Abstract
The pandemic of coronavirus disease 2019 (COVID-19) has unique implications for the anesthetic management of endovascular therapy for acute ischemic stroke. The Society for Neuroscience in Anesthesiology and Critical Care appointed a task force to provide timely, consensus-based expert recommendations using available evidence for the safe and effective anesthetic management of endovascular therapy for acute ischemic stroke during the COVID-19 pandemic. The goal of this consensus statement is to provide recommendations for anesthetic management considering the following (and they are): (1) optimal neurological outcomes for patients; (2) minimizing the risk for health care professionals, and (3) facilitating judicious use of resources while accounting for existing variability in care. It provides a framework for selecting the optimal anesthetic technique (general anesthesia or monitored anesthesia care) for a given patient and offers suggestions for best practices for anesthesia care during the pandemic. Institutions and health care providers are encouraged to adapt these recommendations to best suit local needs, considering existing practice standards and resource availability to ensure safety of patients and providers.
Key Words: conscious sedation, COVID-19 pandemic, general anesthesia, monitored anesthesia care, patient-to-professional transmission, stroke, thrombectomy
At the time of writing (April 2, 2020), anesthesiologists worldwide are involved closely in caring for patients impacted by the coronavirus disease 2019 (COVID-19) pandemic caused by the novel severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2).1 A number of health care personnel have been reported to have contracted COVID-19. Cardiovascular or cerebrovascular disease has been reported in 16.4% and ischemic stroke in 5% of COVID-19 patients.2,3 It is likely that patients with COVID-19 may require endovascular therapy (EVT) for acute ischemic stroke (AIS). In addition, patients requiring EVT may be carriers of SARS-CoV-2 from community exposure.
The choice of anesthetic technique, specifically the preference for general anesthesia (GA) or monitored anesthesia care (MAC), for EVT of AIS is controversial.4–15 Current evidence, based on randomized control trials, indicates potential advantages of GA over MAC for neurological outcomes.16–18 However, there is considerable variability in practice with some institutions routinely using GA, others routinely using MAC, and yet others offering either anesthetic technique.19,20 Essentially, although many patients receive MAC for EVT, urgent conversion to GA is undesirable especially when COVID-19 is suspected. The current pandemic has significant implications for anesthesiology and perioperative care generally.1,21–29 Specific to EVT, there is significant concern for potential risk to health care providers, as AIS patients are rapidly transported between various hospital locations (emergency departments, imaging, intervention suites, intensive care, and postanesthesia care units) over a brief period of time, with little or no opportunity for testing for infection. Although the Society for Neuroscience in Anesthesiology and Critical Care (SNACC) has previously published consensus recommendations for the anesthetic management of EVT,30 the current situation warrants an urgent need for expert recommendations using best available evidence to provide guidance to health care professionals during the COVID-19 pandemic.
AIM
The aim of this work was to make consensus-based expert recommendations using available evidence for safe and effective anesthetic management of endovascular treatment of AIS during the COVID-19 pandemic to achieve the following (and they are): (1) provide best neurological outcomes for patients; (2) minimize the risk for health care professionals; and (3) facilitate judicious use of resources.
TASK FORCE MEMBERS
The task force responsible for sourcing the evidence and writing this consensus statement was appointed by SNACC. It comprises experienced neuroanesthesiologists with expertise in stroke who have published original research in the field of AIS and who currently work at high-volume stroke centers. The team has representation from North America, Europe, and Asia. These consensus guidelines were made available to SNACC members for review and approved by the Board of Directors of SNACC before publication. The recommendations were also critically reviewed by official representatives of the Society of Neurointerventional Surgery, Society of Vascular and Interventional Neurology, European Society of Minimally Invasive Neurological Therapy, and the Neurocritical Care Society, who provided inputs to the consensus before preparation of the final document and its formal endorsement.
SCOPE
This consensus statement was generated in a time-sensitive manner, and its scope is limited to recommendations during the COVID-19 pandemic. It is not intended to be a comprehensive recommendation or guideline for the anesthetic management of EVT during ordinary circumstances. The document also does not comprehensively cover all aspects of the general principles and practices of anesthetic management and exposure prevention during the pandemic. The recommendations provided herein reflect expert consensus opinion based on the information available at the time of writing. The recommendations are designed to provide guidance in the context of the current pandemic and should not be interpreted as standards of care. The key recommendations are summarized in Table 1. Institutions and providers are encouraged to adapt these recommendations to suit local needs, considering existing practice standards and resource availability to ensure safety of patients and providers.
