Abstract
ERECTA gene family encodes leucine-rich repeat receptor-like kinases that control major aspects of plant development such as elongation of aboveground organs, leaf initiation, development of flowers, and epidermis differentiation. To clarify the importance of ERECTA signaling for the development of soybean (Glycine max), we expressed the dominant-negative ERECTA gene from Arabidopsis thaliana that is truncated in the kinase domain (AtΔKinase). Expression of AtΔKinase in soybean resulted in the short stature, reduced number of leaves, reduced leaf surface area and enhanced branching in the transgenic plants. The transgenic AtΔKinase soybean plants exhibited increased tolerance to water deficit stress due to the reduction of total leaf area and reduced transpiration compared to the wild-type plants. Production of seeds in AtΔKinase lines was higher compared to wild type at regular conditions of cultivation and after exposure to drought stress. Transgenic seedlings expressing AtΔKinase were also able to withstand salt stress better than the wild-type. Established results demonstrated the significance of native soybean genes (GmER and GmERL) in development and stress response of soybean, and suggested that the truncated ERECTA gene of Arabidopsis thaliana can be used to manipulate the growth and stress response of different crop species.
Introduction
One of the major questions of developmental biology is how the body and organ size of multicellular organisms is controlled by intrinsic factors [1] The ERECTA gene family of leucine-rich repeat receptor-like kinases (LRR-RLK) is a pleiotropic regulator of various developmental processes [2]. In Arabidopsis thaliana, the synergistic action of three ERECTA-gene family, ERECTA(ER),ERECTA-LIKE 1(ERL1) and ERECTA-LIKE 2(ERL2), controls aboveground organ growth and flower development. These genes regulate shoot apical meristem size, help to establish phyllotaxy, and promote cotyledon [3,4]. Arabidopsis thaliana ERECTA gene family inhibits the differentiation of the protodermal cells into meristemoid mother cells, thereby preventing the formation of guard mother cells [3]. Further, through expression in the epidermis, ERECTA gene family controls stomatal formation. These genes decrease the stomatal density in leaves, reducing the overall stomatal conductance and the water loss [5].
ERECTA signaling has been extensively studied. It has been demonstrated that the ERECTA family receptors are localized in the plasma membrane, where they sense small extracellular peptide [6]. The ER family receptors after perceiving the secreted peptide ligands, epidermal patterning factor 1(EPF1) and epidermal patterning factor 2 (EPF2) modify stomatal patterning [7–9]. Recently, two more peptides (EPF4 and EPF6) were also identified as ligands in the signaling process [10,11]. Shpak et al., (2005) suggested that TMM (TOO MANY MOUTHS) LRR-receptor-like protein forms a heterodimer with the ER family RLK and prevents signaling, thereby negatively regulating stomatal differentiation. A MAP kinase cascade consisting of YODA (YDA), MKK4/MKK5 and MPK3/MPK6 operate downstream of ER receptors [12]. It was suggested that MAPKc, cascade involved in regulating inflorescence architecture is based on both gain- and loss-of-function data [13–15].
Up to date, the functions of ERECTA gene family were mostly characterized in Arabidopsis, which is considered as a model plant for all genetic studies. ERECTA plays an important role in regulating the size of the aboveground organs of Arabidopsis [14]. Loss of function of ERECTA leads to plants with short stature and tightly clustered inflorescences which is due to the reduced internode and pedicel lengths [16]. Also mutant ERECTA (er) plants of Arabidopsis also display shorter hypocotyls, smaller cotyledons, and leaves with short petioles and wider flowers with short and blunt siliques, with a reduced number of cortex cells in the stems and pedicels but more expanded cells than the wild-type [6,13,15,17,18]. The truncated ERECTA protein that lacks the cytoplasmic kinase domain (ΔKinase) confers dominant-negative effects in Arabidopsis including compact inflorescence and short, blunt siliques [13]. Analysis of the ERECTA gene family phylogenetic tree suggested that this group of genes are quite conserved between Arabidopsis and other plant species [25]. Thus, Arabidopsis er phenotypes serve as evidence that modifications of ERECTA signaling in valuable crop species could lead to desirable phenotypical traits that can be achieved through overexpression or suppression of ERECTA genes.
However, studies of ERECTA gene family other than Arabidopsis plants are still limited. Involvement of an ER homolog in Sorghum bicolor, SbRLK1 in the regulation of specific processes in the mesophyll cells was reported [19]. In Zea mays, ERECTA genes play a role in controlling plant growth, organ size and yield of plants [20]. Lately, ERECTA signaling in Solanum lycopersicum was manipulated by expressing AtΔKinase from Arabidopsis using two different promoters [25]. Thus, the expression of AtΔKinase under the control of 35S promoter dramatically reduced vegetative growth and production of seeds in the transgenic tomato lines [25]. Expression of AtΔKinase under the control of its own promoter, on the other hand, resulted in relatively moderate inhibition of tomato plant height but significant decrease in the number of leaves and total leaf area [25].
Several research studies [21, 22, 23, 25] reported links between ERECTA signaling and plant response to abiotic stress. For example, two homologs of ERECTA in the Triticum aestivum genome, TaER1 and TaER2, were recently connected with the improvement of transpiration efficiency and yield in bread wheat [21]. The role of ERECTA gene family in regulating thermotolerance in Oryza sativa and tomato has recently been characterized [22]. Thermotolerance was reduced in a loss-of-function ER homolog rice mutant and tomato with reduced expression of a tomato ER allele [22]. Lately, it has been shown that an ER homolog in wild common bean might be associated with drought [23]. Authors reasoned that the reduction of total leaf area (evaporating surface area) in the transgenic tomato plants, caused by the expression of AtΔKinase, conferred drought tolerance [25]. Most importantly, these transgenic tomato lines did not suffer yield loss as determined by fruit size and number per plant. Described traits will be very desirable for many crops including soybean (Glycine max). Du et al., [24] found that several soybean ER homologs can be upregulated by water stress. Based on the sequence similarity between Arabidopsis ER genes and the predicted soybean ER and ERL genes [25], it is logical to assume that Arabidopsis and soybean ER signaling pathways are quite conserved. Therefore, Arabidopsis ER gene can be used to manipulate the development and stress response in soybean as was previously documented for tomato plants [25].
Here, we determined the role of ERECTA signaling in the development and stress response of soybean plants by disrupting ERECTA signaling using the transgenic approach. Soybean lines expressing the truncated ERECTA protein from Arabidopsis (AtΔKinase) were established and analyzed. AtΔKinase expressing transgenic soybean lines exhibited short stature, reduced leaf area, and a significant increase in tolerance to increased salinity and water deficit stress. We demonstrated that the establishment of more compact and stress-tolerant crop plants with no yield penalty can be achieved successfully by suppressing ERECTA signaling.
