Background: The coronavirus disease 2019 (COVID-19) pandemic has rapidly spread around the world, and the lack of effective treatment has fueled a global search for the “magic bullet” against the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of 29 April 2020, a total of 997 COVID-19 trials were registered at ClinicalTrials.gov, of which more than 30 are investigating the effects of lopinavir and ritonavir.
Lopinavir is the main antiviral agent, whereas ritonavir acts as a pharmacokinetic “booster” that increases lopinavir plasma concentrations by inhibiting cytochrome 3A4 enzymes (1). The combination is an approved antiretroviral therapy for adults with HIV-1 infection. However, in vitro data also suggest antiviral activity of lopinavir against SARS-CoV-1, with a half-maximal effective concentration (EC50) of 4.1 µg/mL; Middle East respiratory syndrome-CoV, with an EC50 of 10.8 µg/mL; and, only recently, SARS-CoV-2, with an EC50 of 16.4 µg/mL (2). Importantly, the EC50 for HIV-1 is 0.07 µg/mL, more than 200-fold lower than for SARS-CoV-2 (1). Recently, Cao and colleagues published negative results of a placebo-controlled trial in patients hospitalized with COVID-19 in which lopinavir treatment did not affect mortality, clinical performance, or viral loads (3).
Objective: To measure trough levels of lopinavir and ritonavir in patients with COVID-19.
Methods and Findings: In this series of 8 patients who were admitted to a “normal care” ward because of COVID-19, we quantified trough plasma concentrations of lopinavir and ritonavir by liquid chromatography–tandem mass spectrometry. Patients with concomitant intake of relevant CYP3A4 inducers or inhibitors were excluded. Patients were hospitalized and received 400 mg of lopinavir and 100 mg of ritonavir twice daily for 3 to 10 days before analysis, which was done in the morning shortly before the next dose. Assuming a half-life of 4 to 6 hours for lopinavir, steady-state conditions may be assumed for all patients. Analysis of trough levels was chosen because they provide more robust pharmacokinetic information for single time–point assessments and were frequently investigated in HIV trials, which makes the data comparable.
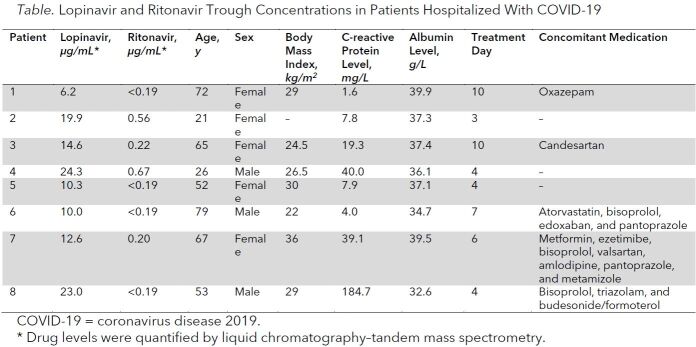
Trough concentrations of lopinavir ranged from 6.2 to 24.3 µg/mL (median, 13.6 µg/mL) (Table). Interestingly, in this small sample, trough concentrations seemed to be associated with C-reactive protein (Spearman correlation coefficient rS = 0.81). All patients had an unremarkable disease course and were discharged from the normal care ward. Specific adverse effects of lopinavir and ritonavir were not observed.
Table. Lopinavir and Ritonavir Trough Concentrations in Patients Hospitalized With COVID-19.
Discussion: We report the first pharmacokinetic data of lopinavir and ritonavir in patients hospitalized with COVID-19. Lopinavir trough levels were approximately 2-fold higher in our population than in patients with HIV receiving the same dose (7.1 µg/mL) (1). This may have been caused by inflammation-induced downregulation of cytochrome P450 enzyme activity and reduced drug metabolism, which is mediated by proinflammatory cytokines, including interleukin (IL)-1, IL-6, and tumor necrosis factor-α (4). The observed correlation of drug concentrations with C-reactive protein, a downstream marker of IL-6, supports this hypothesis and is notable because C-reactive protein levels were not exceedingly high in our population. High levels of IL-6 are associated with disease severity (5), and IL-6–blocking therapies are being investigated in several clinical trials. Thus, drug metabolism may be even more impaired in more severe cases of COVID-19. Furthermore, clinicians should be aware that anti-inflammatory treatments may severely affect the pharmacokinetics of cytochrome enzyme–dependent drugs.
This analysis has limitations. Only trough levels were quantified, and more detailed pharmacokinetics were not available. Also, there are no data on the half-maximal effective dose of lopinavir for SARS-CoV-2 in vivo.
Currently, more than 30 trials of lopinavir and ritonavir treatment of COVID-19 are registered, and according to ClinicalTrials.gov, doses range from 200 to 400 mg of lopinavir and from 50 to 100 mg of ritonavir twice daily (the patients in our analysis received the latter dose for each drug). Unfortunately, lopinavir is almost completely bound by plasma proteins, and only 1% to 2% are free and active (1). To translate drug concentrations in vitro to unbound (and therefore active) concentrations in vivo, a correction for protein binding is required (1, 2). Unbound drug concentrations of lopinavir are far from reaching the EC50 of SARS-CoV-2 (16.4 µg/mL), although they clearly suffice to inhibit HIV-1.
In conclusion, despite the approximately 2-fold higher lopinavir trough concentrations in our sample of patients with COVID-19 compared with patients with HIV, approximately 60- to 120-fold higher concentrations are required to reach the assumed EC50 at trough levels, making effective treatment of COVID-19 with lopinavir and ritonavir at the currently used doses unlikely.
Biography
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-1550.
Reproducible Research Statement: Study protocol: Available from Dr. Jilma (e-mail, Bernd.jilma@meduniwien.ac.at). Statistical code: Not applicable. Data set: Available on request from Dr. Jilma (e-mail, Bernd.jilma@meduniwien.ac.at) and at www.cloudius.meduniwien.ac.at.
Corresponding Author: Bernd Jilma, MD, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail, Bernd.jilma@meduniwien.ac.at.
Footnotes
This article was published at Annals.org on 12 May 2020.
References
- 1. doi: 10.2165/11204950-000000000-00000. Croxtall JD, Perry CM. Lopinavir/ritonavir: a review of its use in the management of HIV-1 infection. Drugs. 2010;70:1885-915. [PMID: 20836579] doi:10.2165/11204950-000000000-00000. [DOI] [PubMed]
- 2. doi: 10.1016/j.antiviral.2020.104786. Choy KT, Wong AY, Kaewpreedee P, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. [PMID: 32251767] doi:10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed]
- 3. doi: 10.1056/NEJMoa2001282. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-1799. [PMID: 32187464] doi:10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed]
- 4. doi: 10.1002/cpt.878. Schoergenhofer C, Hobl EL, Schellongowski P, et al. Clopidogrel in critically ill patients. Clin Pharmacol Ther. 2018;103:217-223. [PMID: 28913918] doi:10.1002/cpt.878. [DOI] [PMC free article] [PubMed]
- 5. doi: 10.1016/j.jcv.2020.104370. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. [PMID: 32344321] doi:10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed]