Abstract
Recent advances in biofabrication technologies, such as cell culture systems, and biomaterials have led to the development of three-dimensional (3D) cell culture platforms, such as tumor organoids. Tumor organoids are more physiologically accurate to the in vivo system, which they are intended to model, compared with traditional 2D cancer cell culture systems. Tumor organoids can mimic pathological and physical characteristics of tumors as well as maintain genetic stability of the cancer cells. Furthermore tumor organoids have advantage over animal models, being made from human cells and easily controlled in the laboratory to attain the desired tissue characteristics. In this section, we describe general tumor organoid technologies, the importance of the tumor microenvironment (TME) in model culture systems, and the use of tumor organoids in drug development and precision medicine. Organoid technologies continue to develop rapidly for applications in academic, clinical, and pharmaceutical settings.
Keywords: Tumor Organoids, Cancer Modelling, Tumor Microenvironment, Extracellular Matrix, 3D Cell Culture
Graphical Abstract
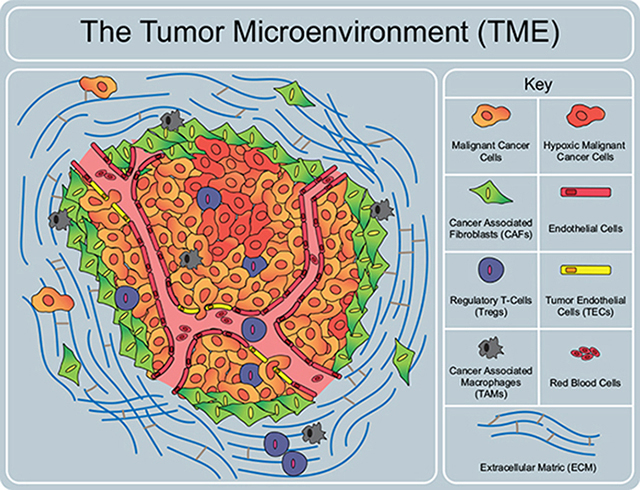
Components of the tumor microenvironment. The TME is composed of many different cell types, signaling factors, extracellular matrix components and vasculature that all significantly affect the tumor. These components must be considered when developing a tumor model system.
1.1. Introduction
Advances in tissue engineering and biomedical techniques over the past decade has given rise to the development of three-dimensional (3D) cell culture systems such as tumor organoids. Organoids are broadly described as small, cellular constructs that represent their in vivo counterparts to a much higher degree compared to conventional 2D methods. Despite having yielded extensive breakthroughs in cancer research, traditional 2D cell cultures have limitations in studying cancer disease and progression causing need for more complex in vitro models. Tumor organoids can be assembled from embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), somatic stem cells, cancer cell lines, primary cancer cells and/or stromal cells in a specific 3D culture matrix; however, most models for precision medicine consist of a mixture of patient cancer and stromal cells[1–4]. The cell type(s) that is incorporated in the tumor organoid system is determined by the experiment at hand. For example, determining the effect of chemotherapy agents on normal colorectal organoids will require stem cell incorporation and crypt self-assembly whereas testing the metastatic nature of cancer in differing ECM environments will most likely involve controlled cancer cell lines with specific mutations. 2D monolayer cell cultures lacks the diversity of cell types, spatial organization, and overall 3D microenvironment seen in tumors and overcome in tumor organoids. Current 2D methods commonly look at the cancer cells in isolation whereas organoids allow the incorporation of many factors (gradients, cell-cell and cell-matrix interactions) that are present in vivo.
Tumor organoids occupy a range of distinct forms, each with their own strengths and weaknesses, and appropriateness for particular applications and can be combined when necessary. Collectively, the bioengineered platforms better mimic structure and cellular heterogeneity of in vivo tissue and are therefore more suitable for cancer research. In cancer, the tumor microenvironment (TME) is the dynamic space in and around the tumor. It contains a myriad of cell types, growth and paracrine factors, and structural components, each contributing to the progression of a tumor. The combination of these interactions determines the final fate of a cancer and has a massive impact on the severity of a patient’s disease. In this section, we will describe the clinical considerations for cancer treatment, the major components within the human TME, and how this can be modeled using tumor organoids.
1.2. Aspects of the tumor microenvironment
Tumors are more than merely a homogenous accumulation of cancer cells. They consist of a convoluted organization of cancerous and healthy cellular elements along with noncellular components. The tumor microenvironment includes the extracellular matrix (ECM), a variety of secreted factors, and different types of cells surrounding the central tumor tissue, has a significant role in regulating tumor growth, cancer malignancy and treatment response (Graph. Abstract) [5].
Tumor organoids have the capability to incorporate TME components to recapitulate some aspects of this complex dynamic depending on the cancer model. The ECM comprises of collagens, proteoglycans, laminins and fibronectin.[6] The ECM is crucial for both tissue function and maintenance. Due to its role on cellular behavior, an unregulated ECM can lead to dramatic consequences such as fibrosis and cancer [7]. ECM abnormality in tumors is an excess in degradation and/or deposition of any of the ECM components described and can result in cancer cell growth, survival, migration, and chemoresistance.[8–11] These changes in the ECM are modulated by the surrounding cells that have the ability to synthesis, degrade, align and crosslink the matrix and can regulate various cellular process such as gene expression, differentiation, and proliferation.[12–14] The 3D nature of tumor organoids enables them to reconstruct certain aspects of the ECM and determine involvement in cancer; however, it remains difficult to isolate the function of each ECM component on tumor progression due to the vast interdependent relationship each component has on one another.
There is a vast array of chemical signals, mainly growth factors (GF), found throughout the microenvironment, each with their own outcomes and signaling pathways. Altered levels of these different factors exert dramatic effects on cancer cell behavior. Of the many cytokines and GF, transforming growth factor β (TGF-β) is a major GF produced by cancer cells and the stromal environment. In a normal, homeostatic environment, TGF-β can induce cell cycle arrest; however, in many cancer types, an increase in TGF-β production is seen, suppressing immune and other reactive cells while inducing EMT in cancer cells [15]. TGF-β also stimulates CAFs to produce and release ECM, making it a key factor in tumor fibrosis and metastasis.
Major classes of cells present in the tumor microenvironment include cancer associated fibroblasts (CAFs), tumor endothelial cells (TECs), tumor associated macrophages (TAMs), and regulatory T cells (Tregs). Recent studies show that organoids can be used to gain insight on ECM interaction with stromal cells and identify function of stromal cells on cancer cells. One group developed organoids using pancreatic stellate cells and pancreatic cancer cells leading to the finding of a pancreatic CAF subtype that expresses less aSMA and secretes interleukins to support organoid growth.[16] Another group shows that IL-1 induces activation of signaling pathways that produce inflammatory CAFs and demonstrates that TGF-R antagonizes this process in adenocarcinomas.[17] This development can suggest multiple strategies to target these cells in vivo. Tumor organoid research has yet to answer larger questions concerning the TMEs’ involvement with cancer progression and drug resistance; however, these major discoveries is on the horizon and will likely emerge from tumor organoid models.
1.3. In vitro models of cancer
Major categories of tumor organoids and the methods by which they are biofabricated have become well established and include tumor spheroids, conventional organoids, device or membrane-based co-cultures (Figure 1). Tumor spheroids are created using U-bottom non-adherent culture plates or via the hanging drop method [18]. Tumor cell populations (homogenous or heterogenous) are added in suspension to the specified plates and allowed to self-assemble overtime (often just 3 days). As spheroids, tumor cells can create their own ECM to further resemble the in vivo microenvironment. The tumor spheroids can be used alone as 3D models of cancer or can be hydrogel encapsulated for additional complexity. Encapsulation allows for physical influences to impact tumor cell behavior and can facilitate the addition of stromal cells and ECM factors both soluble and in-soluble [19]. The use of hydrogels is not limited to spheroid encapsulation and can also be utilized to encapsulate stromal and tumor cell suspensions to study self-organization amongst other things. The use of membranes to create 3D tumor co-cultures systems additionally allow for the study stromal/tumor cell communication and impact on disease progression. In these systems, tumor cells and stromal cells are seeded opposite each other on a thin membrane and cultured together over time. These systems can be further integrated into tumor-on-a-chip devices which allow for the addition of flow into the model. Selection between each of the 3D tumor cell culture methods described ultimately relies on the goals and intended outcomes of the study but offer significant advantages over 2D culture [20].
