Abstract
Due to the close relationship between the incidence of infectious diseases by epidemics and environmental conditions, this research explores the temperature, evaporation, precipitation and regional climate effects on the local transmission of coronavirus SARS-CoV-2 inside 31 states and capital of Mexico since February 29 (national onset) to March 31, 2020. Statistical analysis was conducted to explore the association between the daily local COVID-19 confirmed positive cases (LCPC) and both climate characteristics and the daily weather reported by the regional meteorological stations. In this work, the local transmission ratio (LTR) was calculated with the regional LCPC divided by the number of the effective contagion days since regional onset in each state. The results showed a negative association between temperature (mean, max and min) and climate classification with both LCPC and LTR variables. The precipitation associated positively with LCPC and LTR. The associations between the climate classification with LCPC and LTR are statistically significant. The tropical climate (mean temperature around 25.95 °C and mean precipitation around 8.74 mm) delayed the regional onset. However, the regional onset in dry climates emerged earlier as consequence of the lower temperatures and higher precipitations (20.57 °C and 20.87 mm respectively) than the observed in the tropical climate. The fastest regional onsets were observed in tempered climates in states where the lowest temperatures and lowest precipitations were registered (19.65 °C and 8.48 mm respectively). Meteorological factors influenced the trend on the regional outbreaks in Mexican's states likely by the host predisposition and susceptibility during the cold winter season. In Mexico, the climate characteristics played a crucial role on the local infection during the phase 1 being the tempered regions (as Michoacán, Jalisco, Puebla, etc.) more vulnerable than the dry (as Chihuahua, Durango or Zacatecas, etc.) or tropical areas (as Colima, Campeche, Morelos etc.).
Keywords: SARS-CoV-2, Mexico, Local transmission rate, Climate effect
Graphical abstract
Highlights
-
•
Tropical climate delays the SARS-CoV-2 local transmission onset.
-
•
SARS-CoV-2 local transmission onset was rapidly present in tempered climates.
-
•
Temperature associates negatively with the local confirmed COVID-19 positive cases.
-
•
Regional climate associates significantly with the COVID-19 local transmission rate.
1. Introduction
Coronaviruses were not previously considered highly pathogenic to humans until the outbreak of the severe acute respiratory syndrome (SARS) observed in 2002–2003 in Guangdong province of China and up to the outbreak emerged ten years later in the Middle Eastern countries known as the Middle East respiratory syndrome disease (MERS) (Cui et al., 2019). In December 31, 2019, the official Chinese authorities announced that a novel coronavirus capable of infecting humans had emerged from the Huanan Seafood Market in Wuhan, China. The new coronavirus was termed SARS-CoV-2 and COVID-19 as its respiratory infectious disease. Since that, reports of positive confirmed COVID-19 cases have been informed in >205 countries worldwide (https://coronavirus.jhu.edu/map.html, May 14-2020). In Mexico, the official announcement on the first infected patient was on Feb 29, 2020. To date, there were informed 42,595 cumulated confirmed cases, 26,746 suspected cases, 10,057 confirmed active cases, and 4477 deceases alongside the 31 provinces belonging to Mexico and its capital (https://www.gob.mx/salud, May 14-2020). For previous historical outbreaks of SARS, MERS or influenza viruses some epidemiological studies have suggested that environmental conditions altered the spread pattern. For instance, in the case of influenza infections, Tamerius and cols., assured that in temperate regions the virus exhibited a strong occurrence according to the seasonal cycle: May to September (annual summer) in the southern hemisphere and November to March (annual winter with the minimal levels of specific humidity and temperature) in the northern hemisphere (Tamerius et al., 2013). They concluded that the virus is highly sensitive to high temperature. Lowen and cols., have showed in experiments with guinea pigs as host model at laboratory scale, that both cold (low-temperature) and dry (low-humidity) conditions favor transmission of influenza increasing its infectivity (Lowen et al., 2007). In contrast, if temperature and relative humidity increased, the influenza transmission was entirely blocked. Regarding to SARS-CoV in studies developed at laboratory scale, Chan and cols., founded that such virus was active for at least 5 days at 22–25 °C within a relative humidity between 40 and 50%. In opposite, once the temperature reached 38 °C with a relative humidity of 95%, the virus lost its activity. At 56 °C, virus survival lasted only 15 min (Chan et al., 2011). Casanova and cols., employed the TGEV (transmissible gastroenteritis virus) and MHV (mouse hepatitis virus) as potential surrogates of SARS-CoV to determine the air temperature and relative humidity effects on the viruses survival once deposited in metallic surfaces (Casanova et al., 2010). At 4 °C, viruses persisted as long as 28 days. The lowest level of inactivation occurred at 20% of relative humidity. Inactivation was faster at 20 °C than at 4 °C and the fastest inactivation was observed at 40 °C. Tan and cols., showed a close statistical association between environmental temperature and the SARS outbreak observed in 2003 in 4 cities in China (Tan et al., 2005). The optimum environmental temperature associated with the SARS outbreak was observed between 16 °C and 28 °C. Yuan and cols., confirmed a significant SARS transmission in correlation to 3 principal environmental factors: air temperature, humidity and wind speed (Yuan et al., 2006). The above-mentioned studies predicted the spring as the most suitable environmental seasonal condition for reoccurrence of SARS. In contrast, Cai and cols., suggested that the SARS outbreak was significantly associated with wind speed but only to some extent with the pressure, relative humidity and UV solar irradiation (Cai et al., 2007). Additionally, the same authors did not find association between SARS transmission to neither air temperature nor air pollution. Regarding MERS virus, Doremalen and cols., observed that the virus activity persisted for long time in low-temperature and low-humidity stable and controlled conditions at laboratory scale (Doremalen et al., 2013). Concerning the new COVID-19 disease, recent statistical researches have demonstrated a solid relationship between the total number of positive cases and the cold-dry environment (between −4 °C and 15,58 °C and low humidity) registered in several countries during January and February 2020 (Sajadi Mohammad et al., 2020; Wang et al., 2020; Keshavarzi, 2020; Nazari et al., 2020). According to the above-mentioned authors, spread of the new virus was associated with the latitude and longitude as geographical indicators and seasonal dynamics (Wang et al., 2020; Keshavarzi, 2020). Additionally, Araujo and cols., assured that tropical countries have been marginally affected by SARS-CoV-2 virus and that a high COVID-19 transmission has been observed in countries with cold-dry weather in temperate climates (Araujo and Naimi, 2020). Similarly, Bukary and cols., estimated that the low number of positive COVID-19 confirmed cases observed in tropical countries might be explained due to their usual warm-humid environmental conditions. They also assessed that countries experiencing high absolute humidity above 10 g/m3 could see in a short time a slowdown in the COVID-19 transmissions (Bukhari and Jameel, 2020). Ma and cols., assured that temperature and humidity were affecting factors related to the number of COVID-19 mortality in Wuhan, China (Ma, 2020). Table 1 recapitulates the above-mentioned researches regarding to the effect of ambient factors on the survival, the transmission and/or the infectivity of influenza, SARS-CoV, MERS and the novel SARS-CoV-2 viruses. As possible to see in Table 1, most of the reports rarely have considered neither the well-differentiated stages of the epidemic evolution (phases 1, 2 or 3) nor the origin type of affected patients (imported or local). Therefore, the present study shows the effects of the air temperature, precipitation and regional climate on the daily evolution of the local confirmed COVID-19 positive cases (LCPC) documented in 31 provinces and capital of Mexico since February 29, 2020 to March 31, 2020, i.e. during the phase 1 of the epidemic. This study contributes to elucidate on eventual SARS-CoV-2 reoccurrence considering weather conditions, environmental local characteristic and seasonal cycles in each regional climate existing in Mexico.
Table 1.
Environmental effect on the survival, transmission and infection of H1N1, SARS-CoV, MERS and SARS-CoV-2 viruses.