TABLE 1.
Key Recommendations

RELEVANT GENERAL CONSIDERATIONS
COVID-19 is a serious viral infection with a high risk of spreading through droplets, aerosols, or contaminated surfaces.1,31,32 It is controversial whether COVID-19 can be transmitted via an airborne route (small particles that remain aloft in the air for longer periods of time). However, a recent study demonstrated the ability of the virus to persist in aerosols for hours, making aerosol transmission plausible.33
There is lack of agreement between guidelines with regard to the use of airborne precautions during routine care, although airborne precautions are universally recommended for aerosol-generating procedures.
In the setting of AIS requiring emergent EVT, testing for and confirming COVID-19 is currently not practical. Therefore, the majority of patients presenting for EVT are expected to be either “unknown” or “suspected” COVID-19.
Bag-mask ventilation, intubation, extubation, airway suctioning, and cardiopulmonary resuscitation may result in aerosolization of respiratory secretions increasing the likelihood of exposure to health care personnel.26,29 The American Society of Anesthesiologists has highlighted these issues relevant to anesthesia care.34
Leaks from tracheal tube cuffs, manipulation or adjustment of tracheal tubes, and disconnection of breathing circuits may lead to aerosolization and should be avoided unless essential.
Sneezing may produce as many as 40,000 droplets 0.5 to 12 μm in diameter that may be expelled at speeds up to 100 m/s; coughing may produce up to 3000 droplet nuclei.35–37 According to the Centers for Disease Control, the contribution of small respirable particles (aerosols or droplet nuclei) to close proximity transmission of COVID-19 is currently uncertain.38 However, coughing and sneezing in spontaneously breathing COVID-19-positive patients may increase aerosolization and increase both the distance that viral particles spread and the time they remain airborne, posing potential risk to health care workers in proximity. This may increase the risk of exposure to health care personnel not only during EVT (eg, anesthesia providers, interventional neuroradiology staff) but also during early management in the emergency department, and during imaging studies and transport between hospital locations before and after EVT.
Working in close proximity of the airway, and airway interventions such as chin lift or jaw thrust, may expose anesthesia providers to increased risk of airborne infection.
Increasing oxygen flow rates increases aerosol dispersion with both nasal cannula and simple mask.39,40 High-flow oxygen was associated with increased transmission of the SARS-CoV.41 It is possible that high-flow oxygen in a spontaneously breathing COVID-19-positive patient may result in aerosolization with increased likelihood of exposure to health care personnel.
Covering a patient’s nose and mouth with a surgical mask decreases the distance of aerosol spread during coughing, which can reduce the transmission of airborne infections.42,43
Coughing during EVT is unsafe for the patient (due to possible movement-related vascular complications) and the interventional and anesthesia teams working in close proximity.
Emergent conversion from MAC to GA during EVT is undesirable given the risk of producing aerosol contamination in an uncontrolled situation.
On the basis of data from randomized control trials, GA is noninferior to MAC for neurological outcomes after EVT for AIS, and may be associated with better neurological outcomes as long as hemodynamic stability is maintained.15–18
Outside of the EVT setting, not all COVID-19-positive patients require intubation and mechanical ventilation. The risk of infection to health care personnel providing care to these patients can be reduced using airborne personal protective equipment (PPE).34
During pandemic situations, there is the possibility of resource limitations, including ventilators and PPE.
Drastic changes in clinical care and workflow are typically not desirable, particularly in the absence of strong evidence. However, unprecedented situations such as this pandemic will require flexibility and careful consideration of changes in practices and workflow. Protection of health care staff is critical to the overall ability to manage the pandemic.
RECOMMENDATIONS FOR CHOICE OF ANESTHETIC TECHNIQUE
The vast majority of patients will have to be considered “suspected COVID-19” or “unknown COVID-19” when presenting for EVT. Irrespective of the choice of anesthetic technique, we recommend airborne precautions for all these patients. Testing to rule out COVID-19 should occur as soon as feasible without delaying EVT. The task force is aware of rare cases where patients received nasal swabs for COVID-19 testing in the emergency department and had significant epistaxis following administration of heparin during thrombectomy. Although this is uncommon, we believe that it is important to alert all involved in the care of AIS patients about the possible risk. COVID-19 testing and its timing should account for this possibility.
