Abstract
Neurological diseases and injuries have profound impact on a patient’s lifespan and functional capabilities, but often lack effective intervention strategies to address the underlying neuropathology. The blood-brain barrier (BBB) is a major hurdle in the effective delivery of therapeutics to the brain. Recent discoveries in BBB maintenance reveal a dynamic system where time of day, disease progression, and even biological variables all strongly influence its permeability and flux of molecules. Nanoparticles can be used to improve the efficacy of therapeutics by increasing circulation time, bioavailability, selectivity, and controlling the rate of payload release. Considering these recent findings, the next generation of pharmacological paradigms are evolving to leverage nanotechnology to turn therapeutic intervention to meet the needs of a specific patient (i.e. personalized medicine).
Keywords: Blood-brain barrier (BBB), Nanoparticle (NP), traumatic brain injury (TBI), circadian rhythm, drug delivery
1. Introduction
Neurological disorders afflict approximately 50 million Americans annually and cost hundreds of billions of dollars in expenses and lost productivity [1]. Effective clinical therapies that address the underlying pathology for many of these conditions are limited. The main obstacle for central nervous system (CNS) pharmaceutical therapies is robust administration to the brain/spinal cord parenchyma. Potential delivery routes to the brain include three main approaches: delivery across the blood-brain barrier (BBB), intranasal delivery, and intrathecal delivery (cerebral spinal fluid; CSF). Intranasal delivery bypasses the BBB via direct absorption through the olfactory and trigeminal nerves. Uptake and delivery are limited in this method since the drug carrier must reach but not pass the olfactory region of the nasal cavity. Therefore, the drug carrier size and the patient’s strength of breath significantly impact pharmacokinetics/pharmacodynamics [2]. Intrathecal injection directly into the CSF allows for rapid and efficient bulk drug delivery with limited systemic dispersion. While this route is an emerging form of CNS drug delivery [3], intrathecal injection is relatively invasive, and rapid drug clearance from the CSF along with limited penetration into the brain parenchyma are current obstacles in drug administration [4]. Arguably the most well studied route of drug delivery is directly crossing the BBB. Drug delivery across the BBB has wide-ranging appeal because of its minimally invasive nature, but the inherent structure and function of the BBB does an incredible job of impeding drug delivery. Here, the objective of this opinion piece is to highlight recent advances in BBB penetrating nanoparticle drug delivery systems, reflect on recent findings of fundamental BBB maintenance/permeability with respect to biological variables, and examine how disease progression may impact pharmacokinetics/pharmacodynamics.
2. Blood-Brain Barrier
2.1. Basic Structure
First discovered in 1885 by Paul Ehrlich, the BBB structure consists of three main components (Figure 1A) [5]: (1) Endothelial cells intricately connected through cell-cell receptors including tight junctions providing a selective barrier separating the brain parenchyma and blood, (2) Pericytes envelope endothelial cells providing a second layer to the BBB as well as vascular flow control, and (3) Astrocyte foot ends extend around the pericytes and endothelial cells further stabilizing the BBB and providing critical factors for construction and maintenance of the interstitial cell space and tight junctions. The BBB structure also filters out foreign compounds, including small molecule drugs, via tight regulatory mechanism that include minimal paracellular diffusion, receptor mediated transcytosis (i.e., transferrin), carrier mediated transport (i.e., glucose transport protein (GLUT-1), absorptive mediated transport (i.e., albumin), and active influx/efflux pumps (i.e., ATP-binding cassette transporters such as breast cancer resistance protein (BCRP), multidrug resistance protein 1 (MRP1), and P-glycoprotein (P-gp)) (Figure 1B) [6]–[8]. Many features of the BBB structure are evolutionarily conserved across species from fruit flies to mammals [9], [10]. Therefore, a wide range of models exist to probe key aspects of BBB regulation, maintenance, and permeability to increase our understanding of drug delivery to the brain.
Figure 1:
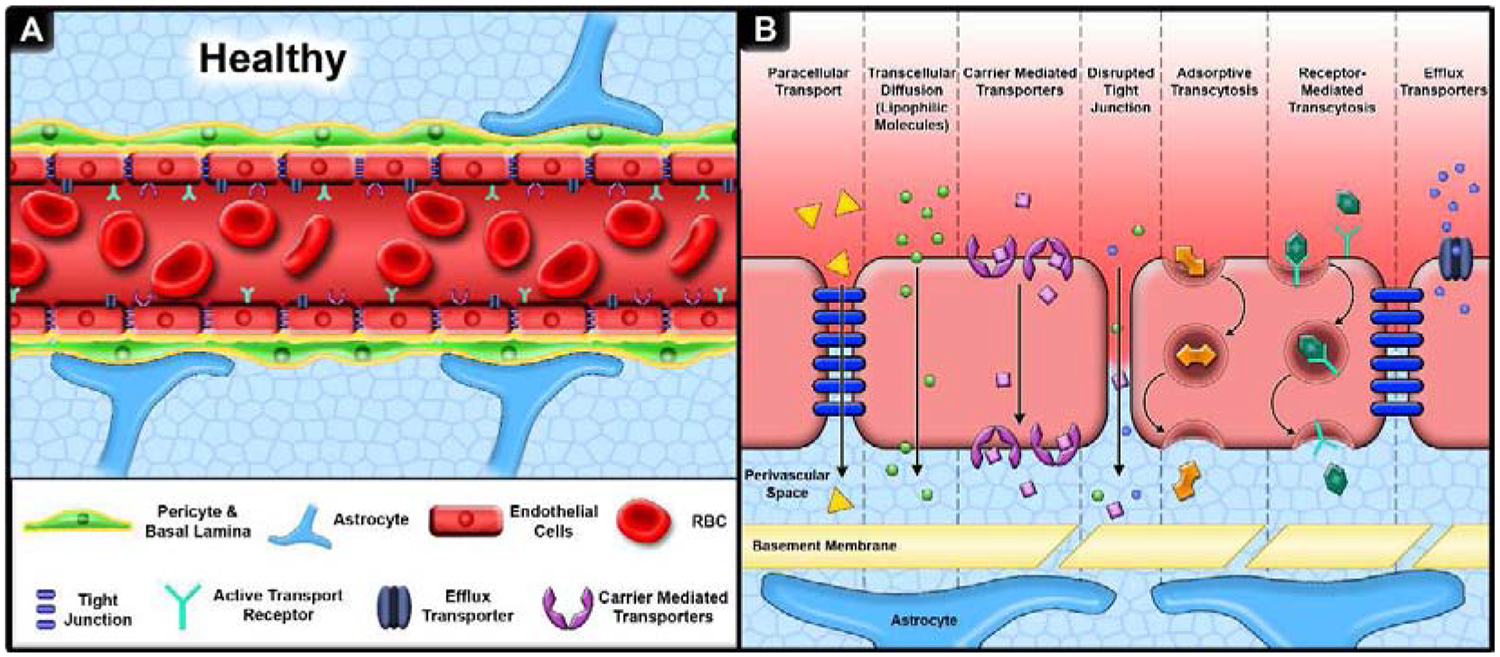
Overview of key elements of the BBB. A. The BBB consists of three primary layers, (1) endothelial cells connected by tight junctions surrounded by (2) pericytes and basal lamina with (3) astrocytic feet extending to and wrapping around the BBB. B. Molecular transport across the BBB is mediated by paracellular diffusion (hydrophilc molecules), transcellular diffusion (lipophilic and gas molecules), carrier mediated transport (glucose), absorptive transcytosis (albumin), receptor mediated transport (transferrin, insulin), and efflux transporters (P-gp). Alterations in homeostatic conditions such as trauma, neurodegenerative disease, or prolonged sleep deprivation can lead to the loss of tight junctions and altered active transport systems thereby increasing BBB permeability.
