Abstract
Diabetes mellitus (DM), a complex metabolic disease, has become a global threat to human health worldwide. Over the past decades, an enormous amount of effort has been devoted to understand how microRNAs (miRNAs), a class of small non-coding RNA regulators of gene expression at the post-transcriptional level, are implicated in DM pathology. Growing evidence suggests that the expression signature of a specific set of miRNAs has been altered in the progression of DM. In the present review, we summarize the recent investigations on the miRNA profiles as novel DM biomarkers in clinical studies and in animal models, and highlight recent discoveries on the complex regulatory effect and functional role of miRNAs in DM.
Keywords: Diabetes Mellitus, microRNA
Introduction
Diabetes mellitus (DM) is an age-related metabolic disorder affecting 347 million people in modern society. Expanding its prevalence beyond developed countries, DM has emerged as a global public health issue associated with a high morbidity and mortality. According to an estimation by the International Diabetes Federation (IDF), the global population affected by diabetes will reach 552 million by the year 2030 [1]. DM is a complex disease characterized by an insufficient secretion of insulin from pancreatic β-cells that prevents the normal maintenance of blood glucose homeostasis. There are two major forms of diabetes. Type 1 diabetes mellitus (T1DM) is due to lack of insulin hormone production from pancreatic β-cells, while type 2 diabetes mellitus (T2DM) results from ineffective insulin response [2]. The clinical manifestations of DM restrict its timely diagnosis, which may be delayed by several years. This delayed prediction often results in chronic complications, including cardiovascular disease associated with DM [3].
According to a recent estimation, there are more than 2000 mature human miRNAs that have been recognized since the first miRNA was identified in 1993 [4]. MiRNAs have emerged as major regulators of gene expression and are involved in the onset and progression of various diseases [5–7]. A growing body of evidence indicates that a specific set of miRNAs has an altered expression profile in the progression of DM [8–11], making these biomolecules potential biomarkers for the prognosis, diagnosis and management of disease. Individual miRNAs or whole miRNA clusters associated with diabetes have been observed to be dysregulated in expression and activity, therefore generating a rising interest in their therapeutic use as clinical targets. The diverse role of miRNAs in the etiology and pathogenesis of DM has been widely explored [12–14], and new findings are continuously emerging. In this present review, we summarize the recent findings on the potential role of miRNAs as biomarkers in the settings of diabetes, and the dysregulation of miRNAs and their molecular targets, to obtain a better understanding of the application of miRNAs in the development of DM.
Role of miRNAs in Regulating Gene Expression
MiRNAs are small single-stranded non-coding RNAs involved in nearly every stage of biological processes. They are best known to regulate gene expression at the post-transcriptional level [15]. Indeed more than 60% of protein-coding genes in mammalian cells are conserved targets of miRNAs, which partially base pair with the 3’UTR of the mRNA targets through their 5’-proximal seeding region [16]. This complex targeting process is tightly regulated under different cellular conditions, and eventually leads to the reduced expression of the target gene [17,18].
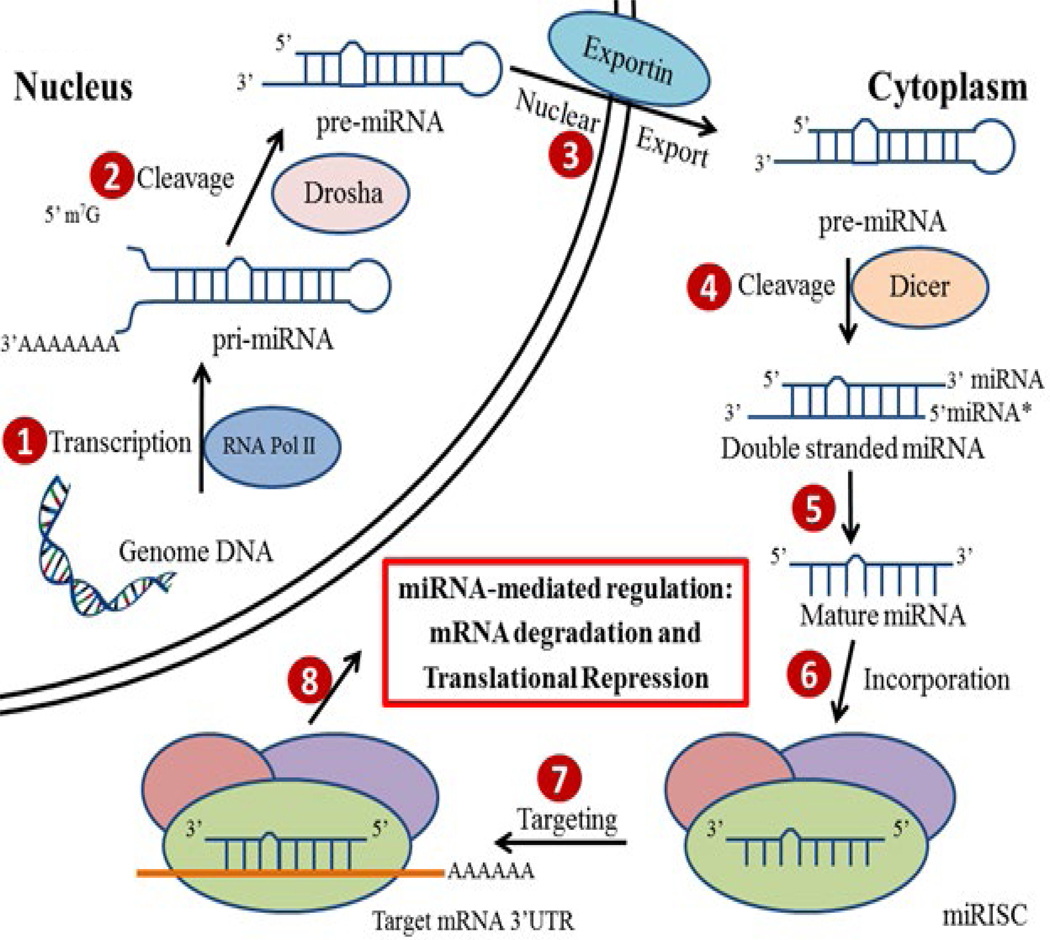
MiRNAs are encoded within the genomes of various species, ranging from protozoans to mammals [19]. Even though most of the mature miRNAs are approximately 22 nucleotides in length, they are first transcribed into long precursor molecules (~1000 nucleotides in length) called primary miRNAs (pri-miRNAs) by RNA polymerase II enzyme. Then with the help of nuclear microprocessor complex consisting of RNase III enzyme Drosha, the pri-miRNAs are further transformed into smaller (<100 nucleotides in length), hairpin-shaped precursor miRNAs (pre-miRNAs) and transported from nucleus into the cytoplasm [20]. In the cytoplasm, the pre-miRNAs are cleaved by RNase III-type endonuclease Dicer, and then are further processed into single stranded mature miRNAs [21]. However, the miRNAs alone are not functional until they are incorporated into the miRNA-induced silencing complex (miRISC). With the recruitment of a group of RNA binding proteins (RBPs), miRISCs are delivered to their mRNA targets in order to regulate the expression of these genes [22] (Figure 1).
Figure 1: Biogenesis and function of miRNAs in the regulation of gene expression.
The pri-miRNAs are transcribed in the nucleus by RNA Pol II (1). Then pre-miRNAs are generated by the Rnase III enzyme Drosha mediated cleavage (2). The pre-miRNAs are transported into the cytoplasm through Exportin (3), cleaved by Dicer to yield double stranded miRNAs (4), and are further processed into single stranded mature miRNAs (5). Mature miRNAs are incorporated into miRISC (6), which is the functional units for targeting mRNAs (7) and reducing gene expression through either mRNA degradation or translational repression (8).
