Abstract
Coronavirus disease 2019 (COVID-19) is caused by the novel coronavirus SARS-CoV-2, which affects the lung and other organs. After an incubation period of 3-14 days, the infection presents with symptoms of variable severity, from mild flu-like disease to severe pneumonia and cytokine storm with increased mortality. Immunosuppressed patients may have higher risk of adverse outcomes; hence, there is an urgent need to evaluate the immune response and clinical outcomes of SARS-CoV-2 infection in these patients. Here, we report a 59-year-old woman with aquaporin-4-positive (AQPR4+) neuromyelitis Optica treated with rituximab who developed mild respiratory symptoms with COVID-19, despite B cell depletion at the time of infection.
Keywords: COVID-19, Neuromyelitis Optica Spectrum Disorder (NMOSD), Multiple Sclerosis, pandemic, coronaviruses, SARS-CoV-2
Introduction
SARS-CoV-2, a novel coronavirus that originated in Wuhan, China, has produced a global pandemic known as COVID-19 with substantial mortality and morbidity (Guan et al., 2020). To infect the host, SARS-CoV-2 uses the viral receptors ACE2 and TMPRSS2, which are membrane associated proteins expressed in many cells throughout the body, particularly the respiratory system (Hoffmann et al., 2020). Knowledge about the immunopathology of COVID-19 is evolving, and there is heterogeneity of symptoms among the affected population. Most cases are mild, but in a number of patients, the disease evolves into an acute respiratory distress syndrome (ARDS) (Wu et al., 2020) or a dysregulated immune system state leading to cytokine storm, most often in older adults, requiring intensive care and resulting in increased mortality (Mehta et al., 2020). There is growing evidence that some younger patients may have worse outcomes as well, which may be related to host factors, including an altered immune system, genetics, and comorbidities like obesity and smoking (Guan et al., 2020). Based on these observations, there is concern that patients treated with immunosuppressive drugs may be at a higher risk of developing poor outcomes during a SARS-CoV-2 infection. Therefore, there is great interest in determining the impact of COVID-19 in patients on disease-modifying therapies (DMTs) for autoimmune neurological diseases like multiple sclerosis (MS), neuromyelitis Optica spectrum disorder (NMOSD) and others. An important concern is how B cell depletion modifies the host ability to mount an effective response to SARS-CoV-2. Here, we describe an AQPR4+ NMOSD patient treated with rituximab who developed mild COVID-19 infection despite B cell depletion. We also discuss the emergent evidence of the host cellular and humoral immunity against SARS-CoV-2.
Case report
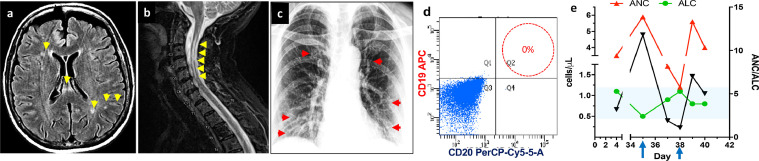
This report was considered exempt research by the UConn Health IRB. A 59-year old woman presented with transverse myelitis in 2006 and was initially diagnosed with multiple sclerosis (MS). She had subsequent relapses with optic neuritis in each eye separately and once simultaneously. MRI of the spine and brain in 2017 showed a left-sided long segment cord signal abnormality extending from C2 to C6 with associated mild cord atrophy, stable since 1 year prior, stable short segment myelitis of the thoracic cord at the level of T2-T3 without evidence of restricted diffusion or abnormal enhancement, and multiple small foci of T2/FLAIR signal hyperintensity within the cerebral white matter bilaterally. She was recategorized in 2014 as NMOSD, AQPR4-positive based on white matter disease in the brain, longitudinal transverse myelitis in the cervical spine, and positive serology for AQPR4 antibody (Fig. 1 A, B). She was initially started on azathioprine, then transitioned to rituximab in early 2014 resulting in chronic B cell depletion and stable disease. In early February 2020 (day 1), the patient developed chills and fever of 102.4 F and low 100s for four days, with no cough, pharyngitis, dyspnea. She went to her PCP on day 5. Rapid influenza A and B tests were negative, other labs were normal, and she was diagnosed with a "fever of unspecified cause." Fever, cough, and malaise persisted, and in early March 2020 (day 26), she presented to Neurology for a routine follow-up clinic visit. She reported continued flu-like symptoms and malaise. Temperature was 99.5 °F and O2 saturation was 96%. She denied international travel to areas of high incidence of COVID-19 or contact with infected individuals. Tamiflu was prescribed. Despite concern for COVID-19, she could not get SARS-CoV-2 PCR testing because of limited availability. A chest x-ray was normal. She was instructed to self-quarantine and monitor for worsening respiratory status. On day 28, she developed sinus congestion with postnasal drip. She was afebrile. Her PCP diagnosed acute viral sinusitis and told her to continue Tamiflu for a total of 5 days. A week later, she had worsening fever of 103.5 °F, rigors, headache, myalgias, and mild dyspnea. She presented to the emergency department, where she was tachycardic with a temperature of 100.4 °F, and O2 saturation was 94% on room air. Tests for influenza, adenovirus, enterovirus, parainfluenza virus types 1-4, rhinovirus, human metapneumovirus, and RSV were negative. SARS-CoV-2 RNA PCR was positive, and she was admitted. During her hospital stay, she developed mild headaches and chest tightness but no dyspnea. Chest x-ray showed only mild prominence of the bronchovascular markings (Fig. 1C). CD19/CD20 quantification by flow cytometry indicated depletion of B cell (Fig. 1D). Absolute neutrophil count (ANC) was elevated and was associated with a decreased of the Absolute lymphocyte count (ALC) at 500 per μL at the time of her most severe symptoms and fever (Fig. 1E). She did not require mechanical ventilation and responded to supportive therapy and was discharged home after 3 days.
