Abstract
Objectives:
To determine the effects of doxorubicin and/or trastuzumab on diastolic function and the relationship between diastolic function and systolic dysfunction.
Background:
Doxorubicin and trastuzumab, commonly used in breast cancer, can result in left ventricular ejection fraction (LVEF) declines. However, the effects of these therapies on diastolic function remain incompletely defined.
Methods:
In a rigorously phenotyped, longitudinal cohort study of 362 breast cancer participants treated with doxorubicin, doxorubicin followed by trastuzumab, or trastuzumb alone, changes in diastolic function were evaluated using linear models estimated via Generalized Estimating Equations (GEE). Associations between baseline and changes in diastolic function with LVEF and longitudinal strain were estimated using GEE. Cox proportional hazards models estimated the associations between baseline characteristics and risk of diastolic dysfunction, and between diastolic function and risk of cancer therapy-related cardiac dysfunction (CTRCD), as defined by an LVEF decline of ≥10% to <50%.
Results:
Over a median of 2.1 (interquartile range [IQR] 1.3, 4.2) years, participants treated with doxorubicin or doxorubicin followed by trastuzumab demonstrated a persistent worsening in diastolic function, with reductions in the E/A ratio, lateral and septal e’ velocities, and increases in E/e’ (p<0.01). These changes were not observed with trastuzumab alone. Abnormal diastolic function grade was present in 60 % at 1 year, 70% by 2 years, and 80% by 3 years. Abnormal diastolic function grade was associated with a subsequent decrease in LVEF (−2.1%, 95% CI - 3.1, −1.2, p<0.001) and worsening in longitudinal strain (0.6%, 95% CI 0.1, 1.1, p=0.013) over time. Changes in E/e’ ratio were also modestly associated with subsequent worsening of longitudinal strain (0.1%, 95% CI 0.0, 0.2, p=0.022).
Conclusions:
A modest, persistent worsening of diastolic function is observed with contemporary breast cancer therapy. Abnormal and worsening diastolic dysfunction is associated with a small risk of subsequent systolic dysfunction.
Keywords: Anthracyclines, trastuzumab, cardiotoxicity, chemotherapy, diastolic dysfunction, cardio-oncology
Condensed Abstract
Doxorubicin and trastuzumab, commonly used in breast cancer, can result in left ventricular ejection fraction (LVEF) declines. However, the effects of these therapies on diastolic function remain incompletely defined. In a rigorously phenotyped cohort study of 362 breast cancer participants, we determined that doxorubicin with or without trastuzumab is associated with mild, persistent abnormalities in diastolic function. Abnormal diastolic function is associated with a small risk of subsequent systolic dysfunction. These findings suggest aggressive cardiovascular risk factor modification may be important prior to or with the onset of diastolic dysfunction, as these strategies may delay the progression of cardiac dysfunction.
Introduction
Advances in screening and treatment have increased survival for patients with breast cancer(1); however, cardiotoxicity from commonly used cancer therapies remains a significant cause of long-term morbidity and mortality(2, 3). Anthracycline chemotherapy, including doxorubicin, results in oxidative and nitrosative stress, mitochondrial dysfunction, and cardiomyocyte apoptosis leading to reductions in left ventricular ejection fraction (LVEF) and heart failure (HF)(4–6). Monoclonal antibodies that block ErbB2 (HER2/neu) signaling, such as trastuzumab, disrupt cardiac homeostasis and myocardial repair and also are cardiotoxic(7). Chest radiation is also believed to result in myocardial fibrosis and microvascular disease contributing to HF development(8).
In the general population, echocardiographic measures of diastolic dysfunction are associated with an increased risk of incident HF and mortality(9–12), are accepted as clinical trial endpoints (13, 14), and are considered fundamental to the pathophysiology of HF(15, 16). In community-based studies, participants with persistent or progression to mild diastolic dysfunction had a 7.8% HF incidence, and those with persistent or progression to moderate-severe diastolic dysfunction had a 12.2% HF incidence(10). In a retrospective analysis of 1,065 outpatients, worsening of diastolic function was associated with a 1.78-fold mortality increase(11). Detailed assessment of diastolic function is thus of considerable importance to cardiovascular disease classification and prognosis.
Within the field of cardio-oncology, prior studies have shown that changes in diastolic function occur with anthracycline cancer therapy. However, these have focused primarily on anthracyclines alone, included limited follow-up time, and have not incorporated newer classifications of diastolic function grade or associations with subsequent declines in systolic function(17–24).
As such, there are important unanswered questions about how modern-day breast cancer therapies can affect diastolic function, and whether pre-treatment diastolic dysfunction or change in diastolic function with breast cancer therapy is of any clinical significance. For example, how common are changes in diastolic function with breast cancer therapy, and do these changes predict subsequent risk of systolic dysfunction? In order to gain insight into these critical questions, we performed a comprehensive evaluation of the changes in echocardiographic measures of diastolic function in a rigorously phenotyped cohort of 362 participants with breast cancer undergoing therapy with doxorubicin and/or trastuzumab over a maximum follow-up time of 6.5 years. We evaluated both individual parameters of diastolic function and diastolic function grade(25) with a focus on the longitudinal changes over time, and determined their associations with subsequent systolic dysfunction.
Methods
Study Population
The Cardiotoxicity of Cancer Therapy (CCT) study is a longitudinal, prospective cohort study of participants with breast cancer undergoing treatment with doxorubicin and/or trastuzumab therapy at the Rena Rowan Breast Cancer Center at the University of Pennsylvania (Philadelphia, PA)(NCT01173341). The study protocol has previously been described(4). Briefly, participants at least 18 years of age with a diagnosis of breast cancer with planned treatment with doxorubicin and/or trastuzumab are eligible. The current analyses are restricted to participants enrolled from August 2010 to April 2017 who had an analyzed baseline echocardiogram and at least one follow-up echocardiogram. The study was approved by the local Institutional Review Board, and all participants provided written informed consent.
Cancer Treatment and Clinical Characteristics
Breast cancer treatment was determined by the oncologist and consisted of: 1) doxorubicin (240mg/m2) with concurrent cyclophosphamide, followed by paclitaxel (Doxorubicin); 2) trastuzumab with docetaxel and either cyclophosphamide or carboplatin (Trastuzumab); or 3) doxorubicin (240mg/m2) with concurrent cyclophosphamide, followed by trastuzumab and paclitaxel (Doxorubicin+Trastuzumab). Radiation laterality and treatment dates were recorded for relevant participants. Cancer-related clinical variables such as clinical stage, hormone receptor status and surgical therapy were collected by clinical chart review. Clinical characteristics such as hypertension, diabetes, hyperlipidemia, tobacco use, and body mass index (BMI) were assessed by participant report, clinical chart review and provider assessment.
Echocardiographic Assessment
Transthoracic echocardiograms were performed by dedicated sonographers at an Intersocietal Accreditation Commission laboratory using primarily GE Vivid E7, E9, or E95 machines (GE Healthcare, Milwaukee, WI)(4). Echocardiograms were obtained at baseline for all participants. For those in the Doxorubicin arm, echocardiograms were obtained at the completion of paclitaxel and then annually. For those in the Trastuzumab arm, echocardiograms were obtained every 3 months during trastuzumab therapy and then annually. In the Doxorubicin+Trastuzumab arm, echocardiograms were obtained after completion of doxorubicin, every 3 months while receiving trastuzumab and then annually.
Echocardiography images were archived, with core-lab quantitation of LVEF via Simpson’s method, longitudinal strain, and diastolic function parameters (TomTec Imaging Systems, Unterschleissheim, Germany)(4). Mitral valve (MV) peak E- and A-wave velocities (cm/sec) were assessed using pulse-wave (PW) Doppler with a 1–3mm sample volume between the mitral valve leaflets in the apical four-chamber view. The MV E/A was calculated as the ratio of these 2 velocities. Tissue Doppler mitral annular e’ velocity (cm/sec) was assessed using a PW Doppler sample volume at the lateral and septal annuli. The average E/e’ was calculated as the MV peak E-wave velocity divided by the average of the septal and lateral e’. Left atrial (LA) volume index was calculated using apical 4- and 2- chamber views immediately prior to MV opening using the area-length method divided by body surface area (BSA). MV deceleration time (DT) was measured as the time interval from the peak E-wave along the slope of LV filling extrapolated to the zero-velocity baseline. Isovolumic relaxation time (IVRT) was measured as the time from aortic valve closure to MV opening using continuous wave (CW) Doppler at the level of the left ventricular outflow tract, optimizing visualization of end of aortic ejection and onset of mitral inflow. Peak tricuspid regurgitation (TR) systolic jet velocity was assessed with CW Doppler across the tricuspid valve. The intraobserver coefficient of variation (CV) for LVEF was 4.4% and 10.9% for longitudinal strain. The intraobserver CVs for mitral inflow and tissue Doppler velocities were 2.3–5.4%.
Diastolic Dysfunction Definitions
In addition to a detailed evaluation of individual measures indicative of diastolic function (E/A, septal and lateral e’, indexed LA volume, TR velocity, and E/e’), in our statistical analyses, we evaluated: 1) E/e’ as a continuous variable; 2) E/e’>14 as a categorical variable; and 3) diastolic function grade according to the 2016 American Society of Echocardiography and European Association of Cardiovascular Imaging(ASE/EACI) guidelines(25).
Cardiovascular Outcome Measures of Dysfunction
Cancer therapeutics-related cardiac dysfunction(CTRCD) was defined as a quantitated LVEF decline of ≥10% to a value of <50%(26). 2D Longitudinal strain was also quantified, as previously described (4).
Statistical Analysis
Baseline characteristics were summarized according to cancer treatment regimen using median (IQR) for continuous variables and proportions for categorical variables. The longitudinal change over time for each echocardiographic variable was assessed graphically according to treatment arm using LOESS curves with pointwise 95% confidence intervals (CIs). The mean change in each echocardiographic parameter at 6, 12, 24 and 36 months since initiation of cancer therapy for each regimen was estimated using linear regression via generalized estimating equations (GEE). These models were adjusted for the baseline value of each parameter and time since initiation of cancer therapy, with separate models for each treatment regimen. A robust variance estimator was used to account for clustering within subjects, and time since initiation of cancer therapy was modeled using a cubic spline with 3 degrees of freedom. Hypothesis tests for change in each parameter at each time point relative to baseline were conducted using Wald tests.
We used the Kaplan-Meier estimator to derive the proportion of patients experiencing incident diastolic dysfunction. This was defined as the development of an abnormal diastolic function grade during follow-up in a participant with normal or indeterminate diastolic function grade at baseline. Univariable Cox proportional hazards models were used to assess the association between baseline demographic and clinical variables and the development of incident diastolic dysfunction among all participants with normal diastolic function at baseline. Variables for multivariable Cox models were prespecified based on hypothesized clinical and pathophysiologic relevance and included age, African American race, hypertension, diabetes, current smoking status, BMI, treatment regimen, and radiation therapy. Radiation therapy was included in models as a time-varying binary indicator, which was coded as absent until the date of therapy initiation and present thereafter. Additional analyses were performed using the outcome of time to the development of elevated filling pressures, defined as an average E/e’ >14.
The association between baseline or changes in diastolic function and the development of subsequent systolic dysfunction was comprehensively assessed in multiple complementary ways. First, GEE estimated the associations between baseline diastolic function grade and subsequent change in LVEF and change in longitudinal strain. Second, this analysis was repeated with abnormal diastolic function grade at any follow-up visit as the predictor and subsequent change in LVEF or change in longitudinal strain as the outcome. Third, we evaluated E/e’ at both baseline and follow-up visits as continuous variables instead of diastolic function grade with change in LVEF or longitudinal strain as the outcomes. All models were adjusted for a parsimonious set of potential confounders, given concerns over sample size limitations in the lagged analyses and the limited number of events. These included: treatment regimen, baseline LVEF (or longitudinal strain), time since cancer therapy initiation (modeled as cubic splines interacted with treatment), age, hypertension, tobacco use and BMI. For the aforementioned models incorporating follow-up visit data, the follow-up predictor occurred subsequent to baseline and just prior to the outcome assessment. For example, the follow-up visit predictors were assessed at visit 2 with outcomes at visit 3; assessed at visit 3 and outcomes at visit 4, and so forth. We also explored worsening in diastolic function grade from baseline(defined categorically) as the exposure variable.
The above analyses were repeated using the same predictors as above with the outcome CTRCD using Cox proportional hazards models. All models were adjusted for baseline LVEF, age, hypertension, tobacco use, and BMI and used baseline hazards stratified by treatment regimen.
In regression models including variables with missing data we used complete case analysis, excluding observations with missing values for any of the covariates included in the model. Statistical significance was evaluated at a two-sided alpha level of 0.05. We did not perform a formal correction for multiple comparisons(27). All analyses were conducted using R 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population
In 362 participants followed for a median of 2.1 (IQR 1.1, 4.1) years, a median of 5 (IQR 4,7) echocardiograms per individual were quantified. Baseline demographic, clinical and echocardiographic characteristics are summarized in Table 1. The median age was 49 years, 70% were Caucasian and the median body mass index (BMI) was 26 kg/m2. Cardiac risk factors hypertension, hyperlipidemia, diabetes and current/former tobacco use were common, occurring in 32%, 21%, 9% and 38% of participants, respectively.
Table 1.
Baseline Demographic, Clinical and Echocardiographic Variables According to Treatment Regimen
| Overall (N = 362) | Doxorubicin (N = 219) | Trastuzumab (N = 83) | Doxorubicin+Trastuzumab (N = 60) | |
|---|---|---|---|---|
| Age (years), median (IQR) | 49.0 (41.0, 57.0) | 49.0 (41.0, 57.0) | 53.0 (44.0, 60.5) | 45.5 (38.8, 55.0) |
| Race, N(%) | ||||
| Caucasian | 253 (70) | 147 (67) | 69 (83) | 37 (62) |
| African American | 89 (25) | 59 (27) | 10 (12) | 20 (33) |
| Asian | 13 (4) | 6 (3) | 4 (5) | 3 (5) |
| Hispanic | 7 (2) | 7 (3) | 0 (0) | 0 (0) |
| BMI (kg/m2), median (IQR) | 26.4 (23.4, 31.4) | 26.5 (23.4, 31.8) | 26.1 (23.8, 30.5) | 27.1 (23.4, 31.2) |
| SBP (mmHg), median (IQR) | 126.0 (116.0, 135.0) | 125.0 (115.0, 134.8) | 129.0 (117.0, 140.0) | 124.0 (115.2, 132.2) |
| DBP (mmHg), median (IQR) | 74.0 (69.0, 81.0) | 75.0 (69.0, 82.0) | 74.0 (68.5, 81.0) | 74.0 (70.0, 79.2) |
|
Heart rate, median (IQR) |
79.0 (72.0, 89.0) | 79.5 (72.0, 89.0) | 77.0 (70.0, 89.0) | 79.5 (74.0, 88.0) |
| Hypertension, N (%) | 115 (32) | 66 (30) | 35 (42) | 14 (23) |
| Diabetes, N (%) | 33 (9) | 21 (10) | 9 (11) | 3 (5) |
| Hyperlipidemia, N (%) | 76 (21) | 47 (22) | 16 (20) | 13 (22) |
| Tobacco use, N (%) | ||||
| Never | 220 (62) | 130 (60) | 51(64) | 39 (66) |
| Current | 25 (7) | 12 (6) | 6 (8) | 7 (12) |
| Former | 110 (31) | 74 (34) | 23 (29) | 13 (22) |
| Angiotensin receptor blocker, N (%) | 26 (7) | 16 (7) | 9 (11) | 1 (2) |
| ACE inhibitor, N (%) | 32 (9) | 20 (9) | 8 (10) | 4 (7) |
| Beta blocker, N (%) | 28 (8) | 18 (8) | 7 (8) | 3 (5) |
| Calcium channel blocker, N (%) | 34 (9) | 17 (8) | 10 (12) | 7 (12) |
| HMG CoA reductase inhibitor, N (%) | 38 (11) | 22 (10) | 11 (13) | 5 (8) |
| Diuretics, N (%) | 42 (12) | 26 (12) | 12 (15) | 4 (7) |
| Disease site, N (%) | ||||
| Bilateral | 17 (5) | 10 (5) | 1 (1) | 6 (10) |
| Left-Sided | 171 (47) | 106(48) | 38 (46) | 27 (45) |
| Right-sided | 173 (48) | 103 (47) | 43 (52) | 27 (45) |
| Stage, N (%) | ||||
| 1 | 80 (22) | 33 (15) | 36 (43) | 11 (18) |
| 2 | 198 (55) | 135 (62) | 32 (39) | 31 (52) |
| 3 | 78 (22) | 50 (23) | 10 (12) | 18 (30) |
| 4 | 6 (2) | 1 (0.5) | 5 (6) | 0 |
| Radiation therapy, N (%) | 229 (65) | 137 (65) | 52 (63) | 40 (69) |
| Echocardiographic parameters, median (IQR) | ||||
| E/A | 1.2 (0.9, 1.4) | 1.2 (0.9, 1.4) | 1.2 (0.9, 1.4) | 1.2 (1.0, 1.5) |
| E/e’ average | 6.9 (5.6, 8.6) | 6.9 (5.4, 8.5) | 7.2 (6.2, 9.3) | 6.4 (5.7, 7.6) |
| LA volume index (mL/BSA) | 29.8 (23.3, 37.7) | 31.0 (24.0, 38.6) | 28.3 (23.1, 35.5) | 28.0 (22.7, 36.0) |
| Deceleration time (msec) | 170.0 (148.0,194.2) | 170.0 (141.8,194.2) | 175.0 (150.8,200.0) | 163.0 (148.0,190.0) |
| Septal e’ (cm/sec) | 9.0 (7.0, 11.0) | 9.0 (7.0, 11.0) | 9.0 (7.0, 10.0) | 10.0 (8.0, 11.0) |
| Lateral e’ (cm/sec) | 12.0 (9.0, 14.0) | 12.0 (9.0, 14.0) | 11.0 (9.0, 13.0) | 12.0 (10.0, 14.2) |
| TR velocity (m/sec) | 2.3 (2.1, 2.4) | 2.2 (2.1, 2.4) | 2.4 (2.2, 2.6) | 2.3 (2.1, 2.4) |
| IVRT (msec) | 94.0 (87.8, 104.0) | 94.5 (88.0, 104.0) | 93.0 (87.5, 96.8) | 96.0 (87.8, 104.5) |
| Diastolic dysfunction grade, N (%) | ||||
| 0 | 192 (53.0) | 110 (50.2) | 46 (55.4) | 36 (60.0) |
| Indeterminate | 25 (6.9) | 14 (6.4) | 5 (6.0) | 6(10.0) |
| 1 | 88 (24.3) | 56 (25.6) | 18 (21.7) | 14 (23.3) |
| 2 | 4 (1.1) | 3 (1.4) | 1 (12) | 0 (0.0) |
| 3 | 2 (0.6) | 2 (0.9) | 0 (0.0) | 0 (0.0) |
| Uncategorizable* | 51 (14.1) | 34 (15.5) | 13 (15.7) | 4 (6.7) |
| LVEF, Median (IQR) | 53.8 (51.2, 56.6) | 53.6 (50.5, 56.0) | 53.4 (51.8, 56.5) | 54.3 (51.8, 57.1) |
BMI refers to body mass index, CTRCD cancer therapeutics related cardiac dysfunction, SBP systolic blood pressure, DBP diastolic blood pressure, E early mitral inflow peak velocity (cm/sec), A late mitral inflow peak velocity,e’ pulse wave tissue Doppler of the lateral and septal annulus, IVRT isovolumic relaxation time, LA left atrium, LVEF left ventricular ejection fraction, TR tricuspid regurgitation
Uncategorizable values secondary to mitral inflow merging, tachycardia, or missingness in more than 1 diastolic function parameter
Numbers expressed as percentage may not equal 100% secondary to rounding
Sixty percent of the participants received doxorubicin without trastuzumab (Doxorubicin), 23% received trastuzumab without doxorubicin (Trastuzumab) and 17% received doxorubicin followed by trastuzumab (Doxorubicin+Trastuzumab). Sixty-five percent of participants also received radiation therapy. Compared to the Doxorubicin group, participants receiving Trastuzumab tended to be older and were more likely Caucasian, while participants in the Doxorubicin+Trastuzumab group tended to be younger with fewer comorbid risk factors such as hypertension and diabetes.
Baseline values of E/A, average E/e’ and LA volume index are noted in Table 1. A total of 94 (26%) participants had abnormal diastolic function grade at baseline, with the majority of these (24%) having grade 1 diastolic dysfunction. Four participants (1.3%) had grade 2 diastolic dysfunction prior to cancer therapy initiation; 0.6% had grade 3 diastolic dysfunction; 7% had indeterminate diastolic function and 14% were unable to be categorized secondary to tachycardia, mitral inflow merging, arrhythmias or inability to quantify greater than 2 diastolic function measures.
Longitudinal Changes in Echocardiographic Measures of Diastolic Function and Incident Diastolic Dysfunction with Cancer Therapy
Over time, there was a sustained, modest decrease in the E/A ratio from baseline in the Doxorubicin group, an initial increase then decrease in the Doxorubicin+Trastuzumab group, and no significant change in the Trastuzumab alone group (Central Illustration, Table 2). There were also modest, statistically significant, sustained reductions in lateral e’ and septal e’ and increases in E/e’ ratio in the Doxorubicin and Doxorubicin+Trastuzumab groups that were evident at 6 months and persisted at 3 years. In contrast, there were no significant changes in e’ or E/e’ ratio in the Trastuzumab group. Left atrial volume index decreased over 3 years of follow-up in all treatment groups, but initially increased in the Doxorubicin+Trastuzumab group. There were no consistent changes in deceleration time, IVRT, or TR velocity over time among the 3 treatment groups (Supplemental Table 1). Over a maximum follow-up time of 6.5 years, incident diastolic dysfunction developed in 184 of 258 participants with normal or indeterminate diastolic function grade at baseline and non-missing follow-up diastolic function grade (Figure 1). Based on Kaplan-Meier analysis, abnormal diastolic function grade was present in 60 % at 1 year; 70% by 2 years, and 80% by 3 years. A total of 18 participants (5% of the 349 participants analyzable baseline E/e’) developed an abnormal E/e’ ratio >14 over the course of follow-up, 104 participants developed a septal e’<7cm/sec and 128 participants developed lateral e’<10cm/sec.
Central Illustration: Changes in Diastolic Function with Doxorubicin and Trastuzumab Cancer Therapy.