Mechanistically, miRNAs regulate gene expression via two distinct and independent pathways, either through mRNA deadenylation activation, which subsequently leads to transcript degradation, and/or through translational repression [22–24]. In previous years, many studies have shown that the elements of the miRISC can functionally interact with a group of RNA binding proteins, facilitating the accurate anchor of the miRISC to the cis-acting regulatory sites of mRNA targets [25]. Interestingly, each mRNA could have multiple miRNA targeting sites at the 3’UTR, whereas each miRNA could base-pair with multiple mRNA targets, resulting in a complex miRNA-mediated gene expression regulatory network [23]. Thus, miRNAs demonstrate a strong role as contributors to the development of DM in humans.
MicroRNA Profiling in Diabetes
In the past decades, the specific profiling of miRNAs has been studied extensively in both blood and pancreatic islets, demonstrating a signature of miRNA alteration is implicated in the context of DM.
Circulating miRNAs as biomarkers in predicting diabetes
In recent years, multiple studies have suggested that circulating miRNAs are correlated with various human diseases, including diabetes. Profiling miRNA content in circulation may reflect the dynamic changes of circulating cells in response to disease states. Therefore, their potential as biomarkers for the prediction and diagnosis of DM has become increasingly appreciated.
A large number of studies reporting the dysregulation of miRNAs in the serum/plasma of patients with diabetes have emerged in the past decade. A recent systematic review has identified eleven circulating miRNAs consistently dysregulated in T1D patients compared to controls: miR-21–5p, miR-24–3p, miR-100–5p, miR-146a-5p, miR-148a-3p, miR-150–5p, miR-181a-5p, miR-210–5p, miR-342–3p, miR-375 and miR-1275 [26]. One interesting study compared the expression levels of serum miRNAs from new onset T1D children and age-matched healthy controls identified twelve up-regulated miRNAs in T1D patients, including miR-24, miR-25, miR-26a, miR-27a, miR-27b, miR-29a, miR-30a-5p, miR-148a, miR-152, miR-181a, miR-200a and miR-210 [27]. Another study providing additional information on the profile of circulating miRNAs in children with recent onset of T1D has detected a significant increase in miR-144–5p, miR222–3p, miR-345–5p and miR-454–3p levels in the serum of children with T1D versus non-diabetic controls [28]. Additionally, the plasma levels of miR-21, miR-24, miR29a, miR-30d, miR-34a, miR-126, miR-146, and miR148a showed a significant up-regulation in one study comparing 16 adults T1D patients with 27 healthy controls [29]. Evaluated by qRT-PCR, higher levels of miR-21 and miR-210 were confirmed by Osipova et al., in the plasma of T1D patients [30]. Nabih et al., has also revealed that miR-181a expression was up-regulated in the serum of subjects with T1D [31]. As it is not uncommon in a rapidly evolving field, there have been numerous conflicting reports regarding the expression signature of miRNAs in T1D patients. Increased miR-375 in the serum of subjects with T1D has been shown in several studies [29,32], while decreased miR-375 was also reported in the serum of T1D children compared to age-matched controls [33]. Among these miRNAs reviewed above, miR-21, miR-24, miR-148a, miR-181a-5p and miR-210–5p have been confirmed to be up-regulated in T1D in more than one independent study. This consistent data strongly supports the potential of miRNAs as circulating biomarkers of T1D (Table 1).
Table 1:
Circulating microRNA profile changes reported in clinical study on diabetic patients.
| Study Design | Source | miRNA Detection Methods | miRNA Expression Alteration | Reference | ||
|---|---|---|---|---|---|---|
| Non-Diabetic Control | Diabetes Patients | |||||
| Type 1 Diabetes Mellitus | ||||||
| 54 | 54 | Serum | miRNA microarray, confirmed by qRT-PCR | Up | miR-24, miR-25, miR-26a, miR-27a, miR-27b, miR-29a, miR-30a-5p, miR-148a, miR-152, miR-181a, miR-200a, miR-210, | 27 |
| 7 | 10 | miRNA qPCR platform | Up | let-7e, let-7g, miR-18a, miR23b, miR-24, miR-25, miR-30e, miR-93, miR-103a, miR-125a, miR-140, miR-144, miR-182, miR-183, miR-192, miR-214, miR-221, miR-222, miR-324–3p, miR324–5p, miR-331, miR-345, miR-377, miR-454, miR-500a, miR-502, miR1468, | 28 | |
| Down | miR-100, miR-154, miR-490, miR-630, miR-636, miR-639, miR-675, miR-720 | |||||
| 40 | 40 | qRT-PCR | Up | miR-144, miR-222, miR-345, miR-454 | ||
| 10 | 22 | Up | miR-181a | 31 | ||
| 51 | 38 | Plasma | Down | miR-375 | 33 | |
| 79 | 68 | Up | miR-375 | 32 | ||
| 27 | 16 | Up | miR-21, miR-210 | 30 | ||
| Up | miR-21, miR-24, miR-30d, miR-34a, miR-126, miR146, miR-148a, miR-375 | 29 | ||||
| Type 2 Diabetes Mellitus | ||||||
| 24 | 24 | Serum | qRT-PCR | Up | miR-571, miR-661, miR-770–5p, miR892b, miR-1303 | 47 |
| 82 | 101 | Up | miR-15b, miR-146b, miR-486 | 46 | ||
| 20 | 24 | Down | let-7i, miR-23a, miR-96, miR-186, miR-191, miR-192, miR-486 | 44 | ||
| 19 | 18 | Up | miR-9, miR-29a, miR-30d, miR34a, miR-124a, miR-146a, miR-375 | 42 | ||
| 100 | 100 | Down | miR-126 | 36 | ||
| 40 | 56 | Down | miR-146a | 45 | ||
| 80 | 80 | Plasma | miRNA microarray, confirmed by qRTPCR | Up | miR-28–3p | 38 |
| Down | miR-15a, miR-29b, miR-126, miR-223 | |||||
| 20 | 20 | qRT-PCR | Down | miR-126 | 37 | |
| 136 | 193 | Down | miR-126–3p | 39 | ||
| 90 | 90 | Up | miR-146a | 43 | ||
| 30 | 30 | Down | miR-126 | 40 | ||
| 107 | 193 | Down | miR-21–5p, miR-126–3p | 41 | ||
| 20 | 61 | Down | miR-191, miR-200b | 49 | ||
| 27 | 31 | Up | miR-21, miR-24, miR-30d, miR-34a, miR-126, miR146, miR-148a, miR-375 | 29 | ||
| 46 | 50 | Blood | miRNA microarray, confirmed by qRTPCR | Up | miR-150, −192, −27a, −320a, and −375 | 68 |
| Down | miR-17, miR-92a, miR-130a, miR-195, miR-197, miR-509–5p, miR-652 | |||||
| 15 | 21 | Up | miR-29a, miR-144, miR-150, miR-192, miR-320 | 34 | ||
| Down | miR-15a, miR-30d, miR-182 | |||||
| 24 | 24 | qRT-PCR | Down | miR-15a | 50 | |
| 46 | 127 | Platelet | qRT-PCR | Down | miR-103b | 51 |
In a global profile study focusing on T2D circulating miRNAs, approximately 70 miRNAs showed elevated levels and about 100 miRNAs showed reduced levels in blood samples of T2D patients [34]. A meta-analysis confirmed 40 significantly dysregulated miRNAs in T2D patients, and highlighted that circulating miR-29a, miR34a, miR-103, miR-107, miR-132, miR-142–3p,miR-144 and miR-375, levels may serve as potential biomarkers for T2D [35]. In addition, the most down-regulated miRNA is miR-126a, which has been validated by several other studies in both the serum [36] and plasma [37–41] of T2D patients. Interestingly, when plasma miR-126 levels were determined in three study groups, including a healthy normal control, T2D-susceptible, and T2D patients, miRNA-126 was significantly reduced in both susceptible and T2D individuals, indicating miR-126 is tightly associated with the manifestation of T2D and could be a potential circulating biomarker for the early identification of individuals susceptible to T2D [40]. Another T2D-related miRNA signature change evaluated by several groups is miR-146a. Surprisingly, the expression pattern of miR-146a determined in different experiment settings is not consistent. Some showed miR146a levels to be elevated in serum [42] and plasma [43], while other studies demonstrated lower levels of miR-146a in serum [44,45] or whole blood [34] from T2D patients. While differences in study populations might have caused this discrepancy, future research will require additional effort to understand the subtleties of the regulation of this miRNA. In serum, age-based comparisons between a control group with normal glucose tolerance and T2D individuals demonstrated significantly increased levels of miR-9, miR-15b, miR-27a, miR-29a, miR-30d, miR34a, miR-124a, miR-146b, miR-150, miR-192, miR-320a, miR-375, miR-486, miR-571, miR-661, miR-770, miR892b and miR-1303 [42,46–48], and decreased expression levels of let-7i, miR-23a, miR-96, miR-186, miR-191, miR192, miR-486 [44]. Altered expression levels of miRNAs in plasma have also been assessed, in which miR-28–3p was highly expressed [38], while miR-15a, miR-21–5p, miR29b, miR-191, miR-200b and miR223 displayed reduced levels [38,41,49] in T2D patients. Additional studies revealed that miR-29a, miR-144, miR-150, miR-192, and miR-320 were up-regulated, whereas miR-15a, miR30d and miR-182 were down-regulated in whole blood evaluations [34,50], and the expression of miR-103b is decreased in platelets of patients with T2D [51] (Table 1).
Although human studies provide clinically disease relevant information to understand the onset of diabetes, major limitations must be considered, such as the limited amount of material from donors, relatively small cohort numbers, and difficulties in finding of non-diabetic controls matched for age, gender and ethnicity. Moreover, the interpretation of these data can be affected by the delayed diagnosis of the disease and the use of various medications on these patients. As a result, the use of animal models can provide valuable insight to understanding the manifestation of diabetes and its underlying molecular mechanisms. Streptozotocin (STZ) is a chemical that destroys insulin-producing cells and is commonly used for the generation of T1D phenotype in mice, while nonobese diabetic (NOD) mice is another rodent model for T1D due to insulitis, a leukocytic infiltrate of the pancreatic islets. The level of miR-375 in plasma showed a significant increase in both STZ-treated mice and NOD mice before diabetes onset, indicating miR-375 could be a suitable blood marker to predict diabetes [52]. In one study on high fat diet fed (HFD) T2D rat model, miR-30d, 146a and miR182 showed reduced expression, while miR-29a, miR-144, miR-150, miR-192 and miR-320a were found to be highly elevated [34]. The Zucker diabetic fatty (ZDF) rat model reflects many characteristics of human conditions, making it an ideal model to observe the natural progression of T2D [53]. In the ZDF rat model, several miRNAs were found to be elevated over the course of T2D, such as miR-122, miR133, miR-210 and miR-375, while others miRNAs including miR-140, miR-151–3p, miR-185, miR-203, miR-434–3p and miR-450a were found to be decreased [54]. The db/db mouse, another rodent T2D model with leptin receptor deficiency, obesity, insulin resistance, hyperglycemia and hyperinsulinemia, is also used to investigate circulating miRNA profiling changes [55]. The serum miR-16b, miR146b and miR-486 showed significantly higher levels in db/db mice compared to those in age-matched male C57BL/6J mice [46] (Table 2).
Table 2:
Circulating microRNA profile changes reported in animal models of diabetes mellitus.
| Animal Models | Source | miRNA Detection Methods | miRNA Expression Alteration | Reference | ||
|---|---|---|---|---|---|---|
| T1DM Model | ||||||
| Streptozotocin (STZ)-induced mice | Plasma | qRT-PCR | Up | miR-375 | 52 | |
| Non-obese diabetic (NOD) mice | ||||||
| T2DM Model | ||||||
| High fat diet (HFD), STZ-induced rats | Blood | miRNA microarray, confirmed by qRT-PCR | Up | miR-29a, miR-144, miR-150, miR-192, miR-320a | 34 | |
| Down | miR-30d, miR-146a, miR-182 | |||||
| Zucker diabetic fatty (ZDF) rats | Plasma | qRT-PCR | Up | miR-122, miR-133, miR-210 and miR-375 | 54 | |
| Down | miR-140, miR-151–3p, miR-185, miR-203, miR-434–3p, miR-450a | |||||
| db/db mice | Serum | qRT-PCR | Up | miR-15b, miR-146b, miR-486 | 46 | |
miRNAs Signature in Pancreatic Islets
Many miRNAs are known to be cell-type or tissue specific. It has been shown that a group of islet-enriched miRNAs participate in the development of the pancreatic islet, the regulation of islet mass, insulin secretion and β-cell proliferation and apoptosis, thus suggesting an important role of these miRNAs in pancreatic islet function. For example, miR-7, miR-9, miR-375 and miR-376 have been shown to be expressed at high levels in the human pancreas during the development and maturation of pancreatic islets [56–58]. To identify the individual miRNAs and how their expression patterns are dynamically regulated throughout the development of diabetes may be of diagnostic and therapeutic interest. Although changes in the profile of miRNAs in pancreatic islets are less investigated than those in circulating miRNAs, scientific knowledge in this area is rapidly increasing.
MiRNA expression profiles between human islets isolated from donors with diabetes and non-diabetic subjects provide valuable insights into the discovery of miRNAs associated with diabetes. In one recent study, miR-125a-5p showed elevated expression levels in donors with T1D compared to donors without diabetes [59]. The miRNA expression profile in T2D has also been widely analyzed in human pancreatic islets. A meta-analysis performed by Zhu et al., suggested the dysregulation of two highly pancreas-specific miRNAs, miR-199a-3p and miR-223, could potentially be tissue biomarkers of T2D [35]. MiR-124a expression was found to be significantly increased [60], and miR-187 hyperexpression was identified in human islets tissue from individuals with T2D versus matched controls [61]. On the other hand, some miRNAs have been found to have lower expression levels. MiR-7a, which regulates pancreatic β-cell function, showed a reduced expression in T2D islets [62]. Tattikota et al., additionally showed a dramatic down-regulation of miR-184 in 12 diabetic individuals [63] compared to pancreatic islets from 15 non-diabetic donors. A cluster of miRNAs highly and specifically expressed in human β-cells, including miR-127, miR-136, miR-369, miR-411, miR-432, miR-487, miR-495, miR-543, miR-589, miR-655 and miR-656, is significantly decreased in islets from T2D organ donors [64] (Table 3).