When caring for patients with known or suspected COVID-19, and when performing intubation or other procedures that may generate aerosolized particles, anesthesia personnel should use properly fitted N95 masks or, for those who are not fit-tested, have facial hair, or fail N95 fit-testing, a powered air purifier respirator (PAPR). Surgical face masks protect against droplet transmission but do not protect against aerosolized particles. Given the possible shortage of N95 masks, N95 masks may have to be reused according to individual institutional guidance. In addition, surgical cap, eye protection (goggles and face shield), full gown, and double gloves should be used. Proper donning and doffing practices should be practiced.
The choice of anesthetic technique should be individualized, accounting for the patient’s neurological and medical status, and for the risk of infection to health care personnel. The threshold for tracheal intubation will need to be altered by the situation presented and is likely to be impacted by availability of equipment and personnel. In general, the threshold for the use of GA for EVT may be reduced during the COVID-19 pandemic. If the anesthesiologist has any concerns for possible urgent conversion from MAC to GA during EVT, it is advisable to use GA from the outset. However, not all patients undergoing EVT need to be intubated solely for the purpose of reducing the risk to health care personnel. In fact, intubation may increase the risk of aerosolization and, hence, exposure.
- Not all COVID-19-positive or suspected-positive patients require GA for EVT because of the following reasons:
- Most COVID-19-positive patients (including those not suffering from AIS) do NOT require intubation/mechanical ventilation unless they are in respiratory failure. Infection risk to health care personnel providing care to patients who are stable and not intubated can be managed using PPE.
- Bag-mask ventilation, intubation, extubation, and airway interventions result in aerosolization of respiratory secretions, thereby increasing the likelihood of exposure to the anesthesiologists and other personnel in the room. Airway interventions require airborne precaution including the possible use of PAPR and, hence, extra time, which may delay puncture time and revascularization.
Figure 1 outlines a suggested scheme to decide between GA versus MAC during the COVID-19 pandemic.
- The following criteria may be used to identify patients who may be preferred candidates for GA during the pandemic:
- Known or suspected COVID-19-positive patients with AIS who have the following (and they are):
- Acute respiratory distress/hypoxemia/requiring high-flow oxygen.
- Active cough.
- Inability to protect airway.
- Active vomiting.
- Posterior circulation/dominant cerebral hemisphere occlusions.
- Severe stroke (National Institutes of Health Stroke Scale>15) or Glasgow Coma Score<9.
- Agitated/uncooperative/aphasic patients.
- The following criteria may be used to identify patients who may be suitable candidates for MAC during the COVID-19 pandemic:
- Those who do not have acute respiratory distress or hypoxemia requiring high-flow oxygen, are not actively coughing or vomiting, and are able to protect their airway.
- Anterior circulation/nondominant cerebral hemisphere occlusions.
- National Institutes of Health Stroke Scale<15 and Glasgow Coma Score>9.
The decision to intubate and use GA should be made early, based on close communication between anesthesiologist, interventionalist, neurologist, and the emergency medicine team. Ideally, induction of GA and intubation should be performed in an airborne isolation room that has negative pressure relative to the surrounding area. This may have to be performed in the emergency department to avoid exposure to personnel in subsequent locations (computed tomography [CT] scanner, during transport, interventional radiology [IR] suite). Importantly, this should be viewed as induction of anesthesia in the emergency department (as opposed to emergent intubation), with careful attention paid to strict maintenance of hemodynamic and ventilation goals. The airway should be managed by the most experienced person available. However, it is recognized that induction of anesthesia in the emergency department may not be logistically feasible or safe for many institutions, and intubation will need to occur in an alternative negative pressure location or in the IR suite.
Patients suffering from AIS while already in the hospital and requiring GA for EVT based on the above criteria should be intubated safely in a suitable negative pressure location while minimizing delays in cerebral reperfusion.
At some centers that receive transfers for EVT from other hospitals, patients are sometimes brought directly to the IR suite by Emergency Medical Services personnel. In such circumstances, it is recommended that the patient is received into a suitable negative pressure location where anesthetic technique decision and induction of anesthesia can be performed if needed.