| Reference | Virus, disease | Study type | Preferential conditions |
Disadvantageous conditions | Remarks |
|---|---|---|---|---|---|
| Lowen et al., 2007 | H1N1,Influenza | Experimental. Arrangement of infected and exposed pigs in environmental chamber | favor transmission: cold and dry environment (low RH of 20%–35%) conditions | Transmission completely blocked: High RH of 80%. No transmission at 30 °C. |
Range tested 5, 20 and 30 °C RH 20% to 80%. Low RH produced by indoor heating and cold temperatures as features of winter that favor virus spread in humans. |
| Tamerius et al., 2013 | H1N1, Influenza | Modeling epidemiological and climatic information. 78 sites sampled Globally. |
Template regions show seasonal cycle with low humidity conditions in the winter (increase virus survival and enable the transmission). In some tropical locations occurs during the rainy season or there is not a well-defined influenza season |
NA | Low SH conditions facilitate the airborne survival and transmission in temperate regions during the cold-dry season (i.e., winter) when SH and T are at minimal levels. For sites where specific humidity and temperature do not decrease 11–12 g/kg and 18–21 °C, seasonal influenza activity peaks when precipitation is >150 mm per month. |
| Chan et al., 2011 | SARS, CoV | Experimental. Individual plastic plate representing nonporous surfaces. | Low temperature and low humidity support prolonged survival of virus on contaminated surfaces. | High temperature at high RH has a synergistic effect on inactivation 38 °C and RH >95%, virus viability was rapidly lost 56 °C lasted only 15 min. |
Ranges tested (38 °C, 33 °C, 28 °C), (>95%, 80–89%), (3, 7, 11, 13, 24 h) and room temperature (22–25 °C), RH 40–50%, 4 weeks (i.e., conditions prevailing in a typical air-conditioned room or environments in subtropical areas during spring). Asian countries in tropical area with high temperature and high RH environment did not have major community outbreaks of SARS. |
| Casanova et al., 2010 | TGEV and MHV as surrogate SARS virus, CoV | Experimental. Risks posed on stainless steel surfaces related to infectivity of coronaviruses. | At 4 °C, infectious virus persisted for as long as 28 days. 20% RH slowest inactivation. Greater survival at low RH (20%) and high RH (80%) than at moderate RH (50%). |
Inactivation was more rapid at 20 °C than at 4 °C at all humidity levels. Inactivation more rapidly at 40 °C than at 20 °C. |
When high numbers of viruses are deposited, TGEV and MHV may survive for days on surfaces at ATs and RHs typical of indoor environments |
| Tan et al., 2005 | SARS, CoV | Meteorological data and statistical analysis. 4 cities in China Daily incidence. |
Optimal temperature for prevalence was from 16 °C to 28 °C. Comparatively dry season might be a favorable condition for prevalence. |
NA | *Significant correlation between the SARS cases and the environmental temperature seven days before the onset and the seven-day time lag (incubation period). |
| Yuan et al., 2006 | SARS, CoV | Meteorological data and statistical analysis. I city (Beijing) Daily incidence. |
Peak spread at mean temperature 16.9 °C, mean RH 52.2% and wind speed 2.8 m/s | NA | Temperature, relative humidity, and wind velocity were the three key meteorological determinants affecting the transmission. |
| Cai et al., 2007 | SARS, CoV | Meteorological data and statistical analysis. China. Secondary attack rate |
Significantly associated to wind speed. Outbreak is some extent associated with daily RH, air pressure (AP) and hours of sunshine (HS) | Outbreak is not influenced by daily temperature and air pollution. Heaters and air conditioning keep the room temperature within 18–22 °C and contributed to the long-lasting outbreak |
|
| Lin et al., 2006 | SARS, CoV | Meteorological data and statistical analysis. 1 city (HK) Daily incidence. |
Lower air temperature during the epidemic increase daily the risk of a larger epidemic in the community: 12.82 fold (or 18·18-fold) higher than that in days with higher air temperature >24.6 °C | Air temperature >24.6 °C | |
| Doremalen et al., 2013 | MERS, CoV | Experimental | Stability for a long time (as droplets on solid surface and as aerosol) as long as in low-temperature, low-humidity environment: 20 °C – 40% RH; 30 °C – 30% RH and 30 °C – 80% RH. | Either warm or humid conditions are favorable | Potential to be transmitted via contact or fomite transmission due to prolonged environmental presence |
| Sajadi Mohammad et al., 2020 | SARS-CoV-2 COVID-19 | Meteorological data and statics analysis. 20 cities. Community spread. |
Significant community spread along east west distribution 30–50 N″ at consistently similar weather patterns (5–11 °C and low specific and absolute humidity). | NA | |
| Wang et al., 2020 | SARS-CoV-2 COVID-19 | Meteorological data and statistical analysis. 429 cities Cumulative number of confirmed cases |
lower temperature contributes to the growth and transmission of the virus | NA | “Day3” new variable was defined for the analysis as impact of the number of imported confirmed cases |
| Doremalen et al., 2020 | SARS-CoV-2 COVID-19 | Experimental. Stability in aerosol and surfaces (plastic, stainless steel, copper, and cardboard) | Stable on plastic and stainless steel (65% RH and 21–23 °C) | Poor stable on copper and cardboard (65% RH and 21–23 °C) | Viable virus was detected up to 72 h after application in all surfaces |
| Keshavarzi, 2020 | SARS-CoV-2 COVID-19 | Meteorological data and 4 statistical analysis. 1 city (Wuhan) 482 cases |
Evidence of geographical signals and seasonal dynamics with respect to the effects of latitude and longitude. | NA | |
| Bukhari and Jameel, 2020 | SARS-CoV-2 COVID-19 | Meteorological data and statistical analysis. 12 countries, 9 US north states, 8 impacted regions Total number of cases and new cases. |
Effect on 83% non-tropical countries (30 N and above) and 90% within a temperature range of 3 to 17 °C. ~72% with humidity between 3 and 9 g/m3 and 90% within the same range of absolute humidity | Lower number of cases in tropical countries due to warm-humid conditions. | High absolute humidity (>10 g/m3) could see a slowdown in transmissions |
| Ma, 2020 | SARS-CoV-2 COVID-19 | Meteorological data and statistical analysis. 1 city (Wuhuan) Mortality ratio |
Positive association is found between daily death counts and temperature | Absolute humidity is negatively associated with daily death counts | Stable and comfortable environment for the patients during therapy. |
| Araujo and Naimi, 2020 | SARS-CoV-2 COVID-19 | Meteorological data and statistical analysis. Climate regions. All reported cases |
Most outbreaks in relatively cool and dry areas. Temperate warm and cold climates are more favorable to spread of the virus. Coronavirus display preference for cool and dry conditions (−4,01 °C to 15,58 °C and 4,68 mm to 116,06 mm) |
Arid and tropical climates are less favorable to spread of the virus. Unsuitable climates cause the virus to destabilize quickly |
Uncertainties in sub- Saharan Africa, Latin Amrica and South East Asia. Climate suitability for outbreak: temperate warm> arid > tropics (high temperatures and precipitation) > polar |
| Méndez-Arriaga, 2020 (this study) | SARS-CoV-2 COVID-19 | Meteorological data and climate classification. Statistical analysis. 31 Mexican states and Mexico city. Phase 1. Local confirmed positive cases. Local Transmission Ratio. |
Regional onset in dry climate came early as the effect of the lower temperatures and higher precipitations (20.57 °C and 20.87 mm respectively). The fastest regional onsets were in tempered climates with the lowest temperatures and precipitations registered (19.65 °C and 8.48 mm respectively). | The tropical climate (mean temperature around 25.95 °C and mean precipitation around 8.74 mm) delayed the regional onset. | Enclosed specific phase of the epidemic and specificity on the contagion types (local o imported) are important factors for weather and climate association studies. |
2. Methodology
Onset data and the cumulative number of positive confirmed COVID-19 cases, alongside with age, sex, preceding type (local or imported), confirmation date, number of day with symptoms and deceases per state were daily collected from the Mexican Health Commission's website (https://www.gob.mx/salud). 1215 COVID-19 positive total confirmed cases (TCPC) were included in this study and they were classified as local COVID-19 confirmed positive cases (LCPC) or imported COVID-19 confirmed positive cases (ICPC) i.e. patients with recent previous stay or travel abroad. Climate regional categorizations according to the Köppen-Geiger climate classification for each state and capital of Mexico were obtained by INEGI database (http://en.www.inegi.org.mx/temas/climatologia/default.html#Mapa). Full-meteorological statistical data (daily highest, lowest and mean temperature, monthly mean atmospheric evaporation and precipitation) were obtained from the historical climatology database of CONAGUA (https://smn.conagua.gob.mx/). The daily average air temperature at the height of 2 m (T2m) and specific humidity at 1000 hPa (SH1000hPa) was provided by the Copernicus Climate Change Service database (https://climate.copernicus.eu/). Development of a dynamic database on Excel spreadsheet was designed in order to compile and handle the last information. The statistical association and qualitative correlation of both the regional climate and daily weather conditions with LCPC for each Mexican state were calculated by the Spearman's non-parametric test (rs). rs determines the strength or weakness of monotonic association between variables through of positive or negative correlation (between −1 and +1) with a concomitant statistical significance (p). All the statistical analyses were two-sided at a 5% level of significance using the R software, version 3.5.3.