Table 3:
Pancreatic islets microRNA profile changes reported in clinical study on diabetic patients.
| Study Design | miRNA Detection Methods | miRNA Expression Alteration | References | ||
|---|---|---|---|---|---|
| Non-Diabetic Donors | Diabetes Patients | ||||
| Type 1 Diabetes Mellitus | |||||
| 4 | 2 | qRT-PCR | Up | miR-125a-5p | 59 |
| Type 2 Diabetes Mellitus | |||||
| 10 | 5 | qRT-PCR | Up | miR-124a | 60 |
| 9 | 11 | Global profilingTaqMan array, comfirmed by qRT-PCR |
Up | miR-187 | 61 |
| 10 | 9 | qRT-PCR | Down | miR-7a | 62 |
| 19 | 12 | Down | miR-184 | 63 | |
| 3 | 4 | Global miRNA Seq | Up | miR-187, miR-216a, miR-589 | 64 |
| Down | miR-7–1, miR-7–3, miR-23c, miR-30a, miR-369, miR-487a, miR-487b, miR-495, miR-539, miR-544a, miR-656, miR-4716 | ||||
| 14 | 10 | qRT-PCR | Down | miR-136, miR-369, miR411, miR-432, miR-487a, miR-487b, miR-655, miR-656 | |
The miRNA expression signature in pancreatic islets has been explored in animal models as well. In one recent study, 64 up-regulated and 72 down-regulated pancreatic miRNAs were detected in streptozotocin (STZ)-induced T1D mice compared to normal controls via a miRNA microarray, and several of them, including let-7a-5p, let-7b5p, let-7f-5p, miR-7a-5p, miR-7b-5p, miR-26a-5p, miR26b-5p, miR-27a-3p and miR-148b-3p, were confirmed by qRT-PCR to have decreased expression levels in diabetic mice [65]. By using another T1D murine model, Ma et al, has shown the down-regulated expression of miR-26a-5p in pancreatic tissues from NOD mice [66]. Other studies have also been done to evaluate miRNA expression pattern in pancreas from rodent models of T2D. A global miRNA profile analyzed in the islets of non-obese T2D model Goto-Kakiz (GK) rats, which spontaneously develop T2D unrelated to obesity [67], identified 30 miRNAs with different expression patterns compared to Wistar controls; GK rat miRNAs clustered into 6 miRNAs with lower expression and 24 miRNAs with higher expression [68]. Another study compared the miRNA expression signature in a T2D rat model and showed that in pancreatic tissue, miR-29a, miR-144, miR-150, miR-192 and miR-320a were highly up-regulated, while miR-30d, miR-146a and miR-182 were highly down-regulated [34]. Regarding T2D mice model, elevated levels of the miR-200 family, which consists of miR-141, miR-200a, miR-200b, miR-200c and miR-429, were observed in islets of db/db diabetic mice at 12 weeks of age [69]. Additional studies showed the expression levels of let-7b, miR-21, miR-34a, miR-132, miR-146, miR-199a-3p and miR-199a-5p to be strongly induced in the pancreatic islets of diabetic mice [70–72]. The miRNAs reported to have reduced expression levels, detected by qRT-PCR in diabetic islets, are miR-30d, miR-184, miR-203, miR-210, miR-338–3p and miR-383 [71–73]. Interestingly, reduced miR-184 was also observed in the islets of 12-week-old leptin receptor-deficient db/db mice, which showed a similar loss of expression in human diabetic individuals [63]. Diet-induced obesity (DIO) mice, which are fed a HFD to induce obesity and insulin resistance, are a valuable model of pre-diabetes or the early phases of T2D [74]. A loss of expression of mature miR-184 in the islets of mice on a HFD was observed [63], making miR-184 a consistently down-regulated miRNA in more than one mouse model of obesity and insulin resistance. qRT-PCR analysis also revealed elevated levels of miR-132, miR-199a-3p and miR-199a-5p, and decreased levels of miR-7a, miR-184, miR-203, miR-210 and miR383 in islets of DIO mice [62,72] (Table 4).
Table 4:
Pancreatic islets microRNA profile changes reported in animal models of diabetes mellitus.
| Animal Models | miRNA Detection Methods | miRNA Expression Alteration | Reference | |
|---|---|---|---|---|
| T1DM Model | ||||
| Streptozotocin (STZ)induced mice | miRNA microarray, confirmed by qRT-PCR | Down | let-7f-5p, let-7b-5p, let-7a-5p, miR-7b-5p, miR-7a-5p, miR-26a-5p, miR-26b-5p, miR-27a-3p, miR-148b-3p | 65 |
| Non-obese diabetic (NOD) mice | qRT-PCR | Down | miR-26a-5p | 66 |
| T2DM Model | ||||
| Goto-Kakizaki (GK) rats | miRNA microarray | Up | let-7i*, miR-7b, miR-124, miR-127, miR-130a, miR-132, miR-136*, miR-142–3p, miR-142–5p, miR-152, miR-199a*−3p, miR-199a-5p, miR-212,miR −335, miR-369–3p, miR-376a, miR-376a*, miR-376b-3p, miR-376c, miR-409–3p, miR-410, miR-411, miR433, miR-434 | 68 |
| Down | miR-28*, miR-216, miR-217, miR-493, miR-503, miR-708 | |||
| High fat diet (HFD), STZ-induced Rats | miRNA microarray, confirmed by qRT-PCR | Up | miR-29a, miR-144, miR-150, miR-192, miR-320a | 34 |
| Down | miR-30d, miR-146a, miR-182 | |||
| qRT-PCR | Up | miR-141, miR-200a, miR-200b, miR-200c, miR-429 | 69 | |
| in situ hybridization | Up | let-7b | 70 | |
| Down | miR-30d | |||
| miRNA microarray | Up | miR-10a, miR-10b, miR-21, miR-22*, miR-34a, miR-34b-5p, miR-34c, miR-99a, miR100, miR-126–3p, miR-132, miR-139–5p, miR-143, miR-146a, miR-146b, miR-152, miR-181c, miR-195, miR-199a-3p, miR-199a-5p, miR-199b*, miR-212, miR-320, miR-322, miR-337–5p, miR-365, miR-455*, miR-497, miR-676, miR-721, miR-802, miR-1224 | 72 | |
| Down | miR-23b, miR-26a, miR-27b, miR-30e, miR-30e*, miR-30d, miR-31, miR-103, miR-129–3p, miR-129–5p, miR-184, miR203, miR-204, miR-210, miR-301a, miR-324–3p,miR-324–5p, miR-325, miR-328, miR-331–3p, miR-338–3p, miR-341, miR-374, miR-378, miR-381, miR-383, miR384–5p, miR-434–3p, miR-652, miR-872 | |||
| qRT-PCR | Up | miR-21, miR-132, miR-199a-3p, miR-199a5p | ||
| Down | miR-184, miR-203, miR-210, miR-383 | |||
| Up | miR-34a, miR-146 | 71 | ||
| Down | miR-338–3p | 73 | ||
| Down | miR-7a | 62 | ||
| Down | miR-184 | 63 | ||
| Diet induced obesity (DIO) mice | miRNA microarray | Up | let-7d*, miR-7a-1*, miR-34c, miR-101b, miR-125a-3p, miR-130b*, miR-132, miR152, miR-182, miR-193, miR-200c*, miR-205, miR-211, miR-216b, miR-221, miR322, miR-323–3p, miR-337–3p, miR-362–5p, miR-380–3p, miR-433, miR-455*, miR-484, miR-485*, miR-494, miR-540–3p, miR-615–3p, miR-670, miR-671–5p, miR-680, miR-702, miR-705, miR-714, miR-770–3p, miR-802, miR-1224, miR-1894–5p, miR-1897–5p, miR-1904, miR-1906 | 72 |
| Down | let-7b*, miR-10a, miR-24–1*, miR-28, miR29a*, miR-30b*, miR-30c-1*, miR-31*, miR-32, miR-33, miR-100, miR-148a*, miR-181d,miR-184, miR-199a-3p, miR202–3p, miR-203, miR-210, miR-215, miR-218, miR-223, miR-301b, miR-328, miR-335–5p, miR-344b, miR-378, miR-383, miR-384–5p, miR-539–5p, miR-541, miR-543, miR-676, miR-690, miR-697, miR-700, miR-1187, miR-1198–5p, miR-1892 | |||
| qRT-PCR | Up | miR-132, miR-199a-3p, miR-199a-5p | ||
| Down | miR-184, miR-203, miR-210, miR-383 | |||
Although the signature pattern of miRNAs in diabetes has been widely studied, the biological correlation between circulatory miRNA and pancreatic islets miRNA expression has not been established. To reveal the detailed molecular mechanisms underlying the regulation on the changes of miRNAs in the global large scale studies is a primary step towards using them as predictive and diagnostic biomarkers in real clinical practice.