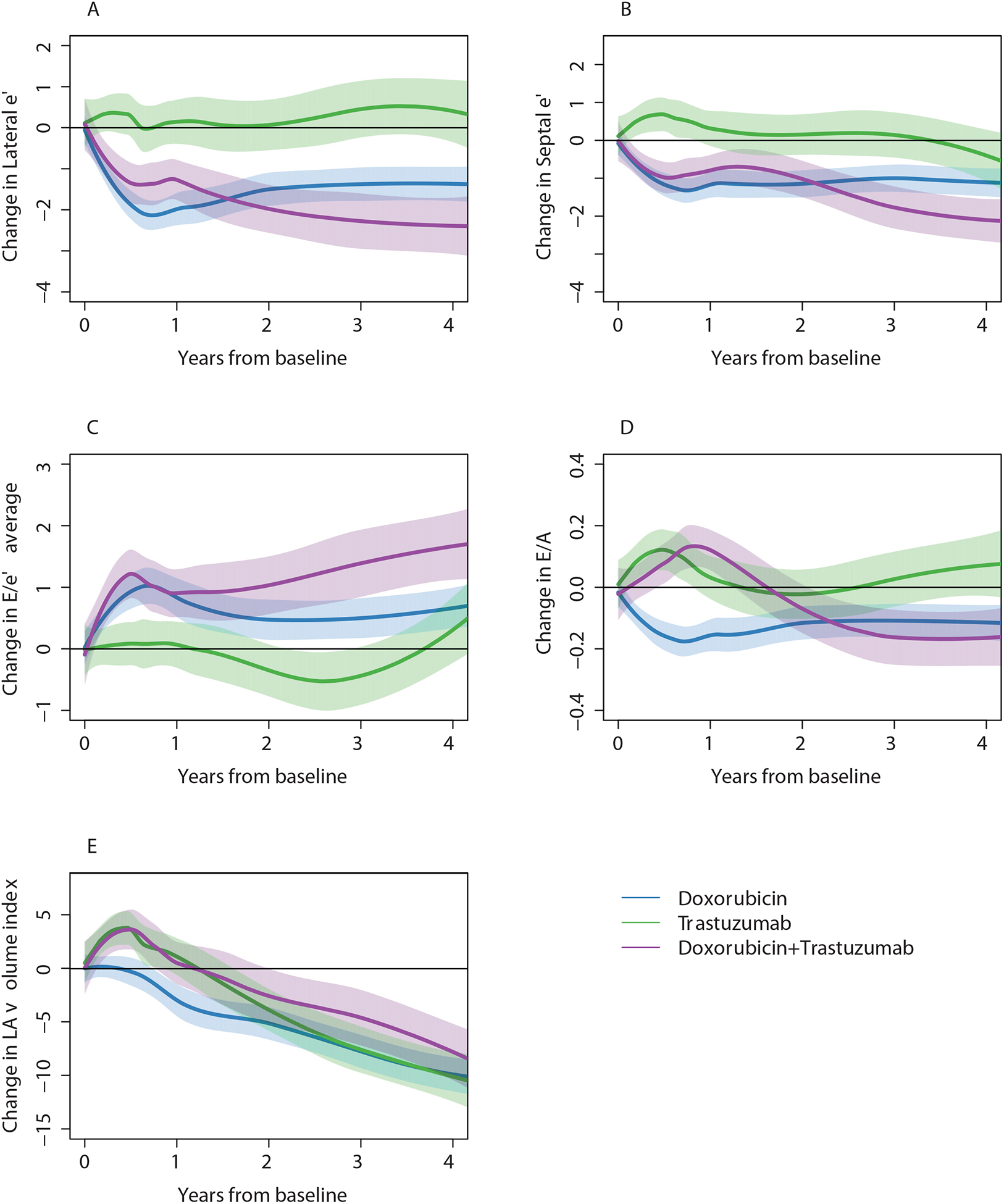
Smoothing splines and 95% CIs for change in diastolic parameters according to breast cancer treatment groups. E refers to early mitral inflow peak velocity (cm/sec), e’ to pulse wave tissue Doppler velocity (cm/sec), E/e’ is the average of septal and lateral E/e’, LA to left atrial volume index (mL/BSA). Blue denotes doxorubicin without trastuzumab; Green is trastuzumab without doxorubicin; Purple is doxorubicin and trastuzumab.
Table 2.
Estimated Changes in Diastolic Function Parameters Over Time According to Treatment Regimen
| Echocardiographic Parameter | Time since initiation of chemotherapy | Doxorubicin Mean Change (95% CI) | P-value | Trastuzumab Mean Change (95% CI) | P-value | Doxorubicin+ Trastuzumab Mean Change (95% CI) | P-value |
|---|---|---|---|---|---|---|---|
| E/A | 6 months | −0.1 (−0.2, −0.1) | <0.001 | 0.1 (0.0, 0.2) | 0.002 | 0.1 (0.0, 0.1) | 0.067 |
| 12 months | −0.1 (−0.2, −0.1) | <0.001 | 0.0 (−0.0, 0.1) | 0.419 | 0.1 (0.0, 0.2) | 0.012 | |
| 24 months | −0.1 (−0.2, −0.1) | <0.001 | 0.0 (−0.1, 0.1) | 0.764 | −0.1 (−0.2, −0.0) | 0.041 | |
| 36 months | −0.1 (−0.2, −0.0) | 0.001 | 0.0 (−0.2, 0.2) | 0.923 | −0.2 (−0.3, −0.1) | <0.001 | |
| Septal e’ (cm/sec) | 6 months | −1.0 (−1.3, −0.8) | <0.001 | 0.6 (0.3, 1.0) | 0.001 | −0.8 (−1.2, −0.4) | <0.001 |
| 12 months | −1.0 (−14, −0.7) | <0.001 | 0.1 (−0.4, 0.6) | 0.692 | −1.0 (−1.4, −0.5) | <0.001 | |
| 24 months | −1.1 (−1.4, −0.8) | <0.001 | −0.2 (−0.8, 0.4) | 0.546 | −1.5 (−1.9, −1.1) | <0.001 | |
| 36 months | −1.2 (−1.6, −0.8) | <0.001 | −0.4 (−1.1, 0.4) | 0.331 | −1.8 (−2.2, −1.3) | <0.001 | |
| Lateral e’ (cm/sec) | 6 months | −1.6 (−1.9, −1.3) | <0.001 | 0.3 (−0.1, 0.7) | 0.170 | −1.1 (−1.6, −0.6) | <0.001 |
| 12 months | −1.8 (−2.2, −1.4) | <0.001 | −0.2 (−0.9, 0.4) | 0.517 | −1.6 (−2.2, −1.0) | <0.001 | |
| 24 months | −1.6 (−2.0, −1.2) | <0.001 | −0.1 (−1.1, 0.9) | 0.815 | −2.0 (−2.8, −1.3) | <0.001 | |
| 36 months | −1.5 (−2.0, −1.0) | <0.001 | 0.1 (−1.0, 1.3) | 0.816 | −2.3 (−3.2, −1.5) | <0.001 | |
| E/e’ average | 6 months | 0.7 (0.4, 1.0) | <0.001 | 0.1 (−0.2, 0.5) | 0.502 | 0.9 (0.5, 1.3) | <0.001 |
| 12 months | 0.6 (0.3, 1.0) | <0.001 | 0.1 (−0.3, 0.6) | 0.600 | 1.1 (0.7, 1.6) | <0.001 | |
| 24 months | 0.5 (0.2, 0.9) | 0.001 | −0.3 (−0.9, 0.2) | 0.195 | 1.2 (0.6, 1.8) | <0.001 | |
| 36 months | 0.6 (0.2, 1.0) | 0.002 | −0.3 (−0.9, 0.3) | 0.283 | 1.3 (0.6, 2.0) | <0.001 | |
| LA volume index (mL/BSA) | 6 months | −0.9 (−2.1, 0.3) | 0.127 | 3.2 (1.5, 4.8) | <0.001 | 2.9 (0.7, 5.2) | 0.011 |
| 12 months | −2.5 (−4.0, −1.0) | 0.001 | 1.1 (−0.8, 2.9) | 0.255 | 1.9 (−0.5, 4.3) | 0.120 | |
| 24 months | −4.6 (−5.9, −3.2) | <0.001 | −2.5 (−4.9, −0.1) | 0.044 | −0.8 (−3.7, 2.0) | 0.570 | |
| 36 months | −5.9 (−7 7 −4 1) | <0.001 | −5.3 (−8 2 −2 5) | <0.001 | −3.8 (−7 1 −0 6) | 0.022 |
E refers to early mitral inflow peak velocity (cm/sec), A late mitral inflow peak velocity, e’ average mean pulse wave tissue Doppler of the lateral and septal annulus, LA left atrium.
