Abstract
Purpose:
Staphylococcus aureus osteomyelitis remains a major clinical problem. Anti-glucosaminidase (Gmd) antibodies (1C11) are efficacious in prophylactic and therapeutic murine models. Towards clinical trials, we assess the feasibility, safety, and pharmacokinetics of 1C11 passive immunization in sheep, and quantified endogenous anti-Gmd levels in osteomyelitis patients.
Main method:
Sheep (n=3) received a 500mg intravenous bolus of 1C11, and levels in sera over 52 days were determined by ELISA. A humanized anti-Gmd monoclonal antibody was made by grafting the Fab portion of 1C11 onto the Fc of human IgG1, which was used to make a standard curve of mean fluorescent intensity versus concentration of anti-Gmd via Luminex. Anti-Gmd serum levels were determined in 297 patients with culture-confirmed S. aureus osteomyelitis, and 40 healthy controls.
Main results:
There were no complications or adverse events associated with the 1C11 intravenous infusion of the sheep, and the estimated circulating half-life of 1C11 was 23.7 days. Endogenous anti-Gmd antibody levels in sera of osteomyelitis patients ranged from <1ng/ml to 300μg/ml, and the mean concentration was 21.7μg/ml. The grossly estimated circulating half-life of endogenous anti-Gmd antibodies in sera of 12 patients with cured osteomyelitis was determined to be 120.4 days.
Conclusions:
A clinically relevant administration of anti-Gmd (500mg i.v. = 7mg/kg/70kg human) is safe in sheep. This dose is >8x endogenous anti-Gmd levels observed in osteomyelitis patients, and is predicted to have a half-life of >3-weeks.
Clinical relevance:
Anti-Gmd passive immunization has potential to prevent and treat S. aureus osteomyelitis. Further clinical development is warranted.
Keywords: Orthopaedic Infections, Immunoassay, Staphylococcus aureus, Osteomyelitis, Peri-prosthetic Joint Infection, 2-Stage Revision Surgery, Passive Immunization
Introduction
Infection of bone, known as osteomyelitis, remains a catastrophic complication of orthopaedic surgery, the majority of which is caused by Staphylococcus aureus (Darouiche, 2004). Although well-established clinical algorithms have been established to prevent these biofilm infections that are refractory to antibiotic therapy (Saeed et al., 2019), the current incidence of peri-prosthetic joint infection (PJI) ranges from 0.2–1.5% (Schwarz et al., 2019). Moreover, it appears that significant improvements are not possible, as implementing the most rigorous protocols (e.g. outcomes from the Surgical Care Improvement Project (SCIP) (Stulberg et al., 2010)) have demonstrated that infection rates for elective surgery cannot be reduced below 1–2% (Cram et al., 2012). Additionally, on top of the clinical complications, the enormous costs for treating osteomyelitis threaten our healthcare systems, as they are projected to exceed $1.62 billion in the USA by 2020 (Kurtz et al., 2012).
The prevalence of S. aureus osteomyelitis is due to the various pathogenic mechanisms that this commensal pathogen has evolved to facilitate immune evasion, including: 1) biofilm formation on the implant (Nishitani et al., 2015b) and necrotic bone (Birt et al., 2017; Lew and Waldvogel, 2004), 2) generation of Staphylococcus abscess communities (SACs) in soft tissues and bone marrow (Cheng et al., 2009; Varrone et al., 2014; Yokogawa et al., 2018), and the ability to colonize the osteocytic-canalicular network of live cortical bone (de Mesy Bentley et al., 2018; de Mesy Bentley et al., 2017). Thus, reinfection rates following surgery for S. aureus osteomyelitis are very high (15–40%), and often require an implant-exchange surgery to remedy the problem (Azzam et al., 2009; Ferry et al., 2009; Ghanem et al., 2009; Parvizi et al., 2009; Salgado et al., 2007).
Although active immunizations are the most cost-effective intervention for infectious diseases, enormous efforts to develop a S. aureus vaccine have been unsuccessful for various reasons (Jansen et al., 2013; Proctor, 2012; Proctor, 2015). Moreover, active vaccinations targeting endogenous pathobionts such as S. aureus may boost pre-existing immune responses, which may be ineffective or may even have debilitating effects. These failures have led to conflict in the field with regard to the role of humoral immunity during S. aureus infections, and reservations about activation vaccination therapy’s potential to treat serious surgical site infections (Bagnoli et al., 2012; Fowler and Proctor, 2014; Pier, 2013; Proctor, 2012; Projan et al., 2006). This raging controversy has been amplified by patient deaths in a large phase 2 clinical trial of the V710 vaccine (Fowler et al., 2013; McNeely et al., 2014), and wonder about how the extensive pre-clinical research program leading up to a clinical trial with 8,000 patients could have reached the wrong conclusions regarding the vaccines safety and efficacy for the intended clinical indication (Harro et al., 2012; Kim et al., 2010). However, this pessimism is tempered by the recent transformative successes of cancer monoclonal antibody-based passive immunotherapies after decades of disappointments, for which the 2018 Nobel Prize in Physiology or Medicine was awarded to Drs. James Allison and Tasuku Honjo for their seminal discoveries of immune checkpoint inhibitors (Smyth and Teng, 2018). Thus, the quest for a S. aureus vaccine continues.
The overall hypothesis of our passive immunotherapy program has been that the most effective monoclonal antibodies (mAb) would have dual-acting mechanisms of action that both directly inhibit functions critical to S. aureus, and also mediate immunomodulatory activity to boost the host response and bacterial clearance. By using a non-biased research approach to test this hypothesis, we identified the glucosaminidase (Gmd) subunit of S. aureus autolysin (Atl) as our lead target (Gedbjerg et al., 2013; Varrone et al., 2014; Varrone et al., 2011b; Yokogawa et al., 2018). Of note is that other groups have also identified Atl as an immunodominant antigen (Brady et al., 2011; Gotz et al., 2014; Holtfreter et al., 2010). Functionally, Atl is known to be essential for cell wall biosynthesis and degradation during binary fission (Oshida et al., 1995; Sugai et al., 1995; Yamada et al., 1996). It has also been demonstrated that Atl acts as an adhesin (Heilmann et al., 2005), a biofilm enzyme (Brady et al., 2006), and a facilitator of host cellular internalization/immune evasion (Hirschhausen et al., 2010). Remarkably, S. aureus amidase (Amd), which is the other subunit of Atl, known to activate platelet activation and aggregation (Binsker et al., 2018), was shown to be a molecular target of vancomycin (Eirich et al., 2011), which is the most common antibiotic used to treatment methicillin-resistant S. aureus (MRSA) infections. Importantly, anti-Gmd passive immunization has been shown to synergize with vancomycin therapy in rabbit and murine models of infection (Brady et al., 2011; Kalali et al., 2018; Yokogawa et al., 2018). Additionally, our clinical research studies to assess humoral immunity in patients with osteomyelitis from PJI, trauma and diabetic foot ulcers have identified anti-Gmd antibodies in patients that recover from these serious infections (Gedbjerg et al., 2013; Nishitani et al., 2015a; Oh et al., 2018).