Role of miRNAs in Diabetes
Various studies have shed light on the miRNA-mediated pathways controlling glucose homeostasis. The regulation of glucose by islet-enriched miRNAs principally occurs through the production and secretion of insulin and the survival and proliferation of β-cells. The aberrant expression and activity of these pancreatic miRNAs may have significant consequences on these regulatory pathways, potentially driving clinical hyperglycemia associated with T1D and T2D (Table 5).
Table 5:
Role of miRNAs in diabetes.
| miRNA | Models Used | Function | References |
|---|---|---|---|
| miR-375 | MIN6, Nit-1, INS1E | Inhibits GSIS by targeting Mtpn and blocking the fusion and exocytosis of insuiln vesicles at β-cell membrane; also possibly downregulates NF-κB activity. Reduces insulin production and β-cell proliferation by targeting PDK1 and inhibiting PI3K signaling axis | 58, 82 |
| miR-124a | MIN6 | Increases basal insuline secretion but decreases GSIS by upregulating SNAP25, Rab3A, and synapsin-1A while decreasing Rab27A and Noc2. Rab27A is a direct target. | 79 |
| miR-96 | MIN6 | Inhibits GSIS by upregulating the expression of granuphilin and decreasing Noc2 levels | 79 |
| miR-145 | MIN6 | Decreases GSIS by targeting ABCA1 and decreasing the efflux of cholesterol from the β-cell | 81 |
| miR-33 | MIN6 | Decreases GSIS by targeting ABCA1 and causing accumulation of cholesterol in the β-cell | 80 |
| miR-7 | MIN6 | Negative regulator of GSIS by directly regulating genes involved in distal stages of the fusion of the insulin vesicle with cell membrane and interaction with ternary SNARE complex. | 62 |
| miR-204 | INS-1, MIN6 | Downregulates insulin transcription by directly targeting insulin transcription factor MAFA. Also decreases insulin secretion by targeting GLP1R. | 83, 84 |
| miR-21 | INS-1, MIN6 | Promotes β-cell proliferation. Overexpression increases β-cell proliferation but also activates apoptosis, impairing net β-cell survival. Also downregulates GSIS via indirect inhibition of VAMP2 but does not affect basal insulin secretion | 85, 86 |
| miR-34a | INS-1, MIN6 | Promotes β-cell death by downregulating SIRT1 and enhancing p53-mediated apoptosis | 85, 86 |
| miR-200 | MIN5, INS-1E | Contributes to β-cell apoptosis by downregulating antiapoptotic and stress-resistance pathways. Also activates Trp53 pathway and concomitant expression of pro-apoptotic genes. | 87 |
Secretion of insulin
Islet-enriched miRNAs act on a diverse array of downstream targets influencing the secretion of insulin. One review has established a profile of human islet derived miRNAs that control insulin secretion by targeting the exocytosis machinery of the β-cell. Notably, all of the miRNAs profiled in this study inhibit the secretion of insulin, suggesting an evolutionarily conserved role of islet miRNAs in preventing lethal hypoglycemia [75].
Through qRT-PCR methods, miR-375 was found to be the most highly expressed miRNA in human pancreatic islet and is known to have a well-defined role in down-regulating insulin secretion [75]. MiR-375 is also highly expressed in murine insulinoma MIN6 cells and one study revealed that miR-375 targets the 3’ UTR of the Myotrophin (Mtpn) mRNA [58]. Mtpn is actively involved in the cytoskeletal remodeling process by depolymerizing actin filaments and allowing for the fusion of insulin vesicles at the β-cell membrane [76]. A miR-375-mediated regulation of the Mtpn exocytosis pathway helps to explain an observed decrease in glucose-stimulated insulin secretion (GSIS) in this model [58]. Mtpn is also known to up-regulate the nuclear transcription factor NF-κB, which may subsequently activate the expression of proteins involved in the trafficking of insulin vesicles to the membrane [77]. Supporting this study’s findings, another group developed an in vitro miR-375 overexpression system in mouse insulinoma Nit-1 cells and verified a reduction in GSIS via the miR-375/Mtpn targeted interaction [78].
MiR-124a and miR-96 have also been found to influence the exocytosis machinery in MIN6 cells [79]. Interestingly, miR-124a increases insulin secretion at basal glucose levels while decreasing GSIS. The variable expression of pro-secretory proteins in a miR-124a overexpression system can help explain these findings: SNAP25, Rab3A, and synapsin-1A levels increased while Rab27a and Noc2 levels decreased. Because Rab27a is a GTPase that facilitates the transport of vesicles to the cell membrane and was specifically found to be a direct target of miR124a, the diminished expression of Rab27a helps to explain the reduced cellular capacity to respond to high glucose conditions. MiR-96 also decreases GSIS by indirectly inhibiting Noc2 expression while increasing the expression of granuphilin, a protein that inhibits insulin exocytosis (79).
The ATP-binding cassette transporter A1 (ABCA1), a cholesterol efflux facilitator, is also implicated in decreased insulin secretion in murine models. MiR-33a and miR-145 were found to target ABCA1, mediating the accumulation of cholesterol in murine islets and decreasing insulin secretion [80,81].
MiR-7 has been shown to down-regulate GSIS by modulating the distal stages of the insulin exocytosis pathway. Mechanistically, miR-7 represses the expression of SNCA and concomitantly inhibits the formation of the SNARE ternary complex, blocking the exocytosis of insulin granules already docked at the β-cell membrane [62].
Production of insulin
In addition to targeting components of the secretory machinery in the β-cell, miRNAs can also influence the production of insulin. The role of miR-375 in glucose homeostasis extends beyond insulin trafficking as it also targets 3’-phosphoinositide-dependent protein kinase-1 (PDK1), a key component of the phosphatidylinositol 3-kinase (PI3K) cascade [82]. Reduced PDK1 levels are associated with decreased insulin gene expression in response to glucose stimulation. Interestingly, high glucose conditions yielded a decrease in precursor miR375 expression and an associated increase in PDK1 and insulin levels [82].
MiR-204 has also been found to play a negative role in insulin production. Thioredoxin-interacting protein (TXNIP), a regulator of redox states in the β-cell, induces the expression of miR-204. Mechanistically, TXNIP decreases the phosphorylation and activity of signal transducer and activator of transcription 3 (STAT3), a known repressor of the miR-204 promoter. TXNIP is upregulated in diabetes and a concomitant increase in miR204 expression allows for the increased direct targeting and degradation of MAFA, an established transcription factor for insulin [83]. Additionally, miR-204 directly targets the 3’ UTR of Glucagon-like peptide 1 receptor (GLP1R) and down-regulates GSIS, demonstrating another connection between TXNIP and glucose homeostasis that is mediated by miR-204 [84].
