Abstract
Knowledge as to the taxonomic status of enigmatic bat species often is hindered by limited availability of specimens. This is particularly true for aerial-hawking bats that are difficult to catch. One such species, “Hypsugo” joffrei, was originally described in Nyctalus due to its long and slender wings, but subsequently transferred to Pipistrellus, and most recently to Hypsugo, on the basis of morphology. Analysis of newly available material, which more than doubles the known specimens of this taxon, demonstrates that it is morphologically and genetically distinct from all other bat genera. We accordingly describe it as belonging to a new, monotypic genus. We provide a detailed description of its external and craniodental traits, measurements, and assessment of genetic relationships, including barcode sequences to facilitate its rapid identification in future. The new genus belongs to a group that includes the recently described Cassistrellus, as well as Tylonycteris, and its closest relative, Philetor. We also describe the echolocation calls emitted by members of the taxon in different situations, which may facilitate finding them in previously unsampled locations. Based on the new data, the species occurs from Nepal to North Vietnam and China, which suggests that it could be more widespread than previously thought.
Keywords: Indomalayan region, mtDNA, nuDNA, phylogeny, systematics, Vespertilionini
The taxonomic assignment of Hypsugo joffrei (Thomas, 1915) and its presumed allies has long posed a challenge to bat taxonomists. The taxon was originally described from the Kachin Hills of Myanmar (extending as a N–S spine from ca. 27.07°N to 25.47°N at elevations from ca. 400 to 3,411 m a.s.l.) and placed in the genus Nyctalus on account of the proportions of the digits. It was subsequently transferred to Pipistrellus by Tate (1942), where it was the nominate species of the Pipistrellus joffrei group, constituted by P. anthonyi (described in the same work), P. stenopterus, and Philetor brachypterus. Tate (1942:252) also remarked that the group might later be elevated to subgeneric rank. Ellerman and Morrison-Scott (1951) returned joffrei to Nyctalus based on the original description, although the authors retained the closely related anthonyi in its original genus, Pipistrellus. Hill (1966:387), in his review of the genus Philetor, followed Tate (1942) in retaining joffrei in Pipistrellus, and stated that “a review of the structural features of Philetor and of other genera and species to which relationship has been postulated hitherto indicates that its affinities are with the joffrei group of Pipistrellus and with the genus Tylonycteris.” The view that joffrei belongs in Pipistrellus also was accepted by Koopman (1973) and supported by Hill and Harrison (1987), the latter of whom introduced the stenopterus group as part of the subgenus Hypsugo of Pipistrellus based on the bacular characters of the nominate species, P. stenopterus. However, because they were only able to study the penial bone of stenopterus, the two other members of this group, anthonyi and joffrei, were allocated on the basis of craniodental similarities alone. This arrangement was followed by Corbet and Hill (1992), whereas Koopman (1994) placed joffrei in Nyctalus, together with anthonyi and stenopterus. Subsequently, Simmons (2005) placed joffrei and anthonyi in Hypsugo and retained stenopterus in Pipistrellus. On the basis of investigation of all material then available, Saikia et al. (2017) synonymized anthonyi with H. joffrei. Most recently, however, in detailing the taxonomic status of Pipistrellus stenopterus, Kruskop et al. (2018) showed definitively that stenopterus and joffrei belong to quite distant phylogenetic lineages within the Vespertilioninae, and included molecular data from the mitochondrial Co1 locus to the effect that separation of joffrei from genuine Hypsugo was warranted.
Until four specimens were captured in the Hoan Lien Son mountain range of North Vietnam by Kruskop and Shchinov (2010), H. joffrei (including anthonyi) had been known for almost a century only from Myanmar (Bates et al. 2005). The former authors—based on morphological investigations—noted the ambiguous generic status of the species and called for an in-depth taxonomic reassessment. A further range extension was published by Saikia et al. (2017), who revised the South Asian records of P. brachypterus and showed that individuals previously reported from Nepal and Sikkim in fact represented H. joffrei. They also reported a new record from Shillong, Meghalaya, India (25.559433°N, 91.89885°E, ca. 1,500 m a.s.l.—Saikia et al. 2017). However, their study excluded material from a Vietnamese expedition that in 2016 collected over a dozen individuals, thereby more than doubling the number of known specimens. Our examination of this new and previously existing material provides novel insights into the phylogenetic relationships, morphometrics, and echolocation call characteristics of this enigmatic taxon. Because the combined evidence indicates a deep divergence between “H.” joffrei and all other bat taxa presently recognized, we describe herein a new genus for the species.
Materials and Methods
Field sampling
Specimens were collected during a field trip to Mu Cang Chai Nature Reserve, Che Tao commune, Yen Bai Province, Vietnam (21.764465°N, 104.043192°E, 2,038 m a.s.l.) by TG, VTT, and PE. Mist nets stacked vertically to a height of 10 m above ground level across the road that crosses the “Che Tao” mountain range through a mountain pass named “Gate to Heaven” (Cong Troi in Vietnamese). Eighteen individuals of “H.” joffrei were captured between 25–28 September 2016; several other individuals of the same species (determined on the basis of their size, silhouette, and call characteristics) were observed through the pass during the same period. Identification, determination of sex and age, forearm length, and weight measurements, echolocation recordings, and tissue sampling were undertaken in the field. Five males and one female were kept as voucher specimens. Wing punches or liver were sampled from the released or euthanized bats, respectively. An additional specimen was captured on 21 March 2017 by NTS in Tay Con Linh Mountain, Vi Xuyen district, Ha Giang Province, Vietnam (22.766°N, 104.816°E, 1,979 m a.s.l.). Field work and handling of bats was undertaken following the guidelines of Sikes et al. (2016), with the permission of Ministry of Agriculture and Rural Development, Hanoi, Vietnam. Specimens are deposited in the collections of the IEBR and the HNHM.
Museum acronyms
AMNH: American Museum of Natural History, New York, United States; BM(NH): The Natural History Museum, London, United Kingdom, formerly British Museum (Natural History); FMNH: Field Museum of Natural History, Chicago, United States; HNHM: Hungarian Natural History Museum, Budapest, Hungary; IEBR: Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi, Vietnam; MNHN: Muséum national d’Histoire naturelle, Paris, France; MSB: Museum of Southwestern Biology, University of New Mexico, Albuquerque, United States; NMP: National Museum of Prague, Prague, Czech Republic; RMNH: Naturalis Biodiversity Center, Leiden, The Netherlands, formerly Rijksmuseum van Natuurlijke Historie; ROM: Royal Ontario Museum, Toronto, Canada; ZMB: Museum für Naturkunde, Berlin, Germany, formerly Zoological Museum Berlin; ZMMU: Zoological Museum of Moscow State University, Moscow, Russian Federation; ZSI: Zoological Survey of India, Shillong, India.
Specimens examined
See Appendix I.
Measurements
Abbreviations and definitions for external and craniodental measurements are as follows, with mass in grams and all lengths in mm; WT: body mass; FA: forearm length—from the extremity of the elbow to the extremity of the carpus with the wings folded; TIB: tibia length—from the knee joint to the ankle; GTL: greatest length of skull—from the front of the first upper incisor to the most projecting point of the occipital region; STOTL: total length of skull—from the anterior rim of alveolus of the first upper incisor to the most projecting point of the occipital region; CCL: condylo-canine length—from the exoccipital condyle to the most anterior part of the canine; UCCW: width across the upper canines—greatest width across the outer borders of the upper canines; UM3M3W: width across the upper molars—greatest width across the outer crowns of the last upper molars; RW_lac: lacrimal rostrum width—the greatest width of the rostrum between the lacrimal openings; RW_sup: rostrum width at the processus supraorbitale—the greatest width across the supraorbital tubercles; IOW: interorbital width—least width of the interorbital constriction; ZYW: zygomatic width—greatest width of the skull across the zygomatic arches; MAW: mastoid width—greatest distance across the mastoid region; BCW: braincase width—greatest width of the braincase; BCH: braincase height—from the basisphenoid at the level of the hamular processes to the highest part of the skull, including the sagittal crest (if present); AOB: antorbital width—the distance by which the antorbital foramen is separated from orbit, measured from the foramen infraorbitale to the foramen lacrimale; UCM3L: maxillary toothrow length—from the front of the upper canine to the back of the crown of the third molar; UCP4L: upper canine–premolar length—from the front of the upper canine to the back of the crown of the last premolar; MANL: mandible length—from the anterior rim of the alveolus of the first lower incisor to the most posterior part of the condyle; LCM3L: mandibular toothrow length—from the front of the lower canine to the back of the crown of the third lower molar; LCP4L: lower canine–premolar length—from the front of the lower canine to the back of the crown of the last premolar; and CPH: least height of the coronoid process—from the tip of the coronoid process to the apex of the indentation on the inferior surface of the ramus adjacent to the angular process. Absolute crown height was used in all height comparisons for individual teeth (e.g., C versus P4).