2.2. Dynamic BBB
Classic depictions of the BBB create visions of a stagnate, impenetrable wall; however, an increasing number of studies are finding the BBB to be regionally dynamic in composition and function. For example, cellular distribution expression of the drug efflux pumps P-gp and MRP1 that contribute to drug uptake and distribution in the brain reportedly differs both regionally and within the cellular compartments of the BBB [11], [12]. Specifically, P-gp was predominantly expressed in endothelial cells, whereas MRP1 expression was prominent in parenchyma astrocytes, glia limitans lining the meninges, and ependymal cells in choroidal structures (blood-cerebrospinal fluid interface). Regionally throughout the cerebrovascular system, Scherrmann et al. reported limited P-gp expression along large, penetrating arterioles, and high ion expression along capillary beds and venules [12]. Additionally, Unadkat et al. measured transporter protein levels in human subjects and identified regional variation in transporter proteins and interindividual variability [13]. They found the primary visual cortex (BA17) had an increased expression of transporter proteins glucose transporter 1 (GLUT1), BCRP, P-gp, equilibrative nucleoside transporter 1 (ENT1), and organic anion transporting polypeptide 2B1 (OATP2B1) compared to the parietal lobe (BA39) [13]. Additionally, they found males had a significantly lower expression of P-gp in both regions compared to females. It is important to note that regional distributions needs confirmed with subsequent studies as uneven enrichment due to sample preparation was cited as a limitation of this study. Another recent study identified a 2.5-fold increased expression in canines of human peptide transporter 2 (PEPT2) in the brain stem compared to the cerebellum, with pronounced altered expression of 18 transporter and receptors between the brain capillaries and the choroid plexus [14]. PEPT2 is an excellent target for drug delivery since it can work against the concentration gradient taking in molecules of a varying size, hydrophobicity and charge [15]. Collectively, these studies demonstrate differential expression and distribution of classic BBB transporters that may readily contribute differential regional drug targeting and distribution within the brain.
More recent studies highlight the impact of daily homeostatic patterns such as the circadian rhythm on the molecular transport in and out of the brain [16]. During periods of wakefulness, molecules such as norepinephrine reportedly preferentially accumulate within the brain parenchyma and are more readily removed while at rest [16]. Similar reports from G. J. Kress et al. also demonstrated that toward the end of wake cycles, the BBB becomes more penetrable to both active ATP-based transport as well as passive carrier-mediated transport mechanisms [17]. Moreover, sleep, tangentially regulated by circadian rhythm and master biological clock in the brain (suprachiasmatic nucleus; SCN), itself reportedly regulates BBB permeability. In the context of neuropathology, β-amyloid, a key molecule involved in Alzheimer’s disease (AD) and normally cleared by low density lipoprotein receptor-related protein 1 (LRP-1),is found to peak in interstitial fluid during the deepest stages of sleep, and disrupting sleep results in increased amyloid plaque deposition [17], [18]. Prolonged lack of sleep is linked to increased BBB permeability via passive diffusion through structural breakdown of tight junctions such as claudin-5 and occludin (Figure 1). Hurtado-Avarado et al. demonstrated that Evans blue—a normally impenetrable dye—infiltrated the brain in 10 day sleep restricted rats, likely due to inflammation disrupting tight junctions [19]. In sum, these studies highlight dynamic nature of the BBB and how both inherent circadian rhythm as well as sleep disturbances may influence BBB permeability thereby contributing to neurological disease progression but may also be exploited for drug delivery applications to optimize pharmacokinetics [20]. For further reading on the dynamic nature of the BBB see Cambell et al [21].
3. Traumatic Brain Injury
Understanding the dynamics of the BBB is paramount to treat neurological disease/injury conditions such as traumatic brain injury (TBI). TBI is a prominent neurological condition with minimal therapeutic interventions that afflicts over 1.4 million people across the U.S. annually [22]. TBI occurs when a mechanical force is delivered to the brain, resulting in rapid damage [23], [24]. The initial phase of the injury results in heterogenous and direct damage to the tissue and axonal shearing of neurons. The primary injury releases a myriad of pathophysiological and biochemical signaling cascades referred to as the secondary injury phase. These include hypo- and hyper-perfusion, edema, BBB dysfunction and inflammation [25], [26][Figure 2]. Throughout this process, both resident microglia, astrocytes and systemic inflammatory cells are activated, resulting in recruitment of other peripheral inflammatory/immune cells. Microglia respond in part to damage-associated molecular patterns, proteins or molecules that under normal physiological conditions reside/function intracellularly but release extracellularly after injury by stressed or damaged cells. Cellular signaling attempts to restore homeostasis in the injured tissue but can often exacerbate the injury [26], [27].
