Abstract
Background
The growing understanding of the importance of a healthy microbiome is challenging traditional thinking that resulted in the general acceptance of the Germ Theory of Disease. We propose a more encompassing Microbial Theory of Health that will have implications for the way that we address our relationship with microbes, including hygiene policy and community-based infection control practices.
Methods
This paper considers theories over the last 30 years that have impacted hygiene policy and consumer practice, from the Germ Theory of Disease and the Hygiene Hypothesis, to the Microbial Theory of Health, including the concept of Bidirectional Hygiene. Here we present a high-level review of the literature on pathogen transmission and the cycle of infection in the home and everyday settings.
Results
Targeted hygiene is an evidence-based hygiene policy that is employed to prevent transmission of pathogens and the transmission of infectious diseases through targeting only sites, surfaces, and practices that are considered high risk for pathogen transmission. Targeted hygiene also discourages the indiscriminate use of broad-spectrum microbicides for lower-risk activities and surfaces.
Conclusions
The Microbial Theory of Health, including age-appropriate and health-appropriate hygiene practices for home and everyday life, should usher in a new era in which pathogen reduction can be accomplished without indiscriminate elimination of potentially beneficial microbes from the human and environmental microbiomes.
Key Words: Bidirectional hygiene, Common-touch surfaces, Microbiome, Probiotic cleaner, Targeted hygiene, Targeted microbicidal products, Pathogen transmission
Introduction
Due to significant advances in microbiome science over the past 2 decades, we are at the brink of a paradigm shift regarding the role of microbes in disease and health, from the Germ Theory of Disease to the Microbial Theory of Health.1 , 2 This shift will necessitate a change in the approaches that we take to design targeted infection control. In particular, we will need to leverage our knowledge of the microbiome when attempting to reduce the risk posed by infectious agents through use of targeted hygiene, and by fostering/balancing exposure to naturally diverse microbial communities. Interestingly, health-care providers have started shifting their own emphasis in this direction by promoting critical care microbiome research/applications that treat dysbiosis in intensive care patients, an ambitious but encouraging goal.3 Recently, a more pragmatic use of antibiotics for treatment/prevention of infectious diseases has also been suggested by Rook and coworkers.4
The revised viewpoints mentioned here are likely to have a profound effect on hygiene policies.5 On the one hand, community-based infections—including respiratory, gastrointestinal, and skin infections—continue to exert a heavy toll on human health and prosperity.6 We use the term “community” in a broad sense to include both home environments as well as school, workplace, and even recreational settings. This problem is exacerbated by the aging of the population as a whole, and the associated increase in percentage (now ∼20%) of immunocompromised individuals living in the community. The latter are often cared for at home.7 Contrary to optimistic predictions made during the mid-20th century,8 infectious diseases clearly have not been eradicated. Rather, new infectious agents continue to emerge and/or reemerge globally. These have included emerging antibiotic-resistant pathogens (eg, methicillin-resistant Staphylococcus aureus and carbapenem-resistant Enterobacteriaceae). It is hard to overstate the risk associated with the emergence of such multidrug-resistant pathogens. The government of the United Kingdom has referred to this issue as a “postantibiotic apocalypse,” which threatens to kill 10 million people globally by 2050.9
There also currently is much concern about the rapid rise in allergies (especially asthma and food allergies) and other chronic inflammatory diseases in the population. The Hygiene Hypothesis proposed by Strachan10 postulated that a lower incidence of early childhood infection (predominantly in first-world countries) might explain the rapid rise of allergic diseases during the 20th century. As discussed in a 2016 review by Bloomfield et al,11 our current understanding of host-microbiome interactions and immune dysfunction suggests that increases in chronic inflammatory diseases are, instead, the combined result of lifestyle, nutrition, medical, and public health (hygiene and sanitation) changes. These changes are thought to have deprived humans of exposure to potentially beneficial microbial agents (described as Old Friends [OF]), particularly in early life.11 These OF microbes are not pathogenic, as argued by Strachan,10 but rather include nonharmful diverse microbial species that inhabit the human gastrointestinal tract and our natural environment. While the identity of the most important OF microbes may not be clear, our attitudes towards such nonpathogenic microbes must change. Thus, the concept of age-appropriate and health-appropriate hygiene practices for home and everyday life has emerged.11 Such practices include age-appropriate vaccination and exposure to nonharmful microbes that beneficially prime the developing immune system.12 , 13 This strategy has the goal of balancing targeted hygiene with maintaining the natural diversity of the human and indoor microbiomes.
