Abstract
Background
Infectious diseases can be transmitted via fomites (contaminated surfaces/objects); disinfection can interrupt this transmission route. However, disinfection guidelines for low-resource outbreak settings are inconsistent and not evidence-based.
Methods
A systematic review of surface disinfection efficacy studies was conducted to inform low-resource outbreak guideline development. Due to variation in experimental procedures, outcomes were synthesized in a narrative summary focusing on chlorine-based disinfection against 7 pathogens with potential to produce outbreaks in low-resource settings (Mycobacterium tuberculosis, Vibrio cholerae, Salmonella spp., hepatitis A virus, rotavirus, norovirus, and Ebola virus).
Results
Data were extracted from 89 laboratory studies and made available, including 20 studies on relevant pathogens used in combination with surrogate data to determine minimum target concentration × time (“CT”) factors. Stainless steel (68%) and chlorine-based disinfectants (56%) were most commonly tested. No consistent trend was seen in the influence of chlorine concentration and exposure time on disinfection efficacy. Disinfectant application mode; soil load; and surface type were frequently identified as influential factors in included studies.
Conclusions
This review highlights that surface disinfection efficacy estimates are strongly influenced by each study's experimental conditions. We therefore recommend laboratory testing to be followed by field-based testing/monitoring to ensure effectiveness is achieved in situ.
Key Words: Fomite, Disinfectant, Chlorination, Epidemic
Contaminated surfaces or objects (“fomites”) can act as vehicles for infectious disease transmission.1 This indirect transmission pathway has been found to play an important role in outbreaks of viral respiratory and enteric diseases, such as avian influenza and norovirus.2, 3, 4 Likewise, contaminated surfaces and needles can contribute to the spread of Ebola in treatment units5, 6, 7 and indirect cholera transmission has been documented in affected communities.8, 9, 10
Cleaning, disinfection, and sterilization can interrupt infectious disease transmission via fomites. Cleaning is “the removal of visible soil from objects or surfaces” and is often used in conjunction with disinfection, defined as “a process that eliminates many or all pathogenic microorganisms, except bacterial spores, on inanimate objects”, while sterilization is “a process that destroys or eliminates all forms of microbial life.”11 , 12 Disinfection interventions in health care facilities, public spaces, or households are usually part of environmental infection prevention and control strategies deployed in response to infectious disease outbreaks.
While a wide range of disinfectants may be used in high-income settings, including chlorine, alcohol-based products, glutaraldehyde, quaternary ammonium compounds, and hydrogen peroxide,12 disinfection in low-income and emergency settings with limited access to basic supplies (“low-resource”) is primarily chlorine-based, as this disinfectant is inexpensive and widely available. Another advantage of chlorine is its broad microbicidal spectrum, while disadvantages include corrosive and irritant properties at high concentrations, potential surface staining, inactivation by organic matter, as well as rapid chlorine decay in solution for some chlorine compounds.12 , 13 Four chlorine compounds are commonly used in emergency response. Sodium hypochlorite (NaOCl), also referred to as household bleach, is widely available as a stabilized (pH 9-11), colorless liquid at concentrations 4,000-6,000 ppm and a shelf-life of at least a month at 25-30°C.13 , 14 Electrolyzed water – or generated NaOCl (gNaOCl) – is produced by electrolysis of a salt (NaCl) solution, has a close-to-neutral pH, and is generated on site, as it expires in days to weeks without stabilization.13 In addition to hypochlorous acid, gNaOCl may contain reactive oxygen species that contribute to disinfection.15 Sodium dichloroisocyanurate (NaDCC) is available as a stable powder; once in solution, however, it has a low pH (∼6) and short shelf-life as it decays rapidly (within hours).13 It has been reported to be less sensitive to inactivation by organic matter than other chlorine compounds.16 Finally, calcium hypochlorite or high-test hypochlorite (HTH) is a salt of hypochlorite, like NaOCl, however it is more commonly found in granular form and produces solutions at pH 9-11 that are as stable as NaOCl.13 , 14
The type and amount of chlorine-based disinfectant used will influence disinfection efficacy. Other parameters that influence disinfection efficacy include14 , 17: (1) the target microorganism's resistance to disinfection; (2) the nature of the fomite to disinfect, for example, porous or nonporous surfaces, and presence of organic matter; (3) characteristics of the tested disinfectant such as ingredients, mode of action and concentration; and, (4) the disinfection protocol, as precleaning, disinfectant mode of application, and exposure time can influence efficacy.
Guidelines on how to clean and disinfect surfaces in infectious disease outbreaks lack consistency. For example, during the 2014 Ebola outbreak, different chlorine exposure times (not specified or up to 15 minutes) and practices (precleaning, covering spills, or direct application) were recommended.18, 19, 20 Recommendations for surface disinfection in cholera outbreaks also vary in terms of chlorine concentration (from 0.1% to 2.0%), exposure time (not specified or up to 10 minutes), and practices (precleaning, spraying, or direct application) – both in health care21, 22, 23 and household settings.23, 24, 25, 26 Additionally, despite the widespread availability of chlorine types with different physicochemical characteristics, outbreak response guidelines do not generally specify which chlorine type should be used to prepare disinfection solutions.
Inconsistencies between guidelines are due, in part, to the lack of evidence to inform disinfection protocols in response to outbreaks, as highlighted during the 2014 Ebola virus disease outbreak.27 , 28 Conducting research in humanitarian settings is challenging, and a recent systematic review found no rigorous evaluation of the field effectiveness or health impacts of surface disinfection in response to outbreaks.29 However, there is a large body of literature on the laboratory-based efficacy of surface disinfection – including studies from different research fields such as hospital infection control, food science, or bioterrorism prevention – that has the potential to inform disinfection interventions in response to outbreaks.
To improve consistency in guidelines and inform recommendations for infectious disease outbreak response interventions in low-resource settings, we conducted a systematic review of laboratory-based surface disinfection efficacy studies. Specifically, we sought to synthesize surface disinfection efficacy data across test organisms, surface types, and disinfectants; to provide evidence-based recommendations for disinfection of pathogens that have the potential to cause large outbreaks in low-resource settings; and to identify and discuss cross-cutting themes and methodological considerations in surface disinfection efficacy studies as well as future research needs.
Methods
We conducted a systematic review of scientific publications assessing chlorine-based surface disinfection efficacy following the PRISMA guidelines and reporting requirements for systematic reviews.30 First, a broad search was performed to identify potentially relevant references, and titles and abstracts were successively screened. Minimal information was extracted from the abstracts and used to refine exclusion criteria prior to full text screening. Surface disinfection efficacy data from eligible full texts were then recorded and a synthesis of disinfection efficacy was prepared.
Eligibility criteria
Criteria were defined following the “Population, Interventions, Comparisons, Outcomes, and Study type” framework. The population for this systematic review consisted of microorganisms on surfaces. The studied intervention was, initially, chemical disinfection. Physical disinfection processes, such as ultraviolet light and heat, were not included. Comparisons were possible between different disinfectants or chlorine compounds; different test organisms; and different surfaces.
The outcome of interest was disinfection efficacy, defined as the proportion of pathogens or surrogate organisms that are inactivated by disinfection, estimated as:
No restriction was placed on study type, however only studies providing a quantitative outcome for surface disinfection efficacy were included. Articles published in English, German, French, Spanish, Portuguese, and Italian were included. Other languages were excluded. No restriction was placed on year of publication.
Information sources and search strategy
Three online databases (PubMed, Scopus, and ISI Web of Knowledge) were searched on March 17, 2017 using specific keywords and strategies, including Medical Subject Headings on PubMed and Index terms on Scopus (Table 1 ). The full search was repeated on May 16, 2018 to include more recent publications. On July 25, 2019, the full search was repeated again, however the screening and data extraction processes were modified to target publications relevant to chlorine disinfection against 7 selected pathogens, as described below. In addition to the online searches, references from review articles identified as potentially relevant were screened for the identification of additional eligible studies.
Table 1.
Search terms used on 3 online databases
| PubMed | Scopus | ISI web of knowledge |
|---|---|---|
| (surfac* OR fomit*) AND (resist* OR surviv* OR inactiv*) AND ((disinfect* OR clean* OR decontamina* OR sanitiz*) OR ("Equipment Contamination"[Mesh] OR "Disinfection"[Mesh] OR "Disinfectants"[Mesh] OR "Disease Outbreaks/prevention and control"[Mesh] OR "Disease Outbreaks/transmission"[Mesh]) OR (“infection control” OR “cross infection”)) |
ALL ((surfac* OR fomit*) AND (disinfect* OR sanitiz* OR clean* OR decontamina* OR "infection control" OR "cross infection") AND (resist* OR surviv* OR inactiv*) AND NOT water) AND INDEXTERMS ((surfac* OR fomit*) AND (disinfect* OR sanitiz* OR cleaning OR decontamina* OR "infection control" OR "cross infection")) |
TS= ((surfac* OR fomit*) AND (disinfect* OR sanitiz* OR clean* OR decontamina* OR "infection control" OR "cross infection") AND (resist* OR surviv* OR inactiv*)) NOT TS=(water) |
Screening and data collection process
Online search results were exported into EndNote X7.7.1 (Thomson Reuters, Canada) and duplicates were removed from the initial search results. References were then screened by title, abstract, and full text in Excel 2016 (Microsoft, Washington), based on the exclusion criteria described below for the initial search and first search update.
Titles that suggested the study would be related to chemical disinfection of a surface were included for abstract screening. Abstracts reporting a quantitative assessment of disinfection efficacy initially passed screening. During abstract screening, information was extracted, if available, on study type, test organism, disinfectant, and surface. This information was used to refine inclusion criteria for full text screening. Studies evaluating the following items were excluded prior to the full text screening: (1) disinfectants including surface coatings and gaseous disinfectants, due to their limited applicability in low-resource settings; (2) living surface materials such as vegetables, meat, or skin, which do not qualify as fomite (inanimate); (3) prions and fungi, and environmental sampling where an uncontrolled amount of test organisms was on the test surface prior to disinfection. Additionally, all studies using disinfectants, surfaces, or test organisms that were recorded less than 5 times during abstract screening as well as disinfectants with multiple active ingredients were excluded prior to full text screening due to the expected limited ability to make comparisons between items used in less than 5 studies and the fact that novel or proprietary disinfectants presented in few studies are unlikely to be available in low-resource settings. In cases where it was unclear from the abstract whether a study was eligible for full text screening, it was included for determination during full text screening.
