Abstract
Human vascular microphysiological systems (MPS) represent promising three-dimensional in vitro models of normal and diseased vascular tissue. These systems build upon advances in tissue engineering, microfluidics, and stem cell differentiation and replicate key functional units of organs and tissues. Vascular models have been developed for the microvasculature as well as medium-size arterioles. Key functions of the vascular system have been reproduced and stem cells offer the potential to model genetic diseases and population variation in genes that may increase individual risk for cardiovascular disease. Such systems can be used to evaluate new therapeutics options.
Keywords: cardiovascular disease, in vitro models, endothelium, vascular smooth muscle cells, microfluidics
Introduction
Complications from cardiovascular disease (CVD) represent the leading cause of death in the United States (1) and other developed countries. The underlying cause for most CVD is atherosclerosis, in which cholesterol-laden plaques on the inner wall of the arterial lumen cause loss of vascular elasticity, reduction in blood flow, and narrowing of the arterial lumen. Risk factors for CVD include age, hypertension, type 2 diabetes, obesity, hypercholesterolemia, and smoking that induce oxidative stress resulting in modified forms of low-density lipoprotein (LDL), inflammation, and smooth muscle cell proliferation (2–5). The formation of plaque and oxidation of LDL causes endothelial cell activation and later leads to the recruitment of monocytes. In the presence of oxidative and pro-inflammatory stimuli, monocytes differentiate to macrophages and promote the formation of foam cells. The plaque may eventually rupture, which results in thrombus formation in the blood vessel, causing ischemia, heart attack, or stroke (4). Other diseases that lead to vascular damage including thrombotic disorders (deep vein thrombosis and disseminated intravascular coagulation), Marfan syndrome, aortic aneurysm, heart valve disease, congenital defects, and Progeria. Human immune system and inflammatory pathway activation play key roles in the initiation of atherosclerosis (6).
Mice are commonly used to study the genetic factors in vascular diseases (7). While animal models have provided crucial information about the initiation and progression of atherosclerosis, they still possess many shortcomings and cannot produce many of the features of the pathology found in humans. Wild type mice use high-density lipoprotein to transport cholesterol to tissues while humans uses LDL (8). The size of arteries in mice is much smaller compared to humans and the heart rate is much higher (9), leading to very different hemodynamic conditions. The many interacting polymorphisms identified in genome-wide association studies cannot be replicated in mice. Given these limitations, the response of treatments in mice may differ from that in humans (10, 11).
To overcome these pitfalls with animal models, human microphysiological systems (MPS) have been introduced to improve the accuracy of experimental predictions, minimize experimental time and cost, and reduce patient risk. Experiments that use MPS are highly reproducible. MPS use advanced fluidic fabrication methods to create three-dimensional models of the functional unit of tissue. These systems can be used for functional assays as well as genomic, metabolomic, and histological analysis. The major advantage of MPS is that they can be modified to test single or combination of hypotheses, which allows identification of the key factors in different model systems.
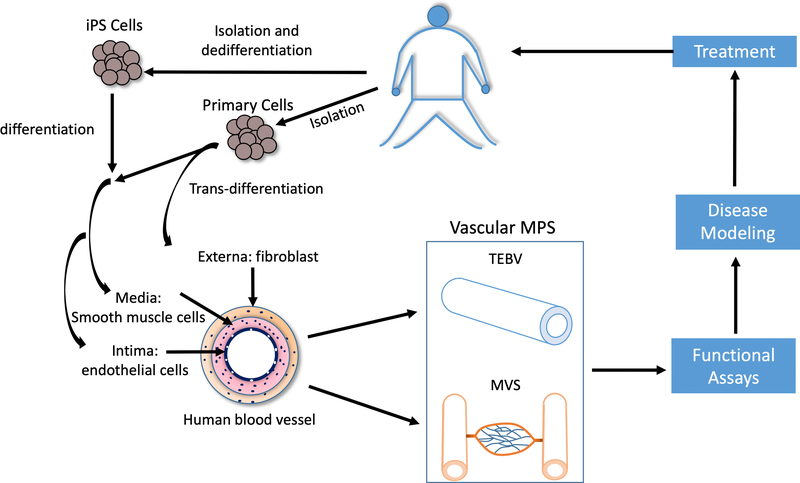
Vascular MPS use microfluidic devices with three-dimensional culture methods to recapitulate many model systems (12) (Figure 1). Furthermore, one or more MPS can be combined together to study systematic effects by the key contributing factors. To study the initiation and progression of vascular disease, tissue engineered blood vessels (TEBVs) have been designed to model the many vascular diseases, including atherosclerosis, progeria, and thrombotic disorders (13–17). The versatility of TEBVs allows the use of these models extended to vasculitis in rheumatoid arthritis and lupus or the role of oxidative stress (18). Given the different features and fabrication strategies of microvascular systems and TEBVs, each will be described separately.
Figure 1.
Overview of in vitro human vascular system. Fibroblasts, smooth muscle cells and endothelial cells that resemble the externa, media and intima layers, respectively, of human blood vessel could be obtained by isolation, differentiation from stem cells, or transdifferentiation from other cell types. Human vascular MPS including TEBV and MVS manufactured from these cells could be applied for functional assays, disease modeling and treatment discovery. These results could potentially translate back to patients.
In this perspective we examine the design criteria to build vascular MPS at the level of microvascular networks and as mimics of arterioles and arteries. Next, we address the various cell sources and the properties needed for adequate differentiation. Disease models are an important application area of MPS and may be used to assess the safety and efficacy of novel therapeutics.
Design Criteria and Fabrication of Arteriole- and Arterial-Scale MPS
The ultimate goal in designing vascular MPS is to reproduce key vascular functions in vivo. The minimum requirements are to allow blood flow under physiological pressures (10– 20 kPa) and shear stress (0–2.5 Pa) without inducing thrombosis or inflammation (19). Given that blood flow is generated by pressure induced by the heart, the large vessel MPS or TEBVs must process two mechanical properties, resistance to rupture (measured by burst pressure) and resistance to plastic deformation (measured by compliance) (19–21). The burst pressure of the human saphenous vein and artery are around 2000 and 3000 mm Hg, respectively (22–24). The compliance of human vessels ranges between 1% and 6% (24–28). Hence, an ideal TEBV should possess mechanical properties similar to the natural vessels.
Other than the mechanical properties of vascular MPS, biological properties also contribute to vessel failure. Protein adsorption on the surface of vascular MPS could subsequently lead to blood clotting. Hence, the non-thrombogenic and non-immunogenic behavior is necessary for designing vascular MPS, usually by incorporating a layer of vascular endothelium. The barrier function of vascular endothelium varies considerably from impermeable in the brain microvascular network to relatively permeable in the kidneys and liver (29, 30).
Current approaches to fabricate TEBVs that are more or less similar to natural vessels can be divided into two major categories, scaffold based or self-assembly. With scaffold-based method, either synthetic polymer or nature extracellular matrix (ECM) could be utilized as scaffold for in-vitro cell seeding. The essential requirements for synthetic polymers are biocompatibility and biodegradability. Polyglycolic acid (PGA) and Poly (lactide-co-glycolide) acid (PLGA) are two of the most widely studies biodegradable polymers used in TEBVs. After a period of growth and maturation, the developing TEBVs is placed in a bioreactor with pulsatile flow and burst pressures above 2000 mm Hg can be achieved after 8–10 weeks (31). Animal studies showed great patency results of these vessels.
Natural biological hydrogels and ECM proteins such as collagen, elastin, fibrin, gelatin, and modified hyaluronic acid (32–34) have been used as scaffold materials. These scaffolds have improved biocompatibility and provide adhesion sites for binding of cell surface integrins or cleavage sites to matrix metalloproteinases (MMPs), which facilitate cell attachment, cell migration and cell proliferation (35). While fibrin scaffolds also produce high burst pressures (36, 37), collagen scaffold TEVBs typically have lower mechanical properties (20, 21, 38, 39).
Although the use of synthetic polymers as scaffold is promising, the long manufacturing time for these vessels poses a great challenge for the use as a disease model system in vitro. One way to increase the burst pressure and rapidly fabricate perfusable vessels is by plastic compression of collagen gels embedded with smooth muscle cells, which increase the collagen density and improves the TEBV (14, 15, 40). Plastic compression generates TEBVs with burst pressures around 1600 mm Hg in a few hours (14). After a one to three week maturation period, these vessels are well-suited for modeling diseases in vitro (15).
Another source of natural scaffold is to decellularize TEBVs by depleting tissue and cells from allogenic or xenogeneic sources. Decellularized scaffolds preserved the natural architecture of the ECM of the vessels (34, 35). Complete depletion of cells is required to avoid the host immune response. To overcome this challenge, two strategies have been used, either by advancing decellularization technique or inactivating immunogenic biomolecules (34).
Self-assembly is another approach to manufacture TEBVs. Self-assembly utilizes the ECM produced by seeded cells as the vessel structural supports. With this method, a confluent layer form by cells in vitro is rolled into a tubular structures to mimic the vessel (22, 24). These vessels achieve a burst pressure over 2000 mm Hg. The major pitfall for TEBVs generated by this method is the long manufacturing time of several months, which makes it difficult to use as disease modeling purpose. Acellular grafts represent one approach to overcome this challenge.
Extrusion of a Matrigel solution containing endothelial cells and smooth muscle cells enabled self-organized of an arteriole-like structure, termed vesseloids (41). The vesseloids exhibit key vessel properties including a restrictive endothelial barrier and smooth muscle cell contractility. The vessels respond to inflammatory stimuli causing the endothelial cells to express the leukocyte adhesion molecules VCAM-1 and ICAM-1. The benefit of this novel approach is that vessels can be rapidly produced without a thick layer of extra cellular matrix, in contrast to most methods to produce tissue-engineered blood vessels.
Design Criteria and Fabrication of Microvascular Models
In addition to models for diseases of large and medium-size arteries, microvascular systems (MVS) have been developed for drug screening, discovery, delivery, and modeling diseases in microvasculature. MVS are often combined with engineered solid organs to allow long-term maintenance (42). For example, human blood-brain barrier (BBB) tissue chips have been designed to model the BBB dysfunctions in neurological disorders (43) and Alzheimer’s disease (44). Animal models do not recapitulate the whole disease state. The use of in vitro MVS with human cells or stem cells allows scientists to model the disease progression and drug response, which would result in better response prediction and reduce the use of live animals for disease modeling and drug testing (45, 46).
The structure of human micro-scale vessels (arterioles, capillaries and venules) are quite different from large vessels (arteries and veins). While arterioles and venules contain all three layers, the media and externa layers are very thin compared to arteries and veins, respectively. The capillaries consist of a layer of endothelial cells that function in tissue-vessel material exchanges. Pericytes are attached to the endothelium, regulating vessel dimensions and permeability.
