Abstract
In December 2019, a pathogenic novel human coronavirus (HCoV), termed SARS-CoV-2, was recognized in Wuhan, China, causing significant morbidity and mortality. The illness caused by SARS-CoV-2 is labelled coronavirus disease-2019 (COVID-19) by the World Health Organization. We report the first case of COVID-19 in an adult congenital heart disease patient with single ventricle physiology S/P Fontan palliation. (Level of Difficulty: Advanced.)
Key Words: cyanotic heart disease, hypoxemia, tricuspid valve
Abbreviations and Acronyms: ALT, alanine aminotransferase; AST, aspartate transaminase
Graphical abstract
In December 2019, a pathogenic novel human coronavirus (HCoV), termed SARS-CoV-2, was recognized in Wuhan, China, causing significant morbidity and mortality…
History of Presentation
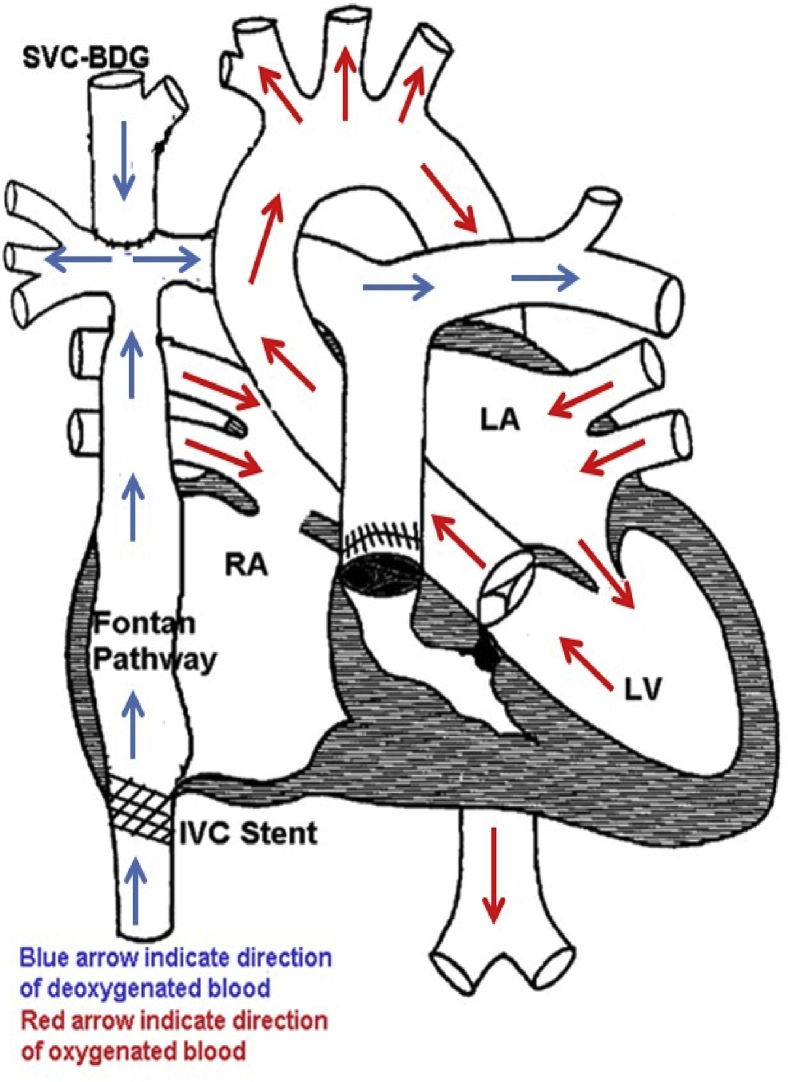
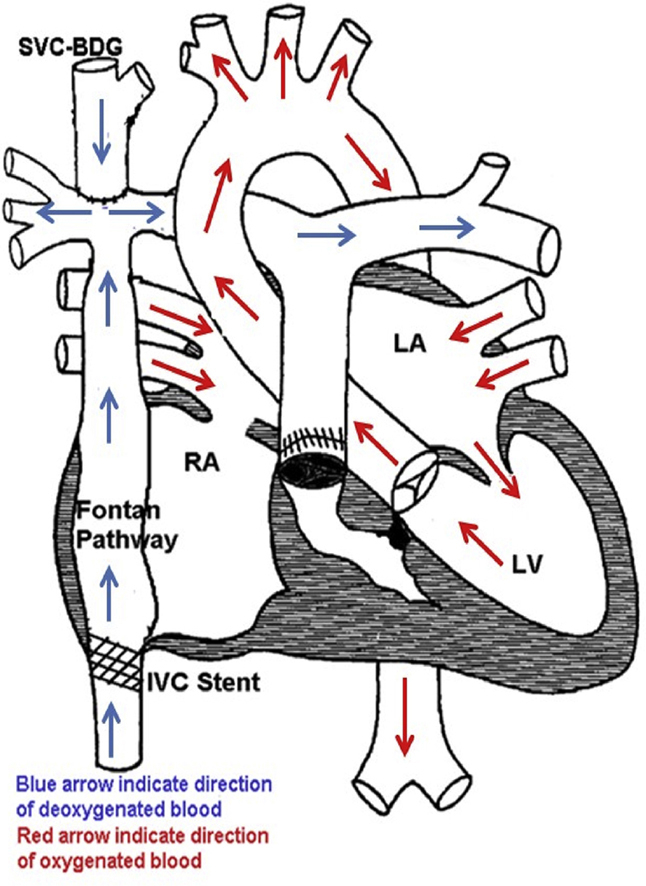
A 29-year-old man with a tricuspid atresia and multiple cardiac surgical palliations with a single ventricle S/P Fontan circulation (Figure 1), presented to the emergency room with 2 days of low-grade fevers, nonproductive cough, easy fatigability, and progressive shortness of breath. He had known exposure to a family member with coronavirus disease-2019 (COVID-19). He did not have anosmia, taste disturbances, or gastrointestinal or neurological symptoms. His vital signs revealed a temperature of 38.2°C, heart rate 79 beats/min, and SpO2 86% on room air. COVID-19 testing was positive. A chest x-ray revealed bilateral patch opacities suggestive of a pneumonic process. Given that his room air oxygen saturations were <94% and chest x-ray findings were consistent with pneumonia, he was diagnosed with moderate COVID-19 infection per hospital guidelines.
Learning Objectives
-
•
To understand the baseline hemodynamics in Fontan patients when managing COVID-19 infection.
Figure 1.
Tricuspid Atresia With Normally Related Great Arteries, S/P Fontan Palliation
BDG = bi-directional Glenn; IVC = inferior vena cava; LA = left atrium; LV = left ventricle; RA = right atrium; SVC = superior vena cava.
Past Medical History
His past medical history was significant for complex cyanotic congenital heart disease. He was born with tricuspid atresia with single ventricle physiology and had undergone multiple palliative surgeries during his childhood. His initial surgery at 3 days of life was a right classic Blalock Tausig shunt (right subclavian artery to the pulmonary artery anastomosis). He had a Glenn procedure performed at 1 year of age, with surgical anastomosis of the right superior vena cava to the pulmonary artery. The final cardiac surgical procedure was a lateral tunnel Fontan (inferior vena cava to pulmonary artery) performed at 4 years of age. He had undergone a cardiac catheterization for systemic desaturation as a child with device closure of a large venovenous collateral from the azygous to the left upper pulmonary vein. He had stenosis of the inferior vena cava pathway of the Fontan circuit, which was treated with balloon dilation and stent placement. A work-up for recurrent epistaxis revealed a sinonasal mass. He underwent endoscopic resection and embolization of bilateral maxillary arteries with coils and onyx through the external carotid artery. He had sporadic long-term follow-up with adult congenital heart disease but had no recent hospitalizations.
Differential Diagnosis
Although Fontan patients commonly have lower oxygen saturations between 90% and 95% in room air, it is important to consider other possible etiologies of systemic oxygen desaturation in a Fontan patient. One reason of baseline desaturation includes markedly deoxygenated coronary sinus vein blood that typically drains into the pulmonary venous chamber and mixes with pulmonary venous blood. Due to absent subpulmonic pumping ventricle, the nonpulsatile pulmonary artery blood flow tends to gravitate in the lower-lung segment, whereas the pulmonary aeration favors upper segments resulting in ventilation-perfusion mismatch. Other differential diagnoses for desaturation in a Fontan patient include venovenous collaterals leading to right to left shunting, which allows deoxygenated venous blood to mix with oxygenated arterial blood. These venovenous collaterals may naturally form as a “pop-off” between the high-pressure systemic venous and lower-pressure pulmonary venous chambers. These communications may develop over time, either within the heart or through extracardiac venous collaterals (1). Another cause of desaturation in patients with Fontan circulation, notably those with heterotaxy syndrome, is from development of pulmonary arteriovenous malformations causing intrapulmonary shunting and progressive cyanosis (2). Some Fontan patients develop plastic bronchitis caused by the spillage of protein-rich lymph through lymphatic-to-bronchial communications, which can present with productive cough, dyspnea, and hypoxemia (3). Other causes of systemic oxygen desaturation that are not exclusive to patients with single ventricles include causes that lead to hypoventilation or V/Q mismatch due to pulmonary embolism, pneumonias, asthma or COPD. Other causes are diffusion impairments or circumstances that cause low inspired oxygen.
Investigations
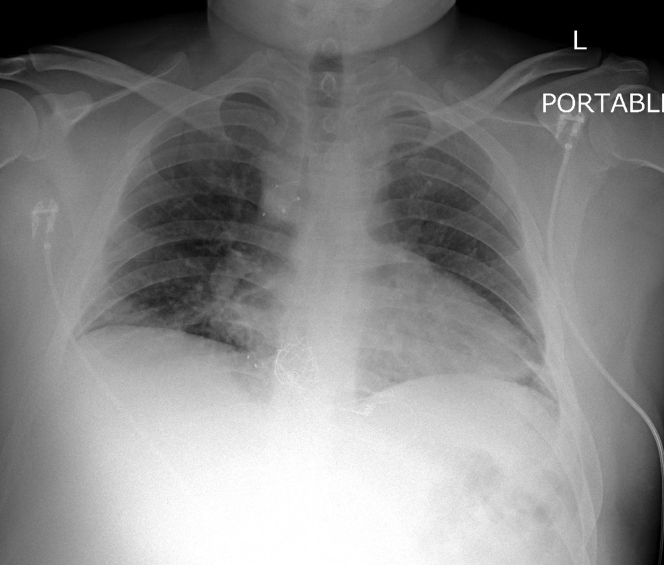
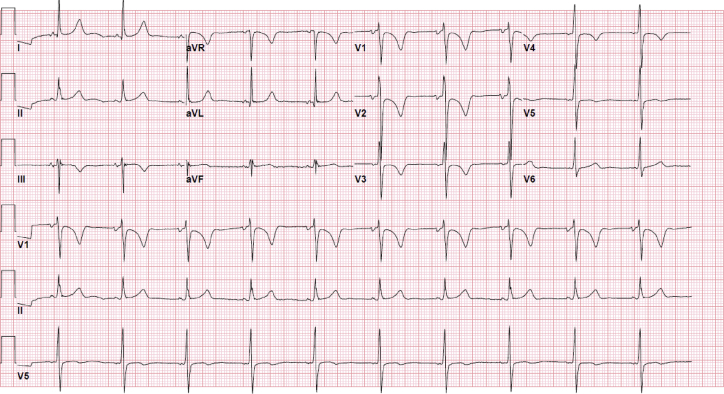
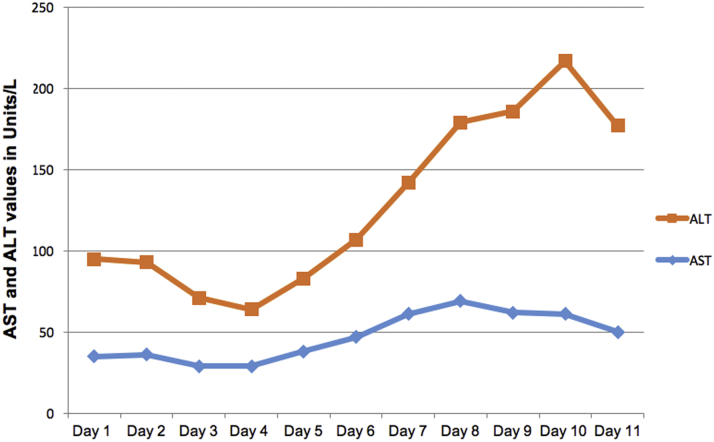
A chest x-ray (Figure 2) showed mild bibasilar opacities at both lung bases. An electrocardiogram (Figure 3) revealed a normal sinus rhythm with a baseline right bundle branch block and QTc interval of 460 ms that was unchanged from prior. A respiratory viral panel was positive for COVID-19. Baseline laboratory results including a complete blood count and chemistries (Table 1) were notable for leukopenia, thrombocytopenia, and elevated hemoglobin. Electrolytes were within normal limits. Renal function was normal with stable baseline creatinine. Cardiac markers including troponin and creatine phosphokinase (CPK) was negative. Inflammatory markers were notable for an elevated C-reactive protein (CRP), normal ferritin, and procalcitonin. Coagulation profile was within normal limits. This included a normal international normalized ratio, fibrinogen, and D-dimer. The Ella Cytokine Storm Panel (Bio-Techne, Minneapolis, Minnesota) was sent to detect cytokine release syndrome in real time. This test included interleukin (IL)-6, IL-8, IL-10, TNFα, and IL-1b sent on all admitted COVID-19 patients at our institution. IL-6 and IL-8 levels were significantly elevated. CRP continued to rise with a peak value of CRP 40.3 mg/l, with an eventual decrement. The d-dimer levels remained normal throughout the admission. Fibrinogen levels, although normal at admission, reached a peak of 488 mg/dl on day 3 of hospitalization. Liver function tests including aspartate transaminase (AST)/alanine aminotransferase (ALT) were mildly elevated at admission and continued to rise with AST, reaching a peak on day 7 of admission. Similarly, ALT peaked on day 9 of admission. By discharge, the transaminase levels were down-trending (Figure 4). An abdominal ultrasound showed normal liver size (15.5 cm) with increased liver echogenicity and a nodular contour suggestive of liver fibrosis, likely due to Fontan Associated Liver Disease (FALD). His CBC was trended with normalization of his platelet count by day 6 of hospitalization.
Figure 2.
Chest Radiograph Posteroanterior View With Bibasilar Mild Opacities
Figure 3.
12-Lead Electrocardiogram
Normal sinus rhythm, nonspecific T-wave abnormality, QTc 460 ms.
Table 1.
Laboratory Values
| Value on Admission | Normal Range | |
|---|---|---|
| WBC | 3.7∗ | 4.5–11.0 × 10−3/ul |
| Hemoglobin | 17.2∗ | 13.9–16.3 g/dl |
| Hematocrit | 53.5∗ | 42.0–52.0% |
| Platelets | 99∗ | 150–450 × 10−3/ul |
| BUN | 9 | 6–23 mg/dl |
| Creatinine | 0.85 | 0.70–1.30 mg/dl |
| AST | 35 | 1–35 U/l |
| ALT | 60∗ | 1–45 U/l |
| PT | 13.5 | 12.3–14.9 s |
| PTT | 32.8 | 25.4–34.9 s |
| INR | 1.1 | Range |
| Fibrinogen | 438 | 175–450 mg/dl |
| D-dimer | 0.36 | 0.00–0.50 μg/ml FEU |
| CPK | 134 | 30–200 U/l |
| Troponin | <0.01 | 0.00–0.03 ng/ml |
| C-reactive protein | 8.7 | 0.0–5.0 mg/l |
| Procalcitonin | 0.06 | <0.49 ng/ml |
| Interleukin-6 | 25.9 | 0.0–5.0 pg/ml |
| Interleukin-8 | 26.1 | 0.0–5.0 pg/ml |
BUN = blood urea nitrogen; CPK = creatine phosphokinase; INR = international normalized ratio; PT = prothrombin time; PTT = partial thromboplastin time; WBC = white blood cell count.
Abnormal values
Figure 4.
Graphical Representation of AST and ALT
ALT = alanine aminotransferase; AST = aspartate transaminase.
Management
In the emergency room, the patient was hypoxic with oxygen saturations of 86% on right atrium with accompanying dyspnea. He was placed on 4 to 6 l of oxygen via a nasal cannula, which led to an improvement in the oxygen saturations to low 90s. High-flow nasal cannula was attempted, which he did not tolerate. He continued to need supplemental oxygen until day 6 of his hospitalization. There was no need for mechanical ventilation. Aggressive intensive spirometry was encouraged. He was seen by the COVID-19 infections disease team and was started on hydroxychloroquine (400 mg orally [PO] twice daily for 1 day, then 400 mg PO daily to complete a 5-day course). A daily electrocardiogram was performed that did not show prolongation of his QTc interval. A 5-day PO course of azithromycin was also given. He was aggressively diuresed with intravenous furosemide. A PDE5i (sildenafil) 20 mg PO three times a day was started. With the initiation of sildenafil, there was a slow improvement in his clinical symptoms. Subcutaneous heparin for deep vein thrombosis prophylaxis was started, which was later switched to full-dose lovenox. The patient was hospitalized for 10 days and was discharged in stable condition on aspirin 81 mg PO daily and sildenafil.
Discussion
There has been no published data on the impact of COVID-19 on adults with a congenital heart defect. This is the first case report of an adult congenital heart disease patient with single ventricle physiology S/P Fontan palliation. At this time, all current management strategies are extrapolated from what is known about the effect of COVID-19 on adult patients with cardiovascular disease. Efforts are underway through the Adult Congenital Heart Association and the International Society of Adult Congenital Heart Disease to gather data on the number of suspected and confirmed cases both in the United States and globally and to better understand outcomes in this population (3). Our patient was diagnosed with moderate COVID-19 disease due to his oxygen saturations <94%; however, it is important to consider baseline hypoxia in a certain type of congenital heart defect and hence prevent inaccurate classification of these patients. The elevated hemoglobin and hematocrit levels are consistent with underlying chronic hypoxia as a compensatory mechanism.
Based on the ACHD Anatomy and Physiological Stage Classification, any patient with complex congenital heart disease (anatomy stage III), which includes Fontan patients, cyanotic congenital heart defect (unrepaired or palliated, all forms), single ventricle, and pulmonary atresia (all forms), should be could be considered high risk for complications related to COVID-19 infection on the basis of decreased functional reserve (3,4). In circumstances when patients are admitted, the treatment management should include symptomatic support, management of respiratory failure, and other signs of end-organ damage. There are no effective therapies for COVID-19 at this point, and for trials of medications including antivirals and other drugs like hydroxychloroquine, caution must be used while using them in patients with congenital heart disease. Clinicians must assess the potential risk versus the uncertain benefits for each individual patient. In cases of mild disease, the patients can be managed by noninvasive forms of supplemental oxygen support. However, patients with severe COVID-19 disease have findings similar to ARDS and often require intubation for improving oxygenation and ventilation. This can be challenging in Fontan patients, as positive pressure ventilation causing increased intrathoracic pressure leads to deleterious effects on the intrapulmonary and intracardiac hemodynamics with decreased pre-load and, ultimately, decreased systemic cardiac output. If intubated, the positive end-expiratory pressure (PEEP) should be within the range to maintain functional residual capacity of the lung and avoid atelectasis and hypoxia-related vasoconstriction. Higher PEEP (9 to 12 cm H2O) has been associated with decreased cardiac index (4). Low respiratory rates, short inspiratory times, low PEEP, and tidal volumes of 5 to 6 ml/kg usually allow adequate pulmonary blood flow, normocarbia, and a low pulmonary vascular resistance. Some studies have shown improvement in exercise capacity and hemodynamics with pulmonary vasodilator therapies for patients with Fontan circulation (5).
As recently reported by Zhang et al. (6), considering the biochemical parameters, elevated levels of infection-related biomarkers and inflammatory cytokines (such as IL-6), neutrophilia and lymphocytopenia (as well as low CD3+ and CD4+ T-cell counts) seem to be correlated with the most severe cases of the infection. Studies have demonstrated a strong correlation between hypertension and nitric oxide (NO), where a high level of blood pressure is a pathological condition characterized by endothelial dysfunction, in which NO availability is impaired with concomitant increased release of IL-6 by the dysfunctional endothelium (7). Extrapolating from the previous points, the COVID-19 virus could determine a more severe cytokines storm (with very high levels of IL-6, but low T cells) in patients in whom the basal levels of cytokines (i.e., IL-6) are higher and NO levels are lower (8). This may be reflective of this patient who had significantly elevated IL-6 levels. Starting from these considerations and focusing attention on the role of IL-6 and NO, we can postulate a possible role for the phosphodiesterase type 5 inhibitors (PDE5-is), such as sildenafil citrate or tadalafil, in increasing the levels of NO. Considering the biochemical mechanisms involved in COVID-19 infection and previous experiences justifying the off-label use of sildenafil citrate (or similar) as antiviral drugs, a possible synergic role of PDE5-i as an early complimentary drug in the treatment of COVID-19 infection should be considered.
Another important aspect of COVID-19 is the presence of abnormal coagulation profile, leading to formation of microthrombi; when combined in a patient with low-flow circuit like Fontan circulation, the outcomes can be catastrophic. The incidence of venous thromboembolism among COVID-19 patients in critically sick patients who are in the intensive care unit appears to be somewhat higher compared with that reported in other studies including such patients with other disease conditions. D-dimer might help in early recognition of these high-risk patients and also predict outcome. Our patient did not have elevated D-dimer through his admission. There is also preliminary data in patients with severe COVID-19 that anticoagulant therapy appears to be associated with lower mortality in the subpopulation meeting sepsis-induced coagulopathy criteria or with markedly elevated d-dimer (9). The predominant predisposing factor is venous stasis, which is often evident in the cavopulmonary circuit in light of the absence of a pump for systemic venous return and pulmonary blood flow. It is now well known that adult patients with Fontan palliation should be considered for long-term anticoagulation (10).