Figure 1:
Three-dimensional organoid techniques that are used in cancer research. These bioengineered 3D organoid platforms better recapitulate structure and cellular heterogeneity of in vivo tumor tissue and are therefore more appropriate to model the desired tumor type.
1.4. In vitro models of different cancers
Models vary for cancer types and the purpose of the model being developed. For any single cancer type no single model is used and variation of models allows for a deeper understanding of the disease state, its progression, and treatment response. Below are examples of 3D models used for different cancer types showing the importance of considering the disease in three dimensions.
Breast
It is beneficial to study breast cancer in 3D, as the disease is associated with specific genetic mutations that behave in a more physiologically relevant manner in organoid models. For example, 15–20% of breast cancer patients carry HER2 mutations. Studies using 3D versus 2D models have shown that HER2 in 3D tumor spheroid cultures exhibits a substantial response to trastuzumab, a HER2-positive treatment, in comparison to 2D models [21]. The 3D models are shown to better mimic the in vivo microenvironment and more closely represent patient response to drug.
Colon
Oncogenic mutations in the epithelial cells lining the lumen of the intestine are the most common cause of colorectal cancer. In this unique environment, the tumor is located between two distinct microenvironments, the luminal space and the submucosa. Tumor cells will migrate towards the submucosa which offers nutrients but is also collagen-rich and primarily populated with smooth muscle cells (SMCs) [22]. 3D tumor spheroids have been encapsulated in collagen with SMCs to replicate this unique environment [22]. Unlike in 2D, the 3D model allows for re-organization of the stroma by stromal cells creating a relevant architecture and physiological cues for the tumor cells to be observed within.
Liver
Liver cancer is closely associated to cirrhosis, making the microenvironment a primary region of focus when understanding disease progression and developing treatments. Cirrhosis of the liver is caused by uncontrolled inflammation and ultimately scarring of the tissue, yielding a collagen and ECM protein rich environment for damaged hepatocytes. This altered microenvironment modulates cell activity and ultimately can be a contributing factor to cancer development and behavior. 3D models of liver cancer are able to incorporate these many cirrhotic components by utilizing collagen-based hydrogels and incorporating hepatic stellate cells to influence the microenvironment and tumor cell behavior [23].
Lung
Trials to develop 3D lung cancer organoids have resulted in models that contain many of the TME cell types to best recapitulate the in vivo tumor. It has been well established that the addition of both fibroblasts and endothelial cells make the model more physiologically representative. As lung cancer is the deadliest cancer in the US, researchers have utilized patient primary samples to create patient and tumor specific microenvironments for the study of disease progression and treatment response in 3D [24]. These methods are mixed culture and preservation of the various cell types within the tumor allow for acini structure development in 3D, which is important for phenotypic modeling [24].
Pancreatic
Similar to other types of cancer, pancreatic cancer treatment has been closely tied to stromal factors, making it important to consider the impact of stellate cells and fibroblasts on disease progression. Researchers have utilized co-culture models of pancreatic cancer to further understand these relationships [25]. Specifically, cancer spheroids have been added on top or within hydrogels containing stromal cells which have yielded physiologically and pharmacologically relevant structures such as lumens which have not been shown in stroma free cultures [25]. These improvements will hopefully allow better drug development to decrease the mortality rate in pancreatic cancer.
Prostate
Prostate cancer is unique in that it is often benign when discovered and left untreated until confirmed to be malignant. One developing treatment of prostate cancer has been the use of nanoparticles which allow for controlled, specific drug delivery to tumor regions, which reduces systemic chemotherapy response and need for invasive surgery. Studies have shown that 2D to in vivo nanoparticle deployment is often times unsuccessful and demands the use of 3D models for predicting efficacy of the treatments [26]. Utilizing custom scaffolds and stroma specific to the prostate microenvironment, researchers have been able to create nanoparticles translational from in vitro to in vivo [26].
1.5. Tumor organoids for clinical treatment predictions
Efforts to utilize patient derived organoids for the prediction of treatment sensitivity has become a driving component of their development [4]. These efforts have demonstrated the ability to predict genetic and proteomic sensitivities to treatments, often in a similar time frame as results from genetic screening. The most direct example of this testing is the screening of approved treatments to attempt to determine which may benefit the patient [27]. There are also efforts to reproduce rare forms of cancer in 3D to elucidate poorly understood cellular mechanisms [28]. Studies are usually aimed at testing large panels of approved and experimental compounds on previously genotyped patients in an attempt to discover novel treatment options [1]. Other studies focus on modeling tumor subtypes of the same cancer, with the ability to predict resistance patterns and suggest optimal treatments based the response of their organoid testing [29]. A more recent focus of the tumor organoid modeling has included intra-tumor sampling to create diverse sets of organoids derived from the same patient [30]. These organoids demonstrate the reality of tumor resistance in different sites of the same tumor. There are also projects to create organoid based biobanks to assist in precision medicine based studies of patient treatment [31]. Finally, implementation of microfabricated devices has allowed for the modeling of both treatment of tumor organoids under flow as well as the ability to prevent tumor cell migration [32]. Overall, there remains a high demand in the utilization of patient derived organoids in therapeutic determination, and many efforts are underway to implement them into clinical settings.
1.6. Tumor microenvironment and immune cell modeling
The ability to increase the functional state of a patient’s anti-tumor immune response by inhibition of normal immune checkpoint signaling, genetic engineering to create chimeric antigen receptor T-cells, or immune supplementation can illicit thorough cytotoxic activity with the potential to be more durable than traditional chemotherapeutics. Main targets for immunotherapy include, but are not limited to, natural killer (NK) cells and cytotoxic T-cells. These cell types operate through different pathways but are both central to anti-tumor immunity. Additionally, the efficacy of these therapies can be further compounded when used synergistically of other therapies such as inorganic photothermal nanoparticle agents that increase the presence of viable tumor antigen or precision medicine targeted therapeutics [33, 34].
We have recently created patient-specific immune-enhances tumor organoids by introducing immune cells from lymph nodes or the peripheral blood along with melanoma cancer cells [35]. We observed significant tumor cell killing in the response to immunotherapy, similar to clinical response in these patients. The creation of organoid models allows for the evaluation of factors that dictate immunotherapy response in a more controlled environment. The number of confounding variables in-vivo makes it much more difficult to assess the immunotherapy induce cytotoxicity and isolation of various factors that may be contributing to immunotherapy resistance. The ability to integrate patient tumor and immune cells into the organoid rather than cell lines can add to precision medicine approaches for patient evaluation. Patient cell inclusion has been performed with NK [36] and T-cells [37], T-cells [34, 38–44], NK cells [34, 38, 41], and B-cell populations [34, 41, 42]. Readouts from these systems can show if a patient will exhibit resistance to immune checkpoint inhibition or other immunotherapies, thereby informing the eligibility of the patient for immunotherapy. Examples include the creation of patient matched cytotoxic T-cells and tumor cells in order to display evidence of direct T-cell mediated killing in vitro using anti-PD-1 [34, 42–44], PD-L1 [34], CTL4 [43], and MICA/B and NKG2A [38] therapies.
1.7. Conclusions and future applications
Tumor organoids from cancer patients can be used to identify the ideal treatment for a specific patient since tumor organoids retain the genetic heterogeneity of the primary tumor as well as their TME, resulting in an attractive tool for precision medicine in oncology. There are many techniques that can be utilized in developing tumor organoids for drug discovery, precision medicine, genomic, or malignancy studies, to name a few; however, researchers all need to consider the TME and ECM when considering which method they wish to use. Models incorporating components of the TME may elucidate new mechanisms behind cancer control leading to novel therapeutics which target the support system of a cancer rather than the cancer itself. Additionally, where the production of organoid models had a relatively low throughput in the past, rapid developments in 3D bioprinting are making organoid models increasingly viable for drug screens [45]. Improvements in related 3D techniques are also allowing expansion in personalized medicine by comparing sequencing data and drug screening results do in vivo models [31]. These strategies could lead to a new landscape of cancer therapies which more directly target cancer cells and their stroma while sparing the body’s healthy tissues.