Beta cell proliferation and survival
Many pancreatic miRNAs also regulate β-cellular pathways driving proliferation and survival, as well as β-cell destruction induced by the presence of proinflammatory cytokines. Through the aforementioned PI3K pathway, miR-375 not only down-regulates PDK expression but also plays an inhibitory role in β-cell proliferation and survival. Supporting this notion, INS-1E cells transfected with a precursor form of miR-375 showed a 25% reduction in proliferation and a 20% reduction in viability, compared to INS-1E cells transfected with a control vector; [methyl3H] thymidine incorporation during DNA synthesis also decreased by 20% in INS-1E cells transfected with precursor miR-375, verifying the aforementioned decrease in cellular proliferation [82]. However, the genetic deletion of miR-375 expression in murine models similarly impaired proliferation and diminished β-cell mass, suggesting that an intermediate and steady state miR-375 level is optimal to promote the adequate survival and proliferative capacity of β-cells [76].
MiR-21 is also involved in the cellular machinery regulating β-cell number [85,86]. Because miR-21 is known to play a pro-proliferative role in β-cell survival, one group overexpressed miR-21 in INS-1 cells and interestingly observed a net decrease in β-cell number despite confirming an increase in cell proliferation [85]. Attributing this net decrease to a hyperactive flux through the cell cycle and a concomitant activation of checkpoint-mediated apoptosis, they indeed found an irregular expression of two cell cycle genes involved in the first apoptosis checkpoint of the G1 phase. The proinflammatory cytokine pathway involving NFκB up-regulates the expression of miR-21, which may play a role in increasing NO synthesis and β-cell apoptosis [85]. In contrast, another study overexpressed miR-21 in MIN6 cells and did not ascertain any effect on cell survival, but did find that decreasing miR-21 expression promoted apoptosis [86]. MiR-34a is known to play the opposite role of miR-21 by targeting protein silent information regulator 1 (SIRT1) and increasing p53-mediated apoptosis. Indeed, the net effect of miR-34a knockdown was an increase in β-cell mass [85].
MiR-200 is strongly linked to β-cell pathology in both T1D and T2D. Specifically, miR-200 suppresses the antiapoptotic and stress-resistance pathways that include Dnajc3, a β-cell heat shock protein, and Xiap, a caspase inhibitor. MiR-200 also facilitates the activation of the tumor suppressor protein Trp53, fostering the expression of pro-apoptotic genes [87].
Summary
Pancreatic miRNAs act through a diverse series of pathways regulating the biological development and function of the β-cell. Disrupting the miRNA expression profile in β-cells has shown to elucidate much of the pathology associated with T1D and T2D. A universal theme that emerges from β-cell miRNA biology is the specific and careful targeting of gene regulatory networks that control glucose homeostasis and β-cell survival and function. Supplementing the role of applying extensive miRNA profiles to predict the onset of diabetes, uncovering the upstream regulation and downstream targets of pancreatic miRNAs can help foster the development of novel clinical therapies that modulate the expression and activity of these miRNAs and potentially restore a normal glucose homeostasis and β-cell function.
Acknowledgments
Funding
This work was supported in part by a research grant AHA_16POST27700029 (to X.Z.).
Abbreviations
- ABCA1
ATP-binding Cassette transporter A1
- DIO
Diet Induced Obesity
- DM
Diabetes Mellitus
- GK
Goto-Kakiz
- GLP1R
Glucagon-like Peptide 1 Receptor
- GSIS
Glucose-stimulated Insulin Secretion
- HFD
High Fat Diet
- IDF
International Diabetes Federation
- miRISC
miRNA-induced Silencing Complex
- miRNAs
microRNAs
- Mtpn
Myotrophin
- NOD
Non-obese Diabetic
- PDK1
3’-phosphoinositide-dependent Protein Kinase-1
- PI3K
Phosphatidylinositol 3-kinase
- pre-miRNAs
precursor miRNAs
- pri-miRNAs
primary miRNAs
- RBPs
RNA Binding Proteins
- SIRT1
Silent Information Regulator
- STAT3
Signal Transducer and Activator of Transcription 3
- STZ
Streptozotocin
- T1DM
Type 1 Diabetes Mellitus
- T2DM
Type 2 Diabetes Mellitus
- TXNIP
Thioredoxin-Interacting Protein
- ZDF
Zucker Diabetic Fatty
Footnotes
Conflict of Interest
The authors report no conflicts of interest to disclose.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes research and clinical practice. 2011;94(3):311–21. [DOI] [PubMed] [Google Scholar]
- 2.Chien HY, Lee TP, Chen CY, Chiu YH, Lin YC, Lee LS, Li WC. Circulating microRNA as a diagnostic marker in populations with type 2 diabetes mellitus and diabetic complications. Journal of the Chinese Medical Association. 2015;78(4):204–11. [DOI] [PubMed] [Google Scholar]
- 3.Samuels TA, Cohen D, Brancati FL, Coresh J, Kao WH. Delayed diagnosis of incident type 2 diabetes mellitus in the ARIC study. The American journal of managed care. 2006;12(12):717–24. [PubMed] [Google Scholar]
- 4.Romaine SP, Tomaszewski M, Condorelli G, Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101(12):921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross JS, Carlson JA, Brock G. miRNA: the new gene silencer. American journal of clinical pathology. 2007;128(5):830–6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Schulze PC. MicroRNAs in heart failure: Non-coding regulators of metabolic function. Biochimica et biophysica acta. 2016;1862(12):2276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Ji R, Liao X, Castillero E, Kennel PJ, Brunjes DL, Franz M, Möbius-Winkler S, Drosatos K, George I, Chen EI. MicroRNA-195 regulates metabolism in failing myocardium via alterations in sirtuin 3 expression and mitochondrial protein acetylation. Circulation. 2018;137(19):2052–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaishya S, Sarwade RD, Seshadri V. MicroRNA, Proteins, and Metabolites as Novel Biomarkers for Prediabetes, Diabetes, and Related Complications. Frontiers in endocrinology. 2018;9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Bunt M, Gaulton KJ, Parts L, Moran I, Johnson PR, Lindgren CM, Ferrer J, Gloyn AL, McCarthy MI. The miRNA profile of human pancreatic islets and betacells and relationship to type 2 diabetes pathogenesis. PloS one. 2013. January 25;8(1):e55272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Vijayan M, Bhatti JS, Reddy PH. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Progress in molecular biology and translational science. 2017;146:47–94. [DOI] [PubMed] [Google Scholar]
- 11.Tana C, Giamberardino MA, Cipollone F. microRNA profiling in atherosclerosis, diabetes, and migraine. Annals of medicine. 2017;49(2):93–105. [DOI] [PubMed] [Google Scholar]
- 12.Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovascular research. 2012;93(4):583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes. 2011;60(7):1832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natarajan R, Putta S, Kato M. MicroRNAs and diabetic complications. Journal of cardiovascular translational research. 2012;5(4):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nature reviews Genetics. 2009;10(2):94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic acids research. 2014;42(Database issue):D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 20.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004. November;432(7014):235. [DOI] [PubMed] [Google Scholar]
- 21.Ha M, Kim VN. Regulation of microRNA biogenesis. Nature reviews Molecular cell biology. 2014;15(8):509–24. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Virtanen A, Kleiman FE. To polyadenylate or to deadenylate: that is the question. Cell cycle. 2010;9(22):4437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature reviews Genetics. 2008;9(2):102–14. [DOI] [PubMed] [Google Scholar]
- 24.Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E. Deadenylation is a widespread effect of miRNA regulation. Rna. 2009;15(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Devany E, Murphy MR, Glazman G, Persaud M, Kleiman FE. PARN deadenylase is involved in miRNA-dependent degradation of TP53 mRNA in mammalian cells. Nucleic acids research. 2015;43(22):10925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assmann TS, Recamonde-Mendoza M, De Souza BM, Crispim D. MicroRNA expression profiles and type 1 diabetes mellitus: systematic review and bioinformatic analysis. Endocrine connections. 2017;6(8):773–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen LB, Wang C, Sørensen K, Bang-Berthelsen CH, Hansen L, Andersen ML, Hougaard P, Juul A, Zhang CY, Pociot F, Mortensen HB. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Experimental diabetes research. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erener S, Marwaha A, Tan R, Panagiotopoulos C, Kieffer TJ. Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. JCI insight. 2017;2(4):e89656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seyhan AA, Lopez YO, Xie H, Yi F, Mathews C, Pasarica M, Pratley RE. Pancreas-enriched miRNAs are altered in the circulation of subjects with diabetes: a pilot cross-sectional study. Scientific reports. 2016;6:31479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osipova J, Fischer DC, Dangwal S, Volkmann I, Widera C, Schwarz K, Lorenzen JM, Schreiver C, Jacoby U, Heimhalt M, Thum T. Diabetes associated microRNAs in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. The Journal of Clinical Endocrinology & Metabolism. 2014;99(9):E1661–5. [DOI] [PubMed] [Google Scholar]
- 31.Nabih ES, Andrawes NG. The Association Between Circulating Levels of miRNA-181a and Pancreatic Beta Cells Dysfunction via SMAD7 in Type 1 Diabetic Children and Adolescents. Journal of clinical laboratory analysis. 2016;30(5):727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latreille M, Herrmanns K, Renwick N, Tuschl T, Malecki MT, McCarthy MI, Owen KR, Rülicke T, Stoffel M. miR-375 gene dosage in pancreatic β-cells: implications for regulation of β-cell mass and biomarker development. Journal of molecular medicine. 2015;93(10):1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchand L, Jalabert A, Meugnier E, Van den Hende K, Fabien N, Nicolino M, Madec AM, Thivolet C, Rome S. miRNA-375 a Sensor of Glucotoxicity Is Altered in the Serum of Children with Newly Diagnosed Type 1 Diabetes. Journal of diabetes research. 2016;2016:1869082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karolina DS, Armugam A, Tavintharan S, Wong MT, Lim SC, Sum CF, Jeyaseelan K. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PloS one. 2011. August 1;6(8):e22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu H, Leung SW. Identification of microRNA biomarkers in type 2 diabetes: a meta-analysis of controlled profiling studies. Diabetologia. 2015;58(5):900–11. [DOI] [PubMed] [Google Scholar]
- 36.Rezk NA, Sabbah NA, Saad MS. Role of MicroRNA 126 in screening, diagnosis, and prognosis of diabetic patients in Egypt. IUBMB life. 2016;68(6):452–8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang T, Li L, Shang Q, Lv C, Wang C, Su B. Circulating miR-126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochemical and biophysical research communications. 2015;463(1–2):60–3. [DOI] [PubMed] [Google Scholar]
- 38.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circulation research. 2010. September 17;107(6):810–7. [DOI] [PubMed] [Google Scholar]
- 39.Olivieri F, Bonafè M, Spazzafumo L, Gobbi M, Prattichizzo F, Recchioni R, Marcheselli F, La Sala L, Galeazzi R, Rippo MR, Fulgenzi G. Age-and glycemiarelated miR-126–3p levels in plasma and endothelial cells. Aging (Albany NY). 2014. September;6(9):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T, Lv C, Li L, Chen S, Liu S, Wang C, Su B. Plasma miR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals. BioMed research international. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olivieri F, Spazzafumo L, Bonafè M, Recchioni R, Prattichizzo F, Marcheselli F, Micolucci L, Mensà E, Giuliani A, Santini G, Gobbi M. MiR-21–5p and miR126a-3p levels in plasma and circulating angiogenic cells: relationship with type 2 diabetes complications. Oncotarget. 2015. November 3;6(34):35372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta diabetologica. 2011. March 1;48(1):61–9. [DOI] [PubMed] [Google Scholar]
- 43.Rong Y, Bao W, Shan Z, Liu J, Yu X, Xia S, Gao H, Wang X, Yao P, Hu FB, Liu L. Increased microRNA-146a levels in plasma of patients with newly diagnosed type 2 diabetes mellitus. PloS one. 2013. September 2;8(9):e73272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z, Chen H, Si H, Li X, Ding X, Sheng Q, Chen P, Zhang H. Serum miR-23a, a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes. Acta diabetologica. 2014. October 1;51(5):823–31. [DOI] [PubMed] [Google Scholar]
- 45.Baldeón L, Weigelt K, De Wit H, Ozcan B, van Oudenaren A, Sempértegui F, Sijbrands E, Grosse L, Freire W, Drexhage HA, Leenen PJ. Decreased serum level of miR-141p06a as sign of chronic inflammation in type 2 diabetic patients. PloS one. 2014. December 12;9(12):e115209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui X, You L, Zhu L, Wang X, Zhou Y, Li Y, Wen J, Xia Y, Wang X, Ji C, Guo X. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metabolism. 2018. January 1;78:95105. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Wan S, Yang T, Niu D, Zhang A, Yang C, Cai J, Wu J, Song J, Zhang CY, Zhang C. Increased serum microRNAs are closely associated with the presence of microvascular complications in type 2 diabetes mellitus. Scientific reports. 2016. February 1;6:20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF, Jeyaseelan K. Circulating miRNA profiles in patients with metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2012;97(12):E2271–6. [DOI] [PubMed] [Google Scholar]
- 49.Dangwal S, Stratmann B, Bang C, Lorenzen JM, Kumarswamy R, Fiedler J, Falk CS, Scholz CJ, Thum T, Tschoepe D. Impairment of wound healing in patients with type 2 diabetes mellitus influences circulating microRNA patterns via inflammatory cytokines. Arteriosclerosis, thrombosis, and vascular biology. 2015. June;35(6):1480–8 [DOI] [PubMed] [Google Scholar]
- 50.Al-Kafaji G, Al-Mahroos G, Alsayed NA, Hasan ZA, Nawaz S, Bakhiet M. Peripheral blood microRNA-15a is a potential biomarker for type 2 diabetes mellitus and pre-diabetes. Molecular medicine reports. 2015;12(5):7485–90. [DOI] [PubMed] [Google Scholar]
- 51.Luo M, Li R, Deng X, Ren M, Chen N, Zeng M, Yan K, Xia J, Liu F, Ma W, Yang Y. Platelet-derived miR-103b as a novel biomarker for the early diagnosis of type 2 diabetes. Acta diabetologica. 2015. October 1;52(5):943–9. [DOI] [PubMed] [Google Scholar]
- 52.Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ. Circulating miR-375 as a biomarker of beta-cell death and diabetes in mice. Endocrinology. 2013;154(2):603–8. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt RE, Dorsey DA, Beaudet LN, Peterson RG. Analysis of the Zucker Diabetic Fatty (ZDF) type 2 diabetic rat model suggests a neurotrophic role for insulin/IGF-I in diabetic autonomic neuropathy. The American journal of pathology. 2003;163(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delic D, Eisele C, Schmid R, Luippold G, Mayoux E, Grempler R. Characterization of Micro-RNA Changes during the Progression of Type 2 Diabetes in Zucker Diabetic Fatty Rats. International journal of molecular sciences. 2016;17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shafrir E, Ziv E, Mosthaf L. Nutritionally induced insulin resistance and receptor defect leading to beta-cell failure in animal models. Annals of the New York Academy of Sciences. 1999;892:223–46. [DOI] [PubMed] [Google Scholar]