Color description
The fur color description was based on digital images using the charts in a ColorChecker Passport (X-Rite, Inc., Grand Rapids, Michigan).
Genetics
Tissue samples (liver) of voucher specimens were preserved in the field in absolute ethanol and stored in a −80°C freezer at HNHM. Total genomic DNA was extracted with DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) according to the instructions of the manufacturer. For phylogenetic analysis, mitochondrial (hereafter mtDNA) Cytochrome c oxidase subunit I (Co1, 657 bp) and Cytochrome b (Cytb, 1,140 bp), and the Recombination activating gene 2 (Rag2, 1,151 bp) nuclear gene were amplified with primers VF1d/VR1d (Ivanova et al. 2007), Molcit-F/Cytb-H (Ibáñez et al. 2006; Weyeneth et al. 2008), and 179F/1458R (Stadelmann et al. 2007), respectively. PCR reactions followed Stadelmann et al. (2007), Csorba et al. (2015), and Lim et al. (2016).
Three “H.” joffrei and one Vespertilio murinus specimen were sequenced for Co1, Cytb, and Rag2 genes; the relevant sequences are deposited in GenBank (accession numbers: MN813969-MN813980); additional pertinent GenBank sequences also were downloaded. Species were selected to represent all major groups within Vespertilioninae as defined by Amador et al. (2018), Koubínová et al. (2013), and Roehrs et al. (2010), including all available species from the hypsugine-group sensu Roehrs et al. (2010). Only species with all three gene sequences available were included in our analyses, and Myotis muricola was used as the outgroup (Appendix II).
Sequences were aligned in MEGA X v10.0.5 (Kumar et al. 2018); missing nucleotides were filled with “N”s, insertions in Rag2 sequences were excluded, and longer sequences truncated to the following lengths: Co1—657 bp; Cytb—1,140 bp; and Rag2—1,017 bp. Separate (Co1, Cytb, and Rag2) as well as concatenated (mtDNA + Rag2) trees were generated using Bayesian inference method (BI). MrBayes v3.2.6 (Ronquist et al. 2012) was run on the CIPRES Science Gateway (Miller et al. 2010) for 10 million generations, sampling every 1,000 generations. Model parameters for single gene trees were determined with jModelTest2 (Darriba et al. 2012) with the following results: Co1—HKY + G + I; Cytb—GTR + G + I; and Rag2—GTR + G + I. For the concatenated tree, model parameters Co1 pos 1—GTR + GI, pos 2—F81 + PI, pos 3—GTR + G; Cytb: pos 1–3—GTR + GI; Rag2 pos 1–2—GTR + GI, pos 3—GTR + G were used, as determined using PartitionFinder2 (Lanfear et al. 2017). Ten percent of the generations were treated as burn-in and discarded. Posterior probabilities were calculated from the consensus of the remaining trees. Two parallel runs were performed on each data set and results were combined. According to Tracer v1.7.1 (Rambaut et al. 2018), the likelihood scores were stabilized and the effective sample size (ESS) values were significantly higher than 200 in each run. Trees were edited with iToL v3 (Letunic and Bork 2016). Pairwise distances were calculated using GTR model in case of concatenated mtDNA (Co1 + Cytb) and Rag2 sequences in PAUP* 4.0a (Swofford 2003).
Acoustic analyses
The echolocation calls of captured bats were recorded in a flight tent (3 × 5 × 1.5 m; four individuals) and during hand release (nine individuals). In addition, calls of free-flying bats (n =8) also were sampled at the capture site. Our recordings were made using a Pettersson D1000X detector (Pettersson Elektronik AB, Uppsala, Sweden) in manual mode, with a sampling rate of 500 kHz. Sound analyses were carried out using BatSound 4.2. (Pettersson Elektronik AB, Sweden), using the following settings: FFT size =512, overlap =96%, Hanning window.
We selected one typical signal of each call type from individual sequences to avoid pseudo-replication. The following parameters were measured from each of the selected calls: call duration, Tdur (duration of a single pulse); pulse interval, PI (time from the start of one call to the beginning of the next); start frequency, Fstart (frequency value at the start of the call); end frequency, Fend (frequency value at the end of the call); frequency with maximum energy, FmaxE (frequency of maximum energy for the whole call). Bandwidth was calculated from Fstart and Fend values. Pulse duration, starting and terminal frequencies were determined where the signals were clearly above the background noise levels.
Results
Morphology
Based on detailed comparisons of all “H.” joffrei specimens currently known with morphologically similar or phylogenetically closely related species in South-East Asia (including available holotypes), we identified the following unique combination of external and craniodental features that this species possesses: conspicuous coloration of dorsal and ventral fur, shortened fifth finger, poorly developed calcar lobe, developed supraorbital tubercles, strongly bicuspid upper canine, and myotodont lower molars (postcristid linking to the entoconid). Taken together, these features strongly suggest the taxon can be distinguished at the level of genus from all other bat taxa currently recognized.
Genetics
No intraspecific variability was detected in mtDNA of “H.” joffrei, although there existed differences among Rag2 sequences (Table 1) that did not result in amino acid changes. Specimens HNHM 26037 and 26040 had several heterozygous nucleotide positions, and the chromatogram of the IEBR VN16-170 Rag2 sequence lacked double peaks.
Table 1.
Heterozygous positions in Rag2 sequences.
| Position | ||||||
|---|---|---|---|---|---|---|
| Specimen | 636 | 662 | 693 | 793 | 831 | 981 |
| IEBR VN16-170 | T | G | A | T | T | T |
| HNHM 26037 | C/T | A/G | A/T | C/T | C/T | C/T |
| HNHM 26040 | C/T | A/G | A/T | C/T | C/T | C/T |
Concatenated (Fig. 1) and separate Co1, Cytb, and Rag2 trees (Supplementary Data SD1) show that “H.” joffrei is only distantly related to the strongly supported monophyletic Hypsugo (sensu stricto) clade, and instead groups with P. brachypterus, Cassistrellus spp., and Tylonycteris spp. Comparing Rag2 sequences, “H.” joffrei has an insertion (CAA, Glutamine) at the positions 31–33 of the sequence, hence the total length is 1,151 bp. The same inserted codon is present in Cassistrellus spp., P. brachypterus, Pipistrellus pipistrellus, and Tylonycteris spp., further supporting the monophyly of this clade.
Fig. 1.
Bayesian inference tree based on concatenated sequences (Co1 + Cytb + Rag2) of selected species of vespertilionid bats. Numbers at splits indicate posterior probabilities. The black line on the right side of the tree indicates a specific codon insertion in Rag2 at positions 31–33.
In mtDNA, P. brachypterus was the most similar to “H.” joffrei, with a genetic distance of 19.7%, whereas in Rag2, Cassistrellus spp. were the closest, with a sequence divergence of 3.85–3.95% (Table 2).
Table 2.
Estimates of genetic distances (GTR model) among sequences, in %. Values in the lower left part of the matrix indicate combined mtDNA differences; upper right values are those of the Rag2 data set.
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cassistrellus dimissus | 0.69 | 4.03 | 3.85 | 3.85 | 6.66 | 2.60 | 2.78 | |
| 2 | Cassistrellus yokdonensis | 10.02 | 4.13 | 3.95 | 3.95 | 7.10 | 3.05 | 2.98 | |
| 3 | Mirostrellus joffrei IEBR VN16-170 | 23.65 | 21.62 | 0.00 | 0.00 | 7.83 | 5.67 | 5.36 | |
| 4 | Mirostrellus joffrei HNHM 26037 | 23.65 | 21.62 | 0.00 | 0.00 | 7.70 | 5.53 | 5.18 | |
| 5 | Mirostrellus joffrei HNHM 26040 | 23.65 | 21.62 | 0.00 | 0.00 | 7.70 | 5.53 | 5.18 | |
| 6 | Philetor brachypterus | 22.59 | 21.83 | 19.70 | 19.70 | 19.70 | 6.80 | 6.67 | |
| 7 | Tylonycteris malayana | 20.39 | 19.94 | 20.14 | 20.14 | 20.14 | 20.02 | 0.14 | |
| 8 | Tylonycteris tonkinensis | 21.56 | 21.44 | 19.94 | 19.94 | 19.94 | 18.95 | 9.27 |
Based on the phylogenetic reconstructions, which are fully supported by anatomical traits, “H.” joffrei is separable at the level of genus both from Hypsugo as well as all other bat genera.
