Abstract
Background
No study has compared the clinical impact of indexation of left ventricular mass (LVM) on adverse clinical outcomes in pre-dialysis patients with chronic kidney disease (CKD).
Methods
We reviewed 2,101 patients from a large-scale multi-center prospective study that gathered anthropometric and echocardiographic measurements and clinical outcomes. The LVM was indexed as body surface area (LVMI-BSA) and height raised to the power of 2.7 (LVMI-H2.7). The main outcomes were composite renal and cardiovascular events and all-cause mortality. Left ventricular hypertrophy (LVH) was defined as the highest sex-specific quartile of LVMI-BSA or LVMI-H2.7.
Results
During a mean period of 3.5 years, 692 patients developed composite outcomes (32.9%). The area under the curve at 5 year of LVM (60.6%) for composite outcome was smaller than that for LVMI-BSA (63.2%, P <0.001) and LVMI-H2.7 (63.4%, P <0.001). The hazard ratio (HR) and 95% confidence interval (CI) per one unit increase in LVM (g), LVMI-BSA (g/m2), and LVMI-H2.7 (g/m2.7) for composite outcomes were 1.004 (1.002–1.005, P <0.001), 1.011 (1.006–1.016, P <0.001), and 1.023 (1.012–1.035, P <0.001), respectively. Patients with LVH determined by LVMI-BSA and LVMI-H2.7 (HR 1.352, 95% CI 1.123–1.626, P = 0.001) and LVH determined by only LVMI-BSA (HR 1.908, 95% CI 1.233–2.953, P = 0.004) showed an independent increase in the risk of composite-outcome development, when compared with patients without LVH, according to LVMI-BSA and LVMI-H2.7.
Conclusion
Indexation of LVM improved the prediction of adverse outcomes. BSA may be as useful as height2.7 in indexing of LVM for predicting adverse outcomes in pre-dialysis patients with CKD.
Introduction
Left ventricular (LV) mass (LVM) increases in response to pathophysiological stresses, resulting in LV hypertrophy (LVH) [1]. Pressure overload (i.e., hypertension) causing concentric LVH and volume overload (i.e., valvular disease) causing eccentric LVH are two major forms of stress [1]. LVH is associated with an increased risk of cardiovascular events and mortality [2–4], and regression of LVH is associated with a reduction in cardiovascular morbidity and mortality [2]. Patients with chronic kidney disease (CKD) are at higher risk of cardiovascular events [5]. LVH is a common problem in these patients [6], causing significant morbidity and mortality [7–9].
The size of a normal heart is influenced by sex, exercise, age, and ethnicity (1). LVM is influenced by body size. Appropriate indexing of LVM is necessary to minimize over- or under-estimation of LVH (1). Body surface area (BSA) and height raised to the power of 2.7 power (height2.7) are common indexing parameters [10–13]. Although the American Society of Echocardiography (ASE) guideline [1, 14] has defined LVH using LVM indexed with BSA (g/m2), indexing LVM (LVMI) with BSA in patients with CKD is questionable, because body-fluid volume status is unstable in such patients [15]. Height2.7 has been recommended as a more appropriate method for indexation in patients with CKD than that using BSA, because Zoccali et al. reported better prognostic impact of LVMI-H2.7 than LVMI-BSA in patients undergoing dialysis [16]. Nevertheless, the results from patients with CKD undergoing dialysis cannot be directly applied to patients with CKD in pre-dialysis, because clinical conditions vary according to the stage of CKD [17]. Therefore, we identified the best indexation for LVM for predicting adverse clinical outcomes in patients with CKD in pre-dialysis using a large number of adults enrolled in the KoreaN cohort study for Outcome in patients With Chronic Kidney Disease (KNOW-CKD).
Material and methods
Participants
The KNOW-CKD was a multi-center prospective cohort study that included 2,238 patients with CKD stages 1–5 who were in pre-dialysis, enrolled between 2011 and 2016 in Korea. The detailed design and methods of the KNOW-CKD have been published earlier (NCT01630486 at http://www.clinicaltrials.gov) [18]. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology collaboration creatinine equation [19]. CKD and its stages were defined using the Kidney Disease Improving Global Outcomes 2012 guidelines [20].
We excluded 137 patients from the cohort of 2,238 participants because of missing echocardiographic measures, anthropometric measures, and clinical outcomes in 101, 14, and 22 patients, respectively. Finally, 2,101 patients were included.
