Abstract
Context
Individuals with cystic fibrosis (CF) develop a distinct form of diabetes characterized by β-cell dysfunction and islet amyloid accumulation similar to type 2 diabetes (T2D), but generally have normal insulin sensitivity. CF-related diabetes (CFRD) risk is determined by both CFTR, the gene responsible for CF, and other genetic variants.
Objective
To identify genetic modifiers of CFRD and determine the genetic overlap with other types of diabetes.
Design and Patients
A genome-wide association study was conducted for CFRD onset on 5740 individuals with CF. Weighted polygenic risk scores (PRSs) for type 1 diabetes (T1D), T2D, and diabetes endophenotypes were tested for association with CFRD.
Results
Genome-wide significance was obtained for variants at a novel locus (PTMA) and 2 known CFRD genetic modifiers (TCF7L2 and SLC26A9). PTMA and SLC26A9 variants were CF-specific; TCF7L2 variants also associated with T2D. CFRD was strongly associated with PRSs for T2D, insulin secretion, postchallenge glucose concentration, and fasting plasma glucose, and less strongly with T1D PRSs. CFRD was inconsistently associated with PRSs for insulin sensitivity and was not associated with a PRS for islet autoimmunity. A CFRD PRS comprising variants selected from these PRSs (with a false discovery rate < 0.1) and the genome-wide significant variants was associated with CFRD in a replication population.
Conclusions
CFRD and T2D have more etiologic and mechanistic overlap than previously known, aligning along pathways involving β-cell function rather than insulin sensitivity. Two CFRD risk loci are unrelated to T2D and may affect multiple aspects of CF. An 18-variant PRS stratifies risk of CFRD in an independent population.
Diabetes is a frequent complication of cystic fibrosis (CF), an autosomal recessive disorder affecting more than 70 000 individuals worldwide. CF is caused by loss-of-function mutations in the CF transmembrane conductance regulator (CFTR) gene which is expressed in a variety of epithelial tissues including lungs and pancreas. CF-related diabetes (CFRD) has some aspects in common with type 1 diabetes (T1D) and type 2 diabetes (T2D), but is distinct from both. Development of CFRD involves a generally slow decline in β-cell function and increased production of islet amyloid (1, 2), as is the case for T2D. However, unlike T2D, individuals with CFRD generally have normal insulin sensitivity (except during pulmonary disease exacerbations or glucocorticoid treatment) (3). Although diabetes in CF is associated with some of the same long-term complications of T1D and T2D (3), the more important complications are more rapid decline in lung function and reduced survival (4).
The prevalence of CFRD increases with age, affecting more than 90% of individuals with common CFTR genotypes causing severe dysfunction by their sixth decade (5). However, age at onset varies considerably (eg, from 10 to 50 years old (5)), and it has been shown that CFRD onset is highly heritable independent of CFTR genotype (h2 = 0.98, with 95% confidence interval, 0.4–1.0) (6), indicating an important role for genetic modifiers. Evidence of shared heritability between CFRD and T2D was demonstrated by a family study in which a history of T2D in adult non-CF family members significantly increased the risk of CFRD (odds ratio, 3.1), and T2D susceptibility variants in TCF7L2 associated with CFRD (7).
In a genome-wide association study (GWAS) involving 3059 unrelated individuals with CF (644 with CFRD) (“GWAS phase 1” (8)), the International CF Gene Modifier Consortium identified genetic variants that associated with age at onset of CFRD within and 5′ of the SLC26A9 gene (hazard ratio [HR], 1.38; P value = 3.6e-8). In addition, the previous association of CFRD with variants in TCF7L2 based on candidate gene studies was supported and T2D-associated variants at 3 candidate loci CDKAL1, CDKN2A/B, and IGF2BP2 associated with CFRD (P value < 0.004). These 5 loci were estimated to account for 8.3% of the phenotypic variance in CFRD onset and had a combined population-attributable risk of 68% (8).
Given the partial phenotypic overlap between CFRD and T2D and the much earlier age at onset of diabetes in CF, we explored whether severe reduction in CFTR function in the presence of T2D risk alleles sensitizes individuals to the development of diabetes. To do this, we analyzed 2697 new subjects, updated the phenotypes of previously studied individuals (total 5740 individuals with CF; 5364 unrelated), and tested for association with CFRD using genome-wide markers. Comparison of association statistics with those for T2D and T1D, and polygenic risk scores (PRSs) for T1D, T2D, and other diabetes-related traits revealed deep commonality but also distinct endophenotypic differences in genetic architecture of T2D and CFRD.
Methods
Samples, genotyping, and quality control
A total of 5740 individuals with CF who have 2 severe CFTR mutations and/or exocrine pancreatic insufficiency were recruited in 2 phases. Phase 1 subjects are those included in the prior CFRD GWAS (8), excluding 16 subjects after additional data cleaning (n = 3043). These individuals were recruited from 3 cohorts (Johns Hopkins Twin and Sibling Study [TSS], Canadian CF Gene Modifier Study [CGS], and Genetic Modifier Study [GMS]) (8–10). Phase 2 subjects included individuals from the 3 cohorts (TSS, CGS, and GMS) who were recruited or acquired CFRD phenotype information since phase 1, or who were excluded from phase 1 for relatedness. Phase 2 also includes an additional cohort from the French CF Gene Modifier Consortium (phase 2, n = 2697). The GMS subjects in phase 1 were recruited for an extremes-of-phenotype study (severe vs mild CF lung disease), and were all F508del homozygotes; GMS subjects in phase 2 included all listed plus additional participants recruited without regard to genotype or lung function recruited by the GMS study, or by CF centers at Children’s Hospital of Denver, Wisconsin, and Boston (see (11) for more information). CGS participants were recruited from CF centers in Canada without regard to genotype or lung function. TSS participants were recruited from CF centers mostly in the United States based on having a surviving affected CF sibling. The French CF Gene Modifier Consortium recruited patients from 48 CF centers with both parents born in European countries. Subjects all met diagnostic criteria for CF and were required to have 2 severe CFTR mutations and/or exocrine pancreatic insufficiency.
An independent dataset of 591 individuals was included as a replication dataset (phase 2 replication dataset). These individuals were recruited in phase 1 and phase 2 as a part of the Johns Hopkins TSS, but not included in the test dataset because of a lack of phenotype information at the time of analysis. Updated phenotype information from the 2017 CFF Patient Registry revealed that 204 of these individuals had CFRD and 387 did not. This dataset was genotyped and imputed together with the phase 1 and phase 2 datasets.
Written informed consent was obtained from each participant and/or parents/guardians. Studies were approved by institutional review boards at participating sites and include: Committee on Clinical Investigation, Boston Children’s Hospital; Institutional Review Board at Children’s Hospital of Wisconsin; Colorado Multiple Institutional Review Board; Johns Hopkins School of Medicine eIRB2 (Committee: IRB-3); Research Ethics Board of The Hospital for Sick Children; Biomedical Institutional Review Board, Office of Human Research, University of North Carolina at Chapel Hill; and University Hospitals Case Medical Center, Institutional Review Board for Human Investigation. In France, the study was approved by the French ethical committee (CPP no. 2004/15) and the information collection was approved by Commission nationale de l’informatique et des libertés (no. 04.404).
Phenotypes were obtained from extracted medical charts and CF Foundation Patient Registry through 2011. CFRD was defined by clinician diagnosis of diabetes plus insulin treatment for at least 1 year. The onset of CFRD was defined as the date at which insulin was started, if it was subsequently continued for at least 1 year. In approximately 50% of the participants, independent laboratory data (such as oral glucose tolerance test or hemoglobin A1c) were able to independently confirm the diagnosis of CFRD. Diabetes data were censored at the last clinic visit or date of solid organ transplant. Participants diagnosed with T1D were excluded. Because phenotype data collection was done before clinical use of CFTR modulators, no participants were treated with CFTR modulators during this study.
Genotyping for phase 1 was performed on the Illumina Quad 610 platform. Genotyping for phase 2 was performed on the Illumina Quad 610 platform (for individuals typed for phase 1 but excluded from the CFRD analysis for relatedness), and on the Illumina 660W and Omni5 platforms (TSS, CGS, and GMS cohorts). The French cohort was genotyped on the Illumina 370K and 660K platforms (11). Genotype calling was performed using GenomeStudio V2011.1. Individuals were removed if the initial call rates were <95% or had extreme heterozygosity rates in which the threshold was described as in (9). Duplicated individuals/identical twins were identified using identity-by-descent/identity-by-state (IBD/IBS) estimation, and for each pair the one with a higher genotype call rate was kept.