To evaluate the potential of anti-Gmd mAb passive immunization in murine models of osteomyelitis, we screened 36 anti-Gmd producing murine hybridomas for their ability to bind recombinant Gmd, inhibit its enzymatic activity in a cell wall digestion assay, and precipitate S. aureus out of culture (Gedbjerg et al., 2013; Varrone et al., 2014; Varrone et al., 2011a). Of these, we identified one IgG1 mAb (1C11) with superior properties based on its: 1) clinically relevant affinity (kf = 3.1 × 104 M−1s−1; kr = 5.0×10−5 s-1; and KD = 1.6 × 10−9 M) (Varrone et al., 2014), 2) stoichiometric neutralizing activity (Gedbjerg et al., 2013), 3) phenocopy of Gmd deficient S. aureus mutants (Varrone et al., 2014), 4) ability to mediate S. aureus megacluster formation and opsonophagocytosis in vitro (Varrone et al., 2014), 5) protect mice from MRSA osteomyelitis (8 out of 17 animals demonstrated undetectable MRSA levels in the 1C11 group compared to 1 out of 15 in the placebo group) (Varrone et al., 2014), and 6) synergize with vancomycin to cure mice with established osteomyelitis (combination therapy yielded a 6.5-fold reduction in MRSA levels (7 out of 10 animals) compared to untreated animals ) (Yokogawa et al., 2018). Although 1C11 does not inhibit bacteria within biofilms, these promising results warrant further research to transform the established rodent dosing regimen (40 mg/kg via intraperitoneal injection), to that of FDA-approved biologics (e.g. infliximab Remicade®, trastuzumab Herceptin®, rituximab Rituxan®, which are chimeric mAb) administered as an intravenous (i.v.) infusion on the order of 500mg in one liter of saline (7mg/kg for 70kg patient). Clinical research is also needed to assess endogenous anti-Gmd levels in osteomyelitis patients. Thus, we evaluated 1C11 passive immunization of sheep, and quantified the concentration of circulating anti-Gmd antibodies in patients with S. aureus osteomyelitis.
Materials and Methods
Large scale production of 1C11 mAb.
A pilot study to assess the feasibility, safety and pharmacokinetics of passively immunizing adult sheep was performed at the AO Research Institute (Davos, Switzerland) on IACUC-approved protocols. To generate the test material, 2g of 1C11 mAb was produced from a well-characterized 1C11 hybridoma cell line (Gedbjerg et al., 2013; Varrone et al., 2014), in the Upstate Stem cGMP Facility (USCGF, University of Rochester, Rochester, NY). Following adaptation to HyClone SFM4Mab media with 1% fetal bovine serum (Thermo Fisher Scientific), an initial 1.0 L inoculum of 1C11 hybridoma cells (1.5 × 105 viable cells/mL, [1C11] = 18.6 ug/mL) was placed in a 5.0 L WAVE bag (GE Healthcare Life Sciences, Pittsburgh, USA) at 37°C. As the viable cell count reached ~8.2 × 105 cells/mL, [1C11] =56.8 ug/mL), an additional 1.5 L of 1.0% serum-containing media was added. Following dilution of the culture with additional media, the viable cell count expanded to ~1.0 × 106 cells/mL, [1C11] = 109.2 ug/mL). At that point 2.0 L of culture was harvested, and 2.0 L of 1% serum media was added to replace the volume in the WAVE bag. As the culture increased in the cell population (1.8 × 106 cells/mL, 33.0 ug/mL), an additional 2.0 L of culture was harvested and 1.0 L of serum-free media was added, bringing the total serum concentration to approximately 0.2%. As the cell count remained stationary at ~7.0 × 105 cells/mL, another 1.5 L of 0.2% serum media was added. After an initial lag in cell growth, the population of 1C11 hybridoma cells resumed growth, and 2.0 L of product was harvested, followed by the addition of 1.0 L of serum-free media, bringing the effective media serum concentration to 0%. At this point the 1C11 cells had been fully adapted to serum-free media (3.9 × 105 cells/mL). With minimal lag, the cells reached a cell count of 1.09 × 106 cells/mL (45.3 ug/mL), so an additional 1.0 L serum-free media was added, followed shortly thereafter with harvesting of the entire 2.0 L contents of the WAVE bag (1.6 × 106 cells/mL, 74.4 ug/mL). In total, 8.0 L of product were obtained from the entire WAVE bag adaptation run, with an estimated antibody production of ~90 ug/mL. The 8.0 L of culture was harvested in 4 independent 2.0L harvests. The culture was processed via microfiltration using a Millipore filtration system (Millipore, Bedford, USA) with Pellicon filter (0.22 um Durapore microfiltration membrane) to separate the cells, yielding ~2.0 L of clarified culture supernatant. The clarified culture supernatant was subsequently concentrated ~5.7-fold to a final volume of ~350 mL using a 30 kDa molecular weight cutoff ultrafiltration membrane. The concentrate was aliquoted and stored at −20°C.
Purification of the 1C11 mAb.
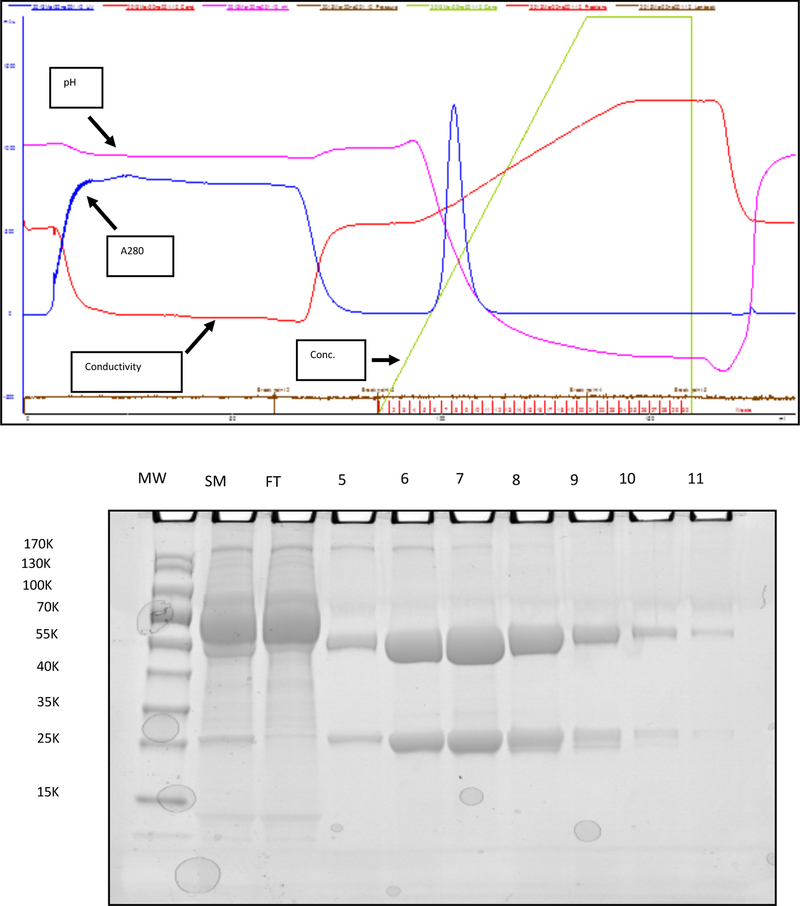
A 60 mL aliquot of concentrated culture supernatant (~30 mg of 1C11 mAb) was thawed and loaded directly onto a MAbSelect SuRE LX Protein A column (GE Healthcare Life Sciences) equilibrated in 25 mM NaPO4 / 0.15 M NaCl (pH = 7.2) using an ÄKTAprime chromatography system (GE Healthcare Life Sciences, Pittsburgh, USA), and a flow rate of 2.5 mL/min. Post loading and washing of the column with equilibration buffer (pH = 7.2), 60 mL of the 1C11 antibody culture supernatant was again added to the column. Following loading, the unbound protein was washed through the column with five column volumes of equilibration buffer (CVs, 1 CV = 5 mL). Following the column wash, elution of the 1C11 product was accomplished by application of a linear gradient (0– 0.1M) of Na citrate (pH = 3.0). Elution of the bound 1C11 mAb is effected with a pH gradient from pH 7.2 to 3.0 over 10 CVs (2.5 mL fractions were collected). Figure 1 describes the results of a typical run in which ~ 30.8 mg of 1C11 antibody in concentrated culture supernatant was loaded onto the column, and ~23.0 mg of 99% pure mAb was recovered, resulting in an overall yield of 74.7%. This process was repeated until 2g of 1C11 antibody was purified. The protein integrity was confirmed by SDS-PAGE, and its antigen binding activity was confirmed by ELISA.
Figure 1. Large Scale Purification of 1C11 mAb.
Large scale protein purification of 1C11 mAb (~30mg in 60ml of concentrated hybridoma culture supernatant) was performed using an ÄKTAprime chromatography system as described in Materials and Methods, and biochemistry data on a representative run are shown to illustrate the process, and purity and integrity of the resulting product. (Top) Realtime data acquisition of the eluate was performed to assess pH, protein concentration (A280), chemoelectrical conductivity, and Na citrate elution buffer (Conc.), during the chromatography run, and the results are presented in an overlaid graph format. Of note is the sharp elution of the antibody (narrow blue peak of A280 midway through the run), which corresponds to the 2.5ml elution fractions 6 to 9. (Bottom) An image of a Coomassie Brilliant Blue stained denatured SDS-PAGE gel of molecular weight standard (MW), starting material (SM), flow through (FT), and elution fractions 5–11, is shown to illustrate the relative purification of the heavy (53 KDa) and light (25 KDa) chains of the 1C11 mAb. In this run, 30.8mg of protein was loaded on to the column, and 23.0 mg of 99% pure mAb was recovered, giving a 74.7% yield.
Passive immunization of sheep and pharmacokinetics assessment in sera.
Three adult (2 years), healthy (based on clinical examination and blood work) Swiss Alpine female sheep weighing 56 to 57.5 kg were acclimatized for two weeks. During this time, as well as after the infusion, they were group housed and fed twice per day with hay and mineral lick at their disposal. Each sheep was given a 500 mg i.v. bolus of mouse IgG1 1C11 mAb in 1.0 L of Ringer`s solution over a period of 3 hours. During infusion the sheep`s physiological parameters were observed by a veterinarian. These include heart rate, respiration rate, temperature and anaphylactic reaction. Post infusion, the sheep were monitored daily for the first 10 days and weekly thereafter using a customized score sheet (temperature increases, potential infusion site infections, weight gain/loss, and feces changes). Blood was withdrawn for hematological analyses including complete blood count, total protein, serum electrolytes, blood urea nitrogen and creatinine before and after infusion as well as twice per week thereafter.
Additionally, blood was taken at one-hour post infusion (to determine C0), daily for two weeks and then weekly for two months. Serum was prepared from each blood sample and stored frozen at −20 ˚C until all samples were collected. Two mL aliquots of the entire collection were then assayed for anti-Gmd antibody activity by sandwich ELISA using recombinant His-Gmd protein for capture, and horseradish peroxidase-conjugated anti-murine IgG1antibody for detection, as previously described (Varrone et al., 2014).
All in vivo work was carried out at the AO Research Institute Davos, Switzerland, an AAALAC International approved facility and according to the Swiss animal protection law and regulations (approval number 05_2013).
Assessment of anti-Gmd antibody levels in human sera via Luminex.
All human subject research was performed on IRB approved protocols. A worldwide clinical registry of 297 patients with culture confirmed S. aureus osteomyelitis was established in 2013, and was completed in 2018 (Kates et al., 2019). This registry contains three serum samples (0 month – diagnosis of infected implant, 6 months – Re-implantation, 12 months – Follow up) from the patients. For this study, anti-Gmd antibody titers were determined from 297 of the patients in the AO5 registry, and 40 uninfected healthy controls. To quantify the relative concentration of anti-Gmd antibodies in the sera, a mouse-human chimeric 1C11 mAb was generated by replacing the murine Fc with human Fc IgG1, while retaining the murine V region of the mAb, using a proprietary method (US Patent 9,683,054 BioAtla Inc., San Diego, CA, USA). Thus, this chimeric mAb has the same antigen-binding characteristics as 1C11, but can be recognized by the anti-human IgG secondary antibody used in the Luminex assay.