From the hygiene hypothesis to the Microbial Theory of Health
The Hygiene Hypothesis of Strachan10 has resulted in the inherently dangerous concept of our being too clean, an idea that has persisted in the media and in the minds of the public. More recently, it has been argued that the Hygiene Hypothesis is flawed, but despite this, broad acceptance of the theory had been encouraged by a phenomenon known as citation bias. Namely, it was found that publications supportive of the Hygiene Hypothesis were cited more often than nonsupportive publications.14 We believe it is time to restore public understanding of hygiene, and specifically targeted hygiene, as a tool for preventing transmission of pathogens (breaking the chain of infection) and, consequently, transmission of infectious diseases. 11 This is consistent with efforts of the infection control communities and public health agencies worldwide toward emphasizing the necessity of basic hygiene practices, at both the individual and community levels, for infection control. Most importantly, hygiene is now being seen as a key component of strategies intended to tackle the global problem of antibiotic resistance.15 , 16 It is hoped that, by reducing the level of infectious agent exposure, fewer people will need to seek antibiotic treatment–thereby limiting the selective pressure for generating antibiotic-resistant genes and the associated antibiotic-resistant strains of pathogens.17, 18, 19
Pathogen transmission and the cycle of infection
Before considering the concept of targeted hygiene, we need to identify the highest risk factors for pathogen transmission. Globally, the home captures a large cross-section of the human population in terms of age, health, nutritional status, and susceptibility to infectious agents. The home is therefore representative of many other community settings in terms of the necessity for hygiene practices. In fact, a constant dynamic exists between the home and other community settings (eg, day care, work, school, travel, leisure, healthcare, etc.) in terms of the dissemination of infectious agents from infected individuals, contaminated food, and domestic animals to surfaces and, via the intermediacy of human hands, through the entire cycle of reinfection.1 Day care settings represent an especially high risk, as young children are immunologically immature and exhibit behaviors that actually encourage transmission of infectious agents (eg, poor personal hygiene and mouthing of objects).
In determining the role that environmental surfaces play in the transmission of infectious agents, it is important first to develop a working definition of surface contamination. The chain of events leading to the occurrence and spread of infectious agents must then be considered. The prudent use of effective targeted hygienic approaches as a possible means of reducing the burden of infectious agents is described, keeping in mind that decontamination of all environmental surfaces on a continuous basis may not be necessary in all instances.
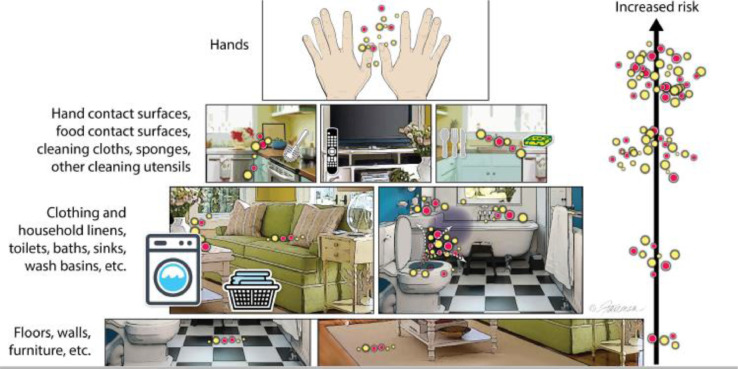
To define the risk associated with surface contamination, we must consider the types of surfaces that are most likely to become contaminated during activities of daily living (Fig 1 ).
Fig 1.
Examples of surfaces in the home ordered by risk for pathogen transmission (from Scott et al.1; modified from Bloomfield et al11) The red and yellow dots represent pathogenic and nonpathogenic microorganisms, respectively.
Pathogens travel via well-defined routes from an infected source to a new host.20, 21, 22 Numerous sampling studies have recorded the presence of both pathogenic bacteria, fungi, parasites, and viruses, as well as nonpathogens, on environmental surfaces in home and community settings.6 Both laboratory and field studies have evaluated the rates of transfer of pathogens via hands and contaminated surfaces, as reviewed by Bloomfield et al.7 These studies demonstrate that the surfaces with the highest risk of transmitting pathogens, and which are therefore the critical control points in the transmission of infection, are the hands and common-touch surfaces, food-contact surfaces, and the cleaning utensils used on these surfaces, as shown in Fig 1.
