Abstract
Background and Objectives.
Young adults with childhood maltreatment (CM) histories are particularly vulnerable to depressive symptoms and alcohol use problems. Research suggest that maltreated youth may misuse alcohol in part to alleviate depressive symptoms. However, many youths with depressive symptoms exercise self-control and abstain from heavy alcohol use. The present study aimed to examine the influence of heart rate variability reactivity (HRV-R), a psychophysiological biomarker of self-regulation, in the indirect link between CM and alcohol use problems via depressive symptoms among low socioeconomic-status rural young adults.
Methods.
Two waves of data were collected from a community sample of 225 low socioeconomic-status non-metropolitan young adults (Mage = 21.56, 52.9% female). HRV data were obtained with an electrocardiogram during a social stress task. CM was assessed through the Childhood Trauma Questionnaire. Alcohol use problems were measured using the Alcohol Use Disorders Identification Test.
Results.
The indirect effect of CM on alcohol use problems via elevated depressive symptoms was positive and significant (α*β = .159, p < .001). Self-regulation indicated by high HRV-R (i.e., vagal withdrawal) was found to significantly buffer the link between depressive symptoms and alcohol use problems (β = .193, p = .022).
Discussion and Conclusions.
Adequate self-regulation capacities can protect maltreated youths from self-medicating alcohol use problems.
Scientific Significance.
This study will advance researchers’ understanding of the development of alcohol use problems through unwrapping the risk and protective mechanisms underlying the association between young adults’ early life stress and alcohol use behaviors.
Keywords: Child Maltreatment, Depressive Symptoms, Alcohol Use Problems, Heart Rate Variability, Self-Regulation, Young Adults
Introduction
Young adults who experienced childhood maltreatment (CM) are at significant risk for substance use problems1. The self-medication hypothesis2 suggests that depressive symptomology is a key mediator in the indirect link between CM and alcohol problems. Specifically, maltreated young adults may use alcohol partly to soothe their depressive symptoms. However, research testing the self-medication hypothesis has yielded inconsistent findings3,4, which might stem from the individual variability in self-regulation while feeling depressed, particularly in a social context. The focus on self-regulation in socially stressful contexts is especially pertinent to young adulthood, a time when youth are more sensitive to social context influences5. Further, self-regulation, as indicated by heart rate variability reactivity (HRV-R), is an emerging transdiagnostic biomarker of psychopathology that is increasingly linked to addictive behaviors6. The current longitudinal study tested the moderating role of HRV-R in the link between CM and increases in alcohol use problems via depressive symptoms among a low socioeconomic-status (SES) non-metropolitan sample of young adults.
Child Maltreatment, Depressive Symptoms, and Alcohol Use Problems
Toxic rearing environments, such as child maltreatment, are linked to significant increases in alcohol use problems during young adulthood1. This association between CM and increased addictive behaviors has been shown to be mediated by elevations in depressive symptoms. Research has documented a strong relation between CM and elevated depressive symptoms among young adults7. Further, the self-medication hypothesis2 suggests that depressed youths are more likely to use alcohol as a coping strategy to soothe negative affect, especially those induced by early adverse experiences. A large body of research supports the self-medication hypothesis by suggesting that depressive symptoms may mediate the indirect link between CM and addictive behaviors.
Despite evidence supporting the self-medication hypothesis, there are inconsistent reports on the link between depressive symptoms and alcohol use problems. Some studies find this association to be statistically significant4, and others find it to be weak or non-significant3. These different findings can be partially explained by the individual heterogeneity in youths’ abilities to self-regulate the urge to drink when feeling depressed. Self-regulation is a critical socio-emotional and neurobiological trait that modulates a range of emotional responses to psychosocial stressors and is associated with reduced risk behaviors among youth8. Research shows that higher levels of self-regulation abilities protect youth from developing addictive behaviors9. In contrast, reduced self-regulation may be particularly harmful among youth who report elevated depressive symptoms because depressed youth are more susceptible to using alcohol as a coping strategy for their negative affect10.
HRV as a Biomarker Addiction Risk
Adequate self-regulation, in particular during situations of acute social stress, is a protective factor for youth’s risky behaviors such as increased alcohol use9. However, the large majority of this research is based on survey data8 and has multiple methodological caveats such as reporter bias and reduced ecological validity. Studies that use experimentally-induced acute stress tasks and obtain physiological parameters of self-regulation might offer complementary information about self-regulation during socially stressful situations. The current study obtained HRV as an indirect biomarker of young adults’ self-regulation, which has been increasingly linked to addictive behaviors in recent literature11.
The polyvagal theory12 and the neurovisceral integration model13 suggest that HRV is a reliable biomarker for cognitive, emotional, and behavioral self-regulation during acute stress. HRV is primarily mediated via vagal nerve activity, which is the central locus of the parasympathetic branch of the ANS responsible for maintaining homeostasis12,13. In young adult samples, it is essential to investigate the HRV response to acute social stress because research suggests that emerging adults, similar to adolescents, are highly sensitive to the influences of peers and social stress14,15. Indeed, a meta-analysis reports that adolescents and young adults exhibited more significant HRV responses to negative social interactions compared to other developmental periods16. Thus, in the current study, we examined HRV-R in response to an experimentally induced stress task modified from the arithmetic component of the Trier Social Stress Test17 in a laboratory setting.
HRV, as an indicator of self-regulation abilities, has been reported to have a strong association with substance use behaviors18. For example, higher HRV-R (vagal withdrawal, a decrease in HRV from baseline to stress) is associated with reduced alcohol use risk19 and can buffer the associations between early life stress and problem behaviors20, including alcohol-related risks21. In contrast, low HRV-R (vagal augmentation, an increase in HRV from baseline to the stress) is associated with hypervigilance and youths’ risk for behavioral problems22. However, the role of HRV-R in the associations between young adults’ depressive symptoms and alcohol use problems in the context of CM is still unclear. It remains to be empirically determined if maltreated young adults who report elevated depressive symptoms and have high HRV-R can effectively regulate their negative affect and therefore abstain from increased risky drinking behaviors.