Fig. 1.
Brain MRI shows lack of typical periventricular T2 lesions but shows sparse scattered T2 hyperintensities in the deep white matter and corpus callosum, atypical for MS (arrows). B) Cervical spine MRI demonstrates of longitudinal extensive T2 hyperintensities from C2-C6 (arrows). C) Anteroposterior chest radiographs during impatient hospitalization that showed alteration in lung fields with increasing markings but not consolidation. D) FACS data demonstrating absence of CD20 population in circulating peripheral blood mononuclear cells due to rituximab E) Quantification of ANC, ALC and ANC/ALC ratio during the symptomatic phase of the infection. Arrows indicate points of maximal decline on ALC that correspondent to increase in ANC and vice versa.
Discussion
Our patient had a prolonged flu-like illness that was either a prolonged course of COVID-19 or more likely a different viral respiratory infection followed by SARS-CoV-2. We cannot distinguish between these two possibilities since she was not tested for SARS-CoV-2 early in her presentation. During the COVID19-positive phase of her illness, she had only mild respiratory symptoms with moderate constitutional symptoms. She did not develop overt respiratory insufficiency and changes in the chest X-ray were mild, with minimal lab abnormalities. She had severe lymphopenia that recovered (Fig 1E), indeed the reappearance of CD8+ T cells precede the resolution of symptoms and is consistent with an effective immune response to SARS-CoV-2 in non-severe cases. (Irani Thevarajan et al., 2020) Lymphopenia is seen frequently, and recent work suggests that sustained lymphopenia, especially CD8+ T cells is an independent predictor for COVID-19 severity (Wang et al., 2020) Her neutrophil population (ANC) increased at the time of increased disease severity, and then declined as her lymphocyte population partially recovered (Fig 1C). The ratio of ANC/ALC has been suggested as a readily available marker of disease severity in COVID infection (Lagunas-Rangel, 2020).
There is a suggestion that in some patients the severe disease is due to immune dysregulation, in particular, cytokine storm, therefore targeting cytokines such as IL6 can be beneficial (Mehta et al., 2020). This suggestion is supported by reports of cases of mild disease in a patient with long term corticosteroid use (Han et al., 2020, Cameron Smail et al., 2019) but there is a need for prospective randomized trials to determine if immunosuppression or immunomodulation may be protective from worse outcome of COVID-19.