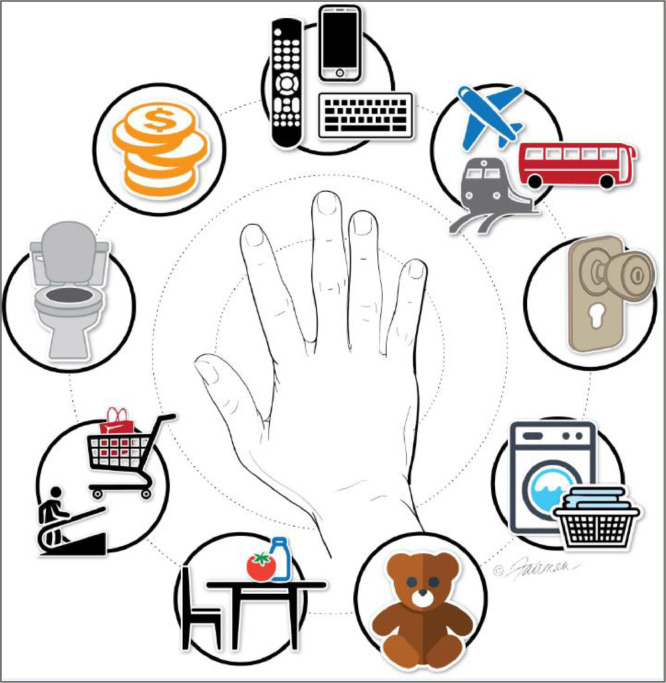
These high-risk environmental surfaces may serve as reservoirs for infectious agents deposited following shedding from humans or domestic animals as aerosols, or infectious agents arising from contaminated raw meats, fruits, and vegetables, as well as pathogen-contaminated air and water. Common-touch surfaces (Fig 2 ) such as door knobs, toilet flush handles, faucet handles, remote control devices, shared desks and other furniture (ie, in schools and offices), digital devices, and light switches represent an especially high risk of dissemination of these infectious agents throughout indoor spaces. Infectious agents have been found to survive on indoor environmental surfaces for extended periods of time, in many instances remaining for days to months at populations high enough to initiate infection of a new host. The duration of persistence is determined by the characteristics of the pathogen and of the surface itself, and other environmental factors, such as temperature and humidity and the organic matrix associated with the pathogens. The health implications of such persisting pathogen loads are dependent, to some extent, on the human infectious doses for the microbes, which can range from 1 to 10 infectious units for some hemorrhagic fever viruses23 (eg, Ebola virus) to thousands of infectious units for Staphylococcus aureus.1
Fig 2.
High-risk common-touch surfaces for transmission of pathogens in the home or outside of the home (school, workplace, recreational settings; from Scott et al1; adapted from Alum et al 20).
The cycle of infection and reinfection involves dissemination, primarily through the intermediacy of the hand, from common-touch surfaces to new surfaces and/or other hosts. This cycle includes: (1) pathogen release from an infected source; (2) contamination of common-touch surfaces by released pathogens; (3) persistence of the pathogen on the contaminated surface; (4) transfer of pathogens to secondary common touch surfaces; (5) pathogen transfer to a new host; and (6) infection of the new host with consequent spread of the associated disease. This cycle may be interrupted through the timely use of effective targeted hygiene practices and the judicial use of effective microbicides. The global outbreak of SARS-CoV-2 and the associated disease COVID-19, emerging late in 2019, is a good example of the need for microbicidal agents to interrupt the cycle of infection. COVID-19 is primarily a lower respiratory syndrome, with some enteric and multisystem impacts, therefore SARS-CoV-2 is disseminated through the very same steps discussed above. Pathogens such as SARS-CoV-2 must be targeted for decontamination using the various microbicidal agents having adequate efficacy (see section below on microbicidal efficacy).
The points to be considered in developing targeted surface decontamination practices include: (1) the probability of significant pathogen contamination at the targeted high-risk (common-touch) surface under consideration; (2) the types of pathogens that are most likely to survive on surfaces and the time periods over which these might remain infectious at levels in excess of the minimum human infectious dose; (3) the likelihood of pathogen transfer from the contaminated surface to human hands and to other surfaces and hosts; and (4) the susceptibility of the new hosts to acquiring infection.