Mirostrellus gen. nov.
Type species
Nyctalus joffrei Thomas, 1915.
Etymology
From the Latin “mirus” meaning “surprise, marvel,” which reflects that both the systematic position and the wide distribution of this bat (previously thought to be extremely rare) were pleasant surprises for the authors.
Diagnosis
A medium-sized vespertilionid, with a FA of 35.7–40.2 mm. The fifth finger of the wing is shortened (on average 20 mm shorter than the fourth finger) and the pelage is sparse and velvety. The supraorbital tubercles are well-developed, protruding for 1.47–1.76 mm measured from the lachrymal opening; the sagittal crest is barely visible, being only approximately 0.1 mm high. The upper canine is characterized by a developed posterior secondary cusp. The taxon has two upper and lower premolars and its lower molars are myotodont.
Description
The only known species of the genus, Mirostrellus joffrei, has a FA between 35.7–40.2 mm, CCL 13.65–14.72 mm, and UCM3L 5.12–5.30 mm (Table 3). The body is covered with moderately long (5.5–7.5 mm on the back and 4.5–5.4 mm on the ventrum) and sparse velvety fur. On the dorsum the lower portion of individual hairs is dark black (PANTONE 440 C), whereas the upper section gradually lightens to warm brownish black (PANTONE 411 C); the overall impression is brownish black (PANTONE 412 C). On the ventral surface the individual hair is uniform sandbrown (PANTONE 728C; Fig. 2). Wings and hindfoot are black. The muzzle blunt and massive (12–14 mm wide), with pronounced buccal glands. The ears are broad (7.5–9.5 mm in width) and rounded, 11–14 mm in length, and have a pale antitragal lobe (Fig. 3). The tragi are short (2.1–3.0 mm) and broad (2.0–2.9 mm) and turned forward, so their upper half appears much wider than the lower portion. The wings are narrow (a common feature for fast-flying bats, i.e., Mimetillus, Philetor, and Nyctalus), the third finger is about 21–23 mm longer than the fifth (which itself is 37–43 mm in length), aspect ratio is 2.4–2.6. In the tail, only distal half of the last vertebra emerges for 1.0–2.5 mm from the uropatagium. The calcars are extending less than half of the free margin of the tail membrane and distally bear a narrow and elongated (1.2–1.5 mm wide and 4.21–5.38 mm long) calcar lobe.
Table 3.
Selected external and craniodental measurements (in mm) of Mirostrellus joffrei specimens. Values are given as mean ± SD; min–max (n). Acronyms and definitions for measurements are given in the text.
| Character | Measurement |
|---|---|
| WT | 16.0 ± 1.95; 13.0–19.0 (18) |
| FA | 38.6 ± 1.08; 35.7–40.2 (28) |
| TIBIA | 15.4 ± 0.74; 13.8–16.4 (12) |
| GTL | 15.33 ± 0.27; 14.86–15.77 (12) |
| STOTL | 14.91 ± 0.30; 14.47–15.35 (15) |
| CCL | 14.21 ± 0.24; 13.65–14.72 (17) |
| UCCW | 5.15 ± 0.11; 4.96–5.34 (16) |
| UM3M3W | 7.08 ± 0.15; 6.79–7.34 (18) |
| RW_lac | 7.25 ± 0.61; 6.67–8.59 (18) |
| RW_sup | 8.04 ± 0.35; 7.62–8.62 (10) |
| IOW | 4.68 ± 0.17; 4.34–5.02 (20) |
| ZYW | 10.47 ± 0.31; 9.95–10.99 (13) |
| MAW | 8.90 ± 0.18; 8.58–9.28 (17) |
| BCW | 8.04 ± 0.25; 7.64–8.58 (17) |
| BCH | 5.57 ± 0.19; 5.36–6.00 (10) |
| AOB | 0.55 ± 0.18; 0.36–0.86 (14) |
| UCM3L | 5.19 ± 0.06; 5.12–5.30 (16) |
| UCP4L | 2.05 ± 0.07; 1.90–2.18 (13) |
| MANL | 10.89 ± 0.25; 10.53–11.45 (19) |
| LCM3L | 5.55 ± 0.10; 5.29–5.70 (19) |
| LCP4L | 1.66 ± 0.17; 1.44–1.80 (4) |
| CPH | 3.66 ± 0.10; 3.54–3.90 (14) |
Fig. 2.
Habitus of a live adult female Mirostrellus joffrei from Mu Cang Chai, Vietnam (HNHM 26034). Note the coloration of the dorsal and ventral side, which is unique for this species.
Fig. 3.
Close up of the head of an adult male Mirostrellus joffrei from Mu Cang Chai, Vietnam (HNHM 26040).
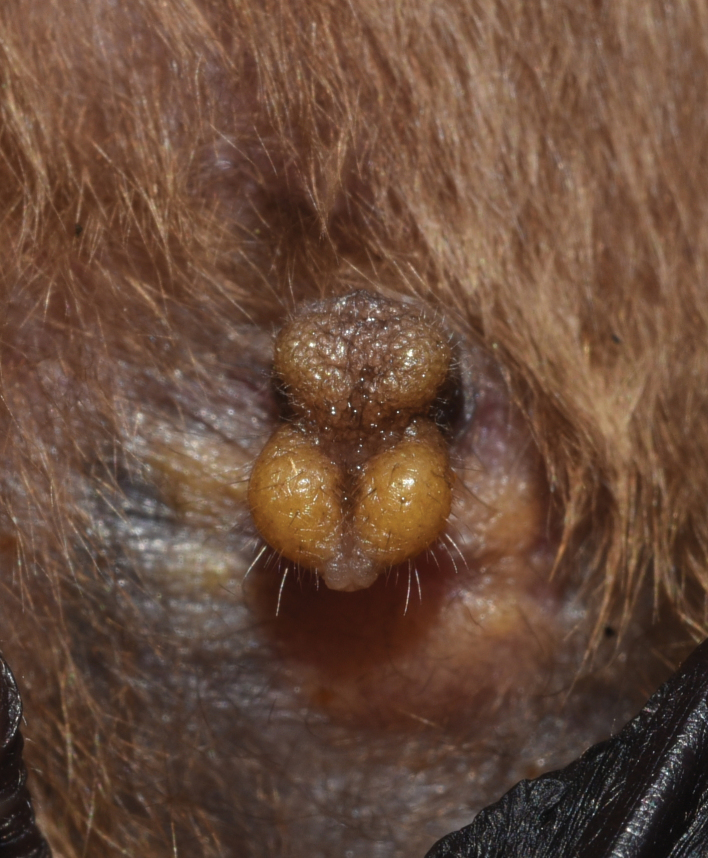
Both the male and female external genitalia are similar to those of Philetor. The penis has a complex structure with a long shaft, which on the dorsal surface bears a cushion-like pad divided by a longitudinal median depression, and a separated, prominent glans (Fig. 4). The female external genitalia is similarly complicated, the transverse vulval opening is separated from the anus by a swollen perineal pad, and is anteriorly almost fully concealed by a wide, triangular cushion. The vulva itself is anteriorly prolonged as a narrow slit.
Fig. 4.
Penis of Mirostrellus joffrei from Mu Cang Chai, Vietnam (HNHM 26041). Not to scale.
The skull has distinct supraorbital projections that protrude beyond the outline of the skull (Figs. 5-7). The occiput is abruptly elevated and its upper point is turned somewhat forward against the condyles. The opening of the antorbital canal is situated just behind the level of the first root of M1. The lacrimal foramen is well-developed and close to the antorbital one, being divided from the latter by a thin bony wall. The anterior palatal emargination is heart-shaped, and protrudes backward to the middle of the upper large premolars. The posterior palatal emargination has a pointed middle projection. The basial pits are well-demarcated. The sagittal crest is very low but present, and anteriorly is continuous with the similarly weakly developed supraorbital crests. The mandible is moderately robust, with a long angular process that is curved distally. The coronoid process is moderately high (3.54–3.90 mm).
Fig. 5.
Lateral view of skull of Mirostrellus joffrei from Mu Cang Chai, Vietnam (HNHM 26041).
Fig. 6.
Lateral views of skulls of a) Mirostrellus joffrei (ZMMU S-186691); b) Philetor brachypterus (ROM 102019); c) Pipistrellus stenopterus (ZMMU S-103149); d) Hypsugo pulveratus (ZMMU S-167186); e) H. macrotis (MHNG 1486.94); f) H. alaschanicus (ZMMU S-108373); g) Tylonycteris malayana (ZMMU S-186637); h) Cassistrellus dimissus (MHNG 1926.053); i) Nyctalus leisleri (ZMMU S-176068). Scale bar =5 mm.
