Abstract
Foraging by animals is hypothesized to be state-dependent, that is, varying with physiological condition of individuals. State often is defined by energy reserves, but state also can reflect differences in nutritional requirements (e.g., for reproduction, lactation, growth, etc.). Testing hypotheses about state-dependent foraging in ungulates is difficult because fine-scale data needed to evaluate these hypotheses generally are lacking. To evaluate whether foraging by caribou (Rangifer tarandus) was state-dependent, we compared bite and intake rates, travel rates, dietary quality, forage selection, daily foraging time, and foraging strategies of caribou with three levels of nutritional requirements (lactating adults, nonlactating adults, subadults 1–2 years old). Only daily foraging times and daily nutrient intakes differed among nutritional classes of caribou. Lactating caribou foraged longer per day than nonlactating caribou—a difference that was greatest at the highest rates of intake, but which persisted even when intake was below requirements. Further, at sites where caribou achieved high rates of intake, caribou in each nutritional class continued foraging even after satisfying daily nutritional requirements, which was consistent with a foraging strategy to maximize energy intake. Foraging time by caribou was partially state-dependent, highlighting the importance of accounting for physiological state in studies of animal behavior. Fine-scale foraging behaviors may influence larger-scale behavioral strategies, with potential implications for conservation and management.
Keywords: bite rate, diet quality, diet selection, energy maximizing, foraging time, intake rate, lactation, Rangifer tarandus, time minimizing, travel rate
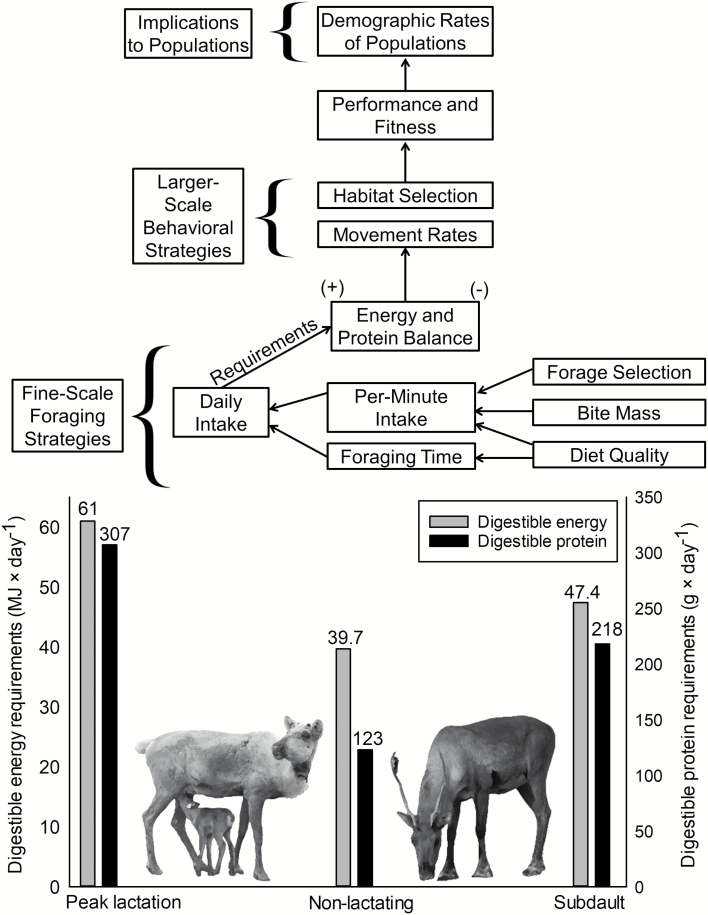
Foraging is a complex, hierarchical process that largely controls the nutritional balance of animals and hence constitutes a fundamental link between animals and food supplies available in their environments (Senft et al. 1987; Parker et al. 2009). At fine scales, animals select bites from acceptable forages; the quality, size, and quantity of bites taken determine short-term rates of intake (Fig. 1). Foraging decisions at fine spatial and temporal scales (e.g., ≤ 1 day) can compound across longer time scales to have multiplier effects on nutritional outcomes such as nutritional condition, body mass, juvenile growth, reproduction, and survival (White 1983; Cook et al. 2004; Shipley 2007; Fig. 1). The need to achieve specific foraging goals at fine scales (e.g., bite mass, nutrient intakes) can motivate larger-scale processes such as seasonal movements, distributions, and habitat selection (White and Trudell 1980; Short 1985; Schaefer et al. 2000; Hobbs et al. 2003; Briand et al. 2009; Massé and Côté 2012). Thus, a holistic understanding of nutritional mechanisms underpinning large-scale processes that ultimately influence fitness necessitates understanding variation in fine-scale foraging behaviors. Yet for many free-ranging ungulates, fine-scale foraging behaviors have not been quantified directly, thereby limiting our understanding of large-scale processes, including those that ultimately influence individual fitness and population productivity (e.g., White 1983; Dussault et al. 2012). Because of potential multiplier effects of foraging (White 1983), knowledge of fine-scale foraging strategies may be particularly important for conservation and recovery planning for species like woodland caribou (Rangifer tarandus caribou) that are at risk of extinction (Johnson et al. 2015).
Fig. 1.
—Conceptual model depicting that differences in energy and protein requirements among lactating, nonlactating, and adult caribou (Rangifer tarandus) could scale up from fine-scale foraging decisions to influence larger-scale behaviors and processes. Digestible energy and digestible protein requirements for adults (assuming a mass of 115 kg) are from National Research Council (2007), plus an additional mass gain of 10 kg over summer from Robbins (1993). Crude protein requirements were converted to digestible protein requirements using values from Denryter (2017). Digestible energy and digestible protein requirements for subadults are the maintenance requirements for an 88-kg nonlactating adult caribou, plus an additional mass gain of 25 kg over summer (Robbins 1993; National Research Council 2007). We assumed gain was 90% fat and 10% protein over a 100-day period (coinciding with the length of our field season) and that fat was energetically equivalent to 39.3 kJ × g−1, muscle was energetically equivalent to 22.6 kJ × g−1, and gain was 65% efficient (Boertje 1985).
Foraging behaviors are directly influenced by characteristics of the available forage base (e.g., Wickstrom et al. 1984; Cook et al. 2016; Denryter 2017), but they also can be influenced by an animal’s state (Stephens 1981; McNamara and Houston 1986; Olsson et al. 2002; Rands et al. 2011; Liesenjohann et al. 2015; Shuai et al. 2017). Conventionally, state includes any variable(s) that reflect the current physiological condition of the animal (though state can change depending on the animals’ environment, its subsequent states, and its decisions—Mangel and Clark 1986). State may refer to nutritional condition (i.e., the physiological state of an animal that is an outcome of nutrient intake and energy expenditures, often indicated by levels of body fat) or production state (e.g., pregnant, lactating, growing, maintaining). Among production states in ungulates, nutritional requirements can vary 2-fold or more (summarized by Robbins 1993; Fig. 1).
Ungulates can modify behavior to satisfy elevated nutritional requirements associated with various production states (Fig. 1). At fine scales, ungulates can increase bite rate or select for forages that offer large bite mass, ease of acquisition, or greater nutrient content (Fig. 1). At moderate scales, ungulates may select vegetation community types that offer high quality and quantity of forage, increase time spent foraging each day, and reduce vigilance. The extent to which ungulates can modify foraging behavior to satisfy elevated nutritional requirements is constrained by physiological changes that occur in various production states. For example, capacity and absorptive surface area of the rumen are greater in lactating than nonlactating females, which allow for greater rates of food processing (Tulloh 1966; Campling 1970; Owen-Smith 2002; Zimmerman et al. 2006; Luna and Weckerly 2013). In addition, lactating females may ruminate faster than nonlactating females (Blanchard 2005). Digestive constraints that differ relative to animal state, such as changes in ruminal capacity, must be considered when comparing foraging behaviors of animals in different states.
To maximize fitness, animals have two optimal foraging strategies from which to choose: time minimizing or energy maximizing (Schoener 1971; although maximizing other or multiple nutrients may occur—Simpson et al. 2004). Time minimizers forage just long enough to satisfy their nutritional requirements, thereby maintaining time for activities that may enhance fitness more than additional foraging (e.g., vigilance). Energy maximizers continue foraging even after they have achieved energy intake needed to satisfy their requirements, presumably because doing so usually confers greater benefits than other nonforaging activities (Belovsky 1986; Stephens and Krebs 1986). Ungulates have been hypothesized to be time minimizers (Schoener 1971), energy maximizers (Vivas and Saether 1987; Forchhammer and Boomsma 1995; Illius et al. 1995; Kohli et al. 2014), or some combination (Bergman et al. 2001). Whether foragers should behave as time minimizers or energy maximizers may be scale-dependent (Bergman et al. 2001), but the nutritional environment animals occupy may, to a much larger degree, determine which strategy is most advantageous. For example, in environments where energy supplies are limited or highly variable, inadequate acquisition and assimilation of energy may be the predominant threat to survival and reproduction. Individuals in these environments should consume energy in excess of requirements because subsequent opportunities to meet or exceed energy requirements may be rare. Hence, maximizing intake of energy should be the more advantageous strategy. In contrast, in environments where foragers can reliably satisfy nutritional requirements each day, and energy acquisition is not the predominant threat to survival and reproduction, a time-minimizing strategy may be more advantageous. Data needed to evaluate predictions arising from optimal foraging theory are scarce for ungulates, which has made it difficult to relate foraging theory to on-the-ground conditions.