Targeting those surfaces at high risk for pathogen transmission/acquisition by hosts, and applying appropriate decontamination practices, form the basis for an evidence-based hygiene policy known as targeted hygiene. The historical approach to pathogen reduction on environmental surfaces has involved attempts to indiscriminately reduce microbes naturally present in those areas without regard for whether these represent low- or high-risk surfaces. For instance, advertisements in the popular media have implied that, for maintaining health, all germs must be eradicated from our environments. Such an impractical approach has not accounted for the possible beneficial impact of nonpathogenic (OF) microorganisms comprising a naturally diverse vs dysbiotic indoor microbiome. We still have much to learn about such beneficial OF microbes. Targeted hygiene, on the other hand, is intended to manage the natural microbial diversity24 of environmental surfaces as well as the human microbiome,11 incorporating the novel concept of bygiene or bi-directional hygiene. 25 How is this to be accomplished?
Bidirectional hygiene (bygiene)
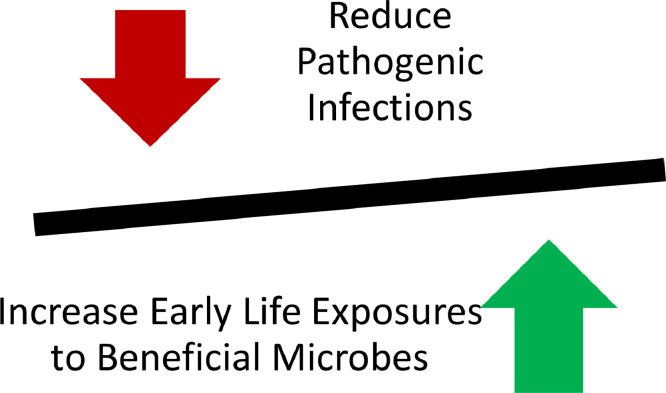
The use of hygiene practices, such as surface decontamination or hand antisepsis, to reduce pathogen burden on a surface must be balanced against the need to maintain the natural microbial diversity. This is the essence of bidirectional hygiene or bygiene, a concept introduced by Al-Ghalith and Knights.25 This concept is similar to that expressed in the phrase “targeted hygiene is smart hygiene.”1 The bygiene approach (Fig 3 ) is designed to reduce the risk of infection and therefore the need for antibiotics, while maintaining exposure to beneficial (OF) microbes. This is in distinct contrast to the Hygiene Hypothesis, which maintains that exposure to microbes (including some pathogens) is necessary, and certain health issues have arisen due to our overemphasis on sanitation. Another way of properly considering hygiene has been formulated by Vandegrift et al.26 as “those actions and practices that reduce the spread or transmission of pathogenic microorganisms, and thus reduce the incidence of disease.”
Fig 3.
Bidirectional hygiene (bygiene) approach.
There are at least 2 approaches to pathogen reduction that are consistent with the principles of bygiene. The first of these is embodied in the term targeted hygiene. Targeted hygiene takes into account the importance of high-risk surfaces and situations (Fig 2) in the transmission of infectious diseases, and emphasizes the use of either hygienic cleaning of hands with soap or detergent and rinsing and the use of broad-spectrum microbicides on such high-risk surfaces selectively.
It follows that high-risk surfaces/situations should be addressed through cleaning and microbicide application, while such interventions do not need to be used for low-risk surfaces.