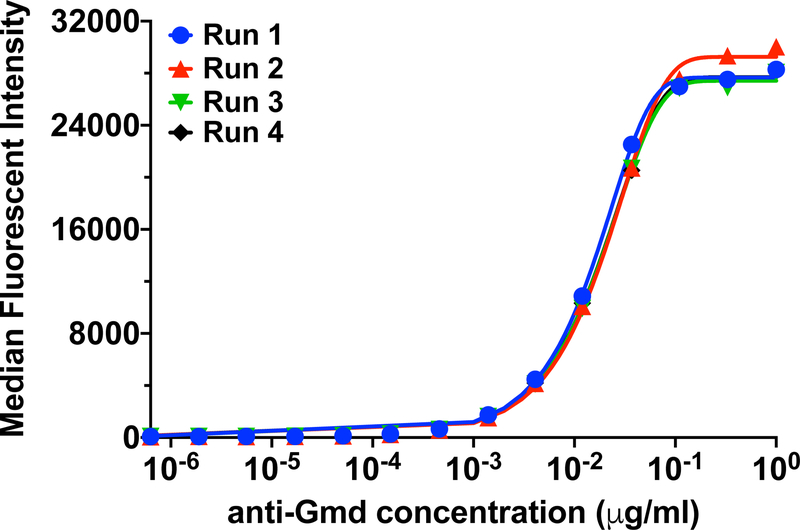
The Luminex assay was performed as previously described (Nishitani et al., 2015a). Briefly, 6.5 μm avidin-coated magnetic beads (MagPlex-Avidin Microspheres, Luminex Corp, Austin, TX) were coupled to recombinant biotinylated glucosaminidase protein (GenScript USA Inc., Piscataway, NJ). A standard curve was generated with the chimeric 1C11 mAb, in which the starting concentration of 1μg/mL was serially diluted three-fold down 14 times. The mAb was incubated with 1000 Gmd-coupled magnetic microspheres per well of a Luminex immunoassay 96-well plate. After the 2-hour incubation, the plate was washed using an automatic plate washer (BioTek 405TS microplate washer, BioTek Instruments, Inc., Winooski, VT)), and then 100 μL of the secondary phycoerythrin (PE) – conjugated anti-human IgG (Southern Biotech, Birmingham, AL) was added to each well for 1-hour incubation. Duplicate samples were analyzed on a flow cytometer (Luminex 200, Luminex Corporation, Austin, TX), to generate the standard cure of anti-Gmd titer (median fluorescent intensity (MFI)) versus concentration of chimeric anti-Gmd mAb (μg/mL) (Figure 2). The aggregate lower limit of detection (LLOD) for anti-Gmd antibody titers was utilized to define the threshold of detection. LLOD was calculated using the formula LLOD = median fluorescent intensity (MFI) of Assay buffer + 2X SEM of Assay buffer MFI (Nishitani et al., 2015a; Oh et al., 2018). An MFI of 95 when projected on the anti-Gmd 1C11 standard curve translates to 0.0139 ng/ml. Thus, although Luminex assays typically have detection thresholds in the pg/mL range, similar to what we found with our mAb, we used a more conservative approach to set the limit of detection at 1ng/mL, which takes the large dilution factor (1:10,000) into consideration. Therefore, the values reflected here are the true anti-Gmd 1C11 levels in serum of patients with S. aureus osteomyelitis. This experiment was performed 4 independent times, and high significant reproducibility was achieved between the experiments, as illustrated by the intraclass correlation coefficient (ICC) of 0.9984 [95% CI: 0.9964 – 0.9994, p=1e−23].
Figure 2. Standard curves of the humanized 1C11 chimeric anti-Gmd mAb.
standard curves of the median fluorescent intensity (MFI) vs. concentration of the chimeric 1C11 anti-Gmd antibody were generated using Luminex immunoassay starting at 1 μg/mL and serially diluting three-fold down 14 times. This experiment was performed four independent times (N=4), and the data from the four runs are superimposed in the graph. Highly significant reproducibility was achieved between the experiments, as illustrated by the intraclass correlation coefficient (ICC) of 0.9984 [95% CI: 0.9964 – 0.9994, p=1e−23].
To determine the anti-Gmd antibody titers in human sera, this same Luminex assay by incubating 100 μL of 1:10,000 diluted serum in phosphate buffered saline (PBS) with the Gmd-MagPlex-Avidin Microspheres, and then the secondary phycoerythrin (PE)-conjugated anti-human IgG. The MFI values were interpolated from the chimeric 1C11 standard curve considering the 1:10,000 dilution factor to determine the concentration of anti-Gmd antibody in each serum sample.
Data Analyses.
Decay curves to determine antibody half-life of 1C11 in sheep (n=3) were generated in GraphPad Prism version 8.0, in which the mean concentration at each time point was used to generate the best fit curve, and the time at which 50% of the C0 was observed was determined to be the circulating antibody half-life. To determine the proportion of A05 sera with an anti-Gmd concentration above uninfected control levels, upper confidence bound (UCB) analyses were performed in which the 95% UCB for the control group was used as a threshold.
Results
Circulating half-life of murine 1C11 anti-Gmd mAb in sheep
Based on our demonstration of the safety and efficacy of 1C11 anti-Gmd passive immunization in prophylactic (Varrone et al., 2014) and therapeutic (Yokogawa et al., 2018) models of implant-associated osteomyelitis, we aimed to establish preliminary safety and pharmacokinetic data in sheep, whose total body mass (~56kg) and dosing capacity (~500mg of mAb in 1 liter of saline, i.v.) is similar to humans. Thus, we manufactured 2g of 1C11 mAb, which was administered to three adult sheep in a single i.v. infusion, and circulating levels of murine IgG1 (1C11 mAb) were assessed over the next 52 days (Figure 3). There were no complications or adverse events associated with the 1C11 mAb passive immunization in any of the sheep, and the serology results demonstrated a steady decay with a half-life of ~23.7 days. Thus, we find this dosing regimen to be safe, and appropriate for assessment of 1C11 mAb efficacy in the sheep model of implant-associated osteomyelitis (Moriarty et al., 2017).
