Abstract
A novel human coronavirus SARS‐CoV‐2 (also referred to as CoV-19) that emerged in late 2019 causes Covid-19 disease a respiratory tract infection which provokes about 4 million deaths per year. Unfortunately, to date, there is no specific antiviral treatment for COVID-19. Mast cells (MCs) are immune cells implicated in the pathogenesis of viral infections, where they mediate inflammation. Microbes, including virus, activate MCs through TLR releasing chemical pro-inflammatory compounds and cytokines. Although, in biomedical literature there are only few reports on MCs activation by SARS-CoV-2 infection. The production of pro-inflammatory cytokines by MC viral activation leads to increase pulmonary inflammation and fibrosis. Sodium Chromo-Glycate (SCG) described as a MC stabilizer, prevents the release of inflammatory chemical compounds, improve mouse survival and respiratory pathological changes in lung viral infection and suppresses inflammation. Furthermore, palmitoylethanolamide (PEA) a nuclear factor agonist, an endogenous fatty acid amide, which exerts a variety of biological effects, related to chronic inflammation and pain, is involved also in MCs homeostasis with an inhibitory and protective effect on the respiratory tract during viral infections. Here, we hypothesize for the first time, that SCG and/or PEA suppress MC activation and pro-inflammatory mediators release, playing an anti-inflammatory therapeutic role in the inflamed lung of patients with COVID-19.
Dear Editors
In recent months, an outbreak of interstitial pneumonia (SARS-CoV-2) known as COVID-19 that started in Wuhan (China) has been affecting the world's population [1]. COVID-19 causes acute respiratory distress syndrome (ARDS), a disease where the lungs are unable to provide enough oxygen for the body [2]. Indeed, SARS patients have been shown acute inflammation with pulmonary edema, pleural effusions, focal bleeding and mucopurulent material in the trachea-bronchial tree [3]. In addition, other changes may occur, including hyaline membrane formation, alveolar hemorrhage, and fibrin exudation in alveolar spaces with alveolar fibrosis which appears during the later stages [4]. Histological examination of the lungs of patients who died of SARS revealed extensive immune cell infiltration in the interstitium and alveoli [5]. Indeed, coronavirus infection causes a severe inflammatory response in the lung, accompanied by interstitial mononuclear inflammatory infiltrates, dominated by lymphocytes [6]. The innate immune response signaling cascade starts with the recognition of pathogen-associated molecular patterns by pattern recognition receptors (PRRs). For RNA viruses in the lungs, the Toll-like receptors (TLRs) 3, 4, 7 and 8, which are expressed on several immune cells, are important PRRs [7]. Also, intracellular cytosolic PRRs such as MDA5 and RIG-I, which are present in virtually any cell type including those of the lung, have been shown to be relevant for respiratory infections [8]. In this way, the innate immune system induces transcription factors in the nucleus which in turn stimulate expression of types I and III interferons (IFNs) and other proinflammatory cytokines [9].