Another alternative to microbicidal formulations for targeted infection control is the use of smart surfaces27 , 28 for high-risk activities such as food preparation. Application have been described for mitigation of the formation of biofilms29 and for the use of copper surfaces for high risk surfaces (eg, door knobs). It has been reported, however, that microorganisms may acquire a variety of defense mechanisms to such microbicidal metals.30
Knowledge of the impacts of disruption of microbiomes on health also can inform the use of microbicides. For instance, recent studies of the oral microbiome have suggested that altering the natural diversity of microorganisms (dysbiosis) may lead to periodontal disease.31 This argues for treatments aimed at tailoring the oral microbiome, rather than simply attempting to eliminate oral microbial populations altogether. Similarly, perturbations in the microbiome within the gastrointestinal tract have been proposed to lead to inflammatory diseases.32 The human skin microbiome of healthy adults is relatively stable and resilient.33 However, there appears to be a much more highly diverse skin microbiome associated with isolated populations such as the Yanomami Amerindians of Venezuela, who have been devoid of contact with individuals from developed countries and to the hygiene practices of those countries.34 The comparative study of such isolated communities suggests that westernization and urbanization has led to a substantial reduction in exposure to the diversity of beneficial environmental microorganisms our ancestors likely were exposed to for millennia. Such exposure is thought to be essential for appropriate development of our immune systems and prevention of acquiring allergies.25 Susceptibility to disruption of the skin microbiome of individuals living in megacities has been reported by Kim et al.35 Air pollutants present in certain megacities may also impact the outdoor microbiome, though the potential impacts have yet to be elucidated. Impacts of pollutants on the pharyngeal microbiome are being investigated, and indicate alterations in taxonomic composition.36 The skin microbiomes were shown to be impacted by factors such as environment and socioeconomic status. Outdoor environment physical activity may also contribute to the acquisition of the human microbiome via skin and airways.11
On the other hand, the composition of the indoor microbiome seems to be more transient than the human microbiome.37 The modern home should not be considered a natural setting, and the composition of the indoor microbiome in such dwellings is not stable, being heavily influenced by the daily activities of the human, domestic animal, and plant inhabitants, as well as by external factors such as pollution and other chemical contributors to our exposome.38 The types of shelters or habitats in which our ancestors lived in the distant past would have provided continuous exposure to external elements such as dirt, animals, plants, and natural water sources. Exposure to this natural outdoor microbiome has now been reduced in our modern dwellings. The consequent impact to health is thought to include an increased incidence of asthma and other manifestations of allergies. In support of this, children growing up on traditional Amish farms in contact with animals have been shown to be less prone to asthma and other types of allergic reactions.39 The reduced incidence of allergic reactivity in such children has been attributed to the inhalation of air containing bacterial endotoxin (lipopolysaccharide), which is thought to reduce the overall reactivity of the immune system.39 This is consistent with reports demonstrating reduction in development of house dust mite–related asthma induced by an allergic stimulus in mice chronically exposed to bacterial endotoxin.40
A beneficial indoor microbiome product of the future might make the indoors more like the outdoors, with respect to composition of the microbiome. As of this time, however, it is not clear exactly what constitutes a healthy indoor microbiome.41 Management of the microbiome, whether that of environment, the oral cavity, or the skin, may represent an important intervention for optimizing human health and appropriate development of the immune system. For instance, in the future there may become available products containing probiotics, beneficial environmental bacteria, or components of these bacteria capable of interacting with the developing immune system in a manner that reduces the types of allergic reactions now being seen in children. This type of product might also provide a constant exposure to such bacteria in order to keep the immune system functioning appropriately.
Probiotics have also been used to manage the indoor microbiome. The first example of such a product, the probiotic-based cleaning hygiene system42 is now commercially available. Probiotic-based cleaning hygiene system is based on the use of nonpathogenic probiotic bacterial spores from the Bacillus genus, and has been evaluated in a hospital setting. It is claimed to allow nonpathogenic bacteria to outcompete pathogens, including antimicrobial-resistant microbes.18 , 42 The mechanism of action is thought to include the inhibition of quorum-sensing molecules of certain pathogenic bacteria.17 , 18 Additional proposed mechanisms include competitive antagonism of pathogen growth,42 and production of antibacterial compounds such as bacteriocins.18 This cleaning system is not intended for use in surgical suites or other areas that need to be aseptic. While environmental microorganism-based cleaning systems are becoming available in the market, with few exceptions the efficacy of such systems have not been demonstrated with experimental rigor, appropriate field studies, or long term analysis of sustainability of any changes in the indoor microbiome and subsequent health outcomes in populations. An example of such a study might include the identification of the microbiome of a prototypic high-risk environment (bathroom, kitchen sink, etc.) prior to and after the application of a probiotic product. Future research should also be conducted to identify the best mix of probiotics; the appropriate or optimal delivery systems; the safety profiles/considerations; potential for causing or preventing infections; the potential impacts of age (particularly the benefit for young children, infants, newborns, as well as older children/adults); and the potential risks for the immunocompromised, aging, and malnourished populations.