3. Results
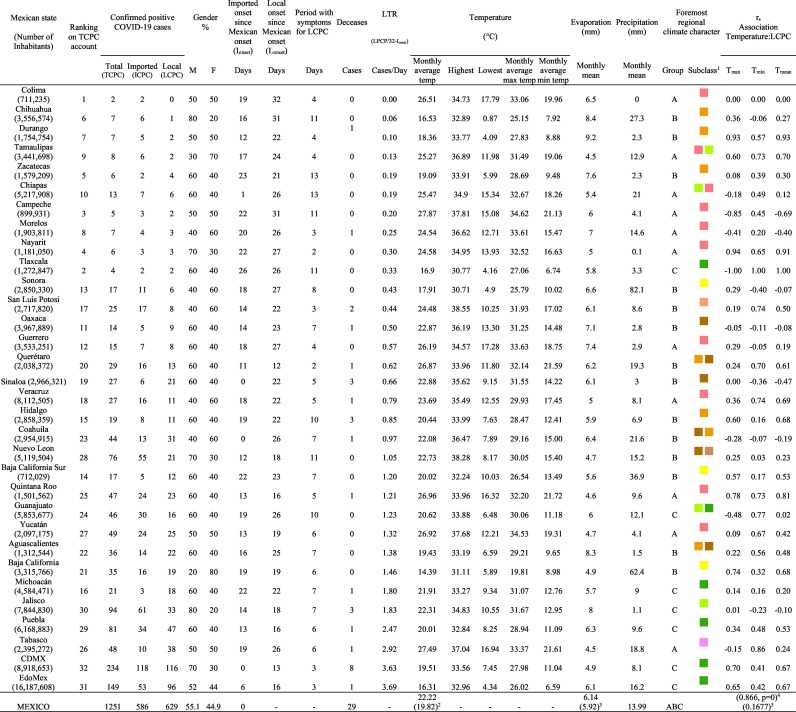
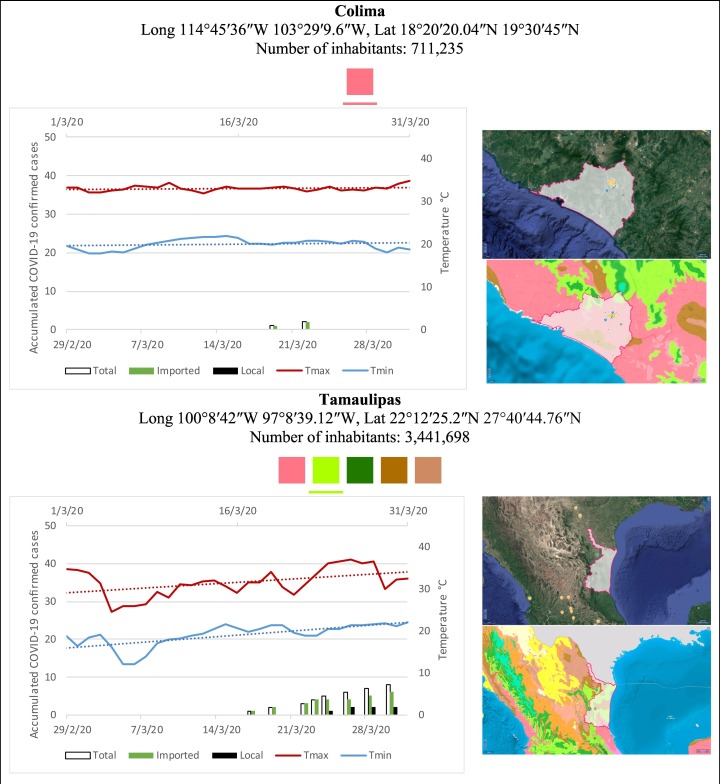
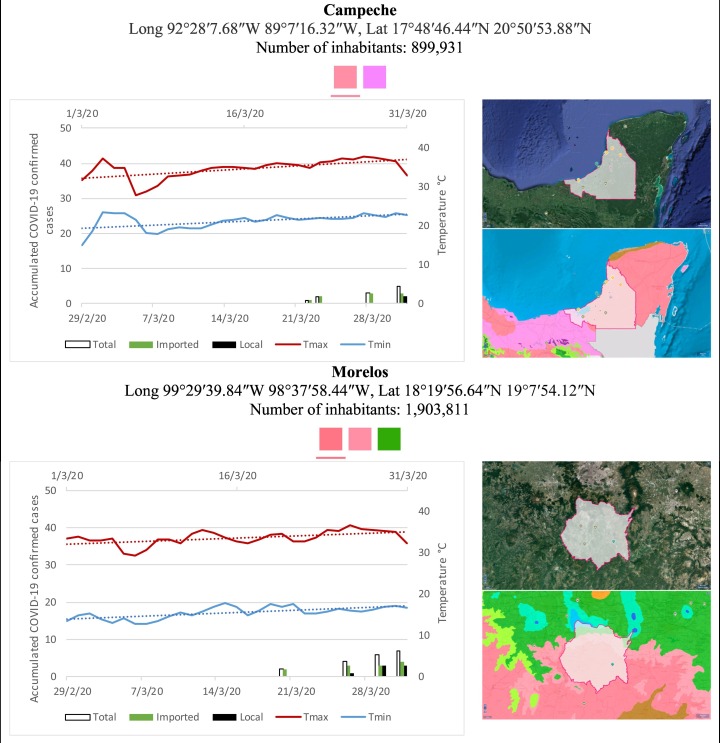
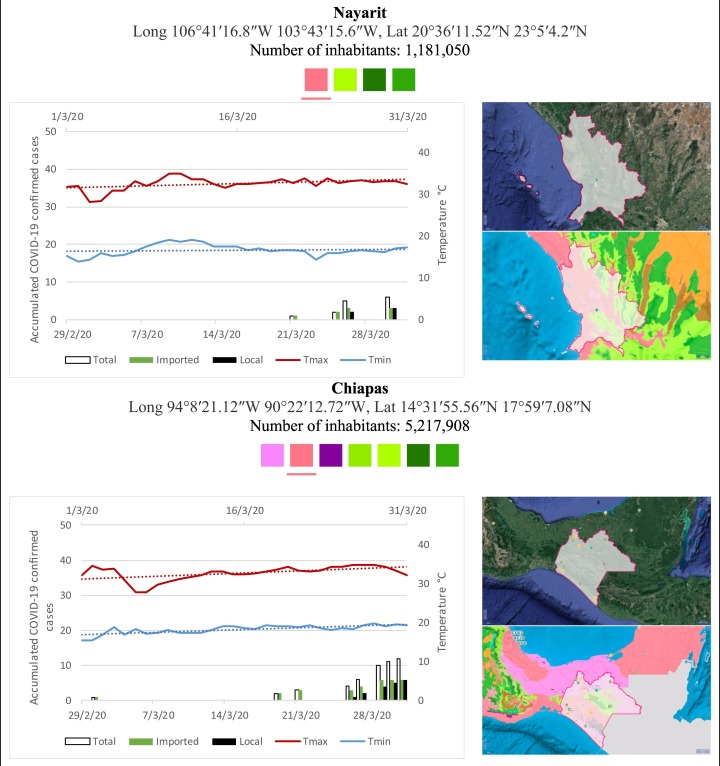
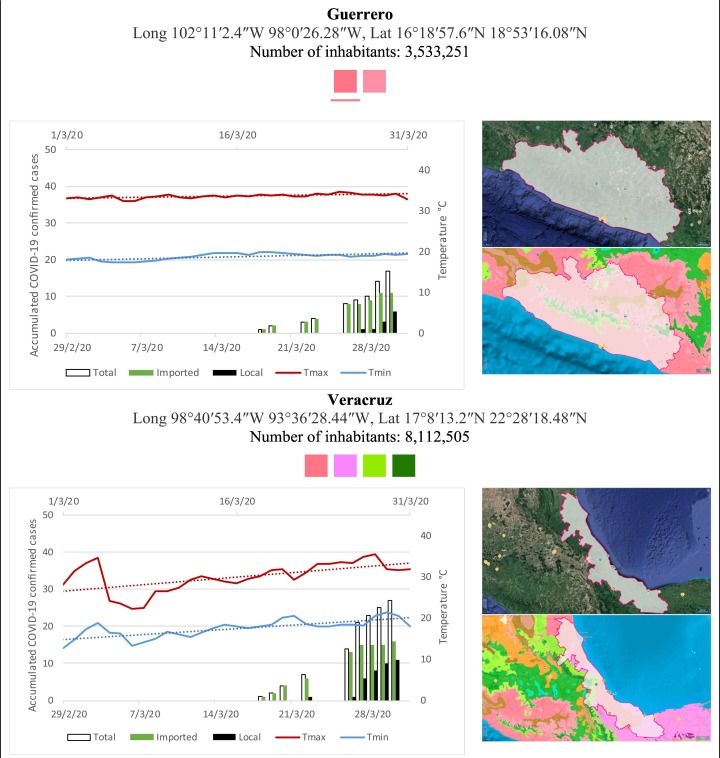
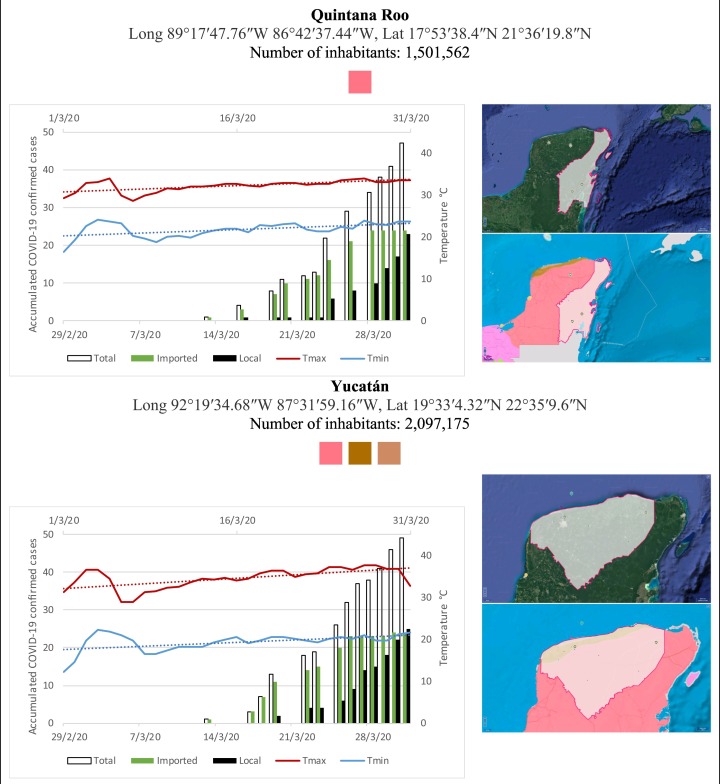
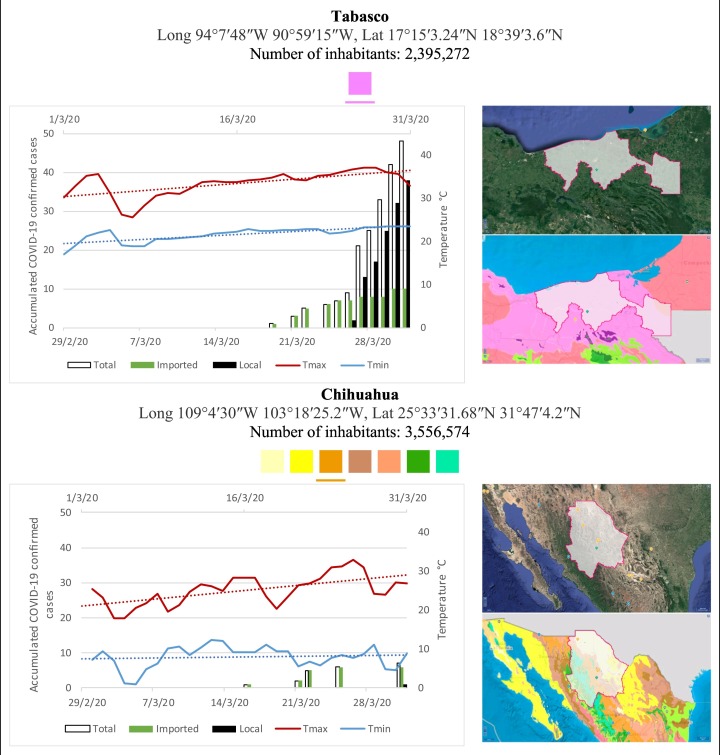
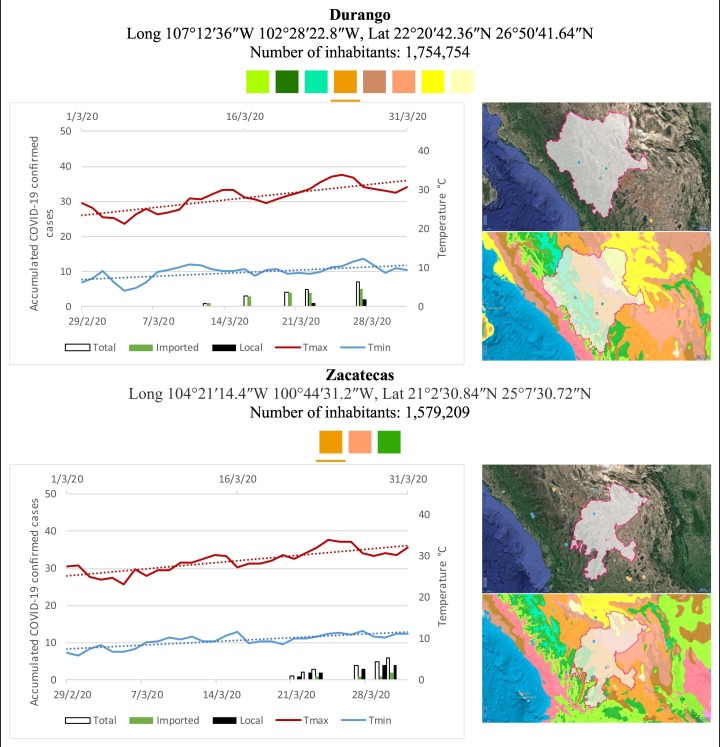
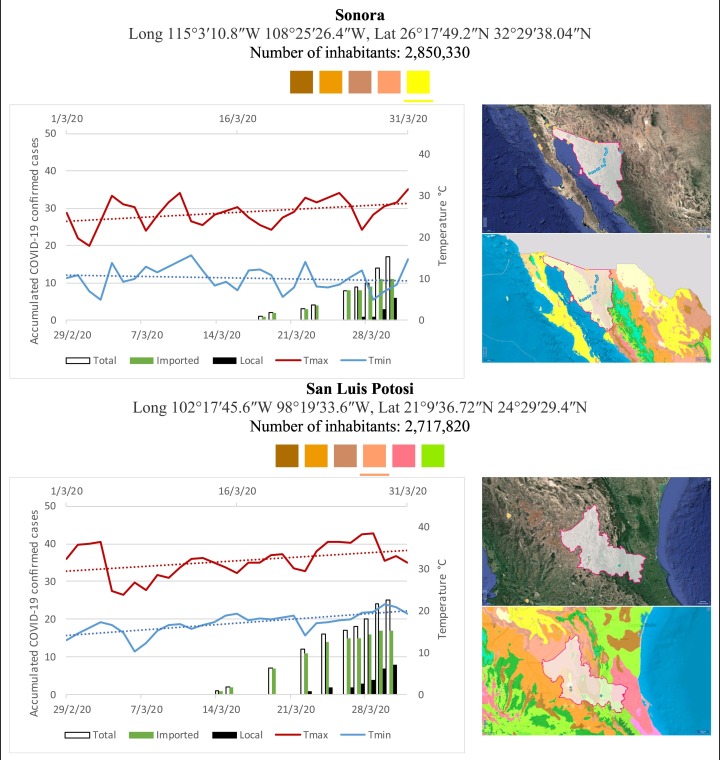
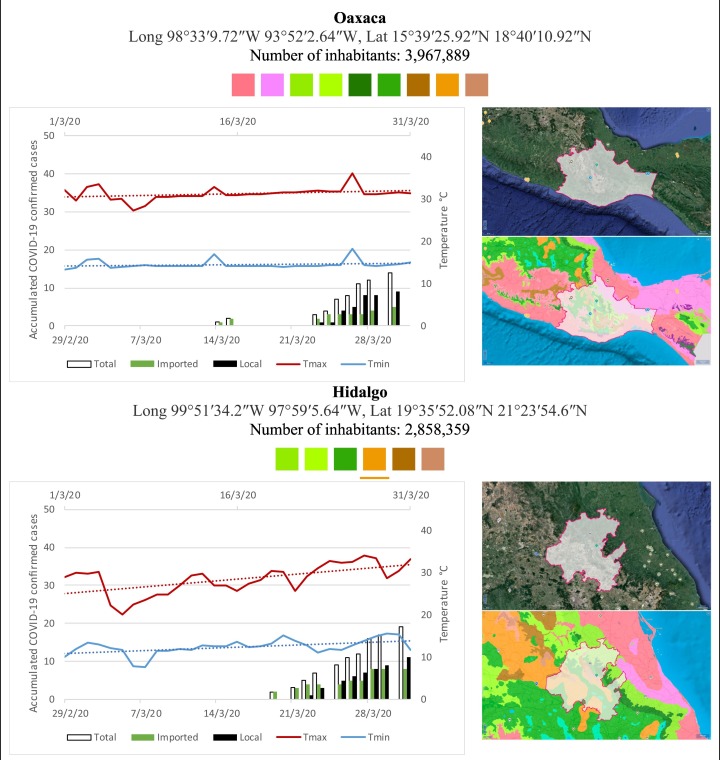
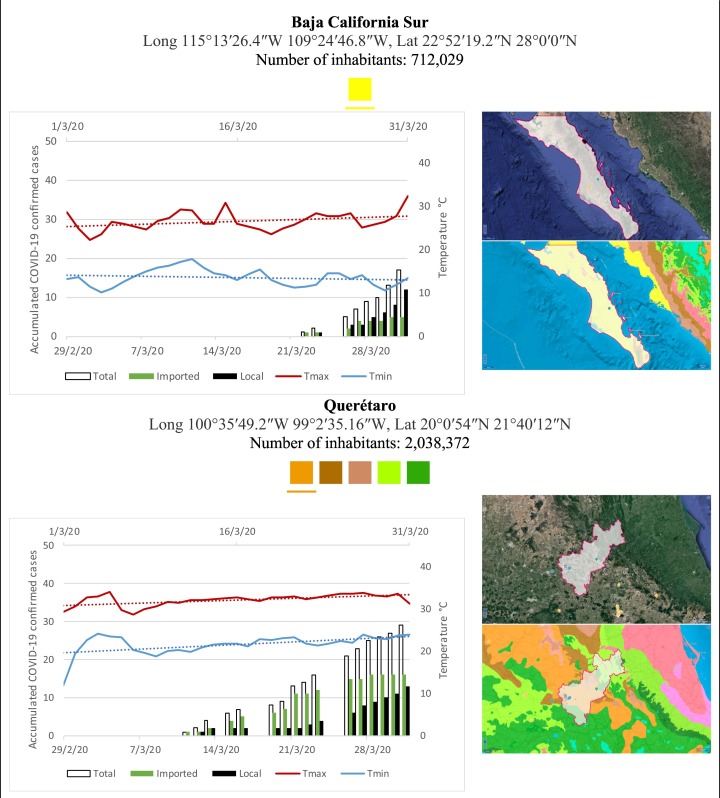
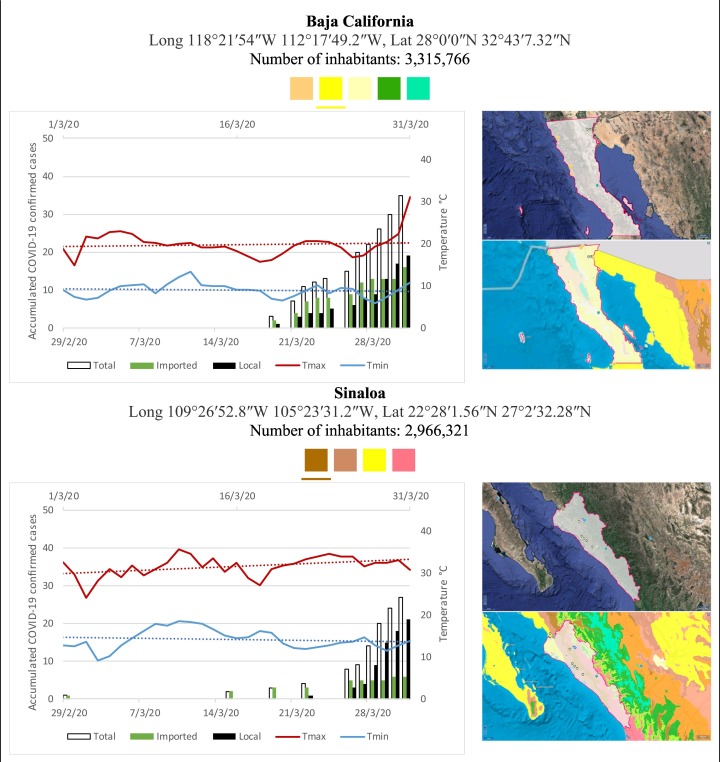
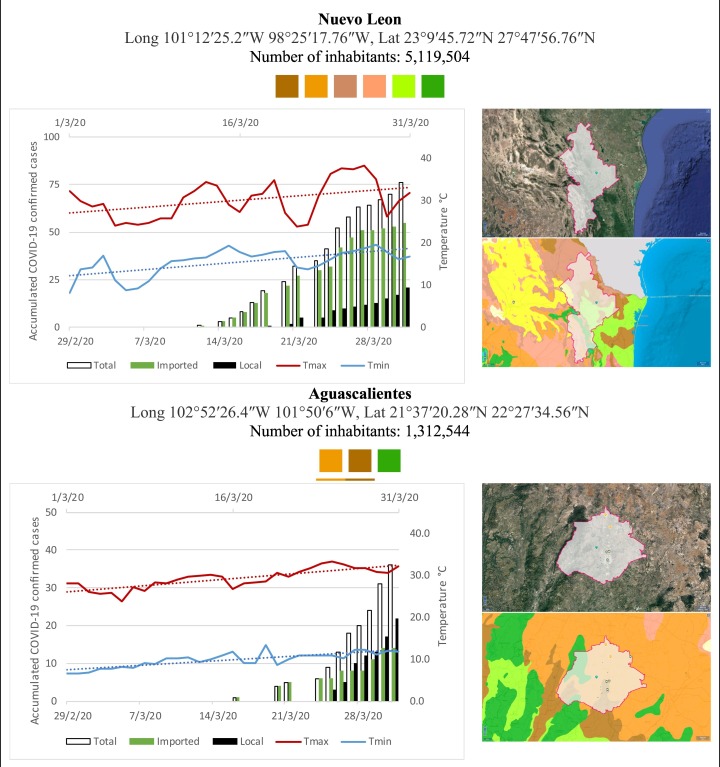
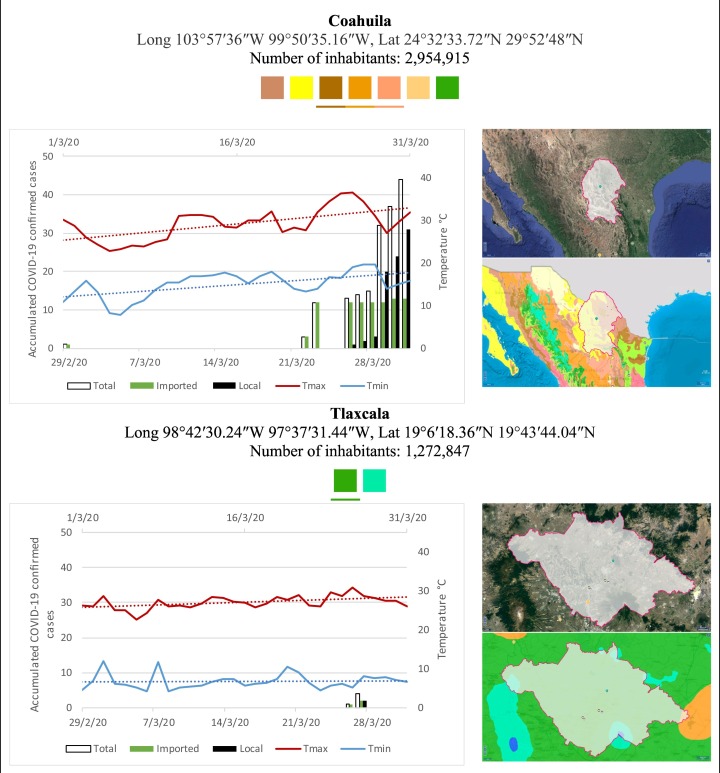