Figure 2:
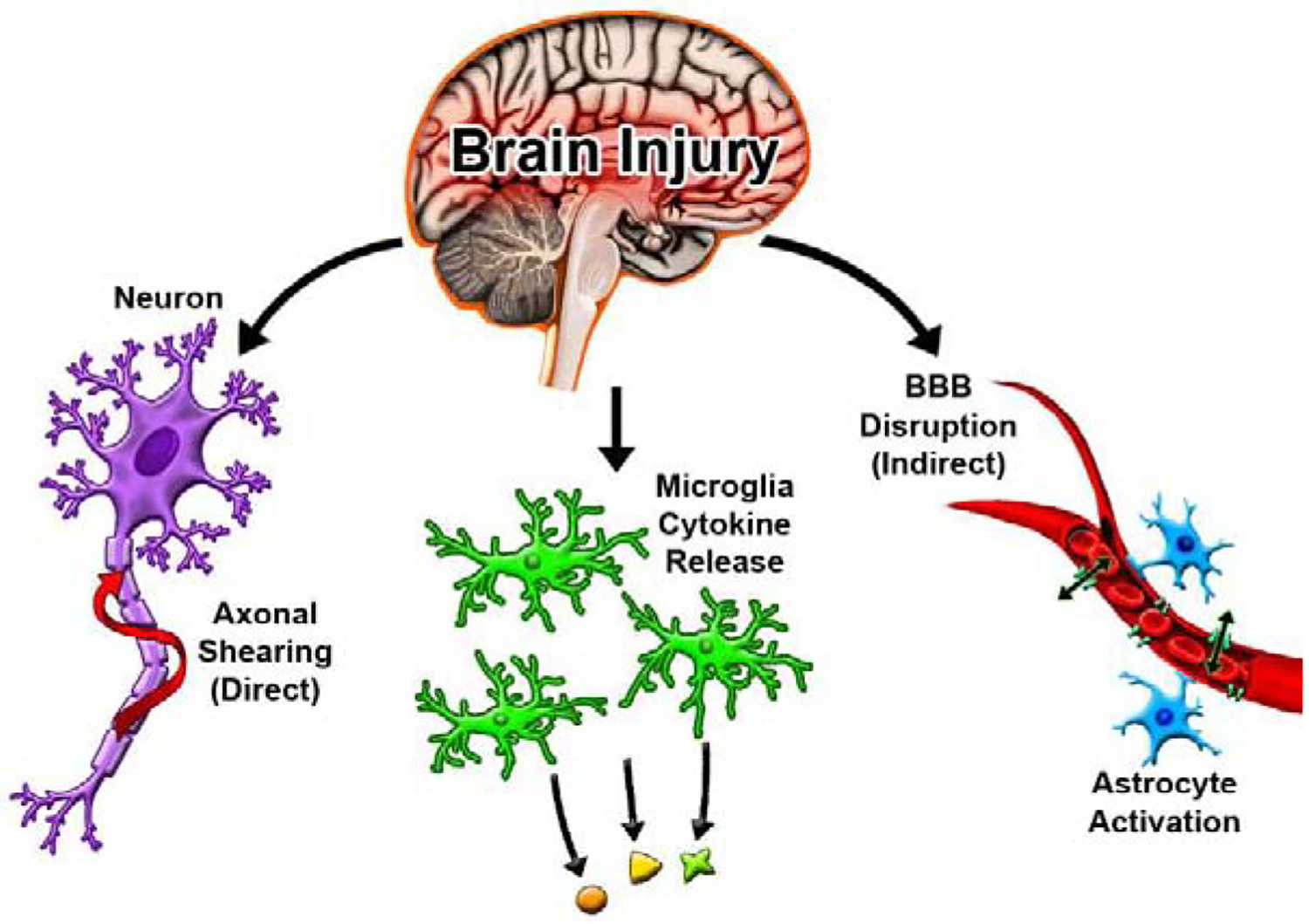
Immediately following a TBI, cells experience direct damage resulting in axonal shearing and the release of cellular debris. Microglia then initiate cytokine release and a cascading inflammatory response is triggered further disrupting the BBB and activating immune cells
3.1. BBB Disruption following TBI
One prominent and potentially persistent homeostatic alteration after TBI is the disruption of the BBB [23]. Intracranial bleeding, even microbleeds, following a TBI is associated with poor recovery and more frequent patient death [26], [28]. Moreover, evidence of chronic BBB dysfunction and deposition of bloodborne proteins is recognized as a hallmark pathology in chronic traumatic encephalopathy [29]. BBB permeability in TBI pre-clinical models occurs immediately after injury lasting up to 7 days. Biphasic BBB permeability peaks occur <6 hours followed by the second peak ~3 days in focal pre-clinical TBI models [30]. Ultimately the extravasation of these blood constituents (e.g. fibrinogen, platelets, and leukocytes) result in increased exacerbation of neuroinflammation [31]–[33] further contributing to BBB permeability [34]–[36].
3.2. Influence of biological factors BBB disruption following TBI
Pathologically divergent sex-dependent responses are well documented for TBI [[24], [37]–[39]. Clinically, females report poorer clinical outcomes than males following TBI, though paradoxically pre-clinical studies show a female neuroprotective response compared to males [40]–[42]. Pre-clinical models of TBI demonstrate a sex-dependent divergent inflammatory and hormonal response following a TBI [24], [37]. One example is that males suppress testosterone while females promote testosterone following TBI [43]. Broadly, testosterone increases BBB permeability while estrogen decreases BBB permeability [44]. Interestingly, our lab recently discovered a sex-dependent response in BBB permeability following TBI (mouse focal TBI model), with males demonstrating the previously reported biphasic permeability at 3 hours and 3 days after the injury compared to female [30], [45]. Conversely, females demonstrated a gradual decrease in BBB permeability with a nearly 2.5-fold greater level of permeability than their male counterparts at the 24 hours post-injury. The underlying mechanisms of this sex-dependent phenomenon is not clear, but its relevance to and potential impact on pharmacokinetics and pharmacodynamics is profound. Understanding the molecular underpinnings of this sex-dependent response may help guide intervention strategies as therapeutic windows and relevant pathological treatments for males and females suffering a TBI or other similar pathologies may diverge.