The Present Study
The present study aimed to test the moderating role of HRV-R in the indirect effect of CM on increased alcohol use problems through elevated depressive symptoms, among a sample of low-SES non-metropolitan young adults. We focus on this rural sample of non-college-educated young adults to distinguish from college-seeking young adults, who are often from a higher-SES background with less racial/ethnic diversity. Low-SES rural children are at higher risk of experiencing child maltreatment compared to urban or high-SES youth23. Additionally, rural young adults are at increased risk for socioeconomic adversity and alcohol use problems24. In order to generate knowledge on the development of alcohol use behaviors and inform prevention and intervention programs targeting rural young adults, it is imperative to investigate the mechanisms underlying the development of substance use problems among this sample. Two hypotheses were tested: First, we hypothesized that CM would be associated with increased alcohol use via elevated depressive symptoms (Hypothesis 1 – self-medication hypothesis). Secondly, we hypothesized that the indirect effect of CM on increased alcohol use problems via elevated depressive symptoms would be attenuated by high HRV-R (Hypothesis 2).
Methods
Participants
Data were obtained from a low-SES non-metropolitan community sample of 225 young adults aged 18 to 25 years old (Mage = 21.56, SDage = 2.24). Eligibility criteria included not being or having been enrolled in a four-year college/university nor in high school and having no history of heart problems at the first time-point (T1). Among the participants, 46.7% were male, 52.9% were female, and 0.4% identified as transgender. The sample was racially and ethnically diverse, with 59.6% Caucasian, 30.7% African American, 5.8% Hispanic/Latino, and 4.0% others. Most of the participants (76.9%) made less than $20,000 a year. In addition, 66.9% of participants were unemployed at T1.
Procedures
Per the approval of Institutional Review Board (IRB) of the sponsoring university, we collected data at two time-points, one year apart. To collect HRV data, we used a Lead II configuration electrocardiogram (ECG) with three dermal electrodes that were attached to both sides of the lower rib cage and the right clavicle. Respiration was obtained using cardiac impedance data collected from impedance cardiography (ICG) with an additional four dermal electrodes attached to the left clavicle, the sternum, and the upper and lower spine. The MindWare Biolab 3.0.1 Software module (MindWare Technologies, Ltd., Gahanna, OH) was used for digitizing the data. HRV baseline data were collected when participants were watching a 3-minute relaxing video. Participants’ relaxation status was confirmed physiologically (i.e., reduction in heart rate and respiration rate) as well as by their self-report rating: 94.0% of participants reported that the video segment was relaxing. Then, following procedure modified from the arithmetic component of the Trier Social Stress Test17, participants were asked to perform a five-minute arithmetic task. During the task, participants were asked to quickly and accurately answer a series of increasingly difficult arithmetic questions verbally, using their mental math, in front of an audience of research assistants of their similar age. The difficulty of math problems was adjusted based on participants’ accuracy and speed to account for differential math abilities. The HRV stress data were collected during the arithmetic task. This task has been reported to produce a significant emotional and physiological reaction and has been extensively used in the literature17. Participants’ stress status was confirmed physiologically, and through their self-report rating: 87.5% of participants responded that the task was stressful. Upon completion of the study procedures, participants were debriefed about the purpose of the arithmetic task. Approximately 10–13 months after T1, participants were re-contacted to complete a brief online follow-up survey. Approximately 65.3% of participants completed the follow-up survey at the second time-point (T2).
Measures
CM experiences (T1).
Participants retrospectively reported their CM experiences through 28 items from the Childhood Trauma Questionnaire CTQ;25. The CTQ assesses five types of maltreatment: physical abuse (α = .86), sexual abuse (α =.94), emotional abuse (α = .90), physical neglect (α = .83), and emotional neglect (α = .88). Participants reported on the frequency of different types of adverse family experiences while they were growing up, and responses ranged from 1 (never true) to 5 (very often true).
Depressive symptoms (T1).
Depressive symptoms were assessed via 11 items from the Center for Epidemiological Studies Depression-Short Scale (CES-D)26 with possible responses ranging from 0 (not at all true) to 3 (nearly every day). Participants reported on their past week depressive symptoms with 11 items, and the total score was used in analyses (α = .88).
Alcohol use problems (T1 & T2).
Young adults’ alcohol use problems at both time-points were assessed with 10 items (αT1 = .77, αT2 = .84) on the Alcohol Use Disorders Identification Test (AUDIT)27. A total score of 8 or higher has been suggested as a cut point to detect harmful alcohol use. The raw (continuous) scores of three subscales (alcohol consumption, dependence, and alcohol-related problems) were derived from the AUDIT.
HRV-R (T1).
HRV data were derived via high-frequency HRV (HF-HRV)28. Data were analyzed using the Mindware HRV 3.1.4 Software module. Spectral analysis of thoracic impedance was used to calculate baseline cardiography and respiration in order to account for noise during data extraction. Inter-beat intervals (IBIs; i.e., time in milliseconds between sequential R-waves) were converted into 120-second segments using an interpolation algorithm. Physiologically improbable IBIs were detected by MindWare software using the MAD/MED artifact detection algorithm. Trained research assistants used video recordings to cross-inspect and correct abnormal R-R intervals, such as inadvertent cardiac fluctuations and ectopic beats due to physical movement or breathing. HRV was then calculated as the natural logarithm of the variance of heart period within the high-frequency bandpass associated with respiration (.15–.40 Hz), a validated proxy to the parasympathetic vagal influence on the heart28. The mean values of HRV across the 120-second segments during baseline and the stress arithmetic task were calculated. Overall, the approach we adopted accords with current guidelines for measuring HRV and is well suited for short-term recordings. In order to quantify HRV-R, a residualized change score (ΔHRV) was computed by regressing HRV stress task mean scores on baseline mean scores and retaining the residual. The residualized change score represents the sample-specific standardized HRV change that allows for the adjustment of baseline HRV levels. Negative ΔHRV scores indicate decreases from baseline to the stress task (i.e., vagal withdrawal), and thus suggests high levels of HRV-R and better self-regulation capacities. Positive ΔHRV scores, instead, indicate increases of HRV level from baseline to stress task (i.e., vagal augmentation) and suggest low HRV-R and lack of self-regulation.