Materials and methods
Vector construction and transgenic soybean lines development
The 8.018 kb EcoR1 –BamH1 fragment from pESH454 consisting of Arabidopsis ERECTA gene truncated in the region encoding the kinase domain (AtΔKinase) [13] was cloned into soybean transformation vector pTF101.1 [26] obtained from Iowa State University using the standard cloning techniques. The truncated ER gene in pNS37 contains native Arabidopsis ER (At2g26330) promoter (1.8 kb), gene fragment (4.2 kb), and the native transcription terminator (1.9 kb). The 6 kb EcoR1 –BamH1 fragment from pESH454 was cloned between EcoR1 and BamH1 sites of pTF101.1 followed by the introduction of 2 kb BamH1 fragment to build pNS37. The ER gene fragment consists of exons and introns for LRR repeats and transmembrane (TM) region. There is a stop codon immediately after TM sequence followed by BamH1 site and ER terminator. The pTF101.1 vector contains 2 x 35S promoter-driven Bar gene as the selection marker (S1 Fig). The resulting pNS37 vector was submitted to Iowa State University for developing transgenic soybean lines using the Williams 82 genotype, where Agrobacterium-mediated transformation protocol [27,28] was pursued to develop putative transgenic lines.
Expression analysis of transgene and native soybean ERECTA genes using real-time RT-PCR
Total RNA was isolated from the apex and young leaves of 21-day-old soybean plants, flowers from 35-d-old mature plants and siliques of 45-d-old plants using an RNeasy Plant Mini kit (Qiagen, Germany). The cDNA was generated from 1μg of total RNA using a SuperScript III First-Strand Synthesis System (Invitrogen, USA) with a dT20 oligonucleotide as primer according to the manufacturer’s protocol. cDNA samples were diluted and used for real-time quantitative PCR analysis with SYBR Green PCR master mix (Thermo Fisher Scientific, UK) in an iCycler iQ Multicolor Real-Time PCR detection system (Bio-Rad, USA). AtΔKinase gene was amplified using primers: 5’-ATGGTATGACATTTGACTCCAAAC -3’ and 5’-GTGGATCTATTCCTCCGATTCTC -3’. House-keeping gene (18S) was amplified using primers: 5’-AGGCCGCGGAAGTTTGAGGC-3’ and 5’-ATCAGTGTAGCGCGCGTGGG -3’. The primers used to amplify the native GmERL1 gene (NP_001237639) were: 5’-GCTCGGAATAGGCTCAGTGG -3’(Forward) and 5’-ACGATATGTCCAGGTACTGCAA-3’(Reverse). To amplify the native GmERL2 gene (NP_001235330), 5’-CTAGTGGAACTGGGCAAGG-3’(Forward) and 5’-TGGGTGGCCATAATAATACTAAGCA-3’(Reverse) primers were used. The native GmERL3 gene (XP_003534036) was amplified using the primers: 5’-TGTTGGCTTTTGTGGGCAAG-3’(Forward) and 5’-TCGCTGAGTGGTGAAGCAAA-3’(Reverse). House-keeping gene (18S) was amplified using primers: 5’-AGGCCGCGGAAGTTTGAGGC-3’(Forward) and 5’-ATCAGTGTAGCGCGCGTGGG -3’(Reverse). Three independent biological replicates were used in the analysis. For each biological replica, three technical replicas were run. The real-time PCR data analyzed by the ‘comparative count’ method to obtain relative mRNA expression of each tissue as described in the iCycle manual (Bio-Rad).
Plant growth conditions and phenotypical analysis of transgenic soybean plants
Seedlings (10-day-old) of wild-type and the selected transgenic soybean lines (3–1, 2–1, 2–4 and 1–2) were transferred into small pots containing sterile Sun Shine Redi-earth Professional growing mix. All plants were grown in a growth chamber under conditions of 12 h light (25 °C) and 12 h dark (20 °C), 45% humidity, and 500 μmol m–2 s–1 light intensity and were watered once a day. Three-week-old seedlings were transferred to the greenhouse in a bigger pot. Seven mature plants from each experimental group (WT, 3–1, 2–1, 2–4 and 1–2) were phenotypically analyzed, and the following data were recorded: length of the leaves, the total number of leaves and number of branches in 66-d-old plants, number of mature siliques and number of seeds in 80-day-old plants. One-way ANOVA (Analysis of Variance) with post-hoc Tukey HSD (Honestly significant difference) using SAS software (SAS Studio 3.8) [29] was used to assess the significance of differences in the data between groups of plants. In a separate experiment, plant height was recorded every 5 days starting from 30-day-old plants, up to the state of full maturity (70 days). Total leaf surface area was measured in 66-day-old plants by removing all the leaves from a plant and then measuring the area using the portable leaf area meter android [Biovis Leaf Av (Android version), Expert Visions Labs Pvt. Ltd, India].
Water loss assay and drought stress experiment
To examine the ability of transgenic plants to lose water, three flag leaves were excised from each 4-week-old wild-type and transgenic plants grown in the greenhouse, and the fresh weight was immediately determined. All the leaves were placed on a laboratory bench at room temperature for 300 min. Every 30 min, leaf weight was recorded. Water loss was calculated as the percentage of the initial fresh weight at each time point. For each genotype (WT, 3–1, 2–1, 2–4 and 1–2), five plants were tested. The relative water content of the detached leaves was measured by the leaf disc method [30]. Six leaves were cut from each 4-week plant grown in the greenhouse. Leaf discs were made from each leaf with the help of a cork borer and fresh weight was immediately measured. The discs were dipped in distilled water and kept in the refrigerator (4 °C) for 24 hours to reach full turgor. The turgid weight and later the dry weight after drying the discs at 70°C for 24 hours was measured. The RWC in % was calculated by the formula: RWC (%) = [(fresh weight-dry weight)/(turgid weight-dry weight)] x100. To test drought tolerance at the adult stage, wild type and a transgenic line were grown under normal watering conditions for 4 weeks and then subjected to drought stress by withdrawing irrigation for the next 6 weeks. After 14 days of drought stress, drought tolerance phenotypes were examined, and pictures of transgenic lines and wild-type were captured. A total of 10 plants for each of WT and transgenic lines were evaluated in water deficit experiment. During the drought stress experiment, the volumetric water content of the soil was measured at 0, 5, 7 and 9 days using the ProCheck decagon device (Decagon Devices, Inc., USA). The photosynthetic activity and the stomatal conductance was also measured using a portable photosynthesis system (Li-Cor LI-6400XT) (LI-COR Biosciences, USA). Three measurements were made for each plant, and 7 plants were used for both the wild type and the transgenic plants.
Salt stress experiments
To estimate the working concentration of NaCl for salt stress experiments, the wild type seeds were grown on both Murashige and Skoog medium (MS) without NaCl supplement (control) and MS media supplemented with different concentrations of NaCl (50mM, 100mM, 150mM, 200mM, 300mM, and 400mM). The shoot and root lengths, as well as fresh and dry biomass of shoots and roots of 10-day-old germinated seedlings, were measured and the data were analyzed statistically using One-way ANOVA (Analysis of Variance) with post-hoc Tukey HSD (Honestly significant difference) using SAS software. In the salt experiment involving transgenic lines, the seeds of wild type and transgenic lines were germinated in the presence of NaCl. Seeds were surface-sterilized using chlorine gas in a desiccator and planted on both pure MS medium (control) and medium supplemented with 100 mM NaCl. Seeds were germinated under continuous light and the germination rate was recorded daily for 10 days. To test salt tolerance at the seedling stage, 10-day old seedlings were photographed to visualize the phenotypes. The root and shoot lengths, as well as whole fresh and dry biomass, were also measured. All tests were repeated thrice, and One-way ANOVA (Analysis of Variance) with post-hoc Tukey HSD (Honestly significant difference) using SAS software was used for statistical analysis.
Results
Development of transgenic soybean plants expressing AtΔKinase transgene
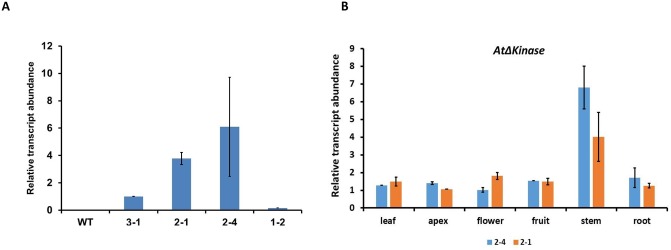
To disrupt ERECTA signaling in soybean, we took the dominant-negative approach and expressed a truncated version of the Arabidopsis ERECTA gene (AtΔKinase) under the control of native ER promoter (AtERECTApro::AtΔKinase) in soybean plants. The truncated ERECTA binds with its ligands and forms heterodimers; however, due to the deletion in the kinase domain, it is unable to phosphorylate and trigger signaling [13]. Therefore, the expression of AtΔKinase is expected to suppress ER signaling. A similar approach was successfully used in the tomato model plant [25], where expression of AtΔKinase resulted in drastic modification of tomato phenotype, including dwarfing of tomato plants and reduction in total leaf area. To determine the effect of AtΔKinase expression in soybean (cv. Williams 82), T1 seeds of 4 putative independent lines established as a result of transformation with AtERpro:AtΔKinase plasmid were obtained from Plant Transformation Facility, Iowa State University. PCR analysis on germinated T1 seedlings of each line showed the presence of the AtΔKinase gene. Low transformation efficiency can be explained by the strong effect of AtΔKinase transgene resulting in developmental defects. The expression of the transgene (AtΔKinase) in four established lines was confirmed by qPCR analysis (Fig 1A). The highest level of AtΔKinase was found in two transgenic lines (2–1 and 2–4). Both lines were selected for monitoring of AtΔKinase expression in different organs of transgenic lines (Fig 1B). It was confirmed that the AtΔKinase gene was expressed in leaves, apex, stem, root, flowers, and fruits of both selected lines. The highest expression of the transgene was observed in the stems of transgenic plants. Same transgenic lines (2–1 and 2–4) were used for the analysis of the expression of three native soybean ERECTA genes (GmERL1, GmERL2, GmERL3) in different organs (leaves, apex, stem, root, flowers, and fruits) of the wild type and AtΔKinase expressing lines (S2 Fig). We documented that the expression of all three native soybean ERECTA genes was enhanced in all analyzed tissues of the transgenic lines compared to wild type.
Fig 1. Analysis of AtΔKinase gene expression in the four generated transgenic lines (A) and different organs of selected AtERpro:AtΔKinase lines (B).
The analysis was performed using quantitative RT-PCR. 18S was used as an internal control. For A- Samples collected from 14-day-old plants. For B- Leaves, apex, roots, stems were collected from 21-day-old plants, flowers were collected from 35-day-old plants, siliques were collected from 45-day-old plants. Results are shown as means ±SE of three biological replicates.
Effects of suppression of ERECTA signaling on the phenotype of transgenic plants
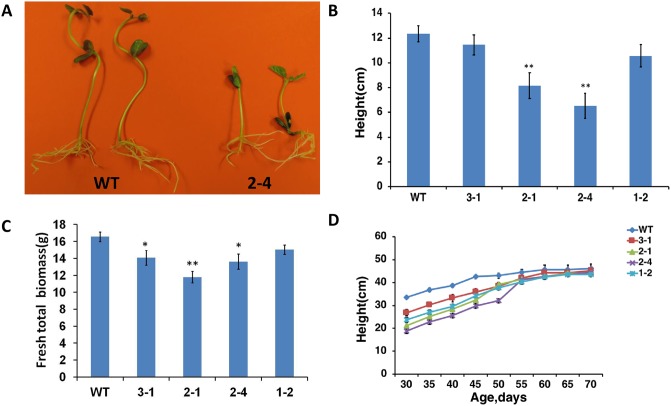
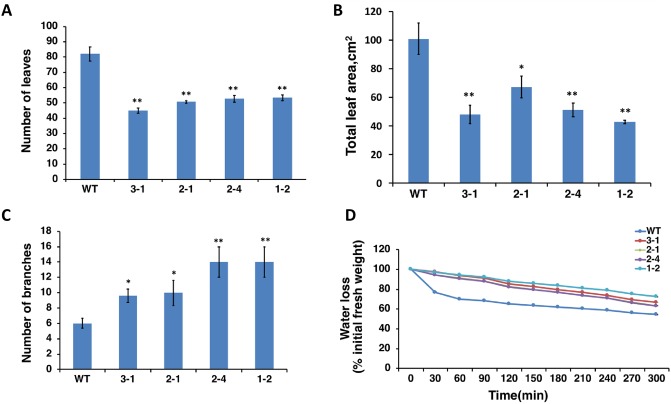
At the stage of young seedlings (10-day-old plants) and young plants (40-day-old), AtERpro:AtΔKinase expressing soybean lines grew slowly and appeared short compared to the wild-type (Fig 2A and 2B). As expected, the reduction in the size of transgenic plants resulted in a decrease in total biomass compared with the wild-type (Fig 2C). However, AtERpro:AtΔKinase lines reached the size of the wild-type plants at the maturity stage (Fig 2D). Thus, 70-day-old fully mature transgenic plants of four independent lines (3–1; 2–1; 2–4; 1–2) had the same height as the wild-type soybean plants (Fig 2D). At the same time, mature transgenic plants developed less number of leaves (Fig 3A) with decreased total leaf area per plant (Fig 3B) compared with the wild-type. On the contrary, the transgenic plants were found to have more branches (Fig 3C) than the wild-type, giving them short, compact and bushy appearance. It is important to note that AtERpro:ΔKinase soybean lines produced more seeds per plant than wild type. Thus, 80-day-old transgenic lines 3–1, 2–1, 2–4, 1–2 produced 12.5%, 39.2%, 23%, and 35.5% more seeds respectively compared to wild type plants of the same age (S3 Fig). Thus, we can conclude that the expression of a truncated version of Arabidopsis ERECTA (AtΔKinase) interfered with native soybean ERECTA signaling and inhibited the vegetative growth and development of transgenic soybean plants. These results were consistent with the results that were established for tomato expressing AtΔKinase [25]. It is interesting that the leaves detached from the AtERpro:ΔKinase transgenic lines were able to retain water and maintain initial weight than the leaves detached from the wild-type plants (Fig 3D). Previously, we reported that the disruption of ERECTA signaling in tomato plants through the expression of Arabidopsis ΔKinase led to significant improvement of drought tolerance in the transgenic tomato plants [25]. We also hypothesized that tolerance to water deficit was improved due to the considerable reduction of evaporating surface area (total leaf area) that may contribute to the reduction of transpiration in transgenic tomato plants expressing AtΔKinase. Since we have observed a decrease of leaf number and leaf area in AtERpro:ΔKinase transgenic soybean lines, we next addressed the response of AtERpro:ΔKinase transgenic lines on water deficit stress.
Fig 2. AtERpro:AtΔKinase transgenic soybean plants grow slowly compared with wild-type plants but can reach the same height at maturity.
(A) Phenotypical comparison of 10-day-old seedlings of wild-type and AtERpro:AtΔKinase plants. (B) Height (n = 10) in 40-day-old wild-type soybean plants and AtERpro:AtΔKinase expressing lines. (C) Accumulation of fresh biomass (n = 6) of wild-type and AtERpro:AtΔKinase soybean transgenic lines after 90 days of cultivation in a growth chamber (D) Gradual changes in the height (n = 4) of wild-type and four transgenic soybean lines during 70 days of cultivation in a growth chamber. Results are shown as means ±SE (*P<0.05;**P <0.01). T1 generation of transgenic lines was analyzed in the presented phenotypical tests.
Fig 3.
AtERpro:AtΔKinase transgenic plants generate less number of leaves (A) with smaller leaf area (B) but produce more branches (C) compared with wild-type soybean plants. Total leaf area per plant and a total number of leaves were calculated for 66-day-old wild-type and four AtERpro:AtΔKinase transgenic lines (n = 7; **P <0.01; *P <0.05). The number of branches was estimated for 62-day-old wild type and transgenic lines cultivated in greenhouse conditions. Water loss (D) in fully expanded and detached leaves was calculated for 58-day-old wild type and four transgenic lines. T3 generation of transgenic lines was used in stress experiments. Results are shown as means ±SE.
Expression of AtERpro: AtΔKinase alters the response to water deficit stress in transgenic soybean plants
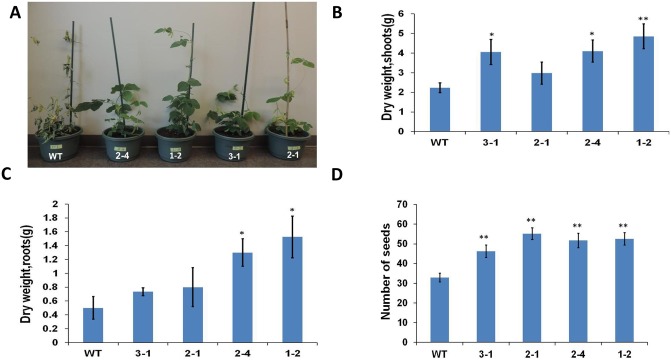
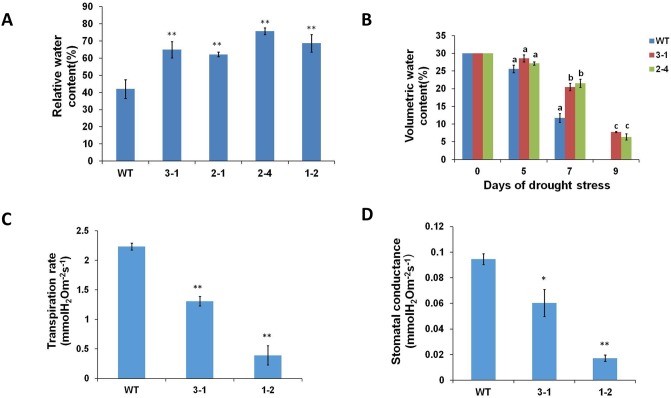
We exposed AtERpro:ΔKinase transgenic soybean lines to water deficit stress by terminating water supply for 6 weeks. Soil moisture in pots was measured as volumetric water content (%) before the experiment and during the stress experiment. Initial volumetric water content (before water withholding) content was equal between pots used for the cultivation of wild-type and transgenic lines. After seven days of water termination, the wild-type plants exhibited typical symptoms of stress such as wilting and rolling of leaves, while the AtERpro:AtΔKinase transgenic lines appeared unstressed. The first symptoms of drought stress in the transgenic plants were observed after 2 weeks of drought stress, and such symptoms were less obvious compared to that in the wild-type (Fig 4A). At the end of stress experiment, the number of seeds per plant, as well as dry weight of the shoot and root biomass and number of produced seeds, was recorded (Fig 4B, 4C and 4D). We found that transgenic soybean lines produced more dry biomass of roots and shoots under water deficit conditions compared to the wild-type plants (Fig 4B and 4C). This can be explained by the ability of transgenic plants to retain water and continue growth for a longer time during the water withholding period than the wild-type control plants. Seed yield is the most valuable trait of soybean [31]. Previously, we have noticed that the expression of AtΔKinase in tomato plants did not cause a reduction of photosynthetic ability and suppression of the reproductive system (production of fruits/seeds per plant) [25]. Similarly, transgenic soybean plants expressing the same gene (AtΔKinase) and exposed to prolonged water deficit stress produced more seeds per plant compared with the wild-type (Fig 4D). To clarify the links between transpiration and the observed resistance to water deficit, we measured the stomatal conductance as well as the transpiration rate during the water deficit stress experiment (Fig 5). We did not detect any statistically significant differences in the number of stomata per leaf between wild-type and the transgenic lines (S4 Fig) However, both the stomatal conductance and transpiration rate were significantly decreased in the two selected transgenic lines (lines 3–1 and 1–2) compared to that of wild type plants (Fig 5C and 5D). As a result of decreased transpiration, the relative water content in the leaves was higher in AtΔKinase lines than in the wild-type (Fig 5A). To evaluate remaining soil moisture during the water deficit experiment, we measured volumetric water content in pots used for plant cultivation. As showed in Fig 5B, pots used for cultivation of transgenic plants maintained more moisture than pots with wild-type plants during the first 9 days of cultivation without watering. Based on these experimental data, we hypothesized that the increased drought tolerance of established transgenic lines is related to lower transpiration surface resulting from the smaller total leaf area of the transgenic AtERpro:AtΔKinase plants. To understand if ERECTA signaling is also linked with plant stress signaling pathways at the molecular level, we have selected two soybean genes (GmRD22-Like and GmPIP1-2) that can be used as markers for plant response to osmotic stress. GmRD22-Like is an apoplast‐localized BURP‐domain protein-encoding gene [42] and GmPIP1-2 is the soybean water channel protein-encoding gene [44]. Expression of both selected genes was monitored by q-PCR in leaves of soybean wild type and all four AtERpro:AtΔKinase lines exposed to drought stress for seven days (S5 Fig). We have noticed a significant increase of expression of both genes in three transgenic lines (2–1; 2–4; 1–2) exposed to water deficit stress (S5 Fig).
Fig 4. AtERpro:AtΔKinase transgenic plants are more tolerant to water deficit stress.
52-day-old AtERpro:AtΔKinase plants exhibited fewer visible stress symptoms after two weeks of incubation without watering (A). Dry biomass of shoots (B), roots (C) and the number of seeds (D) produced by 82-day-old wild type and transgenic lines after cultivation in water deficit conditions. n = 5; **P <0.01; *P <0.05. T3 generation of transgenic lines was used in stress experiments. Results are shown as means ±SE.
Fig 5. AtERpro:AtΔKinase transgenic plants transpire less water under water deficit conditions compared to the wild type.
(A) Relative water content (leaves) in 58-day-old wild type and transgenic lines grown in regular greenhouse conditions. (B) Soil moisture expressed as volumetric water content (n = 7) in pots used for cultivation of wild-type and transgenic lines during 0, 5, 7 and 9 days of drought stress. Letter ‘a’ denote insignificant difference compared to that of wild type. Letters b and c denote significant differences compared to that of wild type. Letter b denotes p < 0.05, Letter c denotes p < 0.01 (C) Transpiration rate of wild-type and transgenic plants in day 7 of water deficit stress. (D) Stomatal conductance (n = 3) in leaves of wild type and transgenic AtERpro:AtΔKinase lines in day 7 of water deficit stress (n = 7). Results are shown as means ±SE.
Expression of AtERpro:AtΔKinase results in enhancement of tolerance of transgenic soybean plants to salt stress
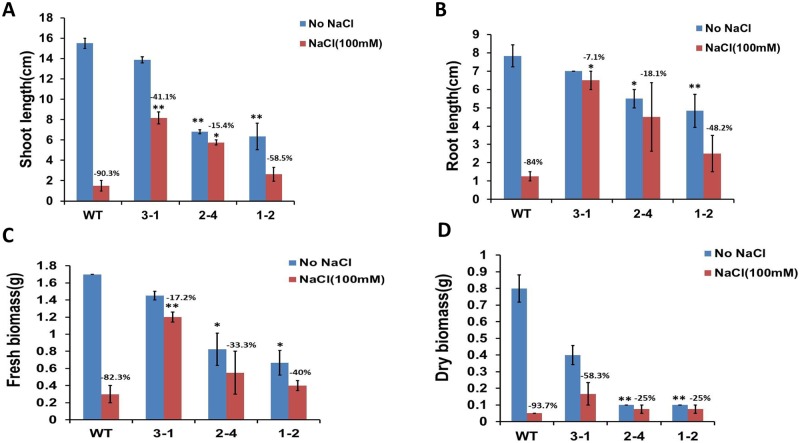
To estimate the appropriate concentration of NaCl for salt stress experiments involving transgenic lines, NaCl in a wide range of concentrations was applied to the seeds of wild-type soybean (S6 Fig). 10-day-old wild-type seedlings were subjected for measurement of the length of shoots and estimation of the total biomass of NaCl-treated seedlings. Based on such measurements we found that NaCl in a concentration of 100 mM was toxic for soybean plants but the damage caused to the seedlings was less as seen at higher NaCl doses (S6 Fig). As the next step, seeds of wild-type and two AtΔKinase expressing lines (3–1,1–2) were exposed to regular MS medium and MS medium supplemented with 100mM NaCl. Seed germination rate, as well as accumulation of biomass of transgenic and control lines during 10 days of cultivation on regular MS medium and MS medium supplemented with salt, were recorded. As shown in Fig 6 and S7 Fig, AtERpro:AtΔKinase lines exhibited higher tolerance to salt stress compared to the wild-type. Thus, two selected transgenic lines (3–1 and 1–2) had a higher germination rate on NaCl-supplemented medium (S7 Fig). When the seeds were germinated and cultivated under salt stress (100 mM NaCl) for 10 days, AtERpro:AtΔKinase lines developed longer shoots and roots, and accumulated more total fresh and dry biomass than the wild-type seedlings exposed to the same salt stress (Fig 6A, 6B, 6C and 6D; S7 Fig). However, if seedlings were grown in regular MS medium (without NaCl supplement), transgenic plants were smaller and produced less biomass than wild-type seedlings (Fig 6A, 6B, 6C and 6D).
Fig 6. AtERpro:AtΔKinase transgenic soybean seedlings produce longer shoots, roots and total biomass than wild type plants under salt stress (NaCl, 100 mM).
(A) Comparison of the shoot length in 10-day-old seedlings of wild type and transgenic lines grown under salt stress. (B) Comparison of the root length in 10-day-old wild type and transgenic seedlings grown under salt stress. (C) Comparison of the weight of total fresh biomass in the 10-day-old wild type and transgenic lines grown under salt stress. (D). Comparison of the weight of total dry biomass in the 10-day-old wild type and transgenic lines grown under salt stress. The seedlings (10-day-old) were grown both on regular MS medium (control) and in MS medium supplemented with NaCl (100mM) (n = 10; **P <0.01; *P <0.05). Results are shown as means ±SE.
Discussion
ERECTA is a leucine-rich repeat RLKs, which in turn are the plasma membrane-localized receptors that sense the extracellular signals in plants [32]. Phylogenetic analysis of the ERECTA gene family suggested that such a group of genes originated at an early stage of evolution. According to the constructed phylogenetic tree of the ERECTA family, three soybean ERECTA genes (GmERL1, GmERL2, GmERL3) are evolutionarily very close to Arabidopsis (AtERL2, AtERL1) and tomato (SlERL) ERECTA genes [25]. Thus, it is logical to expect the existence of functional similarity between Arabidopsis, tomato, and soybean ERECTA genes. Here, we took a dominant-negative approach to terminate soybean ERECTA signaling through the expression of Arabidopsis thaliana ΔKinase gene controlled with the Arabidopsis (AtERpro) promoter in soybean plants. The observed modification to architecture, productivity and stress response in AtERpro:AtΔKinase expressing soybean lines was very similar to changes that were earlier observed in AtERpro:AtΔKinase expressing tomato lines [25]. Transgenic soybean plants exhibited a significant reduction of height at a young stage and restored size at the stage of maturity. The total leaf number was decreased and branching was enhanced in the mature transgenic plants.
