Summary
The Warburg effect is one of the metabolic hallmarks of cancer cells, characterized by enhanced glycolysis even under aerobic conditions. While this physiological adaptation is associated with metastatic behavior, we still have a superficial understanding of how it affects cell behavior during embryonic development. Here we report that the neural crest, a migratory stem cell population in vertebrate embryos, undergoes an extensive metabolic remodeling to engage in aerobic glycolysis prior to delamination. This increase in glycolytic flux promotes Yap/Tead signaling, which directly activates the expression of a set of transcription factors to drive epithelial-to-mesenchymal transition. Our results demonstrate how shifts in carbon metabolism can trigger the gene regulatory circuits that control complex cell behaviors. These findings support the hypothesis that the Warburg effect is a precisely regulated developmental mechanism that is anomalously reactivated during tumorigenesis and metastasis.
eTOC blurb
Cancer cells display the Warburg effect, a metabolic adaptation characterized by enhanced glycolysis under aerobic conditions. Bhattacharya and colleagues demonstrate that this physiological adaptation is required for migration of neural crest cells in vertebrate embryos. Their results indicate aerobic glycolysis is a developmental mechanism that is co-opted by metastatic cells.
Introduction
Shifts in carbon metabolism play essential roles in the regulation of cellular properties in eukaryotes. Under well-defined conditions, cells employ distinct strategies for energy production, with consequences that extend beyond changes in ATP levels. In stem cells, heightened levels of glycolysis stimulate histone acetylation to promote cell proliferation and inhibit differentiation (Cha et al., 2017; Moussaieff et al., 2015). Cancer cells also display high glycolytic activity, which is linked to key features of oncogenesis such as rapid cell division and tissue growth (Hsu and Sabatini, 2008; Vander Heiden et al., 2009). This metabolic adaptation, known as the Warburg effect, is characterized by preferential activation of glycolysis even in environments that are rich in oxygen. While the Warburg effect was initially described as an anomalous mode of metabolism (Warburg, 1925), growing evidence points to glycolysis as a crucial regulator of cell identity and behavior under physiological conditions. Recent studies indicate that aerobic glycolysis is deployed in a tissue-specific manner to influence the activity of signaling pathways in vertebrate embryos (Bulusu et al., 2017; Oginuma et al., 2017). These findings reinforce the notion that metabolism has an active role in the regulation of cell identity and behavior during embryonic development.
A process that may be especially prone to metabolic regulation is cell motility. The association between the Warburg effect and metastatic behavior is well documented (Lu, 2019; Lu et al., 2015), and motile cells from the immune system display enhanced glycolytic flux (Kaushik et al., 2019; Kishore et al., 2017). Despite this, we still have a limited understanding of the importance of metabolism for cell migration in embryonic development. To explore a possible link between energy production and cellular movement, we examined the metabolic shifts that take place during the development of neural crest cells. The neural crest is a migratory stem cell population that contributes to a variety of derivatives including peripheral neurons, glia, facial cartilage, and skin pigments. During early vertebrate development, neural crest cells delaminate from the dorsal neural tube, undergo epithelial-to-mesenchymal transition (EMT) and migrate extensively within the developing embryo (Simoes-Costa and Bronner, 2013). This striking migratory ability has made it an important in vivo model for investigating processes such as EMT, cell motility and migration (Theveneau and Mayor, 2012). Furthermore, numerous studies have drawn parallels between neural crest migration and cancer metastasis to show that the two processes are physiologically similar (Acloque et al., 2009). Neural crest-derived cancers like melanoma and neuroblastoma de-differentiate into a neural crest-like state before metastasis (Tsoi et al., 2018). Similar to cancer cells, neural crest cells are also highly sensitive to oxidative stress during migration (Chen et al., 2013; Laforgia et al., 2018), suggesting a role of energy metabolism in the regulation of this process.
Here we show that carbon metabolism is a major regulator of cell delamination and migration during embryonic development. Upon specification, neural crest cells display an increase in the expression of glycolytic enzymes and engage in aerobic glycolysis. This metabolic transition is necessary for proper cell movement since the chemical inhibition of the Warburg effect prevented EMT and migration. We found that an increase in glycolytic flux promotes the interaction between the YAP1 and TEAD1 proteins, resulting in the activation of the Yap/Tead signaling pathway. YAP/TEAD directly binds to tissue-specific enhancers and promotes the expression of EMT factors in neural crest cells. While enhanced glycolysis may be important for faster ATP production during migration (Epstein et al., 2017), our results indicate that the Warburg effect precedes delamination and activates the gene regulatory circuit that controls EMT. Taken together, these findings demonstrate how intracellular shifts in bioenergetics can modulate gene regulatory networks to ultimately control cell state transitions during embryonic development.
Results
Neural crest cells display the Warburg effect at the onset of migration
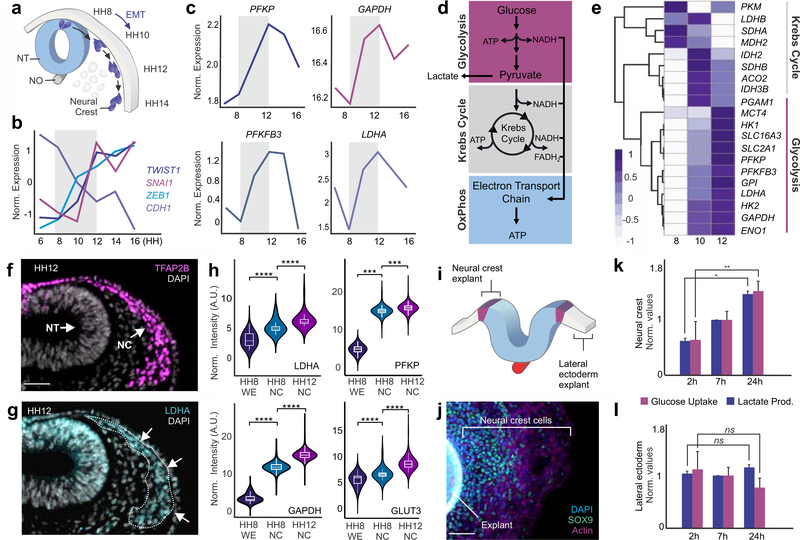
To identify shifts in gene expression that are important for neural crest EMT and migration, we performed an extensive transcriptome analysis of FACS sorted cells at six developmental time-points (Hamburger Hamilton stages 6–16, Fig. 1a) (Hamburger and Hamilton, 1951). The RNA-seq data recapitulated the expected transcriptional changes, such as a marked increase in the expression of EMT factors such as SNAI1, SNAI2, TWIST, and ZEB1 during migration (Fig. 1b). We also observed an unexpected increase in the expression of rate-limiting glycolytic enzymes such as PFKP, GAPDH, PFKFB3, and LDHA, at the onset of migration (Fig. 1c). Hierarchical clustering analysis with a set of carbon metabolism genes indicated that glycolytic enzymes were upregulated during EMT and migration, whereas most components of the Krebs Cycle displayed a distinct pattern of gene expression (Fig. 1d–e). Immunohistochemistry for glycolytic enzymes (Fig. 1f–g) and flow cytometry analysis (Fig. 1h) confirmed that protein expression levels reflected these transcriptomic changes. LDHA, PFKP, GAPDH, and GLUT3 were enriched in neural crest cells, and we observed a significant rise in the levels of these glycolytic enzymes between stages HH8 and HH12 (Fig. 1h). Thus, neural crest migration involves a transient increase in the expression of glycolytic enzymes. Since chick embryos develop in a normoxic environment (Philips, 1941; Romanoff and Romanoff, 1949), these changes in gene expression were reminiscent of the Warburg effect observed in cancer cells (Ngo et al., 2015; Warburg et al., 1924).
Figure 1. The expression of glycolytic enzymes is upregulated in migratory neural crest cells.
(a) Diagram of avian neural tube transverse section, showing the position of cranial neural crest cells at distinct developmental stages. (b) Transcriptional profiles of EMT regulators in developing neural crest cells. Expression of TWIST1, SNAI1, ZEB1 increases during migration while there is a reduction in CDH1 (E-cadherin) transcript levels. (c) Expression levels of rate-limiting glycolytic enzymes increase during neural crest EMT. (d) Simplified schematic of carbohydrate metabolism in eukaryotic cells. (e) Clustering analysis showing an increase in the expression of glycolytic enzymes in pre-migratory (HH8), migratory (HH10) and late migratory (HH12) neural crest cells. The heatmap shows z-score normalized expression levels of each metabolic genes. (f-g) Immunostaining for glycolytic enzyme LDHA reveals high protein expression in TFAP2B+ migratory neural crest cells. (h) The protein levels of glycolytic enzymes LDHA, PFKP, GAPDH, and GLUT3 are enriched in pre-migratory neural crest cells (HH8) and increases further upon neural crest migration. (i) Diagram indicating the regions of the ectoderm that were dissected for explant culture. (j) Neural fold explant with a halo of migratory SOX9+ neural crest cells. (k-l) Cultured neural crest cells increase in glucose uptake and lactate production during migration, while these parameters remain constant in lateral ectoderm explants. Ect: Ectoderm; EMT: epithelial-mesenchymal transition; HH: Hamburger and Hamilton stage; NC: Neural Crest; NT: neural tube; NO: notochord. Error bars represent ± S.E.M. Scale bar : 50 um (f-g), 200um (j).