Conclusions
Shifting the paradigm from a Germ Theory of Disease toward a Microbial Theory of Health, wellness, and disease prevention should not be allowed to undermine the critical role that targeted personal and surface hygiene practices play in interrupting the dissemination of infectious agents. As discussed above, targeted hygiene focuses on high-risk activities and surfaces, and discourages use of broad-spectrum microbicides for lower-risk activities and surfaces. It is the responsibility of subject matter experts (infection control communities, public health agencies, environmental hygienists, and manufacturers of microbicides) to remain current on the advances being made in microbiome science and the impact of microbicides on these microbiomes. In particular, the impact of microbicide use on natural microbial flora, including OF as well as pathogenic species, should be assessed. It is critical to restore the public understanding of the basic principles of good hygiene practices and the importance of the concept of targeted hygiene as a means of minimizing the dissemination of infectious agents. There is an educational component of this as well: in devising best practices for informing the public in both developed and developing countries, it will be wise to draw on field studies43 from low- and middle- income countries. These have demonstrated the critical importance of hygiene education in addition to the use of appropriate and targeted microbicidal “tools” to achieve the desired goal of limiting infectious agents transmission. In explaining targeted hygiene to the public of developed countries with reference to common-touch surfaces such as those outlined in Fig 4 , there are a number of key questions to consider:
-
1.
What are the key targets for microbicidal product use?
-
2.
When is the right time and place to act?
-
3.
Who is most at risk in home and community settings?
-
4.
Which targeted hygiene interventions can be employed?
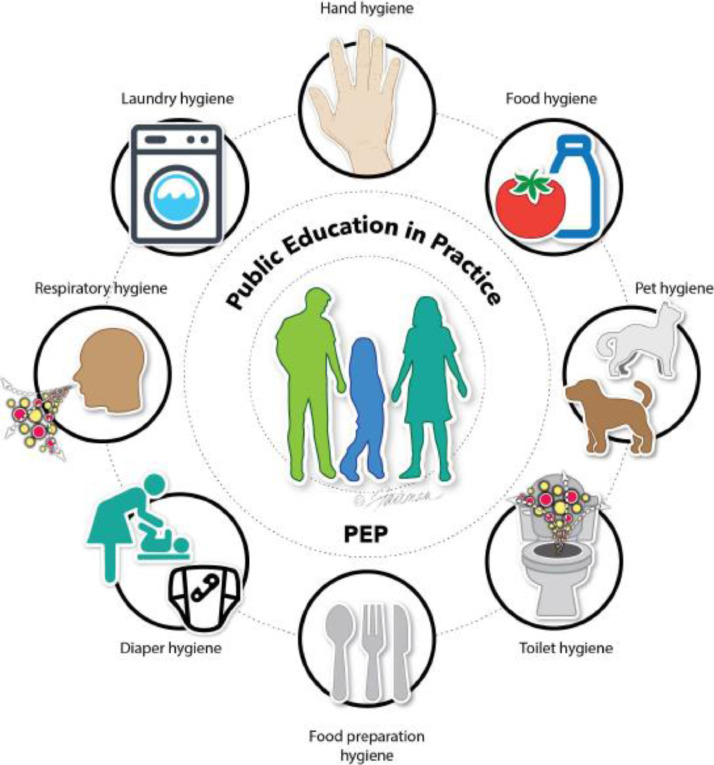
Fig 4.
Infection control and prevention education should highlight the common-touch surfaces for practicing targeted hygiene. Practicing hygiene is particularly important during and after activities such as handling food or eating, handling raw foods (such as meat, poultry, fish, eggs, fruits, and vegetables), using the toilet, diapering, contacting blood or body fluids, touching contaminated porous or nonporous surfaces, dressing a wound or administering medications, touching animals, or performing outdoor activities such as gardening (Source: Scott et al1).
Education of the public within developing countries may need to include more basic microbiological information:
-
5.
The role of pathogens in infectious disease causation and spread.
-
6.
Where are pathogens found and what are the routes of infection?
-
7.
How can infrastructural deficiencies be accommodated while practicing the hygiene principles articulated within this paper?
Focusing on selected behaviors, such as hand washing with soap and clean water, is particularly impactful. Encouraging hand washing at appropriate times before and after high-risk activities (Fig 1) can help to significantly reduce the risk of exposure to infectious agents.