- Situations when intubation may have to be performed in the IR suite include the following (and they are):
- COVID-19-positive patients actively coughing or in respiratory distress/hypoxemic who are not already intubated.
- Need to convert from planned or ongoing MAC to GA due to changes in a patient’s respiratory condition, acute neurological deterioration, or a procedure-related complication.
Each institution should carefully adapt the above recommendations to optimally suit local workflow. Institutional adaptions of these recommendations should balance timeliness of EVT, safety of health care personnel, and available resources while accounting for possible implications on institutional workflow.
FIGURE 1.
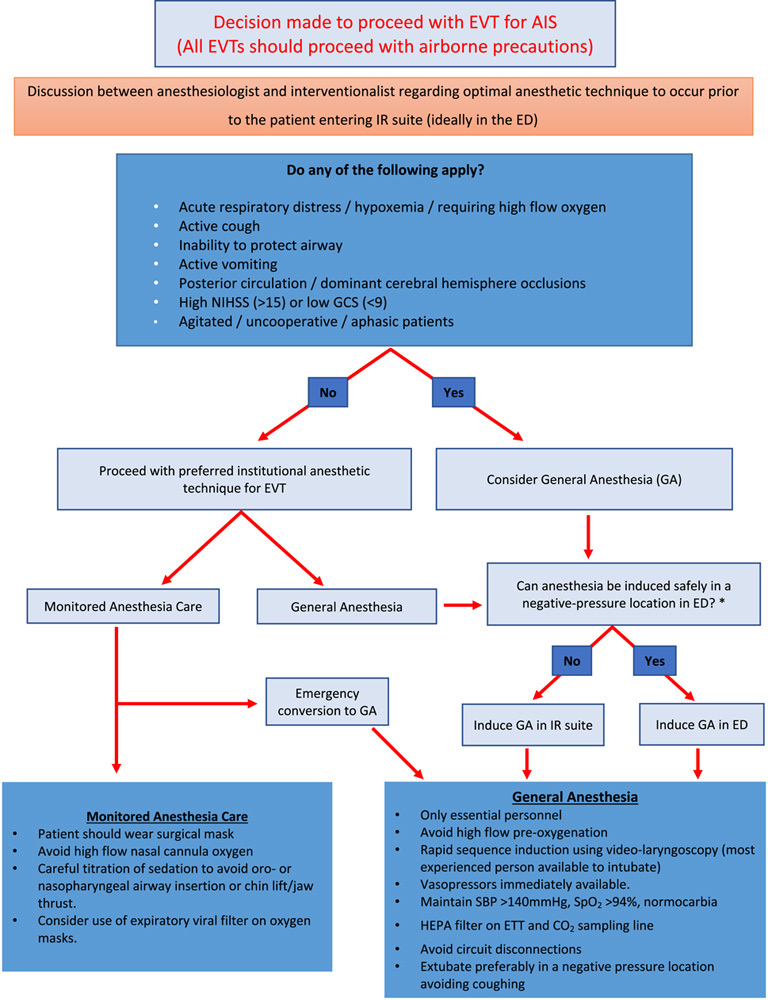
Flowchart to guide the anesthetic management of patients presenting for EVT of AIS during the pandemic of coronavirus disease 2019. *It is recognized that patients in acute respiratory distress or hypoxemia may require emergent intubation in the ED. Patients suffering from AIS while already in hospital and requiring GA for EVT should be intubated safely in a suitable negative pressure location while minimizing delays in reperfusion. AIS indicates acute ischemic stroke; ED, emergency department; ETT, endotracheal tube; EVT, endovascular therapy; GCS, Glasgow Coma Score; HEPA, high-efficiency particulate air; IR, interventional radiology; SBP, systolic blood pressure; SpO2, arterial oxygen saturation; NIHSS, National Institutes of Health Stroke Scale/Score.
GENERAL RECOMMENDATIONS FOR ANESTHETIC MANAGEMENT OF EVT IN KNOWN/SUSPECTED COVID-19-POSITIVE PATIENTS (IRRESPECTIVE OF ANESTHETIC TECHNIQUE)
Previously published general recommendations for anesthetic management should be followed.25–29 Below are some specific considerations relevant to patients requiring emergent EVT.30,44
Airborne precautions should be used for all patients, and the number of personnel should be reduced to essential; any patient may potentially be an asymptomatic carrier of SARS-CoV-2, even in the absence of concerning clinical symptoms for severe viral infection or aerosolization. Lead aprons should be worn before “donning” PPE.