To investigate if higher levels of glycolytic enzymes were linked to aerobic glycolysis, we measured the glycolytic flux at different stages of neural crest migration. First, we employed colorimetric and luminescent assays to quantify lactate production and glucose consumption. In these experiments, we performed explant cultures to isolate populations of migratory neural crest cells (Coles et al., 2007). Dorsal neural folds of HH9 avian embryos were dissected and cultured on fibronectin-coated plates. Within 2h, the neural folds attach to the substrate; by 7h of incubation most neural crest cells have delaminated, and by 24h migratory cells form a halo around the explant (Fig. 1i–j). Consistent with high glycolytic flux, we observed a gradual increase in both glucose uptake and lactate levels as neural crest cells transition from the pre-migratory to the migratory stages in the explant system (Fig. 1k). This metabolic shift was specific to neural crest cells since it was not observed in lateral ectoderm explants maintained under identical culture conditions (Fig. 1l).
Next, we used the Seahorse XFp to measure the levels of glycolysis vs. oxidative phosphorylation in neural crest cells. We compared the basal Extracellular Acidification Rate (ECAR) and the Oxygen Consumption Rate (OCR) of neural crest progenitors at the onset of specification (HH7), and delamination (HH9). Quantification of the ECAR and OCR rates revealed that, during EMT, neural crest cells transition from quiescence to a state of enhanced glycolysis (Fig. 2a–c). This shift was again specific to neural crest cells, as ectodermal cells obtained from HH9 embryos were metabolically quiescent (Fig. 2a,b). Since neural crest-derived melanocytes engage in aerobic respiration and display a high OCR to ECAR ratio (Hall et al., 2013), these results indicate that the metabolic state of neural crest cells is highly dynamic through development.
Figure 2. Cranial neural crest cells display the Warburg effect at the onset of migration.
(a) Basal oxygen consumption and extracellular acidification rates measured using Seahorse XF indicate that metabolically quiescent neural crest cells (NC HH7) become highly glycolytic during delamination (NC HH9). Lateral ectoderm cells remain quiescent (Ect HH9), whereas neural crest-derived melanocytes have been reported to be highly aerobic. (b-c) Despite the increased rates of extracellular acidification (ECAR) and proton efflux (PER) in HH9 neural crest, the oxygen consumption rates (OCR) in these cells is not significantly different from other tissues (b). This manifests in reduced OCR/ECAR ratio in HH9 cells compared to HH7 neural crest (c). (d) Diagram showing the electroporation scheme for assessing the activity of metabolic reporters in avian neural crest cells and neural progenitors at different developmental stages. (e-h) Transverse sections of HH10 transgenic embryos transfected with reporters of intracellular NAD+/NADH ratio (Rex-YFP) and Tfap2aE1:Cherry (e-f) and cytoplasmic glucose levels (Green Glifon50) and Sox2N2:Cherry (g-h). (i-j) Flow cytometry quantification of Rex:YFP (i) and Green Glifon50 (j) activity in isolated neural crest (NC) and neural cells at two developmental stages. Both metabolic reporters have high activity in Tfap2E1+ neural crest cells and display low overlap with Sox2N2+ neural progenitor cells. (k) Diagram depicting Tfap2aE1:Cherry transgenic embryos treatment with fluorescent glucose analog 2-NBDG. (l-m) Transverse section of an HH10 embryo showing colocalization of 2-NBDG and Tfap2aE1+ neural crest cells. (n) Boxplots showing the intensity of 2-NBDG in Tfap2aE1+ neural crest cells and Tfap2aE1- whole embryo cells at stages HH8 and HH10.che: Cherry ECAR: Extracellular Acidification Rate; Ect: Ectoderm; EMT: epithelial-mesenchymal transition; HH: Hamburger and Hamilton stage; PER: Proton Efflux Rate, NC: Neural Crest; Ne: Neural, OCR: Oxygen Consumption Rate. Error bars represent ± S.E.M. Also see Fig. S1. Scale bar : 50 um.
To confirm that these metabolic changes occur during neural crest migration in vivo, we employed a combination of molecular sensors to monitor the glycolytic flux in developing avian embryos(Fig 2d). An increased glycolytic flux raises the cellular NAD+/NADH ratio, which is up to five to ten-fold higher in cancer cells compared to the normal cells (Ngo et al., 2015). Thus, we first examined the NAD+/NADH ratio of ectodermal cells using the Rex:YFP reporter (Bilan et al., 2014). We co-transfected gastrula stage (HH5) avian embryos with pCI-Rex-YFP (Fig. 2d) and either a neural crest (Tfap2aE1:mCherry) (Fig. 2f) or a neural (Sox2N2:mCherry) (Fig. 2h) specific enhancer. Consistent with our previous experiments, transfection of the reporter revealed that neural crest cells have a higher NAD+/NADH ratio than adjacent tissues like the neural tube, as evidenced by microscopy (Fig. 2e–f) and flow cytometry (Fig. 2i). Notably, migratory neural crest cells, which have the highest expression levels of glycolytic genes (Fig. 1e), displayed higher Rex:YFP activity than pre-migratory cells (Fig. 2i). Next, we utilized a Green Glifon50 (green glucose indicating fluorescent protein sensor) to monitor the cytoplasmic glucose levels in neural crest cells. Transfection of avian embryos with a vector encoding Green Glifon50 (Mita et al., 2019) revealed that neural crest cells contain higher levels of intracellular glucose than neural progenitors, and that levels of glucose increase in migratory cells (Fig. 2g–h, 2j). Finally, incubation of embryos with the fluorescent glucose analog 2-NBDG (Fig. 2k) (Oginuma et al., 2017) confirmed that neural crest cells display higher glucose uptake than neighboring cells (Fig. 2l–m) and that this is also exacerbated during migration (Fig. 2n). Taken together, these experiments show that neural crest cells exhibit the Warburg effect, which is characterized by a pronounced increase in lactate production, glucose uptake, high levels of intracellular glucose and low OCR/ECAR but high NAD+/NADH ratios. These observations led us to hypothesize that enhanced glycolysis plays a role in the migration of neural crest cells.
Enhanced glycolysis is necessary for neural crest EMT
To establish the importance of the Warburg effect for neural crest development, we treated these cells with 2-deoxy-D-glucose (2-DG), a competitive inhibitor of glucose transporters and the hexokinase enzyme (Barban and Schulze, 1961). Treatment of neural crest 165 explants with 2-DG drove cells towards aerobic respiration, as evidenced by an increase in the OCR/ECAR ratio (Fig. 3a, Fig. S2a–b), and decreased Rex-YFP fluorescence (Fig. S2c). This also resulted in a decrease in lactate production (Fig. 3b) but did not alter total cellular ATP levels (Fig. 3c), cell proliferation or survival (Fig. S2d–e). Strikingly, we found that inhibition of glycolysis had a strong effect on cell migration. Treatment with 2-DG suppressed neural crest migration, resulting in explants with smaller diameters than controls (Fig. 3d–e). This phenotype was reversible, as removal of the 2-DG prompted cells to resume migration (Fig. S2 f–h). Targeting glycolysis with 3-bromopyruvate (3-BP) and 6-aminonicotinamide (6-AN) yielded similar results (Fig. S3a–e), but inhibition of the electron transport chain with either oligomycin or sodium azide did not affect cell migration or explant size (Fig. S3f–h). Thus, high glycolytic flux is specifically required for proper neural crest migration.
Figure 3. Inhibition of glycolysis disrupts neural crest migration.
(a) Measurement of basal OCR and ECAR show that inhibition of glycolysis with 2-DG drives neural crest cells to adopt aerobic, rather than glycolytic, metabolism. (b) Lactate production by neural crest cells is inhibited by 2-DG treatment in a dose-dependent manner. (c) Despite reducing glycolytic flux, 2-DG treatment does not affect ATP levels in neural crest cells. (d) Images of control and 2-DG-treated neural crest explants after 24h of incubation. (e) Inhibition of glycolysis results in smaller neural crest explants. (f) Overlay of tracks of individual cells from a 12h time-lapse movie of a neural fold explant. Neural crest cells were transfected with vectors expressing H2B-RFP and Actin-GFP for live imaging. Cell trajectories in control and 2-DG treated explants show decreased neural crest migration following inhibition of aerobic glycolysis. (g-i) Treatment with 2-DG results in reduction of the maximum distance traveled, total displacement and mean speed of individual neural crest cells. ECAR: Extracellular Acidification Rate; HH: Hamburger and Hamilton stage; NC: neural crest; OCR: Oxygen Consumption Rate. 2-DG : 2- Deoxy-Glucose, Ctrl: Control, HH: Hamburger and Hamilton stage .Error bar represents ± S.E.M. Scale bar: 200um. Also see Fig. S2 and Fig. S3.
To further characterize this phenotype, we employed time-lapse imaging and quantified cell movement following inhibition of glycolysis (Supplemental Movie 1 and 2). By tracking the nuclei of individual migratory cells (Fig. 3f), we observed that 2-DG treatment significantly decreased the maximum linear distance traveled, the total displacement of the cells, and their mean speed (Fig. 3g–i). These changes in cell migration suggested that 2-DG treated neural crest cells were unable to complete EMT. Immunofluorescence for different EMT markers confirmed this hypothesis. Under control conditions, cells at the migratory front of the explants exhibited mesenchymal characteristics, such as low levels of membrane E-cadherin, Paxillin localized to focal adhesions and elongated actin stress fibers (Fig. 4a). However, 2-DG treatment caused these cells to retain epithelial features, such as high levels of membranelocalized CDH1 (E-cadherin), cytoplasmic Paxillin, and a dense Actin mesh (Fig. 4a). To test if aerobic glycolysis was also important for EMT in vivo, we treated stage HH8 chick embryos with 2-DG in EC culture (Chapman et al., 2001) until they reached HH12. While these embryos had normal gross morphology, staining for neural crest marker TFAP2A revealed an EMT phenotype, with the majority of cells trapped adjacent to the dorsal neural tube (Fig. 4b). Notably, these cells also retained high levels of CDH1, when compared to the neural crest cells from control embryos (Fig. S4).
Figure 4. Aerobic glycolysis is required for neural crest EMT.
(a) Immunofluorescence for different EMT markers reveals that cells in 2-DG treated explants retain epithelial features. (b) Transverse sections of HH12 embryos show that 2-DG treatment inhibits neural crest EMT and migration in vivo, resulting in the majority of cells being trapped adjacent to the dorsal neural tube. (c) Nanostring assay comparing gene expression profiles of control and 2-DG treated explants. Genes below the diagonal lines are significantly downregulated following inhibition of aerobic glycolysis. (d) Neural crest genes related to EMT are more strongly affected by 2-DG treatment, while many markers remain unchanged. (e) qPCR analysis confirms the loss of expression of bona fide EMT markers following inhibition of aerobic glycolysis. (f) Motif enrichment analysis performed with H3K27ac peaks in the loci of glycolysis-responsive genes identified the TEAD1 as one of the motifs to be statistically overrepresented. 2-DG : 2- Deoxy-Glucose, Ctrl: Control, NC: neural crest; NT: neural tube.Error bar represents ± S.E.M. Scale bar : 35 um (a), 50 um (b). Also see Fig. S4.
Next, we sought to identify the molecular mechanism linking the Warburg effect to neural crest EMT. To survey the impact of glycolytic inhibition on neural crest gene expression, we performed a Nanostring analysis with a set of 200 probes targeting genes involved in neural crest, neural and placodal development (Bhattacharya et al., 2018). This analysis showed that neural crest cells treated with 2-DG displayed a robust reduction in the expression of EMT transcription factors such as SNAI2, FOXD3, and ETS1 (Fig. 4c). Expression of many neural crest genes (e.g. TFA2B, CRABP1, LMO4) remained unchanged, indicating that glycolysis was preferentially required for the expression of factors related to EMT (Fig. 4d). This was confirmed by qPCR analysis, which revealed a robust downregulation of the bona fide EMT regulators following treatment with 2-DG (Fig. 4e). These results suggested the existence of a mechanism coupling cell metabolism to the neural crest gene regulatory network (Simoes-Costa and Bronner, 2015). To identify a possible molecular pathway that mediates transcriptional changes in response to glycolytic flux, we examined the regulatory regions of the genes most affected by 2-DG treatment in our Nanostring analysis. We compiled the ten active chromatin regions (H3K27Ac+) closest to glycolysis-responsive genes (Fig. 4f) and performed motif enrichment analysis with TRAP (Bailey et al., 2009). This analysis identified an abundance of TEAD1 binding motifs in these sequences (p-value =5.42e-07), suggesting the involvement of the Yap/Tead signaling pathway in the generation of a transcriptional response to changes in the metabolism of neural crest cells.
High glycolytic flux promotes activation of the Yap/Tead pathway
YAP and TEAD are components of the Hippo signaling system that can respond to a variety of stimuli to regulate transcription (Hansen et al., 2015). YAP lacks a DNA binding domain, but it is able to translocate to the nucleus and interact with TEAD (TEA domain family members 1–4) transcription factors to induce gene expression (Zhao et al., 2008). Yap/Tead 220 signaling has been shown to promote invasiveness and metastasis in several cancer types (Zanconato et al., 2016). Recent studies in zebrafish, mouse and neural crest cell culture models have also implicated this pathway in the control of neural crest migration and differentiation (Dooley et al., 2019; Hindley et al., 2016; Wang et al., 2016). Consistent with this, enrichment of active, non-phosphorylated YAP1 in the nuclei of migratory neural crest cells supported that this pathway was important in chick neural crest development (Fig. 5a–c). To test that YAP1/TEAD1 responds to glycolytic flux in neural crest cells, we employed the HOP-GFP and HOP-FLASH reporter transgenes, which consist of multiple TEAD1 sites upstream of a minimal promoter and a reporter gene (Kim and Gumbiner, 2015). Transfection of HOP-GFP revealed that migratory neural crest cells from explants displayed high YAP/TEAD activity, which was repressed upon inhibition of glycolysis with 2-DG (Fig. 5d). Luciferase assays confirmed that the activity of HOP-FLASH was significantly reduced upon treatment with 2-DG (Fig. 5e), while the HIP-FLASH control construct (which contains mutated TEAD1 binding sites) was not affected by the drug (Fig. S5a).
Figure 5. Glycolysis activates Yap/Tead signaling in neural crest cells by promoting YAP1-TEAD1 interaction.
(a-c) Transverse section showing immunofluorescence for the non-phosphorylated form of YAP1 (Active YAP) and neural crest marker TFAP2B in an HH12 embryo. Arrows show enrichment of Active YAP in migratory neural crest cells. Inserts (b-c) depict nuclei marked by blue arrows. (d) Effect of inhibition of aerobic glycolysis on Yap signaling reporter. Neural crest 460 cells electroporated with HOP-GFP reporter and the transfection control plasmid pCI-CherryRas in control and in the presence of 2-DG. (e) Luminescence assay with luciferase version of the reporter (HOP-Flash) shows a reduction in Yap/Tead signaling activity.
(f)Immunofluorescence for Active YAP1 in control and 2-DG explants. (g) Quantification of the nuclear intensity of active YAP1 staining shows a non-significant difference between control and 2-DG treated cells. (h) Proximity ligation assay (PLA) performed to detect YAP1 and TEAD1 interaction frequency in control vs 2-DG treated explants. (i) Quantification of PLA puncta revealed a significant decrease in YAP1-TEAD1 interaction frequency upon glycolytic inhibition. 2-DG: 2-Deoxy-Glucose, HH: Hamburger and Hamilton, PLA: Proximity Ligation Assay. Error bar represents ± S.E.M. Scale bar: 50 um (a-c,d), 35 um (f,h). Also see Fig. S5.
Next, we examined how YAP/TEAD is regulated by glycolysis. There are two critical steps for the regulation of the pathway: (i) translocation of YAP to the nucleus or (ii) interaction of YAP1 with TEAD1 (Enzo et al., 2015; Meng et al., 2016). We employed immunofluorescence and Proximity Ligation Assays (PLA) (Greenwood et al., 2015) to examine how inhibition of glycolysis affected each of these processes. While immunostaining for active YAP1 showed no significant changes in nuclear translocation upon 2-DG treatment of explants (Fig. 5f–g) or in vivo (Fig. S5b–c), PLA revealed that the inhibitor greatly reduced the number of YAP-TEAD interactions in the nuclei of neural crest cells (Fig. 5h–i). These results demonstrate that the Warburg effect activates of YAP1/TEAD1 signaling by promoting the assembly of its nuclear effector protein complex.
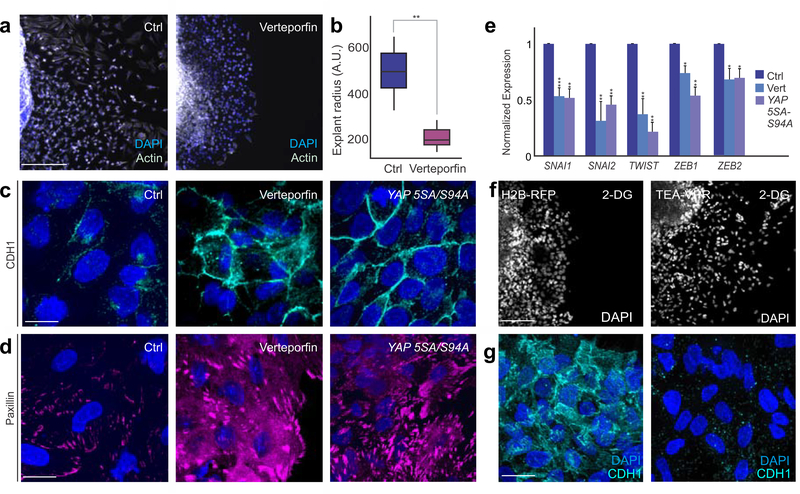
Next, we utilized orthogonal approaches to test if disruption of YAP1-TEAD 1interaction was sufficient to affect neural crest EMT. Treatment of explants with Verteporfin (Feng et al., 2016), a small molecule that inhibits the pathway by preventing YAP1-TEAD1 association (Fig. S6), disrupted neural crest EMT and resulted in a phenotype indistinguishable from 2-DG treatment (Fig. 6a–b,6c–d). Consistent with this, transfection of neural crest cells with a dominant-negative form of YAP1 unable to interact with TEAD (YAP-5SA/S94A), phenocopied 2-DG and Verteporfin treatments (Fig. 6c–d). Both pharmacological and genetic inhibition of YAP1-TEAD1 interaction also resulted in decreased expression of EMT transcription factors in neural crest cells (Fig. 6e). To place Yap/Tead signaling as the metabolism-sensing pathway that promotes EMT, we tested if constitutive activation of the pathway could rescue neural crest cell migration in explants treated with 2-DG. We designed a construct expressing the DNA binding domain of TEAD1 (TEA domain) fused to the activating VPR domain (TEA-VPR). Consistent with our hypothesis, explants expressing TEA-VPR were able to undergo complete EMT and migration, even in the presence of 2-DG (Fig. 6f). TEA-VPR transfected neural crest cells also displayed mesenchymal features like the absence of membrane CDH1 (Fig. 6g). These results show that the Yap/Tead pathway is an essential regulator of neural crest EMT, acting to modulate gene expression and cell behavior in response to glycolytic flux.
Figure 6. Pharmacological inhibition of YAP1-TEAD1 interaction prevents neural crest EMT.
