Abstract
Objective
To investigate the incidence and spectrum of neuroimaging findings and their prognostic role in hospitalized COVID-19 patients in New York City.
Methods
This is a retrospective cohort study of 3218 COVID-19 confirmed patients admitted to a major healthcare system (three hospitals) in New York City between March 1, 2020 and April 13, 2020. Clinical data were extracted from electronic medical records, and particularly data of all neurological symptoms were extracted from the imaging reports. Four neuroradiologists evaluated all neuroimaging studies for acute neuroimaging findings related to COVID-19.
Results
14.1% of admitted COVID-19 patients had neuroimaging and this accounted for only 5.5% of the total imaging studies. Acute stroke was the most common finding on neuro-imaging, seen in 92.5% of patients with positive neuro-imaging studies, and present in 1.1% of hospitalized COVID-19 patients. Patients with acute large ischemic and hemorrhagic stroke had much higher mortality risk adjusted for age, BMI and hypertension compared to those COVID-19 patients without neuroimaging. (Odds Ratio 6.02 by LR; Hazard Ratio 2.28 by CRR).
Conclusions
Our study demonstrates acute stroke is the most common neuroimaging finding among hospitalized COVID-19 patients. Detection of an acute stroke is a strong prognostic marker of poor outcome. Our study also highlights the fact there is limited use of neuroimaging in these patients due to multiple logistical constraints.
Keywords: COVID-19, Neuro-imaging, Stroke
1. Introduction
Involvement of the nervous system with SARS-CoV-2 infection has been documented; [[1], [2], [3], [4]] however, the incidence of neurological complications based on use of neuroimaging and its prognostic role in hospital admitted COVID-19 patients is not well studied. Two mechanisms of virus injury are hypothesized: (1) direct-virus injury and (2) cytokine storm. Angiotensin-converting enzyme 2 (ACE2) is a human cell receptor with a strong binding affinity to the Spike protein of SARS-CoV-2. ACE2 is highly expressed in type II alveolar cells, intestinal epithelia, vascular endothelium, and cardiac muscle. ACE2 is also identified in the nervous system leading to direct-virus injury [1]. A subgroup of patients might have severe cytokine storm consistent with secondary hemophagocytic lymphohistiocytosis causing a fatal hypercytokinemia with multi-organ failure. Indirect mechanism of neurological injury mainly relates to increased coagulation activity, markedly increased D-dimer concentrations in these patients [5,6] leading to increased risk of thromboembolic stroke [7] as well as compromised cardiac status due to associated cardiac injury [8]. We describe use of neuroimaging, incidence and spectrum of acute neuroimaging findings and their prognostic role in hospitalized COVID-19 patients in New York City.
2. Methods
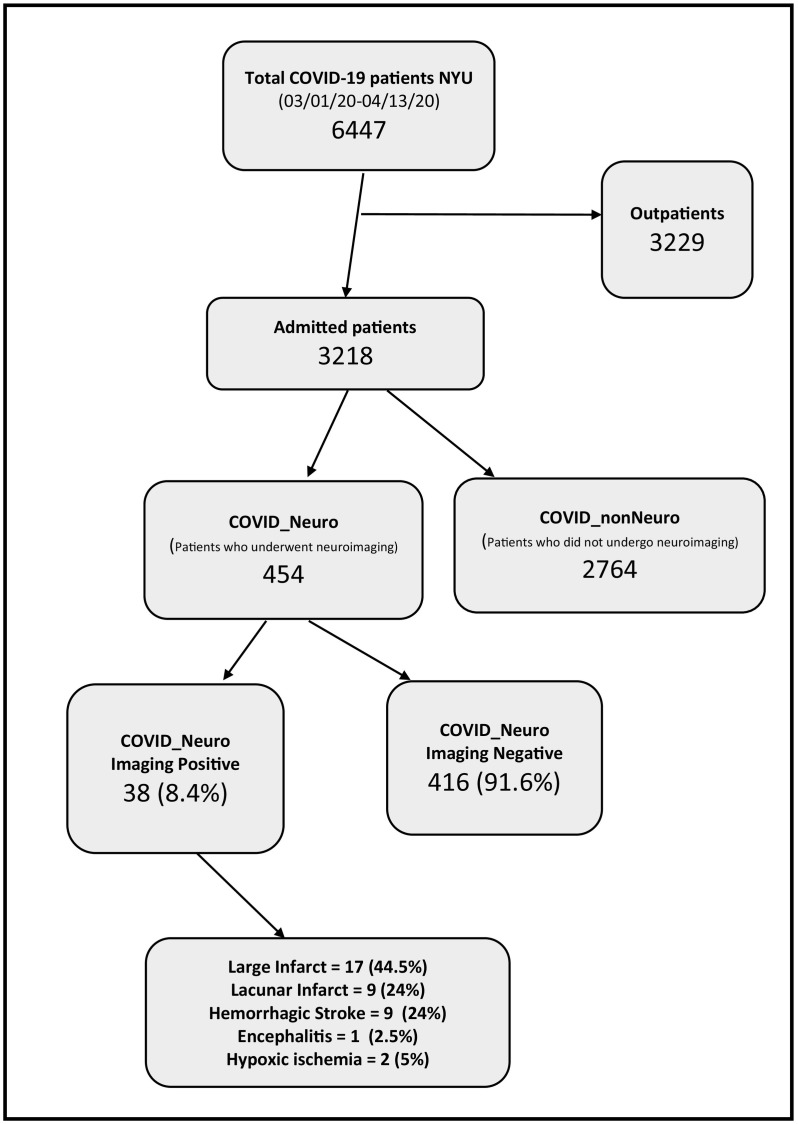
We retrospectively analyzed 3218 COVID-19 confirmed admitted patients from March 1, 2020, to April 13, 2020 (flow chart). A confirmed case of Covid-19 was defined as a positive result on real-time reverse transcriptase-polymerase-chain-reaction (RT-PCR) assay of nasopharyngeal or oropharyngeal swab specimens [9]. Initial tests were conducted by the New York City Department of Health and Mental Hygiene; as of March 16, tests were conducted in our clinical laboratory using the Roche SARS-CoV2 assay in the Cobas 6800 instruments through emergency use authorization (EUA) granted by the FDA. On March 31 we added testing using the SARS-CoV2 Xpert Xpress assay in the Cepheid GeneXpert instruments also under EUA by FDA. The targets amplified by these assays are the ORF1/a and E in the Roche Cobas assay and N2 and E genes in the Cepheid XpertXpress. Since March 16, only pharyngeal samples were collected and tested. Each neuroimaging study was evaluated for acute neuroimaging findings related to COVID-19 and all disagreements were resolved with consensus by the four fellowship-trained neuroradiologists. Descriptive statistics were utilized to characterize the cohort and Student's t-test to compare mean ages. To assess the risk of mortality conferred by positive neuroimaging findings, a multivariable logistic regression (LR) model was adjusted for age, body mass index and hypertension in a sub-group of 2894 patients. These covariates were selected a priori based on known risks for neurologic and COVID-19 complications, while limiting covariates to avoid overfitting the model. As a secondary analysis, to account for right censoring from hospitalizations still in progress, we constructed a competing risk regression survival analysis (CRR) with the competing risk being hospital discharge.
This study was approved by the NYU Grossman School of Medicine Institutional Review Board, which granted both a waiver of informed consent, and a waiver of the Health Information Portability and Privacy Act (IRB # # i20–00485).