Covariates (T1).
Per previous research, we controlled for gender and SES in the examined models. Participants self-reported their gender and past-year household income at T1. Gender was coded as “0” for males and “1” for females.
Analytic Plan
Study hypotheses were tested using Mplus Version 7.4. The missing data rate ranged from 0.0% to 37.8%, with an average of 8.9% across all variables. Missing data were mainly due to attrition at the second time-point. T-tests and chi-square tests showed no significant differences in study variables between participants who remained and who did not respond at T2. Little’s missing completely at random (MCAR) test suggested that the missing data patterns met the MCAR assumption (χ2(80) = 87.48, p = .27). Thus, the full-informative maximum likelihood (FIML) algorithm was used to estimate missing data. Maximum likelihood estimation with robust standard errors was used as a model estimator to remedy the data non-normality issues. Model fit was assessed using indices, including the chi-square test, the comparative fit index (CFI) and the standardized root mean square residual (SRMR).
To account for the common covariance among maltreatment types and obtain a factor that underlies the severity of CM experiences, a confirmatory factor analysis (CFA) was used to assess the latent construct of CM using five CTQ subscales as indicators. A latent change model was used to assess changes in alcohol use problems from T1 to T2. Then, structural equation modeling was employed to examine the study hypotheses. The conditional indirect effect was tested through the R-mediation software, which produces a confidence interval for the product of two normal random variables using three methods: the distribution of the product of coefficients, Monte Carlo, and asymptotic normal theory with the multivariate-delta standard error (asymptotic-delta) method. The Johnson-Neyman technique was used to probe the conditional indirect effect.
Results
Descriptive and Correlation Analyses
Descriptive statistics and correlation coefficients of study variables are presented in Table 1. The current sample manifested significant risk for CM experiences and alcohol use problems. There were 31.6% of participants who reported emotional abuse, 28.6% who reported physical abuse, 20.9% who reported sexual abuse, 31.6% who reported emotional neglect, and 23.1% who reported physical neglect. Further, 28.9% of participants in the sample at T1 and 29.3% at T2 were indicated to have hazardous alcohol use problems.
Table 1.
Descriptive Statistics and Correlation Coefficients of Study Variables (N = 225)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CM Emotional Abuse T1 | -- | |||||||||||||||
| 2. CM Physical Abuse T1 | .72** | -- | ||||||||||||||
| 3. CM Sexual Abuse T1 | .43** | .36** | -- | |||||||||||||
| 4. CM Emotional Neglect T1 | .67** | .58** | .31** | -- | ||||||||||||
| 5. CM Physical Neglect T1 | .64** | .63** | .35** | .66** | -- | |||||||||||
| 6. Depressive Symptoms T1 | .46** | .28** | .22** | .46** | .33** | -- | ||||||||||
| 7. Alcohol Consumption T1 | −.10 | −.11 | −.08 | −.15* | −.07 | −.02 | -- | |||||||||
| 8. Alcohol Dependence T1 | −.05 | −.09 | −.04 | −.05 | −.08 | −.02 | .56** | -- | ||||||||
| 9. Alcohol Problems T1 | .02 | −.02 | −.02 | −.01 | −.01 | .04 | .55** | .70** | -- | |||||||
| 10. Alcohol Consumption T2 | −.15 | −.19* | −.06 | −.19* | −.07 | .04 | .50** | .38** | .38** | -- | ||||||
| 11. Alcohol Dependence T2 | −.14 | −.18* | −.03 | −.15 | −.15 | .04 | .29** | .38** | .35** | .50** | -- | |||||
| 12. Alcohol Problems T2 | −.13 | −.19* | −.15 | −.10 | −.12 | .16 | .41** | .42** | .51** | .55** | .61** | -- | ||||
| 13. ΔHRV T1 | −.11 | −.04 | −.05 | −.10 | −.09 | −.01 | −.04 | .08 | .01 | .00 | .08 | .08 | -- | |||
| 14. Gender | .16* | .07 | .28** | .18** | .03 | .14* | −.32** | −.21** | −.18** | −.18* | .04 | −.04 | −.03 | -- | ||
| 15. Age | −.06 | .00 | .01 | −.02 | .00 | −.03 | .04 | −.08 | −.03 | −.16 | −.11 | −.09 | −.11 | −.01 | -- | |
| 16. Family Income | −.24** | −.32** | −.23** | −.34** | −.30** | −.23** | .22** | .21** | .22** | .24** | .08 | .15 | .10 | −.16* | .00 | -- |
| Minimum | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | −6.13 | .00 | 18.00 | 10.00 |
| Maximum | 25.00 | 25.00 | 25.00 | 25.00 | 23.00 | 30.00 | 12.00 | 6.00 | 14.00 | 12.00 | 8.00 | 16.00 | 4.29 | 1.00 | 30.00 | 120.00 |
| Mean | 11.14 | 8.96 | 7.31 | 12.07 | 7.70 | 10.37 | 3.88 | .62 | 1.56 | 3.55 | .66 | 1.71 | .67 | .53 | 21.56 | 49.08 |
| SD | 5.81 | 4.76 | 4.93 | 5.11 | 3.68 | 7.06 | 2.71 | 1.22 | 2.27 | 2.86 | 1.52 | 2.73 | 1.34 | .50 | 2.24 | 31.32 |
| Skewness | .90 | 1.61 | 2.26 | .49 | 1.74 | .85 | .59 | 2.16 | 2.06 | .41 | 3.09 | 2.23 | −.64 | −.13 | .13 | .72 |
| Kurtosis | −.21 | 1.93 | 4.00 | −.66 | 2.92 | .13 | −.22 | 4.13 | 5.33 | −.61 | 10.29 | 6.18 | 3.24 | −2.00 | −.45 | −.11 |
Note. T1 = Time-point 1; T2 = Time-point 2; SD = Standard deviation; CM = Child maltreatment; ΔHRV = HRV Residualized change score; SD = Standard deviation. Gender was coded as 1 for females and 0 for males. Family income was assessed via participant-report past-year household income at baseline (T1) and was coded in $1,000.
The correlation analysis suggested that five CM types were all positively associated with elevated depressive symptoms at T1. The correlations between depressive symptoms and young adults’ alcohol use were positive but not significant. HRV-R was not significantly associated with CM, concurrent depressive symptoms, or alcohol problems. Additionally, being female and low household income were related to higher levels of CM and depressive symptoms but lower levels of alcohol use problems.
Measurement Model
A CFA was conducted to confirm a latent factor of CM consisting of five indicators (i.e., physical abuse, emotional abuse, sexual abuse, physical neglect, and emotional neglect; Table 1). All factors loadings were moderate to high (λ > .45) and significant (p < .001). The resulting model fit was excellent: χ2 (4) = 3.951, CFI = 1.000, SRMR = .012.
To assess alcohol use increases over time, a latent change model was used to examine the inter-individual differences in alcohol use from T1 to T2 (Table 2). At each time-point, alcohol use consisted of three indicators: alcohol consumption, dependence, and alcohol-related problems. Strong measurement invariance across two time-points was confirmed (Δχ2 (5) =3.729, p = .589). The regression coefficients of alcohol use at T1 and the alcohol-use latent change variable on the follow-up alcohol use were fixed to one, and the residual of T2 was fixed to zero. The mean of the alcohol use latent change variable was negative but not significantly different from zero (M = −.081, p = .675). However, the estimated variance was significantly larger than zero (Σ2 = 2.786, p < .001), suggesting individual differences in the alcohol-use latent change scores of alcohol use from T1 to T2. The measurement model fit was excellent: χ2 (9) = 19.143. CFI = .977, SRMR = .042.
Table 2.
Measurement Models (N = 225)
| (A) Latent Factor of Child Maltreatment | ||||
|---|---|---|---|---|
| Factors and Indicators | B (SE) | λ (SE) | 95%CI of λ | R2 |
| CM → Emotional Abuse | 1.000 (.000) | .888 (.030) | [.829, .947]*** | .211 |
| CM → Physical Abuse | .747 (.071) | .810 (.041) | [.729, .891]*** | .343 |
| CM → Sexual Abuse | .439 (.091) | .460 (.077) | [.310, .610]*** | .789 |
| CM → Emotional Neglect | .731 (.066) | .737 (.042) | [.656, .819]*** | .456 |
| CM → Physical Neglect | .526 (.070) | .737 (.050) | [.639, .836]*** | .456 |
| (B) Latent Change Model of Substance Use Behaviors | ||||
| Factors and Indicators | Λ (SE) | λ (SE) | 95%CI of Λ | p value |
| Alcohol – T1 → Consumption – T1 | 1.000 (.000) | .724 (.052) | [1.000, 1.000]*** | < .001 |
| Alcohol – T1 → Dependence – T1 | .528 (.062) | .733 (.047) | [.407, .649]*** | < .001 |
| Alcohol – T1 → Problems – T1 | .978 (.117) | .773 (.049) | [.749, 1.208]*** | < .001 |
| Alcohol – T2 → Consumption – T2 | 1.000 (.000) | .688 (.051) | [1.000, 1.000]*** | < .001 |
| Alcohol – T2 → Dependence – T2 | .528 (.062) | .798 (.056) | [.407, .649]*** | < .001 |
| Alcohol – T1 → Problems – T2 | .978 (.117) | .788 (.056) | [.749, 1.208]*** | < .001 |
| Paths & Covariance | B (SE) | β(SE) | 95%CI of B | p value |
| Alcohol – T1 → Alcohol – T2 | 1.000 (.000) | .876 (.076) | [1.000, 1.000]*** | < .001 |
| ΔAlcohol → Alcohol – T2 | 1.000 (.000) | .796 (.090) | [1.000, 1.000]*** | < .001 |
| ΔAlcohol & Alcohol – T1 | −.883 (.410) | −.288 (.115) | [−1.688, −.079]* | .031 |
| Means | M (SE) | μ (SE) | 95%CI of M | p value |
| Alcohol – T1 | 3.765 (.167) | 2.048 (.202) | [3.436, 4.093]*** | < .001 |
| Δ Alcohol | −.081 (.194) | −.049 (.116) | [−.461, .298] | .675 |
| Variances | Σ2 (SE) | σ2 (SE) | 95%CI of Σ2 | p value |
| Alcohol – T1 | 3.379 (.613) | 1.000 (.000) | [2.178, 4.580]*** | < .001 |
| Δ Alcohol | 2.786 (.271) | 1.000 (.000) | [1.550, 4.022]*** | < .001 |
Note. CM = Childhood maltreatment experiences; SE = Standard error; CI = Confidence interval; R2 = Residual variance; Alcohol = Alcohol use problems latent factors at different waves; ΔAlcohol = Alcohol use latent change; Consumption = Alcohol consumption score; Dependence = Alcohol dependence score; Problem = Alcohol-related problems score. Model fit is good: (A) χ2 (4) = 3.951 (p = .413). CFI = 1.000, SRMR = .012. (B) χ2 (9) = 19.143 (p = .024). CFI = .977, SRMR = .042.
p < .05,
p < .001
SEM Models
A mediation model was first examined to test the self-medication hypothesis (Table 3), controlling for gender and past-year household income. The model showed a good fit: χ2 (63) = 113.603, CFI = .952, SRMR = .048. Results indicated that the more severe CM experiences were associated with elevated depressive symptoms (β = .477, p < .001), which was further linked to increased alcohol use problems (β = .333, p = .001). The indirect effect of CM on alcohol use problems via increased depressive symptoms was significant (α*β = .159, p = .002), suggesting a mediating role of depressive symptoms on the link between CM and alcohol use problems.