(a-b) Inhibition of YAP1-TEAD1 interaction with Verteporfin inhibits neural crest migration and results in smaller neural crest explants. (c-d) Images of CDH1 and Paxillin staining in control, Verteporfin treated and dominant negative YAP 5SA-S94A transfected neural crest explants, show retention of epithelial features following inhibition of Yap/Tead signaling.(e) qPCR for EMT transcription factors following pharmacological (Vert) and competitive inhibition (mutant YAP5SA-S94A construct) of YAP1-TEAD1 interaction in neural crest cells.(f) Expression of TEA-VPR construct that mimics constitutively active TEAD1, rescues phenotypes of glycolytic inhibition. DAPI staining of control and TEA-VPR overexpressing explants treated with 2-DG. (g) Immunofluorescence for CDH1 shows successful EMT in TEA-VPR expressing explants, even in the presence of 2-DG. Error bars represents ± S.E.M.Scale bar : 200um (a,f), 35um (c-d,g). Also see Fig. S6.
YAP/TEAD interact with tissue-specific enhancers to drive EMT
The outcome from our Nanostring experiment and motif enrichment analysis (Fig. 4c, f) suggests that Yap/Tead signaling directly activates many glycolysis-responsive genes. To define how this signaling pathway promotes neural crest EMT, we employed CUT&RUN (Skene and Henikoff, 2017) (Fig. 7a, Fig. S7a–b) to map the genomic occupancy of YAP1. YAP1 was preferentially bound to intergenic regions (Fig. 7b, Fig. S7c) of open chromatin that are positive for the H3K27Ac mark (Fig. 7c). Motif enrichment analysis of these regions identified the TEADbinding motif as the top hit (p-value<0.00001) (Fig. 7d), indicating that YAP1 binds to these genomic regions with its canonical partner. GO-term analysis of the genes closest to YAP1bound elements confirmed that the pathway regulates a large number of genes involved in cell-cell adhesion (Fig. 7e, Fig. S7d), including both structural proteins and EMT transcription factors. Accordingly, our CUT&RUN analysis identified several YAP1 peaks in the vicinity of EMT genes such as SOX9, ZEB2, ETS1, SNAI2 and MYCN (Fig. 7f). Thus, the genomic profiling of YAP1 indicates that the Yap/Tead1 signaling pathway directly regulates the expression of factors that drive neural crest delamination and migration.
Figure 7. YAP1 activates neural crest EMT via tissue-specific enhancers.
(a) Diagram of CUT&RUN experiments to map genome occupancy of ActYAP1. (b) YAP-associated regions are mostly intergenic. (c) Heatmaps displaying ATAC-seq and H3K27ac signal at ActYAP1 bound regions show that YAP1 binds to open, active chromatin regions.(d) Transcription factor binding sites identified in YAP1-associated peaks. Tead1 was the enriched motif with the highest confidence score. (e) Gene ontology analysis of the genes closest to the YAP1-associated peaks suggests that Yap/Tead signaling is a major regulator of cell signaling and cell-cell adhesion. The plot shows the four most significant biological processes identified by the GO analysis. (f) Examples of regulatory regions associated with YAP1 in the vicinity of EMT regulators Sox9 and Zeb2. (g) Swarm plots showing GFP intensity driven by YAP-associated regions in neural crest cells. Neural crest cells were identified by a specific reporter (Tfap2E1:Che), and GFP expression was measured by flow cytometry. The regions tested were in the loci of the SOX9(Sox9E1), ETS1(Ets1ECR1), ZEB2(Zeb2E1), SNAI2(Snai2E1) and MYCN(MycNE1) genes. GFP intensity analysis was performed in 3500 Tfap2aE1:Che+ cells. (h) Transgenic chick embryos showing tissue-specific activity of Sox9E1, Ets1ECR1, and Zeb2E1. (i-j) Images of embryos transfected with YAP5SA-S94A mutant construct and Sox9E1, Ets1ECR1 and Zeb2E1 respectively. Embryos were injected with the enhancer and a control vector on the left side and the same enhancer and the Yap5SA-S94A on the right side. The expression of enhancer driven GFP was lost on the embryo side transfected with mutant YAP1 construct. (k) Inhibition of Yap/Tead signaling with YAP5SA-S94A prevents neural crest EMT and migration. HH: Hamburger and Hamilton. Scale bar: 200um. Error bars 505 represents ± S.E.M. Also see Fig. S7.
To test whether the YAP1 bound peaks are active enhancers in neural crest cells, we cloned five of these regions in pTK-GFP (Uchikawa et al., 2003) and performed transient transgenesis assays in avian embryos. One of these putative enhancers (Ets1ECR1) had previously been shown to regulate Ets1 expression during early neural crest development (Barembaum and Bronner, 2013). To quantify enhancer activity, we co-transfected the GFP constructs with a neural crest reporter (Tfap2aE1:mCherry) and used flow cytometry to measure GFP levels in HH10 neural crest cells. All five regions tested were able to induce reporter expression above background levels (Fig. 7g), with the enhancers from the SOX9, ETS1, and ZEB2 loci driving the strongest GFP fluorescence in the neural crest cells (Fig. 7h). To further confirm the requirement of YAP1-TEAD1 interaction for the activity of these regulatory elements, we employed the dominant negative YAP-5SA/S94A construct. Embryos were bilaterally electroporated with each enhancer, whereas only the left side of the embryo was co-transfected with YAP-5SA/S94A expression vector. As expected, we observed a significant loss in enhancer activity of Sox9E1, Zeb2E1, and Ets1ECR1 in the presence of the dominant-negative YAP1 construct (Fig. 7i–j), which also prevented proper neural crest migration (Fig. 7k). Notably, the three enhancers were also strongly affected by treatment with 2-DG (Fig. S7e–g), indicating their activity is dependent on glycolysis. Taken together, these results indicate that Yap/Tead signaling is a direct regulator of the EMT program and that it promotes neural crest delamination by activating tissue-specific enhancers.
Discussion
Here we report that the Warburg effect plays an essential function in the control of cell migration during embryonic development. Our results are consistent with previous studies that describe a large degree of metabolic plasticity of embryonic cells. In Drosophila embryos, rapidly proliferating cells upregulate the expression of enzymes and glucose transporters to increase glycolytic flux (Tennessen et al., 2011). During implantation, mouse embryos preferentially produce ATP via glycolysis (Krisher and Prather, 2012), but subsequently undergo a shift to oxidative phosphorylation during organogenesis (Houghton et al., 1996). Furthermore, recent studies in mouse and chick embryos have shown that progenitor cells in the pre-somitic mesoderm display Warburg-like metabolism. In these cells, increased glycolysis mediates the crosstalk between FGF and Wnt signaling to promote a paraxial mesoderm fate (Bulusu et al., 2017; Oginuma et al., 2017). These studies underscore that cellular metabolism has to be tightly regulated during development. Our results support this observation, while also indicating that aerobic glycolysis itself can regulate cellular behavior by impinging on signaling systems, independent of its role in ATP production.
We also describe how metabolism is coupled with genetic programs that control cell identity and behavior. Neural crest development is orchestrated by a complex gene regulatory network, composed by transcription factors, signaling molecules and epigenetic regulators (Simoes-Costa and Bronner, 2015). This system needs to operate in coordination with extrinsic and intrinsic conditions for the accurate progression of morphogenetic events. Extracellular signaling systems, like Wnts and BMPs (Kleber et al., 2005), relay environmental signals to neural crest cells by directly activating nodes of the network. Our results identify Yap/Tead signaling as an intracellular sensor of metabolism, which modulates gene expression in response to glycolytic flux. Importantly, Yap/Tead signaling targets a specific subset of neural crest genes that are part of a regulatory sub-circuit that promotes EMT. Thus, EMT is robustly linked to the energetic status of the cell. This mechanism may ensure that only cells that have been metabolically primed are able to delaminate to engage in long-range migration.
The identification of a metabolic requirement for neural crest migration also carries important clinical implications. Defects in neural crest development result in neurocristopathies, which are amongst the most common congenital disabilities. These conditions are also associated with metabolic abnormalities. Babies born to insulin-dependent diabetic mothers often have oral clefts and other facial defects (Spilson et al., 2001), which suggests excess glucose disrupts neural crest development. Furthermore, babies suffering from Fetal Alcohol Syndrome (FAS) who have reduced fetal glucose levels (Shibley and Pennington, 1997), also develop craniofacial malformations (Johnson et al., 1996). Our findings shed light on the etiology for these congenital diseases, by showing that improper fluctuations in glucose metabolism can adversely affect neural crest EMT and migration. Fetuses of diabetic mothers are exposed to high levels of insulin that promote excess aerobic respiration and oxidative stress in the embryo (Gabbay-Benziv et al., 2015; Morgan et al., 2008). As indicated by our observations, oxidative phosphorylation inhibits neural crest migration. Conversely, in FAS patients, reduced glucose uptake by neural crest cells can prevent aerobic glycolysis in delaminating neural crest cells, thus delaying EMT. While it remains to be established if disruption of neural crest metabolism is the main cause of these conditions, our study opens new avenues for research into the role of metabolism in neurocristopathies.
Finally, this study also highlights an additional parallel between neural crest development and metastasis. The idea of a link between cancer and embryonic development has gained traction in the last decades, as genomic studies have systematically uncovered shared genetic circuits between embryonic progenitor and neoplastic cells. This has been reported for diverse cancer types such as medulloblastoma, glioblastoma (Azzarelli et al., 2018), and more recently, in neural crest-derived cancers (Kaufman et al., 2016; Maguire et al., 2015). Our results show that cancer cells and neural crest cells share metabolic states and that enhanced glycolytic flux promotes cell invasion by regulating the EMT program. This suggests that the Warburg effect is a developmental mechanism with essential functions in the regulation of cell adhesion and migration. Thus, the cooption of aerobic glycolysis by adult cells may underlie the emergence of cellular behaviors that characterize tumorigenesis and malignancy.