Ethics statement
The protocol of the KNOW-CKD adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Boards (IRB) of each participating hospital, including Seoul National University Hospital (IRB number: 1104-089-359), Yonsei University Severance Hospital (IRB number: 4-2011-0163), Kangbuk Samsung Medical Center (IRB number: 2011-01-076), Seoul St. Mary’s Hospital (IRB number: KC11OIMI0441), Gil Hospital (IRB number: GIRBA2553), Nowon Eulji Medical Center (IRB number: 201105–01), Chonnam National University Hospital (IRB number: CNUH-2011-092), and Busan Paik Hospital (IRB number: 11–091). Written informed consent was obtained from all participants. All data were fully anonymized before we accessed them.
Measurement of left ventricular mass index
Complete two-dimensional M-mode echocardiography and Doppler studies were performed in the standard manner by cardiologists of the participating hospitals, who were blind to the clinical data. M-mode examination was performed according to the American Society of Echocardiography guidelines [14]. Left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), inter-ventricular septum thickness (IVST), left ventricular posterior wall thickness (LVPWT), left atrial diameter (LAD), regional wall motion abnormality (RWMA) and ejection fraction (EF) were recorded with echocardiography. LVM was determined using the Devereux formula [14]: LVM (g) = 0.8 × {1.04 × [(LVEDD + IVST + LVPWT)3 - (LVEDD)3]} + 0.6. LVMI was calculated by normalizing LVM with height2.7 (LVMI-H2.7, g/m2.7) or BSA (LVMI-BSA, g/m2). BSA was calculated using the Du Bois formula: BSA (m2) = 0.007184 × (height in cm) 0.725 × (weight in kg)0.425 [21]. The first, second, third, and fourth quartiles for LVM were 59.0–128.0 g, 128.0–155.9 g, 155.9–186.4 g, and ≥186.4 g, respectively.
Definition of LVH
LVH was defined as the highest sex-specific quartile of LVMI in the study’s patients: ≥48.6 g/m2.7 in men and ≥48.4 g/m2.7 in women defined by LVMI-H2.7 (LVH-H2.7) and ≥109.9 g/m2 in men and ≥100.5 g/m2 in women defined by LVMI-BSA (LVH-BSA). We classified the patients into four combined LVH groups, according to the two LVMIs: no LVH/both group (no LVH with LVMI-H2.7 or LVMI-BSA), LVH/BSA-only group (LVH determined only with LVMI-BSA), LVH/H2.7-only group (LVH determined only with LVMI-H2.7), and LVH/both group (LVH determined with both, LVMI-BSA and LVMI-H2.7).
Definitions of study outcomes
Composite renal and cardiovascular events, and all-cause mortality were the primary outcomes. Outcome measurements were described in detail by previously published protocol [18]. A renal event was defined by a >50% decrease in eGFR from the baseline values, doubling of serum creatinine, or initiation of dialysis or kidney transplantation. A cardiovascular event was defined as any first event of the following since study enrollment: acute myocardial infarction, unstable angina, percutaneous coronary artery intervention or coronary bypass graft surgery, ischemic or hemorrhagic cerebral stroke, congestive heart failure and other major cardiovascular events that required hospitalization, interventions, or therapy during the follow-up. Patients with CKD stage ≥ 3 were under close observation and had been followed up at 1- to 3-month intervals by all participating centers. Patients who reached the endpoints were reported by each center, irrespective of the study protocol. Patients were followed up till December 31, 2018 or until they dropped out or died. All the adverse outcomes were detected and adjudicated annually by the researchers and adjudication committee [18].
Other measurements and definitions
Clinical data, including detailed demographic information and baseline laboratory results, were extracted from the electronic data management system (PhactaX). Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg or diastolic BP ≥90 mmHg or treatment with anti-hypertensive drugs. Diabetes was defined as fasting plasma glucose ≥126 mg/dL, or treatment with insulin or oral anti-diabetic drugs. Body mass index (BMI) was calculated as weight (kg) per square meter of height (m2).
Statistical analysis
The distributions of continuous variables were evaluated using histograms and Q-Q plots. Two variables, high sensitivity C-reactive protein (hsCRP) and urine protein-to-creatinine ratio (UPCR) were not normally distributed. Normally distributed continuous variables, non-normally distributed continuous variables, and categorical variables were expressed as mean ± standard deviation, median (interquartile range), and percentages, respectively. The P-trend was analyzed with a linear-term of one-way analysis of variance (ANOVA for normally distributed continuous variables), with the Jonckheere-Terpstra test for non-normally distributed continuous variables, and with linear-by-linear association for categorical variables. Differences were analyzed using Bonferroni post-hoc analysis of one-way ANOVA for normally distributed continuous variables, Mann–Whitney U tests for non-normally distributed continuous variables, and chi-squared tests for categorical variables. The hazard ratio (HR) and its 95% confidence interval (CI) of LVM and its indexations for study outcomes were assessed using Cox proportional hazard regression analysis. The assumption of proportional hazard was tested using the log minus log plot for categorical variables and interaction analysis with time covariate using time-dependent Cox regression analysis for continuous variables. When the proportional hazard assumption was not met, time-dependent Cox regression analysis was used for primary exposures (LVM, LVMI-BSA, and LVMI-H2.7), while categorization by median values was done for other covariates: systolic BP (median 127 mmHg) and diastolic BP (median 77 mm Hg), cholesterol (median 4.4 mmol/l), eGFR (median 46.3 ml/min/1.73m2), blood urea nitrogen (median 8.6 mmol/l), bilirubin (median 10.3 μmol/l), albumin (median 42 g/l), and haemoglobin (median 12.8 g/dl). A P-value < 0.05 was considered statistically significant. Covariates were chosen based on clinical and statistical relevance for multivariate analysis and only patients without missing values were included in the analysis. The area under the curve (AUC) with the CI of the time-dependent receiver operating characteristic curve (ROC) using Kaplan-Meier estimator was evaluated using R Version 3.6.2 (R Core Team, 2019, R Foundation for Statistical Computing, Vienna, Austria) with “timeROC” package [22]. All analyses (unless specified otherwise) were performed using SPSS Version 22 (IBM Corp. released 2013, Armonk, NY).
Results
Of the 2,101 patients, the mean age was 53.6 years and 61.0% were men. The causes of CKD were diabetic nephropathy in 24.8%, hypertensive nephropathy in 19.8%, glomerulonephritis in 31.7%, and others in 23.7% of patients. The mean LVM, LVMI-BSA, and LVMI-H2.7 were 161.6 g, 93.2 g/m2, and 42.1 g/m2.7, respectively at enrollment. During a mean of 3.5 years, 692 patients developed composite outcomes (32.9%): 568 patients experienced renal events (27.0%), 130 patients experienced cardiovascular events (6.2%), and 80 patients died (3.8%).
We explored the baseline characteristics of the LVM quartile (Table 1). Age and the proportion of men increased with an increase in LVM. The proportion of currents smokers in the higher LVM quartile was greater. The proportion of patients with diabetic and hypertensive nephropathy increased, while that of patients with glomerulonephritis and other etiologies of CKD decreased, with the progression of the LVM quartile. Systolic and diastolic BP, BSA, BMI, fasting glucose, blood urea nitrogen, hsCRP, and UPCR increased, while eGFR, bilirubin, serum albumin, and total cholesterol decreased with an increase in LVM. Hemoglobin levels were not associated with LVM quartiles.
Table 1. Baseline characteristics of patients according to the status of left ventricular mass.
| Quartile of LVM | P-trend | ||||
|---|---|---|---|---|---|
| 1 Quartile 59.0–128.0 g (n = 512) | 2 Quartile 128.0–155.9 g (n = 536) | 3 Quartile 155.9–186.4 g (n = 528) | 4 Quartile 186.4–624.6 g (n = 525) | ||
| Age (years) | 49.4 ± 12.3 | 53.6 ± 12.3* | 54.6 ± 11.5* | 56.4 ± 11.8*† | <0.001 |
| Male sex, n (%) | 168, (32.8) | 307, (57.3)* | 374, (70.8)*† | 433, (82.5)*†‡ | <0.001 |
| Current smoking, n (%) | 45, (8.8) | 82, (15.3)* | 108, (20.5)* | 103, (19.7)* | <0.001 |
| Hypertension, n (%) | 471, (92) | 518, (96.6)* | 522, (98.9)* | 521, (99.2)*† | <0.001 |
| Diabetes, n (%) | 115, (22.7) | 168, (31.6)* | 212, (40.4)*† | 249, (47.6)*† | <0.001 |
| Cause of chronic kidney disease | |||||
| Diabetic nephropathy, n (%) | 65, (12.7) | 111, (20.7)* | 162, (30.7)*† | 183, (34.9)**† | <0.001 |
| Hypertensive nephropathy, n (%) | 73, (14.3) | 111, (20.7)* | 87, (16.5) | 144, (27.5)*†‡ | <0.001 |
| Glomerulonephritis, n (%) | 215, (42.0) | 181, (33.8)* | 167, (31.6)* | 103, (19.7)*†‡ | <0.001 |
| Others (%) | 159, (31.1) | 133, (24.8) | 112, (21.2)* | 94, (17.9)*† | <0.001 |
| Systolic BP (mm Hg) | 122.1 ± 14.8 | 127.0 ± 14.9* | 129.4 ± 15.8* | 132.5 ± 17.4*†‡ | <0.001 |
| Diastolic BP (mm Hg) | 75.7 ± 10.6 | 77.3 ± 10.3 | 76.9 ± 10.8 | 78.0 ± 12.3* | 0.003 |
| Weight (kg) | 58.9 ± 10.0 | 65.6 ± 10.1* | 68.9 ± 10.5*† | 73.3 ± 11.7*†‡ | <0.001 |
| Height (cm) | 160.5 ± 7.8 | 164.3 ± 8.3* | 165.8 ± 8.0* | 167.8 ± 7.9*†‡ | <0.001 |
| Body surface area (m2) | 1.6 ± 0.2 | 1.7 ± 0.2* | 1.8 ± 0.2*† | 1.8 ± 0.2*†‡ | <0.001 |
| BMI (kg/m2) | 22.8 ± 3.3 | 24.3 ± 3.1* | 25.0 ± 3.1*† | 26.0 ± 3.3*†‡ | <0.001 |
| LVM (g) | 109.4 ± 13.7 | 141.5 ± 7.8* | 169.8 ± 8.8*† | 224.8 ± 42.9*†‡ | <0.001 |
| Fasting glucose (mmol/l) | 5.8 ± 1.9 | 6.0 ± 1.8 | 6.3 ± 2.3* | 6.4 ± 2.6* | <0.001 |
| Blood urea nitrogen (mmol/l) | 8.6 ± 4.7 | 9.3 ± 5.3 | 10.5 ± 5.5*† | 11.7 ± 6.2*†‡ | <0.001 |
| Serum creatinine (μmol/l) | 131.3 ± 75.6 | 147.6 ± 92.8 | 164.0 ± 94.3* | 196.1 ± 125.3*†‡ | <0.001 |
| eGFR (ml/min/1.73m2) | 61.8 ± 32.8 | 56.3 ± 30.9 | 51.3 ± 29.2* | 44.3 ± 27.6*†‡ | <0.001 |
| Bilirubin (μmol/l) | 11.7 ± 5.5 | 12.0 ± 5.4 | 11.3 ± 5.0 | 10.8 ± 5.0† | 0.001 |
| Serum albumin (g/l) | 41.9 ± 4.1 | 42.4 ± 3.6 | 41.7 ± 4.5 | 41.1 ± 4.7† | <0.001 |
| Total cholesterol (mmol/l) | 4.6 ± 1.0 | 4.5 ± 1.0 | 4.5 ± 1.1 | 4.4 ± 1.0* | 0.001 |
| Hemoglobin (g/dl) | 12.7 ± 1.8 | 13.1 ± 2* | 12.9 ± 2.1 | 12.6 ± 2.1† | 0.510 |
| hsCRP (nmol/l) | 3.8 (1.4–12.1) | 5.7 (2.1–16.3)* | 5.7 (2.7–14.3)* | 8.2 (3.8–20.7)*†‡ | <0.001 |
| UPCR (g/g Cr) | 0.4 (0.1–1.1) | 0.4 (0.1–1.1) | 0.5 (0.2–1.7)*† | 0.8 (0.2–2.3)*†‡ | <0.001 |
BP, blood pressure; BSA, body surface area; BMI, body mass index; LVM, left ventricular mass; eGFR, estimated glomerular filtration rate; hsCRP, high sensitivity C-reactive protein; UPCR, urine protein-to-creatinine ratio.
Values are expressed as mean ± standard deviation for normally distributed continuous variables, median (interquartile range) for non-normally distributed continuous variables, and percentage for categorical variables. P-trend was analyzed by linear-term of one-way ANOVA for normally distributed continuous variables, Jonckheere-Terpstra test for non-normally distributed continuous variables, and a linear-by-linear association for categorical variables.
*, †, and ‡ meant P < 0.01 when compared to 1Q-3Q of LVM group, respectively, by using Bonferroni post-hoc analysis of one-way ANOVA for normally distributed continuous variables, Mann-Whitney U test for non-normally distributed continuous variables, and chi-square test for categorical variables
We compared the echocardiographic parameters according to CKD staging (Table 2). LV chamber size (LVESD and LVEDD) and chamber thickness (IVST and PWT) increased, resulting in increased LVM, with the progression of CKD. Left atrial size (LAD) was also increased as CKD progressed. The prevalence of RWMA increased with the progression of CKD, although it was decreased in CKD stage 5. However, EF was not associated with the CKD stage.
