyperinsulinemia with cognition and its variations by age in one of the largest studies of mid- to late-life Hispanics/Latinos independent of diabetes. Among diverse Hispanics/Latinos without diabetes, we found that age modified the association between IR and executive functioning/information processing speed. Thus, higher IR was associated with better performance with advanced age in our sample. Age also modified the association between hyperinsulinemia status and B-SEVLT memory performance. Unlike the positive association seen between IR as a continuous variable and cognition, however, advanced age coupled with hyperinsulinemia status resulted in worse memory performance compared to advanced age without hyperinsulinemia. Although our sample is relatively young, our results suggest that age modified the relationship between insulin resistance and hyperinsulinemia with cognition and suggested differential associations to specific tests of cognition when considering insulin resistance as a continuous versus a categorical variable.
Reasons for the differential associations of HOMA-IR and hyperinsulinemia to specific tests of cognition with advanced age may have several origins. First, associations of HOMA-IR and hyperinsulinemia with higher executive functioning/information processing speed versus lower memory performance, respectively, may reflect the fact that our different definitions of IR identified different populations of participants with distinct pathophysiology. Additionally, results may also reflect the continuous versus dichotomous nature of predictor variables used in this research. More specifically, as individuals progress from IR to diabetes they lose beta cell secretory capacity relative to glucose levels [30], which may limit HOMA-IR scores. When taken as a continuous variable, HOMA-IR in our sample without diabetes may reflect a restricted range of scores and/or a score captured before sufficient beta cell decline, i.e., when insulin secretion is still maintained and insulin levels remain relatively high. Thus, only when we dichotomized our sample (hyperinsulinemia versus not) were we able to capture the detrimental relationship between IR and cognition. Additionally, in a study that included Hispanics/Latinos with and without diabetes [5], only individuals within the highest insulin quartiles of categorization at baseline had a greater risk of developing Alzheimer’s disease (AD) over time, namely learning and memory deficits. Thus, the fact that higher HOMA-IR among older Latinos was associated with better executive function/information processing performance while hyperinsulinemia was associated with worse memory may reflect distinctions in acute versus chronic states of IR. Whereas chronic IR may be harmful to brain and cognition, acute IR may enhance brain glucose supply [31] and benefit brain and cognition; a finding that is supported across human and non-human primate studies [32]. Given the cross-sectional nature of our work, we cannot determine whether the levels of IR and hyperinsulinemia documented in our study reflect acute or more chronic dysregulation; however, we are actively collecting longitudinal data to investigate this issue further.
This study contributes to the literature on IR and hyperinsulinemia in Hispanics/Latinos in several ways. First, we focused exclusively on a diverse group of Hispanics/Latinos without diabetes. Thus, our study extends the small body of literature investigating IR and cognition in Hispanics/Latinos with and without diabetes by providing results independent of diabetes. Additionally, while our results highlight the relationship between IR and attention/information processing and memory, unlike other studies to date, our results suggest that these relationships may differ based on the nature of the IR metric used as well as age. Thus, a clinical implication of our study may be that when clinicians consider the impact of insulin resistance in adults approximately 60 years of age, they should take into consideration level as well as threshold criterion and query multiple domains of cognitive functioning with a particular focus on learning and memory.
Additional clinical implications for our study surround the investigation of hyperinsulinemia cut points for Hispanics/Latinos of HCHS/SOL. Although measures of IR are thought to vary based on population-specific characteristics [13], we did not find evidence of large differences in participant categorization between clinically relevant and study-specific criterion. Clinically relevant criteria have a natural advantage in terms of offering guidance for clinical translation and applicability in other studies and populations. Thus, our work in HCHS/SOL suggests that clinically relevant cut-points for hyperinsulinemia already used in several other large-scale cohort studies [9, 14] also offer a valid approach to defining hyperinsulinemia in Hispanics/Latinos.
Beyond the fact that this is a cross-sectional study, additional limitations should be noted when considering our results. For example, there is variability in the literature regarding thresholds for hyperinsulinemia that may stem from several factors including sex, race/ethnicity, and cardiovascular disease risk factors [13]. While we attempted to address these issues by investigating clinically relevant versus study-specific thresholds to define our hyperinsulinemia groups and adjusting all analyses for sex, Hispanic/Latino background, and comorbid cardiovascular disease risk factors, we may have unwittingly omitted other key, unmeasured confounders including other medical comorbidities and sleep-related disorders. While our sample reflected a wide age range (45–74 years), the sample as a whole may still be considered quite young given the mean age for all participants fell within the 5th decade. Thus, the positive associations we observed between HOMA-IR and information processing speed in our older adults may become a negative association in adults of older ages. Lastly, given that the focus of the HCHS/SOL study was cardiovascular in nature [16], our cognitive testing was limited; however, it incorporated important cognitive outcomes associated with aging including learning, memory, and attention/executive functioning. Despite these limitations, this study also had its strengths. It represents one of the largest cohort studies of Hispanics/Latinos from six distinct backgrounds and provides information across a comprehensive panel of markers reflecting glycemic dysfunction. Although additional longitudinal study is needed to validate these findings and follow-up in participants with diabetes that includes an investigation of therapeutic regimes and stages of disease progression would extend this work, investigating the relationship between IR and cognition in Hispanics/Latinos without diagnosed diabetes may identify important health targets to consider when discussing brain-behavior wellbeing in vulnerable individuals.
Supplementary Material
Highlights.
Is insulin resistance associated with cognition in Latinos without diabetes?
With advancing age, insulin resistance associated with better processing speed
With advancing age, insulin resistance also associated with more memory problems
Insulin resistance differentially impacts cognition in older Latinos
Acknowledgements
The authors thank the staff and participants of HCHS/SOL for their important contributions. A complete list of staff and investigators has been provided by Sorlie P., et al. in Ann Epidemiol. 2010 Aug;20: 642–649 and is also available on the study website http://www.cscc.unc.edu/hchs/
Funding
This work was supported, as a collaborative study, by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. Additional funding for M.L. was provided by NIA K01-AG040192.
Abbreviations:
- B-SEVLT
Brief Spanish English Verbal Learning Test
- DSST
The Digit Symbol Subtest
- hsCRP
high sensitivity C-reactive protein
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- IR
insulin resistance
Footnotes
Conflict of Interest
None of the authors had any financial or other conflicts of interest.
Data Statement
Data used in this manuscript is available upon request. Additional information may be found at https://sites.cscc.unc.edu/hchs/
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Center for Disease Control and Prevention, CDC (2017) National Diabetes Statistics Report In: Center for Disease Control and Prevention (ed). U.S. Dept of Health and Human Services, Atlanta, GA [Google Scholar]
- [2].Daviglus ML, Talavera GA, Aviles-Santa ML, et al. (2012) Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA : the journal of the American Medical Association 308: 1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schneiderman N, Llabre M, Cowie CC, et al. (2014) Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes care 37: 2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stewart R, Liolitsa D (1999) Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med 16: 93–112 [DOI] [PubMed] [Google Scholar]
- [5].Luchsinger JA, Tang MX, Shea S, Mayeux R (2004) Hyperinsulinemia and risk of Alzheimer disease. Neurology 63: 1187–1192 [DOI] [PubMed] [Google Scholar]
- [6].Lebovitz HE (2001) Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes 109 Suppl 2: S135–148 [DOI] [PubMed] [Google Scholar]
- [7].Bove RM, Brick DJ, Healy BC, et al. (2013) Metabolic and endocrine correlates of cognitive function in healthy young women. Obesity (Silver Spring) 21: 1343–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Messier C, Awad-Shimoon N, Gagnon M, Desrochers A, Tsiakas M (2011) Glucose regulation is associated with cognitive performance in young nondiabetic adults. Behav Brain Res 222: 81–88 [DOI] [PubMed] [Google Scholar]
- [9].Young SE, Mainous AG 3rd, Carnemolla M (2006) Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes care 29: 2688–2693 [DOI] [PubMed] [Google Scholar]
- [10].Laws SM, Gaskin S, Woodfield A, et al. (2017) Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci Rep 7: 9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Haffner SM, D’Agostino R, Saad MF, et al. (1996) Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 45: 742–748 [DOI] [PubMed] [Google Scholar]
- [12].Strizich G, Kaplan RC, Gonzalez HM, et al. (2016) Glycemic control, cognitive function, and family support among middle-aged and older Hispanics with diabetes: The Hispanic Community Health Study/Study of Latinos. Diabetes Res Clin Pract 117: 64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, et al. (2013) Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McAuley KA, Williams SM, Mann JI, et al. (2001) Diagnosing insulin resistance in the general population. Diabetes care 24: 460–464 [DOI] [PubMed] [Google Scholar]
- [15].Lavange LM, Kalsbeek WD, Sorlie PD, et al. (2010) Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Annals of epidemiology 20: 642649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, et al. (2010) Design and implementation of the Hispanic Community Health Study/Study of Latinos. Annals of epidemiology 20: 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].American Diabetes A (2018) Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes care 41: S13–S27 [DOI] [PubMed] [Google Scholar]
- [18].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419 [DOI] [PubMed] [Google Scholar]
- [19].Gonzalez HM, Tarraf W, Gouskova N, et al. (2015) Neurocognitive function among middleaged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 30: 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gonzalez HM, Mungas D, Haan MN (2002) A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. The Clinical neuropsychologist 16: 439–451 [DOI] [PubMed] [Google Scholar]
- [21].Gonzalez HM, Mungas D, Reed BR, Marshall S, Haan MN (2001) A new verbal learning and memory test for English- and Spanish-speaking older people. Journal of the International Neuropsychological Society : JINS 7: 544–555 [DOI] [PubMed] [Google Scholar]
- [22].Benton AL, Hamsher K (1989) Multilingual Aphasia Examination. AJA Associates, Iowa City [Google Scholar]
- [23].Lezak M, Howieson DB, Loring DW (2004) Neuropsychological Assessment. Oxford University Press, New York [Google Scholar]
- [24].Wechsler D (1981) WAIS-R Manual. Psychological Corporation, San Antonio, TX [Google Scholar]
- [25].LaVange L, Davis SM, Hankinson J, et al. (2017) Spirometry Reference Equations from the HCHS/SOL (Hispanic Community Health Study/Study of Latinos). Am J Respir Crit Care Med 196: 993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Korn EL, Graubard BI (1990) Simultaneous testing of regression coefficients with complex survey data: Use of Bonferroni t Statistics. The American Statistician 44: 270–276 [Google Scholar]
- [27].Razali NM, Wah YB (2011) Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors, and Anderson-Darling tests. Journal of Statistical Modeling and analytics 2: 2133 [Google Scholar]
- [28].Cornfield J, Gordon T, Smith W (1961) Quantal response curves for experimentally uncontrolled variables. Bulletin of the International Statistical Institute 38: 97–115 [Google Scholar]
- [29].Kay R, Little S (1987) Transformation of the explanatory variables in the logistic regression model for binary data. Biometrika 74: 495–501 [Google Scholar]
- [30].Saisho Y (2015) beta-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J Diabetes 6: 109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang G (2014) Raison d’etre of insulin resistance: the adjustable threshold hypothesis. J R Soc Interface 11: 20140892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Watson GS, Craft S (2004) Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer’s disease. Eur J Pharmacol 490: 97–113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.