Figure 3. Half-life of murine monoclonal antibody 1C11 in sheep.
To determine basic pharmacokinetic parameters of candidate mouse monoclonal antibodies in circulation of sheep, three candidate sheep were injected with murine 1C11 and the blood (serum) was collected for 52 days in order to measure their concentration of mouse IgG1. Fitting the averaged data to a single decay curve yielded an estimated-circulating half-life of about 23.7 days.
An additional consideration for treatment of at-risk patients with prophylactic anti-Gmd monoclonal antibody 1C11 is the likelihood that treatment will elicit a potentially neutralizing sheep anti-mouse (or anti-human) response. To address this concern, we measured the abundance of sheep anti-mouse IgG (heavy and light chain) antibody present in the serum of each sheep at the time of administration (Day 0) and 4 weeks later (Day 28). Expectedly, we found no evidence for emergence of an anti-mouse response at this relatively early timepoint (data not shown).
Circulating levels and natural decay of endogenous anti-Gmd antibodies in patients with S. aureus osteomyelitis
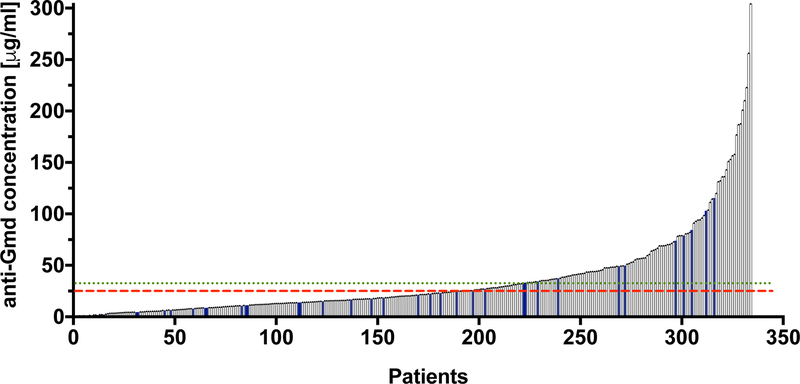
To support a human dose and regimen of anti-Gmd mAb passive immunization as an adjuvant therapy for S. aureus osteomyelitis, we assessed naturally occurring levels in these patients and healthy controls. Figure 4 shows the relative concentration of anti-Gmd antibodies in the serum of 297 patients at the time of their revision surgery (active infection), compared to the mean concentration of anti-Gmd in sera of 40 uninfected healthy controls. Anti-Gmd antibody concentrations in the patient sera ranged from undetectable (<1ng/mL) to 300 μg/mL. The concentration of anti-Gmd in the median patient was 21.7 μg/mL, which is within the range of uninfected control sera (25.6 +/− 28.6 μg/mL). Interestingly, only 104 (35.0%) of the patients have circulating anti-Gmd antibody levels significantly above the mean of healthy people (p<0.05 using a 95% upper confidence bound (UCB) = 33.2 μg /mL), suggesting that most S. aureus osteomyelitis patients fail to make an immune response against this antigen, and that passive-immunization with a protective anti-Gmd mAb is indicated for a large majority of these patients.
Figure 4. Naturally occurring anti-Gmd antibody levels in serum from a large cohort of patients with S. aureus osteomyelitis.
Serum was obtained from 297 patients with culture-confirmed S. aureus osteomyelitis (represented in black), and 40 healthy individuals (represented in blue) with no reported infections. The concentration of anti-Gmd antibodies in each sample was determined via Luminex as described in Figure 2. The anti-Gmd concentrations [μg/mL] are presented in rank order from lowest to the highest, and range from undetectable (<1ng/mL) to 304 μg/mL. The concentration of anti-Gmd in the median patient is 21.7 μg/mL. The red dashed line indicates the mean concentration of anti-Gmd (25.6 +/− 28.6 μg/mL) in sera of 40 uninfected healthy controls, and the green dotted line is the 95% upper confidence boundary = 33.2 μg /mL).
To gain insight on a clinically relevant dosing regimen of anti-Gmd mAb immunotherapy for chronic osteomyelitis, for which a standard of care is 6 to 8-weeks of i.v. antibiotic therapy (Masters et al., 2019), we assessed the decay of circulating anti-Gmd antibodies in the within this patient cohort, over their 1-year treatment period. There were two major prerequisites for inclusion: 1) the patient completed the one-year follow up period, 2) The patient was clinically cured of S. aureus osteomyelitis. Figure 5 shows the results of a post-hoc analysis that identified 12 patients whose immune proteome and clinical profile was consistent with “cured” S. aureus osteomyelitis, in that they had >20 μg/mL of anti-Gmd antibodies in their serum prior to treatment, and displayed no clinical signs or symptoms of infection following treatment. Additionally, their circulating anti-Gmd antibody titers markedly decreased from baseline to 6-months timepoint. Thus, assuming insignificant endogenous anti-Gmd antibody synthesis following treatment, the mean half-life of anti-Gmd antibodies in this cohort is 120.4 days, suggesting an approximately 3-month dosing regimen for passive-immunization with a protective anti-Gmd mAb for this indication. Nonetheless, the primary intention of our anti-Gmd therapy is a one-time preventative treatment in patients lacking anti-Gmd antibodies that are about to undergo TKA/THA.
Figure 5. Analysis of endogenous anti-Gmd antibody level decay in patients with cured S. aureus osteomyelitis.
A post-hoc analysis was performed on the S. aureus infected cohort to identify a “cured” sub-group that had >20 μg/mL of anti-Gmd antibodies in their serum prior to treatment, and who displayed no clinical signs or symptoms of infection following treatment. Anti-Gmd antibody levels in sera of the twelve patients identified in the cured sub-group were determined via Luminex, and normalized values based on each patient’s anti-Gmd antibody level at 0 months (baseline) are presented. Assuming insignificant endogenous anti-Gmd antibody synthesis following treatment, the mean half-life of anti-Gmd antibodies in this cohort is 120.4 days.