The impact of Immunosuppressive medications on the course of COVID-19, especially DMTs like rituximab that deplete circulating B cells, is of particular concern. There are reports of severe and even fatal viral infections in patients on rituximab. These include encephalitis due to Coxsackie A169, Enterovirus (Sham et al., 2019) (Kassab et al., 2013), Powassan (Solomon et al., 2018), Tick-Borne Encephalitis (Steininger et al., 2017), West Nile (Morjaria et al., 2015), JCV neurological disease including PML (Ishikawa et al., 2018) (Berger et al., 2018) and granule cells neuronopathy (Dang et al., 2014). Our patient did not present any new neurological signs or symptoms and there was no indication for a lumbar puncture. Currently, it is unclear if SARS-CoV-2 is neurotropic. The SARS-CoV-2 receptors ACE and TMPRSS2 are not highly expressed in the brain, although preliminary evidence from single cell RNA sequencing data suggest that the olfactory epithelium expresses these receptors. This may explain the cases of anosmia seen in COVID-19. There is preliminary evidence that oligodendrocytes may express COVID receptors, and recently there was a case of encephalitis with SARS-CoV-2 found in the CSF (Moriguchi et al., 2020). Moreover, neurotropism of coronaviruses may be independent of the expression of viral receptor in brain cells (Weiss, 2020). Other neurotrophic coronaviruses use different receptors to cause disease in immunosuppressed patients. Our patient had no encephalitic symptoms and was negative for metapneumovirus, which can cause severe encephalitis. (Bohmwald et al., 2018)
DMTs affect multiple immune pathways that may alter the ability of an individual to mount an effective immune response to COVID-19. However, no prospective or observational studies have evaluated the impact of COVID-19 on MS or NMOSD patients treated with DMTs. The International community has rapidly launched several patient registries that are at an early stage of data acquisition to ascertain the overall impact of the disease in MS and NMO patients: CoViMS in the US, https://www.covims.org/. in UK https://ukmsregister.org/Research/COVID19CRF. However, specific questions regarding the susceptibility and immunological implications of SARS-CoV-2 on MS patients on DMTs will require prospective studies, to evaluate immune parameters and correlated them with the type of DMTs, as well as other clinical and laboratory outcomes measures, including the ability of DMT-treated patients to mount an effective humoral and cellular immune response to an infection of SARS-CoV-2 or to a future COVID-19 vaccine (Yri et al., 2011) .
In summary, our patient developed only a mild course with COVID-19 despite B cell depletion and may be instructive regarding the behavior of SARS-CoV-2 infections in patients with immunomodulation. Long term prospective studies are needed to examine the role of B cells on the immune responses against emerging or endemic human coronaviruses (Weiss, 2020), including SARS-CoV-2.
Acknowledgment
Dr. Imitola has been consultant for Biogen and Novartis and received grants from Biogen.
References
- Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;13 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani Thevarajan T.H.O.N., Marios Koutsakos, Leon Caly Julian J.DruceD., van de Sandt Carolien C.E., Jia Xiaoxiao X., Nicholson Suellen S., Catton Mike M., Cowie Benjamin B., Tong Steven S.Y.C., Sharon, Lewin R., Kedzierska Katherine K. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;16(Mar):1–3. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. COVID-19 in a patient with long-term use of glucocorticoids: a study of a familial cluster. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron Smail R., O’Neill J.H., Andresen D. Brainstem encephalitis caused by Coxsackie A16 virus in a rituximab-immunosuppressed patient. BMJ Case Rep. 2019;12(8) doi: 10.1136/bcr-2019-230177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham L. Treatment of rituximab-associated chronic CNS enterovirus using IVIg and fluoxetine. Neurology. 2019;92:916–918. doi: 10.1212/WNL.0000000000007468. [DOI] [PubMed] [Google Scholar]
- Kassab S. Fatal case of enterovirus 71 infection and rituximab therapy, france, 2012. Emerg. Infect. Dis. 2013;19:1345–1347. doi: 10.3201/eid1908.130202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H. Fatal powassan encephalitis (Deer Tick Virus, Lineage II) in a patient with fever and orchitis receiving rituximab. JAMA Neurol. 2018;75:746–750. doi: 10.1001/jamaneurol.2018.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger P.A. Two cases of severe tick-borne encephalitis in rituximab-treated patients in Germany: implications for diagnosis and prevention. Open Forum Infect. Dis. 2017;4:ofx204. doi: 10.1093/ofid/ofx204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morjaria S. West nile virus central nervous system infection in patients treated with rituximab: implications for diagnosis and prognosis, with a review of literature. Open Forum Infect. Dis. 2015;2:ofv136. doi: 10.1093/ofid/ofv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y., Kasuya T., Ishikawa J., Fujiwara M., Kita Y. A case of developing progressive multifocal leukoencephalopathy while using rituximab and mycophenolate mofetil in refractory systemic lupus erythematosus. Ther. Clin. Risk Manag. 2018;14:1149–1153. doi: 10.2147/TCRM.S167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R., Malik V., Lacey S., Brunetta P., Lehane P.B. Progressive multifocal leukoencephalopathy in rituximab-treated rheumatic diseases: a rare event. J. Neurovirol. 2018;24:323–331. doi: 10.1007/s13365-018-0615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L., Dang X., Koralnik I.J., Todd P.K. JC polyomavirus granule cell neuronopathy in a patient treated with rituximab. JAMA Neurol. 2014;71:487–489. doi: 10.1001/jamaneurol.2013.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R. Forty years with coronaviruses. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmwald K., Galvez N.M.S., Rios M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yri O.E. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769–6771. doi: 10.1182/blood-2011-08-372649. [DOI] [PubMed] [Google Scholar]