It is hoped that the Microbial Theory of Health will usher in a new era in which pathogen reduction can be accomplished without indiscriminate elimination of potentially beneficial/naturally diverse microbes from the human and environmental microbiomes. Implementation of the principles discussed here will depend upon the leveraging of emerging microbiome sciences, development of alternative microbicides, and education of the public in the concept of targeted hygiene.
Acknowledgments
The authors gratefully acknowledge Jennifer Fairman, CMI, FAMI (Fairman Studios, LLC), for creating the illustrations.
Footnotes
Funding: Publication of this manuscript was primarily supported by Reckitt Benckiser LLC, Montvale, New Jersey.
Conflicts of interest: None to report.
References
- 1.Scott E, Bruning E, Ijaz MK. In: Block's Disinfection, Sterilization, and Preservation. 6th edition. McDonnell G, Hansen J, editors. Wolters Kluwer; Philadelphia: 2020. Decontamination of environmental surfaces in everyday settings. in press. [Google Scholar]
- 2.Timmis K, Cavicchioloi R, Garcia JL, et al. The urgent need for microbiology literacy in society. Environ Microbiol. 2019;21:1513–1528. doi: 10.1111/1462-2920.14611. [DOI] [PubMed] [Google Scholar]
- 3.Kitsios GD, Morowitz MJ, Dickson RP, Huffnagle GB, McVerry BJ, Morris A. Dysbiosis in the intensive care unit: microbiome science coming to the bedside. J Crit Care. 2017;38:84–91. doi: 10.1016/j.jcrc.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rook G, Bäckhed F, Levin BR, McFall-Ngai MJ, McLean AR. Evolution, human-microbe interactions, and life history plasticity. Lancet. 2017;390:521–530. doi: 10.1016/S0140-6736(17)30566-4. [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield S. The hygiene hypothesis: identifying microbial friends and protecting against microbial enemies. Perspect Public Health. 2013;133:301–303. doi: 10.1177/1757913913506642. [DOI] [PubMed] [Google Scholar]
- 6.Scott E. Community-based infections and the potential role of common touch surfaces as vectors for the transmission of infectious agents in home and community settings. Am J Infect Control. 2013;41:1087–1092. doi: 10.1016/j.ajic.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Bloomfield SF, Exner M, Nath KJ, Signorelli C, Scott EA. International Scientific Forum on Home Hygiene. 2012. The chain of infection transmission in the home and everyday life settings, and the role of hygiene in reducing the risk of infection.https://www.ifh-homehygiene.org/sites/default/files/publications/IFHinfectiontransmissionreviewFINAL.pdf Available at: [Google Scholar]
- 8.Rappuoli R. From Pasteur to genomics: progress and challenges in infectious diseases. Nature Med. 2004;10:1177–1185. doi: 10.1038/nm1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber S, Swaden-Lewis K. Antimicrobial resistance. Briefing Paper CBP 8141, House of Commons Library, 2017. Available at: https://commonslibrary.parliament.uk/research-briefings/cbp-8141/. Accessed June 24, 2020.
- 10.Strachan DP. Hay fever, hygiene, and household size. Brit Med J. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomfield SF, Rook GA, Scott EA, Shanahan F, Stanwell-Smith R, Turner P. Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health. 2016;136:213–224. doi: 10.1177/1757913916650225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling D. 3rd Annual Translational Microbiome Conference; Boston, MA; 11-13 April, 2017. [Google Scholar]
- 13.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duyx B, Urlings MJE, Swaen GMH, Bouter LM, Zeegers MP. Selective citation in the literature on the hygiene hypothesis: a citation analysis on the association between infections and rhinitis. BMJ Open. 2019;9:e026518. doi: 10.1136/bmjopen-2018-026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Biggest threats and data. 2018. Available at: https://www.cdc.gov/drugresistance/biggest_threats.html. Accessed June 24, 2020.
- 16.World Health Organization. Antimicrobial resistance. 2018. Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed June 24, 2020.