Figure 1: Time to Worsening Diastolic Function Grade or Abnormal Diastolic Function Indices.
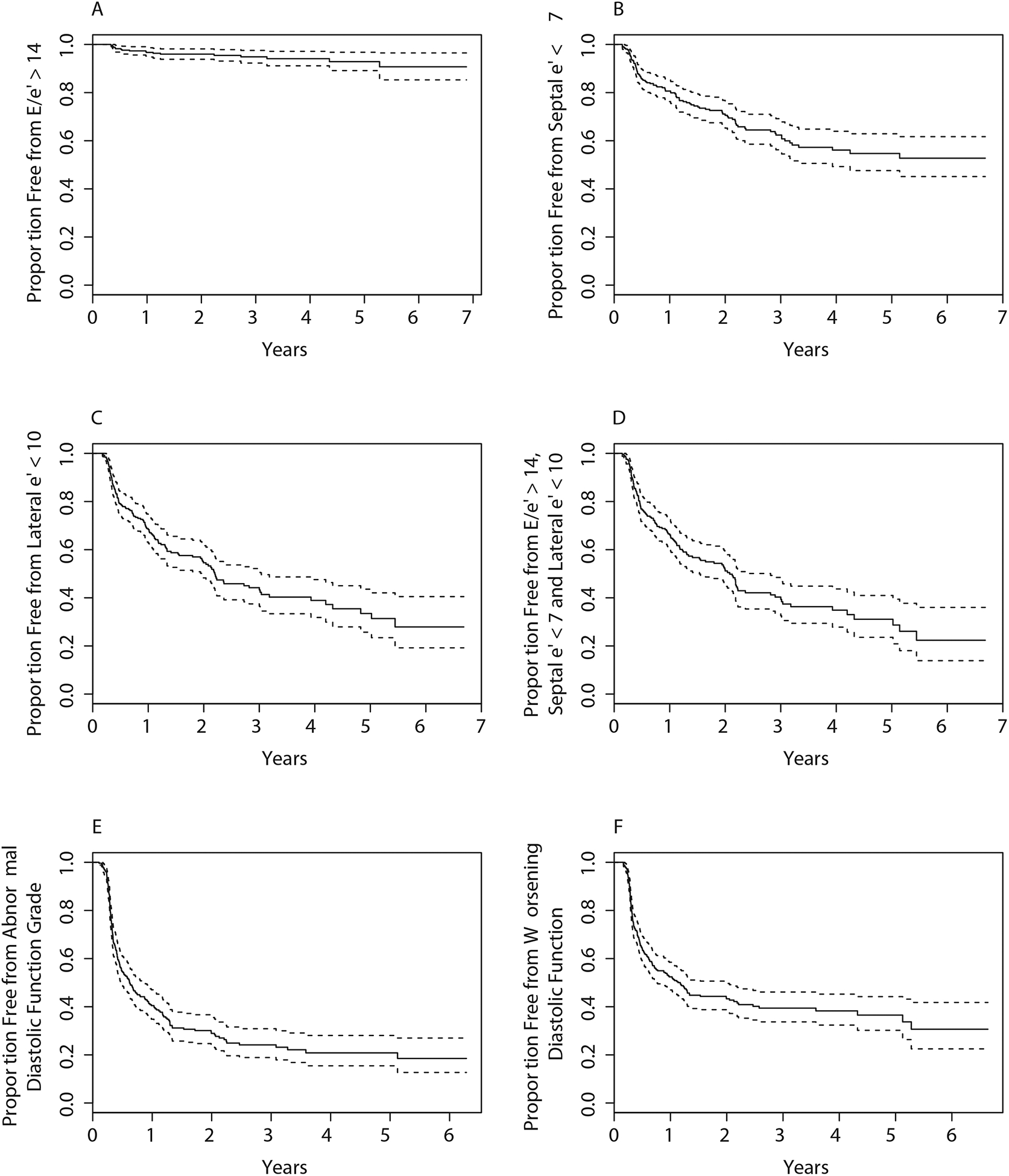
Kaplan-Meier plot illustrating the overall time to worsening diastolic function, as defined by E/e’>14 (A), septal e’<7 (B), lateral e’ <10 (C), E/e’>14 or septal e’<7 or lateral e’<10 (D), development of abnormal diastolic function grade amongst participants with normal or indeterminate diastolic function at baseline (E) or a worsening of grade amongst participants with a diastolic function grade <3 at baseline (F).
Clinical Predictors of Diastolic Dysfunction
In univariable models, no baseline characteristics were significantly associated with incident abnormal diastolic dysfunction grade, except for African American race, calcium channel blocker use, and radiation; the latter was associated with a decreasing hazard (Table 3). In multivariable models, only an inverse association with radiation was significant (p=0.001). In additional analyses evaluating E/e’>14 as the outcome measure, African-American race (HR 4.4, 95% CI 1.5, 13.1, p=0.007) and current tobacco use (HR 4.0, 95% CI 1.2, 12.9, p=0.022) were each associated with an increased hazard of developing diastolic dysfunction (Table 3). Doxorubicin-containing regimens tended to be associated with a greater risk of the development of E/e’> 14, although this was not statistically significant.
Table 3.