Fig. 7.
Dorsal, ventral, and lateral views of the skull and mandible of a male Mirostrellus joffrei from Tram Ton forest station, Vietnam (ZMMU S-186691). Scale bar =5 mm.
The dental formula is I2 C1 P2 M3/i3 c1 p2 m3 × 2 =34. The inner upper incisors are clearly bicuspidate and the outer incisors (I3) do not reach the height of the secondary cusp of I2. The upper canine is large, with a well-developed secondary cusp on the posterior blade. The anterior upper premolar is minute and is displaced lingually from the toothrow, hence in lateral view totally obscured by the canine’s cingulum. Due to the greatly reduced hypocones of M1 and M2 (height and basal dimensions are nearly the half those of the corresponding protocones) the trigon basins of these teeth are nearly open. The cusps of the lower incisors are arranged into a single row. The lower molars are clearly myotodont and their talonids are about one and half times larger than the corresponding trigonids. The lower premolars are similar in shape and size.
The baculum (Fig. 8) is about 1 mm in total length and 0.75 mm in maximum width; the main shaft is narrow (0.14–0.20 mm) and straight. The distal tip is blunt, semicircular, whereas the basal portion has well-developed lateral projections angling at 130° and 230° from the shaft approximately 0.6 mm from the distal tip; the emarginations are somewhat downwardly curved (ca. 140°) and divided by a wide basal emargination, ca. 0.5 mm wide and 0.3 mm deep. The urethral groove on the main shaft is present but very shallow.
Fig. 8.
Baculum of Mirostrellus joffrei from Tram Ton forest station, Vietnam (ZMMU S-186692). Scale bar =1 mm.
Comparisons
Because M. joffrei was initially described as a Nyctalus, and later associated with Pipistrellus and Hypsugo, but is phylogenetically close to Philetor, Cassistrellus, and Tylonycteris, we compare it below in detail with these taxa.
Mirostrellus gen. n. can be distinguished from all species of Pipistrellus and Nyctalus by its poorly developed calcar lobe which lacks a central supporting cartilage, relatively high mandibular coronoid process (over 3.5 mm versus less than 3.3 mm height in the externally similar sized Pipistrellus species and in the even larger N. leisleri and N. azoreum) and myotodont (versus nyctalodont) lower molars. Among the South-East Asian Pipistrellus species, Mirostrellus gen. n. is most similar to P. stenopterus, from which it can be further distinguished by its flat rostral profile and very low cranial crests (versus convex rostral profile and well-pronounced sagittal crest in P. stenopterus; Fig. 6c).
It differs externally from Hypsugo in having a swollen muzzle (Fig. 3) due to the developed buccal glands, and with only half of the last vertebra protruding from uropatagium (in Hypsugo the whole last vertebra is free from the membrane), very shallow urethral grove and developed lateral basal lobes of the baculum (as compared with the typical Hypsugo bacula, see, i.e., figure 6a, 8a, b, c, e, f, in Hill and Harrison 1987), developed supraorbital tubercles, and a strongly bicuspid upper canine.
Compared with Philetor, Mirostrellus gen. n. has a differently shaped baculum (Fig. 8) without the enlarged tip and the elongated, dorsoventrally strongly curved shaft typical of Philetor (see figure 1 in Hill 1966). The female external genitalia of the new genus is less complex as compared with Philetor (Hill 1966: figure 2) with both the anterior and posterior perineal pads are undivided. Mirostrellus gen. n. also has conical upper incisors and a basally nearly quadratical posterior premolar (Philetor is characterized by a blade-like first upper incisor and anterioposteriorly strongly compressed P4 the length of which is equal to approximately one-half of its width), longer toothrow (UCM3L 5.12–5.30 mm versus 4.42–4.98 mm; LCM3L 5.29–5.70 mm versus 4.72–5.22 mm), and myotodont (versus nyctalodont) lower molars.
In contrast with Cassistrellus, Mirostrellus gen. n. is smaller in several dental measurements (UCM3L 5.12–5.30 mm versus 5.8–6.6 mm, LCM3L 5.29–5.70 mm versus 6.29–7.05 mm), has a very low sagittal crest, and lacks an occipital helmet (formed by the well-developed sagittal and lambdoid crests, which meet near the top of the skull in Cassistrellus). It also possesses a second (anterior) upper premolar.
With respect to the closely related Tylonycteris, Mirostrellus gen. n. can be distinguished by its much larger size (FA 35.7–40.2 mm versus 24.4–32 mm, CCL 13.65–14.72 mm versus 9.72–11.82 mm, UCM3L 5.12–5.30 mm versus 3.27–4.31 mm) absence of adhesive disks on pollex and heels, nonflattened skull (Fig. 6a versus 6g), and by the presence of the small upper premolar.
From all other more distantly related Indomalayan Vespertilioninae genera, Mirostrellus gen. n. can generally be distinguished by its shortened fifth finger (where the combined length of the fifth metacarpal and the first phalanx is subequal with the length of the third metacarpal) and the presence of a secondary cusp on the upper canine.
Acoustics
Our sample bats produced different calls depending on the behavior being recorded (Table 4). In the flight tent, M. joffrei emitted very short (Tdur =2.7 ± 1.5 ms) frequency-modulated (FM type) calls with short pulse intervals (PI =69.5 ± 27.5 ms) and highest observed frequencies (FmaxE) of 39.0 ± 2.3 kHz from the three different recording situation. In case of hand releases, they emitted calls with a steep frequency-modulated beginning and a short, quasi-constant frequency ending (FMQCF type) which had lower frequencies (FmaxE =34.6 ± 1.2 kHz) and longer durations (Tdur =6.4 ± 2.1 ms) and pulse intervals (PI =90.3 ± 10.8 ms) than calls recorded in flight tents. The calls of free-flying individuals were mainly FMQCF in structure (Fig. 9), although in some cases the FM part was significantly reduced, resulting in a near QCF structure. The FMQCF calls of free-flying individuals had clearly higher frequency values (FmaxE =30.5 ± 0.7 kHz) and shorter durations (Tdur =11.8 ± 1.6 ms) and pulse intervals (PI =158.4 ± 36.9 ms) than the QCF calls (FmaxE =28.3 ± 0.6 kHz, Tdur =13.3 ± 2.1 ms, PI =199.8 ± 36.2 ms). This call structure is typical for aerial-hawking species that forage in open and edge spaces.
Table 4.
Acoustic parameters. Acronyms and definitions for measurements are given in the text.
| Settings | Call type | n | F start (kHz) | F maxE (kHz) | F end (kHz) | T dur (ms) | PI (ms) | BW (kHz) | |
|---|---|---|---|---|---|---|---|---|---|
| Flight tent | FM | 4 | Mean ± SD Min–max | 66.8 ± 7.8 59.6–77.2 | 39.0 ± 2.3 36.5–41.4 | 32.8 ± 2.1 29.9–34.9 | 2.7 ± 1.5 1.5–4.8 | 69.5 ± 27.5 37.6–103.0 | 34.0 ± 8.7 24.7–43.9 |
| Hand release | FMQCF | 9 | Mean ± SD Min–max | 61.8 ± 7.8 51.1–72.4 | 34.6 ± 1.7 33.1–38.1 | 31.2 ± 1.4 29.4–33.4 | 6.4 ± 2.1 2.2–8.0 | 90.3 ± 10.8 71.6–107.0 | 30.7 ± 7.5 20.7–41.8 |
| Free-flying | FMQCF | 8 | Mean ± SD Min–max | 42.4 ± 4.5 36.3–50.0 | 30.5 ± 0.7 29.5–31.4 | 28.8 ± 0.9 27.4–29.6 | 11.8 ± 1.6 10.0–14.6 | 158.4 ± 36.9 93.3–187.0 | 13.6 ± 3.9 8.8–20.4 |
| Free-flying | QCF | 4 | Mean ± SD Min–max | 32.2 ± 1.3 30.4–33.4 | 28.3 ± 0.6 27.5–28.7 | 26.7 ± 0.8 25.8–27.8 | 13.3 ± 2.1 11.3–16.0 | 199.8 ± 36.2 175.6–253.6 | 5.5 ± 1.0 4.6–6.8 |
Fig. 9.
Echolocation call sequence of a free-flying Mirostrellus joffrei from Mu Cang Chai, Vietnam.