4. Nanoparticles as drug carriers for CNS injury and disease
The development of nanoparticles (NP) can be used to more effectively treat TBI and other neuropathologies. NP are particles ranging from 1nm to 1000nm in diameter. NP exhibit a vast array of shapes, structures and chemical makeups used for a wide variety of applications, including drug delivery. First successfully used to cross the BBB in 2005, NP afford numerous advantages to small molecule drugs and bioactive molecules delivery such as increased bioavailability, increased efficacy, reduced toxicity, among other benefits [46]. Moreover, NP possess additional surface area to display targeting moieties for targeted delivery to a region of interest (Figure 3). Here we will discuss several recent studies that exemplify some advantages of NP for drug delivery, particularly for CNS applications.
Figure 3:
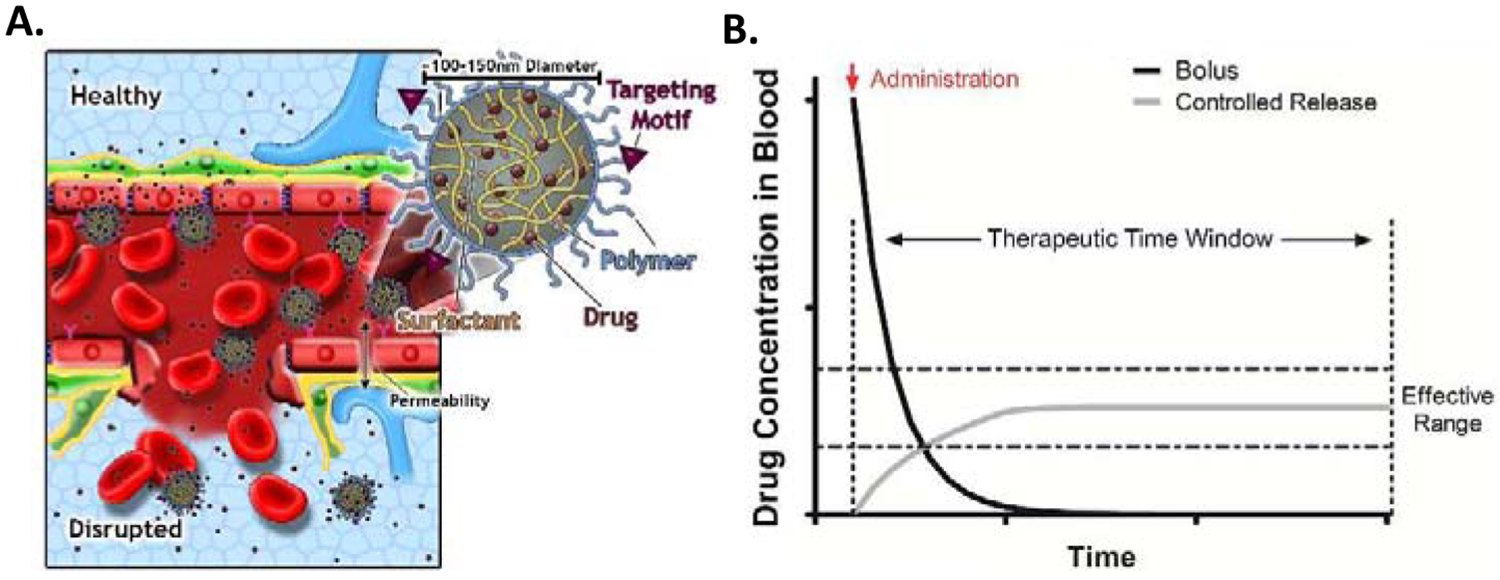
BBB NP delivery strategies. Multiple mechanisms May be employed for NPs to enhance drug delivery to the brain. A. NP surfaces May be functionalized with BBB specific receptors such as transferrin to enable local intravascular NP docking that May either enhance local drug concentrations of BBB permeable therapeutics or engage in active transcytosis of the NP. Secondly, BBB disruption due to trauma/neuropathology May further enhance NP accumulation/extravasation. B. NP encapsulation greatly benefits drug efficacy through prolonged release stabilizing drug concentration within the effective dosing window.
4.1. NP increase bioavailability
Pharmacological drugs can suffer from poor shelf-life, low stability, low bioavailability, and active clearance from the brain through efflux pumps such as P-gp [6]–[8], [47]. These shortcomings make drugs that are otherwise good candidates for TBI and other neurological diseases ineffective in a clinical setting. Encapsulation within a NP can increase the stability and prolong release (Figure 3B). Ruozi et. al used a polylactic-co-glycolide (PLGA) polymer to deliver the peptide mixture cerebrolysin. Cerebrolysin has shown promise for TBI therapy, but suffers from short half-life, poor stability and requires high dosage for treatment. After encapsulating in PLGA, cerebrolysin’s shelf-life and stability increased. Cerebrolysin’s efficacy also improved in a cortical stab injury model, likely from the increase in circulation time and prolonged release resulting in greater bioavailability to the brain [48]. This study demonstrates that often simple encapsulations can greatly improve pharmacokinetics/pharmacodynamics of rapidly cleared therapeutics.
4.2. Surface functionalization for NP targeting
Some drugs, particularly lipophilic drugs, can innately cross the BBB but suffer from low selectivity and rapid clearance. NP can be used to direct molecules to accumulate at the brain—increasing their therapeutic efficacy. Transferrin, a receptor expressed exclusively on brain endothelial cells, has been leveraged as a brain targeting motif [49]. NPs functionalized with transferrin receptor targeting motifs dock the NP locally to facilitate NP transport or enhance local concentrations of BBB permeable therapeutics (Figure 3). Moor et. al demonstrated efficacy of the latter technique by encapsulating the lipophilic drug oxaliplatin within liposome NP functionalized to target transferrin receptors. While the NP never crossed the BBB, drug uptake into the brain was increased with the targeted NP over both non-targeting NP and free drug [49]. Vilaplana et al. completed fundamental mechanistic studies with gold NPs decorated with 8D3 anti-transferrin receptor antibodies to track the NP fate regionally as well as cellularly via transmission electron microscopy. Importantly, and in agreement with Moor et al., they found no NPs beyond the endothelial basal membrane within the brain parenchyma out to 24 hours post-injection. Instead, endothelial vesicle endocytosis engulfed single resulted in one of two outcomes: (1) NPs were temporarily transported and presented on the basal membrane before being re-engulfed into an endothelial vesicle, or (2) the vesicles migrated and reorganized into larger vesicles containing numerous NP. This study demonstrates the usefulness of targeting transferrin to localize NPs and encapsulated payload to the brain BBB. Although the NPs may not readily distribute within the brain parenchyma via this targeting mechanism, NPs may release the encapsulated drug when presented to basal membranes. Additionally, the next generation transferring targeting NPs may employ endosomal escape mechanisms that allow the NP and/or payload to dissociate from the transferrin receptor and/or endocytic re-engulfment [50].
4.3. Biological variables impact NP and drug delivery
As highlighted in previous sections, biological variables such as sex and age impact disease pathology and progression. Sex as a biological variable is well-recognized in clinical pharmacology to strongly impact distribution, clearance, and activity of drugs. Recent studies have begun to probe how and if sex and age as a biological factor may influence NP delivery in the context of cellular uptake and NP toxicity/injury. Cellular NP uptake assessment via in vitro cultures reveal that depending on the cell type, sex and age, directly impact NP uptake and susceptibility to toxicity. Specifically, Foroozandeh et al. demonstrated that PEGylated-quantum dots exposed to senescent cells (fibroblast and epithelial) lead to lysosomal membrane permeabilization and necrosis at a higher rate than young proliferative cells [51]. Sex-dependent variations in NP toxicity have also been reported by Chen et. al showed organ specific sex differences in toxicity from gold NP injections where females had obvious inflammatory responses and heightened toxicity to larger NP while males had higher toxicity to smaller particles [52]. Additionally, Hayashi et al. report differential serum protein adsorption to SiO2 NPs from male versus female samples that in turn directly impacted NP accumulation in lymphoid and myeloid populations, with maximal accumulation with the female derived protein corona NPs regardless of the cellular sex identity [53]. Therefore, biological factors must be considered for future NP delivery and therapeutic approaches.
4.4. Implications on disease pathology for NP delivery to the brain
Alterations in BBB permeability from disease or injury pathology has major implications for NP and drug delivery. A disrupted BBB provides opportunity to deliver otherwise blocked molecules into the brain. Kwon et. al developed a dual membrane interactive and neural targeting peptide-based NP to deliver neuroprotective siRNA into neuronal cells. The NPs infiltrated only through the injured BBB and delivered the siRNA directly into neurons downregulating the apoptosis regulator caspase 3 to induce neuroprotective effects [54]. The disrupted BBB, while deleterious to the patient, can be exploited to deliver interventions that mitigate further neuropathology.
Following severe TBI, patients are often put into anesthesia-induced pharmacological comas to stabilize the brain. Anesthetics can further BBB disruption and increase the activation of microglia caused by TBI. These side effects provide opportunity for enhanced utilization of therapeutic intervention. When glial cells are activated by anesthetics, they can increase their uptake of small molecules. Kannan et. al delivered functionalized poly(amidoamine) dendrimer NP to microglia following the administration of the anesthetic pentobarbital. Glial cell uptake of the NPs was approximately two- fold greater in the presence of pentobarbital [55]. Deeper understanding of the cellular responses of drugs like anesthetics can provide opportunity to turn ostensibly negative side effects into exploitable therapeutic delivery systems.
4.5. Active mechanisms for BBB disruption to enable NP delivery
Techniques inducing transient permeability in the BBB have been used to deliver payloads otherwise blocked by the BBB. One common method involves focused ultrasound (FUS). FUS sends waves that disrupt the tight junctions and general integrity of the BBB, allowing NP to penetrate the brain parenchyma [56]. While increasing the brains uptake of drug is advantageous, careful consideration needs to be taken when purposefully disrupting the BBB. BBB disruption is strongly correlated with many neurological diseases, which may be exasperated by such methodologies [57]. Potential negative side effects of using techniques like FUS need to be studied to ensure that the technique is not inadvertently promoting disease progression.
5. Conclusion
Efficient drug delivery to the brain remains a critical challenge for the medical and pharmaceutical communities. Developing unique NP drug-carrier systems provides multiple avenues of tackling the obstacles of the BBB. Site-specific targeting on the NP surface leads to NP clustering in the region of interest increasing localized drug delivery. Designing NPs that exploit endogenous transport mechanisms across the BBB and/or injury-induced dysfunction of the BBB opens potential opportunities for otherwise impermeable drugs to the brain. Even simply increasing the circulation time of a drug via NP encapsulation provides more stable therapeutic levels within the therapeutic window. However, recognizing and understanding patient variables such as age, sex, and disease state is paramount to maximizing therapeutic efficacy for the right person at the right time. Improving our knowledge of drug response with respect to disease pathology and biological variables will enable the development of the next generation of therapeutics for a myriad of neurological conditions.
Highlights.
The blood-brain barrier (BBB) is dynamic anatomically and temporally
Biological factors such as sex play a large role is disease progression and therapeutic response
Nanoparticles (NP) can be functionalized to target the BBB and enhance drug delivery to the brain.
The BBB plays a critical role in maintaining a health brain, intentionally disrupting it for drug delivery needs to be carefully reviewed to avoid further exacerbating neurological disorders
Acknowledgements
Amanda Witten, Crystal Willingham, and Silina Souvannarath for proof reading. Amanda Witten for graphical support. Funding acknowledgements: NIH, Eunice Kennedy Shriver NICHD (1DP2HD084067; SES), NIH NINDS (R21NS107985; SES), and ASU Ira A. Fulton Schools of Engineering (Dean’s Fellowship; CC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflict of interest.