- 56.Bolmeson C, Esguerra JL, Salehi A, Speidel D, Eliasson L, Cilio CM. Differences in islet-enriched miRNAs in healthy and glucose intolerant human subjects. Biochemical and biophysical research communications. 2011;404(1):16–22. [DOI] [PubMed] [Google Scholar]
- 57.Joglekar MV, Joglekar VM, Hardikar AA. Expression of islet-specific microRNAs during human pancreatic development. Gene expression patterns : GEP. 2009;9(2):109–13. [DOI] [PubMed] [Google Scholar]
- 58.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, MacDonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004. November;432(7014):226. [DOI] [PubMed] [Google Scholar]
- 59.Sebastiani G, Ventriglia G, Stabilini A, Socci C, Morsiani C, Laurenzi A, Nigi L, Formichi C, Mfarrej B, Petrelli A, Fousteri G. Regulatory T-cells from pancreatic lymphnodes of patients with type-1 diabetes express increased levels of microRNA miR-125a-5p that limits CCR2 expression. Scientific reports. 2017. July 31;7(1):6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sebastiani G, Po A, Miele E, Ventriglia G, Ceccarelli E, Bugliani M, Marselli L, Marchetti P, Gulino A, Ferretti E, Dotta F. MicroRNA-124a is hyperexpressed in type 2 diabetic human pancreatic islets and negatively regulates insulin secretion. Acta Diabetologica. 2015. June 1;52(3):523–30. [DOI] [PubMed] [Google Scholar]
- 61.Locke JM, da Silva Xavier G, Dawe HR, Rutter GA, Harries LW. Increased expression of miR-187 in human islets from individuals with type 2 diabetes is associated with reduced glucose-stimulated insulin secretion. Diabetologia. 2014;57(1):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Latreille M, Hausser J, Stützer I, Zhang Q, Hastoy B, Gargani S, Kerr-Conte J, Pattou F, Zavolan M, Esguerra JL, Eliasson L. MicroRNA-7a regulates pancreatic β cell function. The Journal of clinical investigation. 2014. June 2;124(6):2722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tattikota SG, Rathjen T, McAnulty SJ, Wessels HH, Akerman I, Van De Bunt M, Hausser J, Esguerra JL, Musahl A, Pandey AK, You X. Argonaute2 mediates compensatory expansion of the pancreatic β cell. Cell metabolism. 2014. January 7;19(1):122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kameswaran V, Bramswig NC, McKenna LB, Penn M, Schug J, Hand NJ, Chen Y, Choi I, Vourekas A, Won KJ, Liu C. Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell metabolism. 2014. January 7;19(1):135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian C, Ouyang X, Lv Q, Zhang Y, Xie W. Cross-talks between microRNAs and mRNAs in pancreatic tissues of streptozotocin-induced type 1 diabetic mice. Biomedical reports. 2015;3(3):333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma H, Zhang S, Shi D, Mao Y, Cui J. MicroRNA26a Promotes Regulatory T cells and Suppresses Autoimmune Diabetes in Mice. Inflammation. 2016;39(1):1–9. [DOI] [PubMed] [Google Scholar]
- 67.Portha B, Lacraz G, Kergoat M, Homo-Delarche F, Giroix MH, Bailbé D, Gangnerau MN, Dolz M, TourrelCuzin C, Movassat J. The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes?. Molecular and cellular endocrinology. 2009. January 15;297(1–2):73–85. [DOI] [PubMed] [Google Scholar]
- 68.Esguerra JL, Bolmeson C, Cilio CM, Eliasson L. Differential glucose-regulation of microRNAs in pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki rat. PloS one. 2011;6(4):e18613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garfias-Gonzalez K, Organista-Mateos U, Borja-Miranda A, Gomez-Vidales V, Hernandez-Ortega S, Cortez-Maya S, Martínez-García M. High fluorescent porphyrin-PAMAM-fluorene dendrimers. Molecules. 2015. May 13;20(5):8548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lovis P, Roggli E, Laybutt DR, Gattesco S, Yang JY, Widmann C, Abderrahmani A, Regazzi R. Alterations in microRNA expression contribute to fatty acid–induced pancreatic β-cell dysfunction. Diabetes. 2008. October 1;57(10):2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X, Mohan R, Ozcan S, Tang X . MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreatic beta-cells. The Journal of biological chemistry. 2012;287(37):3115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nesca V, Guay C, Jacovetti C, Menoud V, Peyot ML, Laybutt DR, Prentki M, Regazzi R. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia. 2013. October 1;56(10):2203–12. [DOI] [PubMed] [Google Scholar]
- 73.Jacovetti C, Abderrahmani A, Parnaud G, Jonas JC, Peyot ML, Cornu M, Laybutt R, Meugnier E, Rome S, Thorens B, Prentki M. MicroRNAs contribute to compensatory β cell expansion during pregnancy and obesity. The Journal of clinical investigation. 2012. October 1;122(10):3541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SM, Joly E, Prentki M. β-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced β-cell mass. Diabetes. 2010. September 1;59(9):2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filios SR, Shalev A. beta-Cell MicroRNAs: Small but Powerful. Diabetes. 2015;64(11):3631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR375 maintains normal pancreatic α-and β-cell mass. Proceedings of the National Academy of Sciences. 2009. April 7;106(14):5813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hammar EB, Irminger JC, Rickenbach K, Parnaud G, Ribaux P, Bosco D, Rouiller DG, Halban PA. Activation of NF-κB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic beta cells. Journal of Biological Chemistry. 2005. August 26;280(34):30630–7. [DOI] [PubMed] [Google Scholar]
- 78.Xia HQ, Pan Y, Peng J, Lu GX. Over-expression of miR375 reduces glucose-induced insulin secretion in Nit1 cells. Molecular biology reports. 2011;38(5):3061–5. [DOI] [PubMed] [Google Scholar]
- 79.Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biological chemistry. 2008;389(3):305–12. [DOI] [PubMed] [Google Scholar]
- 80.Wijesekara N, Zhang LH, Kang MH, Abraham T, Bhattacharjee A, Warnock GL, Verchere CB, Hayden MR. miR-33a modulates ABCA1 expression, cholesterol accumulation, and insulin secretion in pancreatic islets. Diabetes. 2012. March 1;61(3):653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang MH, Zhang LH, Wijesekara N, de Haan W, Butland S, Bhattacharjee A, Hayden MR. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145. Arteriosclerosis, thrombosis, and vascular biology. 2013. December;33(12):2724–32. [DOI] [PubMed] [Google Scholar]
- 82.El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, van Obberghen E. miR-375 targets 3’-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes. 2008;57(10):2708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu G, Chen J, Jing G, Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nature medicine. 2013;19(9):1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jo S, Chen J, Xu G, Grayson TB, Thielen LA, Shalev A. miR-204 Controls Glucagon-Like Peptide 1 Receptor Expression and Agonist Function. Diabetes. 2018;67(2):256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Backe MB, Novotny GW, Christensen DP, Grunnet LG, Mandrup-Poulsen T. Altering beta-cell number through stable alteration of miR-21 and miR-34a expression. Islets. 2014;6(1):e27754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roggli E, Britan A, Gattesco S, Lin-Marq N, Abderrahmani A, Meda P, Regazzi R. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic β-cells. Diabetes. 2010. April 1;59(4):978–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belgardt BF, Ahmed K, Spranger M, Latreille M, Denzler R, Kondratiuk N, Von Meyenn F, Villena FN, Herrmanns K, Bosco D, Kerr-Conte J. The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nature medicine. 2015. June;21(6):619. [DOI] [PubMed] [Google Scholar]