Flowchart
3. Results
454 (14.1%) patients underwent neuroimaging and 716 neuroimaging studies (median age 64 years, range 2 weeks-105 years, 60.7% males) were done. Neuroimaging examinations constituted only 5.4% of the 13,314 imaging examinations performed in these patients during the study period. Chest imaging examinations were the most common (10,182, 76.5%, 9859 Chest X-rays, 323 CT studies). The most common neuroimaging study performed was CT of the head (586, 81.8%). 323 patients (71.1%) had one examination, 131 patients (28.9%) had more than one imaging examination (2–14 imaging examinations). Other neuroimaging studies performed included CT angiography of the head and neck (34, 4.7%), CT perfusion (14, 2%), MRI brain (48, 6.7%), MRA/MRV (17, 2.4%), and MRI spine (12, 1.7%). Five catheter angiography studies were performed for endovascular treatment of stroke. Common clinical indications for neuroimaging studies performed on COVID-19 patients included altered mental status or delirium (37.6%), stroke (17.3%), and mechanical fall or trauma (25.5%). Less common indications included syncope (4%), headache (3.8%), dizziness (2.8%), seizure (2.1%) and ataxia (1.4%).
38 patients had acute neuroimaging findings (8.4% of those who underwent neuroimaging and 1.2% of the total hospital admitted COVID-19 patients); and 35 patients had stroke (7.7% of those who had neuroimaging and 1.1% of COVID-19 inpatients) (Table 1 ). Thromboembolic ischemic stroke comprised 68.5% of these 38 patients and hemorrhagic stroke was present in 24% (Fig. 1 ). Signs of acute stroke were the initial manifestation of COVID-19 in 63% of these 38 patients. (65% of all ischemic stroke and 44.5% of hemorrhagic strokes). 66.5% of all hemorrhagic stroke were on anti-coagulation therapy. Only 1 patient (2.5%) showed imaging findings consistent with encephalitis and 2 patients (5%) had hypoxic anoxic injury. The 454 patients who underwent neuroimaging were on average older than the 2687 patients who did not undergo neuroimaging (mean age 71 years vs 62 years, p-value <.0001), had lower BMI, and higher rates of hypertension (Table 2 ). However, the subset of patients with acute neuroimaging findings were younger on average than those who had negative neuroimaging studies (mean 66 years vs 75 years, p = .03).
Table 1.
Patient mortality data correlation with neuroimaging findings.
| Acute neuroimaging findings | N (%) | Mortality N (%) |
|---|---|---|
| Stroke | 35 (92.5) | 15 (43) |
| Ischemic stroke | 26 (68.5) | 10 (38.5) |
| Large vessel | 17 (44.5) | 8 (47) |
| Lacunar | 9 (24) | 2 (20) |
| Hemorrhagic stroke | 9 (24) | 5 (55.5) |
| Hypoxic anoxic brain injury | 2 (5) | 1 (50) |
| Encephalitis | 1 (2.5) | 0 |
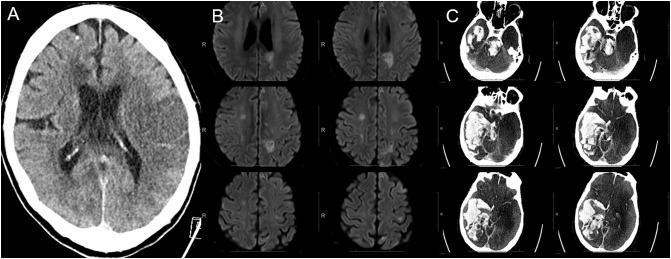
Fig. 1.
Three different COVID-19 patients with spectrum of stroke. A) Non-contrast CT head showing a large acute left MCA infarct in a patient who presented with right hemiplegia and aphasia in the emergency department. B) Diffusion weighted images of another patient with multiple small acute infarcts in both cerebral hemispheres. C) Non-contrast CT head showing large acute hemorrhage in right cerebral hemisphere as well as brainstem in a patient who was on therapeutic anticoagulation due to high D-dimer levels.
Table 2.