Imputation and quality control
MaCH/Minimac software was used for phasing and imputation. The reference used was phase I, version 3, haplotype data from the 1000 Genomes project including all 1000 Genomes reference samples. Genotyped variants with a low minor allele frequency (MAF < 2%) and low call rate (<95%) were excluded before imputation. Imputed variants with a MaCH quality score r2 < 0.30 were excluded from the analysis.
Estimating population structure with principal components
Genotype principal components (PCs) were calculated using the EIGENSOFT package’s EIGENSTRAT method (12). Genotyped variants common to all platforms, with a MAF > 5% and not in linkage disequilibrium (pruned by PLINK indep parameter, with a 50-bp window size that shifts by 5 bases, and prunes based if r2 > 0.5) were included in the PC calculation. For PCs to not be influenced by related individuals, the PCs were calculated on unrelated individuals only (determined by IBD/IBS estimation in PLINK as individuals with a probability of identity <40%, which is individuals that are no more related then first-degree relatives), and projected onto the related individuals using the poplist flag.
Genetic association testing
A combined analysis was conducted on individuals who were included in the first CFRD GWAS (8) (phase 1), in addition to newly genotyped individuals who were not previously reported (phase 2). Because the majority of CF patients with exocrine pancreatic insufficiency are predicted to develop diabetes at some point, but the age at diagnosis of diabetes varies, a Cox proportional hazards (CoxPH) model was used to test for association (event = diagnosis of CFRD; time = age at CFRD diagnosis or age at last clinic visit if no CFRD). Four PCs and site (Johns Hopkins University, Hospital for Sick Children, University of North Carolina/Case Western Reserve University, or University of Pierre and Marie Curie) were included as covariates for the “unadjusted” analysis, and 4 PCs, site and sex were included as covariates for the “adjusted” analysis.
Because of the known presence of first-degree relatives (twins and siblings) and the possibility of additional unknown first-degree relatives within our cohorts, we determined relatedness among individuals based on IBD/IBS estimation in PLINK. Individuals with probability of identity > 40% were considered to be in the same nuclear family. Our analysis showed there were few second- or third-degree relatives in the study, and most relatives were nuclear families. To control for correlation among family members resulting in genomic inflation, we tested several different models on a subset of family samples from the phase 1 TSS patients (396 samples, 288 families): 1) CoxPH with all the samples ignoring within family correlation (13), 2) CoxPH with maximum unrelated individuals, 3) CoxPH with marginal model (cluster), 4) CoxPH with frailty model (random per-family effect), 5) mixed effects Cox model with family-specific random intercept (14), 6) mixed effects Cox model with kinship coefficient matrix as random intercept, and 7) mixed effects model using Martingale residual with kinship coefficient as random intercept (15). Based on the quantile–quantile plots and lambda values (lambda = 1.124, 1.028, 1.086, 1.025, 1, 1.097, 1.097 for the 7 models, respectively) of these analyses, we decided to use the CoxPH with frailty model to control for relatedness in all analyses (16). Using LD-score regression software (17), we determined that our GWAS has a lambda GC of 1.0345, intercept of 1.0288 (standard error [SE], 0.0067), and a ratio of 0.88 (SE, 0.2052), which demonstrates that population stratification has been adequately accounted for.
Analysis included 9 157 530 genotyped and imputed variants with MAF >2% and markers with imputation r2 > 0.3. Genome-wide significance was defined as P value < 5e-8. Suggestive significance was defined as P value < 1.8e-6 (8).
Estimating genetic correlation
Genetic correlation between T2D (taken from Diabetes Genetics Replication And Meta-analysis [DIAGRAM] (18)) and CFRD (our study) was estimated using GWAS summary statistics with the LD-score regression software, v1.0.0 (17, 19), command “-rg.”
Polygenic risk scores
Weighted PRSs were calculated for various phenotypes from published GWAS results, using no more than 1 variant per locus. For T2D, we used 221 primary variants available in our dataset from the body mass index–unadjusted analysis reported in the most recent T2D GWAS from the DIAGRAM consortium (18). For the T1D PRS, we included 48 T1D-associated variants available in our dataset reported by the Type 1 Diabetes Genetics Consortium (20). Of note, the T1D PRS does not include any variants at the human leukocyte antigen (HLA) locus.
For insulin secretion and action, we used PRSs that were previously generated (21) for homeostatic model assessment of β-cell function (HOMA-B; reflects insulin secretion; 20 variants) and homeostatic model assessment for insulin resistance (HOMA-IR; reflects insulin resistance; 10 variants) based on prior genetic and physiologic evidence reported in literature. We also constructed PRSs using subsets of T2D-associated variants that were classified as acting on insulin secretion versus insulin action by a recent study (22) that clustered phenotypic effects using z scores from published GWASs (23).
For postchallenge glucose concentration (2-hour plasma glucose; 2hPG; 9 variants) and fasting plasma glucose (FPG) levels (36 variants), we applied PRSs generated by a previous study (24) that included variants based on their association reported in another study (25). For the autoimmune thyroid disease PRS (7 variants), we used the variants reported in an association study (26); for islet autoimmunity (8 variants), we used associations reported in a recent The Environmental Determinants of Diabetes in the Young study (27); and for hemoglobin A1c (10 variants), we used variants reported from a meta-analysis of 23 GWASs on nondiabetic adults (28).
The PRSs were calculated as a log(OR) or effect size-weighted sum of risk alleles, normalized and scaled from 0 to 10. Lists of the variants included in each PRS are located in a digital research materials repository (29).
Weighted genome-wide T2D polygenic risk scores were constructed using PRSice-2 (30) off of summary statistics with various P value cutoffs for inclusion in the PRS. Clumping was performed with an r2 of 0.1 in 250-kb windows. The PRS with most significantly associating P value cutoff was reported.
PRSs were tested for association with CFRD onset using the CoxPH model with frailty, including 4 PCs and site as covariates in the discovery population, and including 4 PCs as covariates in the replication population (all individuals in the replication population were from Johns Hopkins University).
Direction of effect concordance analysis
P values and HRs of variants in the current study were compared with P values and odds ratios of each variant reported by DIAGRAM (18). Summary statistics for DIAGRAM were downloaded from http://diagram-consortium.org/downloads.html (T2D GWAS meta-analysis - unadjusted for body mass index). Variants at TCF7L2, CDKN2A/B, CDKAL1, and IGF2BP2 were removed. The remaining variants were LD-pruned (r2 > 0.1 within 500-kb windows) and sorted by their T2D and CFRD Fisher-combined P value. The percentage of variants with concordant direction of effect in CFRD and T2D was determined within bins spanning 1500 variants in 750 variant sliding windows. Chi-squared P values of each bin (1500 variants) were calculated in comparison to the expected: 750 variants (50%) concordant, 750 variants (50%) discordant.
Results
Variants at 3 loci were associated with age at onset of CFRD at genome-wide significance
Genome-wide association with CFRD age at onset was performed on 5740 subjects with CF with 2 severe CFTR mutations and/or clinically diagnosed exocrine pancreatic insufficiency, of which 1341 have CFRD (combined phase 1 and phase 2; see Table 1 and Methods). A separate replication dataset (phase 2 replication) was composed of 591 individuals (204 with CFRD). A CoxPH model was used to test for association (event = diagnosis of CFRD; time = age at CFRD diagnosis or age at last clinic visit if no CFRD). Study site and 4 PCs were included as covariates for an “unadjusted” analysis, and sex was included as an additional covariate for an “adjusted” analysis (29).
Table 1.
Characteristics of Patients Enrolled by the Studies Composing the International Cystic Fibrosis Gene Modifier Consortium
| Mean Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase | Study | Site | Total | Unrelated | DF/DF | Female | CFRD | CFRD | No CFRD | Both |
| 1 | TSS | JHU | 285 | 285 | 171 | 147 | 103 | 17.3 | 15.3 | 16 |
| CGS | HSC | 1496 | 1462 | 907 | 819 | 163 | 23.9 | 18.8 | 19.3 | |
| GMS | UNC/CWRU | 1262 | 1247 | 1213 | 674 | 373 | 23.3 | 22.4 | 22.7 | |
| 2 | TSS | JHU | 688 | 403 | 394 | 352 | 149 | 21.5 | 12.4 | 14.4 |
| CGS | HSC | 379 | 351 | 247 | 192 | 55 | 24.4 | 13.4 | 15 | |
| GMS | UNC/CWRU | 740 | 726 | 340 | 403 | 246 | 23.8 | 23.8 | 23.8 | |
| FrGMC | UPMC | 890 | 890 | 588 | 453 | 252 | 24 | 21.2 | 22 | |
| Total phase 1 and 2: | 5740 | 5364 | 3860 | 3040 | 1341 | 23 | 19.1 | 20 | ||
| 2R | TSS | JHU | 591 | 427 | 311 | 267 | 204 | 20.8 | 23.6 | 22.7 |
Abbreviations: 2R, phase 2 replication; CWRU, Case Western Reserve University; DF/DF, individuals homozygous for F508del variant; TSS, Twin and Sibling Study; CGS, Canadian CF Gene Modifier Study; FrGMC, French CF Gene Modifier Consortium; GMS, Genetic Modifier Study; JHU, Johns Hopkins University; HSC, Hospital for Sick Children; UNC, University of North Carolina; UPMC, University of Pierre and Marie Curie.