In the boreal forests and mountains of northeastern British Columbia, we documented and compared foraging behaviors of tame female caribou (Rangifer tarandus) with three levels of nutritional requirements (lactating adults, nonlactating adults, subadults 1–2 years old; hereafter nutritional classes) during summer and early autumn. Our primary objectives were to: 1) evaluate whether fine-scale foraging behaviors of caribou were state-dependent; and 2) determine whether caribou behaved as time minimizers or energy maximizers. First, we hypothesized that foraging by caribou was partially state-dependent (i.e., only some foraging behaviors differ among nutritional classes of caribou). Because there is no advantage to eating more slowly or less efficiently than animals are physically capable of, we predicted that bite rates, per-minute intake rates, dietary qualities, and forage selection patterns would not differ among nutritional classes of caribou. Given differences in daily nutritional requirements and digestive constraints, however, we predicted that daily foraging times and intakes would be greatest for lactating caribou and least for nonlactating caribou, with subadults being intermediate. Second, because caribou should capitalize on annual peaks in quantity and quality of forage (during summer), we hypothesized that caribou were energy maximizers and predicted that they would continue foraging even after satisfying daily nutritional requirements.
Materials and Methods
We collected foraging observations using tame caribou that were hand-reared at the Robert G. White Large Animal Research Station at the University of Alaska, Fairbanks in 2009 (Parker and Barboza 2013). The tame caribou were transferred to a research facility, operated by the National Council for Air and Stream Improvement, near Fort St. John, British Columbia, in April 2013, where they were housed and maintained on a high-quality pelleted ration (Barboza and Parker 2006; Parker and Barboza 2013) when not used in field trials. Some animals born at the facility in 2013 were used in field trials in 2014–2015.
Study area.—
We sampled the predominant plant communities of the mountains and boreal flats of northeastern British Columbia during summer, which is the most nutritionally demanding time of the year for northern ungulates (Cook et al. 2004; Parker et al. 2009). Plant communities in British Columbia are described by the biogeoclimatic ecological classification zone, including several alpine, montane forest, boreal forest, and wetland communities (DeLong et al. 1990; Mackenzie 2012). Descriptions of species composition, biomass, and nutritional value of plant communities were provided in Denryter et al. (2017) and Denryter (2017).
Foraging trials with tame caribou.—
From July to October 2013–2015, caribou were transported in a stock trailer to temporary, electrified enclosures ranging in size from 0.15 to 1.75 ha at 135 remote study sites where we had previously sampled vegetation using destructive sampling techniques (Denryter et al. 2017). Sites were sampled to estimate biomass production of all vascular and nonvascular plants within enclosures. All plant samples were oven-dried to constant mass to estimate mass of dry matter by species at each site (Denryter et al. 2017). Enclosure size was adjusted relative to available biomass so that caribou would consume ≤ 5% of available biomass during their time in the enclosure (up to 48 h plus habituation time), thus minimizing potential effects of patch depression or enclosure size on foraging by caribou. All protocols were approved by the University of Northern British Columbia Animal Care and Use Committee (Protocol Number 2013-9) and followed the guidelines of the American Society of Mammalogists for research on live animals (Sikes et al. 2016).
During foraging trials, two researchers observed foraging by caribou in two separate enclosures (usually n = 4 adults or subadults, plus associated calves per site), using continuous, direct observations. During four 15–20 min foraging trials per caribou per day (75 min per animal per day), we recorded each bite consumed of each plant species and estimated distance traveled (Denryter 2017; Denryter et al. 2017). For intake estimates, we collected bite mass samples of each species consumed (n = 10 per species per site) based on direct observations of intake by caribou (Wallmo and Neff 1970) and oven-dried the samples to constant mass to estimate mean bite mass for each species consumed. We calculated (per-minute) dry matter intake as the product of bite rate (per minute) and bite mass, divided by the length (in minutes) of the foraging trial. For each caribou, we averaged the per-minute intake of all of its trials (up to eight per caribou per site) to determine mean rate of per-minute intake. We determined average bite size for each caribou at each site as shown in equation 1:
| (1) |
where x is a given site, i represents a given species consumed at that site, and n is the total number of species consumed by individual j. Total bites per site were the sum of the number of bites from all foraging trials for each animal-site combination. We multiplied per-minute intakes of dry matter by dietary digestible energy (DDE) and dietary digestible protein (DDP) contents of caribou diets (described below) to estimate per-minute intakes of digestible energy and digestible protein.
To estimate daily foraging time for each caribou, we used a combination of direct observation and accelerometer data from automated activity recorders (Mini-Mitter model AW64; Mini-Mitter Co., Bend, Oregon) that were attached to a radiocollar on each caribou. When we had visual observations, we used those to quantify foraging time; when we did not have visual observations (e.g., at night), we used accelerometer data to estimate foraging time. When directly observing caribou, we grouped activity at 1-min intervals as inactive (e.g., bedded, ruminating, standing still), foraging (e.g., cropping, chewing, ingesting, or searching for food), and hyperactive (e.g., walking, playing, running) behaviors. Accelerometers recorded movement at 2-min intervals. Lowest values of accelerometer data reflected inactive behaviors (infrequent head movement), moderate values reflected foraging behaviors (moderately frequent head movement), and high values reflected hyperactive behaviors (frequent head movement).
To calibrate accelerometer data, we used a combination of the methodology described by Cook et al. (2016) and a series of frequency histograms of accelerometer values for each caribou-accelerometer-enclosure (n = 932 individual graphs—Denryter 2017). Briefly, accelerometer data were paired with visual observations for each caribou and a mean and standard deviation (SD) accelerometer value for foraging were calculated. We then iteratively added up to 2 SDs (at 0.2-SD intervals) and used the interval that most accurately predicted foraging behavior. Due to variability in head movement while foraging in different plant communities (e.g., foraging with head up in shrub-dominated communities versus head down in lichen-dominated communities), we also produced frequency histograms of accelerometer values. From these histograms, we visually estimated foraging values by identifying breaks between peaks in accelerometer values. For example, a typical histogram of accelerometer data included two peaks, one at low values (e.g., < 350) and one at moderate values (e.g., 1,200), with several bins of few to no values in between and several bins of values above the normally distributed values for foraging (Supplementary Data SD1). Using each estimated break for every caribou-enclosure combination, we paired predicted activity with observed activity and calculated accuracy as the percentage of time predicted foraging values agreed with observed foraging values. Overall, our classification of foraging was 93% accurate. After estimating foraging time for each caribou and enclosure, we estimated daily intakes as the product of foraging time, per-minute intake of dry matter, DDE, and DDP. Because not all females bred each year, we used a different mix of nutritional classes each year: 2013: nine (4-year-old) lactating adults, so each enclosure sampled contained only lactating adults; 2014: three lactating and two nonlactating (5-year-old) adults and five yearlings, and enclosures contained a mix of animals in each nutritional class; 2015: one lactating and six nonlactating (6-year-old) adults and three (2-year-old) subadults, and enclosures contained a mix of animals in each nutritional class. For the purposes of this analysis, yearling and 2-year-old caribou were grouped as subadults because intragroup sample sizes were small and, on average, both yearling and 2-year-old caribou grew ~25 kg over summer.
Dietary analyses.—
For each caribou at each site, we estimated dietary composition of plant species by tallying the total number of bites of each species consumed during all of its foraging trials at that site and calculated the proportion of those bites, weighted by bite mass, of each consumed species. Those species with the greatest proportions of bites, which together comprised ~90% of total bites recorded, were collected and combined into a composite diet sample in proportions equaling dietary composition (Denryter 2017). We immediately packed diet samples in ice and later (within 4 days of collection) transferred them to a freezer (Denryter 2017). The Wildlife Habitat and Nutrition Laboratory at Washington State University freeze-dried the samples and conducted assays to estimate energy, fiber, protein, and tannin content of samples with bomb calorimetry, sequential fiber analysis, total elemental N, and tannin precipitation methods (Goering and Van Soest 1970; Martin and Martin 1983). We calculated digestible energy and protein using equations of Robbins et al. (1987a, 1987b).
To assess diet selection for each animal at each site, we calculated Ivlev’s electivity index (equation 2), which scales from −1 (complete avoidance) to +1 (complete selection), as:
| (2) |
where E is Ivlev’s electivity score, U is the proportion of intake composed of a food, and A represents the proportion of available biomass composed of that food (Ivlev 1961).
