Abstract
The ongoing outbreak of COVID-19 has been expanding worldwide. As of 17 April 2020, the death toll stands at a sobering 147,027 and over two million cases, this has been straining the health care systems all over. Respiratory failure has been cited as the major cause of death but here we present a case about a patient who instead succumbed to severe metabolic acidosis with multiple organ failure.
Keywords: COVID-19, Metabolic acidosis, Cytokine storm syndrome
Introduction
The world is in the middle of a pandemic with a new coronavirus, SARS-CoV-2, which has a high mortality in the elderly and people with co-morbidities such as hypertension, chronic heart disease and diabetes. The clinical course is biphasic [1], first a viral infection in the upper or/and lower respiratory tract sometimes followed by a dysregulated inflammatory response (“cytokine storm”) with severe inflammation in the lungs which often require several weeks of mechanical ventilation. It is unclear what triggers the dysregulated inflammatory response which is a clinical feature much more commonly seen than in severe influenza pneumonia, and one suggestion is that it is the viral interaction with its human receptor, the acetylcholinesterase2, ACE2, molecule that could be an explanation [2]. A characteristic of the SARS-CoV-2 infection is lymphopenia and especially a low CD4 + T cell count [3] and the potential to infect CD4 + T cells [4]. It is generally assumed that the mortality is due to lung failure, but here we describe a patient who developed a strong inflammatory response with high C-reactive protein, very high IL-6 and white blood cells (WBC) and went into an irreversible metabolic acidosis.
Case report
A 72-year-old man with diabetes mellitus and G-6PD deficiency presented with 10-day history of fever, dry cough, shortness of breath and nasal congestion. At admission his oxygen saturation was 95 % on 3 L of oxygen. Hemoglobin concentration was 13.6 g/dL(normal range: 11.5–15.5), white blood cell count (WBC) of 4200/uL (normal range: 2200–10000), lymphocyte count of 700 /uL (normal range: 1200–4000), C-reactive protein of 152 mg/L (normal range: <10), alanine aminotransferase (ALT) of 61 IU/L (normal range 10–49), total bilirubin 1.87 mg/dL (normal range:0.1–1.2), renal function test was normal and chest radiograph revealed right lung ground glass opacities. Nasopharyngeal swab for SARS-CoV-2 RNA was positive. He was started on ceftriaxone 2 gm once daily and azithromycin 500 mg once daily according to national guidelines.
On day 12 of his illness (2nd day after admission), his hypoxia worsened. Arterial blood gas showed a pH of 7.48, PaCO2 31 mmHg, PaO2 60 mmHg, bicarbonate 23 mmol/L, lactate 1.5 mmol/L and a C-reactive protein of 237 mg/L (Fig. 1A). He was intubated and started on mechanical ventilation. Hydroxychloroquine, beta interferon and lopinavir/ritonavir were added according to national guidelines. Post intubation, the patient was kept on volume-controlled ventilation and lung protective mode with a tidal volume of 6 mL/kg of predicative body weight (PBW) and positive end expiratory pressure (PEEP) between 16–18 cmH2O. The sedation was maintained with intravenous fentanyl and midazolam with additional intermittent doses of cisatracurium. CO2 accumulation was significant with a pCO2 of 70−80 mmHg. This was attributed to acute lung injury due to SARS-CoV2 and the use of protective lung ventilation strategy. Therefore PEEP was lowered and tidal volume raised. Fraction of inspired oxygen (FiO2) was also successfully weaned to 0.5 with targeted partial pressure of oxygen (PO2) above 60 mmHg.
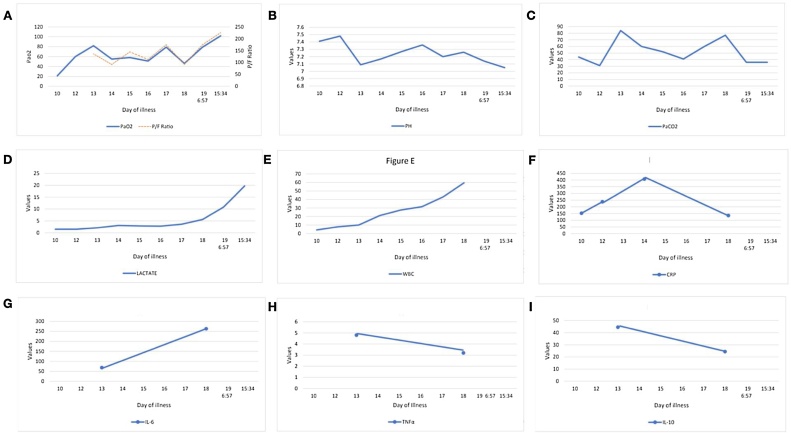
Fig. 1.
A) shows the trend of PaO2,PaO2/FiO2 ratio through the days of illness, B) shows the trend of pH, C) shows values of PCO2, D) shows the minimal followed by the sharp rise of lactate towards 7the end, E) shows the gradual rise in WBC as the disease progressed along, F) shows the rise and fall of CRP, G) shows the rise of interleukin-6, H) shows the trend of tumor necrosis factor alpha (TNFα) and I) shows the level interleukin-10(IL-10) measured.