Irrespective of anesthetic technique, GA or MAC, hemodynamic stability and oxygenation/ventilation should be optimized and maintained in the recommended range. According to current guidelines, systolic blood pressure should be maintained between 140 and 180 mm Hg.30 Blood pressure goals may need to be readjusted after reperfusion in discussion with interventionalists and the stroke team. Normocapnia should be maintained, and inspired oxygen concentration titrated to maintain oxygen saturation >94%.44
Any delays in cerebral reperfusion as a result of changes in practice, specifically due to the increased use of GA, should be minimized while accounting for essential COVID-19 precautions. It is anticipated that door-to-puncture times may be delayed, but every effort should be made to minimize this.
The use of PPE/PAPR and any changes in workflow may create difficulties in communication. Extra care is warranted to ensure effective communication between health care workers and between providers and patients.
It is recognized that anesthesiologists are not routinely involved in EVT at some institutions. It is recommended that such institutions consider using a lower threshold to involve anesthesiologists in EVT during the COVID-19 pandemic, as emergent intubation may be associated with higher risk of exposure for all personnel in the IR suite. Early communication with anesthesia is recommended to better plan workforce in what may be a human resource scarce situation.
RECOMMENDATIONS FOR GA/INTUBATION
Previously published general recommendations for intubation and anesthetic management should be followed.25–29 Below are some special considerations relevant to patients requiring emergent EVT during the COVID-19 pandemic.
Airborne precautions should be used for intubation. These include properly fitted N95 masks/PAPRs, goggles, face shields, protective clothing, and double gloves.
As stated above, intubation/induction of GA should be performed in an airborne isolation room that has a negative pressure relative to the surrounding area.
Any delays in cerebral reperfusion as a result of change in practice, specifically due to the use of GA, should be minimized while accounting for essential COVID-19 precautions. As the preparation for intubation in a known or suspected COVID-19-positive patient is likely to take longer than a regular intubation, it is critical that hemodynamic parameters be strictly maintained in the recommended range while awaiting intubation.
Airway devices, medications (including anesthetic and vasoactive drugs), suction devices, ventilators, and monitors should be prepared before induction of anesthesia. Rapid, focused assessment of neurological status, hemodynamics, and the airway should be performed. Patients with COVID-19 may have associated myocardial injury, exposing them to a greater risk of hemodynamic instability.2,45–47
Following 5-minute preoxygenation with good mask seal, rapid sequence induction should be performed using videolaryngoscopy, carefully avoiding hypotension. It is recommended that vasopressors and/or inotropes be readily available. Two pieces of wet gauze can be considered to cover the mouth and nose of patients.25 Sufficient doses of neuromuscular blocking agent should be given to ensure that there is no cough reflex during intubation.
Avoid the use of laryngeal mask airways for GA, except for rescuing a difficult airway.
A high-efficiency particulate air (HEPA) filter should be placed directly on the tracheal tube immediately after intubation. In addition, viral filters should be placed between the expiratory limb and anesthesia machine to prevent contamination of the machine. Breathing circuits should be carefully discarded after every use.
Disconnections of breathing circuits and changes of ventilators should be avoided to reduce the risk of aerosolization and contamination of multiple ventilators. There may also be a reduced availability of ventilators during the pandemic, requiring conservation of ventilators and anesthesia machines (which may be needed to be deployed as intensive care unit [ICU] ventilators). It may be desirable to use the same ventilator (in some cases, a transport ventilator) during transport, during thrombectomy, and in the ICU. This implies that intravenous anesthesia may have to be used for anesthetic management.
If changes in ventilator or breathing circuits are required, standard precautions should be used to minimize aerosolization during disconnection. These include neuromuscular blockade to ensure that no breaths are taken during the disconnection, and clamping the tracheal tube before the ventilator change. A HEPA filter should remain connected to the tracheal tube while changing the breathing circuit or ventilator.
Capnography should be used throughout the duration of mechanical ventilation to avoid inadvertent hypoventilation or hyperventilation.