Acknowledgments
Funding
The authors acknowledge funding through NIH grants R33CA202822 (S.S.) and T32EB014836. The authors wish to acknowledge the support of the Wake Forest Baptist Comprehensive Cancer Center Tumor Tissue and Pathology Shared Resource supported by the National Cancer Institute’s Cancer Center Support Grant award number P30CA012197. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Abbreviations
- 3D
three-dimensional
- ECM
extracellular matrix
- TME
tumor microenvironment
- CAF
cancer associated fibroblasts
- GF
growth factor
- TGF-β
transforming growth factor-beta
- EMT
epithelial to mesenchymal
- VEGF
vascular endothelial growth factor
- EGF
epidermal growth factor
- PDGF
platelet derived growth factor
- FGF
fibroblast growth factor
- TNF
tumor necrosis factor
- IL
interleukin
- TEC
tumor endothelial cells
- TAM
tumor associated macrophages
- Tregs
regulatory T cells
- MMP
matrix metalloproteases
- HER2
human epidermal growth factor receptor 2
- SMC
smooth muscle cells
- NK
natural killer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Vlachogiannis G, et al. , Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science, 2018. 359(6378): p. 920–926.** This recent review paper compares the responses to chemotheraputic agents Ex vivo in organoids and PDO-based orthotopic mouse tumor xenograph models with the responses of the patients in clinical trials and suggest that PDOs can recapiculate patient responses and could be implemented in personalized medicine.
- 2.Weeber F, et al. , Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci U S A, 2015. 112(43): p. 13308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitra A, Mishra L, and Li S, Technologies for deriving primary tumor cells for use in personalized cancer therapy. Trends Biotechnol, 2013. 31(6): p. 347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsythe S, Pu T, and Skardal A, Using organoid models to predict chemotherapy efficacy: the future of precision oncology? Expert Review of Precision Medicine and Drug Development, 2019. 4(6): p. 317–336. [Google Scholar]
- 5.Balkwill FR, Capasso M, and Hagemann T, The tumor microenvironment at a glance. J Cell Sci, 2012. 125(Pt 23): p. 5591–6. [DOI] [PubMed] [Google Scholar]
- 6.Ozbek S, et al. , The evolution of extracellular matrix. Mol Biol Cell, 2010. 21(24): p. 4300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belgodere JA, et al. , Engineering Breast Cancer Microenvironments and 3D Bioprinting. Front Bioeng Biotechnol, 2018. 6: p. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devarasetty M, et al. , Bioengineered Submucosal Organoids for In Vitro Modeling of Colorectal Cancer. Tissue Engineering Part A, 2017. 23(19–20): p. 1026–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paolillo M and Schinelli S, Extracellular Matrix Alterations in Metastatic Processes. Int J Mol Sci, 2019. 20(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis AL, et al. , Extracellular matrix determinants and the regulation of cancer cell invasion stratagems. J Microsc, 2013. 251(3): p. 250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eble JA and Niland S, The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis, 2019. 36(3): p. 171–198. [DOI] [PubMed] [Google Scholar]
- 12.Walker C, Mojares E, and Del Rio Hernandez A, Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci, 2018. 19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, et al. , Role of tumor microenvironment in tumorigenesis. J Cancer, 2017. 8(5): p. 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Huizen NA, et al. , Up-regulation of collagen proteins in colorectal liver metastasis compared with normal liver tissue. J Biol Chem, 2019. 294(1): p. 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blobe GC, Schiemann WP, and Lodish HF, Role of transforming growth factor beta in human disease. N Engl J Med, 2000. 342(18): p. 1350–8. [DOI] [PubMed] [Google Scholar]
- 16.Ohlund D, et al. , Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med, 2017. 214(3): p. 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biffi G, et al. , IL1-Induced JAK/STAT Signaling Is Antagonized by TGFbeta to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov, 2019. 9(2): p. 282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta G, et al. , Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release, 2012. 164(2): p. 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck JN, et al. , The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials, 2013. 34(37): p. 9486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asghar W, et al. , Engineering cancer microenvironments for in vitro 3-D tumor models. Mater Today (Kidlington), 2015. 18(10): p. 539–553.* This paper discusses the ability and need to model the cancer microenvironment by using 3D organoids and other 3D techniques. They review recent 3D tumor model systems and highlight the direction of biofabricated application for future use in the field of medicine.