Star Methods
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Marcos Simoes-Costa (simoescosta@cornell.edu).
Experimental Models and subject details
Chick embryo collection and electroporation
Fertilized Leghorn White chicken eggs were obtained from the Department of Animal Science, University of Connecticut. Eggs were incubated at 37°C until embryos reached the desired developmental stage. Embryos were collected and cultured according to the EC protocol (Chapman et al., 2001), and staged using the Hamburger and Hamilton staging system (Hamburger and Hamilton, 1951). Metabolic reporters, over-expression constructs and enhancer plasmids were transfected in chick embryos at HH5 by ex ovo electroporation, as previously described (Simoes-Costa et al., 2015). Briefly, expression vectors were injected between the epiblast and vitelline membrane of embryos at a concentration of 1–2ug/ul and electroporated with platinum electrodes (five 50ms pulses of 5.1V, with an interval of 100ms between pulses). Whole embryo injections were performed for neural crest sorting experiments, HOP-GFP transfections, and enhancer reporter assays, while mutant YAP 5SA/S94A and TEA-VPR constructs were transfected in only one side of the embryo. Following electroporation, embryos were cultured in albumin at 37°C until they reached appropriate developmental stages. Embryo survival was >90% and all embryos were screened to ensure uniform electroporation and proper embryo morphology prior to further downstream analysis. For drug treatment experiments, HH8 embryos (4–6 somites) were dissected and incubated in albumin plates containing 2mM of 2-DG, 40 uM of 3-BP and 10uM of Verteporfin. Control embryos were incubated in albumin containing appropriate volumes of the drug solvent (PBS/DMSO). The control and treated embryos were then developed until HH12 and further processed for downstream analysis.
Explant cultures
Neural crest and lateral ectoderm explant cultures were performed as previously described (Coles et al., 2007). Neural crest explants were derived via microdissection of neural folds of HH9 embryos (8ss), and lateral ectoderm was obtained from dissection of ectodermal tissue adjacent to the neural tube. The dissected tissues were then transferred to 6-well cell culture plates or chamber slides coated with fibronectin and containing appropriate volumes of 10%FBS DMEM (10mM glucose + Glutamax) culture media. Unless specified, the explants were incubated for up to 18 hours at 37°C, under normoxic conditions in a CO2 incubator. For drug treatments, the dorsal neural folds were directly plated in DMEM media having a final concentration of 4mM of 2-DG, 40uM of 3-BP, 50uM of 6-NA, 10uM of Verteporfin, 1mM of NaN3 or 1uM of oligomycin (the same volume of vehicle was used for control explants). The explants were also incubated for 18 hours at 37°C in 5% CO2 and normoxic conditions.
Method details
Lactate assays
To obtain samples for this assay, neural crest and ectodermal explants were cultured in 35mm plates for 2h, 7h and 24h. A total of 10 explants were cultured on a plate corresponding to each time point. Following incubation, the media was removed and explants were washed with cold PBS. 100ul of PBS containing 0.5% Tween and protease inhibitors were added to each plate, and the cells were detached by scraping. The lysate was collected and vortexed to ensure complete cell lysis. The samples were immediately frozen in liquid N2 and stored at −80°C, or processed for downstream assay. Cellular lactate levels were measured using the Lactate Assay Kit (Biovision, #K607) in accordance with the manufacturer’s protocol. Values obtained from this assay were normalized to the total protein amount of each sample as measured using the Pierce BCA protein assay kit (Thermo Fisher).
Glucose uptake assays
For performing glucose uptake assay, single dorsal neural folds or ectodermal explants were cultured in individual wells of a clear bottom 96-well plate (Corning). Following appropriate incubation, glucose uptake was measured using Glucose Uptake-GloTM Assay (Promega, #J1341) according to the manufacturer’s instructions. Briefly, glucose-containing media was removed, and explants were incubated in 1mM of 2-DG in PBS for 20 mins at RT. Following cell lysis and neutralization, samples were incubated in 2DG6P detection reagent for 1h and analyzed with a luminometer.
Seahorse assay for measuring basal OCR and ECAR levels in live cells
Chick embryos were incubated until HH7+ (2ss) and HH9+ (8ss), and their dorsal neural folds were micro-dissected in Ringers. 20 neural folds or 15 neural folds were used per HH7+ and HH9+ samples, respectively. For the HH9+ ectodermal samples, adjacent ectodermal regions were dissected in parallel with the neural folds. After collection, the samples were dissociated in 100ul of Accumax for 5 mins. Following dissociation, the samples were centrifuged at 450g for 10 mins, resuspended in 200ul of Seahorse XF complete DMEM media (10mM glucose, 1mM pyruvate, 2mM L-glutamine), and 100ul of the sample was added to an individual well of an 8well Seahorse cell microplate coated with fibronectin (two technical replicates/sample). The total volume of each well was brought up to 180ul with Seahorse XF DMEM, and the microplate was incubated in a non-CO2 37°C incubator for 1h. Following incubation, the seahorse assay was run in Seahorse XFp analyzer according to the manufacturer’s instructions. Post basal ECAR and OCR measurements, the cells from each well were fixed with 4% PFA, stained with DAPI and quantified. The raw OCR and ECAR values were normalized for differences in cell number between individual wells. For ECAR/OCR measurements following 2-DG treatment, explant culture was performed with neural tubes from HH9 embryos in control and 4mM 2-DG conditions. After 18 hours of culture, the explants were dissociated with Accumax, resuspended in 200ul of Seahorse media and plated as described above. Measurements were performed after 1h incubation in a non-CO2 37°C incubator.
Immunohistochemistry
For whole-mount immunohistochemistry, embryos were collected at appropriate developmental stages and fixed in 4% PFA-PB for 20 mins at RT. Post fixation, embryos were dissected from the filter paper and washed in TBS containing 0.1% Triton and 1% DMSO (TBTD). Embryos were blocked at RT for 2 hours in TBTD supplemented with 10% donkey serum and incubated in primary antibody diluted in blocking solution, overnight at 4°C. The following primary antibodies were used: rabbit anti-PFKP (Abcam, 1:200), rabbit anti-LDHA (Abcam, 1:200), mouse anti-GAPDH (Millipore, 1:500), mouse anti-TFAP2B (Santa Cruz Biotechnology, 1:500), rabbit anti-ActYAP1 (Abcam, 1:500), rabbit anti-TFAP2B (Abcam, 1:250), rabbit anti-Sox9 (EMD Millipore, 1:500), mouse anti-E-Cadherin (BD Biosciences, 1:200). Following the primary antibody incubation, embryos were washed, blocked for 30 mins at RT, and stained with appropriate secondary antibodies for 2h at RT. Secondary antibodies used included donkey anti-mouse/rabbit IgG conjugated with Alexa Fluor 488/568/647 or goat anti-mouse Alexa 480/568 (Molecular Probes, 1:3000). Following the secondary antibody step, the embryos were washed, stained with Dapi and post-fixed with 4% PFA for 1h, prior to imaging. Whole-mount images were collected using an upright Zeiss Axio Imager fluorescent microscope and processed as described below.
Cryosectioning
To obtain embryo sections following immunohistochemistry, fixed embryos were washed in 5% sucrose (in PBS) for 3 hours at RT, and in 15% sucrose solution overnight at 4°C. Next, they were incubated in 7.5% porcine gelatin (dissolved in 15% sucrose solution) for 3 hours at 37°C, embedded in silicone molds, snap-frozen in liquid nitrogen and stored at −80oC. 8μM sections were obtained using the CryoStar NX50 (Thermo Fisher). For imaging, the slides were immersed in PBST at 42°C for 15 mins for gelatin removal, washed in PBS and mounted with Vectashield medium containing DAPI (Vector Labs).
Immunohistochemistry of cell suspensions
Immunohistochemistry of dissociated embryos was performed as previously described (Wang et al., 2014). Briefly, the heads of HH8 and HH12 wild type embryos were micro-dissected in Ringers, and dissociated in Accumax Cell dissociation solution (Accutase, SCR006) for 15–20 mins at RT. At least eight HH8 embryo heads and six HH12 heads were used for each antibody staining experiment. Following dissociation, cells were washed in PBS and fixed in 0.4% PFA solution for 15 mins at RT. Post-fixation, cells were permeabilized with PBS+0.3% Triton solution and blocked with 1% BSA solution (in PBS+0.1% Tween 20) for 1h at RT. The cells were subsequently incubated overnight with appropriate dilutions of primary antibody in blocking solution. The antibodies rabbit anti-PFKP, rabbit anti-LDHA, rabbit anti-GLUT3 and rabbit anti-TFAP2B were used at a dilution of 1:200, while mouse anti-GAPDH and mouse anti-TFAP2B were used at a dilution of 1:500. Following primary antibody staining, the cells were washed in PBS+0.1% Tween 20 solution and incubated with appropriate secondary antibodies diluted to a concentration of 1:1000 in blocking solution for 1h at RT. The samples were then washed twice in PBS+0.1% Tween 20 and the antibody staining intensity was measured by FACS analysis using the Attune Nxt flow cytometer at the Cornell Flow facility. The cytometry data was analyzed using the FCS Express 6 software as described below.