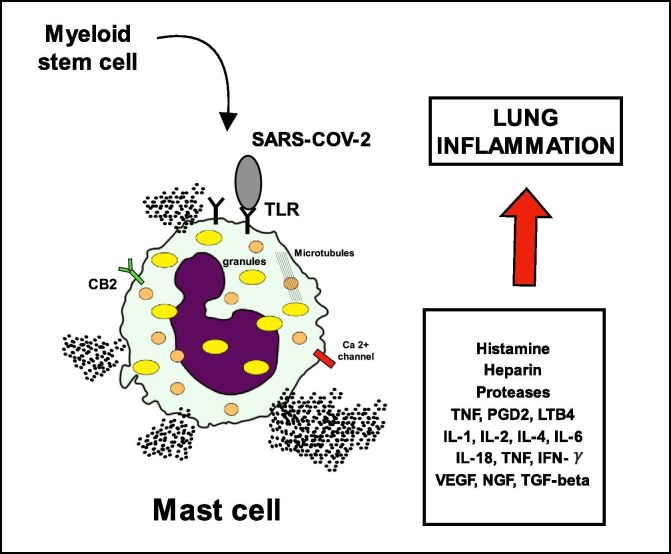
Mast cells (MC) are strategically placed at sites that interface with external environment of the body such as the skin, lung, and intestines [10]. Within such tissues they are predominately below the epithelial layer and closely associated with blood vessels. This location allows them to act as sentinels for tissue damage and pathogen invasion [11]. Indeed, in vitro and in vivo studies demonstrated that mast cells are able to detect DAMPS from a number of viruses including respiratory syncytial virus (RSV), rhinovirus (RV), reovirus, dengue virus (DENV), human immunodeficiency virus (HIV) and influenza [12]. Hu et al., 2012 were the first to demonstrate a direct involvement of MCs in viral infection, showing increased MCs numbers in the nasal mucosa, trachea, and lungs during the early stage of infection with H5N1 influenza virus in mice [13]. Although there are no reports of MCs activation following SARS-CoV or MERS-CoV infection, activation has been observed both in vitro and in vivo following infection with influenza virus [13] and may occur following other severe respiratory infections, including coronaviruses (Fig. 1 ) [14].
Fig. 1.
Degranulation of Mast cells due to TLR-Antigen interaction.
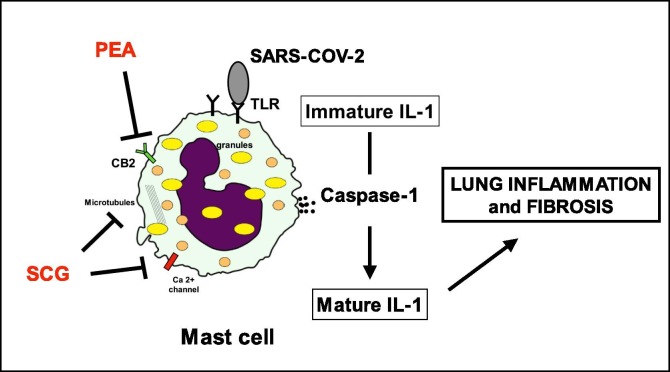
Sodium Chromo-Glycate (SCG) is a natural antiallergic drug that is able to stabilize MCs membranes. Further studies demonstrated that SCG can inhibit degranulation and the release of cytokines and inflammatory mediators from MCs [15]. Interestingly, it has been investigated the role of SCG as protective factor during the initial process of influenza virus infection and the possible mechanism behind any such effects in mouse model. It has been shown that SCG can improve the mouse survival and respiratory pathological changes. Although viral replication was not inhibited, SCG could regulate the expressions of IL-6, TNF-α, TLR3, and TRIF to alleviate the pathological injury to the nose, trachea, and lungs by reducing the inflammatory response [16]. Palmitoylethanolamide (PEA) is another molecule that is involved in mast cells homeostasis and therefore could be of interest in the symptomatic treatment of viral pneumonia. The analgesic and anti-inflammatory action of PEA seems to involve different molecular actors. PEA inhibits the release of both preformed and newly synthesized mast cell mediators, such as histamine and TNF-α [17]. It can down-regulate hyperactive mast cells in a dose-dependent manner [18]. PEA reduces the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) which play a key role in regulating the immune response to infection. In 2013, a systematic review on randomized trials analyzed the efficacy and safety of PEA in the treatment of the common cold and influenza, establishing its protective effects on the respiratory tract during viral infections [19]. Summarizing, to date, there is no specific antiviral treatment recommended for COVID-19, and no vaccine is currently available. For these reasons, it is imperative to look for any other possible strategies that might help the physician in the correct management of these patients. SCG and PEA represent safe, low cost and well-known drugs that might be useful in the management of COVID 19 (Fig. 2 ). Obviously, basic and clinical studies are necessary in order to evaluate SCG and PEA in the current clinical practice.