References:
- [1].“National Institute of Neurological Disorders and Stroke (NIH): Hope Through Research” https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research.
- [2].Dong J, Shang Y, Inthavong K, Chan H-K, and Tu J, “Numerical Comparison of Nasal Aerosol Administration Systems for Efficient Nose-to-Brain Drug Delivery,” Pharm. Res, vol. 35, no. 1, p. 5, Jan. 2018, doi: 10.1007/s11095-017-2280-6. [DOI] [PubMed] [Google Scholar]
- [3].Householder KT, “Nanoparticle Drug Delivery to Brain Tumors: From Intravenous to Intrathecal,” p. 115. [Google Scholar]
- [4].Householder KT, Dharmaraj S, Sandberg DI, Wechsler-Reya RJ, and Sirianni RW, “Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice,” Sci. Rep, vol. 9, no. 1, p. 12587, Dec. 2019, doi: 10.1038/s41598-019-49028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lalatsa A and Butt AM, “Physiology of the Blood–Brain Barrier and Mechanisms of Transport Across the BBB,” in Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors, Elsevier, 2018, pp. 49–74. [Google Scholar]
- [6].Garg P, Dhakne R, and Belekar V, “Role of breast cancer resistance protein (BCRP) as active efflux transporter on blood-brain barrier (BBB) permeability,” Mol. Divers, vol. 19, no. 1, pp. 163–172, Feb. 2015, doi: 10.1007/s11030-014-9562-2. [DOI] [PubMed] [Google Scholar]
- *[7].Lingineni K, Belekar V, Tangadpalliwar SR, and Garg P, “The role of multidrug resistance protein (MRP-1) as an active efflux transporter on blood–brain barrier (BBB) permeability,” Mol. Divers, vol. 21, no. 2, pp. 355–365, May 2017, doi: 10.1007/s11030-016-9715-6. [DOI] [PubMed] [Google Scholar]; Using MRP-1 as an active efflux pump they developed a predictive model with accuracies of 87.39, 93.54 and 80% for training, testing and external data sets respectively. The model shows that even drugs capable of crossing the BBB may suffer low bioavailable from rapid clearance.
- [8].Löscher W and Potschka H, “Blood-brain barrier active efflux transporters: ATP-binding cassette gene family,” NeuroRX, vol. 2, no. 1, pp. 86–98, Jan. 2005, doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Banks WA, “Mouse models of neurological disorders: a view from the blood-brain barrier,” Biochim. Biophys. Acta, vol. 1802, no. 10, pp. 881–888, Oct. 2010, doi: 10.1016/j.bbadis.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].DeSalvo MK et al. , “The Drosophila surface glia transcriptome: evolutionary conserved blood-brain barrier processes,” Front. Neurosci, vol. 8, Nov. 2014, doi: 10.3389/fnins.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mercier C, Masseguin C, Roux F, Gabrion J, and Scherrmann J-M, “Expression of P-glycoprotein (ABCB1) and Mrp1 (ABCC1) in adult rat brain: focus on astrocytes,” Brain Res, vol. 1021, no. 1, pp. 32–40, Sep. 2004, doi: 10.1016/j.brainres.2004.06.034. [DOI] [PubMed] [Google Scholar]
- [12].Saubaméa B, Cochois-Guégan V, Cisternino S, and Scherrmann J-M, “Heterogeneity in the Rat Brain Vasculature Revealed by Quantitative Confocal Analysis of Endothelial Barrier Antigen and P-Glycoprotein Expression,” J. Cereb. Blood Flow Metab, vol. 32, no. 1, pp. 81–92, Jan. 2012, doi: 10.1038/jcbfm.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Billington S et al. , “Interindividual and Regional Variability in Drug Transporter Abundance at the Human Blood–Brain Barrier Measured by Quantitative Targeted Proteomics,” Clin. Pharmacol. Ther, vol. 106, no. 1, pp. 228–237, Jul. 2019, doi: 10.1002/cpt.1373. [DOI] [PubMed] [Google Scholar]
- [14].Braun C et al. , “Quantification of Transporter and Receptor Proteins in Dog Brain Capillaries and Choroid Plexus: Relevance for the Distribution in Brain and CSF of Selected BCRP and P-gp Substrates,” Mol. Pharm, vol. 14, no. 10, pp. 3436–3447, Oct. 2017, doi: 10.1021/acs.molpharmaceut.7b00449. [DOI] [PubMed] [Google Scholar]
- [15].Rubio-Aliaga I and Daniel H, “Mammalian peptide transporters as targets for drug delivery,” Trends Pharmacol. Sci, vol. 23, no. 9, pp. 434–440, Sep. 2002, doi: 10.1016/S0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- *[16].Cuddapah VA, Zhang SL, and Sehgal A, “Regulation of the Blood–Brain Barrier by Circadian Rhythms and Sleep,” Trends Neurosci, vol. 42, no. 7, pp. 500–510, Jul. 2019, doi: 10.1016/j.tins.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review article highlights the growing body of evidence that the circadian rhythm is a major regulator of BBB influx and efflux. Molecules May be more or less prominent inside the brain depending on activity level.
- [17].Kress GJ et al. , “Regulation of amyloid-β dynamics and pathology by the circadian clock,” J. Exp. Med, vol. 215, no. 4, pp. 1059–1068, Apr. 2018, doi: 10.1084/jem.20172347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shibata M et al. , “Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier,” J. Clin. Invest, vol. 106, no. 12, pp. 1489–1499, Dec. 2000, doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hurtado-Alvarado G, Velázquez-Moctezuma J, and Gómez-González B, “Chronic sleep restriction disrupts interendothelial junctions in the hippocampus and increases blood-brain barrier permeability: CHRONIC SLEEP RESTRICTION DISRUPTS INTERENDOTHELIAL JUNCTIONS,” J. Microsc, vol. 268, no. 1, pp. 28–38, Oct. 2017, doi: 10.1111/jmi.12583. [DOI] [PubMed] [Google Scholar]
- [20].Shlosberg D, Benifla M, Kaufer D, and Friedman A, “Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury,” Nat. Rev. Neurol, vol. 6, no. 7, pp. 393–403, Jul. 2010, doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Keaney J and Campbell M, “The dynamic blood-brain barrier,” FEBS J, vol. 282, no. 21, pp. 4067–4079, Nov. 2015, doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- [22].“Brain Injury Statistics.” https://www.brainandspinalcord.org/brain-injury-statistics.