Table 3.
SEM Models of the Associations between child maltreatment, Depressive Symptoms, Alcohol Use Problems, and HRV-R (N = 225)
| Paths | B (SE) | β | 95%CI of B | p value |
|---|---|---|---|---|
| Mediation Models | ||||
| Direct Effects | ||||
| CM (T1) → DEP (T1) | .646 (.110) | .477 | [.429, .861]*** | < .001 |
| CM (T1) → ΔAlcohol | −.142 (.044) | −.467 | [−.228, −.057]** | .001 |
| DEP (T1) → ΔAlcohol | .075 (.024) | .333 | [.029, .121]** | .001 |
| Conditional Indirect Effect | ||||
| CM (T1) → DEP →Δ Alcohol | .048 (.018) | .159 | [.017, .086]* | .002 |
| Control | ||||
| FIncome → DEP – T1 | −.308 (.586) | −.034 | [−1.458, .841] | .599 |
| FIncome → ΔAlcohol | −.096 (.251) | −.047 | [−.587, .395] | .702 |
| Gender → DEP – T1 | .661 (.855) | .047 | [−1.015, 2.338] | .440 |
| Gender → ΔAlcohol | .977 (.431) | .307 | [.132, 1.823]* | .023 |
| Moderated Mediation Model | ||||
| Direct Effects | ||||
| CM (T1) → DEP (T1) | .645 (.110) | .477 | [.429, .861]*** | < .001 |
| CM (T1) → ΔAlcohol | −.148 (.043) | −.488 | [−.233, −.064]** | .001 |
| DEP (T1) → ΔAlcohol | .076 (.022) | .337 | [.033, .118]*** | < .001 |
| ΔHRV (T1) → ΔAlcohol | −.033 (.153) | −.028 | [−.333, .267] | .830 |
| Interaction Effect | ||||
| ΔHRV X DEP → Δ Alcohol | .034 (.015) | .193 | [.005, .063]* | .022 |
| Conditional Indirect Effect | ||||
| CM (T1) → ΔHRV X DEP →Δ Alcohol | .022 (.011) | .092 | [.003, .044]* | .022 |
| Control | ||||
| FIncome → DEP – T1 | −.328 (.585) | −.036 | [−1.474, .818] | .575 |
| FIncome → ΔAlcohol | −.113 (.246) | −.055 | [−.595, .369] | .645 |
| Gender → DEP – T1 | .647 (.850) | .046 | [−1.020, 2.314] | .447 |
| Gender → ΔAlcohol | .877 (.424) | .276 | [.047, 1.708]* | .038 |
Note. T1 = Time 1; SE = Standard error; CI = Confidence interval; CM = Child maltreatment experiences latent factor; DEP = Depressive symptoms; ΔHRV = HRV residualized change score; ΔAlcohol = Alcohol use problems LC score; FIncome = Past-year household income. Gender was coded as 1 for male and 2 for female. Model fit for the mediation model: χ2 (63) = 113.603 (p <.001), CFI = .952, SRMR = .048. Model fit for the moderated mediation model: χ2 (77) = 130.200 (p < .001), CFI = .950, SRMR = .044.
p < .05;
p < .01;
p < .001.
Then, HRV-R was added to the SEM model to examine its buffering effect on the link between depressive symptoms and alcohol use problems (Table 3 and upper panel of Figure 1). The model showed a good fit: χ2 (77) = 130.200, CFI = .950, SRMR = .044. CM was associated with higher depressive symptoms at T1 (β = .477, p < .001), which was further associated with increased alcohol use (β = .337, p < .001). The interaction of ΔHRV and depressive symptoms was significantly related to increased alcohol use (β = .195, p = .022), indicating a significant moderation effect of HRV-R on the associations between depressive symptoms and increased alcohol use. Further, analyses confirmed a significant conditional indirect effect of CM on increases in alcohol use through depressive symptoms contingent upon ΔHRV (α*β = .092, p = .022).
Figure 1.