Statistical analyses.—
All statistical analyses were completed using Stata 14 (StataCorp LP, College Station, Texas, 2016) with α = 0.05 for all tests of significance. We used linear and nonlinear (with a gamma link) multilevel mixed-effects regression (mixed and meglm commands, respectively; hereafter, multilevel models) to analyze foraging behaviors (Skrondal and Rabe-Hesketh 2004). Multilevel models allowed us to track individuals (using both a random slope and intercept) throughout the analysis. We included nutritional class as a covariate and determined the significance of nutritional class across all variables in the model, by varying the reference category for nutritional class. For example, we ran a model using lactating caribou as the reference category and if P < 0.05 for nonlactating or yearling caribou, they were determined to be significantly different from lactating caribou. We then ran the same model again, changing the reference category to nonlactating caribou, which allowed us to compare nonlactating and subadult caribou. If the main analyses suggested differences in foraging behaviors across nutritional classes, we conducted follow-up analyses to better understand the factors underpinning these differences.
Foraging responses are highly variable as a function of attributes of the plant community (e.g., species composition, available biomass, etc.—Trudell and White 1981; Wickstrom et al. 1984; Cook et al. 2016; Denryter 2017); thus, testing for differences across individuals or groups can be misleading unless each was exposed to identical foraging conditions. To illustrate this point, we give the following example: we sampled two enclosures containing the same quantity of accepted biomass (700 kg × ha−1—Denryter 2017), but with significantly different plant composition and significantly different foraging responses: one in a dry alpine site dominated by lichens and the other in a young, productive forest site dominated by forbs and shrubs. At the alpine site, the composition of nutritional classes of caribou in the enclosure was one lactating adult, one nonlactating adult, and two subadult caribou, and mean DDE, DDP, and dry matter intakes were 8.8 kJ × min−1, 1.4 g protein × min−1, and 2.5 g × min−1, respectively. At the forest site, the composition of nutritional classes of caribou in the enclosure was all lactating adults and the mean DDE, DDP, and dry matter intakes were 12.79 kJ × min−1, 6.8 g protein × min−1, and 9.63 g × min−1, respectively. If a traditional analytical framework had been used, wherein biomass was an explanatory variable and nutritional class was a covariate (main effect or interaction), the results would have incorrectly attributed differences in foraging response to nutritional class rather than plant community.
While we had sampling enclosures with each class of caribou represented, we did not have the sample size needed to limit the analysis to this subset. For this reason, we analyzed our data in a way to remove the effects of a plant community (e.g., biomass) by examining relationships between two related foraging behaviors that were mathematically related to a third foraging behavior, with the third foraging behavior being the one of interest. For example, to evaluate whether bite rates differed across nutritional classes of caribou, we examined the relationship between bite size and intake rate. Because bite rate × bite size = per-minute intake rate, the slope of the regression coefficient for bite size (the independent variable) represented bite rate—any differences in the relationship between bite size and intake rate with the covariate of nutritional class were therefore attributable to differences in bite rate. This analysis controlled for the effect of bite size (which is, in part, a function of plant community composition), and because the slope of this regression equation essentially represents bite rate, any significant differences in intake rate among nutritional classes could only be attributable to differences in bite rate (not the influence of plant community). Using the same approach, we also determined if there were differences among nutritional classes of caribou in: travel rates (per-minute intake versus meters traveled per g of food consumed; x versus y); foraging time (per-minute intake versus daily dry matter intake); DDP (daily dry matter intake versus daily digestible protein intake); and DDE (daily dry matter intake versus daily digestible energy intake; Table 2).
Table 2.
Foraging behaviors of caribou (Rangifer tarandus) with three levels of nutritional requirements (lactating, nonlactating, and subadult 1–2 years old) determined from multilevel models, with individuals modeled with a random intercept and a random slope. Differences in foraging behaviors among nutritional classes are indicated by different capital letter superscripts on β coefficients.a
| Dependent variable | Independent variable(s) | Differences in foraging behavior attributable to | Lactating | Nonlactating | Subadult | ||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | Intercept of multilevel model | |||
| Per-minute intake (g × min−1) | Bite size (g) | Bite rate | 15.5A | 0.63 | −0.15A | 0.19 | 0.18A | 0.20 | 0.79 |
| Per-minute intake (g × min−1) | Distance per g of intake (m × g−1) | Travel rate | −0.25A | 0.02 | −0.03A | 0.06 | 0.04A | 0.07 | 1.74 |
| Daily intake (g × day−1) | Per-minute intake (g × min−1) | Foraging time | 692.9A | 15.3 | −253.6B | 81.4 | −70.6A, B | 95.7 | 385.3 |
| Daily digestible protein intake (DP g × day−1)b | Daily intake (g × day−1) | DDPc | 0.07A | 0.004 | −8.97A | 16.1 | −16.1A | 18.6 | −56.7 |
| Daily digestible energy intake (DE kJ × day−1)d | Daily intake (g × day−1), Year | DDEe, Year | 2.99A | 0.02 | −32.2A | 131.7 | −24.1A | 123.3 | −410.9 |
aβ coefficients, standard errors (SE), and intercepts are from multilevel models (with nutritional class as a covariate) for analyses of bite rate, travel rate (nonlinear; fit with a gamma link), foraging time, DDP, and DDE. Nutritional classes sharing the same superscripted letter were not different from each other.
bDP is digestible protein.
cDDP is dietary digestible protein (digestible protein content of diets selected by caribou).
dDE is digestible energy.
eDDE is dietary digestible energy (digestible energy content of diets selected by caribou). The initial analysis of DDE showed that lactating caribou had higher dietary DDE than nonlactating caribou, but a follow-up analysis for DDE that included year as a covariate (to account for annual variation in climatic conditions [e.g., precipitation and temperature] and its effect on plant quality) showed that year rather than nutritional class explained differences in DDE observed in the original analysis.
We did not evaluate bite size because we collected bite mass samples once per enclosure, rather than per individual, and our observations suggested that bite mass on a given plant was consistent among caribou in each enclosure. Summer precipitation varied substantially among years (Denryter et al. 2017), which may have influenced plant chemistry and hence forage quality among years, so we also ran follow-up analyses for DDP and DDE. We reran our models for DDP and DDE with year as an additional covariate and ran analyses of DDP and DDE with data only from 2014 (when all three nutritional classes of caribou were exposed to the same vegetation at the same sites). If DDP and DDE differed across nutritional classes, we expected this difference would be apparent in a model with data only from 2014 or that 2014 would be a significant covariate in the overall model.
Not all plant community bias associated with diet composition for individuals could be removed and hence we did not analyze diet composition relative to nutritional classes. Instead, we assessed forage selection to understand potential differences in dietary choices of caribou relative to nutritional requirements. Forage selection is a function of abundance of food choices and thus, inherently accounts for differences in species composition (i.e., food choices) across sites. We averaged Ivlev scores for animals of the same nutritional class in the same enclosure to avoid inflating the sample size for comparisons of selection in a Kruskal–Wallis test; only plant species with n ≥ 10 for each nutritional class were assessed. Significant main effects of Kruskal–Wallis tests indicated that ranks of Ivlev scores differed among groups, but differences in ranks do not necessarily indicate differences in selection (Denryter et al. 2017). Thus, for any species differing in rank among nutritional classes, we used a Wilcoxon Signed-Rank test to determine if selection changed from avoided (mean Ivlev score significantly < 0; used proportionately less than their availability) to neutral (mean Ivlev score not significantly different from 0; used in proportion to their availability) or avoided to selected (mean Ivlev score significantly > 0; used proportionately greater than their availability—Cook et al. 2016; Denryter et al. 2017).
Results
We sampled ~942 h of foraging, during which we counted almost 1.2 million bites taken by caribou. From these foraging observations we collected ~1,260 bite mass samples and 517 diet samples (for DDP and DDE). After averaging data from foraging trials for each individual caribou (to obtain one observation per animal per site), we analyzed per-minute intake and dietary quality using 512 observations on 24 animals. We had adequate sample sizes (n ≥ 10) of 103 forage species to assess forage selection by at least two nutritional classes of caribou and 86 species for which we could assess selection by all three nutritional classes of caribou. For analyses of foraging time and daily intake, we collected ~1,682 animal-hours of activity via direct observations and had 417 observations on 22 animals available for analyses of foraging time and daily intake (Table 1). We had fewer samples for analyses of daily intake than per-minute intake and dietary quality because several activity recorders malfunctioned in 2014 and 2015.
Table 1.