Discussion
An effective immunotherapy against the primary pathogen responsible for the vast majority of musculoskeletal infections would be transformative for orthopaedic surgery. Unfortunately, none exists and a major contributing factor to failures in S. aureus vaccine development has been the absence of an in vivo model with face and construct validity of surgical site infections (Reizner et al., 2014; Salgado-Pabon and Schlievert, 2014). Thus, our approach to develop a passive immunization has focused on murine models with quantitative outcomes of: in vivo planktonic growth, biofilm bacterial on the implants, Staphylococcus abscess communities, invasion and colonization of the osteocytic-canalicular network of cortical bone, osteolysis and implant osseointegration (de Mesy Bentley et al., 2017; Inzana et al., 2015; Li et al., 2008; Nishitani et al., 2015b; Varrone et al., 2014; Yokogawa et al., 2018). We have also developed a sheep model for a failed two-stage revision of intramedullary nail-related infection by methicillin-resistant S. aureus (Moriarty et al., 2017), which we believe is a suitable model for testing the passive immunization.
Concerning the vaccine’s molecular mechanism of action, our hypothesis has been that an ideal passive immunotherapy would be an mAb with both direct antimicrobial effects through inhibition of a critical S. aureus target, and immunomodulatory activity to enhance the host response and bacterial clearance. From non-biased antigen discovery, in vitro, animal model and clinical research, we have identified Gmd as a validated target for immunotherapy (Gedbjerg et al., 2013; Nishitani et al., 2015a; Oh et al., 2018; Varrone et al., 2014; Varrone et al., 2011b). Additionally, we have developed a lead anti-Gmd mAb (1C11) over 36 candidates, based on its superior in vitro characteristics (Gedbjerg et al., 2013; Nishitani et al., 2015a; Oh et al., 2018; Varrone et al., 2014; Varrone et al., 2011b), and its safety and efficacy in prophylactic and therapeutic murine models of implant-associated MRSA osteomyelitis (Varrone et al., 2014; Yokogawa et al., 2018). Remarkably, the results showed that 1C11 synergizes with the standard of care antibiotic therapy (vancomycin) in the 1-stage exchange model of MRSA via distinct mechanisms of actions, as vancomycin decreased the bacterial burden on the implant, but only anti-Gmd mAb inhibited SACs (Yokogawa et al., 2018). Thus, given its potential as an adjuvant therapy for PJI, here we aimed to further substantiate the feasibility of anti-Gmd mAb passive immunization by: 1) demonstrating safety and favorable pharmacokinetics following a clinically relevant dose in sheep, and 2) defining serum levels of anti-Gmd antibodies in patients with S. aureus osteomyelitis.
From a feasibility standpoint it is important to note that mAb therapies have been broadly adopted into virtually all areas of medicine, and maturity of this form of biologic therapy has recently evolved to generic drugs, known as biosimilars (Ishii-Watabe and Kuwabara, 2019). While subcutaneous mAb therapies exist (Adalimumab, Denosumab, Secukinumab, etc.), most mAb drugs were initially approved as i.v. formulations, partly due to the greater fidelity of this form of dosing regimen for study in clinical trials. Moreover, the “1st generation” chimeric mAb therapies (e.g. infliximab Remicade®, trastuzumab Herceptin®, rituximab Rituxan®) are still administered to patients this way. Based on the well-established formulation of these mAbs, which broadly conform to an i.v. infusion of ~500mg in one liter of saline (7mg/kg for a 70kg patient), we evaluated this bolus dose in sheep whose mass is ~75% of humans. An important initial finding of this experiment was the ease in which we were able to manufacture 1C11 as a drug (Figure 1), and transport it frozen from Rochester, New York, USA to Davos, Switzerland, without significant loss of material or potency (no significant mAb degradation or aggregation). While the absence of any adverse events during and 52 days following the i.v. infusion of 500mg of murine 1C11 mAb into the three sheep was not surprising, the 23.7 day circulating half-life of the mAb in this xenogeneic host (Figure 3) was beyond our expectation based on immunogenicity concerns. However, we acknowledge that our findings in a very small sample size (n=3) cannot be generalized, and that these simple proof of concept studies are not meant to circumvent the very rigorous preclinical safety and toxicology studies required to justify the use of an anti-Gmd mAb in people.
A major safety advantage of anti-Gmd mAb over biologics that target host factors (e.g. anti-cytokine mAb), is that the mAb binds to a bacterial gene product that does not exist in humans. Thus, the potential off-target effects of this mAb are very limited and we expected Gmd to be highly immunogenic in people, as recombinant Gmd protein is highly immunogenic in experimental animals grown in germ free environments (Brady et al., 2011; Varrone et al., 2011a). Thus, a major theoretical concern with anti-Gmd mAb passive immunization was that it would be superfluous to the patient’s endogenous anti-Gmd antibodies. Therefore, our remarkable finding that 65% of patients with active S. aureus osteomyelitis fail to generate circulating anti-Gmd IgG titers above that of healthy control sera (Figure 4), and commercially available i.v. immunoglobulin-G (Rongsheng ® Human Immunoglobulin (pH4) for Intravenous Injection, 50 g/L, Chengdu Rongsheng Pharmaceuticals Co., Ltd. IVIG contains 38.36 μg/mL of anti-Gmd antibody, data not shown), suggests that these patients cannot generate humoral immunity against this critical antigen on their own, and that anti-Gmd passive immunization is indicated for them. While this conclusion is based on our calculations of endogenous anti-Gmd levels in human sera that have been validated with our Luminex assay, it should be noted that a limitation of our approach is the use of a single purified monoclonal antibody (1C11), to quantify polyclonal anti-Gmd antibodies in human sera.
To better understand anti-Gmd immunity as a future direction, we aim to assess the functionality of the anti-Gmd antibodies from the patients with very high titers (>100 μg/mL, n = 23 in Figure 4), as the non-protective effect of these anti-Gmd antibodies could be due to their inability to neutralize Gmd enzymatic activity, or contain an inappropriate Fc (i.e. IgG4) that is incapable of fixing complement and/or mediating opsonophagocytosis by activated leukocytes.
Lastly, as standard of care antibiotic therapy for chronic S. aureus osteomyelitis is given over long periods of time (months), and definitive cure cannot be established with less than 6-weeks of treatment, we aimed to gain insight on the number of anti-Gmd mAb doses needed during the 1-year treatment period following confirmed S. aureus osteomyelitis, by determining the decay of endogenous anti-Gmd antibodies in patients with a cured phenotype (Figure 5). Although our calculated half-life of 120.4 days is an overestimate, as it unreasonably assumes no de novo anti-Gmd antibody production following the baseline blood draw, and autogenous antibodies have greater stability than biologics, the 3 to 4 infusions per year that this predicts is consistent with cancer immunotherapies (ipilimumab Yervoy®, pembrolizumab Keytruda®, nivolumab Opdivo®) (Schwarz et al., 2019). Although more stringent experiments could be performed, formal assessments of biodistribution and circulating anti-Gmd mAb half-life are not warranted until a drug for clinical trials is available.