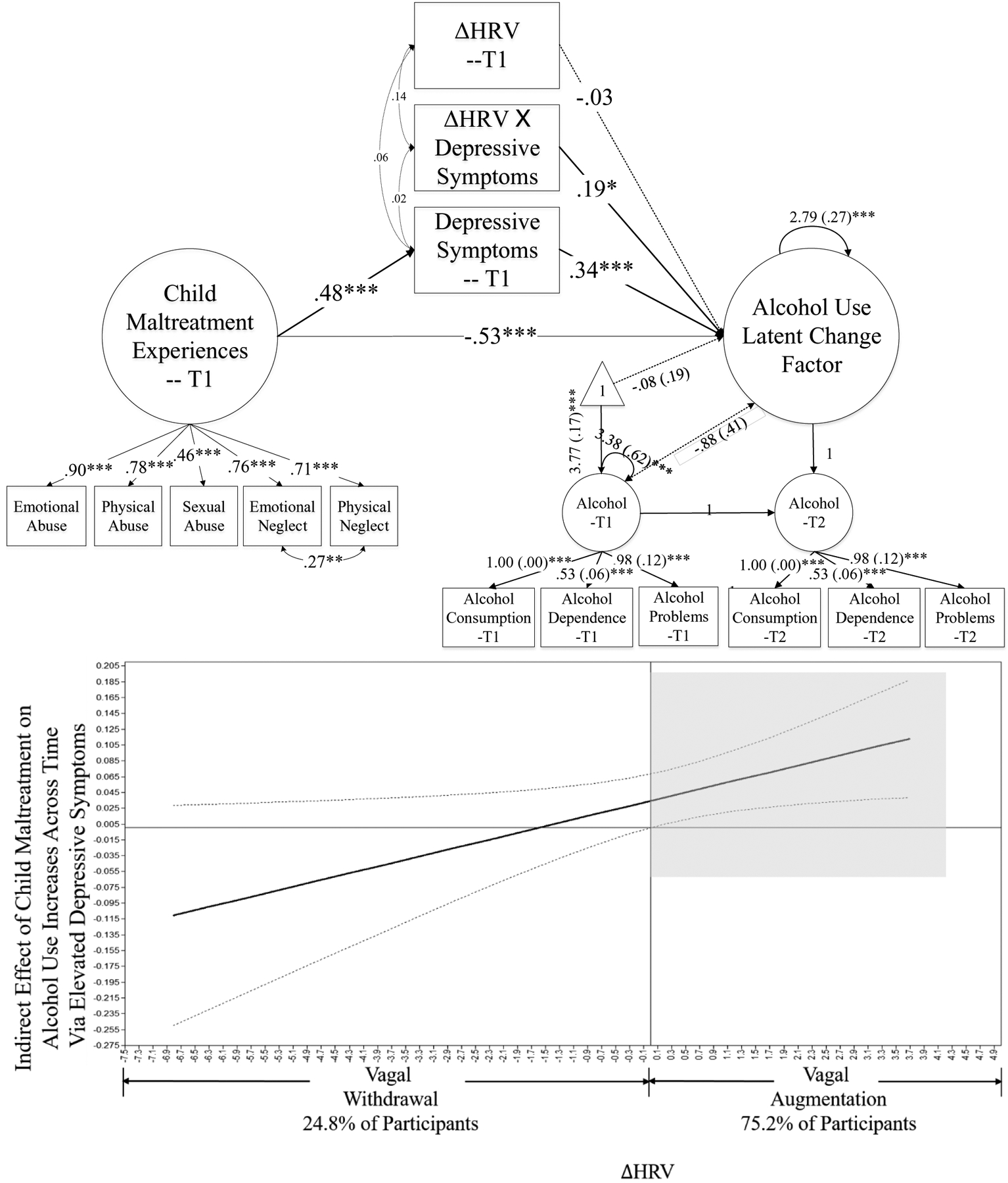
Moderated Mediation Model.
Note. The upper panel presents the structural equation model. The bottom panel presents the interpretation of the Conditional Indirect Effect Between Child Maltreatment and Alcohol Use Problems via Depressive Symptoms Conditional on HRV-R. T1 = Time-point 1, T2 = Time-point 2. Standardized coefficients are presented. Gender and past-year household income were controlled, and their paths are not presented in the figure for clarity. Model fit is good: χ2 (77) = 130.200 (p < .001), CFI = .950, SRMR = .044. *p < .05, **p < .01, ***p < .001. In the bottom panel, Shadowed area indicates that among participants with vagal augmentation (i.e. low HRV-R, 75.2% of participants), the indirect effect of child maltreatment on alcohol use through depressive symptoms was positive and significant.
Johnson-Neyman’s technique was employed to interpret this conditional indirect effect and to probe the regions of significance (lower panel of Figure 1). This Johnson-Neyman plot presents the indirect effect of CM on alcohol use increases via elevated depressive symptoms and its 95% confidence interval contingent on different values of ΔHRV. Accordingly, among participants who exhibited vagal augmentation (i.e., ΔHRV > 0), CM was associated with increased alcohol use through elevated depressive symptoms significantly. Among participants with vagal withdrawal (i.e., ΔHRV < 0), this indirect effect was non-significant, suggesting that high HRV-R can buffer the impact of CM on young adults’ alcohol use problems via elevated depressive symptoms.
Discussion
The present study aimed to examine the underlying mechanisms between CM and increases in alcohol use problems among low-SES rural young adults. Results first confirmed that increased depressive symptoms mediate the impact of CM on young adults’ alcohol use problems, providing support to the self-medication hypothesis. Second, the findings suggest that high HRV-R, a biomarker of self-regulation abilities, buffered the indirect effect of CM on alcohol use through elevated depressive symptoms and thus protected young adults from self-medicated alcohol use.
In support of the first hypothesis, we found that higher severity of CM was significantly associated with elevated depressive symptoms and attendant increases in alcohol use among young adults. From a developmental psychopathology perspective, this effect might reflect problems in self-system processes among maltreated children due to their traumatic rearing experiences. Disrupted self-system processes, in turn, are associated with the manifestation of depressive symptoms29. Similarly, based on the hopelessness theory of depression, negative self-perceptions that are fostered by early adversity could be a proximal cause of depressive symptoms30. Further, the results also corroborated the self-medication hypothesis by suggesting that individuals with depressive symptoms are more likely to consume alcohol as a coping strategy to soothe their negative affect2. This self-medicated alcohol use is most salient for young adults and occurs in socially stressful peer context, putatively due to social pressure to “fit in”, please, and impress peers by conforming to risky behaviors such as alcohol use31. Specifically, as young adults increasingly individuate from the nuclear family, they shift to more leisure time and give more salience and attention to peer environments32. With peers gaining increased salience, young adults are more likely to conform to peer norms of drinking alcohol as a coping strategy to stress and depressive symptoms.