- 21.Pickl M and Ries CH, Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene, 2009. 28(3): p. 461–8. [DOI] [PubMed] [Google Scholar]
- 22.Devarasetty M, et al. , Bioengineered Submucosal Organoids for In Vitro Modeling of Colorectal Cancer. Tissue Eng Part A, 2017. 23(19–20): p. 1026–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devarasetty M, et al. , Mesenchymal stem cells support growth and organization of host-liver colorectal-tumor organoids and possibly resistance to chemotherapy. Biofabrication, 2017. 9(2): p. 021002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzocchi A, et al. , Pleural Effusion Aspirate for Use in 3D Lung Cancer Modeling and Chemotherapy Screening. ACS Biomaterials Science & Engineering, 2019. 5(4): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devarasetty M, et al. , Optical Tracking and Digital Quantification of Beating Behavior in Bioengineered Human Cardiac Organoids. Biosensors (Basel), 2017. 7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, et al. , 3D Porous Chitosan-Alginate Scaffolds as an In Vitro Model for Evaluating Nanoparticle-Mediated Tumor Targeting and Gene Delivery to Prostate Cancer. Biomacromolecules, 2015. 16(10): p. 3362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachs N, et al. , A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell, 2018. 172(1–2): p. 373–386 e10. [DOI] [PubMed] [Google Scholar]
- 28.Puca L, et al. , Patient derived organoids to model rare prostate cancer phenotypes. Nat Commun, 2018. 9(1): p. 2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Votanopoulos KI, et al. , Appendiceal Cancer Patient-Specific Tumor Organoid Model for Predicting Chemotherapy Efficacy Prior to Initiation of Treatment: A Feasibility Study. Ann Surg Oncol, 2019. 26(1): p. 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, et al. , Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight, 2019. 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauli C, et al. , Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov, 2017. 7(5): p. 462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzocchi AR, et al. , In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci Rep, 2018. 8(1): p. 2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, et al. , Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun, 2016. 7: p. 13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neal JT, et al. , Organoid Modeling of the Tumor Immune Microenvironment. Cell, 2018. 175(7): p. 1972–1988 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Votanopoulos KI, et al. , Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann Surg Oncol, 2019.* We determined that the development of 3D mixed immune-enhanced tumor/node organoids is a feasible platform, allowing individual patient immune system and tumor cells to remain viable for studying of personalized immunotherapy response.
- 36.Christakou AE, et al. , Ultrasonic three-dimensional on-chip cell culture for dynamic studies of tumor immune surveillance by natural killer cells. Lab Chip, 2015. 15(15): p. 3222–31. [DOI] [PubMed] [Google Scholar]
- 37.Pavesi A, et al. , A 3D microfluidic model forpreclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight, 2017. 2(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courau T, et al. , Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment. J Immunother Cancer, 2019. 7(1): p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dijkstra KK, et al. , Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell, 2018. 174(6): p. 1586–1598 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finnberg NK, et al. , Application of 3D tumoroid systems to define immune and cytotoxic therapeutic responses based on tumoroid and tissue slice culture molecular signatures. Oncotarget, 2017. 8(40): p. 66747–66757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramamoorthy P, et al. , Metastatic Tumor-in-a-Dish, a Novel Multicellular Organoid to Study Lung Colonization and Predict Therapeutic Response. Cancer Res, 2019. 79(7): p. 1681–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins RW, et al. , Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov, 2018. 8(2): p. 196–215.** Here, they demonstrate feasibility of ex vivo profiling of PD-1 blockade to challenge the tumor immune microenvironment, develop therapeutic combinations, and facilitate precision immuno-oncology efforts.
- 43.Aref AR, et al. , 3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade. Lab Chip, 2018. 18(20): p. 3129–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng J, et al. , CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov, 2018. 8(2): p. 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knowlton S, et al. , Bioprinting for cancer research. Trends Biotechnol, 2015. 33(9): p. 504–13. [DOI] [PubMed] [Google Scholar]