During full text screening, data were extracted from studies providing a quantitative outcome for chemical surface disinfection efficacy using a clearly defined disinfectant, test organism, and surface not previously excluded. The following parameters were recorded for included studies: test organism, preparation, washing or purification if any, and observed inactivation (eg, reported % or log reduction); surface type; disinfectant type, concentration, pH, mode of application, exposure time, neutralization method, and any available information on its safety, stability, and cost; experimental conditions, including temperature, relative humidity, carrier size, addition of soil load, drying time, mode of recovery, types of controls, positive control level, detection limit, and number of replicates; main study conclusions and comments. Please note all log inactivation reported in this manuscript refer to log10.
For the second search update conducted prior to manuscript submission, title screening criteria remained the same. However, given changes to the data analysis strategy and focus on 7 selected pathogens relevant to low-resource settings (as described below), abstract and full text screening were modified to include only studies that quantitatively assessed surface disinfection efficacy against these 7 pathogens. As previously, in cases where it was unclear based on the abstract whether a study contained relevant data, it was included for full text screening.
Throughout full text data extraction, if results were only presented in graphical format without data labels, corresponding authors were contacted via email to request data labels.
A reason for exclusion was recorded for all studies that were not included after title, abstract, or full text screening. All data entry was performed independently by 2 individuals and cross-checked for accuracy. Any discrepancies were discussed and resolved through consensus.
Quality appraisal
Quality appraisal criteria relevant to microbiological laboratory testing were adapted from Yeargin et al.3 Five criteria were defined: (1) adequate controls: at least positive controls (no disinfectant), and cytotoxicity controls if the test organism is a virus quantified using a culture-based assay; (2) methods clearly described; (3) detection method that includes recovery of etiological agent from surface carrier, disinfectant neutralization, and use of culture or propagation method for quantification; (4) at least duplicate experiments; and (5) appropriate statistical analysis (eg, providing a measure of variability). A pass1 or fail (0) score for each criterion was determined for the studies selected for full text review, giving a maximum score of 5 points. Only studies with a score of 3 or more, considered moderate quality and above, were included in the synthesis.
Data analysis
After data extraction, analysis for this systematic review was articulated in 3 phases: (1) mapping of data points across categories of test organisms, surfaces, and disinfectants; (2) synthesis of efficacy outcomes focused only on chlorine-based disinfection and pathogens relevant to low-resource settings; (3) identification of cross-cutting themes and data on factors influencing surface disinfection efficacy. Each phase is described below in more detail.
First, the numbers of data points (ie, reported efficacy outcomes for 1 test condition) recorded for each combination of test organism (bacteria, viruses, and spores), surface type, and disinfectant were mapped in color-coded tables to visualize research trends – ie, identify which items are the most studied, and which ones remain underresearched. This was also completed with the subset of the data including only chlorine compounds to compare the number of data points available.
Second, the synthesis of disinfection efficacy outcomes for this manuscript was restricted to chlorine-based disinfection, as this is the most widely used disinfectant in low-resource outbreak settings. Efficacy outcomes were stratified according to test organism, surface, and chlorine type, and reported or expressed as log reductions for a given chlorine concentration and contact time (or “CT factor,” defined as the multiplication of both). Summary tables were prepared by type of test organism – gram-positive and gram-negative bacteria, viruses, and spores.
Please note that while our initial intent was to perform a meta-analysis, appropriate statistical methods could not be identified given the characteristics of the extracted data. In particular, aggregation categories could not be defined due to the heterogeneity of testing protocols. Data points were plotted using MATLAB R2017a (The MathWorks, Inc., Natick, MA) to visualize the distribution of reported efficacies by surface type, test organism, and disinfectant.
A more qualitative approach was then selected that was in line with the objectives of the work. We identified studies using pathogens with potential to cause outbreaks in low-resource settings from the list of references processed through abstract screening. These studies were included in a summary table and narrative synthesis. The 7 selected pathogens for which data were available were: Mycobacterium tuberculosis, Vibrio cholerae, Salmonella enterica, hepatitis A virus, rotavirus, norovirus, and Ebola virus. M tuberculosis was responsible for 1.6 million deaths in 2017, making it among the global top 10 causes of death, 95% of which in low-income countries.31 V cholerae causes an estimated 95,000 annual cholera deaths among vulnerable populations worldwide.32 Like V cholerae, Salmonella spp. are gram-negative bacteria responsible for diarrheal disease outbreaks. One serovar is particularly important: S enterica serovar Typhi is estimated to cause 11.9 million cases of typhoid fever and 129,000 deaths per year in low- and middle-income countries,33 and the frequency of prolonged, severe outbreaks has been reported to increase across sub-Saharan Africa.34 The hepatitis A virus produces explosive outbreaks, and causes approximately 1.4 million cases per year, primarily in areas without adequate water, sanitation and hygiene.35 , 36 Rotavirus is responsible for approximately half of the global diarrheal disease burden of 215,000 annual deaths in children under 5 years, concentrated in low-income countries.37 , 38 Norovirus is also a major cause of gastroenteritis worldwide, causing approximately 200,000 deaths per year, primarily in low-income countries and among children under 5 years.39 , 40 Ebola virus is among 5 viral hemorrhagic fever agents on the list of ten priority pathogens identified on the World Health Organization (WHO) Blueprint list,41 and is responsible for an ongoing epidemic in the Democratic Republic of the Congo that has been declared a public health emergency of international concern.42 Studies where a surrogate was proposed for one of the 7 selected pathogens were included in the narrative synthesis as well.
In order to guide recommendations for disinfection against the 7 selected pathogens, the US Environmental Protection Agency (US EPA) efficacy criteria for disinfectants to be used on environmental surfaces were taken as a reference43: if possible based on available data, CT factors that could reliably achieve 4 log reduction in M tuberculosis (or surrogates), 5 log reduction in Salmonella spp and other bacterial pathogens, or 3 log reduction in viral pathogens were identified.
Lastly, information recorded on study conclusions during full text screening was used to identify and inform the discussion of parameters relevant to low-resource outbreak settings and, more broadly, to the understanding of parameters influencing surface disinfection efficacy.
Results
Screening process
A total of 12,730 references were retrieved in the initial (2017) and second (2018) online searches on 3 databases. After removal of 2,118 duplicates, 10,612 titles and 1,985 abstracts were screened (Fig 1 ). During abstract screening, 800 studies were identified as likely to provide a quantitative efficacy assessment of surface disinfection efficacy. Twenty-nine additional studies were found by screening references from relevant reviews. In the second search update (in 2019), which focused on 7 pathogens of interest, 1,096 new publications were identified and 3 studies were included for full text screening. During full text screening, data were extracted from 89 studies, for a total of 1,421 data points. Of these, 716 data points were from 57 studies using chlorine-based disinfectants and that had received a quality score >3. Ultimately, 20 studies were included in the narrative synthesis focusing on 7 selected pathogens relevant to low-resource outbreak settings.
Fig 1.
PRISMA systematic review flow chart.
Distribution of data points
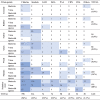
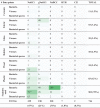
In studies that passed full text screening (1,421 data points), test organisms included bacteria (675 data points, 48%), viruses (495 data points, 35%), and spores (251 data points, 18%) (Table 2 ). The distribution between types of test organisms was similar in studies using chlorine-based disinfectants (Table 3 ): from a total of 716 data points for chlorine-based disinfection, bacteria accounted for 398 data points (56%), viruses for 201 data points (28%), and spores for 117 data points (16%).
Table 2.
Distribution of data points from studies that passed full text. Blue shades intensity increases with increasing numbers
 |
CHX, chlorhexidine; ClO2, chlorine dioxide; GAA, glutaraldehyde; H2O2, hydrogen peroxide; PAA, peracetic acid.
*Including 2 data points from 1 study retrieved during the second search update in 2019.
Table 3.
Distribution of data points included in the synthesis of chlorine-based disinfection efficacy. Green shades intensity increases with increasing numbers
 |
Cl, dilution of a saturated chlorine solution; gNaOCl, electrolyzed water or on-site generated NaOCl; HTH, high-test calcium hypochlorite; NaDCC, sodium dichloroisocyanurate; NaOCl, sodium hypochlorite.
*Including 2 data points from 1 study retrieved during the second search update in 2019.
The most commonly studied surface was stainless steel, with 971 data points (68%) out of 1,421, followed by plastics, wood, glass, fabric, rubber, and ceramic (Table 2). Nonporous surfaces were used in 90% of the tested conditions (1,282 data points). Chlorine-based disinfectants were also studied mostly on nonporous surfaces, with only 12% of the tested conditions (88 data points) on porous surfaces.
Chlorine-based disinfectants were the most studied disinfectants (798 data points, 56%) followed by alcohols (314 data points, 22%). Other disinfectants included glutaraldehyde, hydrogen peroxide, peracetic acid, chlorhexidine, and chlorine dioxide, each of them accounting for less than 5% of all data points. Among chlorine compounds, NaOCl accounted for almost half of the data (339 data points, 47%), followed by NaDCC (218 data points, 30%), and gNaOCl (150 data points, 21%; Table 3). HTH was used in only 2 studies, for a total of 7 data points (1%).
Quality scores
Of the 89 studies that were selected for data extraction after full text screening, almost half (47%, n = 42) had a quality score of 5; a third had a quality score of 4 (34%, n = 30). Seventeen studies (19%) were excluded following quality appraisal, including fifteen that had a score of 3 and 2 with a score of 2 points. Please note studies with a quality score of 3 were included in the narrative summary.
Eighty-five studies (96%) appeared to use appropriate detection methods that included recovery of the etiological agent from surface carrier, disinfectant neutralization, and culture or propagation method for quantitation of the agent; 84 (94%) had adequate controls; and, 82 (92%) performed experiments in duplicate at least. In 16 studies (18%), methods lacked clarity and/or insufficient experimental details were provided, particularly regarding disinfection procedures (eg, disinfectant concentration or recovery). Lastly, 54 (61%) did not obtain the point for “appropriate statistical analysis,” the most common reason being that no measure of variability was provided for the disinfection efficacy outcome.
