Evidence-based medicine is a cornerstone of oncology. As a community we embrace change cautiously, requiring high-quality evidence of improved outcomes before we alter practice. Incorporating results from clinical trials into guidance and routine clinical practice usually takes many months or even years [1], and radiotherapy innovations are often commissioned more slowly than systemic therapies. Once established, oncologists are experts at using this evidence to communicate the relative risks and benefits of cancer treatment to individual patients, taking into account their performance status and co-morbidities.
In early March 2020, reports from Italy and Spain of hospitals becoming overwhelmed with patients with 2019 novel coronavirus disease (COVID-19), together with rising UK infection rates, alerted the medical community to the need to consider radical changes to normal working practice. There was significant concern that cancer patients would be at risk of more serious COVID-19 because of immunosuppression and co-morbidities. The published evidence base was one paper from Wuhan suggesting an excess mortality from COVID-19 in people with cancer [2], but such was the speed of the pandemic that there was an imperative to change treatment pathways within weeks.
Oncology departments began to revise business continuity plans originally drafted in 2008 when a severe acute respiratory syndrome (SARS) pandemic had been expected. Many centres approached the Royal College of Radiologists (RCR) as a professional body to ask for guidance on how to cope with the complex clinical, ethical and practical considerations presented by the conflicting demands of cancer treatment and COVID-19. Could we safely continue life-extending but immunosuppressive therapies? How could services adapt to reduced staff numbers due to redeployment or illness? How could we minimise the risk of exposing vulnerable patients to multiple hospital visits? How could non-surgical oncology treatments be used to mitigate the possibility that operations would cease in order to maximise ventilator capacity?
The RCR responded by opening an online COVID-19 discussion forum for members and Fellows on 13 March 2020 to share ideas and best practice. On the same day, a discussion thread on the existing breast cancer forum asked whether data from the then-unpublished Fast Forward trial could be used to move to hypofractionated radiotherapy for adjuvant breast cancer [3]. This resulted in the lead author for the trial enabling the provision of important radiotherapy quality assurance materials and protocols, essentially enabling revision of departmental radiotherapy protocols for breast cancer within days. Discussions about modifying treatment pathways and protocols to produce guidance documents for other tumour sites followed quickly. Some were initiated on the forum, some on social media (particularly Twitter) [4] and others within existing expert groups.
Royal College of Radiologists' Guidance Repository
On 18 March 2020 the RCR decided to collate these guidance documents into an online repository with the aim of making them widely available to oncologists and their multi-professional teams [5]. We contacted one or two lead authors for each tumour type based on engagement in discussion on the forum or prior known expertise, and asked them to form a team of colleagues from different cancer centres to produce guidance. We deliberately were not proscriptive in how the guidance should be written, as we did not want to risk delaying publication. As a result, documents varied in length, style and content. Some groups produced articles formatted for submission to scientific journals, with associated references to clinical trials and other sources of evidence for alternative fractionations. Others produced shorter, pragmatic summaries with advice for clinicians based upon consensus agreement. We expected the guidance to be rapidly reviewed, and asked authors to contact us with any amendments or updates as they came about, to ensure rapid dissemination of the most up-to-date and relevant advice.
A number of groups referenced the NHS England ‘Clinical guide for the management of non-coronavirus patients requiring acute treatment: cancer’ [6]. This specialty guide was first published on 16 March 2020, and helped clinicians frame their COVID-19-specific guidance documents by providing clear prioritisation structures for cancer patients. The National Institute for Health and Care Excellence (NICE) commissioned COVID-19 rapid guidelines for systemic anti-cancer therapy [7] and radiotherapy [8], which were published on 20 and 28 March 2020, respectively. Prioritisation recommendations within these documents were adapted from NHS England's initial specialty guide and were also referenced within some of the guidance documents. RCR Officers and Fellows contributed to both NICE guidelines and to the NHS-E clinical guide. The NICE radiotherapy guideline included a link to the RCR repository for guidance on modifications to usual care (recommendation 8.8).