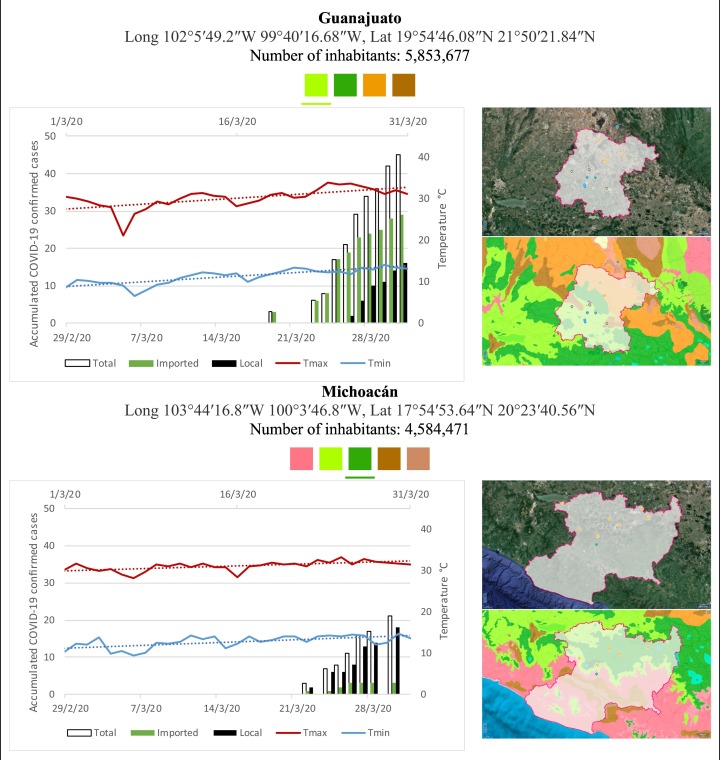
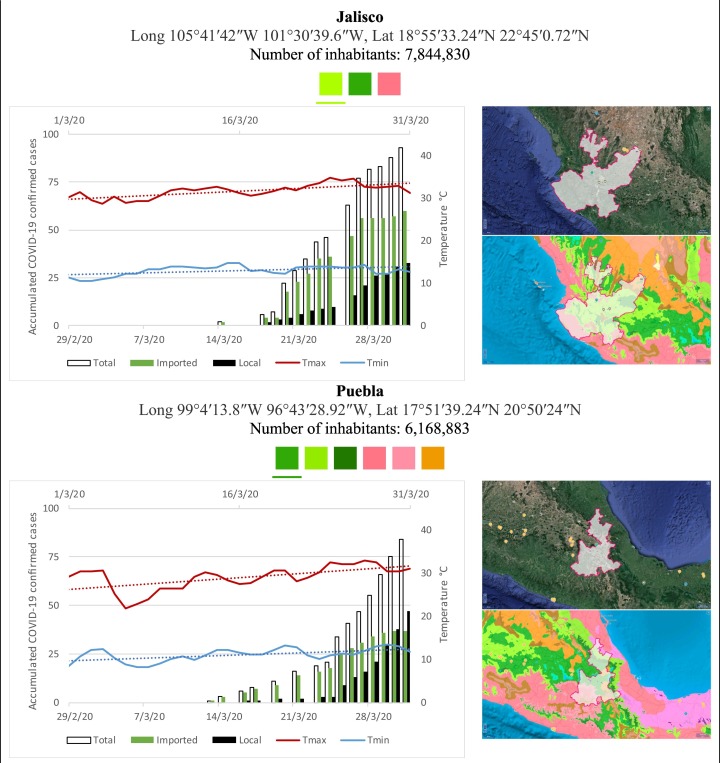
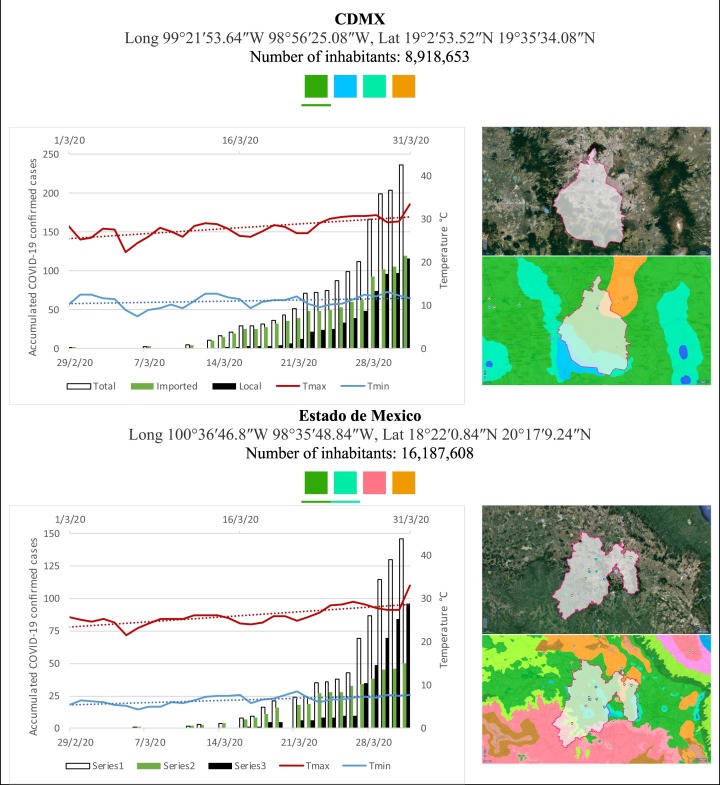
Mexico is geographically part of North America but culturally associated with Latin America. It has around 1,972,550 km2 and 125,959,205 million people (2018). Mexico has almost all the climates according to the Köppen-Geiger climate classification, excepting polar and continental microthermal types. Table 2 shows the TCPC, ICPC, LCPC, gender, average age, days lasting till imported and local onset, the total number of deceases, LTR, monthly average temperature (high, low and mean), monthly mean evaporation and precipitation as well as the predominant climate for each Mexican state and its concomitant number of inhabitants. Moreover, daily cumulative evolution of confirmed positive cases (local, imported and total) since regional onset and daily maximum and minimum temperature recorded for each state alongside detailed climate map and geocoordinate information are depicted in Table 3 . Table 2 lists the data on ascendant LTR values and Table 3 lists the state's information by climate clusters as tropical, dry or tempered classification.