Synthesis of disinfection efficacy
The editable Excel spreadsheet containing all data extracted from 89 studies (1,421 data points) – including chlorine-based and other disinfectants – is made available in supplementary materials (File S1). Additionally, summary tables for studies that provided a quantitative assessment of chlorine-based disinfection efficacy and had a quality score >3 are presented in supplementary materials for gram-positive bacteria (Table S1), gram-negative bacteria (Table S2), viruses (Table S3), and spores (Table S4).
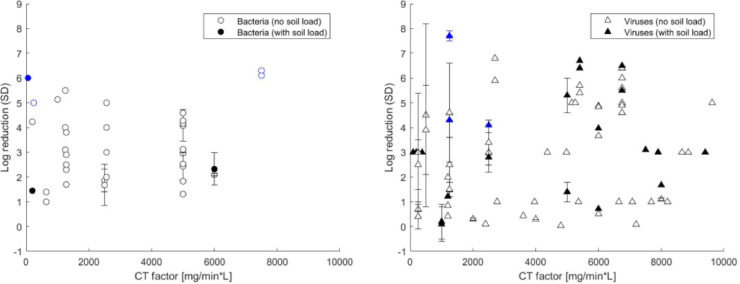
As an example of the observed variability, which precluded meta-analysis, reported log reductions against bacteria and viruses achieved by sodium hypochlorite on stainless steel spread across up to 8 log for a given concentration-exposure time (“CT”) factor (Fig 2 ).
Fig 2.
Reported log reductions for sodium hypochlorite (NaOCl) concentration-exposure time (“CT”) factors up to 10,000 mg min/L against bacteria and viruses. Error bars correspond to standard deviations when reported in the publications. Blue symbols correspond to studies where the limit of detection for the upper bound of the log reduction (y-axis) was reached.
Reported chlorine disinfection efficacy outcomes against the 7 selected pathogens with potential to cause outbreaks in low-resource settings are summarized in Table 4 , and narratively in text below. Please note we found no data in this review to inform surface disinfection efficacy against Shigella spp. or pathogens listed on the WHO Blueprint list of priority pathogens41 other than the Ebola virus.
Table 4.
Selected chlorine disinfection efficacy outcomes against pathogens relevant to low-resource settings
| Test organism | Surface | Study | Disinfectant | Concentration (mg/L) | Exposure time (min) | CT factor (mg × min/L) | Soil load | LRV | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Mycobacterium tuberculosis | Stainless steel | Best et al44 | NaOCl, NaDCC | 6,000-10,000 | 1 | 6,000-10,000 | None | 2.08-3.20 | |
| Best et al44 | NaOCl, NaDCC | 6,000-10,000 | 1 | 6,000-10,000 | Sputum | 2.28-3.22 | |||
| Vibrio cholerae | Aluminum | Calfee et al45 | NaOCl | 6,200 | 15 | 93,000 | None | >6.0 | Spray, pH 6.8 |
| Glass | Calfee et al45 | NaOCl | 6,200 | 15 | 93,000 | None | >6.0 | Spray, pH 6.8 | |
| Carpet | Calfee et al45 | NaOCl | 6,200 | 30 | 186,000 | None | >6.0 | Spray (2x), pH 6.8 | |
| Salmonella spp. | Cotton, fabric | Kusumaningrum et al46 | NaOCl | 800 | 10-30 | 8,000-24,000 | None | 2.0-3.0 | Viscose |
| Ni et al47 | gNaOCl | 40-80 | 5-10 | 200-800 | None | >6.0 | pH 5.70 | ||
| Stainless steel | Riazi et al48 | NaOCl | 128/512 | 5 | 640/2,560 | None | <1.0/5.0 | pH 7.2 | |
| Tuladhar et al49 | NaDCC | 250/1,000 | 20 | 5,000-20,000 | 0.03% BSA | 1.5/2.9 | Wiping | ||
| Tuladhar et al49 | NaDCC | 250/1,000 | 20 | 5,000-20,000 | 1% stool | 1.8/2.9 | Wiping | ||
| Ni et al50 | gNaOCl | 50/70 | 30 | 1,500/2,100 | 20% pig fecal slurry | >5.64 | Spray, pH 6.51-6.56 | ||
| Ni et al50 | gNaOCl | 18 | 30 | 540 | 20% pig fecal slurry | 4.26 | Spray, pH 10.08 | ||
| Hepatitis A virus | Aluminum | Jean et al51 | NaOCl | 3,000 | 5 | 15,000 | None | ∼5 | |
| Plastic | Abad et al52 | NaOCl | 1,250 | 10 | 12,500 | None | 2.58 | Polystyrene | |
| Abad et al52 | NaOCl | 1,250 | 10 | 12,500 | 20% stool | 1.12 | Polystyrene | ||
| Jean et al51 | NaOCl | 3,000 | 5 | 15,000 | None | ∼5 | LDPE, PVC | ||
| Martin et al53 | NaOCl | 10,000 | 30 | 300,000 | 1% BSA, 1% yeast extract | 4.0 | |||
| Stainless steel | Martin et al53 | NaOCl | 10,000 | 30 | 300,000 | 1% BSA, 1% yeast extract | 4.0 | ||
| Jean et al51 | NaOCl | 3,000 | 5 | 15,000 | None | ∼5 | |||
| Sabbah et al54 | NaOCl | 2,500 | 5 | 12,500 | ASTM | 4.41 | |||
| Rotavirus | Plastic | Abad et al52 | NaOCl | 1,250 | 10 | 12,500 | None | 2.76 | Polystyrene |
| Abad et al52 | NaOCl | 1,250 | 10 | 12,500 | 20% stool | 1.62 | Polystyrene | ||
| Stainless steel | Sattar et al55 | NaDCC | 800 | 10 | 8,000 | 10% stool | |||
| Ebola virus | Aluminum | Smither et al56 | NaOCl | 8,000 | 10 | 80,000 | None | >5 | Yambuku variant |
| Seat belt fabric | Smither et al56 | NaOCl | 8,000 | 10 | 80,000 | None | >3 | Yambuku variant | |
| Stainless steel | Cook et al57 | NaOCl | 1,000-5,000 | 5 | 5,000-25,000 | ASTM | >6 | Makona variant | |
| Cook et al58 | NaOCl | 1,000-5,000 | 5 | 5,000-25,000 | ASTM | >6 | Mayinga, Kikwit, Makona variant | ||
| Smither et al59 | NaOCl | 5,000 | 15 | 75,000 | Human blood | 0.9* | Makona variant | ||
| Human norovirus | Ceramic | Park et al60 | gNaOCl | 18.8/188 | 10/1 | 188 | 1% stool | 3.0 | pH 5.5-6.2 |
| Stainless steel | Park et al60 | gNaOCl | 18.8/188 | 5/1 | 94/188 | 1% stool | 3.0 | pH 5.5-6.2 | |
| Tuladhar et al49 | NaDCC | 1,000 | 20 | 20,000 | 1% stool/0.03% BSA | 1.5/1.7 | Wiping | ||
| Moorman et al61 | gNaOCl | 250 ppm | 10-30 min | 2,500-7,500 | None | 1.6-5.0 | pH 7.0 | ||
| Moorman et al61 | gNaOCl | 250 ppm | 10-30 min | 2,500-7,500 | ASTM | <0.3 | pH 7.0 | ||
| Cromeans et al62 | NaOCl | 200-1,000 ppm | 5 min | 1,000-5,000 | 10% FBS | <0.5, <1 | GII.13SP and GI.5SP |
Unclear if results are for aluminum and/or stainless steel.
Mycobacterium tuberculosis
M tuberculosis were used in only 1 study where exposure to 6,000-10,000 ppm NaOCl and NaDCC for 1 minute (CT = 6,000-10,000 mg × min/L) on stainless steel produced 2.08-3.20 log reduction in the absence of soil load and 2.28-3.22 log reductions when sputum was added.44 Best et al44 noted that M tuberculosis is not typically used in disinfection studies due to safety concerns and slow growth, and tested M smegmatitis as a potential nonpathogenic and faster-growing surrogate for M tuberculosis. Varying levels of resistance were observed depending on the tested disinfectant. When exposed to sodium hypochlorite, M smegmatitis appeared less resistant than M tuberculosis, highlighting the need to interpret surrogate testing results with caution.44 Other bacteria from the same genus, M terrae, underwent 8.19 log reduction following exposure to 2,500 ppm sodium hypochlorite for 5 minutes (CT = 12,500 mg × min/L).54 The limited log reduction observed for treatment with CT up to 10,000 mg × min/L for M tuberculosis and the high variation in log reductions for related surrogates highlights the need for further evaluation of effective disinfection strategies. Specifically, there is a data gap on exposure times and concentrations producing CT factors higher than 10,000 mg × min/L against M tuberculosis. Treatments producing CT factors below 10,000 mg × min/L are likely insufficient for M tuberculosis disinfection.
Vibrio cholerae and Salmonella spp
Vibrio cholera and Salmonella spp. are gram-negative bacterial pathogens similar to the more commonly used bacteria Escherichia coli and Pseudomonas spp. The review identified 6 studies investigating disinfection of V cholera (n = 1) and Salmonella spp. (n = 5).
The resistance of V cholerae to chlorine was evaluated in a study focused on improving US preparedness to bioterrorism threats.45 Chlorine (pH-amended bleach, 6,200 ppm, pH 6.8) was dosed by a hand-held sprayer until coupons of glass, aluminum, wood, carpet, and concrete were visibly wetted. Reported reductions were >6 log on glass and aluminum after 15 minutes exposure time (CT = 93,000 mg × min/L) and approximately 5 log on carpet after 2 disinfectant applications and 30 minutes exposure time (CT = 186,000 mg × min/L). Efficacy could not be evaluated on wood and concrete due to poor recoveries.