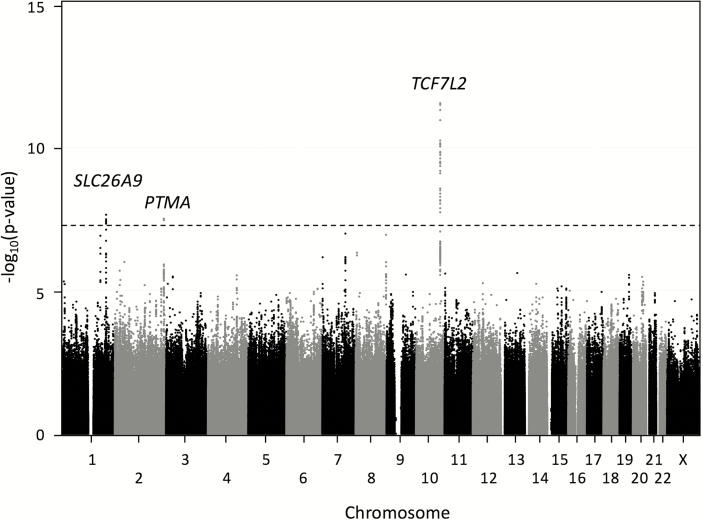
In the unadjusted analysis, variants exhibiting genome-wide significant association with CFRD age at onset were identified on chromosomes 1, 2, and 10 (Fig. 1, Table 2) (29). In the adjusted analysis, the variants on chromosomes 1 and 10 exceeded genome-wide significance (Table 2), and no additional locus exceeded genome-wide significance. Variants in 6 additional loci, RASAL2, UNQ6975, ELFN1, IMMP2L, MCPH1, and CYP11B2, were associated with CFRD with suggestive significance (P value < 1.8e-6) (29). For all loci, results were similar when CoxPH or linear regression using Martingale residuals were used to account for the different ages at onset of diabetes (data not shown). There was no significant heterogeneity in association based on site or platform for any locus (Fig. 2).
Figure 1.
Manhattan plot of phase 1 + 2 combined association analysis. Association analysis was performed on all variants with minor allele frequency (MAF) > 2% that passed quality control criteria. The x-axis indicates chromosomal position, and the y-axis indicates the strength of evidence for association with cystic fibrosis–related diabetes (-log10[P value]) by Cox proportional hazards regression including frailty model to allow for clustering by family. The black line corresponds to the genome-wide significance threshold (P value = 5e-8).
Table 2.
Association Statistics for Top Variant at Each Genome-Wide Significant Locus
| Chr | Position (hg19) | rsID | Risk/Alt Allele | RAF | Unadjusted P Value | HR | Adjusted P Value | Adjusted HR | % Genotyped | Annotation |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 114754071 | rs34872471 | C/T | 0.30 | 2.80e-12 | 1.51 | 1.30e-12 | 1.53 | 0% | TCF7L2 |
| 10 | 114758349 | rs7903146 | T/C | 0.30 | 4.88e-12 | 1.50 | 2.54e-12 | 1.52 | 100% | TCF7L2 |
| 1 | 205914757 | rs4077468 | A/G | 0.59 | 2.25e-8 | 1.38 | 4.12e-8 | 1.38 | 95.8% | SLC26A9 |
| 2 | 232560638 | rs838455 | T/C | 0.08 | 2.98e-8 | 1.75 | 7.60e-8 | 1.74 | 0% | PTMA |
| 2 | 232572011 | rs838440 | G/T | 0.09 | 3.45e-8 | 1.69 | 8.44e-8 | 1.69 | 100% | PTMA |
CFRD onset was analyzed as a censored trait (event = diagnosis of diabetes; censoring = age at last normal diabetes screening test). Unadjusted analysis includes adjustment for 4 principal components and site. Adjusted analysis also includes sex as a covariate. Frailty model was used to account for relatedness. Three loci were genome-wide significant (P value < 5e-8). The most significantly associated variants at each locus and the most significant genotyped variants at each locus are listed.
Abbreviations: % genotyped, percent of individuals who have been genotyped at this locus, as opposed to being imputed; Chr, chromosome; HR, hazard ratio; RAF, risk allele frequency.
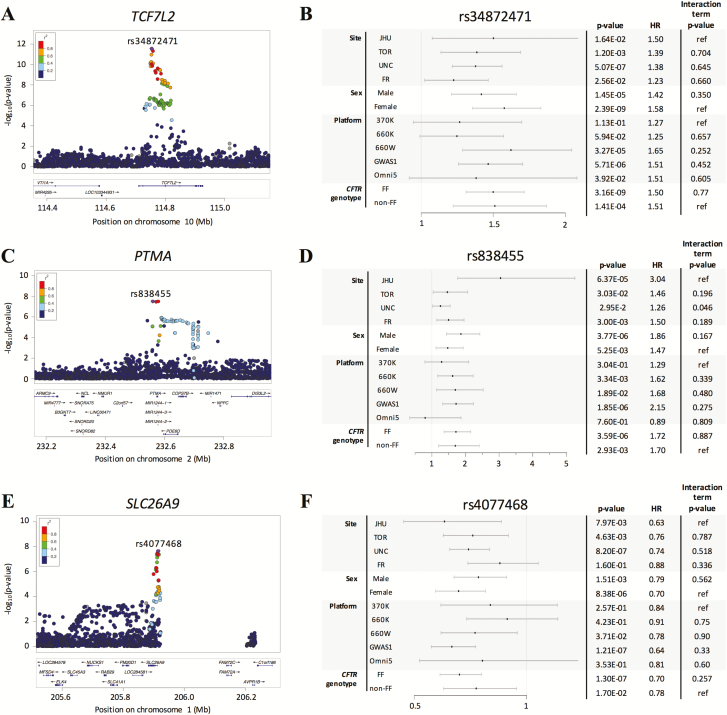
Figure 2.
LocusZoom and Forest plots of genome-wide significant loci, (A-B) TCF7L2, (C-D) PTMA, and (E-F) SLC26A9. Forest plots are shown for the top variant at each locus, rs34872471, rs838455, and rs4077468, respectively. To the right of the Forest plots are subset analyses P values and hazard ratios, in addition to interaction term P values for association of the interaction term between the variable (site, sex, platform, or CFTR genotype) and the variant.
The chromosome 10 locus contains associated variants within and around the TCF7L2 gene that exceed genome-wide significance (eg, rs34872471; combined phase 1 + 2 P value = 2.80e-12; phase 1 P value = 2.58e-06; phase 2 P value = 9.69e-8) (29). The same variants have been shown in several populations to be associated with increased T2D risk with the same direction of effect (31) and were previously reported to be associated with CFRD using a candidate gene-based approach (7, 8). CFRD-associated variants in TCF7L2 all appear to be in high LD (Fig. 2A), and conditional analysis showed that the CFRD-associated variants at this locus (including rs7903146) tag the same genetic association signal as rs34872471 (29). rs7903146 (T allele) and rs34872471 (C allele) showed evidence of association with CFRD in the phase 2 replication dataset (N = 591; 1-sided P values = 1.90e-02 and 2.41e-2, respectively), and an interaction term analysis demonstrated that the association with CFRD in the replication dataset and the test dataset were not significantly different (P values = 0.54 and 0.46, respectively). All variants that exceeded genome-wide significance at this locus fell into open chromatin regions intronic of TCF7L2 (29).
The chromosome 2 locus contains multiple associated variants surrounding genes PTMA, PDE6D, and COPS7B with the most significantly associated variant located 12.5 kb 5′ of PTMA (eg, rs838455; P value = 2.98e-8; HR, 1.75; 95% confidence interval, 1.43–2.13; Fig. 2B). Association of rs838455 with CFRD was replicated in the phase 2 replication dataset (N = 591; 1-sided P value = 2.37e-2; HR, 1.67). In the analysis adjusting for sex, the P values for variants at this locus were less significant in the phase 1 + 2 dataset (P value = 7.60e-8; HR, 1.74; Table 2), and more significant in the replication dataset (1-sided P value = 2.06e-2; HR, 1.71) Individuals from both phase 1 and phase 2 contribute to the association signal (29). Conditional analysis shows all CFRD-associated variants tag the same genetic association signal as rs838455 (29). None of these variants are significant expression quantitative trait loci (eQTL) for any gene within 100 kb in the pancreas, adipose tissue, brain, or muscle (GTEx, version7, dbGaP Accession phs000424.v7.p2) (32). Of the genome-wide significant variants, rs838440 is located in the region predicted to be the promoter for PTMA by chromatin state segmentation using a hidden Markov model from ENCODE/Broad in many cell types. This region has open chromatin based on DNase hypersensitivity tests across 125 cell types by ENCODE (29, 33) and falls on a transcription factor binding site of SMARCA4, VDR, RBL2, CTCF, FOS, and ETS1 according to the Open Regulatory Annotation database (34). Additionally, the ancestral allele at this variant position is conserved across species (Genomic Evolutionary Rate Profiling (GERP) score: 0.768) (35).
The third locus at chromosome 1 encompassed variants located 5′ and intronic of the SLC26A9 gene (Fig. 2C; rs4077468 P value = 2.25e-8; HR, 1.38), that were previously reported to be associated with CFRD in the phase 1 study and replicated in a phase 1 replication population (8). Association of rs4077468 with CFRD onset was demonstrated in analyses of the phase 2 individuals (some of whom were included in the phase 1 replication study (29); rs4077468 phase 2 P value = 1.21e-2; phase 1 P value = 1.20e-7) and the subset of phase 2 individuals who were not in the phase 1 replication study (n = 2170; P value = 1.1e-2). In the phase 2 replication dataset (N = 591), rs4077468 was not significantly associated with CFRD onset (P value = 0.65; HR, 1.06); however, interaction term analyses demonstrated that the effect size did not significantly differ between the replication dataset and test dataset (P value = 0.107), suggesting that the Phase 2 replication may be of limited power to further replicate association at this locus. Conditional analysis demonstrates that all of the significant variants tag the same genetic association signal as rs4077468 (29). The genotyping platforms all contain a 200-kb gap reflecting a former gap in the reference sequence.
To identify genes at the SLC26A9 locus whose expression might be affected by the CFRD modifier variants, eQTL data from GTEx (32) were analyzed. The variants in question are correlated not only with SLC26A9 expression in pancreas (eg, rs4077468, P value = 0.026; beta = 0.19) but also are significant eQTL for the adjacent gene, PM20D1 (rs4077468 eQTL P values: pancreas, 7.1e-3; subcutaneous adipose, 7.5e-6; visceral [omentum] adipose, 6.8e-4), raising the possibility that the CFRD modifier variants could affect SLC26A9 and/or PM20D1 expression. No variant that exceeded genome-wide significance at this locus stood out based on the chromatin state and conservation on that position (29).
CFRD risk correlates strongly with genetic risk of T2D, and weakly with that of T1D
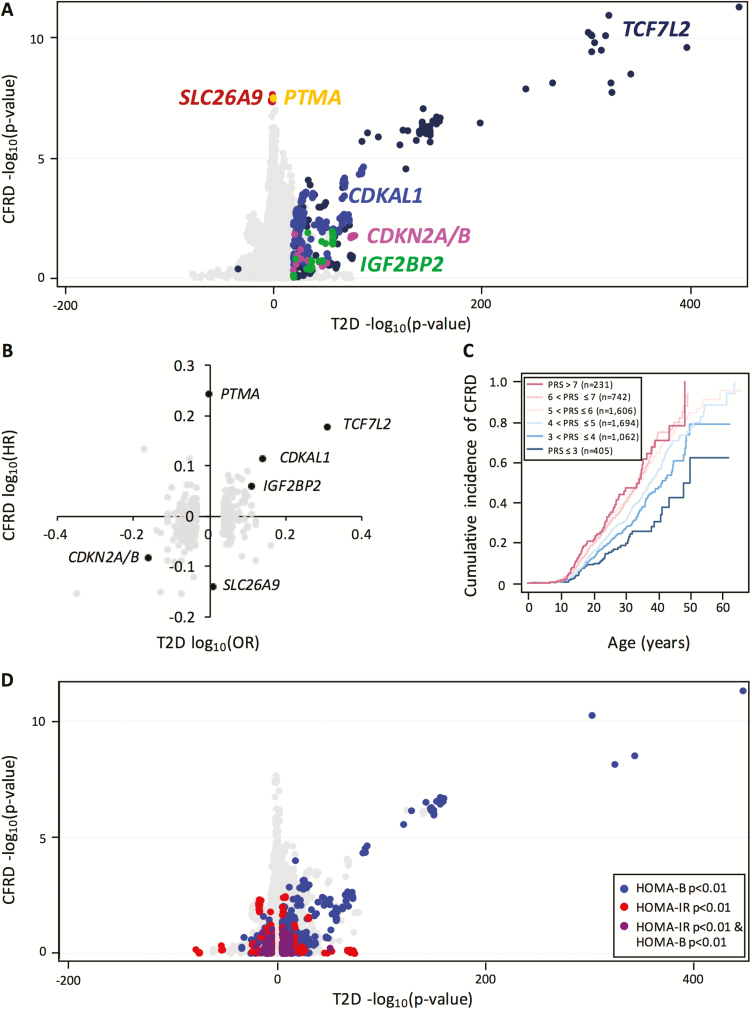
To test broadly for variants associated with both CFRD and T2D, we compared the P values of all variants from a T2D GWAS carried out by the DIAGRAM consortium (18) to the P values from our study (Fig. 3A) (29). The DIAGRAM consortium study is well powered and includes subjects of mostly European descent, similar to our study. Known T2D loci selected using a candidate approach that previously associated with age at onset of CFRD (at TCF7L2, CDKAL1, IGF2BP2, and CDKN2A/B) (7, 8) maintained significant association in this larger study (Fig. 3A) (29). Conversely, CFRD-associated variants at SLC26A9 and PTMA were not significantly associated with T2D (eg, SLC26A9 top variant rs4077468 P value = 0.13; PTMA top variant rs838455 P value = 0.89) (18) (Fig. 3A). Similarly, a comparison with the T2D GWAS (22) demonstrated that the odds ratio (current study) and HR (18) of variants at TCF7L2, CDKAL1, IGF2BP2, and CDKN2A/B were correlated, whereas that of variants at SLC26A9 and PTMA were not (Fig. 3B) (29).
Figure 3.
Comparison of the genetic risk architectures of CFRD and T2D. (A) Comparison of P values for each variant in the T2D (18) and CFRD (current study) genome-wide association studies. All genotyped and imputed variants have been plotted. Variants at known CFRD modifier loci have been colored and labeled. The T2D log10(P value) was defined as positive when the risk alleles were concordant between CFRD and T2D. (B) Comparison of CFRD log-transformed hazard ratios to T2D log-transformed odds ratios. The top variant of the loci that exceeded genome-wide significance (P value < 5e-8) in the T2D GWAS or our study has been plotted. Known CFRD modifiers have been labeled. (C) Cumulative incidence plot for CFRD onset in individuals divided into 6 bins based on their weighted T2D polygenic risk score. (D) Comparison of P values for each variant in the T2D and CFRD genome-wide association studies. All genotyped and imputed variants reported in the T2D (18), HOMA-B (36), and HOMA-IR (36) studies have been plotted. Variants associated with T2D (P value < 0.00001) were colored by association by HOMA-B (P value < 0.01; blue), HOMA-IR (P value < 0.01, red), both (purple). Variants that were not associated with HOMA-B, HOMA-IR, or T2D were colored gray. The T2D log(P value) was defined as positive when the risk alleles were concordant between CFRD and T2D. Abbreviations: CFRD, cystic fibrosis–related diabetes; HOMA-B, homeostatic model assessment of β-cell function; HOMA-IR, homeostatic model assessment for insulin resistance; T2D, type 2 diabetes.