Table 2.
COVID-19 confirmed cases, climate and weather since February 29, 2020 until March 31, 2020.
1Köppen-Geiger classification adapted by INEGI Group A: Tropical/megathermal climates  Warm humid
Warm humid  Warm Sub-humid
Warm Sub-humid  Warm, Semi-warm humid
Warm, Semi-warm humid  Warm, Semi-warm sub-humid Group B: Dry (desert and semi-arid) climates
Warm, Semi-warm sub-humid Group B: Dry (desert and semi-arid) climates  Dry, Warm semi-dry
Dry, Warm semi-dry  Dry, Temperate and semi-dry semi-cold
Dry, Temperate and semi-dry semi-cold  Dry, Warm dry
Dry, Warm dry  Dry, Temperate dry
Dry, Temperate dry  Dry, Temperate dry winter rains
Dry, Temperate dry winter rains  Dry, Warm very dry
Dry, Warm very dry  Dry, Temperate and very dry semi-cold Group C: Temperate/mesothermal climates
Dry, Temperate and very dry semi-cold Group C: Temperate/mesothermal climates  Temperate, Semi-warm humid
Temperate, Semi-warm humid  Temperate, Semi-warm sub-humid
Temperate, Semi-warm sub-humid  Temperate, Humid
Temperate, Humid  Temperate, Sub-humid
Temperate, Sub-humid  Temperate, Semi-cold humid
Temperate, Semi-cold humid  Temperate, Semi-cold sub-humid.
Temperate, Semi-cold sub-humid.
2Based on Monthly average T2m. 3 Based on Monthly average SH1000hPa. 4 T2m vs TCPC. 5SH1000hPa vs TCPC.
Table 3.
Cumulative evolution of confirmed positive cases (local, imported and total) since regional onset and daily maximum and minimum temperature recorded with the concomitant detailed climate map according to Köppen-Geiger classification adapted by INEGI1 alongside the geocoordinate data and number of inhabitants per state.
1Underlined colored squares represent the best climate descriptor in the most populated urban area in each state.
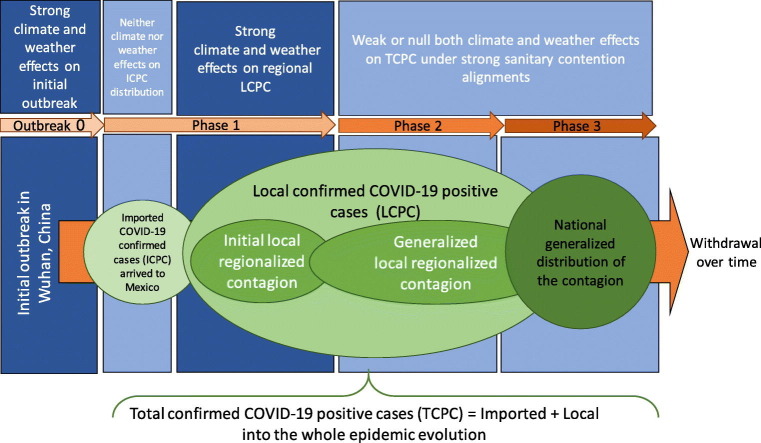
The evolution of ICPC, LCPC and TCPC in each of the 31 Mexico's states and its capital were used to determine whether regional climate and daily weather condition could be a factor in the spread of the COVID-19. This descriptive analysis have considered 3 main thoughts further explained: a) to focus on phase 1 of the epidemic, b) to focus on the communitarian confirmed positive cases (LCPC) observed in each state, and c) to enclose the local transmission rate (LTR) based on the effective regional spread since imported onset (Ionset) case until March 31, 2020. In Mexico and internationally, the COVID-19 disruption has raised high attention to governments and institutions in order to design sanitary emergency actions. The Mexican Health Commission announced the China's epidemic situation in January 3, 2020. Special attention was paid on the international airports where was estimated to detect the earliest infected people arriving to Mexican territory via airplane. The first ICPC was announced on February 28, 2020 in Mexico City arrived from Italy. Since that, Mexican authorities declared phase 1 and the contention strategy emphasized the isolation of all detected imported cases and tracked down their primary near contacts. Few days later, several contagion cases emerged (both imported and local types) in most of Mexico's states. In March 23, 2020 phase 2 was implemented with many major governmental intervention measures (for instance the massive quarantine and massive mobility reduction) in order to deaccelerate the communal infection and to avoid the hospital-system collapse. Since that, the massive quarantine stopped progressively the people's daily activities through the social distance and the confinement. In March 31, 2020, the people's quarantine and the mobility reduction up to 50% decrease were clearly manifested in all of Mexico's states. In this study, the period since February 29, 2020 until March 31, 2020 was of analytic importance from an epidemic-evolution point of view. In phase 1, the human-to-human spread of the virus in both symptomatic and asymptomatic cases was developed predominantly from people with previous stay abroad under usual environmental conditions and unrestricted contention control of the transmission. Neither imported cases nor deceases account were considered as responses related to environmental effects, because the initial imported seed of infection is randomly distributed without reasonable association neither to climate nor weather conditions. On the other hand, during the pandemics events, the peak number of deceases normally take place during phase 2 or 3. Deceases are strongly related to previous individual health condition, comorbidity and/or hospital facilities availability rather than climate and weather conditions. Therefore, LCPC factor was chosen as reasonable variable to analyze the association with weather and regional climate instead of ICPC or TCPC. Moreover, due to the regional onset varied in each state, the Local Transmission Ratio (LTR) was defined as the number of local COVID-19 confirmed cases divided by the effective lapse of dissemination into the cumulated days since regional imported onset (Ionset) until March 31, 2020.
As possible to observe in Table 2, 48.23% and 51.77% were classified as ICPC and LCPC respectively. On average, there were approximately 19.65 LCPC per day. Initial imported cases came from Europe (Spain, Italy, France and Germany), the US and Asia (Singapore). 51.77% and 48.23% were male and female respectively with an average age of 43.32 and 42.84 years old correspondingly. 87.5% of the states reported <50 ICPC, 9% between 51 and 100 ICPC and 3% higher than 100 ICPC. 94% of states shown 66% LCPC and only 2% (2 locations) represented 33% of local contagion including both Mexico City (CDMX) and Estado de Mexico (EdoMex) urban megacities as conjoined metropolitan area with >20 millions of inhabitants. As possible to observe, Colima is the state with the minimum number of ICPC (2) and did not report LCPC in the studied period. Since February 29, 2020 to March 31, 2020, temperatures in Mexico ranged from 0.87 °C to 38.55 °C with a mean temperature of 22.22 °C. The maximum temperature reached between 30.71 and 38.55 °C with an average of 34.61 °C and the minimum between 0.87 and 17.79 °C with an average of 9.97 °C, respectively. Evaporation ranged from 4.5 to 9.2 mm and a mean of 6.14 mm. The average of total precipitation during this period was 13.99 mm. The T2m and SH1000hPa values in Mexico within the studied period were 19.82 °C (13.5 °C < T2m < 24.9 °C) and 5.92 g/kg (3.1 g/kg < SH1000hPa < 8.6 g/kg) respectively. Table 4 shows the correlation parameter rS resulted from association between TCPC, ICPC, LCPC and LTR with both meteorological measures (i.e., monthly average temperature -max, min and mean-, evaporation and precipitation data) and the main climate regional characterization. Qualitative climate factor was associated to numerical equivalences as 1, 2 and 3 for tropical, dry and tempered climates accordingly. The association between of TCPC, ICPC, LCPC and LTR were in all cases negatively associated with temperature (−0.007 < rS < −0.182) and evaporation (−0.221 < rS < −0.317) without statistical significance. The association between ICPC and monthly average Tmax was positive. Association between of TCPC, ICPC, LCPC and LTR were positively associated with precipitation (0.176 < rS < 0.0282) without statistical significance as similar with the TCPC positive association to SH1000hPa (rS = 0.1677). In contrast, a negative association was observed between each TCPC, LCPC and LTR with climate factor with resulted rS values equal to 0.39041, 0.39031 and 0.46377 respectively. All of last associations resulted statistically significant with p values of 0.02716, 0.02721 and 0.00751 concomitantly. ICPC was also negative associated with the climate variable without statistical significance. TCPC and T2m association resulted positive and statistically significant (rS = 0.866 and p = 0): as increasing T2m, increasing TCPC accordingly. Similarly, SH1000hPa associated positively to TCPC (rS = 0.167) without statistics significance.