Similar to the AtERpro:AtΔKinase tomato plants [25], soybean AtERpro:AtΔKinase plants expressed AtΔKinase gene in all analyzed tissues including young leaves and apex. Interestingly, the expression of three soybean native ERECTA genes (GmERL1, GmERL2, GmERL3) was significantly enhanced in AtERpro:AtΔKinase soybean lines compared to the wild type (S2 Fig). This trend was noticed in all analyzed organs including leaf, apex, fruit, stem, and root. Similarly, native tomato ERECTA genes (SlER and SlERL) were overexpressed in AtERpro:AtΔKinase tomato plants compared to tomato wild type [25]. This observation can indicate that termination of ERECTA signaling by expression of AtERpro:AtΔKinase can lead to compensation effect through induction of native ERECTA genes.
It is well known that ERECTA signaling is associated with abiotic and biotic stress response [2]. The petioles in Arabidopsis er mutants are shorter compared to that of the wild-type, and in response to certain environmental changes like flooding, low light, and shade, ERECTA can promote hyponastic growth and petiole elongation due to its effect on petiole morphology [2, 33]. Overexpression of the ERECTA gene from Populus nigra in Arabidopsis led to increased photosynthetic rate, decreased transpiration and increased water use efficiency [34]. ERECTA was also reported to protect against heat stress in Arabidopsis during an adaxial-abaxial polarity formation in the leaves [35], and contribute to disease resistance by reducing pathogen invasion and spread [2]. Such an increase of biotic stress tolerance is most likely linked to the impact of ERECTA on plant morphology, especially on the structure of epidermis and vasculature [2]. Recently, it was discovered that ERECTA interacts with BAK1 (BRASSINOSTEROID INSENSITIVE 1-associated kinase 1) receptor-like kinases, and regulates immune responses in Arabidopsis [36]. Overexpression of ERECTA, improved thermotolerance in rice and tomato [22]. On the contrary, er mutations led to decreased thermotolerance in both tested species [22].
The exact mechanism of links between abiotic stress signaling and ERECTA signaling is not clarified yet. Such a mechanism most likely is associated with drastic morphological changes observed in transgenic lines overexpressing ERECTA genes or in the er mutants. Indeed, it was reported that er mutation can alter leaf morphology including stomatal density, epidermal cell expansion, mesophyll cell proliferation, circadian leaf movements and petiole growth [5, 37, 38]. Other changes in leaf morphology caused by the termination of ERECTA signaling can also contribute to the modified response to environmental stress. Here, we have linked observed reduction of total leaf area in AtΔKinase transgenic soybean lines with a reduction of total transpiration and as a result, enhanced tolerance to water deficit stress (Figs 2, 3 and 4). Grain production is the major soybean trait. AtΔKinase expressing soybean lines produced more seeds per plant after long-term exposure to water deficit stress compared to the wild–type. Thus, genetic modification of ERECTA signaling can be successfully used for the improvement of grain-producing crops without a reduction in the yield. Observed improvement of salt stress tolerance of AtERpro:AtΔKinase is another beneficial trait of soybean plant with terminated ERECTA signaling (Fig 6). Salinity is one of the greatest environmental challenges and one of the best solutions is to create salt-tolerant cultivars [39]. It is predicted that about 50% of the available land will be affected by salinity by 2050 [40]. Taking in account our data demonstrating improvement of drought and salt tolerance of soybean AtERpro:AtΔKinase transgenic lines and early documented thermotolerance of transgenic tomato and rice lines overexpressing Arabidopsis ER gene [22], we can conclude that genetic manipulations with ERECTA signaling may significantly contribute to the creation of crop cultivars with enhanced tolerance to different types of osmotic stress. Here, we have provided experimental evidence that the observed enhanced tolerance of AtERpro:AtΔKinase soybean lines to water deficit stress (Fig 3) can be mainly associated with phenotypical modifications of transgenic leaves including the reduced leaf surface area. However, ERECTA signaling may also be associated with other stress signaling pathways. Although our main focus was not on studies of such molecular links, we have monitored the expression of two soybean stress-inducible genes (GmRD-22 Like and GmPIP1-2) in wild type and AtERpro:AtΔKinase transgenic lines grown under water deficit stress for seven days (S5 Fig). It is known that both selected genes are playing a critical role in the response of plants to osmotic stress and can be used for the improvement of abiotic stress tolerance using a genetic approach [41, 42, 43, 44]. Thus, studies have shown that the triggered ABA production during dehydration stress increases the expression of GmRD22 gene (apoplast‐localized BURP‐domain protein), which protects the plant from major abiotic stresses [41]. Another study has revealed that the expression of GmRD22 in Arabidopsis thaliana and rice during stress could strengthen the cell wall integrity by increasing the lignin contents [42]. There is evidence of the involvement of PIP1 genes (water channel genes) in plant stress tolerance by maintaining the uptake and movement of water in the plant body [43]. It has been shown that the overexpression of GmPIP1-2 gene (soybean water channel protein) in soybean has increased the plant’s abiotic stress tolerance, promote plant growth and finally increase the soybean yields [44]. It is interesting that in our studies both genes (GmRD22-Like and GmPIP1-2) were overexpressed in the transgenic lines after seven days of water deficit stress (S5 Fig). Thus, it is logical to suggest that the termination of ERECTA signaling achieved in our AtERpro:AtΔKinase lines can drastically affect stress signaling pathways and various transcriptional factors. Such influence can contribute to the observed effects of AtΔKinase gene on the response of AtΔKinase transgenic soybean plants on osmotic stress. To clarify the molecular mechanisms of effects of ERECTA genes on plant stress response more mechanistic studies should be done in the future.
Supporting information
The pNS37 construct in pTF101.1 backbone is shown. The 6 kb EcoR1 –BamH1 fragment from pESH454 was cloned between EcoR1 and BamH1 sites of pTF101.1 followed by introduction of 2 kb BamH1 fragment to build pNS37. The ER gene fragment consists of exons and introns for LRR repeats and transmembrane (TM) region. There is a stop codon immediately after TM sequence followed by BamH1 site and ER terminator. The pTF101.1 vector contains 2 x 35S promoter driven Bar gene as the selection marker.
(TIF)
18S was used as an internal control. Samples of leaves, apices, stem and root were collected from 21-day-old plants, flowers were collected from 35-day-old plants and siliques derived from 45-day-old plants. Results are shown as means ±SE of three biological replicates.
(TIF)
n = 10; **P <0.01; *P <0.05. Results are shown as means ±SE.
(TIF)
Six leaves were sampled and analyzed for each line. Values are mean ± SE.