Given the structural differences between large and medium size arteries and capillaries, strategies to manufacture MVS are different from those of TEBVs. Current approaches to manufacture MVS are either Top-Down or Bottom-Up (34, 47, 48). With the Top-Down approach, the pattern or geometry of the vascular systems are designed and then manufactured by 3D-printing (49), mold degradation (50, 51) or multilayer chip (52). While the pre-designed Top-Down approach could provide a controllable vascular structure and allow perfusions, the major disadvantage is that the resolution of these methods does not yet reach the level of capillaries.
In the Bottom-Up approach endothelial cells, pericytes or pericyte-like cells (e.g. fibroblasts or mesenchymal stem cells) are mixed together with a biological hydrogel and local chemical or physical stimuli from the various cell types induce angiogenesis and vasculogenesis. With these approaches, endothelial cells self-organized to form an interconnected network of microvessels (53–56). Growth factors such as VEGF and fibroblast growth factors are also supplemented to promote angiogenesis (57). Pericyte-like cells are needed to stabilize the microvasculature, otherwise the vessels breakdown after 24–48 hours. These methods allow the formation of perfusable capillary-size networks (42, 58) but the structures of these networks are hard to control. Another challenge for these methods is to form perfusable networks.
Cell source
Cells are one of the major components in vascular tissue engineering. Primary autologous cells including vascular smooth muscle cells, endothelial cells and fibroblasts, harvested from the patients, showed great successes for manufacturing vascular MPS. However, there are some challenges to use these cells as source for TEBVs. First, these cells required invasive procedure to harvest. Second, primary cells lost the ability to proliferate after prolonged expansion. Third, these cells are not available or not usable in some of the patients. While primary endothelial cells, which can be isolated from blood-derived endothelial colony forming cells, and fibroblasts are relatively feasible to obtain, obtaining functional and proliferative primary SMCs retains a major challenge given the limited accessibility of donors’ tissue, limited proliferation rate and donor-to-donor variation (59). Human embryonic stem cells (hESCs), mesenchymal stem cells (hMSCs) and induced pluripotent stem cells (iPSCs) show great potentials as cell sources in vascular tissue engineering. While the use of hESCs raises ethical issues, hMSCs and iPSCs seem well-suited for clinical translation and regenerative medicine. hMSCs can be easily obtained from various tissues (60) and their multipotent nature allows them to differentiate into many cell types, including smooth muscle cells (61) and endothelial cells (62). Since the discovery of iPSCs in 2006 by Takahashi and Yamanaka (63), iPSCs have been widely used (64) and showed great potential to develop vascular MPS. iPSCs could be transformed from various adult cells including fibroblasts or blood cells. The pluripotency gives iPSCs the potential to differentiate into cells from all three germ layers (mesoderm, endoderm and ectoderm) (63), which include SMCs (65) and ECs (66). The main pitfalls with iPSCs cells are their tumorigenic potential and immature differentiation. Progress has been made to reduce the risk of tumorigenicity by using non-integrating methods (67–70). Current prevailing non-viral and non-integration approaches include adenoviral vectors (68), Sendai vectors (71), episomal vectors (72), minicircle vectors (73), synthetic mRNAs (74) or small molecule cocktails (75).
A challenge with iPS-derived smooth muscle cells has been limited differentiation which reduced mechanical strength of the TEBVs (76, 77). Optimizing the differentiation protocol (65, 78) or applying cyclical mechanical stimulation (65)promotes SMC differentiation and increases the TEBV mechanical strength. Self-assembled microvascular networks have been developed using human brain microvascular endothelial cells and pericytes have been derived from iPSCs and exhibit the low permeability and high levels of tight junction proteins found in vivo (79).
Transdifferentiation of human adult cells to ECs and SMCs provides another cell source for vascular MPS. Transdifferentiation eliminates the intermediate step of generating iPSC cells and reduce the risk of tumorigenicity and can be achieved by small molecules (80, 81) targeting certain signaling pathways, activation and overexpression of key genes (82, 83) or CRISPR/Cas9-based transcriptional activator systems that force expression of key endogenous transcription factors (84). The introduction of cDNAs or CRISPR/Cas9 systems for gene editing can be achieved by either viral systems or non-viral/non-integration systems (85). In the context of vascular MPS, several groups have shown success in transdifferentiating human fibroblast cells to ECs (80, 82, 83, 86, 87) and SMCs (88) or ECs to SMCs (89).
Disease Models
iPSC cells and transdifferentiated cells offer great potentials for regenerative medicine and personalized medicine with minimum ethical issues. iPSC differentiated smooth muscle cells have been derived from patients with progeria (15), supervalvular aortic stenosis (90), and fibrillin 1 mutations in Marfan syndrome (91). Endothelial cells have been derived from individuals with pulmonary hypertension (92). iPSCs enabled discovery of new biology of these diseases, although only a few have been converted to three-dimensional models. Atchison et al., used Hutchinson-Gilford progeria syndrome (HGPS) patient derived TEVBs and these vessels could reproduce key features of HGPS and the response to drug treatment (15). A recent study used HGPS patient derived TEVBs and identified the contribution of endothelial dysfunction to the progression of atherosclerosis in HGPS (78). A tissue-on-a-chip and bottom-up self-assembly model of the neurovascular unit using primary or iPSCs derived from individuals with various neurological diseases, showed that these disease states alter the blood brain barrier permeability and could be suitable testbeds to assess drug candidates (43, 44).
