Abstract
There is a desperate search to discover effective therapies against coronavirus disease-2019 (COVID-19). Patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) comprise a unique population whose clinical course may provide insights into the effects of antiretroviral therapy on COVID-19. We describe the case of a patient with HIV/AIDS on left ventricular assist device support who was hospitalized and recovered from COVID-19. (Level of Difficulty: Intermediate.)
Key Words: antiretroviral therapy, coronavirus disease, HIV/AIDS, LVAD, mechanical circulatory support
Abbreviations and Acronyms: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; COVID-19, coronavirus disease-2019; HIV, human immunodeficiency virus; IL, interleukin; LVAD, left ventricular assist device; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Graphical abstract
There is a desperate search to discover effective therapies against coronavirus disease-2019 (COVID-19). Patients with human immunodeficiency virus…
Coronavirus disease-2019 (COVID-19) is caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), which belongs to the same family as the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronaviruses. Given the lack of immunity in the world’s population, the initial outbreak of SARS-CoV-2 spread quickly through populations and became a global pandemic. In a desperate search to discover effective therapies against this new pathogen, physicians trialed antimalarials, immune modulating drugs, and antiretroviral drugs with varying degrees of success. SARS-CoV-2 is a single-stranded RNA virus whose genome presents several potential antiviral targets. These include nonstructural proteins (e.g., 3-chymotrypsin-like protease, papain-like protease, RNA-dependent RNA polymerase, and its helicase), structural proteins (e.g., the capsid spike glycoprotein), and accessory proteins (1). We present a case of a patient supported by a durable left ventricular assist device (LVAD) with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) on antiretroviral therapy (ART) and COVID-19.
Learning Objectives
-
•
To describe the presentation of COVID-19 in a patient with HIV/AIDS on ART supported with an LVAD.
-
•
To understand the impact of LVAD, ART, and inflammation on patients with COVID-19.
History of Presentation
A 54-year-old man with HIV/AIDS on ART who received a HeartMate 3 LVAD as destination therapy in 2018 experienced 7 days of fever, myalgia, cough and dyspnea while residing in a nursing facility due to lack of stable housing. Prior to hospital transfer, real-time reverse transcriptase polymerase chain reaction testing from a nasal swab was positive for SAR-CoV-2.
Past Medical History
The patient had a medical history of coronary heart disease, prior coronary artery bypass grafting, and type 2 diabetes mellitus. He was diagnosed with HIV/AIDS in 1991, received radiation therapy in 1995 for treatment of Kaposi’s sarcoma, and has had recurrent thrush. Two months prior to hospitalization, he had a CD4 count of 266 cells/mm2 and an undetectable HIV viral load. He was taking 3 antiretrovirals: emtricitabine-tenofovir, a nucleoside and nucleotide reverse transcriptase inhibitor combination, and dolutegravir, an integrase inhibitor.
Differential Diagnosis
Other viral infections such as influenza and respiratory syncytial virus were considered, but the pre-test probability for COVID-19 was high because other residents at the facility had been diagnosed with COVID-19 recently.
Investigations
In the emergency department, the patient was tachypneic with an initial oxygen (O2) saturation 98%. Table 1 lists the results of his initial laboratory testing including normal levels of ferritin, procalcitonin, interleukin (IL)-1, and IL-6. Levels of C-reactive protein, lactate dehydrogenase, and troponin were elevated. There was a reduced white blood cell count without lymphopenia. A chest radiograph had no air space or interstitial infiltrates (Figure 1). There was a single low-flow LVAD alarm noted 3 days prior to presentation.
Table 1.