Associations between Baseline Demographic and Clinical Variables and Incident Diastolic Function or Elevated E/e’
| Variable | Univariable Association with Incident Diastolic Dysfunction by Grade | Multivariable Association with Incident Diastolic Dysfunction by Grade | Univariable Association with E/e’> 14 | Multivariable Association with E/e’>14 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (years) | 1.0 | (0.8, 1.1) | 0.488 | 1.0 | (0.8, 1.2) | 0.830 | 1.6 | (1.0, 2.5) | 0.031 | 1.5 | (0.9, 2.4) | 0.141 |
| African-American race (Reference = Caucasian) | 1.5 | (1.0, 2.1) | 0.034 | 1.4 | (1.0, 2.1) | 0.083 | 5.0 | (1.9, 12.8) | 0.001 | 4.4 | (1.5, 13.1) | 0.007 |
| Any anti-hypertensive medication | 1.1 | (0.8, 1.6) | 0.572 | 3.4 | (1.3, 8.6) | 0.010 | ||||||
| Angiotensin receptor blocker | 1.3 | (0.7, 2.3) | 0.441 | 2.0 | (0.5, 8.6) | 0.367 | ||||||
| ACE inhibitor | 1.0 | (0.5, 1.8) | 0.886 | 4.8 | (1.7, 13.4) | 0.003 | ||||||
| Beta blocker | 0.7 | (0.4, 1.4) | 0.355 | 5.8 | (2.0, 16.2) | 0.001 | ||||||
| Calcium channel blocker | 1.9 | (11, 3.3) | 0.021 | 7.2 | (2.8, 18.7) | <0.001 | ||||||
| HMG CoA reductase inhibitor | 1.4 | (0.9, 2.3) | 0.183 | 1.8 | (0.5, 6.1) | 0.368 | ||||||
| Diuretics | 1.0 | (0.6, 1.8) | 0.906 | 1.6 | (0.5, 5.4) | 0.479 | ||||||
| Hyperlipidemia | 1.4 | (1.0, 1.9) | 0.090 | 2.3 | (0.9, 6.0) | 0.079 | ||||||
| Hypertension | 1.3 | (0.9, 1.7) | 0.151 | 1.3 | (0.9, 2.0) | 0.151 | 4.9 | (1.8, 13.0) | 0.002 | 2.3 | (0.7, 8.0) | 0.185 |
| Diabetes | 0.8 | (0.4, 1.4) | 0.457 | 0.7 | (0.4, 1.3) | 0.230 | 3.1 | (1.0, 9.5) | 0.045 | 1.5 | (0.4, 5.2) | 0.502 |
| Current smoker | 0.8 | (0.4, 1.7) | 0.647 | 0.7 | (0.3, 1.5) | 0.348 | 6.0 | (1.9, 18.5) | 0.002 | 4.0 | (1.2, 12.9) | 0.022 |
| BMI (5 kg/m2) | 1.0 | (0.9, 1.1) | 0.957 | 1.0 | (0.8, 1.1) | 0.589 | 1.1 | (0.8, 1.5) | 0.626 | 0.7 | (0.5, 1.1) | 0.128 |
| SBP (10 mmHg) | 1.0 | (1.0, 1.1) | 0.366 | 1.5 | (1.1, 2.0) | 0.007 | ||||||
| DBP (10 mmHg) | 1.1 | (1.0, 1.3) | 0.125 | 1.3 | (0.8, 2.2) | 0.218 | ||||||
| Heart rate (10 bpm) | 1.1 | (1.0, 1.2) | 0.085 | 1.1 | (0.8, 1.6) | 0.576 | ||||||
| Doxorubicin (Reference = Trastuzumab) | 0.7 | (0.5, 1.0) | 0.054 | 0.7 | (0.5, 1.1) | 0.092 | 2.2 | (0.5, 10.0) | 0.295 | |||
| Doxorubicin+ Trastuzumab (Reference = Trastuzumab) | 1.0 | (0.6, 1.5) | 0.906 | 0.9 | (0.6, 1.5) | 0.742 | 2.4 | (0.4, 13.0) | 0.318 | |||
| Radiation | 0.5 | (0.3, 0.7) | <0.001 | 0.5 | (0.3, 0.7) | 0.001 | 0.8 | (0.3, 2.2) | 0.657 | |||
BMI refers to body mass index, bpm beats per minute, DBP to diastolic blood pressure, E/e’ to the early mitral inflow peak velocity (cm/sec) divided by the average of the pulsed wave tissue Doppler velocities at the septal and lateral basal regions (cm/sec), HR refers to Hazard Ratio. SBP to systolic blood pressure
Incident diastolic function is defined by abnormal diastolic dysfunction grade amongst participants with normal diastolic function grade at baseline; multivariable analysis includes 249 participants and 184 total abnormal diastolic function grade events. E/e’ refers to time to first E/e’ > 14 in those with and E/e’ 14 at baseline and includes 18 events.
Multivariable model adjusted for age, African American race, hypertension, diabetes, current smoking status, BMI, treatment regimen, and radiation therapy (time-varying).
Associations Between Baseline and Abnormal Diastolic Function and Subsequent Changes in Systolic Function
We then evaluated the associations between baseline diastolic function and systolic dysfunction. Over a maximum follow-up time of 6.5 years, CTRCD occurred in 61 (17%) of the entire cohort, 30 (14%) in the Doxorubicin group, 12 (15%) in the Trastuzumab group and 19 (32%) in the Doxorubicin+Trastuzumab group. Baseline abnormal diastolic function (grade >0) was not associated with a significant change in LVEF (beta=−0.2, 95% −1.4 1.0, p=0.748) or risk of CTRCD (HR 1.2, 95% CI 0.6, 2.3, p=0.647) (Supplementary Table 2). The development of abnormal diastolic function grade over time was however associated with a subsequent decline in LVEF (beta = −2.1%, 95% CI −3.1, −1.2, p<0.001) and worsening in longitudinal strain (beta = 0.6%, 95% CI 0.1, 1.1, p<0.013) (Table 4A). Similarly, a worsening in diastolic function grade from baseline was associated with a 1.4% decrease in LVEF from baseline (p=0.006) and increased hazard of subsequent CTRCD (HR 2.2, 95% CI 1.1, 4.3, p=0.028) (Supplemental Table 3). Neither baseline E/e’ ratio nor follow-up visit E/e’ ratio was associated with a subsequent decline in LVEF or CTRCD, although there was modest association with changes in E/e’ ratio and worsening of longitudinal strain at the subsequent visit (beta=0.1%, 95% CI 0.0, 0.2, p=0.022) (Table 4B). In these analyses, the median time between visits was 10 months (IQR 4,12). Altogether, these findings suggest that abnormalities in diastolic function over time are associated with subsequent systolic dysfunction.
Table 4A:
Associations between Abnormal Diastolic Function Grade and Subsequent Changes in LVEF, Time to CTRCD or Subsequent Changes in Longitudinal Strain
| Variable | Change in LVEF* | Time to Subsequent CTRCD† | Change in Longitudinal Strain* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P-value | HR | 95% CI | P-value | Beta | 95% CI | P-value | |
| Abnormal Diastolic Function Grade | −2.1 | (−3.1, −1.2) | <0.001 | 1.7 | (0.9, 3.5) | 0.127 | 0.6 | (0.1, 1.1) | 0.013 |
| Age (10 years) | 0.1 | (−0.3, 0.5) | 0.616 | 1.0 | (0.7, 1.4) | 0.983 | 0.4 | (0.1, 0.6) | 0.002 |
| Hypertension | −0.2 | (−1.2, 0.8) | 0.743 | 1.0 | (0.5, 2.1) | 0.919 | 0.5 | (0.0, 1.0) | 0.043 |
| Current smoker | 1.4 | (−0.4, 3.2) | 0.119 | 0.9 | (0.2, 3.8) | 0.856 | 1.0 | (0.3, 1.7) | 0.007 |
| BMI (5 kg/m2) | 0.5 | (0.1, 0.9) | 0.021 | 0.9 | (0.7, 1.2) | 0.621 | 0.2 | (−0.0, 0.3) | 0.117 |
| Baseline LVEF | −0.7 | (−0.8, −0.5) | <0.001 | 1.1 | (1.0, 1.2) | 0.038 | - | - | - |
| Baseline Longitudinal Strain | - | - | - | - | - | - | −0.8 | (−0.8, −0.7) | <0.001 |
BMI refers to body mass index, CTRCD to cancer therapeutics-related cardiac dysfunction, LVEF to left ventricular ejection fraction Abnormal diastolic function grade refers to any abnormal diastolic function grade at any follow-up visit prior to LVEF, CTRCD, or longitudinal strain assessment. In all models, outcome evaluated at the subsequent and not the same visit.
Generalized estimating equation beta coefficients reflect the mean difference in LVEF or longitudinal strain relative to baseline for each variable, adjusted for all variables included in this Table plus treatment regimen and time since cancer therapy initiation interacted with treatment, modeled using cubic splines. There are no effect estimates for treatment due to modeling strategies used.