Concordant with the second hypothesis, high HRV-R was found to buffer the association between depressive symptoms and alcohol use problems among young adults. These results provide further support to the protective role of self-regulation in self-medicated substance use problems, especially in stressful social contexts. In order to test our second hypothesis, the present study utilized a psychophysiological biomarker of self-regulation. According to both the polyvagal theory12 and the neurovisceral integration model13, HRV-R is a reliable proxy of self-regulation capacities. Specifically, according to the polyvagal theory12, HRV-R assists youths in directing their attention and increasing their controlled engagement under social stress. In the present study, vagal withdrawal was found to protect young adults from using alcohol as a coping strategy for depressive symptoms. Accordingly, when external conditions elicit negative affect, the capacity to take adaptive actions to regulate emotions is usually associated with less risky behaviors. The neurovisceral integration model further suggests that HRV-R is indirectly associated with self-regulation abilities such as executive functioning13, including inhibitory control (i.e., the ability to suppress or delay the immediate behavioral response) and situation awareness (i.e., cognitive processes to perceive and understand the meaning of a given environment). Inhibitory control delays immediate maladaptive responses to stress by promoting better self-regulatory decisions and inhibiting participation in risk behaviors such as alcohol use13. Situational awareness is critical for an individuals’ ability to make timely and adequate decisions and actions under stressful situations13, thus protecting young adults from choosing alcohol to alleviate depressive symptoms. Overall, the results of the present study are aligned with both the polyvagal theory12 and the neurovisceral integration model13 by showing that self-regulation indexed by vagal withdrawal can protect depressed young adults from alcohol use problems. Vagal augmentation, in contrast, exacerbates young adults’ risk for alcohol misuse.
In support of the second hypothesis, findings also indicated a conditional indirect effect of the association between CM and alcohol use problems via depressive symptoms, contingent upon HRV-R. Vagal withdrawal protects young adults with CM experiences from increased use of alcohol use. Nonetheless, among young adults with vagal augmentation, an indirect effect of CM on alcohol use problems through depressive symptoms was found, which corroborates previous research on the self-medication hypothesis33,34. This result suggests that the consequences of CM could be modified by young adults’ HRV-R under socially induced acute stress. Young adults who have experienced CM may be less likely to seek alcohol as a coping strategy if they have adequate self-regulation capabilities.
It is also important to note that, even though high HRV-R (i.e., vagal withdrawal) has often been shown as an adaptive response of self-regulation among normative samples (such as the current study), it might indicate increased risk for substance-related problems among clinical samples35. For example, Eddie and colleges35 examined the associations between HRV-R, in response to alcohol picture cues, and alcohol expectancies and found that higher HRV-R was associated with reduced risk for non-patient student samples, but was linked to increased alcohol-related risks among the inpatient sample with alcohol use disorders. The mechanisms underlying the influences of HRV-R on addictive behaviors might differ between normative non-patient samples and clinical samples with substance use disorders.
Limitations & Strengths
Several limitations of the current study should be noted. This study used self-report measures of depressive symptoms and alcohol use problems. However, these self-report measures (i.e., CES-D and AUDIT) have all been validated among young adults26,36 and showed high internal consistency in the current sample. The self-report method was also complemented by using more objective data obtained by a task-based psychophysiological indicator. Furthermore, recent research suggests alcohol dependence might affect basal HRV and HRV-R and therefore might induce biased HRV results19. To resolve this issue, we tested the associations among HRV baseline, HRV-R, and concurrent depressive symptoms and alcohol use problems. No significant associations between basal HRV nor HRV-R and depressive symptoms or alcohol problems were found. Further, this study utilized a sample of low-SES non-metropolitan young adults from the southeastern United States, limiting the generalizability to other normative or clinical sample populations in different regions or with higher SES. Lastly, temporal precedence could not be determined on the associations between CM and depressive symptoms because both constructs were assessed during T1. However, given that CTQ asked participants’ experiences before age 18, we assumed that CM preceded the development of depressive symptoms.
Conclusions and Implications
The findings indicate that depressive symptoms underlie the associations between CM and alcohol use problems, contingent on deficits in self-regulation capacities. Early screening for depressive symptoms may benefit substance use prevention programs that target at-risk youth. Additionally, the study found evidence that self-regulation, indexed by higher HRV-R, can protect young adults from alcohol use problems. Harm reduction programs for at-risk youth should include content that aims to improve self-regulation abilities in order to prevent substance use problems. Furthermore, new intervention programs have shown promising results in intervening addiction behaviors via incorporating HRV. For example, Heart Rate Variability Biofeedback, an intervention that uses HRV as a biofeedback tool to train patients via breathing techniques, is effective for treating substance use disorders among young-adult patients as it could help patients buffer stress responses to acute social stressors37. This HRV biofeedback technique might also be effective in programs that aim to prevent substance use problems among at-risk young adults.
Acknowledgment
This work was supported by funding from the University of Georgia (UGA) Owens Institute for Behavioral Research (OIBR, Athens, Georgia; Recipient: Dr. Assaf Oshri); the UGA Office of the Institute of the Vice President for Research (OIVPR; FRG-SE0042; Athens, Georgia; Recipient: Dr. Assaf Oshri); and the National Institute on Drug Abuse (K01DA045219-02; Recipient: Dr. Assaf Oshri).