(TIF)
Leaves were collected from 28-day-old wild type and of transgenic plants grown in conditions of water deficit for 7 days. 18S was used as an internal control. Results are shown as means ±SE of three biological replicates.
(TIF)
Phenotypical comparison of 10-day-old soybean wild-type seedlings unexposed to salt stress and seedlings exposed to different concentrations of NaCl (50mM, 100mM, 150mM, 200mM, 300mM and 400mM) (A, B, C) n = 10; **P <0.01; *P <0.05. Results are shown as means ±SE. (D) Photograph of 10-day-old wild type seedlings grown without NaCl supplement (control) and grown in medium supplemented with a wide range of NaCl concentrations.
(TIF)
(A) The phenotype of wild type and transgenic AtERpro: AtΔKinase seedlings (line 3–1) exposed to salt stress conditions (100mM NaCl). (B) AtERpro:AtΔKinase transgenic plants exhibited higher germination rate on medium supplemented with 100mM of NaCl compared to wild-type.
(TIF)
Acknowledgments
Authors thank Dr. Elena Shpak (University of Tennessee, Knoxville) for valuable discussions and help with the measurement of the stomata index in wild-type and transgenic soybean lines. Authors are to NSF-EPSCoR (RII Track-2) for the establishment of research infrastructure in both participated campuses (University of Arkansas at Little Rock, University of Arkansas, Fayetteville).
Data Availability
All relevant data are at doi:10.5061/dryad.12jm63xtz.
Funding Statement
This study was supported Arkansas Soybean Promotion Board (Award UA AES 0403-61276-01 to VS and MK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gilbert SF. Developmental biology, the stem cell of biological disciplines. PLOS Biol. 2017;15: e2003691 10.1371/journal.pbio.2003691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shpak ED. Diverse Roles of ERECTA Family Genes in Plant Development. J Integr Plant Biol. 2013;55: 1238–1250. 10.1111/jipb.12108 [DOI] [PubMed] [Google Scholar]
- 3.Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal Patterning and Differentiation by Synergistic Interactions of Receptor Kinases. Science. 2005;309: 290–293. 10.1126/science.1109710 [DOI] [PubMed] [Google Scholar]
- 4.Chen M-K, Shpak ED. ERECTA family genes regulate development of cotyledons during embryogenesis. FEBS Lett. 2014;588: 3912–3917. 10.1016/j.febslet.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 5.Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436: 866–870. 10.1038/nature03835 [DOI] [PubMed] [Google Scholar]
- 6.Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, et al. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell. 1996;8: 735–746. 10.1105/tpc.8.4.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt L, Gray JE. The Signaling Peptide EPF2 Controls Asymmetric Cell Divisions during Stomatal Development. Curr Biol. 2009;19: 864–869. 10.1016/j.cub.2009.03.069 [DOI] [PubMed] [Google Scholar]
- 8.Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, et al. Epidermal Cell Density is Autoregulated via a Secretory Peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis Leaves. Plant Cell Physiol. 2009;50: 1019–1031. 10.1093/pcp/pcp068 [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, et al. Direct interaction of ligand-receptor pairs specifying stomatal patterning. Genes Dev. 2012;26: 126–136. 10.1101/gad.179895.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrash EB, Davies KA, Bergmann DC. Generation of Signaling Specificity in Arabidopsis by Spatially Restricted Buffering of Ligand–Receptor Interactions. Plant Cell. 2011;23: 2864–2879. 10.1105/tpc.111.086637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida N, Shimada M, Tasaka M. Modulation of the balance between stem cell proliferation and consumption by ERECTA-family genes. Plant Signal Behav. 2012;7: 1506–1508. 10.4161/psb.22080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng X, Wang H, He Y, Liu Y, Walker JC, Torii K, et al. A MAPK Cascade Downstream of ERECTA Receptor-Like Protein Kinase Regulates Arabidopsis Inflorescence Architecture by Promoting Localized Cell Proliferation. Plant Cell. 2012;24 10.1105/tpc.112.104695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shpak ED, Lakeman MB, Torii KU. Dominant-Negative Receptor Uncovers Redundancy in the Arabidopsis ERECTA Leucine-Rich Repeat Receptor–Like Kinase Signaling Pathway That Regulates Organ Shape. Plant Cell. 2003;15: 1095–1110. 10.1105/tpc.010413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shpak ED, Berthiaume CT, Hill EJ, Torii KU. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development. 2004;131: 1491–1501. 10.1242/dev.01028 [DOI] [PubMed] [Google Scholar]
- 15.Woodward C, Bemis SM, Hill EJ, Sawa S, Koshiba T, Torii KU. Interaction of Auxin and ERECTA in Elaborating Arabidopsis Inflorescence Architecture Revealed by the Activation Tagging of a New Member of the YUCCA Family Putative Flavin Monooxygenases. Plant Physiol. 2005;139: 192–203. 10.1104/pp.105.063495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Zanten M, Snoek LB, Proveniers MCG, Peeters AJM. The many functions of ERECTA. Trends Plant Sci. 2009;14: 214–218. 10.1016/j.tplants.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 17.Rédei GP. Supervital Mutants of Arabidopsis. Genetics. 1962;47: 443–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowman J. Arabidopsis: An Atlas of Morphology and Development. Springer Science & Business Media; 2012. [Google Scholar]
- 19.Annen F, Stockhaus J. SbRLK1, a receptor-like protein kinase of Sorghum bicolor (L.) Moench that is expressed in mesophyll cells. Planta. 1999;208: 420–425. 10.1007/s004250050577 [DOI] [PubMed] [Google Scholar]
- 20.Guo M, Rupe M, Simmons C, Sivasankar S. The maize erecta genes for improving plant growth, transpiration efficiency and drought tolerance in crop plants. WO2008039709A2, 2008. https://patents.google.com/patent/WO2008039709A2/en
- 21.Zheng J, Yang Z, Madgwick PJ, Carmo-Silva E, Parry MAJ, Hu Y-G. TaER Expression Is Associated with Transpiration Efficiency Traits and Yield in Bread Wheat. Ma W, editor. PLOS ONE. 2015;10: e0128415 10.1371/journal.pone.0128415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen H, Zhong X, Zhao F, Wang Y, Yan B, Li Q, et al. Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat Biotechnol. 2015;33: 996–1003. 10.1038/nbt.3321 [DOI] [PubMed] [Google Scholar]
- 23.Darkwa K, Ambachew D, Mohammed H, Asfaw A, Blair MW. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop J. 2016;4: 367–376. 10.1016/j.cj.2016.06.007 [DOI] [Google Scholar]
- 24.Du JunBo, Sun Xin, Sun MengYuan Jiang HengKe, Yan Li, Wan ChuanYin, et al. Identification and expression analysis of ERECTA homologous genes in Glycine max. Int J Agric Biol. 2017;19: 1497–1504. [Google Scholar]
- 25.Villagarcia H, Morin A-C, Shpak ED, Khodakovskaya MV. Modification of tomato growth by expression of truncated ERECTA protein from Arabidopsis thaliana. J Exp Bot. 2012;63: 6493–6504. 10.1093/jxb/ers305 [DOI] [PubMed] [Google Scholar]
- 26.Paz MM, Shou H, Guo Z, Zhang Z, Banerjee AK, Wang K. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica. 2004;136: 167–179. [Google Scholar]
- 27.Paz MMM, Wang K. Soybean transformation and regeneration using half-seed explant. US7473822B1, 2009. https://patents.google.com/patent/US7473822B1/en
- 28.Paz MM, Martinez JC, Kalvig AB, Fonger TM, Wang K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006;25: 206–213. 10.1007/s00299-005-0048-7 [DOI] [PubMed] [Google Scholar]
- 29.SAS Functions by Example, Second Edition.: 62.