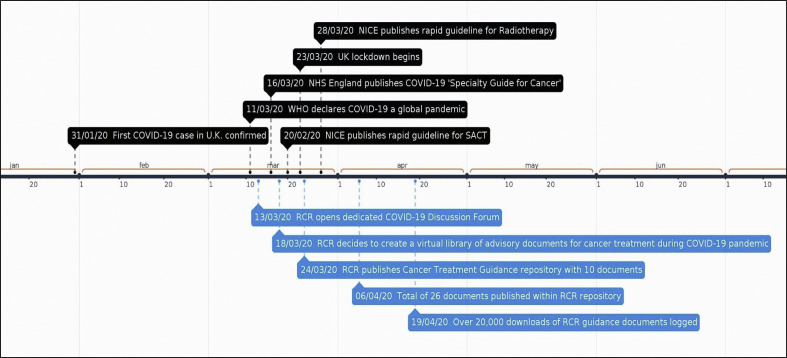
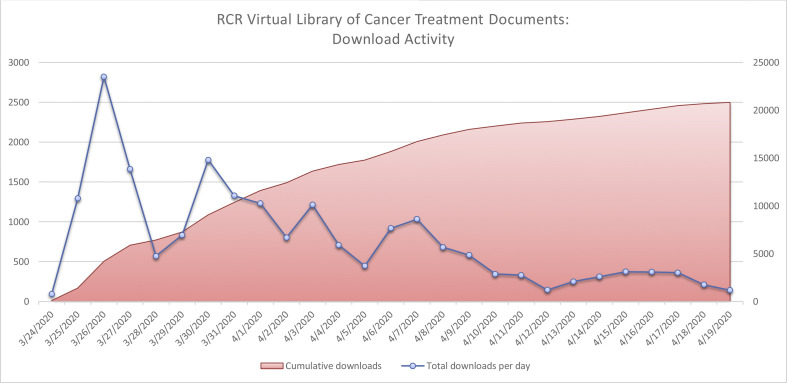
The first guidance documents were uploaded to the repository on 24 March 2020. By 6 April 2020, 26 documents, covering all tumour sites and including guidance for proton beam therapy, paediatric radiotherapy and stereotactic radiosurgery, had been uploaded (see Figure 1 ). As of 19 April 2020 there have been over 20 000 downloads of individual documents (see Figure 2 ). Informal feedback to the RCR has suggested that they have been widely used in clinical practice. Many have been further developed, sometimes with international collaborators, and published in peer-reviewed journals in subsequent weeks [[9], [10], [11], [12], [13]].
Fig 1.
Timeline of events.
Fig 2.
Downloads of documents from Royal College of Radiologists' repository.
There have been challenges. One guidance document was embargoed by a journal before it could be published in the repository, but most authors and publishers have been commendably focussed on early dissemination. Other international groups have occasionally produced guidance with different recommendations. We have attempted to mitigate these risks by encouraging authors to submit updates to their guidance at any time, making the process as responsive as possible.
Next Steps
The focus of the National Health Service is now recovery. The Secretary of State for Health, Matt Hancock, announced on 27 April 2020 that ‘starting tomorrow, we will begin the restoration of other NHS services – starting with … cancer care’. It is to the great credit of the oncology community that radiotherapy and chemotherapy services have continued to operate throughout the initial peak of the pandemic and that the response to COVID-19 has been co-ordinated and evidence-based where possible, and focussed on continuing to treat our patients in a safe environment.