The gas sampling tubing should also be protected by a HEPA filter, and gases exiting the gas analyzer should be scavenged and not allowed to return to room air.48
It is recommended that anesthesiologists continue to use medications with which they are most familiar in this setting to maintain physiological goals.
Nasal/esophageal temperature probes should be avoided. Bladder temperature or skin temperature monitoring are preferred.
Extubation following GA should ideally be performed in an airborne isolation room that has negative pressure relative to the surrounding area. At many centers, this is the ICU, but it could be the postanesthesia care unit, depending on institutional workflow and availability of resources. Sedation and neuromuscular blockade should be titrated to facilitate early extubation under supervision of an anesthesiologist. Extubation should not be delayed unless there is neurological or respiratory deterioration. Standard extubation criteria should be applied. It is recognized that, in resource limitation scenarios, patients may need to be extubated in the IR suite. In such cases, extubation should be carefully performed under airborne precautions, paying special attention to preventing coughing during extubation. The patient should wear a surgical mask after extubation and receive low-flow oxygen, as needed. Droplet and contact precautions should continue until COVID-19 status is confirmed negative.
RECOMMENDATIONS FOR MAC DURING COVID-19 PANDEMIC
The use of MAC according to the criteria recommended above is best suited for experienced anesthesiologists and in centers with a low rate of conversion from MAC to GA. There is a lack of prediction tools or established risk factors for conversion from MAC to GA. Clinicians should exercise judgement and avoid MAC if there is any concern that a patient will require conversion to GA.
While using MAC, the patient should wear a surgical mask.42,43 Surgical mask should be placed on top of the nasal prongs or under a face mask.
Oxygen flow through nasal cannula should be as low as possible to achieve arterial oxygen saturation>94%.44 Oxygen flow rates >5 L/min should be avoided to minimize aerosolization; carefully consider conversion to GA if the patient continues to remain hypoxemic.39 If available, oxygen masks with expiratory viral filters may be used.
Capnography may be feasible during MAC using recommendations provided by the Anesthesia Patient Safety Foundation.48
The minimal necessary sedation should be used to avoid the need for insertion of an oropharyngeal airway or jaw thrust/chin lift. Anesthesiologists should continue to use the pharmacological agents for MAC with which they are most familiar in this setting.
Extra caution is warranted in case of pooling of oral secretions requiring suctioning.
Anesthesiologists should be prepared to safely convert to GA if needed.
RECOMMENDATIONS FOR URGENT CONVERSION FROM MAC TO GA DURING COVID-19 PANDEMIC
Conversion to GA may be required due to changing patient or procedural conditions. Emergency intubations may be associated with a higher risk of aerosolization, and may be linked to higher transmission events.25
In the event that urgent conversion to GA is necessary, all nonessential personnel should leave the room during intubation. Rapid sequence intubation should be performed by the most experienced person available using a videolaryngoscopy and airborne precautions.25–27
As with a planned GA, vasopressors should be immediately available to maintain systolic blood pressure >140 mm Hg. Once the patient is intubated, ventilation should be managed to achieve normoxia and normocapnia.
After urgent conversion to GA, aerosolization may be a risk, but, with routine airborne precautions already in place, it is possible to resume EVT quickly.
RECOMMENDATIONS FOR WITHIN-HOSPITAL TRANSPORT DURING COVID-19 PANDEMIC
Transport for post-EVT imaging should be limited as much as possible. It is recommended that post-EVT imaging is undertaken only in the setting of concern for neurological compromise and to rule out hemorrhagic conversion, and then only if it cannot be performed using a flat-panel CT in the IR suite. Patients receiving GA should remain intubated for imaging.
A HEPA filter should remain connected directly to the tracheal tube for intubated patients, and capnography used throughout transport to avoid inadvertent hypoventilation/hyperventilation.
Coughing/disconnections of breathing circuits should be avoided, as described above.
Hemodynamics should be strictly maintained during transport, according to standard guidelines.30,44
Patients who are not ventilated during transport should wear a surgical mask. Oxygen can be administered during transport either via nasal cannulae under the surgical mask or using an oxygen mask placed over the surgical mask during transport.
Personnel transporting an intubated patient should wear PPE, as contact with patient and equipment is expected. Another member of the care team (not in PPE) should be designated to interact with other personnel and the environment during the transport. The PPE that was used during airborne procedures must be doffed before leaving the room and should not be worn during transport.