Patient age and co-morbidities for various subgroups (this data was available for 2894 out of 3218 patients).
| COVID_nonNeuro (N = 2516) | COVID_Neuro Imaging Negative (N = 351) |
COVID_Neuro Imaging Positive (N = 27) |
|
|---|---|---|---|
|
Age Median (interquartile range) |
62 (49–73) | 75 (66–83) | 66 (61.5–75.5) |
|
BMI Median (interquartile range) |
28.5 (24.7–33.2) | 25.9 (22.6–30.2) | 26.96 (24.5–29.9) |
|
Hypertension Number (percentage) |
906 (36.0%) | 178 (50.7%) | 14 (51.9%) |
|
Mortality Ratios CRR HR (95% CI, p-value) LR OR (95% CI, p-value) |
1.09 (0.88–1.33, 0.440) 1.07 (0.82–1.38, 0.618) |
2.28 (1.35–3.85, 0.002) 6.02 (2.60–14.63, <0.001) |
Sixteen of 38 (42%) patients with acute neuroimaging findings died during hospitalization. 47% of patients who had large vessel acute ischemic stroke and 55.5% of those with hemorrhagic stroke died (Table 1). After adjustment for age, BMI and hypertension, patients with neuroimaging findings had higher risk for mortality than those without neuroimaging (odds ratio [OR] 6.02, 95% confidence interval [CI] 2.6–15.6, p < .001); this risk remained using a competing risk model (hazard ratio [HR] 2.28, 95% CI, 1.35–3.85) (Table 2). On the other hand, patients with no acute neuroimaging findings did not have significantly increased mortality compared to those never imaged as per both the LR (OR 0.07, 95% CI confidence interval 0.82–1.38, p = .618) and CRR (HR 1.09, 95% CI 0.88–1.33, p = .440) models (Table 2).
4. Discussion
This study describes the spectrum of acute neuroimaging findings in admitted COVID-19 patients evaluated in a large tertiary hospital system in NYC. The acute stroke incidence was 1.1% among all hospitalized COVID-19 patients. Our study also demonstrates that an acute stroke finding on neuroimaging is a strong prognostic marker of poor outcome. Only 5.4% of the total imaging examinations performed on COVID-19 patients were neuroimaging studies. Neuroimaging studies have tended to constitute a larger percentage of overall diagnostic imaging use in hospital admitted patients. However, COVID-19 being a pandemic associated primarily with pulmonary disease, has led to the chest being the predominant organ imaged. Neurological manifestations attributed to COVID-19 infection include headache, encephalopathy or delirium, stroke, and seizures [[1], [2], [3], [4]]. Our incidence of stroke as determined by neuroimaging findings is lower than 2.8% incidence found in patients admitted through an emergency department [10]. A recent publication has also shown a decrease in the use of stroke imaging both in patients with severe strokes and in nonelderly patients who may have been at low risk for Covid-19 complications [11]. Nonetheless, it may be difficult to obtain the true incidence of neurological manifestations related to COVID-19 due to the following factors. First, most neurologic complications, such as delirium, encephalopathy, inflammatory neuropathy and autonomic dysfunction, may or may not be associated with abnormalities on neuroimaging. The reported yield of neuroimaging for hospitalized patients with delirium ranges from 2.7% to 14.5% across studies [12]. Second, strict patient isolation and need for extreme contact precautions and stringent infection control measures may have limited the ability to perform detailed neurological examinations and obtain neuroimaging especially MRI, which is the mainstay of acute stroke diagnosis. Moreover, critically ill, intubated patients are difficult to transport and image and hence may have been underdiagnosed. Finally, critically ill patients are often sedated, making it difficult to identify new neurologic complications. Hence, obtaining more expert neurology consultations, detailed neurological examinations and neuroimaging for early and accurate diagnosis of these often fatal neurological complications could improve our understanding of the disease and its neurological manifestations.