Geographic distribution
The only known species of Mirostrellus gen. n. has an Indomalayan distribution, ranging from Nepal, NE India (Sikkim, Meghalaya), through the northern part of Myanmar, to North Vietnam (Saikia et al. 2017). In the National Museum of Prague (Czech Republic), four hitherto unreported specimens from western Yunnan, China (Zao Teng He, 25.31°N, 98.80°E, 1,451 m a.s.l.), were revealed by SVK. The species probably also occurs between these localities as it is difficult to capture and so may be missed during faunal surveys (Fig. 10).
Fig. 10.
Distribution map of Mirostrellus joffrei.
Habitat and ecology
The recent observations from Vietnam provide new insights into the habitat requirements and ecology of this very poorly known bat. All collecting sites (Mu Cang Chai, Tram Ton [22.35°N, 103.77°E, 1,850–1,950 m a.s.l.]; Cat Cat [22.33°N, 103.83°E, 1,290 m a.s.l.]; Tay Con Linh) are located in the high mountains of northern Vietnam. The mean annual temperature of these sites is usually lower than 20°C, and mean precipitation usually over 1,700 mm. In these areas, temperatures can fall below freezing during the coldest days of the winter, usually in December and January. The vegetation of collection sites consists of montane evergreen forests. In Mu Cang Chai and Tay Con Linh, the mature and least disturbed forests are found only at high elevations, whereas little forest remains at lower parts. All of the records are from higher elevations, between 575 and 2,038 m a.s.l., but mostly (nine of 11 known) above 1,000 m a.s.l.
In Tram Ton, bats (males only) were captured over a stream at the elevation of ca. 1,900 m a.s.l. in tall primary mountain forest. Other fast-flying bats such as Miniopterus fuliginosus also were observed in that location.
The site in the vicinity of Cat Cat village is situated at ca. 1,290 m a.s.l. and is mostly deforested. Mirostrellus joffrei were seen here at dawn, foraging 10–20 m above ground level; they resembled small noctules in their flight behavior. The single specimen collected was an adult female. The only other bats observed simultaneously at that location were Pipistrellus coromandra.
The specimen (adult male) from Tay Con Linh was captured in a primary forest with trees ranging from 15 to 20 m. Other bats captured at the same locality were: Myotis altarium, Coelops frithii, Rhinolophus affinis, Arielulus circumdatus, Harpiocephalus harpia, Murina huttoni, Murina chrysochaetes, and Harpiola isodon.
Mirostrellus joffrei is probably a forest-dwelling species given that most of the individuals in Mu Cang Chai were caught just after sunset (ca. 1800 h local time) and no caves or other underground shelters are known in the vicinity, although rock crevices could occur in nearby granite formations. However, Hypsugo cadornae and H. pulveratus were captured in the same mist nets; and these species are thought to primarily roost in caves. Judging from its body and wing proportions, M. joffrei is a fast-flying aerial-hawker; this corresponds with our field observations and the characteristics of echolocation calls produced by free-flying individuals. Like the similarly built noctules, it may make long-range flights to locate high concentrations of night-flying insects.
Discussion
Frequent nomenclatural changes for a given taxon are suggestive of uncertain and likely imperfect taxonomy which—after careful examination and combination of different approaches—can result in novel systematic arrangements (i.e., Görföl and Csorba 2018; Ruedi et al. 2018). In the case of Joffre’s Pipistrelle, integrative taxonomy, including comparative morphology, morphometrics, and multi-locus sequence typing, allowed us to reconstruct the phylogenetic position of this very poorly known bat and to distinguish it from all other currently recognized genera.
Amador et al. (2018) identified a clade in the Vespertilionini that contained Philetor and Tylonycteris. That same clade also was recovered by Ruedi et al. (2018) with the addition of Cassistrellus, a then newly described genus of vespertilionid. Mirostrellus gen. n. is included in this group with high support on the basis of phylogenetic reconstructions. The common features of taxa belonging to this group (all of which are fast-flying aerial-hawkers) are as follows: short tragus, fleshy posterior edge of ear conch that extends toward the corner of mouth, prominent facial glands, developed supraorbital processes, upper canines that tend to have an additional cusp (exception is Cassistrellus), and a special insertion in the Rag2 gene at positions 31–33.
The individuals that were captured and observed during the expedition to Mu Cang Chai provided a unique opportunity to study the echolocation calls of the species under different conditions: in a flight tent, in hand-released individuals, and in free-flying bats. Our description of its call parameters will assist detection of this rarely encountered species in new areas. This is important because the species is difficult to catch with mist nets or harp-traps due to its behavior of fast-flying and foraging at great heights (Kruskop and Shchinov 2010; our personal observations). Thus, our ability to record the species will largely rely on acoustic surveys. Besides the two abovementioned Hypsugo species, other rarely mist-netted bats including Thainycteris aureocollaris, Nyctalus plancyi, Ia io, A. circumdatus, and Scotomanes ornatus, as well as other, more common, aerial-hawkers such as Taphozous melanopogon and M. fuliginosus, also were recorded during our six nights of fieldwork at Mu Cang Chai. This is evidence that passes in large and steep mountain ranges function as important commuting paths between roosts and foraging areas on opposing mountain sides and provide ideal locations to study bats that otherwise are hard to observe.
The current IUCN Red List status for “Hypsugo” joffrei is Data Deficient (Görföl et al. 2016). Because the new localities are fairly close to the previous recorded localities (Hoang Lien Son mountain range is about 40 km from Mu Cang Chai and 120 km from Tay Con Linh, whereas Kachin Hills is in the vicinity of the locality in Yunnan, China), the species’ known distribution area has not changed considerably. Furthermore, while the number of individuals encountered during our expedition was relatively high, the species remains known only from a handful of localities. Our knowledge about its ecology, habitat preferences, and conservation status, therefore, remains largely incomplete. As a consequence, we believe that its present IUCN status should be retained.
Supplementary Data
Supplementary Data SD1.—Bayesian inference tree based on a) Co1, b) Cytb, c) Rag2 sequences of selected species of vespertilionid bats. Numbers at splits indicate posterior probabilities.
Acknowledgments
TG, PE, and GC received support from the Hungarian Scientific Research Fund – OTKA (grant no. K112440), from the National Research, Development and Innovation Fund of Hungary NKFIH (grant no. KH130360), and from the SYNTHESYS Project, financed by the European Community Research Infrastructure Action under the FP7 “Capacities” Program. TG was also supported by the Hungarian Ministry of Human Capacities (grant no. NTP-NFTÖ-17). We are grateful for the help in the field and/or for the local organization for Gábor Kemenesi, Ottó Merkl, Chu Thi Hang, Nguyen Van Sinh, and Dương Anh Tuấn. We thank Petr Benda (NMP), Nancy Simmons (AMNH), Roberto Portela-Miguez and Louise Tomsett (BM[NH]), Larry Heaney (FMNH), Jean-Marc Pons (MNHN), Steven van der Mije, Wendy van Bohemen (RMNH), Burton K. Lim (ROM), and Frieder Mayer (ZMB), for kindly providing access to specimens under their care. We are especially grateful to Neil M. Furey for his expert advice and linguistic corrections. The work of SVK was performed in line with State theme of the ZMMU (АААА-А16-116021660077-3) with financial support from the Russian Foundation for Basic Research (grant no. 17-04-00689a). NTS and VTT were funded by the National Foundation for Science and Technology Development, grant numbers 106‐NN.05‐2016.14 and Vietnamese Academy of Science and Technology - Japan Society for the Promotion of Science (grant no. QTJP01.02/18-20), JSPS Core-to-Core Program, and the NAGAO Natural Environmental Foundation’s Bio-ecological Nature Conservation Project in Mountainous Regions of Northern Vietnam. We thank associate editor Luis A. Ruedas and the two anonymous reviewers who greatly improved the quality of this article.
Appendix I
Specimens Examined
Cassistrellus dimissus—THAILAND: Surat Thani Province; Kao Nawng, Tai Rom Yen National Park, 427 m: BM(NH) 1916.4.21.1 (holotype). LAOS: Phongsaly Province; Ban Naten, Nam Lan Conservation Area: MHNG 1926.053.
Cassistrellus yokdonensis—VIETNAM: Dak Lak Province; Yok Don National Park, Dak Ken River (tributary of the Serepok River), base of Yok Mt., 12.8672N, 107.7075E, 194 m: ROM 107751 (holotype).