ADMINISTRATIVE RECOMMENDATIONS DURING THE COVID-19 PANDEMIC
Changes in institutional practice during the COVID-19 pandemic should be carefully implemented to prevent inadvertent consequences.
Multidisciplinary consensus and education should be organized, accounting for unique local needs.
Quality measures for AIS and patient outcomes should be carefully monitored during the pandemic, and institutions should have a plan to return to regular practice at the end of the pandemic.
ANTICIPATED IMPACT OF RECOMMENDATIONS
Increased utilization of GA for EVT in AIS.
Potential delays in door-to-puncture times and hence reperfusion times in patients receiving GA (attributable largely to precautions necessary for airway management). These delays may be unavoidable in the current extraordinary circumstances.
Enhanced safety of health care providers.
LIMITATIONS
Data directly examining the impact of COVID-19 or other respiratory infections on the outcomes of AIS are unknown.
This recommendation does not address the process for special “COVID-19 only” designation of CT scanners, magnetic resonance imaging, IR suites, or their decontamination after exposure.
The recommendations may not be universally applicable in their entirety. All institutions are expected to adapt to this guidance, accounting for local processes of care and resource availability.
These recommendations assume the current turnaround time of about 2 hours for COVID-19 test results to become available. The recommendations may need to be updated if a rapid diagnostic test for COVID-19 becomes available.
SUMMARY AND CONCLUSIONS
This expert consensus provides a framework for careful selection and implementation of anesthetic technique for EVT for AIS during the COVID-19 pandemic. Institutions currently using GA for all EVTs should continue to do so with added airborne precautions. Institutions using MAC for all or the majority of EVTs should consider lowering the threshold for using GA according to the criteria suggested above. Airborne precautions should be used in all cases. All institutions should carefully implement changes to existing workflows, anticipating impact on both patients and providers. If possible, any drastic change in workflow should be avoided. The issues and solutions described in this guidance may be generalizable to future pandemics, which conceivably could present similar medical issues and resource constraints. In addition, these recommendations may need to be updated as new information about COVID-19 becomes available.
ACKNOWLEDGMENTS
The authors acknowledge the following: Board of Directors of SNACC; Members of SNACC: David S. Liebeskind, MD, FAAN, FAHA, FANA, FSVIN, FWSO, Department of Neurology, University of California Los Angeles, Los Angeles, CA (President, Society for Vascular and Interventional Neurology). Richard P. Klucznik, MD, FACR, Houston Methodist, Houston, TX (President SNIS). William J. Mack, MD, Professor of Neurosurgery, Keck School of Medicine, University of Southern California, Los Angeles, CA (President-elect SNIS). Jose I. Suarez, MD, FNCS, FANA, Departments of Anesthesiology and Critical Care Medicine, Neurology, and Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD (Immediate Past President Neurocritical Care Society). Patrick A. Brouwer, MD, MSc (President, European Society of Minimally Invasive Neurological Therapy).
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electron J. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jumaa MA, Zhang F, Ruiz-Ares G, et al. Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke. 2010;41:1180–1184. [DOI] [PubMed] [Google Scholar]
- 5.Davis MJ, Menon BK, Baghirzada LB, et al. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116:396–405. [DOI] [PubMed] [Google Scholar]
- 6.van den Berg LA, Koelman DL, Berkhemer OA, et al. Type of anesthesia and differences in clinical outcome after intra-arterial treatment for ischemic stroke. Stroke. 2015;46:1257–1262. [DOI] [PubMed] [Google Scholar]
- 7.Berkhemer OA, van den Berg LA, Fransen PS, et al. The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. Neurology. 2016;87:656–664. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Chebl A, Yeatts SD, Yan B, et al. Impact of general anesthesia on safety and outcomes in the endovascular arm of Interventional Management of Stroke (IMS) III Trial. Stroke. 2015;46:2142–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinjikji W, Murad MH, Rabinstein AA, et al. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell BCV, van Zwam WH, Goyal M, et al. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17:47–53. [DOI] [PubMed] [Google Scholar]
- 11.Sivasankar C, Stiefel M, Miano TA, et al. Anesthetic variation and potential impact of anesthetics used during endovascular management of acute ischemic stroke. J Neurointerv Surg. 2016;8:1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316:1986–1996. [DOI] [PubMed] [Google Scholar]