Patient Characteristics
| Reference Values | Patient's Values | |
|---|---|---|
| Temperature, °C | 36.7 | |
| MAP, mm Hg | 98 | |
| Pulse, beats/min | 77 | |
| Resp rate | 33 | |
| SpO2, % | 98 | |
| O2 flow rate, l/min | 2 | |
| LVAD speed, rpm | 4,900 | |
| LVAD flow, l/min | 3.4 | |
| WBC, ×10−3/ul | 4.5–11 | 4.0 |
| Platelet, ×10−3/ul | 150–450 | 123 |
| Absolute neutrophil, ×10−3/ul | 1.9–8.0 | 1.4 |
| Absolute lymphocyte, ×10−3/ul | 1.0–4.5 | 2.1 |
| Creatinine, mg/dl | 0.70–1.30 | 1.12 |
| AST, U/l | 1–35 | 23 |
| ALT, U/l | 1–45 | 20 |
| Total bilirubin, mg/dl | 0.1–1.2 | 0.4 |
| INR | 1.4 | |
| CRP, mg/l | 0–5.0 | 12.1 |
| LDH, U/l | 100–220 | 230 |
| D-dimer, ug/ml | 0.00–0.50 | 1.59 |
| Ferritin, ng/ml | 30–400 | 215 |
| Interleukin-1, pg/ml | ≤36 | 5 |
| Interleukin-6, pg/ml | 0.0–15.5 | 3.4 |
| Procalcitonin, ng/ml | <0.49 | 0.02 |
| Troponin, ng/ml | 0.00–0.03 | 0.08 |
| CK-MB, ng/ml | 0.6–6.3 | 1.0 |
| Creatine kinase, U/l | 30–200 | 112 |
| BNP, pg/ml | 0.0–100.0 | 33.7 |
| proBNP, pg/ml | 300–899 | 235 |
AST = aspartate aminotransferase; ALT = alanine transaminase; BNP = brain natriuretic peptide; CK-MB = creatinine kinase myocardial band; CRP = C-reactive protein; INR = international normalized ratio; LDH = lactate dehydrogenase; MAP = mean arterial pressure (obtained via Doppler); SpO2 = peripheral oxygen saturation.
Figure 1.
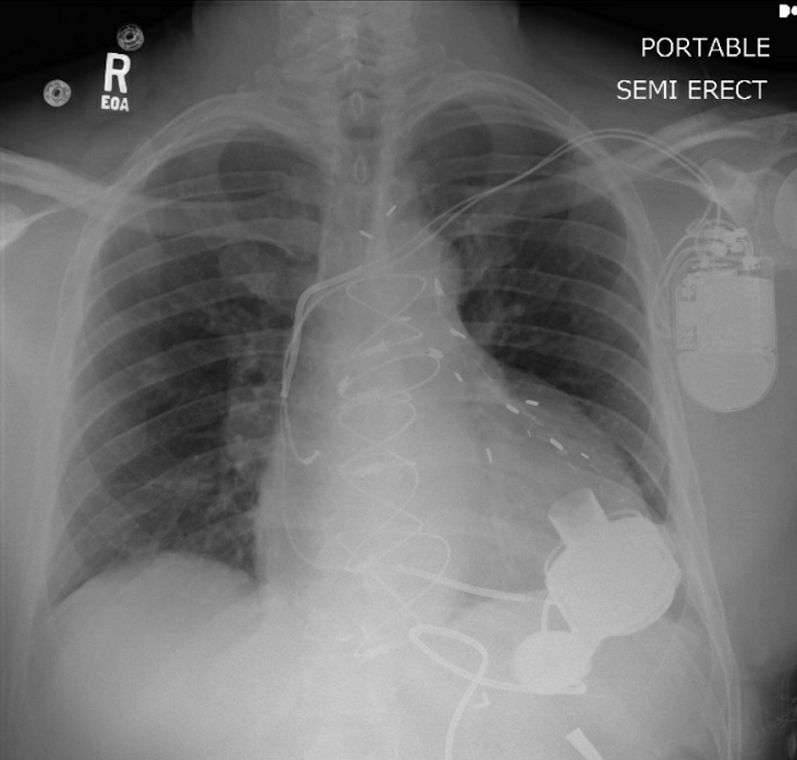
Chest Radiograph With Mild Subsegmental Atelectasis
Management
Based upon the adequate room air saturation, absence of pulmonary infiltrates, and minimally abnormal inflammatory markers, the patient was classified as having a mild case of COVID-19. Due to persistent breathlessness, hydroxychloroquine was initiated on day 2 with QTc monitoring.