The studies investigating surface disinfection efficacy of Salmonella spp. included 4 studies on Salmonella enterica serovar Enteritidis and 1 on S Typhimurium. Exposure of S Enteritidis to NaOCl (800 ppm) resulted in 3 log reduction after 30 minutes on viscose without soil load (CT = 24,000 mg × min/L).46 On stainless steel, Riazi et al48 reported increasing log reductions (up to 5 log) in S Enteritidis with increasing NaOCl concentrations (up to 512 ppm) applied for 5 minutes in absence of soil load (CT = 2,560 mg × min/L), whereas Tuladhar et al,49 who assessed the effect of wiping a surface contaminated with S Enteritidis with a cloth soaked in NaDCC (1,000 ppm), observed a maximum inactivation of 2.9 log after 20 minutes (CT = 20,000 mg × min/L). In contrast, Ni et al47 , 50 observed >6 log reduction in S Typhimurium after applying electrolyzed water (gNaOCl, 40-80 ppm, pH 5.7-6.5) on fabric without soil load for 5 minutes (CT = 200-400 mg × min/L) and >5.6 log reduction when gNaOCl was sprayed on stainless steel with a longer exposure time (30 minutes, CT = 1,200-2,400 mg × min/L) in presence of 20% pig slurry.
E coli and P aeruginosa are 2 gram-negative bacteria widely used in surface disinfection efficacy testing and results from studies using these bacteria may provide insight into disinfection of other gram-negative bacteria including V cholerae and Salmonella spp. (Table S2). Relatively high log reductions (4.7 to >7.3) were reported for E coli across a range of surfaces and CT factors (200-50,000 mg × min/L). The CT factors reported to achieve 5 to >6 log reductions in the only study with V cholerae identified in this review were much higher (93,000-186,000 mg × min/L) than those observed to achieve similar log reduction values (LRVs) in E coli.45 However, in experiments using NaDCC on 6 different surfaces (CT = 200-2,000 mg × min/L), we observed consistently higher reductions in culturable V cholerae (5.4-7.0 log) compared to E coli (1.4 to >6.9).63 E coli and Salmonella spp. exhibit similar sensitivities to gNaOCl.47 , 50 While less than 3 log reductions were observed in Salmonella spp. in 2 studies, with CT factors between 20,000 and 24,000 mg × min/L for NaOCl and NaDCC,46 , 49 Riazi et al48 reported 2, 4, and 5 log reductions in P aeruginosa, E coli, and S Enteritidis, respectively, exposed to NaOCl (CT = 2,560 mg × min/L).
Notably, high variation in LRVs are observed between studies investigating gram-negative bacteria, with no clear relationships to CT factors, in line with findings for all bacteria (Fig 2). Nevertheless, conservative approaches to disinfection (use of high chlorine concentrations with long exposure times) could be selected. For example, CT factors on the order of 200,000 mg × min/L could be targeted for disinfection in cholera outbreaks; however, as this may be challenging to implement in practice and data suggest that lower CT factors may be efficacious, future disinfection efficacy assessments should test lower CT factors against V cholerae. For Salmonella spp., using CT factors >24,000 mg × min/L is likely necessary to ensure 5 log reduction and further studies are needed to confirm the efficacy and reliability of gNaOCl at low CT factors (<3,000 mg × min/L) against gram-negative bacteria.
An additional complication with gram-negative bacteria is the ability to enter a viable but nonculturable (VBNC) stage where they lose the ability to grow on culture media but remain infectious and can withstand greater oxidative stress.64 Recent results from our laboratory and the broader literature suggest that disinfection efficacy may be overestimated for bacteria that can enter VBNC, which includes V cholerae, M tuberculosis, and other pathogenic bacteria.63, 64, 65, 66 This was not investigated in studies identified through this review. We recommend further research be conducted to better understand the resistance of VBNC pathogenic bacteria to surface disinfection and their relevance to disease transmission.
Hepatitis A virus and rotavirus
The hepatitis A virus and rotavirus are 2 viruses causing frequent outbreaks of enteric disease.35 , 38 The hepatitis A virus was used in 4 and rotavirus in 2 surface disinfection studies identified in this review.
In the first study using the hepatitis A virus, 1.12 and 2.58 log reductions were observed after 10 minutes exposure to 1,250 ppm NaOCl (CT factor = 12,500 min × mg/L) on polystyrene with and without 20% stool, respectively.52 On stainless steel, using the American Society for Testing and Materials (ASTM) standard soil load, the hepatitis A virus was exposed to 2,500 ppm for 5 minutes (CT factor = 12,500 min × mg/L), resulting in a 4.4 log reduction.54 In another study using polystyrene and stainless steel carriers, a concentration of 1% NaOCl (10,000 ppm) was required to achieve 4 log reduction in 30 minutes (CT = 300,000 min × mg/L) in the presence of 1% bovine albumin and 1% yeast extract, and it was noted that the hepatitis A virus was particularly resistant to drying.53 Lastly, sodium hypochlorite was identified as the most efficacious disinfectant in 1 study using the hepatitis A virus on stainless steel, aluminum, copper, high-density polyethylene and polyvinyl chloride, with approximately 5 log reduction after 5 minutes exposure to 3,000 ppm NaOCl (CT = 15,000 min × mg/L) across all materials.51 Please note the last 2 studies were initially excluded from the review due to a lack of details in the methods regarding procedures and data analysis (quality scores of 3), but were included in this narrative summary.
Human rotavirus was used as test organism in 2 studies included in this review. With 10% stool, 1.7 log reduction was observed after 10 minutes exposure to 800 ppm NaDCC (CT = 8,000 mg × min/L) on stainless steel.55 On plastic carriers, after 10 minutes exposure to 1,250 ppm NaDCC (CT = 12,500 mg × min/L), 2.8 and 1.6 log reduction in human rotavirus occurred without soil load and with 20% stool, respectively.52
In a study comparing hepatitis A virus, human rotavirus, and bacteriophage B40-8 exposed to NaOCl on polystyrene (CT =12,500 mg × min/L), log reductions were lower for B40-8 (0.7-1.3 log) compared to hepatitis A virus (1.1-2.6 log) and human rotavirus (1.6-2.8) but the differences were not statistically significant.52 Wiping a stainless steel surface with 1,000 ppm NaDCC reduced simian rotavirus by 4.2-5.7 log after 20 minutes (CT = 20,000 mg × min/L) under “clean” (0.03% BSA) and “dirty” (1% stool) conditions.49
These results overall suggest that CT factors higher than 15,000 mg × min/L (and possibly as high as 300,000 mg × min/L on soiled surfaces) should be targeted for disinfection of the hepatitis A virus. The 2 human rotavirus studies included in this review reported a maximum of 2.8 log reduction after exposure to a CT factor of 12,500 mg × min/L; targeting at least 20,000 mg × min/L may be sufficient to achieve 3-log reduction based on surrogate testing, however further confirmation is required given the limited evidence available.
Norovirus
Human norovirus was used in 4 studies, all of which used RNA-based assays to assess disinfection efficacy because no culture-based infectivity assay is available.
Park et al60 noted that 1 minute exposure to gNaOCl (pH 5.6-6.2) at 188 ppm (CT = 188 mg × min/L) and would be sufficient to achieve 3 log reduction on both ceramic and stainless steel with 1% stool as soil load, while Moorman et al61 reported only 1.3 and <0.3 log reduction with and without ASTM standard soil load, respectively, after exposure to 250 ppm gNaOCl at pH 7.0 for 10 minutes on stainless steel (CT = 2,500 mg × min/L). Increasing exposure time only had an effect in absence of soil load, with 5.0 log inactivation in human norovirus reported after 30 minutes (CT = 7,500 mg × min/L).61 Cromeans et al62 noted limited reductions in human norovirus dried on stainless steel after exposure to 1,000 ppm NaOCl for 5 minutes (CT = 5,000 ppm), with <0.5 log reduction in GII.13 norovirus and <1.0 log reduction GI.5 norovirus in presence of 10% fetal bovine serum as soil load. Tuladhar et al49 observed 1.5 (with 1% stool) and 1.7 (without soil load) log reductions 20 minutes after wiping stainless steel with a cloth soaked in 1,000 ppm NaDCC (CT = 20,000 mg × min/L).
Several surrogates have been proposed for human norovirus to overcome the absence of infectivity assay, including murine norovirus and MS2,3 , 15 , 49 , 60 , 62 , 67, 68, 69, 70 feline calicivirus,3 , 62 , 67, 68, 69, 70, 71, 72 and Tulane virus.61 , 62 Comparisons between human norovirus and the proposed surrogates were made in 4 studies. Park and Sobsey69 determined that similar times (CT = 94-380 mg × min/L) were required for a 3-log inactivation in human norovirus, murine norovirus, and MS2 exposed to gNaOCl on stainless steel and noted that MS2 resembles human norovirus in terms of persistence and elution from surfaces. Moorman et al61 also tested gNaOCl (CT = 250-7,500 mg × min/L) on stainless steel and observed similar inactivation in Tulane virus (3.0-4.1 log) and human norovirus (1.6-5.0 log) with increasing exposure times in absence of soil load. Cromeans et al62 observed that murine norovirus and Tulane virus were similarly resistant to human norovirus GII (<0.5 log reduction in RNA levels) and more resistant than feline calicivirus (>2.5 log reduction) to NaOCl on stainless steel (CT = 1,000-5,000 mg × min/L). However, Tuladhar et al49 observed inconsistencies in the correlation between murine norovirus and human norovirus inactivation in experiments where NaDCC was applied on by wiping stainless steel (CT = 5,000-20,000 mg × min/L).
Overall, no single virus stands out as a particularly good surrogate for human norovirus in this review and evidence to support chlorine-based disinfection efficacy against human norovirus remains weak; gNaOCl appears as an interesting option but with inconsistent results reported so far. Further testing of gNaOCl to verify reproducibility and alternative disinfectants should be considered. In the absence of additional data to refine estimates, disinfection for norovirus should be conservative and rely on combinations of chlorine concentrations and exposure times that yield CT factors substantially greater than 20,000 mg × min/L in order to achieve at least 3 log reduction.
Ebola virus
The Ebola virus was evaluated in 4 surface disinfection studies, all published following the 2014 West African Ebola outbreak. In the first study, the Ebola virus Makona variant was exposed to 1,000-5,000 ppm NaOCl on stainless steel for 1 and 5 minutes in presence of the ASTM standard soil load and complete inactivation (>6 log reduction) was observed after 5 minutes at both concentrations (CT = 5,000-25,000 mg × min/L).57 In the second study, similar experimental procedures were used to compare 3 Ebola virus variants, including the Makona variant, isolated during the 2014 West African outbreak, the Mayinga variant, from the first reported Ebola outbreak, in 1976 in Zaire (now DRC), and the Kikwit variant, from an outbreak in 1995. Concentrations of 1,000-5,000 ppm NaOCl achieved complete inactivation of all 3 virus variants within 5 minutes, as observed in the previous study, however the Makona variant was more resistant to lower chlorine concentrations (0.05%-0.1%) than previous variants of the Ebola virus.58 In a third study, where aircraft-relevant surfaces were used, sodium hypochlorite (8,000 ppm, with <1% potassium permanganate) achieved complete inactivation – ie, >5 log reduction on painted aluminum, >3 log reduction on seat-belt strapping material – of the Ebola Yambuku variant (from the 1976 outbreak in the DRC) after 10 minutes (CT = 80,000 mg × L/min).56 In contrast to these 3 studies, incomplete inactivation of the Ebola virus (Makona variant) in a human blood matrix after exposure to 5,000 ppm NaOCl for 15 minutes on aluminum and stainless steel was reported in a recent publication, with a reduction of only 0.9 log compared to complete inactivation when the virus was mixed with cell growth medium.59
In a comparison of 4 bacteriophages, the enveloped Phi6 was found to undergo slightly lower inactivation (4.1 log) than that reported for the Ebola virus when exposed to NaOCl (5,000 ppm) on stainless steel for 5 minutes (CT = 25,000 mg × min/L) and was therefore proposed as an appropriate surrogate for surface disinfection efficacy testing for Ebola settings.73 Further testing using Phi6 showed complete inactivation on 3 surface types following a 15-minute exposure to 5,000 ppm (CT = 75,000 mg × min/L) NaOCl, gNaOCl, HTH, and NaDCC, independently of chlorine type, soil load, and mode of application.63
As complete inactivation of the Ebola virus and surrogate was reported in all studies where testing was conducted without blood (CT = 5,000-80,000 mg × min/L), a conservative recommendation would be to target at least 80,000 mg × min/L for disinfection in Ebola settings when there is no visible blood on surfaces. Alternative disinfectants should be investigated for the disinfection of blood spills from Ebola patients.
Parameters influencing disinfection efficacy
We identified 4 parameters influencing disinfection efficacy: disinfectant application mode, chlorine compound used, surface type, and soil load. Data relevant to each of them are summarized hereafter, followed by a discussion of experimental parameters that likely influenced efficacy results reported in the laboratory studies included in this review.
Disinfectant application mode
Disinfectant application mode, including immersion, spraying, and wiping, is one of the parameters influencing efficacy and a recurrent theme in studies included in this review. Of the 89 studies investigated, the most common disinfectant application modes were pipetting (n = 54, 61%), immersion (n = 20, 22%), spraying (n = 8, 9%), or wiping (n = 5, 6%). Because disinfection is often combined with cleaning procedures, wiping was investigated and was found to have an effect on viruses and spores even in absence of disinfectant, suggesting that the mechanical action of wiping contributes to reducing contamination levels on surfaces.49 , 74 With spraying, ensuring contact between disinfectant and test organisms can be challenging.75 , 76 Additionally, chlorine loss during the process – from spray nozzle to the targeted surface – is a concern.77 Ni et al (2016) found consistent increases in efficacy with increasing disinfectant spraying time from 0.5 to 2 minutes and keeping similar exposure times after spraying. A proposed explanation for variable efficacies observed between studies is the use of different spraying equipment, such as gas-powered pressurized sprayers producing high spray velocities and hand-held spray bottles.78
Although there are likely differences in the impact of disinfectant application mode on efficacy, only 3 studies directly compared application modes.79, 80, 81 Immersion into pH-amended bleach (6,000-6,700 ppm, pH 6.8) was observed to result in higher log reductions compared to spraying, particularly on porous surfaces such as carpet, paper, and wood.81 In contrast, a comparison of wiping and spraying showed similar efficacies against C difficile spores, though spraying was considered less appropriate for health care settings as it required extended drying times and would not remove dirt and debris.74
Lastly, Park et al80 noted an increase in efficacy when surface carriers were subject to shaking after immersion into electrolyzed water, with >3.12 log reduction in S aureus achieved within 5 minutes exposure to electrolyzed water (52.8 ppm, pH 2.55), compared to 1.50-1.67 log reduction without shaking.
Chlorine compounds
Five studies directly compared chlorine compounds, and showed that available chlorine compounds generally achieve similar disinfection efficacies on surfaces. Bloomfield et al82 reported lower LRVs following a 5-minute exposure to 250 ppm NaDCC compared to NaOCl at the same concentration against S aureus (2.4 vs 4.9 to >6.2 log reduction), P aeruginosa (3.7 vs 3.7-4.3 log reduction), and E faecium (2.2 vs 3.1 log reduction) on stainless steel. At 2,500 ppm, both NaDCC and NaOCl achieved at least 6 log reduction in each test organism. Gallandat et al83 observed similar efficacies of NaOCl, gNaOCl, NaDCC, and HTH (5,000 ppm) against both E coli and Phi6 after 10-15 minutes on 3 nonporous surfaces, with minimum 5.9 and 3.1 log reductions, respectively. At higher concentrations, Aarnisalo et al84 observed 3.1 and 2.2 log reductions (without/with 2% pork meat) in L monocytogenes after 30 seconds exposure to 22,400 ppm NaDCC and >3.6 and 2.5 log reductions (without/with 2% pork meat) after 30 seconds exposure to 27,000 ppm NaOCl. Lombardi et al85 reported slightly lower efficacies of NaOCl compared to HTH against low pathogenic avian influenza virus on stainless steel (3.1 vs 3.7 log reductions) and wood (0.0 vs 1.9 log reductions) after 10 minutes exposure to 750 ppm. Julian et al15 found that electrolyzed water and household bleach (NaOCl) were also similar against murine norovirus and MS2 using 500-2,500 ppm for 30 seconds (Table S3).
Surface type
As previously described, identified studies tested a variety of surface materials. Overwhelmingly, the most common material was stainless steel. Among the 89 studies identified, 27 (30%) tested more than one surface, including 13 (15%) that used porous surfaces (ceramic, fabric, rubber or wood). Porous surfaces were identified as more challenging to disinfect in 8 of these 13 studies. For chlorine-based disinfection in particular, Baek et al86 observed lower inactivation in E coli on wood (3.34 log) and rubber (4.69 log) compared to nonporous surfaces such as stainless steel, glass, and polyethylene (5.05-5.18 log) after exposure to NaOCl. While Park et al80 found no difference in gNaOCl efficacy against S aureus on 5 nonporous surfaces (1.7-1.9 log), Kim et al87 reported consistently lower log reductions in S aureus on scratched polyethylene, polypropylene, glass, and stainless steel compared to the same surfaces without scratches after disinfection with NaOCl. The influence of surface structure on disinfection is further affirmed with observations in another study that E coli was more challenging to disinfect on heavy duty tarp compared to smoother surfaces like nitrile and stainless steel.83 Lombardi et al85 observed lower log reductions in low pathogenic avian influenza virus on wood (0.0-1.9) compared to metal (3.1-3.7) and plastic (3.9-4.3) surfaces after exposure to 750 ppm NaOCl or HTH for 10 minutes. Julian et al15 compared gNaOCl and NaOCl on polyvinyl chloride and stainless steel; surface type was statistically significant for murine norovirus inactivation (overall higher on polyvinyl chloride) but not for MS2. Yeargin et al88 also found that glass and polyester were easier to disinfect than cotton using 5,000 ppm NaOCl with a 5-minute exposure time, with 4.5-5.5 log reduction in murine norovirus and feline calicivirus on glass, 4.3-5.1 log reduction on polyester, and 3.1 log reduction on cotton. Additionally, Yeargin et al88 noted that surface porosity and hydrophobicity impacted both disinfection efficacy and viral recovery for testing, with the latter potentially biasing disinfection efficacy estimates.
Soil load
Chlorine is inactivated by organic matter14 and, as a consequence, the selection of a soil load for surface disinfection efficacy testing can affect observed results. A wide range of matrices representing soiled surfaces were used in included studies, from the standard ASTM formulation with tryptone, bovine serum albumin, and bovine mucin to human stool, serum, or shrimp and pork meat (Tables S1-S4). The impact of specific matrices used to represent soil loading on observed efficacies are unclear from this review, with the notable exception of the results reported above for the Ebola virus, which was more resistant to disinfection in human blood compared to other testing media.59 Given the known impacts of chlorine demand on chlorine disinfection efficacy, the large variation in impacts of soil loading on efficacy is surprising. However, 1 potential explanation is the variation in chlorine demand of chosen specific matrices. For example, the formulation recommended in the ASTM standard has a low chlorine demand, and as such may not be appropriate to assess chlorine-based disinfection against pathogens that are shed in bodily fluids such as vomit and stool. 83 The soil load selection is a good example of the trade-off needed between testing conditions that are relevant to real-world applications (eg, working with surfaces in contact with seafood) and generating efficacy data that can be compared to the existing literature and support the development of guidelines.
Experimental parameters
One finding from this systematic review is that, while there is a large body of literature on surface disinfection efficacy with a majority of high-quality studies, experimental protocols, and observed surface disinfection efficacies are highly variable between studies, including the use of different preparation methods for the test organisms (eg, washing bacteria); inoculation methods (eg, liquid vs dry aerosols for spores); surface carrier size (from <1 to 100 cm2); drying times (from no drying to 24 hours) and conditions (temperature, relative humidity); disinfectant application mode (eg, immersion, spraying, or wiping), concentration and exposures time; soil loads; neutralization procedures and media; and recovery methods postdisinfection (eg, vortexing, sonicating, swabbing, and scraping were described).
Discussion
In this systematic review, we extracted 1,421 data points from 89 potentially relevant studies and found that: (1) there is high variability and no clear trends in reported disinfection efficacy of outbreak-relevant pathogens and surrogates; (2) this high variability likely reflects inconsistent and highly variable testing procedures; (3) the majority of included studies evaluated chlorine-based disinfection of stainless steel surfaces, which have limited relevance to low-resource outbreak settings, and pathogens with potential to produce outbreaks in low-income contexts remain underresearched; (4) improvements in consistency, reproducibility, and reporting are necessary in surface disinfection efficacy studies; and (5) the selected qualitative approach to synthesize surface disinfection efficacy data from pathogen and surrogate testing nevertheless informs recommendations for disinfection in outbreak settings.