Cox proportional hazards model, HR for time to CTRCD, adjusted for all variables included in this Table, and using stratified baseline hazards for treatment regimen. There are no effect estimates for treatment due to modeling strategies used. 35 CTRCD events in this analysis.
Table 4B:
Associations between Baseline and Follow-up Visit E/e’ and Subsequent Change in LVEF, Time to CTRCD or Change in Longitudinal Strain
| Variable | Change in LVEF* | Time to Subsequent CTRCD† | Change in Longitudinal Strain* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P-value | HR | 95% CI | P-value | Beta | 95% CI | P-value | |
| Baseline E/e’ | 0.2 | (−0.1, 0.4) | 0.314 | 0.9 | (0.8, 1.1) | 0.294 | −0.1 | (−0.2, 0.0) | 0.147 |
| Follow-up visit E/e’ | −0.0 | (−0.3, 0.2) | 0.716 | 1.0 | (0.9, 1.2) | 0.946 | 0.1 | (0.0, 0.2) | 0.022 |
| Age (10 years) | −0.1 | (−0.7, 0.4) | 0.610 | 0.9 | (0.7, 1.3) | 0.643 | 0.4 | (0.2, 0.7) | <0.001 |
| Hypertension | −0.6 | (−1.7, 0.5) | 0.318 | 1.5 | (0.8, 2.9) | 0.248 | 0.5 | (−0.0, 1.0) | 0.057 |
| Current smoker | 0.5 | (−1.5, 2.5) | 0.619 | 1.0 | (0.3, 3.3) | 0.989 | 0.4 | (−0.4, 1.1) | 0.332 |
| BMI (5 kg/m2) | 0.3 | (−0.2, 0.7) | 0.235 | 0.9 | (0.7, 1.2) | 0.583 | 0.1 | (−0.1, 0.3) | 0.343 |
| Baseline LVEF | −0.4 | (−0.6, −0.3) | <0.001 | 1.1 | (1.0, 1.1) | 0.114 | - | - | - |
| Baseline Longitudinal Strain | - | - | - | - | - | - | −0.8 | (−0.8, −0.7) | <0.001 |
BMI refers to body mass index, CTRCD to cancer therapeutics-related cardiac dysfunction, LVEF to left ventricular ejection fraction Follow-up visit E/e’ refers to the value at the visit after baseline, but prior to measurement of LVEF or longitudinal strain. In all models, outcome evaluated at the subsequent and not the same visit.
Generalized estimating equation beta coefficients reflect the mean difference in LVEF or longitudinal strain relative to baseline for each variable, adjusted for all variables included in this Table plus treatment regimen and time since cancer therapy initiation interacted with treatment, modeled using cubic splines. There are no effect estimates for treatment due to modeling strategies used.
Cox proportional hazards model, HR for time to CTRCD, adjusted for all variables included in this Table, and using stratified baseline hazards for treatment regimen. There are no effect estimates for treatment due to modeling strategies used. 48 CTRCD events in this analysis.
Discussion
Our study is the largest prospective study to date to evaluate changes in diastolic function with breast cancer therapy, to define the clinical factors associated with these changes, and to determine the associations between changes in diastolic function and subsequent systolic dysfunction. Our principal findings are as follows: (1) reductions in E/A ratio and e’, and increases in E/e’ ratio, changes all consistent with worsening diastolic function, occur early with doxorubicin exposure and persist over the long-term; (2) these adverse changes are not observed with trastuzumab alone; (3) abnormal or worsening diastolic function over time is modestly associated with systolic dysfunction, as defined by LVEF, CTRCD risk, and longitudinal strain; (4) baseline diastolic dysfunction is not associated with subsequent systolic dysfunction. Our findings provide definitive evidence that there are significant changes in diastolic function over time with modern breast cancer therapy. Abnormal or worsening in diastolic function precedes systolic dysfunction and is associated with an increased risk of CTRCD. An important clinical implication is that aggressive modification of cardiovascular risk factors associated with diastolic dysfunction in the general population (e.g. aggressive control of blood pressure, weight loss, exercise) be implemented prior to or with the onset of worsening of diastolic function, as these strategies may delay the progression of worsening diastolic and systolic dysfunction(10).
We found modest but statistically significant reductions in E/A, septal, and lateral e’ and an increase in the E/e’ ratio with doxorubicin therapy, all consistent with worsening diastolic function. Multiple large cohort studies have shown an association between diastolic dysfunction and risk of incident heart failure and mortality in the general population(9–12). Our study confirms prior smaller studies and a meta-analysis in anthracycline-treated patients with shorter follow-up that have suggested early changes in some of these parameters(17–24), and provides definitive evidence that early, sustained changes in diastolic function occur. We also specifically evaluated patients exposed to trastuzumab without anthracyclines, where there are less published data(24). In our prospective cohort study of 362 participants, with a median of 5 quantified echocardiograms per participant over a maximum of 6.5 years of follow up, changes in mitral inflow and tissue Doppler parameters occurred and persisted. The magnitude of the changes in e’ and E/e’ seen in the 6 months after anthracycline therapy are similar to the changes seen in a longitudinal population based study of the general population over a median follow-up of 4.7 years(28), suggestive of “accelerated aging” with cancer therapy. In this study by Kuznetsova, et al. of 650 participants, the cardiovascular risk profile (age, hypertension, BMI, and diabetes) was similar to our population. However, over an extended follow-up time of 4.7 years, mean changes in E/A were 0.08 cm/sec and E/e’ 0.4. In contrast, the changes in the doxorubicin subgroup in just 6 months were on the order of 0.1 cm/sec for E/A and 0.7 for E/e’. We postulate that the population with breast cancer exposed to doxorubicin is at risk for HF with preserved ejection fraction due to treatment factors, in combination with age, gender, elevated BMI, and other shared cardiovascular/oncologic risk factors. These early, sustained changes in diastolic function may thus have important long-term implications.
Many of the traditional risk factors for diastolic dysfunction such as older age, hypertension and diabetes(9) were not associated with incident diastolic dysfunction in our cohort. In addition, we did not find a consistent association with radiation therapy and diastolic dysfunction, potentially due to a longer latency period for the cardiac effects of radiation to occur. However, this may also be related to the need for a more granular assessment of radiation therapy delivery (e.g. dose to whole heart or cardiac substructures). Such detailed analyses are the subject of future work.
We found that baseline abnormal diastolic function grade was not associated with increased risk of reduced LVEF and CTRCD. However, abnormal or worsening diastolic function grade assessed after initiation of cancer therapy was associated with subsequent reductions in LVEF, longitudinal strain, or CTRCD. We did also find a modest association between changes in E/e’ ratio and worsening longitudinal strain. This suggests that changes in diastolic function may precede systolic dysfunction over an intermediate follow up period (median 10 months between echocardiographic assessments). Several small studies, on the order of less than 50 patients, have evaluated whether early changes in diastolic function with anthracycline chemotherapy predict later reductions in LVEF. These have yielded mixed results and have limited standardized followup over an extended time, and are thus unable to provide conclusive evidence(18,19,21). Our study provides an important contribution to the current literature by demonstrating that worsening diastolic function with the initiation of cancer therapy is associated with a modest decline in systolic function over an intermediate time interval. Importantly, long-term follow-up is necessary and ongoing to discern if this translates to an increased burden of overt HF, including HF with reduced and preserved LVEF. In the interim, however, an important implication of our findings would be for clinicians to aggressively modify cardiovascular risk factors through blood pressure control, exercise, and dietary modifications (factors related to worse diastolic function in the general population) prior to or at the immediate onset of worsening diastolic function.