Conclusions
Based on its dual mechanisms of action that includes direct antimicrobial effects and immunomodulation, anti-Gmd mAb passive immunization has emerged as a potential prophylaxis for patients at high-risk of surgical-site infections, and as an adjuvant to antibiotic therapy for S. aureus osteomyelitis. Towards clinical trials, here we established that an anti-Gmd mAb has favorable drug manufacturing and storage characteristics, and a clinically relevant dose (8.9mg/kg/i.v.) is safe in sheep. We also find that anti-Gmd mAb therapy is warranted for the majority of patients with active S. aureus osteomyelitis who fail to develop humoral immunity against Gmd.
Acknowledgements
This work was supported by research grants from the National Institutes of Health (P30 AR069655 and P50 AR72000), and AOTrauma Clinical Priority Program.
References:
- Azzam K, McHale K, Austin M, Purtill JJ, Parvizi J (2009) Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin Orthop Relat Res 467: 1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnoli F, Bertholet S, Grandi G (2012) Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binsker U, Palankar R, Wesche J, Kohler TP, Prucha J, Burchhardt G, Rohde M, Schmidt F, Broker BM, Mamat U, Pane-Farre J, Graf A, Ebner P, Greinacher A, Hammerschmidt S (2018) Secreted Immunomodulatory Proteins of Staphylococcus aureus Activate Platelets and Induce Platelet Aggregation. Thrombosis and Haemostasis 118: 745–757. [DOI] [PubMed] [Google Scholar]
- Birt MC, Anderson DW, Bruce Toby E, Wang J (2017) Osteomyelitis: Recent advances in pathophysiology and therapeutic strategies. J Orthop 14: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME (2006) Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infection and Immunity 74: 3415–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RA, O’May GA, Leid JG, Prior ML, Costerton JW, Shirtliff ME (2011) Resolution of Staphylococcus aureus Biofilm Infection Using Vaccination and Antibiotic Treatment. Infection and Immunity 79: 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB Journal 23: 3393–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR (2012) Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA 308: 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darouiche RO (2004) Treatment of infections associated with surgical implants. New England Journal of Medicine 350: 1422–1429. [DOI] [PubMed] [Google Scholar]
- de Mesy Bentley KL, MacDonald A, Schwarz EM, Oh I (2018) Chronic Osteomyelitis with Staphylococcus aureus Deformation in Submicron Canaliculi of Osteocytes: A Case Report. JBJS Case Connect 8: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L, Chung HL, McGrath JL, Daiss JL, Awad HA, Kates SL, Schwarz EM (2017) Evidence of Staphylococcus Aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. Journal of Bone and Mineral Research 32: 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirich J, Orth R, Sieber SA (2011) Unraveling the protein targets of vancomycin in living S. aureus and E. faecalis cells. Journal of the American Chemical Society 133: 12144–12153. [DOI] [PubMed] [Google Scholar]
- Ferry T, Uckay I, Vaudaux P, Francois P, Schrenzel J, Harbarth S, Laurent F, Bernard L, Vandenesch F, Etienne J, Hoffmeyer P, Lew D (2009) Risk factors for treatment failure in orthopedic device-related methicillin-resistant Staphylococcus aureus infection. Eur J Clin Microbiol Infect Dis 29: 171–180. [DOI] [PubMed] [Google Scholar]
- Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A (2013) Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309: 1368–1378. [DOI] [PubMed] [Google Scholar]
- Fowler VG Jr., Proctor RA (2014) Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect 20 Suppl 5: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedbjerg N, Larosa R, Hunter JG, Varrone JJ, Kates SL, Schwarz EM, Daiss JL (2013) Anti-Glucosaminidase IgG in Sera as a Biomarker of Host Immunity Against Staphylococcus aureus in Orthopaedic Surgery Patients. Journal of Bone and Joint Surgery (American Volume) 95: e1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J (2009) Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res 467: 1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz F, Heilmann C, Stehle T (2014) Functional and structural analysis of the major amidase (Atl) in Staphylococcus. International Journal of Medical Microbiology 304: 156–163. [DOI] [PubMed] [Google Scholar]
- Harro CD, Betts RF, Hartzel JS, Onorato MT, Lipka J, Smugar SS, Kartsonis NA (2012) The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two Phase I studies. Vaccine 30: 1729–1736. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Hartleib J, Hussain MS, Peters G (2005) The multifunctional Staphylococcus aureus autolysin aaa mediates adherence to immobilized fibrinogen and fibronectin. Infection and Immunity 73: 4793–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhausen N, Schlesier T, Schmidt MA, Gotz F, Peters G, Heilmann C (2010) A novel staphylococcal internalization mechanism involves the major autolysin Atl and heat shock cognate protein Hsc70 as host cell receptor. Cellular Microbiology 12: 1746–1764. [DOI] [PubMed] [Google Scholar]
- Holtfreter S, Kolata J, Broker BM (2010) Towards the immune proteome of Staphylococcus aureus - The anti-S. aureus antibody response. International Journal of Medical Microbiology 300: 176–192. [DOI] [PubMed] [Google Scholar]
- Inzana JA, Schwarz EM, Kates SL, Awad HA (2015) A novel murine model of established Staphylococcal bone infection in the presence of a fracture fixation plate to study therapies utilizing antibiotic-laden spacers after revision surgery. Bone 72: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii-Watabe A, Kuwabara T (2019) Biosimilarity assessment of biosimilar therapeutic monoclonal antibodies. Drug Metabolism and Pharmacokinetics 34: 64–70. [DOI] [PubMed] [Google Scholar]
- Jansen KU, Girgenti DQ, Scully IL, Anderson AS (2013) Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine 31: 2723–2730. [DOI] [PubMed] [Google Scholar]
- Kalali Y, Haghighat S, Mahdavi M (2018) Passive immunotherapy with specific IgG fraction against autolysin: analogous protectivity in the MRSA infection with antibiotic therapy. Immunology Letters. [DOI] [PubMed] [Google Scholar]
- Kates SL, Hurni S, Chen MS (2019) Development and challenges in setting up an international bone infection registry. Archives of Orthopaedic and Trauma Surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, Schneewind O (2010) IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 28: 6382–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J (2012) Economic burden of periprosthetic joint infection in the United States. The Journal of arthroplasty 27: 61–65. e61. [DOI] [PubMed] [Google Scholar]
- Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364: 369–379. [DOI] [PubMed] [Google Scholar]
- Li D, Gromov K, Soballe K, Puzas JE, O’Keefe RJ, Awad H, Drissi H, Schwarz EM (2008) Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. Journal of Orthopaedic Research 26: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters E, Trombetta RP, de Mesy Bentley KL, Boyce BF, Gill A, Gill SR, Nishitani K, Ishikawa M, Morita Y, Bello-Irizarry SN, Ninomiya M, Brodell JD, Lee CC, Hao SP, Oh I, Xie C, Awad HA, Daiss JL, Owen JR, Kates SL, Schwarz EM, Muthukrishnan G (2019) Evolving concepts in bone infection: redefining “biofilm”, “acute vs chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely TB, Shah NA, Fridman A, Joshi A, Hartzel JS, Keshari RS, Lupu F, DiNubile MJ (2014) Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Human Vaccines & Immunotherapeutics 10: 3513–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty TF, Schmid T, Post V, Samara E, Kates S, Schwarz EM, Zeiter S, Richards RG (2017) A large animal model for a failed two-stage revision of intramedullary nail-related infection by methicillin-resistant Staphylococcus aureus. Eur Cell Mater 34: 83–98. [DOI] [PubMed] [Google Scholar]
- Nishitani K, Beck CA, Rosenberg AF, Kates SL, Schwarz EM, Daiss JL (2015a) A Diagnostic Serum Antibody Test for Patients With Staphylococcus aureus Osteomyelitis. Clinical Orthopaedics and Related Research 473: 2735–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, Varrone JJ, Bello-Irizarry SN, Ito H, Matsuda S, Kates SL, Daiss JL, Schwarz EM (2015b) Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. Journal of Orthopaedic Research 33: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh I, Muthukrishnan G, Ninomiya MJ, Brodell JD Jr., Smith BL, Lee CC, Gill SR, Beck CA, Schwarz EM, Daiss JL (2018) Tracking Anti-Staphylococcus aureus Antibodies Produced In Vivo and Ex Vivo during Foot Salvage Therapy for Diabetic Foot Infections Reveals Prognostic Insights and Evidence of Diversified Humoral Immunity. Infection and Immunity 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshida T, Sugai M, Komatsuzawa H, Hong YM, Suginaka H, Tomasz A (1995) A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proceedings of the National Academy of Sciences of the United States of America 92: 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH (2009) Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res 467: 1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB (2013) Will there ever be a universal Staphylococcus aureus vaccine? Human Vaccines & Immunotherapeutics 9: 1865–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RA (2012) Is there a future for a Staphylococcus aureus vaccine? Vaccine 30: 2921–2927. [DOI] [PubMed] [Google Scholar]
- Proctor RA (2015) Recent developments for Staphylococcus aureus vaccines: clinical and basic science challenges. Eur Cell Mater 30: 315–326. [DOI] [PubMed] [Google Scholar]
- Projan SJ, Nesin M, Dunman PM (2006) Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Current Opinion in Pharmacology 6: 473–479. [DOI] [PubMed] [Google Scholar]
- Reizner W, Hunter JG, O’Malley NT, Southgate RD, Schwarz EM, Kates SL (2014) A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur Cell Mater 27: 196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed K, McLaren AC, Schwarz EM, Antoci V, Arnold WV, Chen AF, Clauss M, Esteban J, Gant V, Hendershot E, Hickok N, Higuera CA, Coraca-Huber DC, Choe H, Jennings JA, Joshi M, Li WT, Noble PC, Phillips KS, Pottinger PS, Restrepo C, Rohde H, Schaer TP, Shen H, Smeltzer M, Stoodley P, Webb JCJ, Witso E (2019) 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. Journal of Orthopaedic Research 37: 1007–1017. [DOI] [PubMed] [Google Scholar]
- Salgado CD, Dash S, Cantey JR, Marculescu CE (2007) Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clinical Orthopaedics and Related Research 461: 48–53. [DOI] [PubMed] [Google Scholar]
- Salgado-Pabon W, Schlievert PM (2014) Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol 12: 585–591. [DOI] [PubMed] [Google Scholar]
- Schwarz EM, Parvizi J, Gehrke T, Aiyer A, Battenberg A, Brown SA, Callaghan JJ, Citak M, Egol K, Garrigues GE, Ghert M, Goswami K, Green A, Hammound S, Kates SL, McLaren AC, Mont MA, Namdari S, Obremskey WT, O’Toole R, Raikin S, Restrepo C, Ricciardi B, Saeed K, Sanchez-Sotelo J, Shohat N, Tan T, Thirukumaran CP, Winters B (2019) 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. Journal of Orthopaedic Research 37: 997–1006. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Teng MW (2018) 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology 7: e1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM (2010) Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA 303: 2479–2485. [DOI] [PubMed] [Google Scholar]
- Sugai M, Komatsuzawa H, Akiyama T, Hong YM, Oshida T, Miyake Y, Yamaguchi T, Suginaka H (1995) Identification of endo-beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. Journal of Bacteriology 177: 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrone JJ, de Mesy Bentley KL, Bello-Irizarry SN, Nishitani K, Mack S, Hunter JG, Kates SL, Daiss JL, Schwarz EM (2014) Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. Journal of Orthopaedic Research 32: 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrone JJ, Li D, Daiss JL, Schwarz EM (2011a) Anti-Glucosaminidase Monoclonal Antibodies as a Passive Immunization for Methicillin-Resistant Staphylococcus aureus (MRSA) Orthopaedic Infections. Bonekey Osteovision 8: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrone JJ, Li D, Daiss JL, Schwarz EM (2011b) Anti-glucosaminidase monoclonal antibodies as a passive immunization for methicillin-resistant Staphylococcus aureus (MRSA) orthopedic infections. IBMS BoneKEy 8: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Sugai M, Komatsuzawa H, Nakashima S, Oshida T, Matsumoto A, Suginaka H (1996) An autolysin ring associated with cell separation of Staphylococcus aureus. Journal of Bacteriology 178: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa N, Ishikawa M, Nishitani K, Beck CA, Tsuchiya H, Mesfin A, Kates SL, Daiss JL, Xie C, Schwarz EM (2018) Immunotherapy synergizes with debridement and antibiotic therapy in a murine 1-stage exchange model of MRSA implant-associated osteomyelitis. Journal of Orthopaedic Research 36: 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]