We identified 20 studies using pathogens particularly relevant to low-resource settings in surface disinfection efficacy testing: M tuberculosis, 1 V cholerae, 1 Salmonella spp.,5 hepatitis A virus,4 rotavirus,2 human norovirus,3 and the Ebola virus.4 Given the variation in study findings, we advocate conservative approaches to field disinfection relying on high CT factors for chlorine, driven largely by the assumption that chlorine-based disinfectants are readily available. We recommend following pathogen-specific target CT factors rather than aggregating pathogens by type (ie, bacteria, virus, and protozoa), as we believe that the latter would misrepresent the complexity of surface disinfection efficacy data. Specifically, the data extracted in this review support use of the following conservative target CT factors: >15,000 mg × min/L for the hepatitis A virus, >20,000 mg × min/L for rotavirus and norovirus, and >80,000 mg × min/L for the Ebola virus, as relatively consistent evidence – although from small numbers of studies – was available for these pathogens. Data for gram-negative bacteria exhibited particularly high variability, leading to a cautious (and possibly unrealistic) recommendation of 200,000 mg × min/L for V cholerae and >24,000 mg × min/L for Salmonella spp. Insufficient data were available to formulate a recommendation for disinfection of M tuberculosis. The appropriate combination of chlorine concentration and exposure time to achieve a given CT factor should be selected depending on safety concerns (as high chlorine concentrations may be irritant and corrosive) and practical considerations with regards to applicable exposure times, in compliance with relevant environmental and safety guidelines. Additional rigorous research is needed to better resolve (lower) recommendations for specific pathogens to ensure adequate protection of human health. Furthermore, studies to inform disinfection against the WHO Blueprint priority viruses41 other than the Ebola virus are needed.
Submission of this manuscript coincided with the COVID-19 pandemic89; surface disinfection efficacy data for coronaviruses and potential surrogates was synthesized in the recent systematic review by Kampf et al,90 where it is noted that exposure of human coronavirus (E229) to 1,000 ppm NaOCl for 1 minute (CT = 1,000 mg × min/L) achieved >3 LRV. In line with the findings in our review, all relevant studies identified by Kampf et al90 were carried out using stainless steel surface carriers, thus highlighting the need for further research to be conducted on other surface types.”
In this review, we also identified themes relevant to outbreak response in surface disinfection efficacy evaluations, including the influence of disinfectant application mode, selected chlorine compound, surface type, and soil load. Each of these is briefly summarized hereafter. Increases in observed inactivation with mechanical action (shaking during immersion, wiping) were reported, as well as specificities of spraying (equipment, spray characteristics, and spraying time matter). Comparisons between the 4 chlorine compounds typically available in emergency response suggest no clear difference in terms of efficacy. Several studies mentioned that porous surfaces are more challenging to disinfect – and to use in laboratory testing – than nonporous surfaces, which is a concern in outbreaks in health care facilities, where mattresses, linens, cloths may need to be disinfected, and in households, where an even wider range of porous materials (wood, ceramic, and carpets) may be found. Soil load is expected to impact chlorine disinfection efficacy, as chlorine is inactivated by organic matter, though this review did not observe systematic reductions in efficacy due to soil load. This may be due to the wide range of substances used as soil loads. Notably, no matrix was identified that is known to accurately mimic bodily fluids excreted by cholera or Ebola patients and the development of appropriate soil load mixtures for laboratory testing is still needed.
Surface disinfection efficacy testing standards such as the ASTM Quantitative Disk Carrier test method91 recommend the use of 1-cm stainless steel disks as surface carriers and chlorine is a widely available disinfectant that has been used for over a century.92 It is therefore not surprising that most studies focus on stainless steel and chlorine. What is striking, however, is the variability in reported efficacies for similar test organisms, surface types, and disinfectants (Fig 2), which made it infeasible to define appropriate aggregation categories to perform a meta-analysis. We hypothesize that this variability reflects the variability in testing procedures, which was also identified as a concern in large-scale, interlaboratory studies for the development of standard methods, where researchers advocated for the use of large numbers of replicates.79 , 82 , 93 For the registration of bactericidal disinfectants, the US EPA requires tests to be carried out following the Association of Official Analytical Collaboration International standard methods using 60 surface carriers on at least 3 different days, with a qualitative outcome after disinfection (positive/negative),43 while the quantitative ASTM standard method requires triplicate controls and a minimum of 5 replicates.91 In this review, the average number of replicates for data points that passed full text screening was 3.9. A quantitative decision-making framework has recently been proposed by Parker et al94 to determine whether the achieved reproducibility for surface disinfection efficacy experiments is acceptable, and we recommend this be more widely adopted in reporting surface disinfection laboratory efficacy results. Lastly, the data extraction spreadsheet (available in Supplementary Materials) includes 1,421 data points that can be filtered by any user depending on the test organism, surface, or disinfectant of interest. We believe this database is a useful tool for those who are designing surface disinfection experiments or searching for specific disinfection efficacy data. This review focused on chlorine-based disinfectants because of their widespread availability and use in low-resource settings, however data were also extracted from studies using other disinfectants (Table 2) and our dataset could be further analyzed to synthesize surface disinfection efficacy outcomes for alternative disinfectants or include additional test organisms.
We recommend further research that integrates components from existing standards (eg, stainless steel) for methods validation, while expanding testing to conditions relevant to low-resource settings (eg, porous surfaces, spraying). In view of the variability in efficacy outcomes observed in this review, replicating experiments over different days or periods of time to account for within-laboratory differences and changes in the resistance of test organisms, as suggested by Bloomfield et al,93 seems sensible. The following experimental information should be reported at a minimum: test organism preparation (eg, washing, growth phase); surface inoculation method, including soil load and test organism concentration, drying time and conditions (eg, temperature, relative humidity); surface carrier size; disinfectant ingredients, preparation, storage, concentration, application mode and exposure time; recovery methods; number of replicates (for both test and control carriers) and time of testing.
Besides the inability to conduct a meta-analysis, the work presented herein had several limitations. While LRVs are the most widespread measure of disinfection efficacy, they are sensitive to the inoculation level and recovery of test organisms in positive controls,81 , 93 which can affect comparisons between studies. A second limitation is the reliance on study descriptors (test organisms, surfaces, and disinfectants) during abstract screening to refine full text inclusion criteria, which may have resulted in the exclusion of some studies relevant to low-resource settings. Similarly, relevant studies published in languages other than those understood by our review team may have been missed. Furthermore, the requirement to only include disinfectants that have 5 or more relevant publications may have limited our ability to identify novel disinfectants potentially usable in outbreak response. Additionally, this systematic review primarily focused on the scientific literature. Alternative approaches such as screening US EPA-registered disinfectants for ease of use, cost, and international availability may allow identification of other relevant information for the development of recommendations for disinfection in low-resource settings.
Conclusions
The variability in reported disinfection efficacies between the studies included in this review highlights how challenging it may be for policy makers to use laboratory results to develop recommendations for surface disinfection in outbreaks, particularly in low-resource contexts. We therefore recommend that laboratory experiments increase reproducibility, for example through replicating experiments over different days or periods of time. More importantly, the high variation in the well-controlled laboratory experiments highlights the need for demonstrating sufficient efficacy under field conditions. We therefore strongly recommend field-based testing and monitoring to ensure effectiveness is achieved in situ. An efficient strategy for laboratory testing could be to conduct studies comparing priority pathogens to test organisms that have been widely studied, such as S aureus and P aeruginosa, feline calicivirus, murine norovirus and MS2, or Bacillus spp., so that existing data (available in Supplementary Materials) could be extrapolated to inform surface disinfection guideline development in low-resource settings with minimal additional laboratory testing.
Acknowledgments
We are thankful to Julia Fasse, Mark Morton, Magnifique Mukundwa, Nathan Pacheco, Alyssa Rose, and Kevin Sung for their assistance with screening and data extraction.
Footnotes
Funding: This work was supported by funding from the Swiss National Science Foundation Doc.Mobility program (grants P1SKP2_174771, P1SKP2_181335) and ELRHA/R2HC Research for Health in Humanitarian Crises.
Conflicts of interest: None to report.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2020.05.014.
Appendix. SUPPLEMENTARY MATERIALS
File S1: link to download our dataset from Open Science Framework and Tables S1-S4
Table S1: Reported chlorine disinfection efficacy outcomes for gram-positive bacteria used in at least 2 studies with a quality score >3.
Table S2: Reported chlorine disinfection efficacy outcomes for gram-negative bacteria used in at least 2 studies with a quality score >3.
Table S3: Reported chlorine disinfection efficacy outcomes for viruses used in at least 2 studies with a quality score >3.
Table S4: Reported chlorine disinfection efficacy outcomes for bacterial spores used in at least 2 studies with a quality score >3.