Sample sizes for observations of tame caribou (Rangifer tarandus) of three different nutritional classes (lactating, nonlactating, and subadult 1–2 years old) for measures of instantaneous (bite rate, travel rate) and daily foraging behaviors (foraging time; daily intakes of dry matter, digestible energy, and digestible protein).
| Nutritional class | 2013 | 2014 | 2015 | Total |
|---|---|---|---|---|
| Instantaneous foraging behaviors | ||||
| Lactating adults | 179 | 44 | 4 | 227 |
| Nonlactating adults | 0 | 44 | 110 | 154 |
| Subadults (1–2 years old) | 0 | 69 | 62 | 131 |
| Daily foraging behaviors | ||||
| Lactating adults | 176 | 40 | 3 | 219 |
| Nonlactating adults | 0 | 20 | 88 | 108 |
| Subadults (1–2 years old) | 0 | 56 | 34 | 90 |
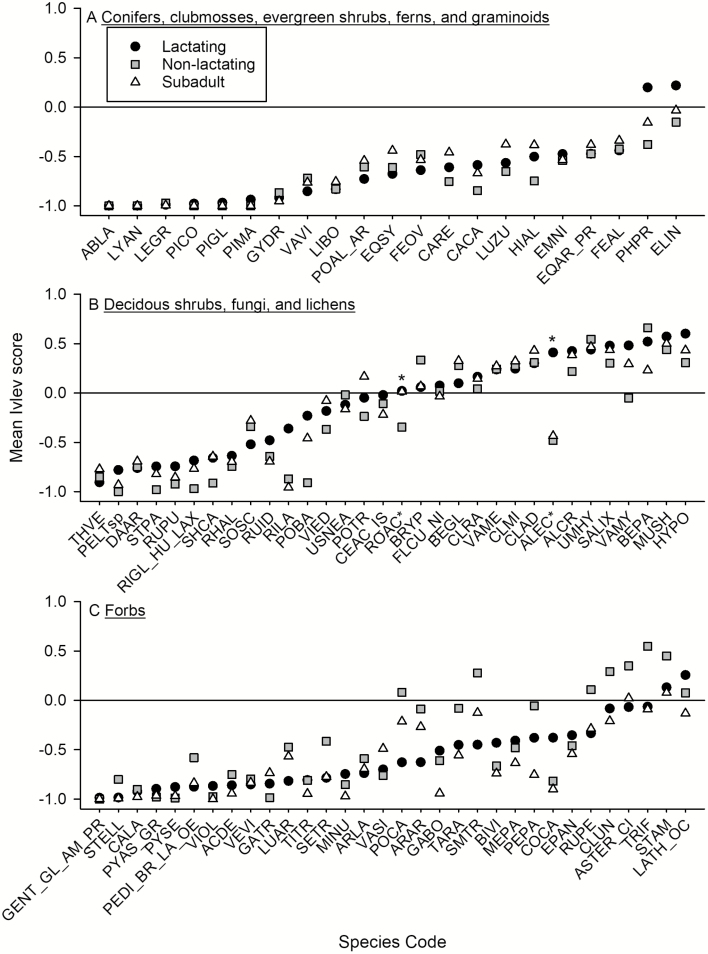
Kruskal–Wallis tests identified potential differences in forage selection for 22 of 103 forage species, but follow-up analyses using Wilcoxon Signed-Rank tests showed that selection differed among nutritional classes only for Alectoria spp. and Rosa acicularis (Fig. 2). Alectoria spp. was neutral to lactating adults but avoided by nonlactating adults and subadults; R. acicularis was neutral to lactating adults and subadults but avoided by nonlactating adults (Supplementary Data SD2). Both species accounted for < 5% of intake.
Fig. 2.
—Mean Ivlev scores for species of (A) conifers, clubmosses, evergreen shrubs, ferns, and graminoids; (B) deciduous shrubs, fungi, and lichens; and (C) forbs encountered by lactating adult, nonlactating adult, and subadult (1–2 years old) tame caribou (Rangifer tarandus) during summer in plant communities of northeastern British Columbia. Sample sizes by nutritional class for each plant species and a key to species codes are provided in Supplementary Data SD2. *indicates selection of these species (ALEC = Alectoria sp., ROAC = Rosa acicularis) was inconsistent among nutritional classes of caribou, and was either neutral or avoided (see text).
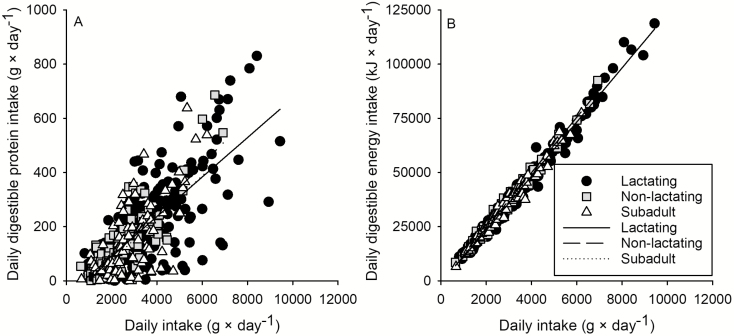
Daily intake of digestible protein by caribou was affected by daily intake of dry matter (P < 0.001; Fig. 3A), but the relationships were highly variable. Slopes of the nutritional class-specific lines (representing DDP because daily digestible protein intake = daily intake × DDP) did not differ (Table 2; all P ≥ 0.388). Daily digestible energy intake was strongly affected by daily intake (P < 0.001; Fig. 3B) and was significantly higher for lactating than nonlactating adult caribou (Table 2), but this difference averaged only ~0.2 kJ × g−1 higher. Including year as a covariate in a follow-up analysis of daily digestible energy intake and daily dry matter intake accounted for the apparent difference in DDE between lactating and nonlactating caribou (all nutritional classes P ≥ 0.807; Table 2). In addition, analysis of data from 2014 only, when all nutritional classes were exposed to identical foraging conditions, showed there were no differences in DDE or DDP across nutritional classes (marginal ± SE for lactating adults, nonlactating adults, and subadults: DDE [kJ × g−1] = 12.0 ± 0.1, 12.2 ± 0.1, 12.0 ± 0.1; DDP [g × 100 g−1] = 0.04 ± 0.01, 0.04 ± 0.01, 0.05 ± 0.01).
Fig. 3.
—Dietary digestible protein (A) and dietary digestible energy intakes (B) as a function of daily intakes of dry matter by lactating adult, nonlactating adult, and subadult (1–2 years old) tame caribou (Rangifer tarandus) during summer in plant communities of northeastern British Columbia. Sample sizes were n = 219, 108, 90 for lactating adult, nonlactating adult, and subadult (1–2 years old) caribou for daily foraging behaviors.
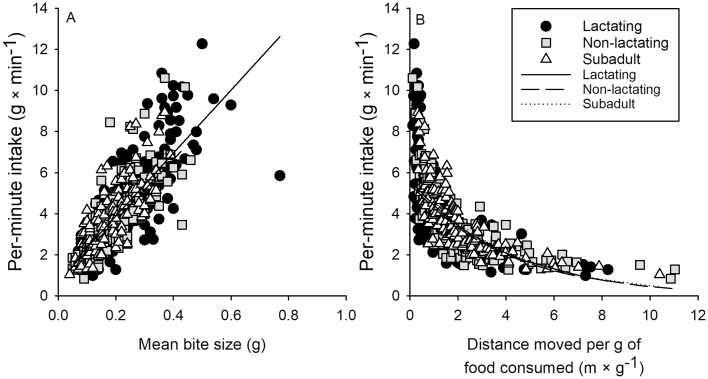
Differences in bite rates were assessed by exploring the relationship between bite size and per-minute intake because per-minute intake = bite size × bite rate. There was a strong linear relationship between bite size and per-minute intake rate (P < 0.001; Fig. 4A). Per-minute dry matter intake rates varied more than 12-fold (0.98–12.3 g × min−1) and average bite sizes obtained by caribou varied more than 19-fold among sites (range 0.04–0.77 g; Fig. 4A), but there were no differences in per-minute intake as a function of bite size among nutritional classes, indicating that bite rates were similar across all nutritional classes (Table 2; all P ≥ 0.120). Caribou moved between 0.1 and 11.0 m for every g of food consumed (P < 0.001; Fig. 4B). When per-minute intakes were low, caribou moved further for each g of food consumed (Fig. 4B), but there were no differences among nutritional classes of caribou in per-minute intakes as a function of distance moved per g of food consumed (Table 2; all P ≥ 0.342), indicating that travel rates were similar across nutritional classes of caribou.
Fig. 4.
—Per-minute intake as a function of (A) mean bite size and (B) distance moved per g of food consumed by lactating adult, nonlactating adult, and subadult (1–2 years old) tame caribou (Rangifer tarandus) during summer in plant communities of northeastern British Columbia. Sample sizes were n = 227, 154, 131 for lactating adults, nonlactating adults, and subadult (1–2 years old) caribou for per-minute foraging behaviors.