- 30.Barrs HD, Weatherley PE. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust J Biol Sci. 1962;15: 413–428. 10.1071/bi9620413 [DOI] [Google Scholar]
- 31.Chaudhary J, Patil GB, Sonah H, Deshmukh RK, Vuong TD, Valliyodan B, et al. Expanding Omics Resources for Improvement of Soybean Seed Composition Traits. Front Plant Sci. 2015;6 10.3389/fpls.2015.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosentka PZ, Zhang L, Simon YA, Satpathy B, Maradiaga R, Mitoubsi O, et al. Identification of critical functional residues of receptor-like kinase ERECTA. J Exp Bot. 2017;68: 1507–1518. 10.1093/jxb/erx022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoyama R, Takahashi T, Kato A, Torii KU, Komeda Y. The Arabidopsis ERECTA gene is expressed in the shoot apical meristem and organ primordia. Plant J. 1998;15: 301–310. 10.1046/j.1365-313x.1998.00203.x [DOI] [PubMed] [Google Scholar]
- 34.Xing HT, Guo P, Xia XL, Yin WL. PdERECTA, a leucine-rich repeat receptor-like kinase of poplar, confers enhanced water use efficiency in Arabidopsis. Planta. 2011;234: 229–241. 10.1007/s00425-011-1389-9 [DOI] [PubMed] [Google Scholar]
- 35.Qi Y, Sun Y, Xu L, Xu Y, Huang H. ERECTA is required for protection against heat-stress in the AS1/AS2 pathway to regulate adaxial–abaxial leaf polarity in Arabidopsis. Planta. 2004;219: 270–276. 10.1007/s00425-004-1248-z [DOI] [PubMed] [Google Scholar]
- 36.Jordá L, Sopeña-Torres S, Escudero V, Nuñez-Corcuera B, Delgado-Cerezo M, Torii KU, et al. ERECTA and BAK1 Receptor Like Kinases Interact to Regulate Immune Responses in Arabidopsis. Front Plant Sci. 2016;7 10.3389/fpls.2016.00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel D, Basu M, Hayes S, Majláth I, Hetherington FM, Tschaplinski TJ, et al. Temperature-dependent shade avoidance involves the receptor-like kinase ERECTA. Plant J. 2013;73: 980–992. 10.1111/tpj.12088 [DOI] [PubMed] [Google Scholar]
- 38.Kasulin L, Agrofoglio Y, Botto JF. The receptor-like kinase ERECTA contributes to the shade-avoidance syndrome in a background-dependent manner. Ann Bot. 2013;111: 811–819. 10.1093/aob/mct038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren S, Lyle C, Jiang G, Penumala A. Soybean Salt Tolerance 1 (GmST1) Reduces ROS Production, Enhances ABA Sensitivity, and Abiotic Stress Tolerance in Arabidopsis thaliana. Front Plant Sci. 2016;7 10.3389/fpls.2016.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halford N. Plant Biotechnology: Current and Future Applications of Genetically Modified Crops. John Wiley & Sons; 2006. [Google Scholar]
- 41.Use of GmRD22-like genes to protect against abiotic stress. [cited 2 Apr 2020]. http://www.freepatentsonline.com/20080184385.pdf
- 42.Wang H, Zhou L, Fu Y, Cheung M-Y, Wong F-L, Phang T-H, et al. Expression of an apoplast-localized BURP-domain protein from soybean (GmRD22) enhances tolerance towards abiotic stress. Plant Cell Environ. 2012;35: 1932–1947. 10.1111/j.1365-3040.2012.02526.x [DOI] [PubMed] [Google Scholar]
- 43.Kapilan R, Vaziri M, Zwiazek JJ. Regulation of aquaporins in plants under stress. Biol Res. 2018;51 10.1186/s40659-018-0152-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soybean water channel protein gene GmPIP1; The application of 2. CN103266130B, 2015. https://patents.google.com/patent/CN103266130B/en
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The pNS37 construct in pTF101.1 backbone is shown. The 6 kb EcoR1 –BamH1 fragment from pESH454 was cloned between EcoR1 and BamH1 sites of pTF101.1 followed by introduction of 2 kb BamH1 fragment to build pNS37. The ER gene fragment consists of exons and introns for LRR repeats and transmembrane (TM) region. There is a stop codon immediately after TM sequence followed by BamH1 site and ER terminator. The pTF101.1 vector contains 2 x 35S promoter driven Bar gene as the selection marker.
(TIF)
18S was used as an internal control. Samples of leaves, apices, stem and root were collected from 21-day-old plants, flowers were collected from 35-day-old plants and siliques derived from 45-day-old plants. Results are shown as means ±SE of three biological replicates.
(TIF)
n = 10; **P <0.01; *P <0.05. Results are shown as means ±SE.
(TIF)
Six leaves were sampled and analyzed for each line. Values are mean ± SE.
(TIF)
Leaves were collected from 28-day-old wild type and of transgenic plants grown in conditions of water deficit for 7 days. 18S was used as an internal control. Results are shown as means ±SE of three biological replicates.
(TIF)
Phenotypical comparison of 10-day-old soybean wild-type seedlings unexposed to salt stress and seedlings exposed to different concentrations of NaCl (50mM, 100mM, 150mM, 200mM, 300mM and 400mM) (A, B, C) n = 10; **P <0.01; *P <0.05. Results are shown as means ±SE. (D) Photograph of 10-day-old wild type seedlings grown without NaCl supplement (control) and grown in medium supplemented with a wide range of NaCl concentrations.
(TIF)
(A) The phenotype of wild type and transgenic AtERpro: AtΔKinase seedlings (line 3–1) exposed to salt stress conditions (100mM NaCl). (B) AtERpro:AtΔKinase transgenic plants exhibited higher germination rate on medium supplemented with 100mM of NaCl compared to wild-type.
(TIF)
Data Availability Statement
All relevant data are at doi:10.5061/dryad.12jm63xtz.