To quantify the shared heritability between CFRD and T2D, we estimated the genetic correlation between the CFRD and T2D (18) GWAS summary statistics using LD-score regression (see Methods). We found that although the genetic correlation was high (0.6477 (ranges from -1 to 1), it was not statistically significant (SE, 0.983, P value = 0.51). To further assess the genetic overlap between T2D and CFRD, we created a log odds ratio–weighted PRS for T2D from distinct loci reported in the most recent T2D GWAS by DIAGRAM (18, 29) (see Methods). This T2D PRS was significantly associated with CFRD (P value = 8.84e-17; HR, 1.29 (29); Fig. 3C), and CFRD onset in individuals with high T2D PRSs (PRS > 6; n = 973) was at a significantly younger age than individuals with low T2D PRSs (PRS ≤ 4; n = 1467) (log-rank P value = 1.03e-13). After excluding the 4 loci associated with CFRD previously and in this study (see previous sections; TCF7L2, CDKAL1, CDKN2A/B, and IGF2BP2), the T2D PRS retained significant association with CFRD (P value = 1.45e-6; HR, 1.19). To assess which variants are responsible for the remaining association signal, we identified the variants that exceeded a false discovery rate (FDR) of 0.1 using the Benjamini-Hochberg procedure. Variants at 13 loci were significant; 10 novel (CEBPB, ADCY5, LTK, SLC2A2, ANK1, BCAR1, GLIS3, ETS1, SHQ1, and SLC30A8), and 3 previously known to influence CFRD (see previous sections; TCF7L2, CDKN2A/B, and CDKAL1) (29).
Two approaches were used to assess genetic overlap between CFRD and T2D beyond the variants associating with either trait with genome-wide significance. First, we constructed an optimized PRS using PRSice-2 (30) including variants with P values < 0.0025 (1067 variants) reported in the most recent T2D GWAS by DIAGRAM (18). This T2D PRS was also significantly associated with CFRD (P value = 1.80e-18; HR, 1.297) indicating that variants beyond those reaching genome-wide significance influence both CFRD and T2D. Second, we tested whether the direction of effect was concordant (ie, having the same risk allele for T2D and CFRD) more often than 50%, which would occur by chance. To do so, variants outside of TCF7L2, CDKAL1, IGF2BP2, and CDKN2A/B were LD-pruned (r2 > 0.1 within 500-kb windows), then sorted by their Fisher-combined P value for association with CFRD (this study) and T2D (DIAGRAM) (18). Among the top ranked 5000 variants, the CFRD and T2D risk alleles were the same for 2643 (instead of 2500 expected by chance; P value = 0.004), demonstrating that there could be >100 independent association signals that are associated with both CFRD and T2D. The percentage of variants within 1500-variant bins with concordant direction of effect in CFRD and T2D (29), and the chi-squared P values in comparison to expected were plotted (29); see Methods.
In contrast, a comparison of the association P values from a T1D GWAS (36) with the current study did not highlight any variants that appeared to associate with both diseases. Variants were either associated with CFRD only (eg, at SLC26A9, PTMA, and TCF7L2), T1D only (eg, at HLA, IGF2, and PTPN22), or neither phenotype (29). A T1D PRS constructed from independent non-HLA T1D variants reported (48 variants (20)) (29) showed weak evidence of association with CFRD (P value = 2.45e-02; HR, 1.08), and CFRD age at onset in individuals with higher T1D PRSs (PRS > 7; n = 535) and lower T1D PRSs (PRS ≤ 4; n = 760) were significantly different (log-rank P value = 0.02) (29). A genome-wide T1D PRS generated using PRSice-2 (30) (1029 variants; P value < 0.06; see Methods) was significantly associated with CFRD (P value = 7.1e-05; HR, 1.13), indicating that there might be some overlap between CFRD and T1D. Of the 1029 variants included in the PRS, 567 associated with CFRD and T1D in the same direction; however, none had an FDR < 0.1 with the Benjamini-Hochberg procedure. Of note, the T1D PRSs did not include variants at the HLA locus, which has a large effect size on T1D. Variants at the HLA locus did not associate with CFRD (29).
CFRD is associated with genetic risk for reduced β-cell function, not insulin resistance
To evaluate the effect of genetic risk for insulin secretion and insulin action on CFRD, we constructed trait-specific PRSs in 3 different ways. First, PRSs were made based on PRSs previously constructed (21) for HOMA-B (20 variants) and HOMA-IR (10 variants). A second pair of PRSs included T2D-associated variants classified as influencing either insulin secretion (14 variants; 10 overlapping with HOMA-B), or insulin action (7 variants; 4 overlapping with HOMA-IR) (22). Third, PRSs were constructed using PRSice-2 (30) from summary statistics of genome-wide association studies on HOMA-B and HOMA-IR (37) (3940 and 233 variants, respectively).
The HOMA-B and the insulin secretion PRSs significantly associated with CFRD onset (P value = 1.47e-09; HR, 1.192, and P value = 7.57e-18; HR, 1.247, respectively). Similar results were obtained after removing variants known to influence CFRD (P value = 9.92e-03; HR, 1.081; and P value = 1.20e-02; HR, 1.078, respectively) (29). Of the variants included in the HOMA-B PRS, variants at 9 loci had an FDR < 0.1 with the Benjamini-Hochberg procedure; 5 were novel loci (ADCY5, SLC30A8, MAEA, GLIS3, and DGKB), of which ADCY5, GLIS3, and SLC30A8 were also significant in the T2D PRS FDR analysis (29). The remaining 4 loci are known to be associated with CFRD (see previous sections; TCF7L2, CDKAL1, CDKN2A/B, IGF2BP2) (29). Of the 14 variants included in the insulin secretion PRS, 7 significantly associated with CFRD (FDR < 0.1), all of which were included in the HOMA-B PRS and had an FDR < 0.1 (at TCF7L2, CDKAL1, CDKN2A/B, IGF2BP2, ADCY5, SLC30A8, and GLIS3). In addition, the HOMA-B PRS constructed using PRSice-2, which includes 3940 variants with P value < 0.02757 was significantly associated with CFRD (P value = 1.50e-03; HR, 1.103), demonstrating overlap in the genetic risk variants for CFRD and insulin secretion outside of the most significantly associated variants.
Both the insulin action PRS and the HOMA-IR PRS did not associate with CFRD (P value = 0.949; HR, 0.998; and P value = 0.903, HR, 1.004, respectively). An optimized PRS constructed using PRSice, including variants with P value < 2.54e-4 in the HOMA-IR GWAS (233 variants) showed weak evidence of association with CFRD (P value = 0.02, HR, 1.070) (29). None of the variants included in the PRS individually associated with CFRD with an FDR < 0.1. Thus, genetic risk for reduced β-cell function (HOMA-B and insulin secretion PRSs) was strongly associated with CFRD, whereas the genetic risk for insulin resistance (HOMA-IR and insulin action PRSs) was associated with CFRD either weakly or not at all. This is also illustrated in Fig. 3D, in which variants associated with HOMA-B (blue) (37) tended to be associated with CFRD, whereas variants associated with HOMA-IR (red) (37) were not.
In addition, we constructed PRSs for postchallenge glucose concentration (2hPG; 9 variants (24)), FPG (36 variants (24)), autoimmune thyroid disease (7 variants; (26)), islet autoimmunity (8 variants (27)), and hemoglobin A1c (10 variants; (28)). Each PRS was constructed from variants reported in previous studies to be associated with the respective phenotype, with 1 variant per locus included (Methods) (29). The 2hPG and FPG PRSs were associated with CFRD onset (P value = 2.99e-8 and 1.25e-4, respectively); however, upon removal of variants previously known to influence CFRD, the association was no longer significant. The autoimmune thyroid, islet autoimmunity, and hemoglobin A1c PRSs were not associated with CFRD onset.