References
- 1.Julian TR. Stanford University; 2010. Fomites in Infectious Disease Transmission: A Modeling, Laboratory, and Field Study on Microbial Transfer Between Skin and Surfaces [Internet]https://stacks.stanford.edu/file/druid:cf347cn1097/Julian_Dissertation_v2Dec2010_AB2-augmented.pdf [cited 2017 Jan 19]. Available at: [Google Scholar]
- 2.Boone SA, Gerba CP. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73:1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeargin T, Buckley D, Fraser A, Jiang X. The survival and inactivation of enteric viruses on soft surfaces: a systematic review of the literature. Am J Infect Control. 2016;44:1365–1373. doi: 10.1016/j.ajic.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 4.WHO . 2007. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Diseases in Health Care: WHO Interim Guidelines.http://apps.who.int/iris/bitstream/10665/112656/1/9789241507134_eng.pdf?ua=1 Available at: [PubMed] [Google Scholar]
- 5.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osterholm MT, Moore KA, Kelley NS, et al. Transmission of Ebola viruses: what we know and what we do not know. mBio. 2015;6 doi: 10.1128/mBio.00137-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youkee D, Brown CS, Lilburn P, et al. Assessment of environmental contamination and environmental decontamination practices within an Ebola Holding Unit, Freetown, Sierra Leone. PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0145167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugimoto JD, Koepke AA, Kenah EE, et al. Household transmission of Vibrio cholerae in Bangladesh. PLoS NTD. 2014;8:e3314. doi: 10.1371/journal.pntd.0003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukandavire Z, Liao S, Wang J, Gaff H, Smith DL, Morris JG. Estimating the reproductive numbers for the 2008–2009 cholera outbreaks in Zimbabwe. Proc Natl Acad Sci. 2011;108:8767–8772. doi: 10.1073/pnas.1019712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukandavire Z, Morris JG. Modeling the epidemiology of cholera to prevent disease transmission in developing countries. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.VE-0011-2014. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4634708/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block SS. 5th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. Disinfection, Sterilization and Preservation. [Google Scholar]
- 12.Rutala WA, Weber DJ, HICPAC . Centers for Disease Control and Prevention; 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities.https://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf Available at: [Google Scholar]
- 13.Iqbal Q, Lubeck-Schricker M, Wells E, Wolfe MK, Lantagne D. Shelf-life of chlorine solutions recommended in Ebola virus disease response. PLOS ONE. 2016;11 doi: 10.1371/journal.pone.0156136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutala WA, Weber D. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Rev. 1997;10:597–610. doi: 10.1128/cmr.10.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julian TR, Trumble JM, Schwab KJ. Evaluating efficacy of field-generated electrochemical oxidants on disinfection of fomites using bacteriophage MS2 and mouse norovirus MNV-1 as pathogenic virus surrogates. Food Environ Virol. 2014;6:145–155. doi: 10.1007/s12560-014-9136-6. [DOI] [PubMed] [Google Scholar]
- 16.Coates D. Comparison of sodium hypochlorite and sodium dichloroisocyanurate disinfectants: neutralization by serum. J Hosp Infect. 1988;11:60–67. doi: 10.1016/0195-6701(88)90040-0. [DOI] [PubMed] [Google Scholar]
- 17.Russell, Hugo, Ayliffe . 5th ed. Wiley-Blackwell; 2013. Principles and Practice of Disinfection, Preservation and Sterilization.https://www.wiley.com/en-us/Russell%2C+Hugo+and+Ayliffe%27s+Principles+and+Practice+of+Disinfection%2C+Preservation+and+Sterilization%2C+5th+Edition-p-9781444333251 Available at: [Google Scholar]
- 18.WHO. Manual for the care and management of patients in Ebola care units. 2015. Available at: http://www.who.int/csr/resources/publications/ebola/patient-care-CCUs/en/. Accessed June 8, 2020.
- 19.MSF. Filovirus haemorrhagic fever guideline. 2008. Available at: https://www.medbox.org/preview/53f1e3e2-a078-464d-ba8e-257e1fcc7b89/doc.pdf. Accessed June 8, 2020.
- 20.CDC. Interim guidance for environmental infection control in hospitals for Ebola virus. 2015. Available at:http://www.cdc.gov/vhf/ebola/healthcare-us/cleaning/hospitals.html. Accessed June 8, 2020.
- 21.WHO, PAHO. Infection control precautions in cholera outbreaks - infection prevention and control in health care. 2010. Available at: https://www.paho.org/hq/dmdocuments/2010/Aide_Mem_Cholera-Eng_4Nov%20.pdf. Accessed June 8, 2020.
- 22.CDC. Infection control for cholera in health care settings. 2017. Available at:http://www.cdc.gov/cholera/infection-control-hcp.html. Accessed June 8, 2020.
- 23.MSF . 2nd ed. 2017. Cholera Guideline.https://samumsf.org/sites/default/files/2018-10/Management%20of%20a%20Cholera%20Epidemic.pdf Available at: [Google Scholar]
- 24.UNICEF. Cholera toolkit. 2013. Available at: https://www.unicef.org/cholera_toolkit/. Accessed June 8, 2020
- 25.Lamond E, Kinyanjui J. OXFAM; 2012. Cholera Outbreak Guidelines. [Google Scholar]
- 26.2018. How Family Members of People With Suspect or Confirmed Cholera Can Prevent Infection | Cholera | CDC.https://www.cdc.gov/cholera/family-infection-control.html Available at: [Google Scholar]
- 27.Bibby K, Casson LW, Stachler E, Haas CN. Response to comment on “Ebola virus persistence in the environment: state of the knowledge and research needs.”. Environ Sci Technol Lett. 2015;2:50–51. doi: 10.1021/acs.estlett.5b00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopman J, Kubilay Z, Allen T, Edrees H, Pittet D, Allegranzi B. Efficacy of chlorine solutions used for hand hygiene and gloves disinfection in Ebola settings: a systematic review. Antimicrob Resist Infect Control. 2015;4(Suppl 1):O13. [Google Scholar]
- 29.Yates T, Vujcic JA, Joseph ML, Gallandat K, Lantagne D. Water, sanitation, and hygiene interventions in outbreak response: a synthesis of evidence. Waterlines. 2018;37:5–30. [Google Scholar]
- 30.2009. PRISMA Checklist [Internet]http://www.prisma-statement.org/ Available at: [Google Scholar]
- 31.WHO . 2018. Tuberculosis (TB) [Internet]http://www.who.int/news-room/fact-sheets/detail/tuberculosis Available at: [Google Scholar]
- 32.GTFCC . 2017. Ending Cholera: A Global Roadmap to 2030.http://www.who.int/cholera/publications/global-roadmap.pdf?ua=1 Available at: [Google Scholar]
- 33.Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2:e570–e580. doi: 10.1016/S2214-109X(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 34.Slayton R, Date K, Mintz E. Vaccination for typhoid fever in Sub-Saharan Africa. Hum Vaccines Immunother. 2013;9:903–906. doi: 10.4161/hv.23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. Immunization, vaccines, and biologicals: hepatitis A. 2015. Available at:http://www.who.int/immunization/diseases/hepatitisA/en/. Accessed June 8, 2020
- 36.WHO. Hepatitis A. 2018. Available at: http://www.who.int/news-room/fact-sheets/detail/hepatitis-a. Accessed June 8, 2020
- 37.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0072788. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3762858/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. Immunization, vaccines and biologicals: rotavirus. 2018. Available at: http://www.who.int/immunization/diseases/rotavirus/en/. Accessed June 8, 2020
- 39.Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob Health. 2015;3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mans J. Norovirus infections and disease in lower-middle- and low-income countries, 1997–2018. Viruses. 2019;11 doi: 10.3390/v11040341. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6521228/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO. 2018 annual review of diseases prioritized under the research and development blueprint. 2018. Available at: https://www.who.int/emergencies/diseases/2018prioritization-report.pdf?ua=1. Accessed June 8, 2020
- 42.World Health Organization (WHO). Ebola outbreak in the Democratic Republic of the Congo declared a public health emergency of international concern. 2019. Available at:https://www.who.int/news-room/detail/17-07-2019-ebola-outbreak-in-the-democratic-republic-of-the-congo-declared-a-public-health-emergency-of-international-concern. Accessed June 8, 2020
- 43.US Environmental Protection Agency (EPA) Guidance for Efficacy Testing. 2018. Product performance test guidelines. Office of Chemical Safety and Pollution Prevention (OCSPP) 810.2200. Disinfectants for use on environmental surfaces.https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0150-0036 Available at: [Google Scholar]
- 44.Best M, Sattar SA, Springthorpe VS, Kennedy ME. Efficacies of selected disinfectants against Mycobacterium tuberculosis. J Clin Microbiol. 1990;28:2234–2239. doi: 10.1128/jcm.28.10.2234-2239.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calfee MW, Wendling M. Inactivation of vegetative bacterial threat agents on environmental surfaces. Sci Total Environ. 2013;443:387–396. doi: 10.1016/j.scitotenv.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Kusumaningrum HD, Paltinaite R, Koomen AJ, Hazeleger WC, Rombouts FM, Beumer RR. Tolerance of Salmonella enteritidis and Staphylococcus aureus to surface cleaning and household bleach. J Food Prot. 2003;66:2289–2295. doi: 10.4315/0362-028x-66.12.2289. [DOI] [PubMed] [Google Scholar]
- 47.Ni L, Cao W, Zheng WC, Zhang Q, Li BM. Reduction of microbial contamination on the surfaces of layer houses using slightly acidic electrolyzed water. Poult Sci. 2015;94:2838–2848. doi: 10.3382/ps/pev261. [DOI] [PubMed] [Google Scholar]
- 48.Riazi S, Matthews KR. Failure of foodborne pathogens to develop resistance to sanitizers following repeated exposure to common sanitizers. Int Biodeterior Biodegrad. 2011;65:374–378. [Google Scholar]
- 49.Tuladhar E, Hazeleger WC, Koopmans M, Zwietering MH, Beumer RR, Duizer E. Residual viral and bacterial contamination of surfaces after cleaning and disinfection. Appl Environ Microbiol. 2012;78:7769–7775. doi: 10.1128/AEM.02144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ni L, Zheng W, Zhang Q, Cao W, Li B. Application of slightly acidic electrolyzed water for decontamination of stainless steel surfaces in animal transport vehicles. Prev Vet Med. 2016;133:42–51. doi: 10.1016/j.prevetmed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Jean J, Vachon JF, Moroni O, Darveau A, Kukavica-Ibrulj I, Fliss I. Effectiveness of commercial disinfectants for inactivating hepatitis A virus on agri-food surfaces. J Food Prot. 2003;66:115–119. doi: 10.4315/0362-028x-66.1.115. [DOI] [PubMed] [Google Scholar]