By day 14 he had a worsening renal function with a GFR of 40 mL/min (normal range >90). The WBC had increased to 21100/uL, with 19700/uL neutrophils and persistent lymphopenia of 900 /uL. C-reactive protein doubled to 406 mg/L. He developed high grade fever and antimicrobials were changed to meropenem, levofloxacin and teicoplanin. He was initiated on continuous veno-venous hemodialysis (CVVHD). The possibility of a nosocomial infection versus cytokine storm was raised. By day 17 he showed significant improvement of his chest x-ray and ventilatory parameters reaching FiO2 of 0.45 and PEEP of 12 with PO2 more than 80 mmHg. His ABG showed pH 7.43, PaCO2 36 mmHg, PaO2 101 mmHg, bicarbonate of 24 mmol/L, lactate of 1.10 mmol/L. However, there was a further increase in WBC to 31500/uL with 26000/uL neutrophils with progressive drop in hemoglobin reaching 10.1 g/dL.
Nasopharyngeal SARS-CoV-2 RNA on day 16 was still positive. Inspite of the initial improvement of ventilatory parameters, his pH was 7.28, PaO2 85 mmHg, PCO2 46 mmHg, HCO3− 22 mmol/L, Na 134 mmol/L,Cl 103 mmol/L, suggestive of metabolic acidosis with increased anion gap of 53mEq/L (normal range 8−16mEq/L), with further rise in WBC to 42900/uL and neutrophils to 34.8 × 109/L. The lactate dehydrogenase (LDH) was 2215 IU/L and haptoglobin less than 10 mg/L and a normal absolute reticulocyte count ruling out drug related hemolysis and suggesting on going cytokine storm.
Over the next few days, the metabolic acidosis progressed despite CVVHD with pH of 6.9, PaO2 68 mmHg, PCO2 33 mmHg, Na 134 mmol/L, CL 102 mmol/L, lactate reaching 19.6 mmol/L, and the WBC increased to 59500/uL with neutrophils of 35000/u L, ALT increased to more than 3300 IU/L, and interleukin-6 level was 262.7 pg/mL (normal range < 16.4) eventually leading to cardiopulmonary arrest and death.
Discussion
IL-6 is a strong predictor of respiratory failure [5]. During the energy producing metabolism that takes place in the cytoplasm of cells, glucose is transformed into pyruvate molecules generating 2 ATP molecules. During this glycolysis process, no oxygen is utilized. If the tissue oxygenation is regular, pyruvate molecules enters the mitochondria where they undergoes a series of enzymatic processes generating ATP, as well as CO2 and H2O molecules. In a situation of tissue hypoxia pyruvate molecules cannot enter the mitochondria but are converted to lactic acid and in this process ATP is released maintaining the energy production. The lactic acid is removed mainly through the process of gluconeogenesis that takes place in the liver and kidney cortex, but also through the oxidation process in various organs. The lactic acid accumulation is the result of increased production and/or its decreased removal [6]. On 18th day of the patient’s illness, the lactic acid levels started to increase significantly but levels of pCO2 were acceptable, it was concluded that he was in metabolic acidosis. It is possible that dysregulated inflammatory response (cytokine storm) caused the metabolic acidosis.This has been described in severe bacterial sepsis [7].
Another reason could be extensive microvascular thrombosis causing the tissue hypoxia which has been described in COVID-19 patients [8]. Significant contribution to high lactic acid levels probably also comes from the high WBC count [9]. However, there is also the possibility of mitochondrial toxicity caused by lopinavir and ritonavir treatment although this is less likely to occur in short treatment duration [10].
Consent for publication
Consent to publish this case report was obtained from the patient’s family and the copy of the consent is available for the journal on request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author’s contribution
Shabnam Chhetri: Writing (original draft preparation).
Faryal Khamis: Conceptualization, Supervision, Writing (Review and editing).
Eskild Peterson: Supervision, Writing (Review and editing).
Nenad Pandak: Writing (Review and editing).
Huda Al Khalili: Writing (Review and editing).
Elias Said: Investigation (performing laboratory test).
All authors read and approved the final manuscript.
Declaration of Competing Interest
All authors have no competing interests to declare.
Contributor Information
Shabnam Chhetri, Email: drshabnamchhetri24@gmail.com.
Faryal Khamis, Email: khami001@gmail.com.
Nenad Pandak, Email: npandak@gmail.com.
Huda Al Khalili, Email: hudalkhalili@gmail.com.
Elias Said, Email: esaid@squ.edu.om.
Eskild Petersen, Email: eskild.peterson@gmail.com.
References
- 1.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transpl. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer, Solomon S.D. Renin-angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X., Xu W., Hu G., Xia S., Sun Z., Liu Z. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 2020:1–3. doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herold T., Jurinovic V., Arnreich C., Hellmuth J., von Bergwelt-Baildon M., Klein M. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. MedRxiv preprint. 2020 doi: 10.1101/2020.04.01.20047381. [DOI] [Google Scholar]
- 6.Kamel K.S., Oh M.S., Halperin M.L. L-lactic acidosis: pathophysiology, classification, and causes; emphasis on biochemical and metabolic basis. Kidney Int. 2020;97:75–88. doi: 10.1016/j.kint.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Kogelmann K., Jarczak D., Scheller M., Drüner M. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017;21:74. doi: 10.1186/s13054-017-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei H., Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002. doi: 10.3760/cma.j.issn.0253-2727.2020.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Backer D. Lactic acidosis. Intensive Care Med. 2003;29:699–702. doi: 10.1007/s00134-003-1746-7. [DOI] [PubMed] [Google Scholar]
- 10.Monnin A., Nagot N., Peries M. Lopinavir/ritonavir induces mitochondrial toxicity in HIV-exposed uninfected children. Poster presented at: Conference on Retroviruses and Opportunistic Infections (CROI); March 4-7. Boston, MA, USA; 2018. [Google Scholar]