Metabolic reporter fluorescence analysis
To measure the ratio of NAD+/NADH in the neural crest and neural tube, HH5 embryos were co-electroporated with 1 ug/ul of pCI-Rex-YFP, which was sub-cloned from the original plasmid provided by Vsevolod Belousov (Addgene plasmid #48247) (Bilan et al., 2014). pCI-Rex-YFP was co-transfected with 1.5ug/ul of either neural crest specific enhancer Tfap2aE1-mCherry (Bhattacharya et al., 2018) or neural tube specific enhancer Sox2N2-mCherry enhancer (Uchikawa et al., 2003). To measure intracellular glucose levels, embryos were electroporated with the glucose reporter Green Glifon50, a gift from Tetsuya Kitaguchi (Addgene plasmid #126206) (Mita et al., 2019), along with the Tfap2aE1 or Sox2N2 reporter constructs. Post electroporation, the embryos were incubated at 37°C ex ovo until they reached HH8 (~8 hours) or HH10 (~10 hours). Embryo heads were dissected in Ringers and rapidly dissociated in Accumax for 15 mins at RT. Following dissociation, the fluorescence intensity of the Rex:YFP and Green Glifon50 in Tfap2aE1-mCherry+ neural crest cells and Sox2N2-mCherry+ neural progenitor cells was measured using the Attune NxT Flow Cytometer (Thermo Fisher). Additionally, HH10 embryos electroporated with each metabolic reporter and enhancers construct combination were also fixed in 4% PFA for 2h at RT and further processed for sectioning and imaging. Both the metabolic reporter constructs were subcloned into the pCIH2B-RFP (Betancur et al., 2010) vector backbone to ensure robust expression in the chick embryo. To confirm that this subcloning does not bias the expression of the constructs, we measured the fluorescence activity of the pCI:EGFP vector in neural crest and neural tube cells of HH8 embryos by flow cytometry analysis. Upon performing this experiment, we observed no significant difference in GFP intensity between the tissues (Fig. S1a).
Validation of metabolic reporters
To validate that the Rex:YFP reporter responds to changes in cellular NAD+/NADH ratio, HH8 embryos transfected with pTK-Tfap2E1-mCherry and the pCI-Rex-YFP plasmids were incubated on albumin plates containing 10μM pyruvate for 20 mins. The embryos were then quickly dissociated in Accumax and the intensity of Rex-YFP in mCherry+ neural crest cells was measured in control vs pyruvate treated samples by flow cytometry. To confirm the activity of Green Glifon50, HH8 embryos co-electroporated with pTK-Tfap2E1-mCherry and the glucose sensor were incubated on albumin plates containing 50μM glucose for 40 mins. Following incubation, the embryos were processed as described above. The intensity of Green Glifon50 in control and glucose treated Tfap2aE1+ neural crest cells was assayed by FACS analysis in the Attune NxT cytometer (Fig. S1b–c).
2-NBDG Uptake assay
20ul of 1mM solution of the fluorescent glucose analog 2-NBDG was applied to the dorsal and ventral side of the HH8 and HH10 chick embryos electroporated with the pTK-Tfap2E1-mCherry enhancer construct. The embryos were incubated in albumin plates for 2h at 37°C. Following incubation, embryos were dissected and processed for flow cytometry analysis as described above. A subset of the HH10 embryos incubated with the 2-NBDG reporter was also fixed in 4% PFA for 2h at RT and further processed for sectioning and imaging.
Luciferase Assays
To quantitatively measure Yap/Tead signaling activity in vivo, HH5 avian embryos were co-electroporated with HOP-flash plasmid or its mutated version HIP flash (Addgene plasmid # 83467) (Kim and Gumbiner, 2015) and the pRL-TK control reporter vector (Promega, #E2231). The embryos were then cultured ex ovo until HH9. The two dorsal neural folds of each embryo were micro-dissected, and one explant was cultured in control media while the other was cultured in 2-DG containing media for 18h, in white, clear-bottom 96 well plates. Following explant culture, a luciferase assay was performed using the Dual-Luciferase Assay kit (Promega, #E1910) according to the manufacturer’s protocol. Post luminometer reading, firefly luciferase values were normalized to Renilla luciferase measurements to account for differences in transfection efficiency and number of cells. Normalized luciferase values were compared between control and 2-DG treated explants obtained from the same embryo.
Live imaging of neural crest explants
HH5 embryos were co-electroporated with pCI-H2B-RFP and pCAG-mGFP-Actin (Addgene plasmid # 21948)(Murakoshi et al., 2008) and cultured ex ovo until HH9-. The dorsal neural fold of each embryo was micro-dissected and plated on fibronectin-coated 4-well chamber slides, with each well containing either control DMEM or DMEM+2-DG. Before imaging, the explants were incubated for 3h until they were completely attached to the plates. Live imaging was performed using the inverted Andor/Olympus Spinning Disk Confocal microscope at the BRC Imaging Facility, Cornell University. The chamber slides were maintained at 37°C at a 5% CO2 condition and imaged for a total of 12h (1 frame/12mins) using the 10X objective. For all live imaging experiments, control and 2-DG treated explants were imaged simultaneously. Post imaging, nuclear tracking analysis was performed using the TrackMate plug-in in Fiji (Tinevez et al., 2017).
Immunohistochemistry of explant cultures and proximity ligations assay (PLA)
For immunohistochemistry experiments, explants were fixed with 4% PFA for 10 mins at RT, followed by permeabilization with 0.1% NP40 for 30 mins at 37°C. Next, the cells were blocked with 1% BSA in PBS for 30 mins at 37°C, prior to incubation with the primary antibody for 1h at 37°C. Primary antibodies were prepared in blocking solution, and were used in the following dilutions: mouse anti-E-cadherin antibody (BD Biosciences) (1:200), mouse anti-Paxillin antibody (BD Biosciences) (1:200), and rabbit anti-Active Yap1 (Abcam)(1:200), anti-pH3(S10) (Abcam, 1:200), anti-Caspase3 (R&D Systems, 1:200). Following primary antibody incubation, the explants were washed 4X times in PBS for 10 mins and incubated in a secondary antibody cocktail for 30 mins at 37°C. Finally, cells were washed 3X times in PBS and stained with DAPI or Phalloidin for 20 mins at RT. Post antibody staining, imaging was performed using Andor/Olympus Spinning Disk Confocal microscope at BRC facility, Cornell University.For Proximity Ligation Assay (PLA), explants were fixed and permeabilized as described above.The primary antibodies used were: Mouse Anti-YAP1 (DSHB) (1:5) and Rabbit Anti-TEAD1 (Abcam) (1:200). Following primary antibody incubation, the remaining steps of PLA were performed using reagents from the Duolink PLA detection kit (Sigma Aldrich, DUO92101) according to the manufacturer’s protocol.
Quantitative reverse transcription PCR (RT-PCR)
To quantify changes in gene expression following 2-DG/verteporfin treatments, we compared neural crest explants (obtained from the two sides of the same embryo), cultured in control vs. drug treatment media. The explants were lysed in the lysis buffer from Power SYBR Green Cells-to-CT Kit (Thermo Fisher). RNA extraction and cDNA preparation were performed according to the suggested protocol. RT-PCR was performed using the Power Sybr Green PCR master mix (Thermo Fisher, 4368577) in an ABI viia7 RT-PCR machine. Ct values of all genes were normalized to reference gene HPRT and presented as a fold-change of the control sample ddCT).
Nanostring analysis
To access the global effect of glycolytic inhibition on the neural crest gene regulatory network, Nanostring analysis was performed for control and 2-DG treated neural crest explants. As described above, pairs of dorsal neural folds were dissected from individual HH9 embryos, and one neural tube was cultured in control DMEM, while the other explant was cultured in DMEM+2-DG for 18 hrs. Following culture, the cells were lysed in 5ul of Cell-to-CT lysis buffer (Cell-to-CT kit, Thermo Fisher). The cell lysate was hybridized to a Nanostring Probe Set containing 200 probes for neural crest, placode and neural genes (Bhattacharya et al., 2018), at 65°C for 16 hrs. Nanostring data was analyzed using the nSolver software package.
Cloning of expression vectors
The TEA-VPR expression vector is a fusion construct of the sequence of the TEA domain of the avian TEAD1 protein and the sequence of VPR activating domains. The sequence of the TEA domain was amplified from a Gallus gallus HH9 cDNA library, and the VPR sequence was amplified from PB-TRE-dcas9-VPR vector, a gift from George Church (Addgene plasmid # 63800) (Chavez et al., 2015). The two sequences were cloned in the pCI-H2B-RFP vector (Betancur et al., 2010), upstream of the H2B-RFP coding sequence. The YAP-5SA/S94A was a gift from Kunliang Guan (Addgene plasmid # 33103) (Zhao et al., 2008). The HOP-GFP vector was derived from the HOP-Flash vector by swapping the Firefly luciferase sequence with the EGFP sequence amplified from pTK-EGFP vector backbone.
CUT&RUN
Neural folds were dissected from HH9+ embryos (n=20 per CUT&RUN experiment). Cells were dissociated in Accumax (Accutase, SCR006) for 20 minutes at RT under mild agitation. CUT&RUN experiments were carried out as described previously described (Rothstein and Simoes-Costa, 2019). Briefly, cells were immobilized on BioMag Plus Concanavalin A magnetic beads (Bangs Laboratories, BP531) and incubated with rabbit anti-Active-Yap1 (Abcam, ab205270) (1:50) or anti-Histone H3 (acetyl K27) (Abcam, ab177178) (1:50) antibody overnight at 4°C. After washing away unbound antibody, protei n A-MNase was added to a final concentration of 700ng/mL and incubated for 1 h at 4°C. Cells were cooled to 0°C and CaCl2 was added to a final concentration of 2 mM to activate the MNase enzyme. MNase digestion was performed for 45 minutes and terminated by the addition of 2XSTOP buffer containing heterologous Saccharomyces cerevisiae spike-in DNA at a concentration of 2pg/mL. The protein-DNA complexes were released by centrifugation and digested with proteinase K for 10 minutes at 70°C. DNA fragments were isolated via ph enol-chloroform extraction and ethanol precipitation. Protein A-MNase and spike-in DNA were kindly provided by Dr. Steven Henikoff (Skene and Henikoff, 2017).