- 17.Caselli E, Arnoldo L, Rognoni C, et al. Impact of a probiotic-based hospital sanitation on antimicrobial resistance and HAI-associated antimicrobial consumption and costs: a multicenter study. Infect Drug Resist. 2019;12:501–510. doi: 10.2147/IDR.S194670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Accolti M, Soffritti I, Mazzacane S, Caselli E. Fighting AMR in the healthcare environment: microbiome-based sanitation approaches and monitoring tools. Int J Mol Sci. 2019;20:1535. doi: 10.3390/ijms20071535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Cao J, Chen QL, et al. Global survey of antibiotic resistance genes in air. Environ Sci Technol. 2018;52:10975–10984. doi: 10.1021/acs.est.8b02204. [DOI] [PubMed] [Google Scholar]
- 20.Alum A, Rubino JR, Ijaz MK. The global war against intestinal parasites – should we use a holistic approach? Int J Infect Dis. 2010;14:e732–e738. doi: 10.1016/j.ijid.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14:1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei H, Li Y, Xiao S, et al. Logistic growth of a surface contamination network and its role in disease spread. Sci Rep. 2017;7:14826. doi: 10.1038/s41598-017-13840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franz DR, Jahrling PB, Friedlander AM, et al. Clinical recognition and management of patients exposed to biological warfare agents. J Am Med Assoc. 1997;278:399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- 24.Velazquez S, Griffiths W, Dietz L, et al. From one species to another: a review on the interaction between chemistry and microbiology in relation to cleaning in the built environment. Indoor Air. 2019;29:880–894. doi: 10.1111/ina.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Ghalith GA, Knights D. Bygiene: the new paradigm of bidirectional hygiene. Yale J Biol Med. 2015;88:359–365. [PMC free article] [PubMed] [Google Scholar]
- 26.Vandegrift R, Bateman AC, Siemens KN, et al. Cleanliness in context: reconciling hygiene with a modern microbial perspective. Microbiome. 2017;5:76. doi: 10.1186/s40168-017-0294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei T, Tang Z, Yu Q, Chen H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. ACS Appl Mater Interfaces. 2017;9:37511–37523. doi: 10.1021/acsami.7b13565. [DOI] [PubMed] [Google Scholar]
- 28.Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo H, Allan RN, Howlin RP, Hall-Stoodley L, Stoodley P. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15:740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruins MR, Kapil S, Oehme FW. Microbial resistance to metals in the environment. Ecotox Environ Safety. 2000;45:198–207. doi: 10.1006/eesa.1999.1860. [DOI] [PubMed] [Google Scholar]
- 31.Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162:22–38. doi: 10.1016/j.imlet.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachnit T, Bosch TCG, Deines P. Exposure of the host-associated microbiome to nutrient-rich conditions may lead to dysbiosis and disease development- an evolutionary perspective. mBio. 2019;10:e00355-19. [DOI] [PMC free article] [PubMed]
- 33.Oh J, Byrd AL, Park M, NISC Comparative Sequencing Program. Kong HH, Segre JA. Temporal stability of the human skin microbiome. Cell. 2016;165:854–866. doi: 10.1016/j.cell.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv. 2015;1 doi: 10.1126/sciadv.1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H-J, Kim H, Kim JJ, et al. Fragile skin microbiomes in megacities are assembled by a predominantly niche-based process. Sci Adv. 2018;4 doi: 10.1126/sciadv.1701581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin T, Zhang F, Zhou H, et al. High-level PM2.5/PM10 exposure is associated with alterations in the human pharyngeal microbiota composition. Front Microbiol. 2019;10:54. doi: 10.3389/fmicb.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lax S, Smith DP, Hampton-Marcell J, et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014;345:1048–1052. doi: 10.1126/science.1254529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai D, Prussin AJ, II, Marr LC, Vikesland PJ, Edwards MA, Pruden A. Factors shaping the human exposome in the built environment: opportunities for engineering control. Environ Sci Technol. 2017;51:7759–7774. doi: 10.1021/acs.est.7b01097. [DOI] [PubMed] [Google Scholar]
- 39.Stein MM, Hrusch CL, Gozdz J, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. New Engl J Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuijs MJ, Willart MA, Vergote K, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 41.Dannemiller KC. Moving towards a robust definition for a “healthy” indoor microbiome. mSystems. 2019;4 doi: 10.1128/mSystems.00074-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caselli E, D'Accolti M, Vandini A, et al. Impact of a probiotic-based cleaning intervention on the microbiota ecosystem of the hospital surfaces: focus on the resistome remodulation. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0148857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole EC, Hawkley M, Rubino JR, et al. Comprehensive family hygiene promotion in peri-urban Cape Town: gastrointestinal and respiratory illness and skin infection reduction in children aged under 5. S Afr J Child Health. 2012;6:109–117. [Google Scholar]