“Hypsugo” joffrei—CHINA: Yunnan Province; Zao Teng He, 25.31N, 98.80E, 1,451 m: NMP CN007, CN008, CN009, CN010. INDIA: Meghalaya State; Shillong, Risa Colony area, 25°33.566′N, 91°53.931′E, 1,500 m: ZSI V/M/ERS/292. INDIA: Sikkim State; Hee-Gyathang, 27°30′N, 88°30′E, 1,846 m: MSB 67466, 67467. MYANMAR: Kachin State; Changyinku, Chipwi River Valley, 2,134 m: AMNH 114849 (anthonyi holotype). MYANMAR: Kachin State; Kachin Hills: BM(NH) 1888.12.1.37 (holotype). MYANMAR: Chin State; Chin Hills: BM(NH) 1916.3.26.2. MYANMAR: Sagaing State; W of Kindat: BM(NH) 1916.3.26.83, 1916.3.26.84. NEPAL: Sindhupalchok District; Bahrabise, approx. 27°78′N, 85°89′E, 575 m: FMNH 114249. NEPAL: Sankhuwasabha District; Num Bridge, approx. 27°54′N, 87°34′E, 850 m: FMNH 114481. VIETNAM, Yen Bai Province, Che Tao commune, Mu Cang Chai Nature Reserve, 21.764465°N, 104.043192°E, 2,038 m: HNHM 26037, 26040, 26041; IEBR VN16-168, VN16-169, VN16-170. VIETNAM: Ha Giang Province; Vi Xuyen District, Tay Con Linh Mountain, 22.766°N, 104.816°E, 1,979 m: IEBR M-6516. VIETNAM: Lao Cai Province; Sa Pa, Tram Ton forest station, 22.35°N, 103.77°E, 1,850–1,950 m: ZMMU S-186691, 186692, 186693. VIETNAM: Lao Cai Province; Sa Pa, Cat Cat, 22.33°N, 103.83°E, 1,290 m: ZMMU 186694.
Hypsugo affinis—CHINA, Yunnan Province, Lijiang: AMNH 44565. INDIA: Uttarakhand State; Kumaon: BM(NH) 1879.11.21.103. INDIA: Kerala State; Wynaad (now Wayanad?): 1982.3.3.2. INDIA: Tamil Nadu State; Nilgiris District, Kotagiri: BM(NH) 1892.4.7.1, 1892.4.7.2. INDIA: West Bengal State; Darjeeling, Gopaldhara: BM(NH) 1916.3.25.5. NEPAL: Central Nepal; Bhaktapur, Nagarkot: BM(NH) 1937.3.14.3.
Hypsugo alaschanicus—RUSSIA: Primorskiy Territory; Khasan District, Slavyanka: ZMMU S-108373.
Hypsugo cadornae—INDIA: West Bengal State; Darjeeling, Pashok: BM(NH) 1916.3.25.6 (holotype).
Hypsugo dolichodon—LAOS: Attapu Province; 10 km east from Ban Paam, Xe Kaman proposed dam site, 14°57′N, 107°08′E, 150 m: ROM 110459 (holotype), 110462 (paratype). LAOS: Attapu Province; 14°58′N, 107°13′E, 150 m: ROM 110464 (paratype). VIETNAM: Dong Nai Province; Cat Tien National Park, 11°25′N, 107°26′E, 100 m: ZMMU S-180563, 180565.
Hypsugo imbricatus—INDONESIA: Java. BM(NH) 1879.11.21.108 (holotype).
Hypsugo kitcheneri—INDONESIA, Borneo, Central Kalimantan, Boentok, Barito River: BM(NH) 1910.4.5.55 (holotype).
Hypsugo lophurus—MYANMAR: Tanintharyi; Maliwun. BM(NH) 1914.12.1.6 (holotype).
Hypsugo macrotis—INDONESIA: West Sumatra; Padang. RMNH 35469–35471 (syntypes). INDONESIA: Sumatra; Riau, Japura, Indragiri. MHNG 1486.94.
Hypsugo mordax—INDONESIA: Java. ZMB 2559 (holotype).
Hypsugo petersi—INDONESIA: Buru Regency; Kayeli. BM(NH) 1897.12.6.2. MALAYSIA: Sabah; Sinsuron, Crocker Range, 1,500 m. BM(NH) 1985.908, 1985.909, 1985.910, 1985.911. PHILIPPINES: Luzon; 0.8 km N and 1.5 km E of the south peak of Mt. Data, 2,128 m. FMNH 188235, 188238. PHILIPPINES: Luzon; 0.5 km N and 0.5 km W of the peak of Mt. Amuyao, 2,530 m. FMNH 193513. PHILIPPINES: Luzon, La Laguna. MNHN 1987-356.
Hypsugo pulveratus—CHINA: Fujien; Amoy (now Xiamen). BM(NH) 1870.7.18.12 (holotype). VIETNAM: Quang Binh Province; Ke Bang. ZMMU S-167186.
Hypsugo vordermanni—INDONESIA: Borneo, Sarawak; Samunson Forest Sanctuary. RMNH 35570 (holotype).
Nyctalus leisleri—RUSSIA: Voronezh Region; Voronezhskiy State Reserve. ZMMU S-176068.
Philetor brachypterus—INDONESIA: West Sumatra; Padang. RMNH 35155 (holotype). INDONESIA: Borneo, Kalimantan, North Kalimantan; Kayan Mentarang Nature Reserve, Lalut Birai Reserve Station, 2.85N, 115.80E. ROM 102165, 102019. MALAYSIA: Johor; Endau Rompin National Park, 2.533N, 103.40E. ROM 113087.
Pipistrellus stenopterus—INDONESIA: North Sumatra; Medan. ZMMU S-103146, 103147, 103148, 103149, 103150.
Tylonycteris fulvida—MYANMAR: Schwe Gyin; valley of Sittang River. BM(NH) 1915.4.29.1, 1915.4.29.2 (syntypes).
Tylonycteris malayana—MALAYSIA: Perak; Batang Padang, Jor. BM(NH) 1947.1433 (holotype). VIETNAM: Binh Phouc Province; Bu Gia Map. ZMMU S-186637.
Tylonycteris pachypus—INDONESIA: Banten; Bantam (now Banten). RMNH 35248, 35249, 35250 (syntypes).
Tylonycteris robustula—MALAYSIA: Borneo, Sarawak; Upper Sarawak. BM(NH) 1911.1.18.8 (holotype).
Appendix II
Species, tissue/voucher number, locality, GenBank accession numbers, and references for sequences used in this study.