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Shin SH, Jiskrova GK, Wills T. Childhood maltreatment and alcohol use in young adulthood: the role of self-regulation processes. Addictive behaviors. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Khantzian EJ. Addiction as a self-regulation disorder and the role of self-medication. Addiction. 2013;108(4):668–669. [DOI] [PubMed] [Google Scholar]
- 3.King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99(12):1548–1559. [DOI] [PubMed] [Google Scholar]
- 4.Bolton JM, Robinson J, Sareen J. Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of affective disorders. 2009;115(3):367–375. [DOI] [PubMed] [Google Scholar]
- 5.Schriber RA, Guyer AE. Adolescent neurobiological susceptibility to social context. Developmental cognitive neuroscience. 2016;19:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchaine TP. Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current opinion in psychology. 2015;3:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, D’arcy C, Meng X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychological medicine. 2016;46(4):717–730. [DOI] [PubMed] [Google Scholar]
- 8.Patock-Peckham JA, Cheong J, Balhorn ME, Nagoshi CT. A social learning perspective: a model of parenting styles, self-regulation, perceived drinking control, and alcohol Use and problems. Alcoholism: clinical and experimental research. 2001;25(9):1284–1292. [PubMed] [Google Scholar]
- 9.Oshri A, Liu S, Duprey EB, MacKillop J. Child maltreatment, delayed reward discounting, and alcohol and other drug use problems: the moderating role of heart rate variability. Alcoholism: clinical and experimental research. 2018;42(10):2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auerbach RP, Abela JR, Ho M-HR. Responding to symptoms of depression and anxiety: Emotion regulation, neuroticism, and engagement in risky behaviors. Behaviour Research and Therapy. 2007;45(9):2182–2191. [DOI] [PubMed] [Google Scholar]
- 11.Park SM, Lee JY, Choi AR, et al. Maladaptive neurovisceral interactions in patients with Internet gaming disorder: A study of heart rate variability and functional neural connectivity using the graph theory approach. Addiction biology. 2019:e12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porges SW. The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation (Norton Series on Interpersonal Neurobiology). WW Norton & Company; 2011. [Google Scholar]
- 13.Thayer JF. Heart rate variability: a neurovisceral integration model. 2009.
- 14.Kudielka B, Buske-Kirschbaum A, Hellhammer D, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83–98. [DOI] [PubMed] [Google Scholar]
- 15.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. International journal of behavioral medicine. 2004;11(2):116–121. [DOI] [PubMed] [Google Scholar]
- 16.Shahrestani S, Stewart EM, Quintana DS, Hickie IB, Guastella AJ. Heart rate variability during adolescent and adult social interactions: A meta-analysis. Biological Psychology. 2015;105:43–50. [DOI] [PubMed] [Google Scholar]
- 17.Kirschbaum C. Trier social stress test. Encyclopedia of psychopharmacology. 2015:1755–1758. [Google Scholar]
- 18.Eddie D, Vaschillo E, Vaschillo B, Lehrer P. Heart rate variability biofeedback: Theoretical basis, delivery, and its potential for the treatment of substance use disorders. Addiction research & theory. 2015;23(4):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boschloo L, Vogelzangs N, Licht CM, et al. Heavy alcohol use, rather than alcohol dependence, is associated with dysregulation of the hypothalamic–pituitary–adrenal axis and the autonomic nervous system. Drug and Alcohol Dependence. 2011;116(1–3):170–176. [DOI] [PubMed] [Google Scholar]
- 20.El-Sheikh M, Erath SA. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology. 2011;23(2):703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colder CR. Life stress, physiological and subjective indexes of negative emotionality, and coping reasons for drinking: Is there evidence for a self-medication model of alcohol use? Psychology of Addictive Behaviors. 2001;15(3):237. [PubMed] [Google Scholar]
- 22.Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological psychology. 2013;94(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getto CR, Pollack D. Meeting the Challenge of Child Maltreatment in Rural Areas. Child L Prac. 2015;34:33. [Google Scholar]
- 24.Mack KA. Illicit Drug Use, Illicit Drug Use Disorders, and Drug Overdose Deaths in Metropolitan and Nonmetropolitan Areas—United States. MMWR Surveillance Summaries. 2017;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(3):340–348. [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of youth and adolescence. 1991;20(2):149–166. [DOI] [PubMed] [Google Scholar]
- 27.Babor TF, de la Fuente JR, Saunders J, Grant M. The Alcohol Use Disorders Identification Test: Guidelines for use in. 2001.
- 28.Berntson GG, Thomas Bigger J, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Cicchetti D. Longitudinal trajectories of self-system processes and depressive symptoms among maltreated and nonmaltreated children. Child development. 2006;77(3):624–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abramson LY, Alloy LB, Metalsky GI. Hopelessness depression. 1995.
- 31.Van Ryzin MJ, Fosco GM, Dishion TJ. Family and peer predictors of substance use from early adolescence to early adulthood: An 11-year prospective analysis. Addictive behaviors. 2012;37(12):1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnett JJ. Emerging adulthood: What is it, and what is it good for? 2007;1(2):68–73. [Google Scholar]
- 33.Paljärvi T, Koskenvuo M, Poikolainen K, Kauhanen J, Sillanmäki L, Mäkelä P. Binge drinking and depressive symptoms: a 5-year population-based cohort study. Addiction. 2009;104(7):1168–1178. [DOI] [PubMed] [Google Scholar]
- 34.Sihvola E, Rose RJ, Dick DM, Pulkkinen L, Marttunen M, Kaprio J. Early-onset depressive disorders predict the use of addictive substances in adolescence: a prospective study of adolescent Finnish twins. Addiction. 2008;103(12):2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eddie D, Buckman J, Mun E-Y, et al. Different associations of alcohol cue reactivity with negative alcohol expectancies in mandated and inpatient samples of young adults. Addictive behaviors. 2013;38(4):2040–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcoholism: clinical and experimental research. 1997;21(4):613–619. [PubMed] [Google Scholar]
- 37.Eddie D, Kim C, Lehrer P, Deneke E, Bates ME. A pilot study of brief heart rate variability biofeedback to reduce craving in young adult men receiving inpatient treatment for substance use disorders. Applied psychophysiology biofeedback. 2014;39(3–4):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]