Translational Insight
The development of vascular MPS provides an effective platform for the investigation of vascular development, vascular disease modeling, and evaluation of drug safety and efficacy. The vascular MPS create physiologically relevant microenvironments that closely model the in vivo environments. By incorporating recent advances in stem cell differentiation, vascular MPS could also be used in tailored medicine to model diseases individually and provide personalized information for each patient. Microvascular systems have already been used to study angiogenesis (54), (93), and the blood-brain barrier (42). Furthermore, the easy modification of vascular MPS enables deconvolution of the complex in vivo systems and testing hypotheses one by one. For example, high levels of LDL, monocyte activation, and accumulation and inflammatory environments all contribute to the initiation and progression of atherosclerosis. However, it is extremely difficult to identify which contributes more to the early stages of atherosclerosis by in vivo system. By using vascular MPS, each factor could be tested individually or combined with other factors to give more information about the underlying mechanisms. Gene editing technology allows creation of specific acquired changes to examine complex conditions such as aging (94) and interactions among polymorphisms associated with CVD. The future of vascular MPS relies on new techniques to 1) manufacture vascular MPS with shorter manufacturing time and more closely mimicking the natural vessels, 2) promote complete cell differentiate from iPSCs or hMSCs to functional ECs and SMCs, and 3) integrate different scales of vessel (artery to capillary) in the same system.
Acknowledgements
This work was supported, in part, by NIH grants RO1HL138252 and UH3TR002142.
Footnotes
Disclosures
The authors have no disclosures or conflicts to declare.
References:
- 1.Heron M. Deaths: Leading Causes for 2017. National Vital Statistics Reports. 2019;68(6). [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–54. Epub 2003/01/23. doi: 10.1161/01.cir.0000048893.62841.f7. PubMed PMID: 12538439. [DOI] [PubMed] [Google Scholar]
- 3.Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13(2):79–98. Epub 2015/10/28. doi: 10.1038/nrcardio.2015.164. PubMed PMID: 26503410. [DOI] [PubMed] [Google Scholar]
- 4.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–55. Epub 2011/05/03. doi: 10.1016/j.cell.2011.04.005. PubMed PMID: 21529710; PubMed Central PMCID: PMCPMC3111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol. 2015;12(4):199–211. Epub 2015/02/11. doi: 10.1038/nrcardio.2015.5. PubMed PMID: 25666404. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116(2):307–11. Epub 2015/01/17. doi: 10.1161/CIRCRESAHA.116.301313. PubMed PMID: 25593275; PubMed Central PMCID: PMCPMC4299915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukhopadhyay R. Mouse models of atherosclerosis: explaining critical roles of lipid metabolism and inflammation. J Appl Genet. 2013;54(2):185–92. Epub 2013/01/31. doi: 10.1007/s13353-013-0134-4. PubMed PMID: 23361320. [DOI] [PubMed] [Google Scholar]
- 8.Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, et al. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13. Epub 2017/05/10. doi: 10.1016/j.ejphar.2017.05.010. PubMed PMID: 28483459. [DOI] [PubMed] [Google Scholar]
- 9.Ho D, Zhao X, Gao S, Hong C, Vatner DE, Vatner SF. Heart Rate and Electrocardiography Monitoring in Mice. Curr Protoc Mouse Biol. 2011;1:123–39. Epub 2011/07/12. doi: 10.1002/9780470942390.mo100159. PubMed PMID: 21743842; PubMed Central PMCID: PMCPMC3130311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oppi S, Luscher TF, Stein S. Mouse Models for Atherosclerosis Research-Which Is My Line? Front Cardiovasc Med. 2019;6:46. Epub 2019/04/30. doi: 10.3389/fcvm.2019.00046. PubMed PMID: 31032262; PubMed Central PMCID: PMCPMC6473202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YT, Lin HY, Chan YW, Li KH, To OT, Yan BP, et al. Mouse models of atherosclerosis: a historical perspective and recent advances. Lipids Health Dis. 2017;16(1):12. Epub 2017/01/18. doi: 10.1186/s12944-016-0402-5. PubMed PMID: 28095860; PubMed Central PMCID: PMCPMC5240327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–72. Epub 2014/08/06. doi: 10.1038/nbt.2989. PubMed PMID: 25093883. [DOI] [PubMed] [Google Scholar]
- 13.Robert J, Weber B, Frese L, Emmert MY, Schmidt D, von Eckardstein A, et al. A three-dimensional engineered artery model for in vitro atherosclerosis research. PLoS One. 2013;8(11):e79821. Epub 2013/11/19. doi: 10.1371/journal.pone.0079821. PubMed PMID: 24244566; PubMed Central PMCID: PMCPMC3828234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez CE, Yen RW, Perez SM, Bedell HW, Povsic TJ, Reichert WM, et al. Human Vascular Microphysiological System for in vitro Drug Screening. Sci Rep. 2016;6:21579. Epub 2016/02/19. doi: 10.1038/srep21579. PubMed PMID: 26888719; PubMed Central PMCID: PMCPMC4757887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atchison L, Zhang H, Cao K, Truskey GA. A Tissue Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome Using Human iPSC-derived Smooth Muscle Cells. Sci Rep. 2017;7(1):8168. Epub 2017/08/16. doi: 10.1038/s41598-017-08632-4. PubMed PMID: 28811655; PubMed Central PMCID: PMCPMC5557922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang YS, Davoudi F, Walch P, Manbachi A, Luo X, Dell’Erba V, et al. Bioprinted thrombosis-on-a-chip. Lab Chip. 2016;16(21):4097–105. Epub 2016/10/19. doi: 10.1039/c6lc00380j. PubMed PMID: 27722710; PubMed Central PMCID: PMCPMC5072176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109(24):9342–7. Epub 2012/05/31. doi: 10.1073/pnas.1201240109. PubMed PMID: 22645376; PubMed Central PMCID: PMCPMC3386137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubattu S, Forte M, Raffa S. Circulating Leukocytes and Oxidative Stress in Cardiovascular Diseases: A State of the Art. Oxid Med Cell Longev. 2019;2019:2650429. Epub 2019/11/19. doi: 10.1155/2019/2650429. PubMed PMID: 31737166; PubMed Central PMCID: PMCPMC6815586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimitrievska S, Niklason LE. Historical Perspective and Future Direction of Blood Vessel Developments. Cold Spring Harb Perspect Med. 2018;8(2). a025742. doi: 10.1101/cshperspect.a025742. PubMed PMID: 28348177; PMCID: PMCPMC5685928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pashneh-Tala S, MacNeil S, Claeyssens F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng Part B Rev. 2016;22(1):68–100. Epub 2015/10/09. doi: 10.1089/ten.teb.2015.0100. PubMed PMID: 26447530; PubMed Central PMCID: PMCPMC4753638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar VA, Brewster LP, Caves JM, Chaikof EL. Tissue Engineering of Blood Vessels: Functional Requirements, Progress, and Future Challenges. Cardiovasc Eng Technol. 2011;2(3):137–48. Epub 2011/09/01. doi: 10.1007/s13239-011-0049-3. PubMed PMID: 23181145; PubMed Central PMCID: PMCPMC3505086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56. Epub 1998/01/23. doi: 10.1096/fasebj.12.1.47. PubMed PMID: 9438410. [DOI] [PubMed] [Google Scholar]
- 23.Lamm P, Juchem G, Milz S, Schuffenhauer M, Reichart B. Autologous endothelialized vein allograft: a solution in the search for small-caliber grafts in coronary artery bypass graft operations. Circulation. 2001;104(12 Suppl 1):I108–14. Epub 2001/09/25. PubMed PMID: 11568040. [PubMed] [Google Scholar]
- 24.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12(3):361–5. Epub 2006/02/24. doi: 10.1038/nm1364. PubMed PMID: 16491087; PubMed Central PMCID: PMCPMC1513140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobrin PB. Mechanical behavior of vascular smooth muscle in cylindrical segments of arteries in vitro. Ann Biomed Eng. 1984;12(5):497–510. Epub 1984/01/01. doi: 10.1007/bf02363919. PubMed PMID: 6534220. [DOI] [PubMed] [Google Scholar]
- 26.Cambria RP, Megerman J, Brewster DC, Warnock DF, Hasson J, Abbott WM. The evolution of morphologic and biomechanical changes in reversed and in-situ vein grafts. Ann Surg. 1987;205(2):167–74. Epub 1987/02/01. doi: 10.1097/00000658-198702000-00011. PubMed PMID: 3813687; PubMed Central PMCID: PMCPMC1492826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamiot-Clerc P, Copie X, Renaud JF, Safar M, Girerd X. Comparative reactivity and mechanical properties of human isolated internal mammary and radial arteries. Cardiovasc Res. 1998;37(3):811–9. Epub 1998/07/11. doi: 10.1016/s0008-6363(97)00267-8. PubMed PMID: 9659466. [DOI] [PubMed] [Google Scholar]
- 28.Girerd XJ, Acar C, Mourad JJ, Boutouyrie P, Safar ME, Laurent S. Incompressibility of the human arterial wall: an in vitro ultrasound study. J Hypertens Suppl. 1992;10(6):S111–4. Epub 1992/08/01. PubMed PMID: 1432310. [PubMed] [Google Scholar]
- 29.Sukriti S, Tauseef M, Yazbeck P, Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ. 2014;4(4):535–51. Epub 2015/01/23. doi: 10.1086/677356. PubMed PMID: 25610592; PubMed Central PMCID: PMCPMC4278616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan SY, Rigor RR. Regulation of Endothelial Barrier Function. Integrated Systems Physiology: From Molecule to Function to Disease; San Rafael (CA)2010. [Google Scholar]
- 31.Niklason LE, Langer RS. Advances in tissue engineering of blood vessels and other tissues. Transpl Immunol. 1997;5(4):303–6. Epub 1998/03/21. doi: 10.1016/s0966-3274(97)80013-5. PubMed PMID: 9504152. [DOI] [PubMed] [Google Scholar]
- 32.Blache U, Ehrbar M. Inspired by Nature: Hydrogels as Versatile Tools for Vascular Engineering. Adv Wound Care (New Rochelle). 2018;7(7):232–46. Epub 2018/07/10. doi: 10.1089/wound.2017.0760. PubMed PMID: 29984113; PubMed Central PMCID: PMCPMC6032659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coenen AMJ, Bernaerts KV, Harings JAW, Jockenhoevel S, Ghazanfari S. Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers. Acta Biomater. 2018;79:60–82. Epub 2018/08/31. doi: 10.1016/j.actbio.2018.08.027. PubMed PMID: 30165203. [DOI] [PubMed] [Google Scholar]