The pace of change means that the guidance documents will continue to need frequent updating, to include recommendations for future care. We need to consider when and how to safely reintroduce deferred treatments such as prostate cancer radiotherapy. We need to consider the role of screening patients and staff to keep cancer departments COVID-protected. We need to re-appraise our treatments as we learn more about the risks of this novel virus to oncology patients, from studies such as the UK Coronavirus Cancer Monitoring Project [14]. Data from the National Cancer Registration and Analysis Service, which includes the systemic anti-cancer treatment and radiotherapy datasets, will describe how the delivery of cancer treatment is changing. Individual cancer centres are collecting information on why treatments have changed in response to COVID-19. National research groups and tumour-specific teams are collating this information. For example, CT-Rad are launching ‘COVID RT’ in collaboration with the RCR, the Society and College of Radiographers and the Institute of Physics and Engineering in Medicine to examine the impact of COVID-19 on radiotherapy [15]. If these data can be linked to meaningful clinical outcomes, we will begin to understand the full impact of COVID-19 on cancer services and patients.
There is also a need and a will to seize the opportunities that the last 2 months have presented; telephone or video appointments for selected patients, better information technology for meetings, including cancer multidisciplinary team working, and improved remote working platforms. But perhaps the most important is the renewed ability of our clinical community to support each other and to innovate and adapt at pace while maintaining a high quality of care for our patients.
Conflicts of interest
There are no actual or potential conflicts of interest to declare.
Acknowledgements
The authors would like to thank all the RCR staff who enabled the RCR COVID-19 guidance repository and fora, particularly Gillian Dollamore, Clinical Oncology Executive Officer, and Beth Skinner, Digital Content Officer, and everyone who has contributed to the guidance documents.
References
- 1.Thompson M.K., Poortmans P., Chalmers A.J. Practice-changing radiation therapy trials for the treatment of cancer: where are we 150 years after the birth of Marie Curie? Br J Cancer. 2018;119(4):389–407. doi: 10.1038/s41416-018-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt A.M., Haviland J.S., Wheatley D.A. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simcock R., Thomas T.V., Estes C. COVID-19: Global radiation oncology's targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020;22:55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royal College of Radiologists Coronavirus (COVID-19): cancer treatment documents. https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-information/coronavirus-covid-19-cancer Available at:
- 6.NHS England Clinical guide for the management of non-coronavirus patients requiring acute treatment: cancer. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-acute-treatment-cancer-23-march-2020.pdf Available at:
- 7.NICE Guidance COVID-19 rapid guideline: delivery of systemic anticancer treatments. https://www.nice.org.uk/guidance/ng161 Available at: [PubMed]
- 8.NICE Guidance COVID-19 rapid guideline: delivery of radiotherapy. https://www.nice.org.uk/guidance/ng162 Available at: [PubMed]
- 9.Yahalom J., Dabaja B., Ricardi U. ILROG emergency guidelines for radiation therapy of hematological malignancies during the COVID-19 pandemic. Blood. 2020 doi: 10.1182/blood.2020006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coles C.E., Aristei C., Bliss J. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32(5):279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssens G.O., Mandeville H.C., Timmermann B. A rapid review of evidence and recommendations from the SIOPE radiation oncology working group to help mitigate for reduced paediatric radiotherapy capacity during the COVID-19 pandemic or other crises. Radiother Oncol. 2020;148:216–222. doi: 10.1016/j.radonc.2020.04.035. pii: S0167-8140(20)30216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones C.M., Hawkins M., Mukherjee S., Radhakrishna, Crosby T. Considerations for the treatment of oesophageal cancer with radiotherapy during the COVID-19 pandemic. Clin Oncol. 2020;32:354–357. doi: 10.1016/j.clon.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel K., Choudhury A., Hoskin P. Clinical guidance for the management of patients with urothelial cancers during the COVID-19 pandemic – rapid review. Clin Oncol. 2020;32:347–353. doi: 10.1016/j.clon.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Coronavirus Cancer Monitoring Project. https://ukcoronaviruscancermonitoring.com/
- 15.NCRI’s CTRad to lead COVID RT: a UK-wide initiative to study the impact of COVID-19 on radiotherapy services and patient outcomes. https://www.ncri.org.uk/news/covid19-radiotherapy-initiative/ Available at: