Dear Editor,
Infection by SARS-CoV-2, known as coronavirus disease 19 (COVID-19) has developed into a pandemic of proportions rarely witnessed before. A significant number of subjects infected with COVID-19 present with gastrointestinal (GI)-related manifestations, particularly acute liver injury (ALI). Reports describe different patterns of liver enzymes elevations in affected individuals ranging from 14% to 78% [1]. Most of the data originates from case reports or case series from specific sub-regions of China and there is little understanding on the significance of ALI during COVID-19 infection [2]. In the following study, we systematically reviewed information related to acute liver injury in subjects infected with COVID-19, further characterizing the hepatic injury patterns and correlation to in-hospital outcomes.
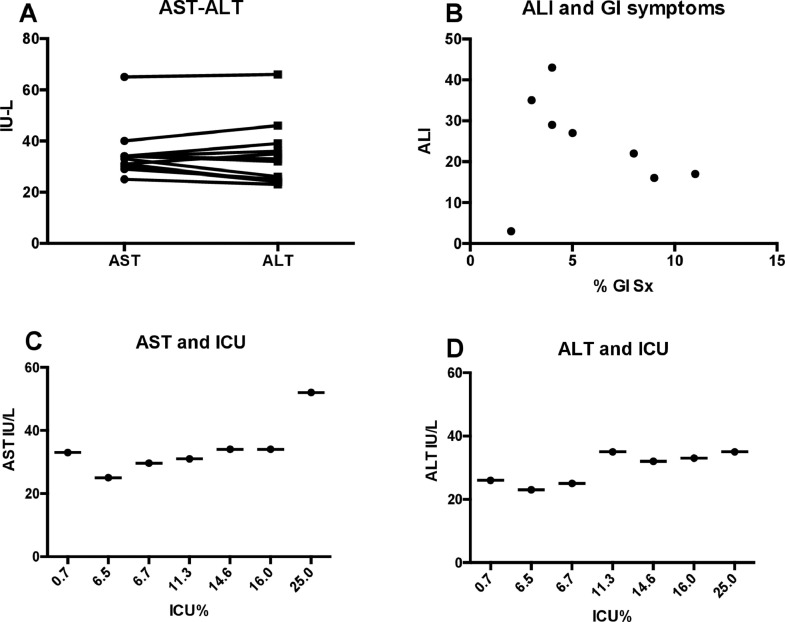
We systematically reviewed all manuscripts related to COVID-19 infections, written in the English language, published in peer-reviewed journals, between December 1st 2019 (the beginning of the pandemic) and March 25th 2020. We included studies that reported information specific to levels of alanine or aspartate aminotransferase (ALT-AST) and/or total bilirubin, presentation of GI symptoms, preexisting liver disease, and in-hospital clinical progression. Authors and institutions in each manuscript were compared to avoid studies reporting on similar patients in different studies. Demographic variables were described by median with interquartile range (IQR). Acute liver injury was defined as an increase in ALT or AST above the upper limit of normal as reported by each study. Statistical analyses were performed via PRISM, using paired T-test aiming for a clinical significance of p<0.05, and correlation coefficients were used to estimate association between clinical variables and transaminase values. Median values with standard deviation from each manuscript related to each variable were used for statistical analyses. A total of 26 articles were evaluated, of which 15 articles met the appropriate criteria, involving a total of 3109 subjects infected with COVID-19 (Table 1 ). All studies originated in China. The median age of affected individuals was 50 years (IQR 47–55) and 56% were males. A total of 6.5% (IQR 3–11.5%) of subjects presented with GI symptoms, most frequently diarrhea and vomiting. A total of 2% (1–9.2%) had evidence of underlying liver disease. The median number of subjects with ALI was 24.5% (IQR 17–36) and the great majority of these injury patterns were characterized by minimal elevations of ALT (median 29 U/L, IQR 24–35) and ALT (median 33 U/L, IQR 30–34) with overall normal values of total bilirubin (median 0.64 mg/dl, IQR 0.57–0.80). There was no statistical difference between levels of ALT and AST in any of the evaluated studies (p = 0.5, Fig. 1 (A)). We found no correlation between the presence of ALI and presentation of GI symptoms (correlation coefficient 0.22, Fig. 1(B)) or presence of underlying liver disease (correlation coefficient 0.12). We found a high degree of correlation between elevated AST or ALT and admission to the intensive care unit (ICU) during hospitalization (correlation coefficients of 0.79 and 0.78 respectively, Fig. 1(C) and (D). Correlation between ALI and mortality could not be performed due to lack of reported data.
Table 1.
Studies providing liver-related data.
| Paper | N | Male (%) | Median age | Location | GI symptoms | Underlying liver disease | ALT (U/L) | AST (U/L) | Total bilirubin (uMol/L) |
|---|---|---|---|---|---|---|---|---|---|
| Xu et al. [6] | 62 | 56 | 41 (32–52) | Zhejiang, China | 9% (diarrhea) | 11% | N/A | N/A | N/A |
| Shi et al. [7] | 81 | 52 | 49 (SD 11) | Wuhan, China | 4.5% (diarrhea-vomiting) | 9% | 29.5% (46) | 17% (40.8) | 11 |
| Cao et al. [8] | 199 | 60 | 58 (49–68) | Beijing, China | N/A | 0% | 33 (22–55) | 34 (26–42) | N/A |
| Chen et al. [3] | 99 | 67 | 55 (SD 13) | Wuhan, China | 3% (diarrhea, vomiting) | 39 (22–53) | 34 (26–48) | 15.1 | |
| Wu et al. [9] | 80 | 49 | 46 (30–61) | Jiangzu, China | 2% (vomiting, diarrhea) | 24 (12–38) | 30 (19–39) | 6.6 | |
| Zhao et al. [10] | 19 | 58 | 48 (27–56) | Anhui, China | 5% (diarrhea) | 1% | 36 (11–85) | 34 (17–103) | N/A |
| Chen et al. [11] | 249 | 50 | 51 (36–64) | Shanghai, China | 3% (diarrhea) | 23 (15–33) | 25 (20–33) | N/A | |
| Yang et al. [12] | 52 | 57 | 59 (SD 13) | Wuhan, China | 4% (vomiting) | N/A | N/A | N/A | N/A |
| Pan et al. [13] | 204 | 52.5 | 51.91 (SD 15.98) | Hubei, China | 18% (diarrhea, vomiting, abdominal pain) | 1% | 35.98 (SD 35.82) | 31.36 (SD 25.55) | 13.65 (SD 10.26) |
| Huang et al. [14] | 41 | 73 | 49 (41–58) | Wuhan, China | 2.6% (diarrhea) | 2% | 32 (21–50) | 34 (26–48) | 11.7 (9.5–13.9) |
| Wang et al. [15] | 138 | 54.3 | 56 (42–68) | Wuhan, China | 10%, (nausea, diarrhea, vomiting, abdominal pain) | 2.9% | 24 (16–40) | 31 (24–51) | 9.8 (8.4–14.1) |
| Guan et al. [16] | 1099 | 58.1 | 47 (35–58) | National Health Commission of China | 8.8% (Nausea/vomiting, diarrhea) | 2% | N/A | N/A | N/A |
| Jin et al. [17] | 651 | 50 | 46.14 (SD 14.19) | Health Commission of Zhejiang Province | 11.4% (nausea, emesis, diarrhea) | 10.8% | b25 (15.75–38.47) | b29.35 (20.87–38.62) | b10 (7.15–13.8) |
| Wan et al. [18] | 135 | 53.3 | 47 (36–55) | Northeast Chongqing | Diarrhea 13.3%, Retching 3.0% | 1.5% | 26 (12.9–33.15) | 33.4 (27.8–43.7) | 8.6 (5.9–13.7) |
| aLuo et al. [19] | 183 | 56 | 53.8 | Wuhan, China | N/A | 66.4 ± 13.2 | 65.8 ± 12.7 | N/A |
Numbers reported were only in patients that presented with GI symptoms and therefore not included in analysis.
Median values in patients without GI symptoms were ALT 21.5 (15–32.8), AST 24.4 (19–32) and bilirubin 9.6 (7.0–13.1). Median age of patients without GI symptoms was 45.09 (SD 14.45) All laboratory data is presented as median (IQR), except for Pan et al., and Luo et al., which are presented as mean ± SD.
Fig. 1.
(A) Median levels of AST and ALT in each study; (B) Correlation between acute liver injury (ALI) and percentage of GI symptoms on presentation each study, y-axis describes percentage of subjects with ALI, x-axis describes percentage of subjects with GI symptoms; (C and D) Correlation between admission to intensive care unit (ICU) and median AST and ALT levels respectively.
Our study found that approximately a quarter of subjects presenting with COVID-19 infection requiring hospitalization have ALI. Liver injury was mainly represented by minimal elevation of ALT and AST with normal values of total bilirubin (Table 1). In most studies, AST showed a slightly higher value than ALT, but not enough to yield a statistical or clinical significant difference. One study found an isolated case of severe liver injury, which was removed from the analysis [3]. Interestingly, our analysis found that cholestatic enzymes remained normal, contradicting the hypothetical notion that angiotensin converting enzyme-2 (ACE-2) receptors present in cholangiocytes, but not hepatocytes could be involved in the physiology underlying liver injury [4]. Our study found no association between GI symptoms and the presence of liver injury. Moreover, we found no correlation between the presence of underlying liver disease and elevated liver enzymes. Interestingly, only 2% of subjects had evidence of a pre-existing liver conditions. This finding alone is of interest as the population of China has a hepatitis B infection prevalence of 7–11% depending on the region and age [5]. We found an association between higher degree of liver injury, expressed by levels of ALT or AST, and admission to the ICU. Although this is an expected finding, it has not been reported systematically across studies. Our study has several limitations, the main one being that it is a systematic analysis of multiple studies and therefore we could not evaluate the specific trend and prognosis of each subject with liver injury within studies, but rather the median of each study. Also, all studies originated in China, limiting the generalization of these findings. Nonetheless, our study provides important insights for specialty providers evaluating COVID-19 individuals and indicates that most COVID-19-related liver disease is expressed by mild alteration of the hepatocellular component, and that cases involving acute liver failure, significant elevation of liver enzymes, or cholestatic disease should be further evaluated for other causes of liver disease.
Footnotes
Source of support: Funded by the Robert Wood Johnson Foundation, Harold Amos Medical Faculty Development Program, and NIH-NCI R21 CA215883-01A1 both to JDD.
References
- 1.Xu L., Liu J., Lu M. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H., Men P., Xiao Y. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:811. doi: 10.1186/s12879-019-4428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J., Liu J., Zhao X. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao D., Yao F., Wang L. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J., Qi T., Liu L. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin X., Lian J.S., Hu J.H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo S., Zhang X., Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;20 doi: 10.1016/j.cgh.2020.03.043. S1542-3565:30401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]