| Species | Tissue/voucher # | Locality | Co1 | Cytb | Rag2 | Reference |
|---|---|---|---|---|---|---|
| Arielulus circumdatus | BNB 055 | Vietnam | MF038573 | MF038474 | MF038336 | Hassanin et al. (2018) |
| Barbastella barbastellus | HNHM 24733 | Hungary | MF038569 | MF038470 | MF038332 | Hassanin et al. (2018) |
| Cassistrellus dimissus | MHNG 1926.053 | Laos | MG194430 | MG194436 | GU328057 | Lack et al. (2010); Ruedi et al. (2018) |
| Cassistrellus yokdonensis | ROM 107765 | Vietnam | HM540266 | MG194435 | MG194433 | Francis et al. (2010); Ruedi et al. (2018) |
| Chalinolobus neocaledonicus | 2000-273 | MF038571 | MF038472 | MF038334 | Hassanin et al. (2018) | |
| Eptesicus serotinus | ZMMU S-183029 | Russia | JF442822 | Kruskop et al. (2012) | ||
| Eptesicus serotinus | ZMMU S-191978 | Russia | GQ272579 | Artyushin et al. (2009) | ||
| Eptesicus serotinus | MHNG 1807.065 | Greece | HM561650 | Roehrs et al. (2010) | ||
| Falsistrellus tasmaniensis | ABTC5974 | Australia | MH753135 | MH753138 | MH753141 | Görföl and Csorba (2018) |
| Glauconycteris superba | MNHN 2016-2803 | DR Congo | MF038653 | MF038552 | MF038390 | Hassanin et al. (2018) |
| Glischropus bucephalus | HNHM 2006.34.37. | Cambodia | KR612334 | KR612331 | MH753142 | Csorba et al. (2015); Görföl and Csorba (2018) |
| Hesperoptenus blandfordi | CPV10-40 | MF038582 | MF038482 | MF038342 | Hassanin et al. (2018) | |
| Hesperoptenus tickelli | CPV10-21 | MF038583 | MF038483 | MF038343 | Hassanin et al. (2018) | |
| Hypsugo cadornae | HNHM 25057 | Vietnam | MH753136 | MH753139 | MH753143 | Görföl and Csorba (2018) |
| Hypsugo dolichodon | CBC02156 | Cambodia | MH234219 | MH234221 | MH753144 | Görföl et al. (2018); Görföl and Csorba (2018) |
| Hypsugo petersi | FMNH 193513 | The Philippines | MG194431 | JX570897 | JX570913 | Heaney et al. (2012); Ruedi et al. (2018) |
| Hypsugo pulveratus | ROM 110689 | Laos | HM540657 | KX429686 | MH753145 | Francis et al. (2010); Lim et al. (2016); Görföl and Csorba (2018) |
| Hypsugo savii | MIBZPL01295 | Italy | FR856654 | Galimberti et al. (2012) | ||
| Hypsugo savii | MHNG 1805.007 | Switzerland | AJ504450 | Stadelmann et al. (2004b) | ||
| Hypsugo savii | MHNG 1804.100 | Switzerland | HM561667 | Roehrs et al. (2010) | ||
| Ia io | VN11-0688 | MF038584 | JX465365 | MF038344 | Hassanin et al. (2018) | |
| Laephotis botswanae | ECJS-11/2009 | MF038572 | MF038473 | MF038335 | Hassanin et al. (2018) | |
| Lasionycteris noctivagans | MVZ 192695 | United States | GU723196 | Streicker et al. (2010) | ||
| Lasionycteris noctivagans | 22Jul09-03-AHH, M37176 | Unites States | KC747682 | Patrick and Stevens (2014) | ||
| Lasionycteris noctivagans | TK24216, TTU 56255 | United States | GU328065 | Lack et al. (2010) | ||
| Lasiurus cinereus | TX6295 | United States | GU722978 | Streicker et al. (2010) | ||
| Lasiurus cinereus | TK78926 | United States | KC747685 | HM561638 | Patrick and Stevens (2014); Roehrs et al. (2010) | |
| Mimetillus moloneyi | HNHM 23243 | Cameroon | MF038570 | MF038471 | MF038333 | Hassanin et al. (2018) |
| Mirostrellus joffrei | IEBR VN16-170 | Vietnam | MN813969 | MN813973 | MN813977 | This study |
| Mirostrellus joffrei | HNHM 26037 | Vietnam | MN813970 | MN813974 | MN813978 | This study |
| Mirostrellus joffrei | HNHM 26040 | Vietnam | MN813971 | MN813975 | MN813979 | This study |
| Myotis muricola | IEBR VN11-1186 | Vietnam | MH137299 | MH137365 | MH137496 | Tu et al. (2018) |
| Neoromicia nanus | ROM 100552 | Cote d’Ivoire | JF444203 | Eger et al. (unpublished) | ||
| Neoromicia nanus | ECJS_45_2009, TM 48486 | Botswana | KM886076 | Goodman et al. (2015) | ||
| Neoromicia nanus | DM 7542 | South Africa | GU328062 | Lack et al. (2010) | ||
| Nyctalus leisleri | ZMMU S-167374 | Russia | JF443043 | Kruskop et al. (2012) | ||
| Nyctalus leisleri | M1473, MHNG 1956.071 | Switzerland | JX570901 | Heaney et al. (2012) | ||
| Nyctalus leisleri | FMNH140374 | Pakistan | HM561657 | Roehrs et al. (2010) | ||
| Nycticeinops schlieffeni | UP 0775 | South Africa | KF452658 | McCulloch (unpublished) | ||
| Nycticeinops schlieffeni | IVB S657 | Senegal | JX276305 | Koubinova et al. (2013) | ||
| Nycticeius humeralis | ASNHC 12501 | United States | GU723247 | Streicker et al. (2010) | ||
| Nycticeius humeralis | LSUMZ M8901 | United States | KC747697 | Patrick and Stevens (2014) | ||
| Nycticeius humeralis | TK26380, TTU 49536 | United States | GU328096 | Lack et al. (2010) | ||
| Parastrellus hesperus | MVZ 198302 | United States | GU723249 | Streicker et al. (2010) | ||
| Parastrellus hesperus | NK32223, MSB 75640 | United States | KC747698 | Patrick and Stevens (2014) | ||
| Parastrellus hesperus | TK78703, TTU 79269 | United States | GU328099 | Lack et al. (2010) | ||
| Perimyotis subflavus | JJ107 | United States | GU723254 | Streicker et al. (2010) | ||
| Perimyotis subflavus | TK90671, TTU 80684 | United States | AJ504449 | GU328103 | Stadelmann et al. (2004a); Lack et al. (2010) | |
| Philetor brachypterus | ROM 102019 | Indonesia | HM541204 | KX429688 | Francis et al. (2010); Lim et al. (2016) | |
| Philetor brachypterus | JLS274, FMNH 180236 | The Philippines | JX570922 | Heaney et al. (2012) | ||
| Pipistrellus pipistrellus | ZMMU S-180542 | Switzerland | JF443078 | Kruskop et al. (2012) | ||
| Pipistrellus pipistrellus | MHNG 1807.052 | Greece | AJ504443 | Stadelmann et al. (2004a) | ||
| Pipistrellus pipistrellus | M1439, MHNG 1956.031 | Switzerland | HM561662 | Roehrs et al. (2010) | ||
| Plecotus macrobullaris | Pi08 | Spain | KR134391 | KR134391 | GU328106 | Alberdi et al. (2015); Lack et al. (2010) |
| Scotomanes ornatus | VN11-0238 | Vietnam | MF038585 | MF038484 | MF038345 | Hassanin et al. (2018) |
| Scotophilus heathii | ROM 107786 | HM541926 | EU750944 | GU328112 | Trujillo et al. (2009); Francis et al. (2010); Lack et al. (2010) | |
| Thainycteris torquatus | GS 20813 | MF038577 | MF038478 | MF038338 | Hassanin et al. (2018) | |
| Tylonycteris malayana | VN11-0022 | Vietnam | KX496402 | KX496401 | Tu et al. (2017) | |
| Tylonycteris malayana | M1538, MHNG 1970.041 | Laos | JX570928 | Heaney et al. (2012) | ||
| Tylonycteris tonkinensis | M1203, MHNG 1926.059 | Laos | KX496442 | KX496441 | HM561673 | Roehrs et al. (2010); Tu et al. (2017) |
| Vespertilio murinus | GTHU757, HNHM 24293 | Hungary | MN813972 | MN813976 | MN813980 | This study |
Nomenclatural statement: A Life Science Identifier (LSID) number was obtained for this publication: urn:lsid:zoobank.org:pub:F6DBAD82-EB6A-48AC-A785-68FF698CE6AB.