- [23].Adelson PD, Whalen MJ, Kochanek PM, Robichaud P, and Carlos TM, “Blood Brain Barrier Permeability and Acute Inflammation in Two Models of Traumatic Brain Injury in the Immature Rat: A Preliminary Report,” in Intracranial Pressure and Neuromonitoring in Brain Injury, Marmarou A, Bullock R, Avezaat C, Baethmann A, Becker D, Brock M, Hoff J, Nagai H, Reulen H-J, and Teasdale G, Eds. Vienna: Springer Vienna, 1998, pp. 104–106. [DOI] [PubMed] [Google Scholar]
- **[24].Villapol S, Loane DJ, and Burns MP, “Sexual dimorphism in the inflammatory response to traumatic brain injury,” Glia, vol. 65, no. 9, pp. 1423–1438, 2017, doi: 10.1002/glia.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]; Following a controlled cortical impact injury male and female mice were accessed for inflammatory response and had a divergent response. Treatments effective for one sex May be ineffective or deleterious to the other suffering from the same condition.
- [25].Masel BE and DeWitt DS, “Traumatic Brain Injury: A Disease Process, Not an Event,” J. Neurotrauma, vol. 27, no. 8, pp. 1529–1540, Aug. 2010, doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- [26].Simon DW, McGeachy MJ, Bayır H, Clark RSB, Loane DJ, and Kochanek PM, “The far-reaching scope of neuroinflammation after traumatic brain injury,” Nat. Rev. Neurol, vol. 13, no. 3, pp. 171–191, Mar. 2017, doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Devoto C et al. , “Inflammation Relates to Chronic Behavioral and Neurological Symptoms in Military Personnel with Traumatic Brain Injuries,” Cell Transplant, vol. 26, no. 7, pp. 1169–1177, Jul. 2017, doi: 10.1177/0963689717714098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].“Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial,” The Lancet, vol. 394, no. 10210, pp. 1713–1723, Nov. 2019, doi: 10.1016/S0140-6736(19)32233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Doherty CP et al. , “Blood–Brain Barrier Dysfunction as a Hallmark Pathology in Chronic Traumatic Encephalopathy,” J. Neuropathol. Exp. Neurol, vol. 75, no. 7, pp. 656–662, Jul. 2016, doi: 10.1093/jnen/nlw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Başkaya MK, Muralikrishna Rao A, Doğan A, Donaldson D, and Dempsey RJ, “The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats,” Neurosci. Lett, vol. 226, no. 1, pp. 33–36, Apr. 1997, doi: 10.1016/S0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- [31].Ryu JK et al. , “Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation,” Nat. Commun, vol. 6, no. 1, p. 8164, Nov. 2015, doi: 10.1038/ncomms9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pankratz S et al. , “The Inflammatory Role of Platelets: Translational Insights from Experimental Studies of Autoimmune Disorders,” Int. J. Mol. Sci, vol. 17, no. 10, p. 1723, Oct. 2016, doi: 10.3390/ijms17101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dziedzic A and Bijak M, “Interactions between platelets and leukocytesin pathogenesis of multiple sclerosis,” Adv. Clin. Exp. Med, vol. 28, no. 2, pp. 277–285, Feb. 2019, doi: 10.17219/acem/83588. [DOI] [PubMed] [Google Scholar]
- [34].Garbuzova-Davis S et al. , “Blood-Brain Barrier Alterations Provide Evidence of Subacute Diaschisis in an Ischemic Stroke Rat Model,” PLoS ONE, vol. 8, no. 5, p. e63553, May 2013, doi: 10.1371/journal.pone.0063553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jones LD, Jackson JW, and Maggirwar SB, “Modeling HIV-1 Induced Neuroinflammation in Mice: Role of Platelets in Mediating Blood-Brain Barrier Dysfunction,” PLOS ONE, vol. 11, no. 3, p. e0151702, Mar. 2016, doi: 10.1371/journal.pone.0151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[36].Bonney S et al. , “Gamma Interferon Alters Junctional Integrity via Rho Kinase, Resulting in Blood-Brain Barrier Leakage in Experimental Viral Encephalitis,” mBio, vol. 10, no. 4, pp. e01675–19, /mbio/10/4/mBio.01675–19.atom, Aug. 2019, doi: 10.1128/mBio.01675-19. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides a model of BBB breakdown through junctional disorganization and cell-cell separation, providing a potential therapeutic target for encephalitis.
- [37].Xu X, Cao S, Chao H, Liu Y, and Ji J, “Sex-related differences in striatal dopaminergic system after traumatic brain injury,” Brain Res. Bull, vol. 124, pp. 214–221, Jun. 2016, doi: 10.1016/j.brainresbull.2016.05.010. [DOI] [PubMed] [Google Scholar]
- [38].McGlade E, Rogowska J, and Yurgelun-Todd D, “Sex differences in orbitofrontal connectivity in male and female veterans with TBI,” Brain Imaging Behav, vol. 9, no. 3, pp. 535–549, Sep. 2015, doi: 10.1007/s11682-015-9379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhong YH, Wu HY, He RH, Zheng BE, and Fan JZ, “Sex Differences in Sex Hormone Profiles and Prediction of Consciousness Recovery After Severe Traumatic Brain Injury,” Front. Endocrinol, vol. 10, p. 261, Apr. 2019, doi: 10.3389/fendo.2019.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Berz K, Divine J, Foss KB, Heyl R, Ford KR, and Myer GD, “Sex-Specific Differences in the Severity of Symptoms and Recovery Rate following Sports-Related Concussion in Young Athletes,” Phys. Sportsmed, vol. 41, no. 2, pp. 58–63, May 2013, doi: 10.3810/psm.2013.05.2015. [DOI] [PubMed] [Google Scholar]
- [41].O’Connor CA, Cernak I, and Vink R, “Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats,” Brain Res, vol. 1062, no. 1–2, pp. 171–174, Nov. 2005, doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- [42].Roof RL, Duvdevani R, and Stein DG, “Gender influences outcome of brain injury: progesterone plays a protective role,” Brain Res, vol. 607, no. 1–2, pp. 333–336, Apr. 1993, doi: 10.1016/0006-8993(93)91526-X. [DOI] [PubMed] [Google Scholar]
- [43].Wagner AK et al. , “Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury,” J. Neurotrauma, vol. 28, no. 6, pp. 871–888, Jun. 2011, doi: 10.1089/neu.2010.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shin JA, Yang SJ, Jeong SI, Park HJ, Choi Y-H, and Park E-M, “Activation of estrogen receptor β reduces blood–brain barrier breakdown following ischemic injury,” Neuroscience, vol. 235, pp. 165–173, Apr. 2013, doi: 10.1016/j.neuroscience.2013.01.031. [DOI] [PubMed] [Google Scholar]
- *[45].Bharadwaj VN et al. , “Sex-dependent macromolecule and nanoparticle delivery in experimental brain injury,” Bioengineering, preprint, Oct. 2019. doi: 10.1101/817296. [DOI] [PMC free article] [PubMed] [Google Scholar]; Following a controlled cortical impact model, mice were injected with nanoparticles and macromolecules at various timepoints. Females and males experienced divergent permeability depending on the timepoint.
- [46].Das D and Lin S, “Double-coated poly (butylcynanoacrylate) nanoparticulate delivery systems for brain targeting of dalargin via oral administration,” J. Pharm. Sci, vol. 94, no. 6, pp. 1343–1353, Jun. 2005, doi: 10.1002/jps.20357. [DOI] [PubMed] [Google Scholar]
- [47].Parrish KE, Pokorny JL, Mittapalli RK, Bakken K, Sarkaria JN, and Elmquist WF, “Abstract C81: BBB efflux pump activity limits brain penetration of palbociclib (PD0332991) in glioblastoma.,” in Drug Metabolism, Transport, and Biodistribution, Nov. 2013, pp. C81–C81, doi: 10.1158/1535-7163.TARG-13-C81. [DOI] [Google Scholar]
- [48].Ruozi B et al. , “PLGA Nanoparticles Loaded Cerebrolysin: Studies on Their Preparation and Investigation of the Effect of Storage and Serum Stability with Reference to Traumatic Brain Injury,” Mol. Neurobiol, vol. 52, no. 2, pp. 899–912, Oct. 2015, doi: 10.1007/s12035-015-9235-x. [DOI] [PubMed] [Google Scholar]
- *[49].Johnsen KB et al. , “Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma,” Sci. Rep, vol. 7, no. 1, p. 10396, Dec. 2017, doi: 10.1038/s41598-017-11220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; The transferrin receptor was targeted to improve drug delivery. NPs accumulated around the BBB allowing drug to diffuse inside the brain as they were released from the NPs.
- [50].Cabezón I, Manich G, Martín-Venegas R, Camins A, Pelegrí C, and Vilaplana J, “Trafficking of Gold Nanoparticles Coated with the 8D3 Anti-Transferrin Receptor Antibody at the Mouse Blood–Brain Barrier,” Mol. Pharm, vol. 12, no. 11, pp. 4137–4145, Nov. 2015, doi: 10.1021/acs.molpharmaceut.5b00597. [DOI] [PubMed] [Google Scholar]
- [51].Foroozandeh P, Aziz AA, and Mahmoudi M, “Effect of Cell Age on Uptake and Toxicity of Nanoparticles: The Overlooked Factor at the Nanobio Interface,” ACS Appl. Mater. Interfaces, vol. 11, no. 43, pp. 39672–39687, Oct. 2019, doi: 10.1021/acsami.9b15533. [DOI] [PubMed] [Google Scholar]
- [52].Zhang X-D et al. , “Sex differences in the toxicity of polyethylene glycol-coated gold nanoparticles in mice,” Int. J. Nanomedicine, p. 2409, Jul. 2013, doi: 10.2147/IJN.S46376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hayashi Y et al. , “Female versus male biological identities of nanoparticles determine the interaction with immune cells in fish,” Environ. Sci. Nano, vol. 4, no. 4, pp. 895–906, 2017, doi: 10.1039/C7EN00071E. [DOI] [Google Scholar]
- [54].Kwon EJ, Skalak M, Lo Bu R, and Bhatia SN, “Neuron-Targeted Nanoparticle for siRNA Delivery to Traumatic Brain Injuries,” ACS Nano, vol. 10, no. 8, pp. 7926–7933, Aug. 2016, doi: 10.1021/acsnano.6b03858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kannan G, Kambhampati SP, and Kudchadkar SR, “Effect of anesthetics on microglial activation and nanoparticle uptake: Implications for drug delivery in traumatic brain injury,” J. Controlled Release, vol. 263, pp. 192–199, Oct. 2017, doi: 10.1016/j.jconrel.2017.03.032. [DOI] [PubMed] [Google Scholar]
- [56].Li Y et al. , “Mechanisms of enhanced antiglioma efficacy of polysorbate 80-modified paclitaxel-loaded PLGA nanoparticles by focused ultrasound,” J. Cell. Mol. Med, vol. 22, no. 9, pp. 4171–4182, 2018, doi: 10.1111/jcmm.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Huang J et al. , “Blood-Brain Barrier Damage as the Starting Point of Leukoaraiosis Caused by Cerebral Chronic Hypoperfusion and Its Involved Mechanisms: Effect of Agrin and Aquaporin-4,” BioMed Res. Int, vol. 2018, pp. 1–10, 2018, doi: 10.1155/2018/2321797. [DOI] [PMC free article] [PubMed] [Google Scholar]