Fig. 2.
PEA and SCG are able to inhibit degranulation of Mast cells and the release of proinflammatory mediators (i.e. caspase-1). PEA through indirect activation of CB2 receptors. Several mechanisms have been proposed for SCG. PEA: palmitoylethanolamide; SCG: sodium cromoglycate.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors gratefully acknowledge Ms. Monica Gigante for the comprehensive bibliographic review and the linguistic revision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.109856.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Liu Y.C., Liao C.H., Chang C.F., Chou C.C., Lin Y.R. A locally transmitted case of SARS-CoV-2 infection in Taiwan. N Engl J Med. 2020;382(11):1070–1072. doi: 10.1056/NEJMc2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conti P., Gallenga C.E., Tetè G. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/Editorial-Conti-2. [DOI] [PubMed] [Google Scholar]
- 3.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection--a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect March 2020:1–14. doi:10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed]
- 4.Rothe C., Schunk M., Sothmann P. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls J.M., Poon L.L.M., Lee K.C. Lung pathology of fatal severe acute respiratory syndrome. Lancet (London, England) 2003;361(9371):1773–1778. doi: 10.1016/s0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma B.K., Kakker N.K., Bhadouriya S., Chhabra R. Effect of TLR agonist on infections bronchitis virus replication and cytokine expression in embryonated chicken eggs. Mol Immunol. 2020;120:52–60. doi: 10.1016/j.molimm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X., Chu H., Wong B.H.-Y. Activation of C-type lectin receptor and (RIG)-I-like receptors contributes to proinflammatory response in middle east respiratory syndrome coronavirus-infected macrophages. J Infect Dis. 2019;221(4):647–659. doi: 10.1093/infdis/jiz483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikkert M. Innate immune evasion by human respiratory RNA viruses. J Innate Immun. 2020;12(1):4–20. doi: 10.1159/000503030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kritas S.K., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents. 2020;34(1) doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 12.Marshall J.S., Portales-Cervantes L., Leong E. Mast cell responses to viruses and pathogen products. Int J Mol Sci. 2019;20(17) doi: 10.3390/ijms20174241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Y., Jin Y., Han D. Mast cell-induced lung injury in mice infected with H5N1 influenza virus. J Virol. 2012;86(6):3347–3356. doi: 10.1128/JVI.06053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio 2018;9(5). doi:10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed]
- 15.Lin Y-Y, Chou Y-L, Chu Y-H, Wu C-C, Wang J-Y, Wang H-W. Effects of cromolyn sodium on isolated rat’s trachea. Allergy Rhinol 2011;2(2):ar.2011.2.0015. doi:10.2500/ar.2011.2.0015. [DOI] [PMC free article] [PubMed]
- 16.Han D., Wei T., Zhang S. The therapeutic effects of sodium cromoglycate against influenza A virus H5N1 in mice. Influenza Other Respi Viruses. 2016;10(1):57–66. doi: 10.1111/irv.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerrato S, Brazis P, della Valle MF, Miolo A, Puigdemont A. Effects of palmitoylethanolamide on immunologically induced histamine, PGD2 and TNFα release from canine skin mast cells. Vet Immunol Immunopathol 2010;133(1):9–15. doi:10.1016/j.vetimm.2009.06.011. [DOI] [PubMed]
- 18.De Filippis D., D’Amico A., Cinelli M.P., Esposito G., Di Marzo V., Iuvone T. Adelmidrol, a palmitoylethanolamide analogue, reduces chronic inflammation in a carrageenin-granuloma model in rats. J Cell Mol Med. 2009;13(6):1086–1095. doi: 10.1111/j.1582-4934.2008.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keppel M, Hesselink JM, de Boer T, Witkamp RF. Palmitoylethanolamide: a natural body-own anti-inflammatory agent, effective and safe against influenza and common cold. Int J Inflam 2013;2013:151028. doi:10.1155/2013/151028. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.