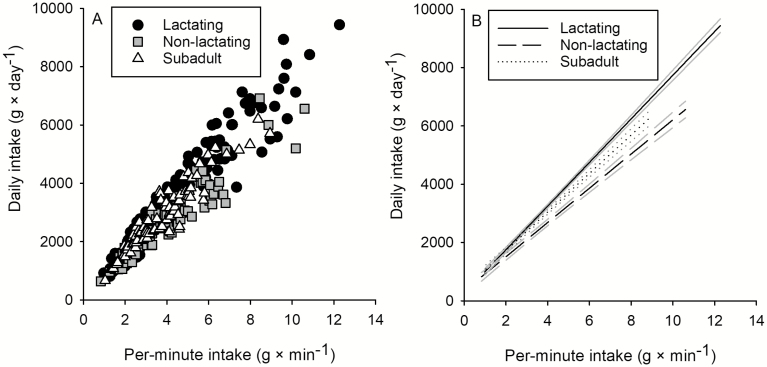
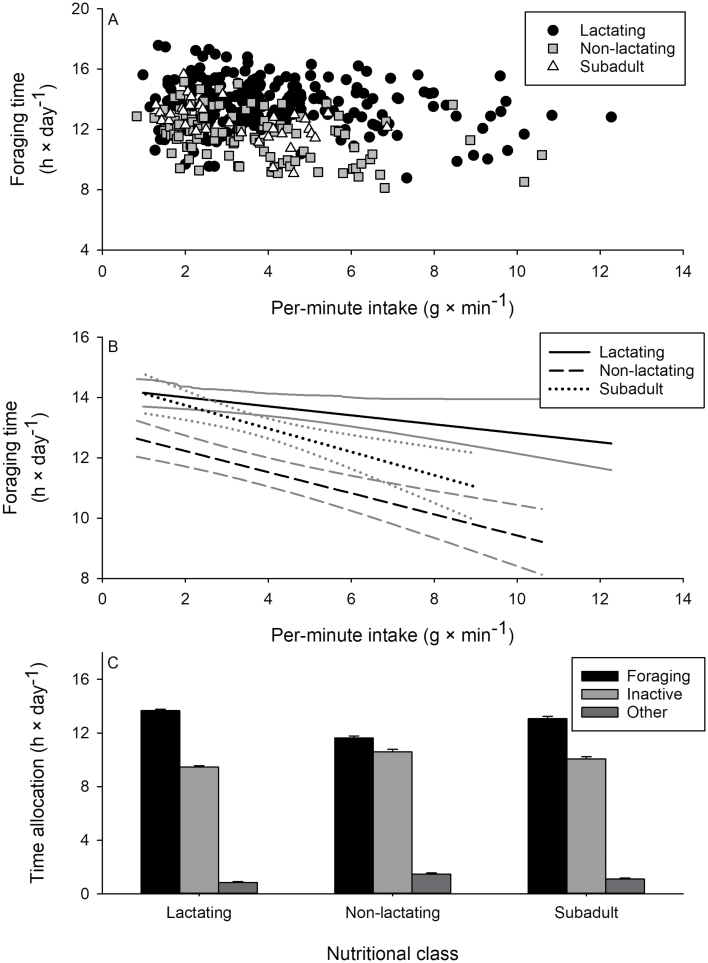
Differences in foraging time were assessed by exploring the relationship between daily dry matter intake and per-minute intake because daily dry matter intake = per-minute intake × foraging time. Daily dry matter intake by caribou increased linearly as a function of increasing per-minute intake and varied more than 14-fold among sites (666 – 9,437 g × day−1; P < 0.001; Fig. 5A). Nonlactating caribou had significantly lower daily intake rates than lactating animals (P = 0.002; Table 2; Fig. 5B)—a difference attributable to differences in foraging time (Fig. 6). Daily intakes by subadults were intermediate to lactating and nonlactating adults but did not significantly differ from either (both P ≥ 0.080; Fig. 5), nor did foraging times of subadults (as indicated by the relationship between daily and per-minute intake rates; Fig. 5; Table 2) differ from lactating or nonlactating adults. Caribou spent 8.1–17.6 h foraging per day, with lactating caribou having the highest maximum foraging time (17.6 h × day−1), followed by subadults (17.0 h × day−1), and nonlactating caribou (15.2 h × day−1; Figs. 6A and 6B). Across all sites sampled, lactating adults allocated an average of 2.1 h more to foraging each day than nonlactating adults and 0.6 h more than subadults ( ± SE: 13.7 ± 0.1 h, 11.6 ± 0.2 h, and 13.1 ± 0.2 h, respectively; Fig. 6C). Daily inactive time (e.g., standing, bedding, ruminating) ranged from 0.4 to 14.8 h and was greatest for nonlactating caribou (± SE: 10.6 ± 0.2 h), followed by subadult (9.9 ± 0.2 h) and lactating (9.4 ± 0.1 h) caribou. Time spent on other behaviors (e.g., vigilance, play, antler rubbing) ranged from 0 to 6.7 h per day and was greatest for nonlactating caribou (± SE: 1.5 ± 0.1 h), followed by subadult (1.1 ± 0.1 h) and lactating (0.9 ± 0.1 h) caribou (Fig. 6C).
Fig. 5.
—Daily intake by lactating adult, nonlactating adult, and subadult (1–2 years old) tame caribou (Rangifer tarandus), during summer in plant communities of northeastern British Columbia, as a function of (A) per-minute intake and (B) fitted lines (black) of daily intake as a function of per-minute intake (shown with 95% confidence intervals [gray] of the predicted values) differed between lactating and nonlactating adult caribou, but subadults did not differ from other classes (see Table 2). Sample sizes were n = 219, 108, 90 for lactating adult, nonlactating adult, and subadult (1–2 years old) caribou for daily foraging behaviors.
Fig. 6.
—Foraging time of lactating adult, nonlactating adult, and subadult (1–2 years old) tame caribou (Rangifer tarandus), during summer in plant communities of northeastern British Columbia, as a function of (A) per-minute intake; differences in foraging time among nutritional classes of caribou are shown with 95% confidence intervals of the predictions in gray (B). Mean ± SE daily activity budgets are shown (C) for lactating adults, nonlactating adults, and subadult caribou. Sample sizes were n = 219, 108, 90 for lactating adult, nonlactating adult, and subadult (1–2 years old) caribou for daily foraging behaviors.
To understand the relative contribution of differences in foraging time between lactating and nonlactating adult caribou (Fig. 6B) to the differences we observed in daily intakes of dry matter, digestible energy, and digestible protein, we conducted a follow-up analysis using a multilevel model. Equation 3 is the difference in foraging time between nonlactating and lactating caribou from that multilevel model:
| (3) |
where y is foraging time (in hours), x is per-minute intake of dry matter, and nutr is nutritional class of caribou with lactating = 0 and nonlactating = 1 (coefficient ± SE: 14.6 ± 0.24, −0.207 ± 0.04, and −2.13 ± 0.28). Adjusting foraging time of nonlactating caribou to that of lactating caribou using equation 3 removed all effects of nutritional class on daily intakes of dry matter, digestible energy, and digestible protein (Supplementary Data SD3).
Discussion
Physiological state is thought to be an important consideration in understanding foraging behavior (Mangel and Clark 1986; Newman et al. 1995). We evaluated whether caribou foraged at or near their maximum capacity regardless of the nutritional resources available to them (energy-maximizing strategy), or only enough to meet requirements (time-minimizing strategy). Fine-scale foraging responses (per-minute intake, per-minute bite rate, travel rate, DDE, DDP, and selection among forage species; Figs. 2–4) did not differ among lactating, nonlactating, and subadult caribou. Instead, effects of nutritional requirements manifested through differences in foraging time. Caribou with lower nutritional requirements spent less time foraging than those with higher requirements, even when they were unable to satisfy nutritional requirements through foraging; hence, foraging time of nonlactating caribou likely was more limited by digestive constraints than was foraging time of lactating caribou. At sites where caribou could meet or exceed nutritional requirements, caribou of all nutritional classes continued foraging even after satisfying nutritional requirements. Combined, these results suggest that foraging by our caribou was partially state-dependent, the ability of caribou to satisfy daily intake requirements was limited by digestive constraints (in addition to attributes of the forage base that influence foraging responses), and our caribou were energy maximizers.
Forage selection, bite rates, per-minute intakes, dietary quality, and travel rates were not state-dependent in our caribou. Regardless of nutritional class, our caribou exhibited strong patterns of selection among plant taxa that resulted in maintaining relatively high-quality diets across most plant communities (Denryter 2017). Highly selective foraging by caribou in our study (Denryter et al. 2017) and by free-ranging caribou across their circumpolar range (e.g., Bergerud 1972; White and Trudell 1980; Boertje 1984; Russell et al. 1993) resulting in high-quality diets may enhance digestion rates and allow for high levels of nutrient intake (Weston and Poppi 1987; Spalinger et al. 1988; Minson and Wilson 1994; Gray and Servello 1995). Selective foraging and its influence on fine-scale foraging behaviors also may scale up to influence larger-scale decisions, such as habitat use and selection (Fig. 1; Cook et al. 2018). As selection is the base of the foraging hierarchy, consistency in selection of forages likely explains why we failed to detect differences in bite rates, per-minute intakes, and quality of diets of caribou with different levels of nutritional requirements.