Table 4.
rS coefficient between the two variables. p value is shown if association is considered statistically significant.
| Temperature |
Monthly mean evaporation | Monthly mean precipitation | Specific humidity |
Climate type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monthly Mean | Max | Min | Monthly AverMax | Monthly AverMin | T2m | SH1000hPa | |||||
| Confirmed COVID-19 positive cases | Total | −0.075 | −0.007 | −0.123 | −0.112 | −0.034 | 0.866, p = 0 | −0.279 | 0.256 | 0.167 | −0.39041, p = 0.02716 |
| Imported | −0.076 | 0.012 | −0.147 | −0.123 | −0.021 | – | −0.221 | 0.282 | – | −0.314 | |
| Local | −0.067 | −0.040 | −0.068 | −0.084 | −0.042 | – | −0.268 | 0.180 | – | −0.39031, p = 0.02721 | |
| LTR | −0.158 | −0.182 | −0.112 | −0.155 | −0.134 | – | −0.317 | 0.176 | −0.46377, p = 0.00751 | ||
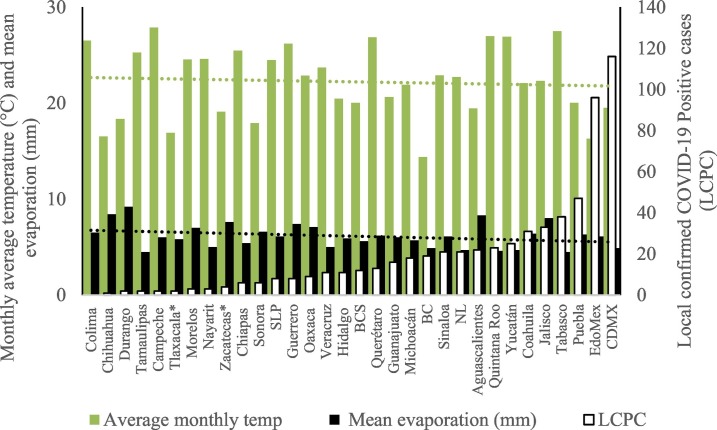
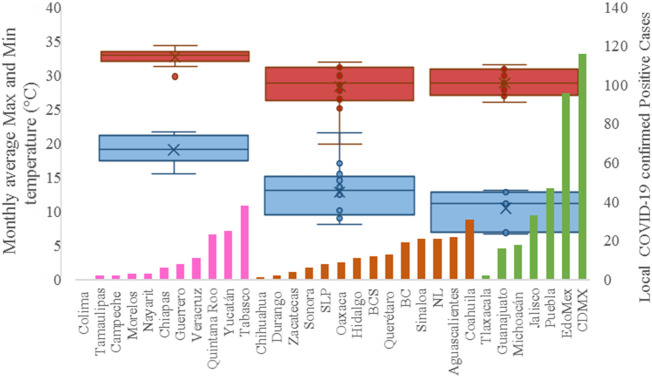
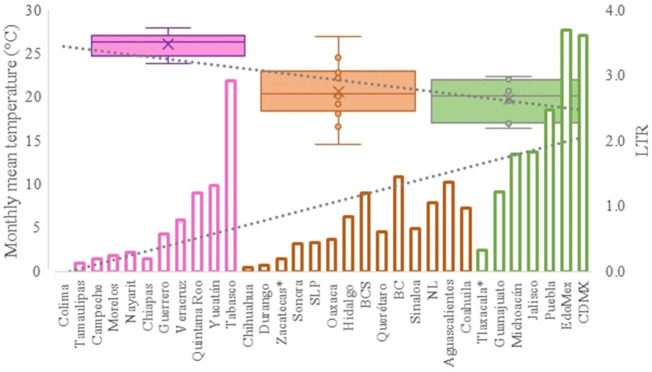
On the other hand, Fig. 1 shows the monthly average temperature (green bars) and monthly mean evaporation (black bars) per state alongside to the regional LCPC (white bars) in ascending order. As possible to observe, in general both monthly average temperature and monthly mean evaporation decrease as LCPC increases. Fig. 2 shows the monthly average max and min temperatures (red and blue boxes respectively) and LCPC values clustered in tropical (pink bars), dry (brown bars) and tempered (green bars) climates. Fig. 3 shows the mean temperature (boxes) and LTR (bars) also classified by climate clusters (tropical in pink color, dry in brown color and temperate in green color). As possible to observe in Fig. 2, Fig. 3, climate clusters pointed highlight out the role of environmental factors on LCPC. In the dry cluster, the average evaporation value was 6.65 mm similar to evaporation observed in tropical and temperate climates, 5.5 mm and 6.11 mm respectively. However, precipitation in the dry cluster (20.87 mm) was around two folds higher, than the observed in tropical and tempered clusters, 8.74 mm and 8.84 mm respectively. Tropical cluster showed the highest mean temperature of 25.95 °C in comparison to 20.57 °C and 19.65 °C for dry and temperate clusters respectively. Similar behaviour was observed for the max and min temperatures with higher values in tropical areas (32.87 °C and 19.03 °C) than in dry (28.39 °C and 12.75 °C) or temperate (28.97 °C and 10.33 °C) regions. Thus in tropical areas infectivity of SARS-CoV-2 disfavour transmission under stable high temperature conditions. In temperate regions, both low temperature and low evaporation with poor precipitation favoured the local infectivity observed in cities such as Tlaxcala, Guanajuato or Michoacan. LTR is statistically significant correlated to climate factor as previously observed in Table 4. LTR describes the dynamics of the epidemics into the effective elapsed time since regional onset. Average onset data occurs later in tropical climates but earlier in the dry and temperate climates (25.09, 22.35 and 19.57 days respectively) as observed in Fig. 3. Thus, the negative association between LCPC and local onset is considered statistically significant with rs = −0.65207 and p = 5E-05. Moreover, the number of days between the Ionset and the Lonset was 8.54, 8.35 and 5.28 days respectively in tropical, dry and tempered climates respectively. According with the last results, high LCPC values would be not expected in tropical climates such as Yucatán or Quintana Roo states. However, as possible to observe in Fig. 2, Fig. 3, both Yucatán and Quintana Roo belonging to the tropical cluster, were states with high LCPC values. The last likely resulted due to the high touristic character of both states where the ICPC were the highest values reported (24 each) for the whole tropical cluster. In temperate climate, the infectivity and virus transmission were facilitated in local community due to the low-temperature and low-humidity reported compared with temperature in the dry and tropical clusters. Mexico city and EdoMex, classified as temperate states, reported the highest national local transmission not only justified to the environmental factors but also by the demographic aspects such as the population density (see Table 2). Therefore, in Mexico, the climate characteristics played a crucial role on the initial local infection during the phase 1 being the tempered regions (as Michoacán, Jalisco, Puebla, CDMX, among others) more vulnerable than the dry (as Chihuahua, Durango or Zacatecas among others) or tropical areas (as Colima, Campeche, Morelos among others). The last assumption agreed with previous evidences observed in tropical areas of Malaysia, Indonesia, and Thailand during the SARS-CoV outbreak in 2003 (Chan et al., 2011).
Fig. 1.