- 34.Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell. 2018;22(3):340–54. Epub 2018/03/03. doi: 10.1016/j.stem.2018.02.009. PubMed PMID: 29499152; PubMed Central PMCID: PMCPMC5849079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truskey GA. Advancing cardiovascular tissue engineering. F1000Res. 2016;5. Epub 2016/06/16. doi: 10.12688/f1000research.8237.1. PubMed PMID: 27303643; PubMed Central PMCID: PMCPMC4890312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grassl ED, Oegema TR, Tranquillo RT. A fibrin-based arterial media equivalent. J Biomed Mater Res A. 2003;66(3):550–61. Epub 2003/08/15. doi: 10.1002/jbm.a.10589. PubMed PMID: 12918038. [DOI] [PubMed] [Google Scholar]
- 37.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32(3):714–22. Epub 2010/10/12. doi: 10.1016/j.biomaterials.2010.09.019. PubMed PMID: 20934214; PubMed Central PMCID: PMCPMC3042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736):397–400. Epub 1986/01/24. doi: 10.1126/science.2934816. PubMed PMID: 2934816. [DOI] [PubMed] [Google Scholar]
- 39.Berglund JD, Mohseni MM, Nerem RM, Sambanis A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials. 2003;24(7):1241–54. Epub 2003/01/16. doi: 10.1016/s0142-9612(02)00506-9. PubMed PMID: 12527265. [DOI] [PubMed] [Google Scholar]
- 40.Ghezzi CE, Risse PA, Marelli B, Muja N, Barralet JE, Martin JG, et al. An airway smooth muscle cell niche under physiological pulsatile flow culture using a tubular dense collagen construct. Biomaterials. 2013;34(8):1954–66. Epub 2012/12/22. doi: 10.1016/j.biomaterials.2012.11.025. PubMed PMID: 23257180. [DOI] [PubMed] [Google Scholar]
- 41.Andrique L, Recher G, Alessandri K, Pujol N, Feyeux M, Bon P, et al. A model of guided cell self-organization for rapid and spontaneous formation of functional vessels. Science Advances. 2019;5(6): 10.1126/sciadv.aau6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osaki T, Sivathanu V, Kamm RD. Vascularized microfluidic organ-chips for drug screening, disease models and tissue engineering. Curr Opin Biotechnol. 2018;52:116–23. Epub 2018/04/16. doi: 10.1016/j.copbio.2018.03.011. PubMed PMID: 29656237; PubMed Central PMCID: PMCPMC6082713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell. 2019;24(6):995–1005 e6. Epub 2019/06/08. doi: 10.1016/j.stem.2019.05.011. PubMed PMID: 31173718. [DOI] [PubMed] [Google Scholar]
- 44.Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, et al. Blood-Brain Barrier Dysfunction in a 3D In Vitro Model of Alzheimer’s Disease. Adv Sci (Weinh). 2019;6(20):1900962. Epub 2019/10/23. doi: 10.1002/advs.201900962. PubMed PMID: 31637161; PubMed Central PMCID: PMCPMC6794630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jorfi M, D’Avanzo C, Kim DY, Irimia D. Three-Dimensional Models of the Human Brain Development and Diseases. Adv Healthc Mater. 2018;7(1). Epub 2017/08/29. doi: 10.1002/adhm.201700723. PubMed PMID: 28845922; PubMed Central PMCID: PMCPMC5762251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shuler ML, Hickman JJ. Toward in vitro models of brain structure and function. Proc Natl Acad Sci U S A. 2014;111(38):13682–3. Epub 2014/09/07. doi: 10.1073/pnas.1414484111. PubMed PMID: 25192937; PubMed Central PMCID: PMCPMC4183344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiruvannamalai-Annamalai R, Armant DR, Matthew HW. A glycosaminoglycan based, modular tissue scaffold system for rapid assembly of perfusable, high cell density, engineered tissues. PLoS One. 2014;9(1):e84287. Epub 2014/01/28. doi: 10.1371/journal.pone.0084287. PubMed PMID: 24465401; PubMed Central PMCID: PMCPMC3896358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nichol JW, Khademhosseini A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter. 2009;5(7):1312–9. Epub 2010/02/25. doi: 10.1039/b814285h. PubMed PMID: 20179781; PubMed Central PMCID: PMCPMC2826124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365(6452):482–7. Epub 2019/08/03. doi: 10.1126/science.aav9051. PubMed PMID: 31371612. [DOI] [PubMed] [Google Scholar]
- 50.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7(6):720–5. Epub 2007/06/01. doi: 10.1039/b618409j. PubMed PMID: 17538713. [DOI] [PubMed] [Google Scholar]
- 51.Heintz KA, Bregenzer ME, Mantle JL, Lee KH, West JL, Slater JH. Fabrication of 3D Biomimetic Microfluidic Networks in Hydrogels. Adv Healthc Mater. 2016;5(17):2153–60. Epub 2016/05/31. doi: 10.1002/adhm.201600351. PubMed PMID: 27239785; PubMed Central PMCID: PMCPMC5014628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater. 2016;15(6):669–78. Epub 2016/03/08. doi: 10.1038/nmat4570. PubMed PMID: 26950595; PubMed Central PMCID: PMCPMC4879054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13(8):1489–500. Epub 2013/02/27. doi: 10.1039/c3lc41320a. PubMed PMID: 23440068. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A. 2013;110(17):6712–7. Epub 2013/04/10. doi: 10.1073/pnas.1221526110. PubMed PMID: 23569284; PubMed Central PMCID: PMCPMC3637738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Natividad-Diaz SL, Browne S, Jha AK, Ma Z, Hossainy S, Kurokawa YK, et al. A combined hiPSC-derived endothelial cell and in vitro microfluidic platform for assessing biomaterial-based angiogenesis. Biomaterials. 2019;194:73–83. Epub 2018/12/26. doi: 10.1016/j.biomaterials.2018.11.032. PubMed PMID: 30583150; PubMed Central PMCID: PMCPMC6453535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters EB, Christoforou N, Leong KW, Truskey GA, West JL. Poly(ethylene glycol) Hydrogel Scaffolds Containing Cell-Adhesive and Protease-Sensitive Peptides Support Microvessel Formation by Endothelial Progenitor Cells. Cell Mol Bioeng. 2016;9(1):38–54. Epub 2016/04/05. doi: 10.1007/s12195-015-0423-6. PubMed PMID: 27042236; PubMed Central PMCID: PMCPMC4812433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28(6):1123–31. Epub 2006/11/23. doi: 10.1016/j.biomaterials.2006.10.029. PubMed PMID: 17113636. [DOI] [PubMed] [Google Scholar]
- 58.Hsu YH, Moya ML, Hughes CC, George SC, Lee AP. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip. 2013;13(15):2990–8. Epub 2013/06/01. doi: 10.1039/c3lc50424g. PubMed PMID: 23723013; PubMed Central PMCID: PMCPMC3734340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, et al. Blood vessels engineered from human cells. Lancet. 2005;365(9477):2122–4. Epub 2005/06/21. doi: 10.1016/S0140-6736(05)66735-9. PubMed PMID: 15964449. [DOI] [PubMed] [Google Scholar]
- 60.Zomer HD, Vidane AS, Goncalves NN, Ambrosio CE. Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives. Stem Cells Cloning. 2015;8:125–34. Epub 2015/10/10. doi: 10.2147/SCCAA.S88036. PubMed PMID: 26451119; PubMed Central PMCID: PMCPMC4592031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu W, Hong X, Le Bras A, Nowak WN, Issa Bhaloo S, Deng J, et al. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J Biol Chem. 2018;293(21):8089–102. Epub 2018/04/13. doi: 10.1074/jbc.RA118.001739. PubMed PMID: 29643181; PubMed Central PMCID: PMCPMC5971462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khaki M, Salmanian AH, Abtahi H, Ganji A, Mosayebi G. Mesenchymal Stem Cells Differentiate to Endothelial Cells Using Recombinant Vascular Endothelial Growth Factor -A. Rep Biochem Mol Biol. 2018;6(2):144–50. Epub 2018/05/16. PubMed PMID: 29761109; PubMed Central PMCID: PMCPMC5940356. [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. Epub 2006/08/15. doi: 10.1016/j.cell.2006.07.024. PubMed PMID: 16904174. [DOI] [PubMed] [Google Scholar]
- 64.Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16(2):115–30. Epub 2016/12/17. doi: 10.1038/nrd.2016.245. PubMed PMID: 27980341; PubMed Central PMCID: PMCPMC6416143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo J, Qin L, Zhao L, Gui L, Ellis MW, Huang Y, et al. Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs. Cell Stem Cell. 2020. Epub 2020/01/21. doi: 10.1016/j.stem.2019.12.012. PubMed PMID: 31956039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. 2015;17(8):994–1003. Epub 2015/07/28. doi: 10.1038/ncb3205. PubMed PMID: 26214132; PubMed Central PMCID: PMCPMC4566857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015;33(1):58–63. Epub 2014/12/02. doi: 10.1038/nbt.3070. PubMed PMID: 25437882; PubMed Central PMCID: PMCPMC4329913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–9. Epub 2008/09/27. doi: 10.1126/science.1162494. PubMed PMID: 18818365; PubMed Central PMCID: PMCPMC3987909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan Y, Ooi S, Wang L. Immunogenicity and tumorigenicity of pluripotent stem cells and their derivatives: genetic and epigenetic perspectives. Curr Stem Cell Res Ther. 2014;9(1):63–72. Epub 2013/10/29. doi: . PubMed PMID: 24160683; PubMed Central PMCID: PMCPMC3873036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panda A, Gurusamy N, Rajasingh S, Carter HK, Thomas EL, Rajasingh J. Non-viral reprogramming and induced pluripotent stem cells for cardiovascular therapy. Differentiation. 2020;112:58–66. Epub 2020/01/19. doi: 10.1016/j.diff.2019.12.001. PubMed PMID: 31954271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–62. Epub 2009/10/20. doi: 10.2183/pjab.85.348. PubMed PMID: 19838014; PubMed Central PMCID: PMCPMC3621571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin, II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. Epub 2009/03/28. doi: 10.1126/science.1172482. PubMed PMID: 19325077; PubMed Central PMCID: PMCPMC2758053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197–9. Epub 2010/02/09. doi: 10.1038/nmeth.1426. PubMed PMID: 20139967; PubMed Central PMCID: PMCPMC2892897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grudzien-Nogalska E, Kowalska J, Su W, Kuhn AN, Slepenkov SV, Darzynkiewicz E, et al. Synthetic mRNAs with superior translation and stability properties. Methods Mol Biol. 2013;969:55–72. Epub 2013/01/09. doi: 10.1007/978-1-62703-260-5_4. PubMed PMID: 23296927. [DOI] [PubMed] [Google Scholar]
- 75.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–4. Epub 2013/07/23. doi: 10.1126/science.1239278. PubMed PMID: 23868920. [DOI] [PubMed] [Google Scholar]