Community acquired pneumonias are known to be associated with hypercoaguability [5,6]. It is no surprise that emerging evidence shows that COVID-19 may also predispose patients to both venous and arterial thromboembolism due to diffuse intravascular coagulation, hypoxia, cytokine storm and immobilization [6]. High D-dimer levels, a marker of inflammation, are known to be associated with poor outcome in COVID-19 [8]. Acute strokes seen in the current study are also likely related to thromboembolic disease and majority were present at the initial admission. Most importantly, our data demonstrates for the first time, that when a neuroimaging shows acute large ischemic stroke or hemorrhagic stroke in admitted COVID-19 patients, it is one of the strongest prognostic markers of poor outcome, even more than age and other previously reported co-morbidities such as hypertension and obesity [8]. COVID-19 patients who have a large ischemic or hemorrhagic stroke on imaging have a 50% mortality in the current study.
In conclusion, our study demonstrates that acute stroke is the most common COVID-19 related neuroimaging finding and its incidence is 1.1% in hospitalized COVID-19 patients. The discovery of acute stroke by neuroimaging is a strong prognostic marker of poor outcome. Our study also highlights the fact there is restricted use of neuroimaging in COVID-19 patients due to multiple logistical constraints including the severity of their illness and the concern for spread of infection.
Acknowledgements
We would like to acknowledge that there are other studies in various stages of publication from our instituional patient data base, which are analyzing patients with neuro-imaging findings and stroke. These studies are being done independent of the current study.
References
- 1.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurol. 2020 Apr 10 doi: 10.1001/jamaneurol.2020.1127. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman C., Mayer K., Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. 2020 Apr;28 doi: 10.1212/WNL.0000000000009673. 32345728 Review. PubMed PMID. [DOI] [PubMed] [Google Scholar]
- 4.Carod-Artal F.J. Neurological complications of coronavirus and COVID-19. Rev. Neurol. 2020 may 1;70(9):311–322. doi: 10.33588/rn.7009.2020179. 32329044 Review. English, Spanish. PubMed PMID. [DOI] [PubMed] [Google Scholar]
- 5.Milbrandt E.B., Reade M.C., Lee M. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol. Med. 2009;15:438–445. doi: 10.2119/molmed.2009.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., DAMPJ Gommers, Kant K.M., FHJ Kaptein, van Paassen J., MAM Stals, Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020 Apr 10 doi: 10.1016/j.thromres.2020.04.013. pii: S0049–3848(20)30120–1. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020 Apr 28 doi: 10.1056/NEJMc2009787. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. 9 March 2020. www.thelancet.com [DOI] [PMC free article] [PubMed]
- 9.Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). Accessed at https://urldefense.proofpoint.com/v2/url?u=http-3A__www.cdc.gov_coronavirus_2019-2DnCoV_lab_guidelines-2Dclinical-2Dspecimens.html&d=DwIFaQ&c=j5oPpO0eBH1iio48DtsedeElZfc04rx3ExJHeIIZuCs&r=yx-R_kKSAE9wSyP2I19abLndpsC9-s8eTc3VgZIgfx8&m=AdfDyE3DURuq-KunmKqrUdvp86Xkr5Meo-X4ine6RPw&s=fODtC7G0rA2uZsEOdtgqmOx44tBm7Bf45OqKTIdKYyc&e=. on 27 March 2020. [Ref list].
- 10.HCUP Clinical Classifications Software (CCS). Healthcare Cost and Utilization Project (HCUP). August 2006. U.S. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- 11.Kansagra A.P., Goyal M.S., Hamilton S., Albers G.W. Collateral Effect of Covid-19 on Stroke Evaluation in the United States. N. Engl. J. Med. 2020 May 8 doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow S., McWilliams A., Kaplan D.M., Stephens J.R. Things we do for no reason: neuroimaging for hospitalized patients with delirium. J. Hosp. Med. 2019 Jul 1;14(7):441–444. doi: 10.12788/jhm.3167. [DOI] [PubMed] [Google Scholar]