Construction of a CFRD PRS
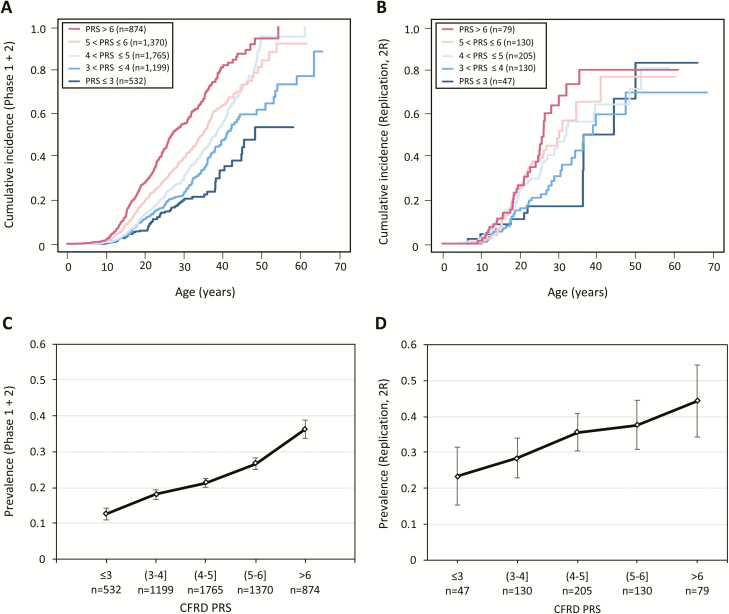
With a more expansive list of variants that modify CFRD, including 3 genome-wide significant loci and 15 variants that exceeded FDR of 0.1 from the either or both of the T2D and HOMA-B PRSs, we constructed a log-HR weighted CFRD PRS from these loci (n = 18) (29), which, as expected, was associated with CFRD in our discovery population (P value = 2.7e-51, HR, 1.54; Fig. 4A and C). This PRS was validated by demonstrating an association with CFRD onset in the independent replication population (n = 591; 204 with CFRD; P value = 5.8e-6; HR, 1.35; Fig. 4B and D), and the received operating characteristic area under the curve was 0.5798. A 16-variant PRS not including the CF-specific loci (at SLC26A9 and PTMA) was also significantly associated with CFRD in the replication population (1.90e-5; HR, 1.29).
Figure 4.
Cumulative incidence and prevalence plots by CFRD PRS in test and replication populations. Cumulative incidence plots of incidence of CFRD in (A) phase 1 + 2 populations and (B) replication population. Individuals are divided into bins based on their CFRD PRS. Prevalence plots of CFRD in (C) phase 1 + 2 populations and (D) replication population, divided by CFRD PRS bin. Abbreviations: CFRD, cystic fibrosis–related diabetes; PRS, polygenic risk score.
In addition, we constructed a CFRD PRS using PRSice-2 (30) which included 188 variants with a P value < 0.001 in the discovery sample. This PRS was also significantly associated with CFRD (P value = 3.1e-03; HR, 1.21), and the receiver operating characteristic area under the curve was 0.5312.
Association at CFRD modifier loci are not CFTR genotype specific
We tested to see whether the modifier variants were acting in a CFTR genotype-specific fashion. F508del is the most common CF-causing variant in CFTR. It causes misfolding of the CFTR protein, leading to degradation and absence of CFTR at the cell surface. We conducted F508del homozygote only (n = 2303) and non-F508del homozygote only (n = 1800) subset association analysis on the genome-wide significant loci to see if association signals were specific to either subgroup. Additionally, we conducted a F508del homozygosity (in which homozygosity defined as 0 or 1) interaction term analysis to determine whether the associations between CFRD and the modifier variants in F508del homozygotes and non-F508del homozygotes are different. Association signals at TCF7L2, SLC26A9, PTMA, CDKAL1, CDKN2A/B, and IGF2BP2 did not show any F508del-specific effect (29).
Discussion
This study provides evidence that genetic modification of CFRD incorporates pathways both in common with and dissimilar to T2D, and that T2D and CFRD have etiologic and mechanistic overlap to greater depth than previously known. We had previously demonstrated that family history of T2D substantially increases the risk for developing CFRD (odds ratio, 3.1) and that variants within TCF7L2, CDKAL1, CDKN2A/B, and IGF2BP2 that influence T2D (38–40) are associated with CFRD (7, 8). The genetic overlap contrasted with clinical and pathophysiologic differences; people with CFRD tend to have normal insulin sensitivity and reduced/abnormal production of insulin unlike in T2D which is due to a combination of reduced insulin sensitivity and insufficient production of insulin.
In the current study, GWAS has identified a novel modifier locus on chromosome 2 (PTMA), and replicated the previous association of modifier variants at the SLC26A9 locus, which were both specific to CFRD. On the other hand, T2D risk variants at TCF7L2 were associated with CFRD with genome-wide significance, and the newly genotyped samples provided replication of variants at IGF2BP2, confirming that there is a genetic overlap between T2D and CFRD. Moreover, multiple lines of evidence demonstrated that additional T2D susceptibility loci influence CFRD.
A key advance from this work was an increased understanding of the relationship between CFRD and related metabolic traits. Because genetic variants known to influence complex phenotypes such as diabetes are now better understood, PRSs can be used to explore the genetic overlap between various diabetes endophenotypes. Here, we demonstrated that PRSs for insulin secretion, HOMA-B, FPG, and 2hPG in the general population are associated with CFRD. An in-depth look revealed that the variants associated with both T2D and CFRD were frequently associated with HOMA-B (β-cell function), and none with HOMA-IR (insulin sensitivity) (Fig. 3D). Taken together, these results indicated that, in general, CFRD is modified by variants that tend to affect β-cell function rather than insulin sensitivity.
TCF7L2 is a well-known T2D-associated locus and has been shown through candidate studies that it also influences CFRD (7, 8). Previous studies have shown that rs7903146, 1 of the most significantly T2D- and CFRD-associated variant in TCF7L2, falls in a FOXA2 binding site (41), is located in islet-selective open chromatin (42), alters enhancer activity (42), and is considered a plausible causal variant for earlier onset of diabetes. Many studies have examined how variants at TCF7L2 contribute to diabetes; however, the mechanism remains unknown (43). TCF7L2 encodes a transcription factor, and it has been shown that reduction of TCF7L2 inhibits insulin secretion (44, 45). This could similarly influence age CFRD onset as well because a key feature of CFRD is decreased insulin secretion. Alternatively, a study has shown that rs7903146 influences the expression of a nearby gene, ACSL5, an acyl-CoA enzyme essential for fatty acid metabolism (46).
Additional T2D susceptibility genes also contribute to CFRD risk. By direction of effect concordance analysis, we found evidence that more than 100 additional independent loci are associated with both CFRD and T2D. Of the variants comprising the T2D PRS, 10 novel variants (in addition to 3 previously identified variants) were significantly associated with CFRD using an FDR-based approach. Of these, the top variant at CEBPB (rs1169802, C allele) is in high LD with the variant (rs2094716, A allele) associated with meconium ileus in individuals with CF (47). A similar FDR-based approach taken with the HOMA-B PRS identified 2 additional loci (MAEA, DGKB) that contribute to this CFRD association.
Three loci were identified by both the T2D and HOMA-B FDR analyses (ADCY5, GLIS3, and SLC30A8). ADCY5 encodes an adenylate cyclase enzyme that catalyzes the production of cyclic adenosine monophosphate, which is a second messenger molecule involved in insulin secretion. A previous study has shown that the risk allele at this locus disrupts an islet enhancer, resulting in reduced ADCY5 expression and impaired insulin secretion (48); it is plausible that variants at this locus are acting on CFRD through the same mechanism. Defective expression of Glis3 has been shown to drive increased levels of beta cell apoptosis and senescence in nonobese diabetic mice (49). SLC30A8 transports zinc from the cytoplasm to insulin secretory granules in the pancreatic β-cells, and variants at this locus are associated with lower β-cell function and lower plasma insulin levels (50). Consistently, all 3 of these loci are thought to influence T2D by affecting insulin secretion; therefore, their association with CFRD demonstrates that genetic predisposition to decreased insulin secretion increases risk for CFRD.
Studies have shown that a T1D PRS is good at discriminating T1D from T2D (51) and can be used to identify patients with T2D who rapidly progress to insulin therapy (52). Interestingly, we found some evidence of overlap between the genetic architecture of CFRD and T1D using PRSs, even though PRSs for the T1D-related endophenotypes islet autoimmunity and autoimmune thyroiditis were not associated with CFRD. Previous studies reported that HLA haplotypes associated with T1D and autoantibody levels are not risk factors for CFRD (53, 54). An FDR approach did not reveal any individual variant within the T1D PRS that associates with CFRD; therefore, a more highly powered analysis on more individuals will be needed to dissect the basis of the genetic overlap between T1D and CFRD.
The newly identified locus on chromosome 2 contains a compelling candidate gene, PTMA. PTMA encodes Prothymosin-α (ProT), which is involved in oxidative stress, inflammation, cell proliferation, and apoptosis, all of which can plausibly be argued to be involved with CFRD pathogenesis. Recently, a study of 185 T2D and nondiabetes subjects showed that the serum PTMA level was higher in T2D patients compared with healthy subjects (55). PTMA may also affect insulin sensitivity by acting as a ligand for Toll-like receptor 4 (56). Additionally, transgenic mice overexpressing ProT had insulin resistance, and silencing hepatic ProT expression in mice ameliorated high-fat diet-induced insulin resistance. Insulin sensitivity in CF can be substantially reduced at times of acute pulmonary exacerbation, which is often a time of increased inflammation. Furthermore, a study has shown that thymosin alpha could rectify the multiple tissue defects of CF mice and cells from subjects with the most common CF-causing mutation, F508del (57), though another group was unable to replicate these findings (58). We hypothesize that this variant is influencing PTMA expression, though it does not appear so in GTEx, perhaps because the eQTL calculations in GTEx are based on whole tissue instead of being cell-type specific. The CFRD-associated variants at this locus are also located within and near 3’ genes COPS7B and PDE6D. COPS7B is a protein that is a component of the COP9 signalosome complex, which is involved in regulation of the ubiquitin conjugation pathway. PDE6D is a phosphodiesterase involved in the phototransduction cascade. Either of these genes could also be implicated in CFRD, although there is no evidence to date of these genes acting on diabetes in the general population.