Literature Cited
- Alberdi A., Gilbert M. T. P., Razgour O., Aizpurua O., Aihartza J., and Garin I... 2015. Contrasting population-level responses to Pleistocene climatic oscillations in an alpine bat revealed by complete mitochondrial genomes and evolutionary history inference. Journal of Biogeography 42:1689–1700. [Google Scholar]
- Amador L. I., Moyers Arévalo R. L., Almeida F. C., Catalano S. A., and Giannini N. P... 2018. Bat systematics in the light of unconstrained analyses of a comprehensive molecular supermatrix. Journal of Mammalian Evolution 25:37–70. [Google Scholar]
- Artyushin I. V., Bannikovs A. A., Lebedev V. S., and Kruskop S. V... 2009. Mitochondrial DNA relationships among North Palaearctic Eptesicus (Vespertilionidae, Chiroptera) and past hybridization between common serotine and northern bat. Zootaxa 2262:40–52. [Google Scholar]
- Bates P. J. J., et al. 2005. A review of the genera Myotis, Ia, Pipistrellus, Hypsugo, and Arielulus (Chiroptera: Vespertilionidae) from Myanmar (Burma), including three species new to the country. Acta Chiropterologica 7:205–236. [Google Scholar]
- Corbet G. B., and Hill J. E... 1992. The mammals of the Indomalayan region. Oxford University Press; Oxford, United Kingdom. [Google Scholar]
- Csorba G., Görföl T., Wiantoro S., Kingston T., Bates P. J., and Huang J. C... 2015. Thumb–pads up-a new species of thick-thumbed bat from Sumatra (Chiroptera: Vespertilionidae: Glischropus). Zootaxa 3980:267–278. [DOI] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., and Posada D... 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerman J. R., and Morrison-Scott T. C. S... 1951. Checklist of Palaearctic and Indian mammals 1758 to 1946. British Museum (Natural History) London, United Kingdom. [Google Scholar]
- Francis C. M., et al. 2010. The role of DNA barcodes in understanding and conservation of mammal diversity in Southeast Asia. PLoS ONE 5:e12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti A., et al. 2012. Integrated Operational Taxonomic Units (IOTUs) in echolocating bats: a bridge between molecular and traditional taxonomy. PLoS ONE 7:e40122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. M., et al. 2015. An integrative approach to characterize Malagasy bats of the subfamily Vespertilioninae Gray, 1821, with the description of a new species of Hypsugo. Zoological Journal of the Linnean Society 173:988–1018. [Google Scholar]
- Görföl T., and Csorba G... 2018. Integrative taxonomy places Asian species of Falsistrellus (Chiroptera: Vespertilionidae) into Hypsugo. Mammalian Biology 93:56–63. [Google Scholar]
- Görföl T., Francis C. M., Bates P. J. J., and Csorba G... 2016. Hypsugo joffrei. The IUCN Red List of Threatened Species. 2016:e.T17345A22127938. 10.2305/IUCN.UK.2016-3.RLTS.T17345A22127938.en. Accessed 24 July 2019. [DOI] [Google Scholar]
- Hassanin A., et al. 2018. Multilocus phylogeny and species delimitation within the genus Glauconycteris (Chiroptera, Vespertilionidae), with the description of a new bat species from the Tshopo Province of the Democratic Republic of the Congo. Journal of Zoological Systematics and Evolutionary Research 56:1–22. [Google Scholar]
- Heaney L. R., Balete D. S., Alviola P., Rickart E. A., and Ruedi M... 2012. Nyctalus plancyi and Falsistrellus petersi (Chiroptera: Vespertilionidae) from northern Luzon, Philippines: ecology, phylogeny, and biogeographic implications. Acta Chiropterologica 14:265–278. [Google Scholar]
- Hill J. E. 1966. A review of the genus Philetor (Chiroptera: Vespertilionidae). Bulletin of the British Museum (Natural History) 14:373–387. [Google Scholar]
- Hill J. E., and Harrison D. L... 1987. The baculum in the Vespertilioninae (Chiroptera: Vespertilionidae) with a systematic review, a synopsis of Pipistrellus and Eptesicus, and the descriptions of a new genus and subgenus. Bulletin of the British Museum (Natural History) 52:225–305. [Google Scholar]
- Ibáñez C., García-Mudarra J. L., Ruedi M., Stadelmann B., and Juste J... 2006. The Iberian contribution to cryptic diversity in European bats. Acta Chiropterologica 8:277–297. [Google Scholar]
- Ivanova N. V., Zemlak T. S., Hanner R. H., and Hebert P. D. N... 2007. Universal primer cocktails for fish DNA barcoding. Molecular Ecology Notes 7:544–548. [Google Scholar]
- Koopman K. F. 1973. Systematics of Indo-Australian pipistrelles. Periodicum Biologorum, Zagreb 75:113–116. [Google Scholar]
- Koopman K. F. 1994. Mammalia: chiroptera. Handbook of Zoology, DeGruyter; Berlin, Germany. [Google Scholar]
- Koubínová D., Irwin N., Hulva P., Koubek P., and Zima J... 2013. Hidden diversity in Senegalese bats and associated findings in the systematics of the family Vespertilionidae. Frontiers in Zoology 10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskop S. V., and Shchinov A. V... 2010. New remarkable bat records in Hoang Lien Son mountain range, northern Vietnam. Russian Journal of Theriology 9:1–8. [Google Scholar]
- Kruskop S. V., Borisenko A. V., Ivanova N. V., Lim B. K., and Eger J. L... 2012. Genetic diversity of Northeastern Palaearctic bats as revealed by DNA barcodes. Acta Chiropterologica 14:1–14. [Google Scholar]
- Kruskop S. V., Solovyeva E. N., and Kaznadzey A. D... 2018. Unusual pipistrelle: taxonomic position of the Malayan Noctule (Pipistrellus stenopterus; Vespertilionidae; Chiroptera). Zoological Studies 57:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., and Tamura K... 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35:1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack J. B., Roehrs Z. P., Stanley C. E., Ruedi M., and van den Bussche R. A... 2010. Molecular phylogenetics of Myotis indicate familial-level divergence for the genus Cistugo (Chiroptera). Journal of Mammalogy 91:976–992. [Google Scholar]
- Lanfear R., Frandsen P. B., Wright A. M., Senfeld T., and Calcott B... 2017. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34:772–773. [DOI] [PubMed] [Google Scholar]
- Letunic I., and Bork P... 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Research 44:W242–W245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L. S., et al. 2016. The systematic position of Hypsugo macrotis (Chiroptera: Vespertilionidae) and a new record from Peninsular Malaysia. Zootaxa 4170:169–177. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Pfeiffer W., and Schwartz T... 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Pp. 1–8 in Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, Louisiana, 14 November 2010 Institute of Electrical and Electronics Engineers (IEEE). Piscataway, New Jersey.
- Patrick L. E., and Stevens R. D... 2014. Investigating sensitivity of phylogenetic community structure metrics using North American desert bats. Journal of Mammalogy 95:1240–1253. [Google Scholar]
- Rambaut A., Drummond A. J., Xie D., Baele G., and Suchard M. A... 2018. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Systematic Biology 67:901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs Z. P., Lack J. B., and van den Bussche R. A... 2010. Tribal phylogenetic relationships within Vespertilioninae (Chiroptera: Vespertilionidae) based on mitochondrial and nuclear sequence data. Journal of Mammalogy 91:1073–1092. [Google Scholar]
- Ronquist F., et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedi M., Eger J. L., Lim B. K., and Csorba G... 2018. A new genus and species of vespertilionid bat from the Indomalayan region. Journal of Mammalogy 99:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia U., Csorba G., and Ruedi M... 2017. First records of Hypsugo joffrei (Thomas, 1915) and the revision of Philetor brachypterus (Temminck, 1840) specimens (Chiroptera: Vespertilionidae) from the Indian subcontinent. Revue suisse de Zoologie 124:83–89. [Google Scholar]
- Sikes R. S., and T he A nimal C are and U se C ommittee of the A merican S ociety of M ammalogists. 2016. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97:663–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons N. B. 2005. Order chiroptera. Pp. 312–529 in Mammal species of the world: a taxonomic and geographic reference. 3rd ed. (D. E. Wilson and D. M. Reeder, eds.). The Johns Hopkins University Press; Baltimore, Maryland. [Google Scholar]
- Stadelmann B., Herrera L. G., Arroyo-Cabrales J., Flores-Martínez J. J., May B. P., and Ruedi M... 2004a. Molecular systematics of the fishing bat Myotis (Pizonyx) vivesi. Journal of Mammalogy 85:133–139. [Google Scholar]
- Stadelmann B., Jacobs D. S., Schoeman C., and Ruedi M... 2004b. Phylogeny of African Myotis bats (Chiroptera, Vespertilionidae) inferred from cytochrome b sequences. Acta Chiropterologica 6:177–192. [Google Scholar]
- Stadelmann B., Lin L. K., Kunz T. H., and Ruedi M... 2007. Molecular phylogeny of New World Myotis (Chiroptera, Vespertilionidae) inferred from mitochondrial and nuclear DNA genes. Molecular Phylogenetics and Evolution 43:32–48. [DOI] [PubMed] [Google Scholar]
- Streicker D. G., Turmelle A. S., Vonhof M. J., Kuzmin I. V., McCracken G. F., and Rupprecht C. E... 2010. Host phylogeny constrains cross-species emergence and establishment of Rabies virus in bats. Science 329:676–679. [DOI] [PubMed] [Google Scholar]
- Swofford D. L. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates; Sunderland, Massachusetts. [Google Scholar]
- Tate G. H. H. 1942. Results of the Archbold expeditions 47. Review of the vespertilionine bats, with special attention to genera and species of the Archbold collections. Bulletin of the American Museum of Natural History 80:221–297. [Google Scholar]
- Thomas O. 1915. On bats of the genera Nyctalus, Tylonycteris, and Pipistrellus. Annals and Magazine of Natural History, Series 8 15:225–232. [Google Scholar]
- Trujillo R. G., Patton J. C., Schlitter D. A., and Bickham J. W... 2009. Molecular phylogenetics of the bat genus Scotophilus (Chiroptera: Vespertilionidae): perspectives from paternally and maternally inherited genomes. Journal of Mammalogy 90:548–560. [Google Scholar]
- Tu V. T., Hassanin A., Furey N. M., Son N. T., and Csorba G... 2018. Four species in one: multigene analyses reveal phylogenetic patterns within Hardwicke’s woolly bat, Kerivoula hardwickii-complex (Chiroptera, Vespertilionidae) in Asia. Hystrix, the Italian Journal of Mammalogy 29:111–121. [Google Scholar]
- Weyeneth N., Goodman S. M., Stanley W. T., and Ruedi M... 2008. The biogeography of Miniopterus bats (Chiroptera: Miniopteridae) from the Comoro Archipelago inferred from mitochondrial DNA. Molecular Ecology 17:5205–5219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.