Unlike dietary quality and per-minute foraging behaviors, daily foraging behaviors (time spent foraging, daily intake) of our caribou were state-dependent. As predicted, caribou with higher nutritional requirements spent more time foraging and as a result achieved higher daily intakes than caribou with lower nutritional requirements. Our finding that foraging time was state-dependent and increased with increasing nutritional requirements is consistent with reports for red deer (Cervus elaphus—Clutton-Brock et al. 1982) and for late-summer and autumn foraging budgets of bighorn sheep (Ovis canadensis—Ruckstuhl and Festa-Bianchet 1998). In enclosures where even nonlactating animals (with the lowest nutritional requirements of any class) could not satisfy their nutritional requirements, they increased foraging time, but still foraged ~1.5 h × day−1 less than lactating caribou. Failure to increase foraging time sufficiently to compensate for low intake rates in nutritionally inadequate plant communities also has been reported for domestic sheep (Ovis aries—Allden and McDWhittaker 1970), cattle (Bos taurus—Chacon and Stobbs 1986), and elk (Cook et al. 2016). Because accepting a negative nutritional balance seems maladaptive, nonlactating caribou likely were physically unable to process as much food as lactating caribou, owing to greater digestive constraints (Tulloh 1966; Owen-Smith 2002; Blanchard 2005; Zimmerman et al. 2006; Luna and Weckerly 2013). Overall, total daily foraging time and the upper daily limit of foraging time were state-dependent, and nonlactating caribou likely were more constrained by digestion than were lactating caribou.
Although foraging time was state-dependent, foraging strategy was not—all our caribou behaved as energy maximizers. Differences in foraging time among nutritional classes (e.g., lactating > nonlactating) could be interpreted as evidence of time minimization, but our caribou continued to forage even after satisfying nutritional requirements. Basing conclusions of foraging strategy solely on foraging time is problematic because it ignores the predominant role of digestive constraints in limiting daily foraging time and intake in ruminants. Rumination is an obligation that requires ≥ 4–12.6 h × day−1 (Renecker and Hudson 1989; Coleman et al. 2003; Schirmann et al. 2013; Soriani et al. 2013; Cook et al. 2016) and, when combined with allocation of time to other behaviors (e.g., moving, lying, standing, vigilance, etc.—Boertje 1985; Russell et al. 1993), sets upper physiological limits on daily foraging time (Penning et al. 1991; Illius 1997) of 7–17 h. Further, ruminants must curtail daily intake to “allow digestion to catch up” (Owen-Smith 2002). The substantial effects of digestion rate on dry matter intake were illustrated by Owen-Smith (2002) as follows: a 100-kg ruminant theoretically could consume 17.3 kg of dry food, but can only digest up to ~3.5 kg (3.5% of its body mass) × day−1; hence, digestion is the limitation. Limits to digestion also may explain why as daily dry matter intake increased, foraging time declined for all caribou; at high intake rates (e.g., 8 g × min−1), caribou consumed three to four times more food each day than at low intake rates (e.g., 2 g × min−1; Fig. 5). Given digestive constraints and upper daily limits of foraging time, our caribou likely spent as much time as possible foraging each day.
One might question whether data from captive animals are relevant to their wild counterparts. Certainly, predation risk and hordes of biting insects that caribou typically face represent strong evolutionary pressures, and wild caribou undoubtedly spend time avoiding both when these threats are imminent to a greater degree than did our caribou. By avoiding confounding effects of predation risk and insect harassment, however, we evaluated whether natural selection favored the inherent tendency of caribou to behave as time minimizers or energy maximizers. Further, we required estimates of bite rate, bite mass, dietary quality, foraging time, daily digestible protein and energy intakes, and nutritional class of the caribou, all of which can be difficult or impossible to measure accurately using wild animals. Hence, tame caribou provided data at the level of detail needed to identify misleading indicators of a time-minimizing strategy. For example, instead of incorrectly concluding that caribou were time minimizers because of differences in foraging time relative to nutritional requirements, we showed that at sites where caribou met or exceeded daily nutritional requirements, caribou of all nutritional classes continued foraging even after satisfying daily nutritional requirements.
Using a time-minimizing strategy when threats are not imminent may be disadvantageous. Certainly, if predation is an imminent threat, time spent foraging will decline in the short-term (e.g., Middleton et al. 2013), but this observation provides little support for the time-minimizing hypothesis. In fact, the more frequently threats are imminent, the more important using an energy-maximizing strategy may become, because in addition to satisfying nutritional requirements for maintenance of body tissues, caribou need to accumulate fat reserves during summer if they are to breed in autumn and survive winter. Maximizing energy intake may represent an adaptation to marginal and variable forage conditions, which may be the norm in northern regions where the growing season is short, nutritional resources are variable, and nutritional resources on summer ranges are often inadequate to support lactation and juvenile growth (Hurley et al. 2014; Cook et al. 2016; Denryter 2017). Rather than employing a time-minimizing strategy to avoid predators, animals may alter broad-scale movement or landscape-use patterns to offset potential costs associated with an energy-maximizing strategy (Fischhoff et al. 2007; Kauffman et al. 2007; Hebblewhite et al. 2008; Middleton et al. 2013; Demars and Boutin 2017).
Understanding how animals forage relative to their requirements remains a relevant topic in ungulate nutritional ecology (e.g., Bergman et al. 2001; Kohli et al. 2014; Long et al. 2014). The importance of considering an animal’s state and how it may influence performance is increasingly recognized (Gerhart et al. 1997; Cook et al. 2004, 2013; Tollefson et al. 2010; Monteith et al. 2014; Jesmer et al. 2017). Our findings demonstrate that interpreting foraging strategy without physiological and ecological contexts (e.g., physiological state of animals and nutritional values of plant communities) could provide misleading results. Such misinterpretations potentially could lead to misunderstandings of the adaptive significance of foraging decisions relative to habitat selection and thus habitat management, forage-predation trade-offs, and recruitment, with implications for conservation and management of populations (Fig. 1). Other studies have reported that lactating and nonlactating ungulates use the landscape in dissimilar ways (Barten et al. 2001; Walker et al. 2006), likely reflecting different nutritional constraints and responses to predation risk. Spacing away from predators may explain higher-order selection (e.g., selection of home ranges) by caribou (Rettie and Messier 2000; Gustine et al. 2006; Demars 2015), but forage quality influences selection within home ranges (Barten et al. 2001; Gustine et al. 2006). Given the highly selective foraging behavior of caribou, and the fact that there was no apparent cost to dietary quality in divergent strategies of habitat use (Barten et al. 2001), it is plausible that differences in daily rates of foraging time and forage intake underpin differences in habitat use and other large-scale processes (Fig. 1). Hence, accounting for physiological state will contribute to a better understanding of why animals use the landscape as they do and will provide an ecological context for conservation of caribou, a species at risk in Canada.
Supplementary Data
Supplementary data are available at Journal of Mammalogy online.
Supplementary Data SD1.—Calibration of activity data from accelerometers with behavior of caribou.
Supplementary Data SD2.—Selection index for forages consumed by caribou with different levels of nutritional requirements.
Supplementary Data SD3.—Foraging time of and intakes by caribou with different levels of nutritional requirements.
Acknowledgments
The National Council for Air and Stream Improvement (NCASI) Canada, Montreal, Quebec and its member companies funded the costs of animal care and field work. BC Habitat Conservation Trust Foundation (HCTF), Sustainable Forestry Initiative (SFI), the University of Northern British Columbia, and the W. Garfield Weston Foundation Fellowship Program, a program of the Wildlife Conservation Society Canada funded by the W. Garfield Weston Foundation provided additional funds. KD was supported by an Industrial Post-graduate Scholarship (2) from the National Science and Engineering Research Council co-funded by NCASI. We thank Y. Allen, S. Black, S. Blake, A. Brar, N. Dimond, E. Garrett, K. Hawkshaw, C. Herc, D. Lancaster, M. Olivos, O. Van Jarrett, D. Walsh, and D. White for field assistance; A. Keim and B. Keim for contributions to animal care; and H. Schwantje, B. Macbeth, O. Slater, and staff at the North Peace Veterinary Clinic for contributions to veterinary care.