The variants associated with CFRD at the SLC26A9 locus are 5′ and intronic (all noncoding), suggesting a role in gene regulation. SLC26A9 encodes a bicarbonate and chloride transport protein (59, 60) and is a good biologic candidate as a modifier of CFRD for several reasons. First, in vitro studies have shown that SLC26A9 interacts with CFTR via its STAS domain and PDZ-binding motif (61). Second, a CFRD-associated variant in SLC26A9 was associated with exocrine pancreatic dysfunction (assessed by immunoreactive trypsinogen levels at birth) (62). Third, the CFRD-associated variants in and near SLC26A9 have also been associated with risk for meconium ileus in CF (9), a complication that requires the presence of pancreatic exocrine insufficiency (63); these modifier variants colocalized with eQTL in the pancreas (47). Finally, Slc26a9 knockout mice with CF manifest increased rates of mortality resulting from intestinal obstruction (64). These CFRD-associated variants are thought to delay age at onset of CFRD by increasing the expression of SLC26A9 in pancreatic ductal cells (65). Although SLC26A9 is a compelling candidate as a causal gene, the CFRD modifier variants at SLC26A9 are also significantly associated with expression of a neighboring gene, PM20D1. The PM20D1 gene is located 63 kb downstream of SLC26A9 and encodes for a secreted enzyme that acts as a regulator of N-acyl amino acids. PM20D1 is expressed across many tissues (32) and has been found to be involved in energy expenditure in brown and beige adipocytes, where it leads to reduced fat mass and lowered glucose (66). Thus, both PM20D1 and SLC26A9 are plausible candidate genes for CFRD modifiers.
There are several limitations of this study. First, diabetes is defined by data available in the clinical chart and/or CF Foundation Patient Registry, which does not always include laboratory confirmation. Our previous analysis found good agreement between the clinical and laboratory-based diagnoses of diabetes (8). Also, annual updates of the patient registry provide increasingly accurate ascertainment of CFRD as the cohort ages. Second, as with any association study, these data do not implicate any specific variant or gene as causal for CFRD. Further investigation is required to identify the molecular mechanisms implicated in this study. Another limitation of the study is sample size; although this is a large group for a Mendelian disease to study complex phenotypes such as diabetes, a much larger sample size would have increased power to detect more genetic variants. Additionally, most of the variants included in this study were imputed, which could have imputation errors. We removed variants that were poorly genotyped or imputed; this could have resulted in missing some associated loci.
Studying modifiers of Mendelian disorders is not only informative of that disorder, but also could be informative for the general population. By comparing summary statistics of T2D and CFRD GWASs, we identified 2 risk loci that are CFRD specific (at SLC26A9 or PTMA), and 16 loci that influence both CFRD and T2D (such as TCF7L2 and CDKAL1). The overlapping variants tend to affect β-cell function rather than insulin sensitivity, supporting the hypothesis that CFRD is less related to insulin resistance than insulin secretion. Investigations of these differences hold the promise of delivering insight into overlapping and nonoverlapping molecular etiologies of CFRD and T2D. Studying sequence data of a larger cohort and conducting functional studies on our variants and genes of interest will be useful to further test hypotheses on variants influencing CFRD.
Acknowledgments
The authors are grateful for the participation of the many CF patients, families, research coordinators, and clinicians in the Cystic Fibrosis Twin and Sibling Study, the Genetic Modifiers of Cystic Fibrosis Study, and the Canadian Consortium for Cystic Fibrosis Genetic Studies. In addition, we would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data from the participants in this study, and the patients, care providers, and clinic coordinators for their contributions to the CF Foundation Patient Registry (67). The authors also thank Jackie Stonebraker, Marci Sontag, Hara Levy, Pauline Touche, Tania Kombi, and Julie Mésinèle for assistance in preparing the samples.
Glossary
Abbreviations
- 2hPG
2-hour plasma glucose
- CF
cystic fibrosis
- CFRD
cystic fibrosis–related diabetes
- CoxPH
Cox proportional hazards
- eQTL
expression quantitative trait loci
- FDR
false discovery rate
- FPG
fasting plasma glucose
- GWAS
genome-wide association study
- HLA
human leukocyte antigen
- HOMA-B
homeostatic model assessment of β-cell function
- HOMA-IR
homeostatic model assessment for insulin resistance
- HR
hazard ratio
- MAF
minor allele frequency
- PC
principal component
- ProT
Prothymosin-α
- PRS
polygenic risk score
- SE
standard error
- T1D
type 1 diabetes
- T2D
type 2 diabetes.
Financial Support: This work was supported in part by the National Institutes of Health (R01 HL068927 to G.R.C.), United States Cystic Fibrosis Foundation (CUTTIN06P0, BLACKMG0, KNOWLE00A0, KNOWLE16XX0, BLACKM16G0, and CORVOL16G0), and the Gilead Sciences Research Scholars Program in Cystic Fibrosis (to S.M.B.). This work was supported in part by Genome Canada through the Ontario Genomics Institute (as per research agreement 2004-OGI-3-05 with the Ontario Research Fund), Research Excellence Program, the Ontario Ministry of Research and Innovation Early Researcher (to L.J.S.), Cystic Fibrosis Canada grant (#2626 to L.J.S.), Canadian Institutes of Health Research (MOP 258916) (to L.J.S.) Institut National de la Santé et de la Recherche Médicale (to H.C.), Sorbonne Université, Assistance Publique-Hôpitaux de Paris (to H.C.), Agence Nationale de la Recherche (to H.C.), DGS (to H.C.), and Association Vaincre La Mucoviscidose (to H.C.). M.A.A. was supported in part by training grant T32GM07814. Funding for genome-wide genotyping was provided by the United States Cystic Fibrosis Foundation.
Disclosure Summary: The authors have nothing to disclose.
Web Resources
All locus zoom plots were created via University of Michigan’s LocusZoom tools.
MaCH/Minimac software, http://www.sph.umich.edu/csg/abecasis/MACH/index.html
Version 3 haplotype data from 1000 Genomes project, ftp://ftp-trace.ncbi.nih.gov/1000genomes/ftp/release/20110521/
Eigensoft, http://www.hsph.harvard.edu/alkes-price/software/
GTEx, https://gtexportal.org
References
- 1. Couce M, O’Brien TD, Moran A, Roche PC, Butler PC. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab. 1996;81(3):1267–1272. [DOI] [PubMed] [Google Scholar]
- 2. Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89(8):3629–3643. [DOI] [PubMed] [Google Scholar]
- 3. Moran A, Becker D, Casella SJ, Gottlieb PA, Kirkman MS, Marshall BC, Slovis B; CFRD Consensus Conference Committee Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care. 2010;33(12):2677–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J, Thomas W, Moran A. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med. 2015;191(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackman SM, Hsu S, Vanscoy LL, Collaco JM, Ritter SE, Naughton K, Cutting GR. Genetic modifiers play a substantial role in diabetes complicating cystic fibrosis. J Clin Endocrinol Metab. 2009;94(4):1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blackman SM, Hsu S, Ritter SE, et al. . A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia. 2009;52(9):1858–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackman SM, Commander CW, Watson C, et al.. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes. 2013;62(10):3627–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun L, Rommens JM, Corvol H, et al. . Multiple apical plasma membrane constituents are associated with susceptibility to meconium ileus in individuals with cystic fibrosis. Nat Genet. 2012;44(5):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright FA, Strug LJ, Doshi VK, et al. . Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nat Genet. 2011;43(6):539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corvol H, Blackman SM, Boëlle PY, et al. . Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat Commun. 2015;6:8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. [DOI] [PubMed] [Google Scholar]
- 13. Therneau TM. A package for survival analysis in S. version 2.38. 2015.
- 14. Therneau TM. Package ‘coxme’, version 2.2–7. 2018.
- 15. Therneau T. The lmekin function. 2018. [Google Scholar]
- 16. Ling H, Zhang P, Pugh EW, Atalar M, Blackman SM. A comparison of methods for identification of genetic variants related to age-of-onset of cystic fibrosis related diabetes. In: The 2017 Annual Meeting of the International Genetic Epidemiology Society; 2017; Queens’ College Cambridge, UK: 644–709. [Google Scholar]
- 17. Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, Daly MJ, Price AL, Neale BM; Schizophrenia Working Group of the Psychiatric Genomics Consortium LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahajan A, Taliun D, Thurner M, et al. . Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bulik-Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Onengut-Gumuscu S, Chen WM, Burren O, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47(4):381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vassy JL, Hivert MF, Porneala B, et al. . Polygenic type 2 diabetes prediction at the limit of common variant detection. Diabetes. 2014;63(6):2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott RA, Scott LJ, Mägi R, et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. 2017;66(11):2888–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manning AK, Hivert MF, Scott RA, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stančáková A, Kuulasmaa T, Kuusisto J, et al. . Genetic risk scores in the prediction of plasma glucose, impaired insulin secretion, insulin resistance and incident type 2 diabetes in the METSIM study. Diabetologia. 2017;60(9):1722–1730. [DOI] [PubMed] [Google Scholar]
- 25. Scott RA, Lagou V, Welch RP, et al. Nat Genet. 2012;44(9):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cooper JD, Simmonds MJ, Walker NM, et al. Seven newly identified loci for autoimmune thyroid disease. Hum Mol Genet. 2012;21(23):5202–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharma A, Liu X, Hadley D, et al. Identification of non-HLA genes associated with development of islet autoimmunity and type 1 diabetes in the prospective TEDDY cohort. J Autoimmun. 2018;89:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soranzo N, Sanna S, Wheeler E, et al. Common variants at 10 genomic loci influence hemoglobin A1(C) l evels via glycemic and nonglycemic pathways. Diabetes. 2010;59(12):3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aksit M. Data from: cystic fibrosis-related diabetes & type 2 diabetes supplemental data. 2019. doi: 10.17605/OSF.IO/AG6ZW, OSF.IO/AG6ZW. [DOI]
- 30. Euesden J, Lewis CM, O’Reilly PF. PRSice: polygenic risk score software. Bioinformatics. 2015;31(9):1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin T. Current understanding on role of the Wnt signaling pathway effector TCF7L2 in glucose homeostasis. Endocr Rev. 2016;37(3):254–277. [DOI] [PubMed] [Google Scholar]
- 32. GTEx Consortium T he genotype-tissue expression (GTEx) project. Nat Genet. 2013;45(6):580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ENCODE Project Consortium A n integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lesurf R, Cotto KC, Wang G, Griffith M, Kasaian K, Jones SJ, Montgomery SB, Griffith OL; Open Regulatory Annotation Consortium ORegAnno 3.0: a community-driven resource for curated regulatory annotation. Nucleic Acids Res. 2016;44(D1):D126–D132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper GM, Stone EA, Asimenos G, et al. Distribution and intensity of constraint in mammalian genomic sequence. Genome Research. 2005;15:901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cauchi S, Froguel P. TCF7L2 genetic defect and type 2 diabetes. Curr Diab Rep. 2008;8(2):149–155. [DOI] [PubMed] [Google Scholar]
- 39. Weedon MN. The importance of TCF7L2. Diabet Med. 2007;24(10):1062–1066. [DOI] [PubMed] [Google Scholar]
- 40. Grant SF, Thorleifsson G, Reynisdottir I, et al. . Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320–323. [DOI] [PubMed] [Google Scholar]
- 41. Gaulton KJ, Ferreira T, Lee Y, et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47(12):1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaulton KJ, Nammo T, Pasquali L, et al. . A map of open chromatin in human pancreatic islets. Nat Genet. 2010;42(3):255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adams JD, Vella A. What can diabetes-associated genetic variation in TCF7L2 teach us about the pathogenesis of type 2 diabetes? Metab Syndr Relat Disord. 2018;16(8):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. da Silva Xavier G, Loder MK, McDonald A, Tarasov AI, Carzaniga R, Kronenberger K, Barg S, Rutter GA. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes. 2009;58(4):894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes. 2008;57(3):645–653. [DOI] [PubMed] [Google Scholar]
- 46. Xia Q, Chesi A, Manduchi E, et al. . The type 2 diabetes presumed causal variant within TCF7L2 resides in an element that controls the expression of ACSL5. Diabetologia. 2016;59(11):2360–2368. [DOI] [PubMed] [Google Scholar]
- 47. Gong J, Wang F, Xiao B, et al. . Genetic association and transcriptome integration identify contributing genes and tissues at cystic fibrosis modifier loci. Plos Genet. 2019;15(2):e1008007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roman TS, Cannon ME, Vadlamudi S, et al. A type 2 diabetes-associated functional regulatory variant in a pancreatic islet enhancer at the ADCY5 locus. Diabetes. 2017;66(9):2521–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dooley J, Tian L, Schonefeldt S, et al. . Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet. 2016;48(5):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rutter GA, Chimienti F. SLC30A8 mutations in type 2 diabetes. Diabetologia. 2015;58(1):31–36. [DOI] [PubMed] [Google Scholar]
- 51. Oram RA, Patel K, Hill A, Shields B, McDonald TJ, Jones A, Hattersley AT, Weedon MN. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016;39(3):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grubb AL, McDonald TJ, Rutters F, et al. . A type 1 diabetes genetic risk score can identify patients with GAD65 autoantibody-positive type 2 diabetes who rapidly progress to insulin therapy. Diabetes Care. 2019;42(2):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gottlieb PA, Yu L, Babu S, et al. . No relation between cystic fibrosis-related diabetes and type 1 diabetes autoimmunity. Diabetes Care. 2012;35(8):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minicucci L, Cotellessa M, Pittaluga L, et al. . Beta-cell autoantibodies and diabetes mellitus family history in cystic fibrosis. J Pediatr Endocrinol Metab. 2005;18(8):755–760. [DOI] [PubMed] [Google Scholar]
- 55. Su YC, Ou HY, Wu HT, et al. . Prothymosin-α overexpression contributes to the development of insulin resistance. J Clin Endocrinol Metab. 2015;100(11):4114–4123. [DOI] [PubMed] [Google Scholar]
- 56. Mosoian A, Teixeira A, Burns CS, et al. . Prothymosin-alpha inhibits HIV-1 via Toll-like receptor 4-mediated type I interferon induction. Proc Natl Acad Sci U S A. 2010;107(22):10178–10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Romani L, Oikonomou V, Moretti S, et al. . Thymosin α1 represents a potential potent single-molecule-based therapy for cystic fibrosis. Nat Med. 2017;23(5):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tomati V, Caci E, Ferrera L, et al. . Thymosin α-1 does not correct F508del-CFTR in cystic fibrosis airway epithelia. JCI Insight. 2018;3(3):e98699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J Biol Chem. 2002;277(16):14246–14254. [DOI] [PubMed] [Google Scholar]
- 60. Loriol C, Dulong S, Avella M, et al. . Characterization of SLC26A9, facilitation of Cl(-) transport by bicarbonate. Cell Physiol Biochem. 2008;22(1-4):15–30. [DOI] [PubMed] [Google Scholar]
- 61. Chang MH, Plata C, Sindic A, et al. . Slc26a9 is inhibited by the R-region of the cystic fibrosis transmembrane conductance regulator via the STAS domain. J Biol Chem. 2009;284(41):28306–28318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Soave D, Miller MR, Keenan K, et al. . Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: a Mendelian randomization study. Diabetes. 2014;63(6):2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Blackman SM, Deering-Brose R, McWilliams R, et al. . Relative contribution of genetic and nongenetic modifiers to intestinal obstruction in cystic fibrosis. Gastroenterology. 2006;131(4):1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu X, Li T, Riederer B, et al. . Loss of Slc26a9 anion transporter alters intestinal electrolyte and HCO3(-) transport and reduces survival in CFTR-deficient mice. Pflugers Arch. 2015;467(6):1261–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lam AN, Aksit MA, Vecchio-Pagan B, et al. . Increased expression of anion transporter SLC26A9 delays diabetes onset in cystic fibrosis. J Clin Invest. 2020;130(1):272–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Long JZ, Svensson KJ, Bateman LA, et al. . The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell. 2016;166(2):424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, Elbert A, Petren KM, Marshall BC. The cystic fibrosis foundation patient registry. Design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13(7):1173–1179. [DOI] [PubMed] [Google Scholar]