Patients with underlying liver disease and those after liver transplantation represent vulnerable patient cohorts with an increased risk of infection and/or a severe course of COVID-19 (11). Liver involvement in patients with Fontan circulation (also called Fontan-associated liver disease or FALD) is well reported, and the pathogenesis includes elevated central venous pressure, lymphatic congestion, and decreased cardiac output. FALD can manifest as mild elevation of transaminases (AST and ALT), with numerous studies showing that Fontan patients on long-term follow-up had demonstrated at least 1 serological liver abnormality with elevated GGT (γ-glutamyl transferase) being the most common (12). This patient had modest transaminitis likely due to the effects of COVID-19 on his underlying FALD (13).
Follow-Up
The patient was discharged after a 10-day hospitalization. At the time of discharge, his oxygen saturations had improved to 92% to 94% on room air, which was his baseline. His symptoms of shortness of breath had completely resolved. Due to his transaminitis with concerns for FALD, he was scheduled for elective hepatic magnetic resonance elastography-derived liver stiffness to evaluate for the extent of underlying liver fibrosis (14).
Conclusions
The COVID-19 pandemic has acutely affected patients with underlying medical conditions. Evaluation and care by ACHD specialists are mandatory in optimizing management strategies for adults with complex congenital heart disease with COVID-19.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Sugiyama H., Yoo S.J., Williams W., Benson L.N. Characterization and treatment of systemic venous to pulmonary venous collaterals seen after the Fontan operation. Cardiol Young. 2003;13 42–30. [PubMed] [Google Scholar]
- 2.Srivastava D., Preminger T., Lock J.E. Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation. 1995;92:1217–1222. doi: 10.1161/01.cir.92.5.1217. [DOI] [PubMed] [Google Scholar]
- 3.Dori Y., Keller M.S., Rychik J., Itkin M. Successful treatment of plastic bronchitis by selective lymphatic embolization in a Fontan patient. Pediatrics. 2014;134:e590–e595. doi: 10.1542/peds.2013-3723. [DOI] [PubMed] [Google Scholar]
- 4.Williams D.B., Kiernan P.D., Metke M.P., Marsh H.M., Danielson G.K. Hemodynamic response to positive end-expiratory pressure following right atrium-pulmonary artery bypass (Fontan procedure) J Thorac Cardiovasc Surg. 1984;87:856–861. [PubMed] [Google Scholar]
- 5.Oldenburger N.J., Mank A., Etnel J., Takkenberg J.J., Helbing W.A. Drug therapy in the prevention of failure of the Fontan circulation: a systematic review. Cardiol Young. 2016;26:842–850. doi: 10.1017/S1047951115002747. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W., Zhao Y., Zhang F. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calo L., Semplicini A., Davis P.A. Cyclosporin-induced endothelial dysfunction and hypertension: are nitric oxide system abnormality and oxidative stress involved? Transpl Int. 2000;13(Suppl 1):S413–S418. doi: 10.1007/s001470050374. [DOI] [PubMed] [Google Scholar]
- 8.Dal Moro F., Livi U. Any possible role of phosphodiesterase type 5 inhibitors in the treatment of severe COVID19 infections? A lesson from urology. Clin Immunol. 2020;214:108414. doi: 10.1016/j.clim.2020.108414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sathananthan G., Johal N., Verma T. clinical importance of Fontan circuit thrombus in the adult population: significant association with increased risk of cardiovascular events. Can J Cardiol. 2019;35:1807–1814. doi: 10.1016/j.cjca.2019.08.038. [DOI] [PubMed] [Google Scholar]
- 11.Boettler T., Newsome P.N., Mondelli M.U. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Nieuwenhuizen R.C., Peters M., Lubbers L.J., Trip M.D., Tijssen J.G., Mulder B.J. Abnormalities in liver function and coagulation profile following the Fontan procedure. Heart. 1999;82:40–46. doi: 10.1136/hrt.82.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egbe A., Miranda W.R., Connolly H.M. Temporal changes in liver stiffness after Fontan operation: Results of serial magnetic resonance elastography. Int J Cardiol. 2018;258:299–304. doi: 10.1016/j.ijcard.2018.01.108. [DOI] [PubMed] [Google Scholar]