Literature Cited
- Allden W. G., and McDWhittaker I. A.. . 1970. The determinants of herbage intake by grazing sheep: the interrelationship of factors influencing herbage intake and availability. Australian Journal of Agricultural Research 21:755–766. [Google Scholar]
- Barboza P. S., and Parker K. L.. . 2006. Body protein stores and isotopic indicators of N balance in female reindeer (Rangifer tarandus) during winter. Physiological and Biochemical Zoology 79:628–644. [DOI] [PubMed] [Google Scholar]
- Barten N. L., Bowyer R. T., and Jenkins K. J.. . 2001. Habitat use by female caribou: tradeoffs associated with parturition. Journal of Wildlife Management 65:77–92. [Google Scholar]
- Belovsky G. E. 1986. Optimal foraging and community structure: implications for a guild of generalist grassland herbivores. Oecologia 70:35–52. [DOI] [PubMed] [Google Scholar]
- Bergerud A. T. 1972. Food habits of Newfoundland caribou. Journal of Wildlife Management 36:913–923. [Google Scholar]
- Bergman C. M., Fryxell J. M., Gates C. C., and Fortin D.. . 2001. Ungulate foraging strategies: energy maximizing or time minimizing? Journal of Animal Ecology 70:289–300. [Google Scholar]
- Blanchard P. 2005. On lactation and rumination in bighorn ewes (Ovis canadensis). Journal of Zoology 265:107–112. [Google Scholar]
- Boertje R. D. 1984. Seasonal diets of the Denali caribou herd, Alaska. Arctic 37:161–165. [Google Scholar]
- Boertje R. D. 1985. Seasonal activity of the Denali caribou herd, Alaska. Rangifer 5:32–42. [Google Scholar]
- Briand Y., Ouellet J. P., Dussault C., and St. Laurent M. H.. . 2009. Fine-scale habitat selection by female forest-dwelling caribou in managed boreal forest: empirical evidence of a seasonal shift between foraging opportunities and antipredator strategies. Ecoscience 16:330–340. [Google Scholar]
- Campling R. C. 1970. Physical regulation of voluntary intake. Pp. 226–234 in Physiology of digestion and metabolism in the ruminant (Phillipson A. J., ed.). Oriel Press; Newcastle, New South Wales, Australia. [Google Scholar]
- Chacon E., and Stobbs T. H.. . 1986. Influence of progressive defoliation of a grass sward on the eating behaviour of cattle. Australian Journal of Agricultural Research 27:709–727. [Google Scholar]
- Clutton-Brock T. H., Iason G. R., Albon S. D., and Guiness F. E.. . 1982. The effects of lactation on feeding behavior in wild red deer hinds. Journal of Zoology 198:227–236. [Google Scholar]
- Coleman S. W., Hart S. P., and Sahlu T.. . 2003. Relationships among forage chemistry, rumination and retention time with intake and digestibility of hay by goats. Small Ruminant Research 50:129–140. [Google Scholar]
- Cook J. G., Johnson B. K., Cook R. C., Riggs R. A., Bryant L. D., and Irwin L. L.. . 2004. Effects of summer-autumn nutrition and parturition date on reproduction and survival of elk. Wildlife Monographs 155:1–61. [Google Scholar]
- Cook R. C., et al. 2013. Regional and seasonal patterns of nutritional condition and reproduction in elk. Wildlife Monographs 184:1–45. [Google Scholar]
- Cook J. G., Cook R. C., Davis R. W., and Irwin L. L.. . 2016. Nutritional ecology of elk during summer and autumn in the Pacific Northwest. Wildlife Monographs 195:1–81. [Google Scholar]
- Cook J. G., et al. 2018. Development and evaluation of a landscape nutrition model for elk in western Oregon and Washington. Pp. 13–30 in Modeling elk nutrition and habitat use in western Oregon and Washington. Wildlife Monographs 199:1–69. [Google Scholar]
- DeLong C. A., MacKinnon A., and Jang L.. . 1990. A field guide for identification and interpretation of ecosystems of the northeast portion of the Prince George forest region: land management handbook No. 22. British Columbia Ministry of Forests; Prince George, British Columbia, Canada. [Google Scholar]
- Demars C. A. 2015. Calving behavior of boreal caribou in a multi-predator, multi-use landscape. Ph.D. dissertation, University of Alberta; Edmonton, Alberta, Canada. [Google Scholar]
- Demars C. A., and Boutin S.. . 2017. Nowhere to hide: effects of linear features on predator–prey dynamics in a large mammal system. Journal of Animal Ecology 87:274–284. [DOI] [PubMed] [Google Scholar]
- Denryter K. 2017. Foraging ecology of woodland caribou in boreal and montane ecosystems of northern British Columbia. Ph.D. dissertation, University of Northern British Columbia; Prince George. British Columbia, Canada. [Google Scholar]
- Denryter K., Cook R. C., Cook J. G., and Parker K. L.. . 2017. Straight from the caribou’s (Rangifer tarandus) mouth: detailed observations of tame caribou reveal new insights into summer–autumn diets. Canadian Journal of Zoology 95:81–94. [Google Scholar]
- Dussault C., Pinard V., Ouellet J. P., Courtois R., and Fortin D.. . 2012. Avoidance of roads and selection for recent cutovers by threatened caribou: fitness-rewarding or maladaptive behaviour? Proceedings of the Royal Society of London, B. Biological Sciences 279:4481–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff I. R., Sundaresan S. R., Cordingley J., and Rubenstein D. I.. . 2007. Habitat use and movements of plains zebra (Equus burchelli) in response to predation danger from lions. Behavioral Ecology 18:725–729. [Google Scholar]
- Forchhammer M. C., and Boomsma J. J.. . 1995. Foraging strategies and seasonal diet optimization of muskoxen in West Greenland. Oecologia 104:169–180. [DOI] [PubMed] [Google Scholar]
- Gerhart K. L., White R. G., Cameron R. D., Russell D. E., and Van De Wetering D.. . 1997. Pregnancy rate as an indicator of nutritional status in Rangifer: implications of lactational infertility. Rangifer 17:21–24. [Google Scholar]
- Goering H. K., and Van Soest P. J.. . 1970. Forage fiber analysis. United States Department of Agriculture; Washington, D.C. [Google Scholar]
- Gray P. B., and Servello F. A.. . 1995. Energy intake relationships for white-tailed deer on winter browse diets. Journal of Wildlife Management 59:147–152. [Google Scholar]
- Gustine D. D., Parker K. L., Lay R. J., Gillingham M. P., and Heard D. C.. . 2006. Calf survival of woodland caribou in a multi-predator ecosystem. Wildlife Monographs 165:1–32. [Google Scholar]
- Hebblewhite M., Merrill E., and McDermid G.. . 2008. A multi-scale test of the forage maturation hypothesis in a partially migratory ungulate population. Ecological Monographs 78:141–166. [Google Scholar]
- Hobbs N. T., Gross J. E., Shipley L. A., Spalinger D. E., and Wunder B. A.. . 2003. Herbivore functional response in heterogeneous environments: a contest among models. Ecology 84:666–681. [Google Scholar]
- Hurley M. A., et al. 2014. Functional analysis of normalized difference vegetation index curves reveals overwinter mule deer survival is driven by both spring and autumn phenology. Philosophical Transactions of the Royal Society of London, B. Biological Sciences 369:20130196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illius A. W. 1997. Advances and retreats in specifying the constraints on intake in grazing ruminants. Proceedings of XVIII International Grassland Congress 3:39–44. [Google Scholar]
- Illius A. W., Albon S. D., Pemberton J. M., and Gordon I. J.. . 1995. Selection for foraging efficiency during a population crash in Soay sheep. Journal of Animal Ecology 64:481–492. [Google Scholar]
- Ivlev V. S. 1961. Experimental ecology of the feeding of fishes. Yale University Press; New Haven, Connecticut. [Google Scholar]
- Jesmer B. R., Goheen J. R., Monteith K. L., and Kauffman M. J.. . 2017. State-dependent behavior alters endocrine-energy relationship: implications for conservation and management. Ecological Applications 27:2303–2312. [DOI] [PubMed] [Google Scholar]
- Johnson C. J., Ehlers L. P. W., and Seip D. R.. . 2015. Witnessing extinction — cumulative impacts across landscapes and the future loss of an evolutionarily significant unit of woodland caribou in Canada. Biological Conservation 186:176–186. [Google Scholar]
- Kauffman M. J., Varley N., Smith D. W., Stahler D. R., MacNulty D. R., and Boyce M. S.. . 2007. Landscape heterogeneity shapes predation in a newly restored predator-prey system. Ecology Letters 10:690–700. [DOI] [PubMed] [Google Scholar]
- Kohli M., Sankaran M., Suryawanshi K. R., and Mishra C.. . 2014. A penny saved is a penny earned: lean season foraging strategy of an alpine ungulate. Animal Behaviour 92:93–100. [Google Scholar]
- Liesenjohann T., et al. 2015. State-dependent foraging: lactating voles adjust their foraging behavior according to the presence of a potential nest predator and season. Behavioral Ecology and Sociobiology 69:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long R. A., Terry Bowyer R., Porter W. P., Mathewson P., Monteith K. L., and Kie J. G.. . 2014. Behavior and nutritional condition buffer a large-bodied endotherm against direct and indirect effects of climate. Ecological Monographs 84:513–532. [Google Scholar]
- Luna R. S., and Weckerly F. W.. . 2013. Variation across years in rumen-reticulum capacity and digesta load in white-tailed deer (Odocoileus virginianus). Southeastern Naturalist 12:283–296. [Google Scholar]
- Mackenzie W. 2012. Biogeoclimatic ecosystem classification of non-forested ecosystems in British Columbia. Technical Report 68. British Columbia Ministry of Forests, Lands, and Natural Resource Operations; Victoria, British Columbia, Canada. [Google Scholar]
- Mangel M., and Clark C. W.. . 1986. Toward a unified foraging theory. Ecology 67:1127–1138. [Google Scholar]
- Martin J. S., and Martin M. M.. . 1983. Tannin assays in ecological studies: precipitation of ribulose-1,5-bisphosphate carboxylase/oxygenase by tannic acid, quebracho, and oak foliage extracts. Journal of Chemical Ecology 9:285–294. [DOI] [PubMed] [Google Scholar]
- Massé A., and Côté S. D.. . 2012. Linking habitat heterogeneity to space use by large herbivores at multiple scales: from habitat mosaics to forest canopy openings. Forest Ecology and Management 285:67–76. [Google Scholar]
- McNamara J. M., and Houston A. I.. . 1986. The common currency for behavioral decisions. The American Naturalist 127:358–378. [Google Scholar]
- Middleton A. D., et al. 2013. Animal migration amid shifting patterns of phenology and predation: lessons from a Yellowstone elk herd. Ecology 94:1245–1256. [DOI] [PubMed] [Google Scholar]
- Minson D. J., and Wilson J. R.. . 1994. Prediction of intake as an element of forage quality. Pp. 533–563 in Forage quality, evaluation and utilization (Fahey G. C., ed.). American Society of Agronomy; Madison, Wisconsin. [Google Scholar]
- Monteith K. L., et al. 2014. Life-history characteristics of mule deer: effects of nutrition in a variable environment. Wildlife Monographs 186:1–61. [Google Scholar]
- National Research Council. 2007. Nutritional requirements of small ruminants: sheep, goats, cervids, and new world camelids. The National Academies Press; Washington DC. [Google Scholar]
- Newman J. A., Parsons A. j, Thornley J. H. M., Penning P. D., and Krebs J. R.. . 1995. Optimal diet selection by a generalist grazing herbivore. Functional Ecology 63:465–478. [Google Scholar]
- Olsson O., Brown J. S., and Smith H. G.. . 2002. Long- and short-term state-dependent foraging under predation risk: an indication of habitat quality. Animal Behaviour 63:981–989. [Google Scholar]
- Owen-Smith N. 2002. Adaptive herbivore ecology: from resources to populations in variable environments. Cambridge University Press; Cambridge, United Kingdom. [Google Scholar]
- Parker K. L., and Barboza P. S.. . 2013. Hand-rearing wild caribou calves for studies of nutritional ecology. Zoo Biology 32:163–171. [DOI] [PubMed] [Google Scholar]
- Parker K. L., Barboza P. S., and Gillingham M. P.. . 2009. Nutrition integrates environmental responses of ungulates. Functional Ecology 23:57–69. [Google Scholar]
- Penning P. D., Parsons A. J., Orr R. J., and Treacher T. T.. . 1991. Intake and behaviour responses by sheep to changes in sward characteristics under continuous stocking. Grass and Forage Science 46:15–28. [Google Scholar]
- Rands S. A., Pettifor R. A., Rowcliffe J. M., and Cowlishaw G.. . 2011. State-dependent foraging rules for social animals in selfish herds. Proceedings of the Royal Society of London, B. Biological Sciences 271:2613–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renecker L. A., and Hudson R. J.. . 1989. Seasonal activity budgets of moose in aspen-dominated boreal forests. Journal of Wildlife Management 53:296–302. [Google Scholar]
- Rettie W. J., and Messier F.. . 2000. Hierarchical habitat selection by woodland caribou: its relationship to limiting factors. Ecography 23:466–478. [Google Scholar]
- Robbins C. T. 1993 Wildlife feeding and nutrition. 2nd ed. Academic Press Inc; San Diego, California. [Google Scholar]
- Robbins C. T., et al. 1987a. Role of tannins in defending plants against ruminants: reduction in protein availability. Ecology 68:98–107. [DOI] [PubMed] [Google Scholar]
- Robbins C. T., Mole S., Hagerman A. E., and Hanley T. A.. . 1987b. Role of Tannins in defending plants against ruminants: reduction in dry matter digestion? Ecology 68:1606–1615. [DOI] [PubMed] [Google Scholar]
- Ruckstuhl K. E., and Festa-Bianchet M.. . 1998. Do reproductive status and lamb gender affect the foraging behavior of bighorn ewes? Ethology 104:941–954. [Google Scholar]
- Russell D. E., Martell A. M., and Nixon W. A. C.. . 1993. Range ecology of the Porcupine caribou herd in Canada. Rangifer Special Issue 13:1–170. [Google Scholar]
- Schaefer J. A., Bergman C. M., and Luttich S. N.. . 2000. Site fidelity of female caribou at multiple spatial scales. Landscape Ecology 15:731–739. [Google Scholar]
- Schirmann K., Chapinal N., Weary D. M., Vickers L., and von Keyserlingk M. A. G.. . 2013. Short communication: rumination and feeding behavior before and after calving in dairy cows. Journal of Dairy Science 96:7088–7092. [DOI] [PubMed] [Google Scholar]
- Schoener T. W. 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics 2:369–404. [Google Scholar]
- Senft R. L., Coughenour M. B., Bailey D. W., Rittenhouse L. R., Sala O. E., and Swift D. M.. . 1987. Large herbivore foraging and ecological hierarchies. BioScience 37:789–799. [Google Scholar]
- Shipley L. A. 2007. The influence of bite size on foraging at larger spatial and temporal scales by mammalian herbivores. Oikos 116:1964–1974. [Google Scholar]
- Short J. 1985. The functional response of kangaroos, sheep, and rabbits in an arid grazing system. Journal of Applied Ecology 22:435–447. [Google Scholar]
- Shuai L. Y., Zhang Z. R., and Zeng Z. G.. . 2017. When should I be aggressive? A state-dependent foraging game between competitors. Behavioral Ecology 28:471–478. [Google Scholar]
- Sikes R. S., and T he A nimal C are and U se C ommittee of the A merican S ociety of M ammalogists. 2016. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97:663–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. J., Sibly R. M., Lee K. P., Behmer S. T., and Raubenheimer D.. . 2004. Optimal foraging when regulating intake of multiple nutrients. Animal Behaviour 68:1299–1311. [Google Scholar]
- Skrondal A., and Rabe-Hesketh S.. . 2004. Generalized latent variable modeling: multilevel, longitudinal, and structural equation models. Chapman & Hall; New York. [Google Scholar]
- Soriani N., Panella G., and Calamari L.. . 2013. Rumination time during the summer season and its relationships with metabolic conditions and milk production. Journal of Dairy Science 96:5082–5094. [DOI] [PubMed] [Google Scholar]
- Spalinger D. E., Hanley T. A., and Robbins C. T.. . 1988. Analysis of the functional response in foraging in the Sitka black-tailed deer. Ecology 69:1166–1175. [Google Scholar]
- Stephens D. W. 1981. The logic of risk-sensitive foraging preferences. Animal Behaviour 29:628–629. [Google Scholar]
- Stephens D. W., and Krebs C. J.. . 1986. Foraging theory. Princeton University Press; Princeton, New Jersey. [Google Scholar]
- Tollefson T. N., Shipley L. A., Myers W. L., and Keisler D. H.. . 2010. Influence of summer and autumn nutrition on body condition and reproduction in lactating mule deer. Journal of Wildlife Management 74:974–986. [Google Scholar]
- Trudell J., and White R. G.. . 1981. The effect of forage structure and availability on food intake, biting rate, bite size, and daily eating time of reindeer. Journal of Applied Ecology 18:63–81. [Google Scholar]
- Tulloh N. M. 1966. Physical studies of the alimentary tract of grazing cattle: IV. Dimensions of the tract in lactating and non-lactating cows. New Zealand Journal of Agricultural Research 9:999–1008. [Google Scholar]
- Vivas H. J., and Saether B. E.. . 1987. Interactions between a generalist herbivore, the moose Alces alces and its food resources: an experimental study of winter foraging behaviour in relation to browse availability. Journal of Animal Ecology 56:509–520. [Google Scholar]
- Walker A. B. D., Parker K. L., and Gillingham M. P.. . 2006. Behaviour, habitat associations, and intrasexual differences of female Stone’s sheep. Canadian Journal of Zoology 84:1187–1201. [Google Scholar]
- Wallmo O. C., and Neff D. J.. . 1970. Direct observations of tamed deer to measure their consumption of natural forage. Pp. 105–110 in Proceedings of Range and Wildlife Habitat Evaluation—A Research Symposium, Flagstaff, Arizona.
- Weston R. H., and Poppi D. P.. . 1987. Comparative aspects of food intake. Pp. 133–161 in The nutrition of herbivores (Hacker J. B. and Ternouth J. H., eds.). Academic Press; Sydney, New South Wales, Australia. [Google Scholar]
- White R. G. 1983. Foraging patterns and their multiplier effects on productivity of northern ungulates. Oikos 40:377–384. [Google Scholar]
- White R. G., and Trudell J.. . 1980. Habitat preference and forage consumption by reindeer and caribou near Atkasook, Alaska. Arctic and Alpine Research 12:511–529. [Google Scholar]
- Wickstrom M. L., Robbins C. T., Hanley T. A., Spalinger D. E., and Parish S. M.. . 1984. Food intake and foraging energetics of elk and mule deer. Journal of Range Management 48:1285–1301. [Google Scholar]
- Zimmerman T. J., Jenks J. A., and Leslie D. M.. . 2006. Gastgrointestinal morphology of female white-tailed and mule deer: effects of fire, reproduction, and feeding type. Journal of Mammalogy 87:598–605. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.