- 52.Abad FX, Pinto RM, Bosch A. Disinfection of human enteric viruses on fomites. Fems Microbiol Lett. 1997;156:107–111. doi: 10.1111/j.1574-6968.1997.tb12713.x. [DOI] [PubMed] [Google Scholar]
- 53.Martin H, Soumet C, Fresnel R, et al. Comparison of the virucidal efficiency of peracetic acid, potassium monopersulfate and sodium hypochlorite on hepatitis A and enteric cytopathogenic bovine orphan virus. J Appl Microbiol. 2013;115:955–968. doi: 10.1111/jam.12297. [DOI] [PubMed] [Google Scholar]
- 54.Sabbah S, Springthorpe S, Sattar SA. Use of a mixture of surrogates for infectious bioagents in a standard approach to assessing disinfection of environmental surfaces. Appl Environ Microbiol. 2010;76:6020–6022. doi: 10.1128/AEM.00246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sattar SA, Jacobsen H, Rahman H, Cusack TM, Rubino JR. Interruption of rotavirus spread through chemical disinfection. Infect Control Hosp Epidemiol. 1994;15:751–756. doi: 10.1086/646852. [DOI] [PubMed] [Google Scholar]
- 56.Smither S, Phelps A, Eastaugh L, et al. Effectiveness of four disinfectants against Ebola virus on different materials. Viruses. 2016;8:185. doi: 10.3390/v8070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook B, Cutts T, Nikiforuk A, et al. Evaluating environmental persistence and disinfection of the Ebola virus Makona variant. Viruses. 2015;7:1975–1986. doi: 10.3390/v7041975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cook B, Cutts TA, Nikiforuk AM, Leung A, Kobasa D, Theriault SS. The disinfection characteristics of Ebola virus outbreak variants. Sci Rep. 2016;6:38293. doi: 10.1038/srep38293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smither SJ, Eastaugh L, Filone CM, et al. Two-center evaluation of disinfectant efficacy against Ebola virus in clinical and laboratory matrices. Emerg Infect Dis. 2018;24 doi: 10.3201/eid2401.170504. http://wwwnc.cdc.gov/eid/article/24/1/17-0504_article.htm Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park GW, Boston DM, Kase JA, Sampson MN, Sobsey MD. Evaluation of liquid- and fog-based application of Sterilox hypochlorous acid solution for surface inactivation of human norovirus. Appl Environ Microbiol. 2007;73:4463–4468. doi: 10.1128/AEM.02839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moorman E, Montazeri N, Jaykus L-A. Efficacy of neutral electrolyzed water for inactivation of human norovirus. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.00653-17. https://aem.asm.org/content/83/16/e00653-17 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cromeans T, Park GW, Costantini V, et al. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl Environ Microbiol. 2014;80:5743–5751. doi: 10.1128/AEM.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gallandat K. Tufts University; Medford, MA: 2018. Surface Disinfection Efficacy and Effectiveness Assessment for Low-Resource Outbreak Settings. [Google Scholar]
- 64.Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2010;34:415–425. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 65.Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S. Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front Public Health. 2014;2 doi: 10.3389/fpubh.2014.00103. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4116801/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaiyanan S, Chaiyanan S, Huq A, Maugel T, Colwell RR. Viability of the nonculturable Vibrio cholerae O1 and O139. Syst Appl Microbiol. 2001;24:331–341. doi: 10.1078/0723-2020-00032. [DOI] [PubMed] [Google Scholar]
- 67.D'Souza DH, Su X. Efficacy of chemical treatments against murine norovirus, feline calicivirus, and MS2 bacteriophage. Foodborne Pathog Dis. 2010;7:319–326. doi: 10.1089/fpd.2009.0426. [DOI] [PubMed] [Google Scholar]
- 68.Zonta W, Mauroy A, Farnir F, Thiry E. Comparative virucidal efficacy of seven disinfectants against murine norovirus and feline calicivirus, surrogates of human norovirus. Food Environ Virol. 2016;8:1–12. doi: 10.1007/s12560-015-9216-2. [DOI] [PubMed] [Google Scholar]
- 69.Park GW, Sobsey MD. Simultaneous comparison of murine norovirus, feline calicivirus, coliphage MS2, and GII.4 norovirus to evaluate the efficacy of sodium hypochlorite against human norovirus on a fecally soiled stainless steel surface. Foodborne Pathog Dis. 2011;8:1005–1010. doi: 10.1089/fpd.2010.0782. [DOI] [PubMed] [Google Scholar]
- 70.Chiu S, Skura B, Petric M, McIntyre L, Gamage B, Isaac-Renton J. Efficacy of common disinfectant/cleaning agents in inactivating murine norovirus and feline calicivirus as surrogate viruses for human norovirus. Am J Infect Control. 2015;43:1208–1212. doi: 10.1016/j.ajic.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 71.Chander Y, Johnson T, Goyal SM, Russell RJ. Antiviral activity of Ecasol against feline calicivirus, a surrogate of human norovirus. J Infect Public Health. 2012;5:420–424. doi: 10.1016/j.jiph.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Whitehead K, McCue KA. Virucidal efficacy of disinfectant actives against feline calicivirus, a surrogate for norovirus, in a short contact time. Am J Infect Control. 2010;38:26–30. doi: 10.1016/j.ajic.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 73.Gallandat K, Lantagne D. Selection of a biosafety level 1 (BSL-1) surrogate for the Ebola virus: comparison of bacteriophages MS2, M13, PR772 and Phi6. PLoS ONE. 2017;12:e0177943. doi: 10.1371/journal.pone.0177943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rutala WA, Gergen MF, Weber DJ. Efficacy of different cleaning and disinfection methods against Clostridium difficile spores: importance of physical removal versus sporicidal inactivation. Infect Control Hosp Epidemiol. 2012;33:1255–1258. doi: 10.1086/668434. [DOI] [PubMed] [Google Scholar]
- 75.Hakim H, Alam MS, Sangsriratanakul N, et al. Inactivation of bacteria on surfaces by sprayed slightly acidic hypochlorous acid water: in vitro experiments. J Vet Med Sci. 2016;78:1123–1128. doi: 10.1292/jvms.16-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tyan K, Jin K, Kang J, Kyle AM. Novel color additive for chlorine disinfectants corrects deficiencies in spray surface coverage and wet-contact time and checks for correct chlorine concentration. Am J Infect Control. 2018;46:1188–1191. doi: 10.1016/j.ajic.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Hakim H, Thammakarn C, Suguro A, et al. Evaluation of sprayed hypochlorous acid solutions for their virucidal activity against avian influenza virus through in vitro experiments. J Vet Med Sci. 2015;77:211–215. doi: 10.1292/jvms.14-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calfee MW, Ryan SP, Wood JP, et al. Laboratory evaluation of large-scale decontamination approaches. J Appl Microbiol. 2012;112:874–882. doi: 10.1111/j.1365-2672.2012.05259.x. [DOI] [PubMed] [Google Scholar]
- 79.Klingeren B, Koller W, Bloomfield SF, et al. Assessment of the efficacy of disinfectants on surfaces. Int Biodeterior Biodegrad. 1998;41:289–296. [Google Scholar]
- 80.Park H, Hung Y-C, Kim C. Effectiveness of electrolyzed water as a sanitizer for treating different surfaces. J Food Prot. 2002;65:1276–1280. doi: 10.4315/0362-028x-65.8.1276. [DOI] [PubMed] [Google Scholar]
- 81.Ryan SP, Lee SD, Calfee MW, et al. Effect of inoculation method on the determination of decontamination efficacy against Bacillus spores. World J Microbiol Biotechnol. 2014;30:2609–2623. doi: 10.1007/s11274-014-1684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bloomfield SF, Arthur M, Begun K, Patel H. Comparative testing of disinfectants using proposed European surface test methods. Lett Appl Microbiol. 1993;17:119–125. [Google Scholar]
- 83.Gallandat K, Wolfe MK, Lantagne D. Surface cleaning and disinfection: efficacy assessment of four chlorine types using escherichia coli and the Ebola surrogate Phi6. Env Sci Technol. 2017;51:4624–4631. doi: 10.1021/acs.est.6b06014. [DOI] [PubMed] [Google Scholar]
- 84.Aarnisalo K, Salo S, Miettinen H, et al. Bactericidal efficiencies of commercial disinfectants against Listeria monocytogenes on surfaces. J Food Saf. 2000;20:237–250. [Google Scholar]
- 85.Lombardi ME, Ladman BS, Alphin RL, Benson ER. Inactivation of avian influenza virus using common detergents and chemicals. Avian Dis. 2008;52:118–123. doi: 10.1637/8055-070907-Reg. [DOI] [PubMed] [Google Scholar]
- 86.Baek SB, Kim SW, Ha SD. Reduction of Escherichia coli on surfaces of utensils and development of a predictive model as a function of concentration and exposure time of chlorine. Foodborne Pathog Dis. 2012;9:1–6. doi: 10.1089/fpd.2011.0910. [DOI] [PubMed] [Google Scholar]
- 87.Kim CY, Ryu GJ, Park HY, Ryu K. Resistance of Staphylococcus aureus on food contact surfaces with different surface characteristics to chemical sanitizers. J Food Saf. 2017;37:e12354. [Google Scholar]
- 88.Yeargin T, Fraser A, Huang G, Jiang X. Recovery and disinfection of two human norovirus surrogates, feline calicivirus and murine norovirus, from hard nonporous and soft porous surfaces. J Food Prot. 2015;78:1842–1850. doi: 10.4315/0362-028X.JFP-14-515. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organization. Coronavirus disease (COVID-19) pandemic—outbreak situation. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed June 8, 2020
- 90.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ASTM International . 2011. Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals. [Google Scholar]
- 92.Rutala WA, Weber D. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Rev. 1997;10:597–610. doi: 10.1128/cmr.10.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bloomfield SF, Arthur M, Vanklingeren B, Pullen W, Holah JT, Elton R. An evaluation of the repeatability and reproducibility of a surface test for the activity of disinfectants. J Appl Bacteriol. 1994;76:86–94. doi: 10.1111/j.1365-2672.1994.tb04420.x. [DOI] [PubMed] [Google Scholar]
- 94.Parker AE, Hamilton MA, Goeres DM. Reproducibility of antimicrobial test methods. Sci Rep. 2018 doi: 10.1038/s41598-018-30282-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6105646/ [cited 2019 Oct 31];8. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1: link to download our dataset from Open Science Framework and Tables S1-S4
Table S1: Reported chlorine disinfection efficacy outcomes for gram-positive bacteria used in at least 2 studies with a quality score >3.
Table S2: Reported chlorine disinfection efficacy outcomes for gram-negative bacteria used in at least 2 studies with a quality score >3.
Table S3: Reported chlorine disinfection efficacy outcomes for viruses used in at least 2 studies with a quality score >3.
Table S4: Reported chlorine disinfection efficacy outcomes for bacterial spores used in at least 2 studies with a quality score >3.