Discussion
To the best of our knowledge, this is the first reported case of COVID-19 in a patient with HIV/AIDS on ART supported with an LVAD. COVID-19 is a viral-induced illness whose outcomes seem to be determined by the exaggerated immune response producing tissue damage and multiorgan dysfunction. The spectrum of disease varies, but comorbid conditions confer a 2.85- to 21.4-fold increased risk of in-hospital mortality (2). Therefore, it was surprising to see a relatively benign course in our patient given his immunocompromised state and complex cardiovascular history. Because the majority of patients with COVID-19 will have mild symptoms and recover, it is likely that this applies to our patient as well, but we hypothesize that LVAD support and ART reduced the severity of COVID-19 manifestation in our vulnerable patient through its immunomodulatory effects.
Cardiac Injury Mitigated by LVAD?
Cardiac injury is associated with increased risk of hospital death. In a study of 416 hospitalized patients in Wuhan, China, cardiac injury, as reflected by elevations in troponin-I, was associated with a 4-fold increase risk of in-hospital mortality (3). The mechanism(s) producing myocardial injury in COVID-19 disease are debatable, but include ischemia, resulting in either type 1 or 2 myocardial infarction, injury from decompensated heart failure, or the direct result of viral myocarditis. Our patient had mild elevations in cardiac enzymes that were diagnostic of injury and fell within the interquartile range of troponin-I as reported in the cohort from Wuhan, China (3). It is possible that the continuous left ventricular unloading and cardiac output produced by the LVAD reduced wall stress, myocardial oxygen demand, and subsequent injury.
Inflammation Reduced by LVAD Support?
Advanced heart failure patients live in a pro-inflammatory state and by restoring end-organ perfusion and reversing the heart failure state, LVADs improve the inflammatory profile, reduce cytokine levels, and lower leukocyte counts (4). Although the devices’ biomaterials may themselves be pro-inflammatory, the net effect of LVAD support is to decrease inflammatory cytokines compared with their pre-implant levels (4). We hypothesize that the hemodynamic support provided by the LVAD in our patient with extensive cardiovascular comorbidities produced a favorable immunomodulatory effect in the setting of COVID-19 disease.
Inflammation and HIV/AIDS
Persons with HIV/AIDS have persistent immune activation despite the viral suppression produced by ART (5). In a nested case-control study within the SMART (Strategies for Management of Antiretroviral Therapy) trial, researchers demonstrated that higher plasma levels of IL-6, C-reactive protein, and D-dimer strongly predicted higher mortality and cardiovascular events. These findings suggest that HIV-induced inflammation increases mortality risk among HIV patients and that interrupting ART further increases this risk (6). Because ART is associated with lowering the levels of these biomarkers, it may mitigate the exaggerated inflammatory response seen in patients with COVID-19 and affect disease severity.
Impact of HIV Antiretroviral Therapy
There are currently no approved antiviral drugs to treat COVID-19 patients, but combination therapies used to treat HIV may mitigate other coronavirus diseases such as SARS and Middle East respiratory syndrome. In 2004, during the SARS epidemic in China, it was observed that 0 of 19 patients with HIV/AIDS contracted SARS even though they were in close contact and hospitalized with SARS patients. Most were receiving ART. This compares to 6 of 28 health care workers with similar exposure who did contract SARS. This observation leads to a hypothesis that existing HIV infection might interfere with SARS virus replication in the same host or that ART might prevent SARS development (7). These initial observations were supported by a retrospective matched-cohort study from Hong Kong where the addition of lopinavir/ritonavir, a protease inhibitor, to standard SARS treatment protocols appeared to be associated with improved clinical outcomes (8).
In the current COVID-19 pandemic, lopinavir/ritonavir was studied in a randomized, controlled trial in Wuhan, China, but produced no benefit in patients with severe COVID-19 (9). Currently, the Centers for Disease Control and Prevention recommends that people with HIV not switch their HIV medicines in an attempt to prevent or treat COVID-19 (10). Multiple clinical trials evaluating efficacy of different ARTs for COVID-19 are underway and their results are likely to inform future management decisions (Table 2).
Table 2.
Clinical Trials Using ARTs in COVID-19
| Study Title | Interventions | Location | Status |
|---|---|---|---|
| Evaluation of Ganovo (Danoprevir) Combined With Ritonavir in the Treatment of Novel Coronavirus Infection | Ganovo/ritonavir, interferon nebulization | China | Completed |
| The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection | Lopinavir/ritonavir, arbidol | China | Recruiting |
| Efficacy and Safety of Darunavir and Cobicistat for Treatment of Pneumonia Caused by 2019-nCoV | Darunavir, cobicistat | China | Recruiting |
| Treatment and Prevention of Traditional Chinese Medicines (TCMs) on 2019-nCoV Infection | Oxygen therapy, alfa interferon via aerosol inhalation, lopinavir/ritonavir, TCM granules | China | Recruiting |
| Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | Lopinavir/ritonavir, ribavirin, interferon beta-1B | Hong Kong | Recruiting |
| Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) | Lopinavir/ritonavir, hydroxychloroquine sulfate | South Korea | Recruiting |
| Trial of Treatments for COVID-19 in Hospitalized Adults | Remdesivir, lopinavir/ritonavir, interferon beta-1A, hydroxychloroquine | France | Recruiting |
| COVID-19 Ring-based Prevention Trial With Lopinavir/Ritonavir | Lopinavir/ritonavir | Canada | Not yet recruiting |
| Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID19: A Randomized Control Trial | Oseltamivir, hydroxychloroquine, lopinavir/ritonavir, darunavir, favipiravir | Thailand | Not yet recruiting |
| The Clinical Study of Carrimycin on Treatment Patients With COVID-19 | Carrimycin, lopinavir/ritonavir, arbidol, chloroquine phosphate | Not yet recruiting | |
| Treatment of Moderate to Severe Coronavirus Disease (COVID-19) in Hospitalized Patients | Lopinavir/ritonavir, hydroxychloroquine sulfate, baricitinib, sarilumab | Not yet recruiting | |
| Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection | ASC09/ritonavir, lopinavir/ritonavir | Not yet recruiting | |
| Xiyanping Injection for the Treatment of New Coronavirus Infected Pneumonia | Xiyanping injection, lopinavir/ritonavir, alpha-interferon nebulization | Not yet recruiting | |
| Multicenter Clinical Study on the Efficacy and Safety of Xiyanping Injection in the Treatment of New Coronavirus Infection Pneumonia (General and Severe) | Lopinavir/ritonavir, xiyanping injection | Not yet recruiting |
COVID-19 = coronavirus disease-2019; TCM = traditional Chinese medicine.
Follow-Up
The patient was discharged to his nursing facility on hospital day 5 once a room was available where he could be quarantined, and he continued to feel well 10 days later.
Conclusions
We describe the first case of a patient on LVAD support with HIV/AIDS on ART who recovered from mild COVID-19. Further clinical experience and research will be required to understand the full impact of LVADs, HIV/AIDS, and ART on COVID-19.
Footnotes
Dr. Pinney has received consulting fees from Abbott, CareDx, Medtronic, and Procyrion. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; Treasure Island, FL: 2020. Features, evaluation and treatment Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020:e200950. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radley G., Pieper I.L., Ali S., Bhatti F., Thornton C.A. The inflammatory response to ventricular assist devices. Front Immunol. 2018;9:2651. doi: 10.3389/fimmu.2018.02651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt P.W. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 6.Kuller L.H., Tracy R., Belloso W. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X.P., Cao Y. Consideration of highly active antiretroviral therapy in the prevention and treatment of severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1030–1032. doi: 10.1086/386340. [DOI] [PubMed] [Google Scholar]
- 8.Chan K.S., Lai S.T., Tsui E. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 9.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19): people with HIV. March 2020. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/hiv.html Available at:


