Dear Editor,
We note with interest the study of Liu et al.1 where they used routine laboratory test parameters to derive a potentially useful, independent measure (neutrophil – lymphocyte ratio – NLR) of hospital-based mortality for male patients with COVID-19.
Indeed, many routinely performed tests can offer useful insights into the status and evolving epidemiology of COVID-19 patients in local patient populations.2 Here, we use our SARS-CoV-2 testing data to derive several useful epidemiological parameters, which can be applied simply by any team, to gain greater insight into the characteristics of their COVID-19-infected populations, which may help to target additional interventions and investigations, as appropriate.
Between 3 March 2020 and 29 April 2020, we performed a total of 8774 SARS-CoV-2 PCR tests (mostly from nose and throat swabs), either via the local Public Health England (PHE) Birmingham reference laboratory or in-house assays.3 , 4 No asymptomatic testing was routinely performed during this period and those tested exhibited at least one or more of the following typical COVID-19 symptoms at the time of testing: fever, cough, myalgia, shortness of breath, sore throat, headache, chest tightness, fatigue, loss of taste and/or smell, abdominal discomfort and/or diarrhoea.
Hospital staff were mostly self-isolating at the time of testing or performing non-patient-facing duties. The community patient samples were collected by a mobile team consisting mostly of general practitioners, supported by East Midlands PHE team. This team travelled to the homes (during the day and out of hours) and took nose and throat swab samples of suspected COVID-19 cases who had called NHS 111, reporting COVID-19 compatible symptoms – including any recent relevant travel history to COVID-19 endemic areas. We decided to exclude children from the final analysis, as it is still uncertain what role they play in the transmission of SARS-CoV-2.5 This meant that we excluded 658 test results from patients aged 0-17 years, of which only 30 were positive.
Thus, swab samples were obtained from symptomatic hospitalised and community–based patients, and hospital staff, with positivity rates as follows: 1674 positive cases (21.35%) out of 7840 hospitalised patients (HOSP) tested; 200 positive cases (48.70%) out of 411 community patients (COMM) tested; 152 positive cases (29.10%) out of 523 hospital staff (STAF) tested.
Our positivity rate for the cumulative total number of tests performed on hospitalised patients and hospital staff (21.35-29.10%) is slightly higher than that published by PHE on 5 May 2020 (under “Pillar 1: swab testing in PHE labs and NHS hospitals for those with a clinical need, and health and care workers”) of 18.5% (155,567/842,903).6 The high positivity rate in our community patients is likely a result of a very targeted local COVID-19 testing program led by the NHS 111 and East Midlands PHE teams, where the COVID-19 case definition became relatively more sensitive and specific outside of our normal seasonal influenza period. The latter had ended earlier than usual this year in Leicester (effectively by the end of February 2020), making it more likely that any acute febrile influenza-like illness was due to COVID-19.
We can use these positivity rates obtained from these initially wholly suceptible populations,7 during this exponential growth stage of the evolving COVID-19 epidemic (Fig. S1) to define the ‘attack rate’ (AR) as the proportion of infected individuals who develop symptoms during the early phase of an outbreak, in the absence of any intervention, by the formula:
where R0 is the basic reproductive number, which is the average number of secondary cases generated by a single index case in a wholly susceptible population, and ln is the natural logarithm.8
Thus, by solving the equation above for each of those populations we can derive a value for R0 for each of these populations: R0(HOSP) =1.13; R0(COMM) =1.38; R0(STAF)=1.21
During this early stage of the COVID-19 epidemic in this population, in the absence of any pre-existing immunity, we can equate R0 to Rt, the effective reproductive number,7 , 8 which describes the number of secondary cases in an evolving outbreak, taking into account various interventional factors, such as the use of hand-washing, personal protective equipment, social distancing, as well as a non-uniformly distributed susceptible population.9
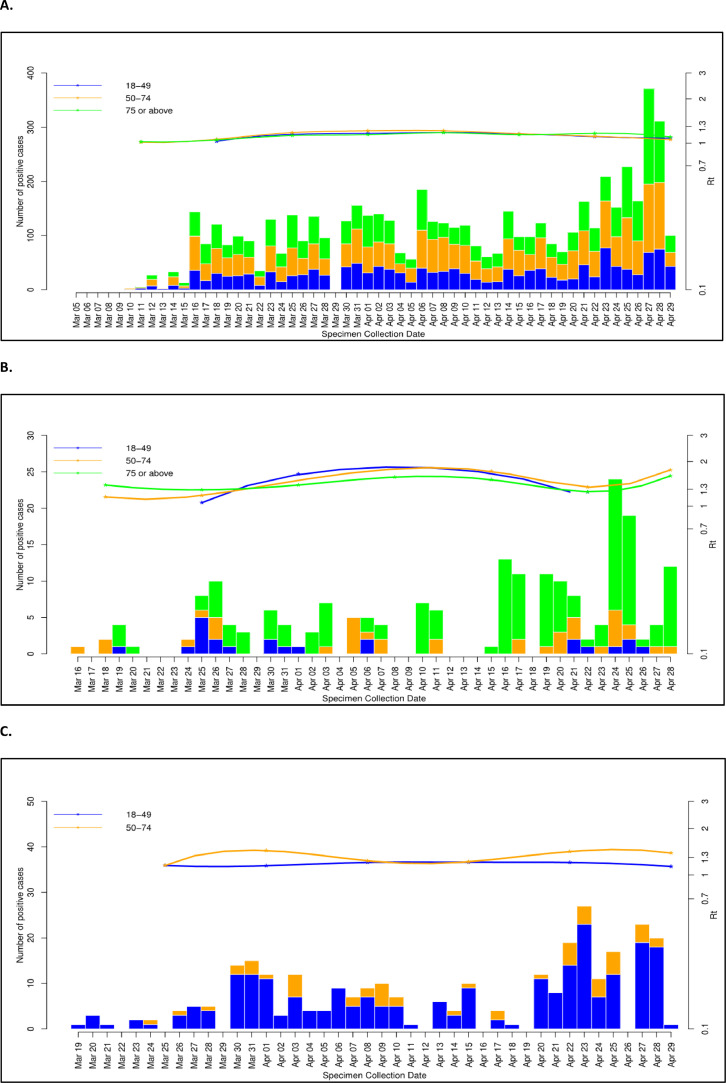
The data within each group (HOSP, COMM, STAF) was further age-stratified (18-49 years, 50-74 years, >75 years) and plotted with the changing value of Rt (Fig. 1 ).
Fig. 1.
Age-stratified (18-49 yrs, 50-74 yrs, >75 yrs) SARS-CoV-2 (COVID-19) positive cases (left vertical axis – linear scale) in hospitalised (A - HOSP) and community (B - COMM) patients, and staff (C - STAF) populations plotted over time. Effective reproductive number (Rt –right vertical axis – logarithmic scale) is plotted for each group with the same age-related colour code.
From these plots we can see that the Rt value generally remains between 1-2 for all these populations, with wider fluctuations in the COMM population across all age groups, compared to the HOSP or STAF populations.
A value of Rt>1 indicates that the epidemic is still self-propagating, and further interventions are required to reduce the ongoing transmission of SARS-CoV-2 in that population, e.g. by increased social distancing measures (in the community patients), or more stringent adherence and/or additional infection control measures, such as improved ventilation (for hospital staff working within a hospital environment).
For the hospitalised patients, as for the hospital staff, Rt lies very close to 1, indicating that for these populations, the rate of new COVID-19 diagnoses – either from new COVID-19 patients being admitted or additional hospital staff getting infected – is still just around that required to maintain the ongoing COVID-19 epidemic, locally.
In summary, we have used our routinely collected diagnostic data on SARS-CoV-2 testing in suspected COVID-19 patients to derive some useful epidemiological parameters to understand better the characteristics of the evolving COVID-19 epidemic during its early exponential growth phase in our local population. These methods are easily applied by other teams where such SARS-CoV-2 testing data from the initial phase of the epidemic is available.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.05.029.
Appendix. Supplementary materials
References
- 1.Liu Y., Du X., Chen J., Jin Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020 Apr 10 doi: 10.1016/j.jinf.2020.04.002. pii: S0163-4453(20)30208-5[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J., Litvinova M., Wang W. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis. 2020 Apr 2 doi: 10.1016/S1473-3099(20)30230-9. pii: S1473-3099(20)30230-9[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England (PHE). Novel coronavirus diagnostic test rolled out across UK. https://www.gov.uk/government/news/phe-novel-coronavirus-diagnostic-test-rolled-out-across-uk(Accessed 5 May, 2020)
- 4.National Health Service England (NHSE). NHS to ramp up coronavirus testing labshttps://www.england.nhs.uk/2020/03/nhs-to-ramp-up-coronavirus-testing-labs/(Accessed 5 May, 2020)
- 5.Nature News How do children spread the coronavirus? The science still isn't clear. 7 May 2020 doi: 10.1038/d41586-020-01354-0. https://www.nature.com/articles/d41586-020-01354-0 [DOI] [PubMed] [Google Scholar]
- 6.Public Health England. Number of coronavirus (COVID-19) cases and risk in the UK. https://www.gov.uk/guidance/coronavirus-covid-19-information-for-the-public (Accessed 5 May 2020)
- 7.Ridenhour B., Kowalik J.M., Shay D.K. Unraveling R0: considerations for public health applications. Am J Public Health. 2014 Feb;104(2):e32–e41. doi: 10.2105/AJPH.2013.301704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obadia T., Haneef R., Boëlle P.Y. The R0 package: a toolbox to estimate reproduction numbers for epidemic outbreaks. BMC Med Inform Decis Mak. 2012 Dec 18;12:147. doi: 10.1186/1472-6947-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwok K.O., Lai F., Wei W.I., Wong S.Y.S., Tang J.W.T. Herd immunity - estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect. 2020 Mar 21 doi: 10.1016/j.jinf.2020.03.027. pii: S0163-4453(20)30154-7[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.