Monthly average temperature (green bars) and monthly mean evaporation (black bars) per state alongside to the regional LCPC (white bars) in ascending order. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Monthly average max (red boxes) and min (blue boxes) temperatures and LCPC clustered in tropical (pink bars), dry (brown bars) and tempered (green bars) climates. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Mean temperature (boxes) and LTR (bars) classified by climate clusters: tropical in pink color, dry in brown color and tempered in green color. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The most accepted scientific consensus agrees that the low temperature and the low humidity favor the outbreak, the virus survival and the human-to-human transmission as previously observed in historical epidemic events. However, currently there is no consensus of what climates or weather conditions constitute the lower or higher limit to be considered as key-level factors for pandemics control or spread prediction. In the literature, is common to find several parameters that could be not equivalent for a precise comparison. For instance 1) the chosen response of the epidemics to be monitored i.e. total confirmed cases, suspected cases, mortality, hospital collapse, etc., 2) the chosen time period for evaluation (whole epidemic evolution, weekly, monthly or any arbitrary lapse), and 3) the reference of environmental factors for analysis as indoor or outdoor contexts. The vast majority of the reports are based on either laboratory results -through in situ viral activity observations- or meteorological statistical evidences and mathematical adjusting models. At laboratory conditions, there exist specific protocols at extreme well-defined experimental environments (incubation time, precise room temperature, controlled room humidity, host specimen specification, etc.). Such studies evaluated the effect of “specific controlled environmental conditions” on general infectivity of targeted viruses. Results of such studies normally pointed out precise values of room temperature or humidity as valid limits for virus survival or inactivation (Lowen et al., 2007; Chan et al., 2011; Casanova et al., 2010; Doremalen et al., 2013, Doremalen et al., 2020). However, such experimental information cannot fully explain why epidemics are also reached in localities where temperature of humidity are out of the range experimentally observed at laboratory conditions. On the other hand, most of the statistical methods and mathematical models have employed data from different lapses or periods of pandemics. Such information is acquired with more or less detailed and in-depth data according its availability (Wang et al., 2020; Sajadi Mohammad et al., 2020; Keshavarzi, 2020; Bukhari and Jameel, 2020; Ma, 2020; Araujo and Naimi, 2020). The vast majority of such studies disregard both the origin of the transmission (local or imported) -assuming the total infection cases as the suitable variable to monitor- and the well-differentiated phases of the epidemic -assuming the whole pandemic lapsed as the most valid range for evaluation-. Such studies are not clearly conclusive respect to the environmental effects on epidemic's overall outcomes. Inappropriate focus on the epidemic lapse could release contradictory conclusions as pointed out by Cai et al. (2007). For instance, in this study, the association of temperature with confirmed cases resulted positive with perfect statistical significance (p = 0): as increases temperature, increases the number of infected people as well (see Table 2). However, this expected result comes from the ordinary seasonal cycles that cross from winter to spring season with a concomitant temperature increase. Therefore, the positive association between temperature and TCPC with statistical significance could be mistakenly interpreted. In contrast, in the present analysis was possible to evaluate the correct negative association between environmental factors and LCPC similar than the high correlation coefficient statistically significant between LPCP and TCPC with the climatic character. Moreover, the LTR, which takes into account the effective number of days when the epidemics is regionally active, showed the highest correlation coefficients in association with the climate variable (see Table 4). The last results were observed taking in consideration that the principal effect of environmental factors on virus transmission occurred only in the early stage of the epidemics before any imposed contention strategy. Therefore, both the initial lapse of the epidemic and the contagion origin-type are of the most significant highlights here founded useful to assure that the regional climate character in each state did influence the initial transmission of the COVID-19 disease in Mexico. Finally, it is important underline that experimental results under controlled conditions at laboratory scale offer extremely useful information to be employed during confinement phases 2, 3 and beyond. As shown in Table 1, SARS-CoV-2 is stable at low temperatures (between 5 and 11 °C or generally below 20 °C) and low humidity (generally dry condition) (Doremalen et al., 2020). Commonly, in tropical and warm-dry climates the indoor environment is artificially adjusted by air-condition apparatus that reduces the temperature and the humidity for comfortable spaces. In the dry cluster analyzed in this study, the LCPC was high in states such BC and BCS likely linked to the intensive use of air-conditioning instrument in agreement with observations previously reported in Singapore and Hong Kong by Chan and cols. (Chan et al., 2011). The use of air-condition apparatus promotes the most appropriate conditions for survival of the SARS-CoV-2. Therefore it is important avoid the low temperature and low humidity in both indoor hospitals and room-home level spaces during phase 2 and 3 under implemented strict contention strategies. In Mexico, the quarantine obligation in phase 2 and 3 resulted in reduction until up 70% of the mobility in metropolitan areas with >20 million of inhabitants. People stayed at home in a massive extent. Taking into account that the virus infectivity decreases as temperature and humidity increases some public health recommendations could generate massive non-contagion indoor areas. The employment of commercial humidifiers and heaters or even better, natural solar warm and fresh air circulation when possible, could be effective massive sanitary strategies in order to reduce the virus transmission as well as to decelerate the hospital system collapse.
5. Conclusion
The occurrence of confirmed local cases of COVID-19 in Mexico has been analyzed taking into account the weather and climate of each of 31 states and the capital during the phase 1 of the epidemic progress. The evolution of COVID-19 was analyzed through in the number of LCPC rather than TCPC or ICPC. Neither ICPC nor deceases described the environmental effect on transmission in phase 1. COVID-19 local spread was strongly associated with both the climate and daily environmental conditions due to during phase 1 the transmission occurred freely without contention strategy influences. Thus, the regional climate in each Mexico's state has a central role in the initial local SARS-CoV-2 transmission. TCPC (i.e. ICPC+LCPC) associates positively with daily average air temperature. High temperature and high evaporation in tropical climates are the best-combined predictors as a disadvantaged condition for SARS-CoV-2 survival. Daily incidence of COVID-19 increased slowly in states belonging to tropical cluster. Template condition promotes the highest LCPC observed. Temperature and evaporation were negatively associated with the LCPC without statistical significance. Regional climate was statistically significantly associated with LCPC and LTR. Dry and temperate cold climates are the more suitable environmental conditions for transmission. The tropical climate (mean temperature around 25.95 °C and mean precipitation around 8.74 mm) delayed the regional onset. However, the regional onset in dry climates emerged earlier as consequence of the lower temperatures and higher precipitations (20.57 °C and 20.87 mm respectively) than the observed in the tropical climate. The fastest regional onsets were observed in tempered climates with the lowest temperatures and precipitations registered (19.65 °C and 8.48 mm respectively). Therefore, the regional climate was strongly associated with initial COVID-19 local contagion during the phase 1 being the tempered regions (as Tlaxcala, Guanajuato, Jalisco, among others) more vulnerable than the dry (as Aguascalientes, Sinaloa, Queretaro, among others) or tropical areas (as Veracruz, Guerrero, Chiapas, among others). Any attempt related to evaluate the effect of outdoor environmental factors on the spread, transmission or virus survival should take into account both the phase of the epidemic evolution and the effective active period of the virus since regional onset on communitarian transmission.
Availability of supporting data
The data used during the current study are available from the websites.
Funding
This work was supported by the National Council of Science and Technology, Mexico through Catedras-CONACyT Program.
CRediT authorship contribution statement
Fabiola Méndez-Arriaga:Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
To National Council of Science and Technology through Catedras Program. In memorial to the COVID-19's deceases worldwide.
References
- Araujo B., Naimi Babak. 2020. Spread of SARS-CoV-2 Coronavirus Likely to Be Constrained by Climate. [DOI] [Google Scholar]
- Bukhari, Qasim and Jameel, Yusuf, 2020. Will coronavirus pandemic diminish by summer? (March 17, 2020). Available at SSRN: https://ssrn.com/abstract=3556998 or doi: 10.2139/ssrn.3556998. [DOI]
- Cai Q.C., Lu J., Xu Q.F. Influence of meteorological factors and air pollution on the outbreak of severe acute respiratory syndrome. Public Health. 2007;121(4):258–265. doi: 10.1016/j.puhe.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76(9):2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Malik Peiris J.S., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Advances in Virology. 2011;2011 doi: 10.1155/2011/734690. (Hindawi Publishing Corporation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li Fang, Shi Zheng-Li. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. (PMCID: PMC7097006, PMID: 30531947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eurosurveillance. 2013;18(38) doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- Doremalen N., Dylan H., Morris M., Phil N.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. https://www.nejm.org/doi/pdf/10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi Ali. 2020. Coronavirus Infectious Disease (COVID-19) Modeling: Evidence of Geographical Signals. SSRN-id3568425. [DOI] [Google Scholar]
- Lin K., Yee-Tak Fong D., Zhu B., Karlberg J. Environmental factors on the SARS epidemic: air temperature, passage of time and multiplicative effect of hospital infection. Epidemiol. Infect. 2006;134(2):223–230. doi: 10.1017/S0950268805005054. 2006 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen, A. C., Mubareka, S., Steel, J. and Palese, P., 2007. Influenza virus transmission is dependent on relative humidity and temperature, PLoS Pathog., 3(10), e151, doi: 10.1371/journal.ppat.0030151 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed]
- Ma Y. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020:138226. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari N., Harmooshi, Shirbandi Kiarash, Rahim Fakher. 2020. Environmental Concern Regarding the Effect of Humidity and Temperature on SARS-COV-2 (COVID-19) Survival: Fact or Fiction. SSRN-id3563403.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi Mohammad M., Habibzadeh Parham, Vintzileos Augustin, Shokouhi Shervin, Miralles-Wilhelm Fernando, Amoroso Anthony. 2020. Temperature and Latitude Analysis to Predict Potential Spread and Seasonality for COVID-19. SSRN-id3550308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius J.D., Shaman J., Alonso W.J., Bloom-Feshbach K., Uejio C.K., Comrie A., Viboud C. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates, edited by S. Riley. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Mu L., Huang J., Yu S., Chen B., Yin J. An initial investigation of the association between the SARS outbreak and weather: with the view of the environmental temperature and its variation. J. Epidemiol Community Health. Mar. 2005;59(3):186–192. doi: 10.1136/jech.2004.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Jiang Aili, Gong Lijuan, Luo Lina, Guo Wenbin, Li Chuyi, Zheng Jing, Li Chaoyong, Yang Bixing, Zeng Jietong, Chen Youping, Ke Zheng, Li Hongyan. 2020. Temperature Significant Change COVID19 Transmission in 429 Cities. medRxiv 2020.02.22.20025791. [DOI] [Google Scholar]
- Yuan J., Yun H., Lan W. A climatologic investigation of the SARS-CoV outbreak in Beijing, China. Am. J. Infect. Control. 2006;34(4):234–236. doi: 10.1016/j.ajic.2005.12.006. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used during the current study are available from the websites.