There are limitations to this analysis that should be considered. First, although this is the largest cohort study to evaluate diastolic function changes in patients undergoing chemotherapy, we acknowledge that these effect sizes are small, and the number of severe HF events (urgent outpatient visits or hospitalizations) and elevated filling pressures (E/e’ ratio >14) are low. Moreover, we evaluated the relationship between diastolic function and LVEF changes at the immediate subsequent visit, which occurred at a median time of 10 months. Additional participants and follow up time will be necessary to establish the longer term clinical implications. Second, while we collected data on radiation treatment, we did not contour the cardiac substructures to determine the mean heart dose to the left ventricle or segments. This is the subject of future work. Third, although we carefully controlled for confounders using multivariable regression, as in any observational study we cannot exclude the presence of residual confounding. Given the limited number of CTRCD events, we could not fully assess the impact of additional confounders, including the initiation of cardiac medications during follow-up in these models. We believe that confounding and the definition of diastolic function grade are the most likely explanations behind our results demonstrating a HR<1 for the association between radiation and incident diastolic dysfunction grade. These analyses also excluded participants with baseline abnormal diastolic function grade - a population that may be more susceptible to diastolic dysfunction with radiation. Fourth, we did not have a control group without cancer therapy. However, in our analyses detailing the longitudinal changes over time, the trastuzumab group did not demonstrate significant changes compared to the doxorubicin groups. Lastly, we performed several analyses and did not correct for multiple comparisons, thus all significant associations should be considered in this context.
In summary, doxorubicin-based cancer therapy regimens are associated with a modest but sustained worsening of diastolic function parameters over a long duration of follow up. While abnormal diastolic function grade at baseline is not a marker of increased risk of LVEF declines, abnormal or worsening of diastolic function with therapy is associated with a small increased risk of systolic dysfunction, as defined by LVEF declines, worse longitudinal strain, and an increased risk of CTRCD. Further study is needed to determine whether the changes in diastolic function are associated with a longer-term risk of overt HF.
Supplementary Material
Funding:
This work is supported by NHLBI R01-HL118018 (Ky), McCabe Fellow Award (Philadelphia, PA, Ky), American Cancer Society Institutional Research Grant -78-002-30 (Atlanta, Georgia, Ky), NHLBI K23-HL095661 (Ky).
Abbreviations:
- LVEF
Left ventricular ejection fraction
- HF
Heart failure
- BMI
Body mass index
- MV
Mitral valve
- PW
Pulse-wave
- LA
Left atrial
- BSA
Body surface area
- DT
Deceleration time
- IVRT
Isovolumic relaxation time
- CW
Continuous wave
- TR
Tricuspid regurgitation
- CTRCD
Cancer therapeutics-related cardiac dysfunction
- GEE
Generalized estimating equations
- HR
Hazard ratio
Footnotes
Disclosures: No relevant disclosures.
References:
- 1.DeSantis CE,Lin CC,Mariotto AB,et al. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians.2014;64(4):252–71. [DOI] [PubMed] [Google Scholar]
- 2.Pinder MC,Duan Z,Goodwin JS,et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. Journal of clinical oncology. 2007;25(25):3808–15. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw PT,Stevens J,Khankari N,et al. Cardiovascular Disease Mortality Among Breast Cancer Survivors.Epidemiology.2016;27(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan HK,Finkelman B,French B,et al. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations With Ejection Fraction Decline, Recovery, and Heart Failure Symptoms Over 3 Years of Follow-Up.Circulation.2017;135(15):1397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardinale D,Colombo A,Bacchiani G,et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy.Circulation.2015;131(22):1981–8. [DOI] [PubMed] [Google Scholar]
- 6.Finkelman BS,Putt M,Wang T,et al. Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients With Breast Cancer.JACC.2017;70(2):152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez-MacGregor M,Zhang N,Buchholz TA,et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer.Journal of clinical oncology.2013;31(33):4222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiki H,Petersen IA,Scott CG,et al. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer.Circulation.2017;135(15):1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redfield MM,Jacobsen SJ,Burnett JC Jr.,et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic.JAMA.2003;289(2):194–202. [DOI] [PubMed] [Google Scholar]
- 10.Kane GC,Karon BL,Mahoney DW,et al. Progression of left ventricular diastolic dysfunction and risk of heart failure.JAMA.2011;306(8):856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aljaroudi W,Alraies MC,Halley C,et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction.Circulation.2012;125(6):782–8. [DOI] [PubMed] [Google Scholar]
- 12.Shah AM,Claggett B,Kitzman D,et al. Contemporary Assessment of Left Ventricular Diastolic Function in Older Adults:The Atherosclerosis Risk in Communities Study.Circulation.2017;135(5):426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledwidge M,Gallagher J,Conlon C,et al. Natriuretic peptide-based screening and collaborative care for heart failure:the STOP-HF randomized trial.JAMA.2013;310(1):66–74. [DOI] [PubMed] [Google Scholar]
- 14.Edelmann F,Wachter R,Schmidt AG,et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial.JAMA.2013;309(8):781–91. [DOI] [PubMed] [Google Scholar]
- 15.Faris R,Coats AJ,Henein MY.Echocardiography-derived variables predict outcome in patients with nonischemic dilated cardiomyopathy with or without a restrictive filling pattern.American heart journal.2002;144(2):343–50. [DOI] [PubMed] [Google Scholar]
- 16.Shah AM,Claggett B,Sweitzer NK,et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction:findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial.Circulation Heart failure.2014;7(5):740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho E,Brown A,Barrett P,et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors:a speckle tracking echocardiographic study.Heart.2010;96(9):701–7. [DOI] [PubMed] [Google Scholar]
- 18.Serrano JM,Gonzalez I,Del Castillo S,et al. Diastolic Dysfunction Following Anthracycline-Based Chemotherapy in Breast Cancer Patients:Incidence and Predictors.The oncologist.2015;20(8):864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Florescu M,Magda LS,Enescu OA,et al. Early detection of epirubicin-induced cardiotoxicity in patients with breast cancer.Journal of the American Society of Echocardiography:official publication of the American Society of Echocardiography. 2014;27(1):83–92. [DOI] [PubMed] [Google Scholar]
- 20.Stoodley PW,Richards DA,Boyd A,et al. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy.European heart journal cardiovascular Imaging.2013;14(3):228–34. [DOI] [PubMed] [Google Scholar]
- 21.Ganame J,Claus P,Eyskens B,et al. Acute cardiac functional and morphological changes after Anthracycline infusions in children.The American journal of cardiology.2007;99(7):974–7. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese V,Menna P,Annibali O,et al. Early Diastolic Dysfunction after Cancer Chemotherapy: Primary Endpoint Results of a Multicenter Cardio-Oncology Study.Chemotherapy.2018;63(2):55–63. [DOI] [PubMed] [Google Scholar]
- 23.Boyd A,Stoodley P,Richards D, et al. Anthracyclines induce early changes in left ventricular systolic and diastolic function:A single centre study.PloS one.2017;12(4):e0175544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagiub M,Nixon JV,Kontos MC.Ability of Nonstrain Diastolic Parameters to Predict Doxorubicin-Induced Cardiomyopathy:A Systematic Review With Meta-Analysis.Cardiology in review.2018;26(1):29–34. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF,Smiseth OA,Appleton CP,et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography:An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.European heart journal cardiovascular Imaging.2016;17(12):1321–60. [DOI] [PubMed] [Google Scholar]
- 26.Cardinale D,Colombo A,Sandri MT,et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition.Circulation.2006;114(23):2474–81. [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ.No adjustments are needed for multiple comparisons.Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 28.Kuznetsova T,Thijs L,Knez J,et al. Longitudinal changes in left ventricular diastolic function in a general population.Circulation Cardiovascular imaging.2015;8(4):e002882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