CUT&RUN library preparation
CUT&RUN libraries were prepared using the NEBNext Ultra II DNA Library Prep Kit (New England Biolabs, E7645) using the suggested protocol. Fragment analysis was performed with ABI 3730xl DNA Analyzer to perform quality control for the libraries. Equimolar concentrations of the libraries were pooled using the KAPA Library Quantification Kit - ROX Low (Roche, 07960336001) and sequenced with paired-end 37bp reads on an Illumina NextSeq500 instrument.
Enhancer analysis
Yap-bound putative enhancers in the loci of to neural crest genes were amplified from HH10 chicken gDNA and cloned in the pTK-EGFP (Uchikawa et al., 2003). To assess enhancer activity, HH5 embryos were co-electroporated with Tfap2aE1-mCherry and the pTK-EGFP constructs, and incubated until HH9. To measure the activity of each enhancer in mCherry+ neural crest cells, embryos were dissociated and analyzed in the Attune NxT cytometer. These values were plotted to determine the specificity and strength of each enhancer in delaminating neural crest cells.
Functional analysis of enhancer activity
To assess the effect of 2-DG treatment on the activity of neural crest specific enhancers, HH5 avian embryos were electroporated with Ets1ECR1, Sox9E1 or Zeb2E1 along with control plasmid pCI-Cherry-Ras. The embryos were cultured ex ovo until HH9. The two dorsal neural folds of each embryo were micro-dissected, and one explant was cultured in control media while the other was cultured in 2-DG containing media in chamber slides. Following 18h of incubation, the explants were fixed in 4% PFA for 10 mins at RT, stained with DAPI and imaged using an inverted Olympus Spinning Disk Confocal microscope.
Quantification and Statistical Analysis
Flow cytometry data analysis
Flow cytometry data obtained from the metabolic reporter assays and the antibody-staining experiments were analyzed using the FCS Express 6 software package. After assigning appropriate forward scatter and side scatter gates, the neural crest and/or neural populations were identified as cells having enhancer reporter fluorescence or antibody staining intensity above the threshold that was set based on the negative and single-color controls. Next, we obtained the intensity values of individual cells in the population of interest (neural crest, neural or whole embryo). To compare samples and replicates across different experiments, the intensity values were log-transformed and normalized by the variance of each sample.
Live imaging analysis
Analysis of live-cell imaging experiments was performed using the Trackmate plugin in ImageJ.To track the movement of individual cell nuclei, the program was provided with 60 frames/movie of the H2B-RFP channel (frames were captured every twelve minutes). Minimum threshold values of track duration and track displacement were set in order to restrict the analysis to (i) nuclei that could be tracked for the entire duration of the movie and (ii) cells with an average displacement that was above background levels. From this analysis, we obtained values for total displacement (in pixels) and the mean speed of each nucleus (in pixels/frame) for the two conditions. Furthermore, we measured the maximum distance each nucleus travels in a straight line before changing direction (maximum distance traveled). The values for these parameters were obtained for all tracked nuclei from a total of 5 movies/condition. We used these values to plot the graphs depicted in Fig. 3 and to perform the corresponding statistical analysis.
CUT&RUN data analysis
Paired-end sequencing reads from the CUT&RUN libraries were trimmed using Cutadapt (Martin, 2011). Reads were filtered for those with a minimum length of 25bp or longer and aligned to the reference chicken Galgal5 assembly using Bowtie2 (Langmead and Salzberg, 2012). Picard MarkDuplicates tool was used to mark duplicate reads and BAM files were filtered with SAMtools to discard unmapped reads (those that were not the primary alignment, reads failing platform/vendor quality checks, and PCR/optical duplicates (-f 2 -F 1804)). Peak calling was performed using MACS version 2.1 with a p-value cutoff of 0.01. Representative heatmaps showing the H3K27ac and ATAC-seq signal at YAP1 bound peaks were generated using the deepTools2 package (Ramirez et al., 2016). We also utilized this program to determine the correlation between the CUT&RUN replicates for ActYAP1. To identify transcription factor motifs in the genomic regions occupied by Yap1, motif enrichment analysis was performed using the TRAP web tools (Thomas-Chollier et al., 2011) on 250bp sequences flanking the summit of each peak on either side. We also used the Bioconductor package ChIPseeker to annotate the Yap1 bound peaks and determine their occupancy genome-wide compared to a random peak-set (Yu et al., 2015). Lastly, to identify putative genes regulated by Yap/Tead signaling, the closest gene was assigned to each peak using the Bedtools closest function. The GO-category analysis was performed on these putative Yap targets by utilizing the ClusterProfiler package (Yu et al., 2012) to assay for over-represented Biological Processes (BP) having a p-value cut off of 0.01 post Bonferonni correction.
Additional Statistical Analyses
The metabolic assays including lactate production and Seahorse analysis were performed with 3 independent biological replicates (each iteration having two technical replicates). The glucose uptake assay was performed with eight individual neural crest and lateral ectoderm replicates. The flow cytometry analysis for metabolic reporter activity included at least six embryos per stage per reporter construct/enhancer, and the data from multiple embryos were pooled to plot the graphs. The immunohistochemistry experiments in the embryo were performed with at least 5 embryos, and the functional experiments following 2-DG and 3-BP drug treatment included six control and six treated embryos. For all functional experiments utilizing neural crest explants at least 10 explants were analyzed per condition in each experiment. The Nanostring experiment was performed with three replicates of control and 2-DG treated neural fold explants. The CUT&RUN experiment for ActYAP1 was repeated three times, while CUT&RUN for H3K27ac was replicated twice. The n and p values of all quantitative experiments are listed in Table S1. Student’s t-test (one-tailed) was performed to calculate p-values for functional experiments and p<0.05 were considered to be significant. Wilcoxon test was used to calculate p-values for all flow cytometry data analysis and for comparing the distributions of mean speed and total distance traveled (as obtained from the live imaging experiments), to account for the nonparametric distribution of these values.
Data and Software availability
The CUT&RUN datasets for ActYAP1 and H3K27ac in chick embryos have been deposited to the Gene Expression Omnibus GSE142101.
Supplementary Material
Supplemental Movie 1. (Related to Figure 3) Time-lapse movie of control neural crest explant.
Supplemental Movie 2. (Related to Figure 3) Time-lapse movie of neural crest explant treated with 2-DG.
Supplemental Table 1. (Related to Star Methods) Number of biological replicates and p-values for quantitative experiments.
Highlights.
Neural crest cells display the metabolic adaptation known as the Warburg effect
Enhanced glycolysis is required for neural crest epithelial-to-mesenchymal transition
Aerobic glycolysis promotes cell motility via the Yap/Tead signaling pathway
Yap/Tead interact with tissue-specific enhancers to promote neural crest migration
Acknowledgments
We are indebted to Adam Wojno for cell-sorting assistance at the BRC Flow Cytometry Cell Sorting Facility in Cornell University. We thank Dr. Rebecca M. Williams and Carol Bayles for support in image acquisition through the BRC Imaging Facility. We are also thankful to Austin Hovland for support with RNA-seq data analysis. This work was supported by NIH grants R00DE024232 and R01DE028576 to M.S.-C, a Basil O’Connor Starter Scholar Award from March of Dimes to M.S.-C, and a Cornell CVG Scholars Award to D.B.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, and Nieto MA (2009). Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. The Journal of clinical investigation 119, 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzarelli R, Simons BD, and Philpott A (2018). The developmental origin of brain tumours: a cellular and molecular framework. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, and Noble WS (2009). MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37, W202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barban S, and Schulze HO (1961). The effects of 2-deoxyglucose on the growth and metabolism of cultured human cells. J Biol Chem 236, 1887–1890. [PubMed] [Google Scholar]
- Barembaum M, and Bronner ME (2013). Identification and dissection of a key enhancer mediating cranial neural crest specific expression of transcription factor, Ets-1. Dev Biol 382, 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, and Sauka-Spengler T (2010). Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proceedings of the 900 National Academy of Sciences of the United States of America 107, 3570–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Rothstein M, Azambuja AP, and Simoes-Costa M (2018). Control of neural crest multipotency by Wnt signaling and the Lin28/let-7 axis. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilan DS, Matlashov ME, Gorokhovatsky AY, Schultz C, Enikolopov G, and Belousov VV (2014). Genetically encoded fluorescent indicator for imaging NAD(+)/NADH ratio changes in different cellular compartments. Biochim Biophys Acta 1840, 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulusu V, Prior N, Snaebjornsson MT, Kuehne A, Sonnen KF, Kress J, Stein F, Schultz C, Sauer U, and Aulehla A (2017). Spatiotemporal Analysis of a Glycolytic Activity Gradient Linked to Mouse Embryo Mesoderm Development. Developmental cell 40, 331–341 e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y, Han MJ, Cha HJ, Zoldan J, Burkart A, Jung JH, Jang Y, Kim CH, Jeong HC, Kim BG, et al. (2017). Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis. Nat Cell Biol 19, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, and Lumsden A (2001). Improved method for chick whole-embryo culture using a filter paper carrier. Developmental dynamics : an official publication of the American Association of Anatomists 220, 284–289. [DOI] [PubMed] [Google Scholar]
- Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, E PRI, Lin S, Kiani S, Guzman CD, Wiegand DJ, et al. (2015). Highly efficient Cas9-mediated transcriptional programming. Nat Methods 12, 326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu J, and Chen SY (2013). Over-expression of Nrf2 diminishes ethanol-induced oxidative stress and apoptosis in neural crest cells by inducing an antioxidant response. Reprod Toxicol 42, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles EG, Taneyhill LA, and Bronner-Fraser M (2007). A critical role for Cadherin6B in regulating avian neural crest emigration. Developmental biology 312, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley CM, Wali N, Sealy IM, White RJ, Stemple DL, Collins JE, and Busch-Nentwich EM (2019). The gene regulatory basis of genetic compensation during neural crest induction. PLoS Genet 15, e1008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, et al. (2015). Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J 34, 1349–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein T, Gatenby RA, and Brown JS (2017). The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PLoS One 12, e0185085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Gou J, Jia J, Yi T, Cui T, and Li Z (2016). Verteporfin, a suppressor of YAP-TEAD complex, presents promising antitumor properties on ovarian cancer. Onco Targets Ther 9, 5371–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay-Benziv R, Reece EA, Wang F, and Yang P (2015). Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World J Diabetes 6, 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C, Ruff D, Kirvell S, Johnson G, Dhillon HS, and Bustin SA (2015). Proximity assays for sensitive quantification of proteins. Biomol Detect Quantif 4, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Meyle KD, Lange MK, Klima M, Sanderhoff M, Dahl C, Abildgaard C, Thorup K, Moghimi SM, Jensen PB, et al. (2013). Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget 4, 584–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, and Hamilton HL (1951). A series of normal stages in the development of the chick embryo. Journal of morphology 88, 49–92. [PubMed] [Google Scholar]
- Hansen CG, Moroishi T, and Guan KL (2015). YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol 25, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley CJ, Condurat AL, Menon V, Thomas R, Azmitia LM, Davis JA, and Pruszak J (2016). The Hippo pathway member YAP enhances human neural crest cell fate and migration. Sci Rep 6, 23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton FD, Thompson JG, Kennedy CJ, and Leese HJ (1996). Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev 44, 476–485. [DOI] [PubMed] [Google Scholar]
- Hsu PP, and Sabatini DM (2008). Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707. [DOI] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW II, Sato Y, and Andreasen NC (1996). Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am J Med Genet 61, 329–339. [DOI] [PubMed] [Google Scholar]
- Kaufman CK, Mosimann C, Fan ZP, Yang S, Thomas AJ, Ablain J, Tan JL, Fogley RD, van Rooijen E, Hagedorn EJ, et al. (2016). A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science 351, aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik DK, Bhattacharya A, Mirzaei R, Rawji KS, Ahn Y, Rho JM, and Yong VW (2019). Enhanced glycolytic metabolism supports transmigration of brain-infiltrating macrophages in multiple sclerosis. The Journal of clinical investigation 129, 3277–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NG, and Gumbiner BM (2015). Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol 210, 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore M, Cheung KCP, Fu H, Bonacina F, Wang G, Coe D, Ward EJ, Colamatteo A, Jangani M, Baragetti A, et al. (2017). Regulatory T Cell Migration Is Dependent on Glucokinase-Mediated Glycolysis. Immunity 47, 875–889 e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber M, Lee HY, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, and Sommer L (2005). Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol 169, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisher RL, and Prather RS (2012). A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol Reprod Dev 79, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforgia N, Di Mauro A, Favia Guarnieri G, Varvara D, De Cosmo L, Panza R, Capozza M, Baldassarre ME, and Resta N (2018). The Role of Oxidative Stress in the Pathomechanism of Congenital Malformations. Oxid Med Cell Longev 2018, 7404082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J (2019). The Warburg metabolism fuels tumor metastasis. Cancer Metastasis Rev 38, 157164. [DOI] [PubMed] [Google Scholar]
- Lu J, Tan M, and Cai Q (2015). The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett 356, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire LH, Thomas AR, and Goldstein AM (2015). Tumors of the neural crest: Common themes in development and cancer. Dev Dyn 244, 311–322. [DOI] [PubMed] [Google Scholar]
- Martin M (2011). Cutadapt removes adapter sequences from high-throughput sequencing 985 reads. EMBnetjournal 17, 10–12. [Google Scholar]
- Meng Z, Moroishi T, and Guan KL (2016). Mechanisms of Hippo pathway regulation. Genes Dev 30, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita M, Ito M, Harada K, Sugawara I, Ueda H, Tsuboi T, and Kitaguchi T (2019). Green Fluorescent Protein-Based Glucose Indicators Report Glucose Dynamics in Living Cells. Anal Chem 91, 4821–4830. [DOI] [PubMed] [Google Scholar]
- Morgan SC, Relaix F, Sandell LL, and Loeken MR (2008). Oxidative stress during diabetic pregnancy disrupts cardiac neural crest migration and causes outflow tract defects. Birth Defects Res A Clin Mol Teratol 82, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M, et al. (2015). Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21, 392–402. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Lee SJ, and Yasuda R (2008). Highly sensitive and quantitative FRET-FLIM imaging in single dendritic spines using improved non-radiative YFP. Brain Cell Biol 36, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo H, Tortorella SM, Ververis K, and Karagiannis TC (2015). The Warburg effect: molecular aspects and therapeutic possibilities. Mol Biol Rep 42, 825–834. [DOI] [PubMed] [Google Scholar]
- Oginuma M, Moncuquet P, Xiong F, Karoly E, Chal J, Guevorkian K, and Pourquie O (2017). A Gradient of Glycolytic Activity Coordinates FGF and Wnt Signaling during Elongation of the Body Axis in Amniote Embryos. Dev Cell 40, 342–353 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips FS (1941). The oxygen consumption of the early chick embryo at various stages of development. J Exp Zool 86, 257–289. [Google Scholar]
- Ramirez F, Ryan DP, Gruning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dundar F, and Manke T (2016). deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44, W160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanoff AL, and Romanoff AJ (1949). The avian egg (New York,: J. Wiley; ). [Google Scholar]
- Rothstein M, and Simoes-Costa M (2019). Heterodimerization of TFAP2 pioneer factors drives epigenomic remodeling during neural crest specification. Genome research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibley IA Jr., and Pennington SN (1997). Metabolic and mitotic changes associated with the fetal alcohol syndrome. Alcohol Alcohol 32, 423–434. [DOI] [PubMed] [Google Scholar]
- Simoes-Costa M, and Bronner ME (2013). Insights into neural crest development and evolution from genomic analysis. Genome research 23, 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, and Bronner ME (2015). Establishing neural crest identity: a gene regulatory recipe. Development 142, 242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, Stone M, and Bronner ME (2015). Axud1 Integrates Wnt Signaling and Transcriptional Inputs to Drive Neural Crest Formation. Developmental cell 34, 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, and Henikoff S (2017). An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilson SV, Kim HJ, and Chung KC (2001). Association between maternal diabetes mellitus and newborn oral cleft. Ann Plast Surg 47, 477–481. [DOI] [PubMed] [Google Scholar]
- Tennessen JM, Baker KD, Lam G, Evans J, and Thummel CS (2011). The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab 13, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, and Mayor R (2012). Neural crest delamination and migration: from epitheliumto-mesenchyme transition to collective cell migration. Developmental biology 366, 34–54. [DOI] [PubMed] [Google Scholar]
- Thomas-Chollier M, Hufton A, Heinig M, O’Keeffe S, Masri NE, Roider HG, Manke T, and Vingron M (2011). Transcription factor binding predictions using TRAP for the analysis of ChIP-seq data and regulatory SNPs. Nat Protoc 6, 1860–1869. [DOI] [PubMed] [Google Scholar]
- Tinevez JY, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E, Bednarek SY, Shorte SL, and Eliceiri KW (2017). TrackMate: An open and extensible platform for single-particle tracking. Methods 115, 80–90. [DOI] [PubMed] [Google Scholar]
- Tsoi J, Robert L, Paraiso K, Galvan C, Sheu KM, Lay J, Wong DJL, Atefi M, Shirazi R, Wang X, et al. (2018). Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 33, 890–904 e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchikawa M, Ishida Y, Takemoto T, Kamachi Y, and Kondoh H (2003). Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Developmental cell 4, 509–519. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, and Thompson CB (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Bajikar SS, Jamal L, Atkins KA, and Janes KA (2014). A time- and matrixdependent TGFBR3-JUND-KRT5 regulatory circuit in single breast epithelial cells and basallike premalignancies. Nat Cell Biol 16, 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xiao Y, Hsu CW, Martinez-Traverso IM, Zhang M, Bai Y, Ishii M, Maxson RE, Olson EN, Dickinson ME, et al. (2016). Yap and Taz play a crucial role in neural crest-derived craniofacial development. Development 143, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O (1925). The metabolism of carcinoma cells. The Journal of Cancer Research 9, 148–163. [Google Scholar]
- Warburg O, Posener K, and Negelein E (1924). On the metabolism of carcinoma cells. Biochem Z 152, 309–344. [Google Scholar]
- Yu G, Wang LG, Han Y, and He QY (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, and He QY (2015). ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383. [DOI] [PubMed] [Google Scholar]
- Zanconato F, Cordenonsi M, and Piccolo S (2016). YAP/TAZ at the Roots of Cancer. Cancer Cell 29, 783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al. (2008). TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22, 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1. (Related to Figure 3) Time-lapse movie of control neural crest explant.
Supplemental Movie 2. (Related to Figure 3) Time-lapse movie of neural crest explant treated with 2-DG.
Supplemental Table 1. (Related to Star Methods) Number of biological replicates and p-values for quantitative experiments.
Data Availability Statement
The CUT&RUN datasets for ActYAP1 and H3K27ac in chick embryos have been deposited to the Gene Expression Omnibus GSE142101.