- 76.Gui L, Dash BC, Luo J, Qin L, Zhao L, Yamamoto K, et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials. 2016;102:120–9. Epub 2016/06/24. doi: 10.1016/j.biomaterials.2016.06.010. PubMed PMID: 27336184; PubMed Central PMCID: PMCPMC4939127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elliott MB, Ginn B, Fukunishi T, Bedja D, Suresh A, Chen T, et al. Regenerative and durable small-diameter graft as an arterial conduit. Proc Natl Acad Sci U S A. 2019;116(26):12710–9. Epub 2019/06/12. doi: 10.1073/pnas.1905966116. PubMed PMID: 31182572; PubMed Central PMCID: PMCPMC6601275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atchison L, Abutaleb NO, Snyder-Mounts E, Gete Y, Ladha A, Ribar T, et al. iPSC-Derived Endothelial Cells Affect Vascular Function in a Tissue-Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome. Stem Cell Reports. 2020;14(2):325–37. Epub 02/06/2020. doi: 10.1016/j.stemcr.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–29. Epub 2018/07/23. doi: 10.1016/j.biomaterials.2018.07.014. PubMed PMID: 30032046; PubMed Central PMCID: PMCPMC6201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sayed N, Wong WT, Ospino F, Meng S, Lee J, Jha A, et al. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation. 2015;131(3):300–9. Epub 2014/11/02. doi: 10.1161/CIRCULATIONAHA.113.007394. PubMed PMID: 25359165; PubMed Central PMCID: PMCPMC4309381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu JD, et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016;352(6290):1216–20. Epub 2016/04/30. doi: 10.1126/science.aaf1502. PubMed PMID: 27127239. [DOI] [PubMed] [Google Scholar]
- 82.Lee S, Park C, Han JW, Kim JY, Cho K, Kim EJ, et al. Direct Reprogramming of Human Dermal Fibroblasts Into Endothelial Cells Using ER71/ETV2. Circ Res. 2017;120(5):848–61. Epub 2016/12/23. doi: 10.1161/CIRCRESAHA.116.309833. PubMed PMID: 28003219; PubMed Central PMCID: PMCPMC5336520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morita R, Suzuki M, Kasahara H, Shimizu N, Shichita T, Sekiya T, et al. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci U S A. 2015;112(1):160–5. Epub 2014/12/30. doi: 10.1073/pnas.1413234112. PubMed PMID: 25540418; PubMed Central PMCID: PMCPMC4291653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Black JB, Adler AF, Wang HG, D’Ippolito AM, Hutchinson HA, Reddy TE, et al. Targeted Epigenetic Remodeling of Endogenous Loci by CRISPR/Cas9-Based Transcriptional Activators Directly Converts Fibroblasts to Neuronal Cells. Cell Stem Cell. 2016;19(3):406–14. Epub 2016/08/16. doi: 10.1016/j.stem.2016.07.001. PubMed PMID: 27524438; PubMed Central PMCID: PMCPMC5010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grath A, Dai G. Direct cell reprogramming for tissue engineering and regenerative medicine. J Biol Eng. 2019;13:14. Epub 2019/02/26. doi: 10.1186/s13036-019-0144-9. PubMed PMID: 30805026; PubMed Central PMCID: PMCPMC6373087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012;109(34):13793–8. Epub 2012/08/08. doi: 10.1073/pnas.1205526109. PubMed PMID: 22869753; PubMed Central PMCID: PMCPMC3427074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Junker JP, Lonnqvist S, Rakar J, Karlsson LK, Grenegard M, Kratz G. Differentiation of human dermal fibroblasts towards endothelial cells. Differentiation. 2013;85(3):67–77. Epub 2013/05/07. doi: 10.1016/j.diff.2013.01.005. PubMed PMID: 23644553. [DOI] [PubMed] [Google Scholar]
- 88.Karamariti E, Margariti A, Winkler B, Wang X, Hong X, Baban D, et al. Smooth muscle cells differentiated from reprogrammed embryonic lung fibroblasts through DKK3 signaling are potent for tissue engineering of vascular grafts. Circ Res. 2013;112(11):1433–43. Epub 2013/03/27. doi: 10.1161/CIRCRESAHA.111.300415. PubMed PMID: 23529184. [DOI] [PubMed] [Google Scholar]
- 89.Ji H, Atchison L, Chen Z, Chakraborty S, Jung Y, Truskey GA, et al. Transdifferentiation of human endothelial progenitors into smooth muscle cells. Biomaterials. 2016;85:180–94. Epub 2016/02/14. doi: 10.1016/j.biomaterials.2016.01.066. PubMed PMID: 26874281; PubMed Central PMCID: PMCPMC4763719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ge X, Ren Y, Bartulos O, Lee Min Y, Yue Z, Kim K-Y, et al. Modeling Supravalvular Aortic Stenosis Syndrome With Human Induced Pluripotent Stem Cells. Circulation. 2012;126(14):1695–704. doi: 10.1161/CIRCULATIONAHA.112.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Granata A, Serrano F, Bernard WG, McNamara M, Low L, Sastry P, et al. An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nature Genetics. 2017;49(1):97–109. doi: 10.1038/ng.3723. [DOI] [PubMed] [Google Scholar]
- 92.Gu M, Shao NY, Sa S, Li D, Termglinchan V, Ameen M, et al. Patient-Specific iPSC-Derived Endothelial Cells Uncover Pathways that Protect against Pulmonary Hypertension in BMPR2 Mutation Carriers. Cell Stem Cell. 2017;20(4):490–504 e5. Epub 2016/12/27. doi: 10.1016/j.stem.2016.08.019. PubMed PMID: 28017794; PubMed Central PMCID: PMCPMC5500296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shirure VS, Bi Y, Curtis MB, Lezia A, Goedegebuure MM, Goedegebuure SP, et al. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip. 2018;18(23):3687–702. Epub 2018/11/06. doi: 10.1039/c8lc00596f. PubMed PMID: 30393802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Acun A, Zorlutuna P. CRISPR/Cas9 Edited Induced Pluripotent Stem Cell-Based Vascular Tissues to Model Aging and Disease-Dependent Impairment. Tissue Engineering Part A. 2019;25(9–10):759–72. doi: 10.1089/ten.tea.2018.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]