- 13.Lowhagen Henden P, Rentzos A, Karlsson JE, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke Trial (Anesthesia During Stroke). Stroke. 2017;48:1601–1607. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen CZ, Yoo AJ, Sorensen LH, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen M, Simonsen CZ, Sharma D. Letter by Rasmussen et al regarding article, “Anesthesia-related outcomes for endovascular stroke revascularization: a systematic review and meta-analysis”. Stroke. 2018;49:e20. [DOI] [PubMed] [Google Scholar]
- 16.Schonenberger S, Henden PL, Simonsen CZ, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA. 2019;322:1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell D, Diprose WK, Deng C, et al. General anesthesia versus conscious sedation in endovascular thrombectomy for stroke: a meta-analysis of 4 randomized controlled trials. J Neurosurg Anesthesiol. 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Jia L, Fang F, et al. General anesthesia versus conscious sedation for intracranial mechanical thrombectomy: a systematic review and meta-analysis of randomized clinical trials. J Am Heart Assoc. 2019;8:e011754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rusy DA, Hofer A, Rasmussen M, et al. Assessment of anesthesia practice patterns for endovascular therapy for acute ischemic stroke: a Society for Neuroscience in Anesthesiology and Critical Care (SNACC) Member Survey. J Neurosurg Anesthesiol. 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen M, Simonsen CZ, Sorensen LH, et al. Anaesthesia practices for endovascular therapy of acute ischaemic stroke: a Nordic survey. Acta Anaesthesiol Scand. 2017;61:885–894. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Johns Hopkins Center for Systems Science and Engineering. Coronavirus COVID-19 global cases. 2020. Available at: https://coronavirus.jhu.edu/map.html Accessed April 2, 2020.
- 23.Kharasch ED, Jiang Y. Novel coronavirus 2019 and anesthesiology. Anesthesiology. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowdle A, Munoz-Price LS. Preventing infection of patients and healthcare workers should be the new normal in the era of novel coronavirus epidemics. Anesthesiology. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists. Anesthesiology. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo MZ, Huang YG, Ma WH, et al. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin Med Sci J. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland JR, Michelow MD, Wang L, et al. COVID-19 infection: implications for perioperative and critical care physicians. Anesthesiology. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo M, Cao S, Wei L, et al. Precautions for intubating patients with COVID-19. Anesthesiology. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talke PO, Sharma D, Heyer EJ, et al. Society for Neuroscience in Anesthesiology and Critical Care Expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke: endorsed by the Society of NeuroInterventional Surgery and the Neurocritical Care Society. J Neurosurg Anesthesiol. 2014;26:95–108. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Guan X, Wu P, et al. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Rio C, Malani PN. COVID-19-new insights on a rapidly changing epidemic. JAMA. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ASA Committee on Occupational Health. Coronavirus resources for anesthesiologists. Available at: www.asahq.org/about-asa/governance-and-committees/asa-committees/committee-on-occupational-health/coronavirus Accessed April 2, 2020.
- 35.Cole EC, Cook CE. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am J Infect Control. 1998;26:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang JW, Li Y, Eames I, et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson J. World Health Organization. Natural Ventilation for Infection Control in Health-Care Settings. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. 2020. Available at: www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html Accessed April 2, 2020.
- 39.Hui DS, Chow BK, Chu L, et al. Exhaled air dispersion and removal is influenced by isolation room size and ventilation settings during oxygen delivery via nasal cannula. Respirology. 2011;16:1005–1013. [DOI] [PubMed] [Google Scholar]
- 40.Hui DS, Hall SD, Chan MT, et al. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest. 2007;132:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu IT, Xie ZH, Tsoi KK, et al. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang JW, Liebner TJ, Craven BA, et al. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6(suppl 6):S727–S736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui DS, Chow BK, Chu L, et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. 2012;7:e50845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. [DOI] [PubMed] [Google Scholar]
- 45.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonow RO, Fonarow GC, O’Gara PT, et al. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 47.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anesthesia Patient Safety Foundation. FAQ on anestheisa machine use, protection, and decontamination during the COVID-19 pandemic. 2020. Available at: www.apsf.org/faq-on-anesthesia-machine-use-protection-and-decontamination-during-the-covid-19-pandemic/#gas Accessed April 2, 2020.