Table 2. Trends of echocardiographic parameter according to CKD stages.
| CKD Stage (n = 2,101) | P-trend | ||||||
|---|---|---|---|---|---|---|---|
| Stage 1(n = 344) | Stage 2 (n = 398) | Stage 3a (n = 344) | Stage 3b (n = 445) | Stage 4 (n = 444) | Stage 5 (n = 126) | ||
| SBP (mmHg) | 126 ± 14.3 | 126.4 ± 14.7 | 126.5 ± 15.8 | 126.8 ± 15.8 | 130.2 ± 17.1*†‡ | 135.7 ± 20.9*†‡¶§ | <0.001 |
| DBP (mmHg) | 78.4 ± 10.8 | 77.6 ± 10.5 | 76.9 ± 10.5 | 75.9 ± 10.4 | 76.3 ± 12.2 | 77.4 ± 12.5 | 0.004 |
| LVESD (cm) | 3.0 ± 0.4 | 3.0 ± 0.4 | 3.0 ± 0.4 | 3.0 ± 0.4 | 3.1 ± 0.5† | 3.1 ± 0.4 | 0.004 |
| LVEDD (cm) | 4.8 ± 0.4 | 4.8 ± 0.4 | 4.9 ± 0.4 | 4.9 ± 0.5 | 4.9 ± 0.5*† | 4.9 ± 0.5 | <0.001 |
| IVST (cm) | 0.9 ± 0.2 | 0.9 ± 0.2* | 0.9 ± 0.2* | 1.0 ± 0.2*† | 1.0 ± 0.2*† | 1.0 ± 0.2*†‡ | <0.001 |
| PWT (cm) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1* | 0.9 ± 0.1*† | 1.0 ± 0.2*†‡ | 1.0 ± 0.2*†‡ | <0.001 |
| RWT | 0.36 ± 0.06 | 0.38 ± 0.07 | 0.38 ± 0.06* | 0.39 ± 0.07* | 0.39 ± 0.08* | 0.41 ± 0.09*†‡ | <0.001 |
| LVM (g) | 145.7 ± 44.0 | 153.7 ± 40.7 | 158.6 ± 40.3* | 165.4 ± 45.2*† | 174.2 ± 57.6*†‡ | 180.0 ± 55.8*†‡ | <0.001 |
| LVMI-BSA (g/m2) | 83.4 ± 20.5 | 87.4 ± 20.7 | 90.8 ± 20.4* | 95.7 ± 22.8*† | 101.3 ± 28.6*†‡¶ | 106.9 ± 29.0*†‡¶ | <0.001 |
| LVMI-H2.7 (g/m2.7) | 37.1 ± 9.8 | 39.0 ± 10.0 | 41.0 ± 10.0* | 43.5 ± 11.3*† | 46.0 ± 13.4*†‡ | 49.0 ± 13.8*†‡¶ | <0.001 |
| EF (%) | 64.0 ± 5.6 | 64.2 ± 6 | 63.9 ± 5.5 | 64.5 ± 5.8 | 63.8 ± 6.8 | 65.6 ± 6.2 | 0.288 |
| RWMA, n (%) | 4, (1.2) | 8, (2.0) | 10, (2.9) | 18, (4.0) | 22, (5.0)* | 3, (2.4) | 0.004 |
| LAD (cm) | 3.6 ± 0.5 | 3.7 ± 0.5 | 3.8 ± 0.6* | 3.8 ± 0.6* | 3.9 ± 0.6*† | 3.9 ± 0.6* | <0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; CKD, chronic kidney disease; LVESD, left ventricular end systolic diameter; LVEDD, left ventricular end diastolic diameter; IVST, interventricular septum thickness; PWT, posterior wall thickness; RWT, relative wall thickness; LVM, left ventricular mass; LVMI, left ventricular mass index; BSA, body surface area; H2.7, height to the 2.7 power; EF, ejection fraction; RWMA, regional wall motion abnormality; LAD, left atrial diameter.
Values are expressed as mean ± standard deviation for continuous variables and percentage for categorical variables. P-trend was analyzed by linear-term of one-way ANOVA for continuous variables and a linear-by-linear association for categorical variables.
*, †, ‡, ¶, and § meant P < 0.01 when compared to CKD stage 1, 2, 3a, 3b, and 4 by using Bonferroni post-hoc analysis of one-way ANOVA for continuous variables and chi-square test for categorical variables.
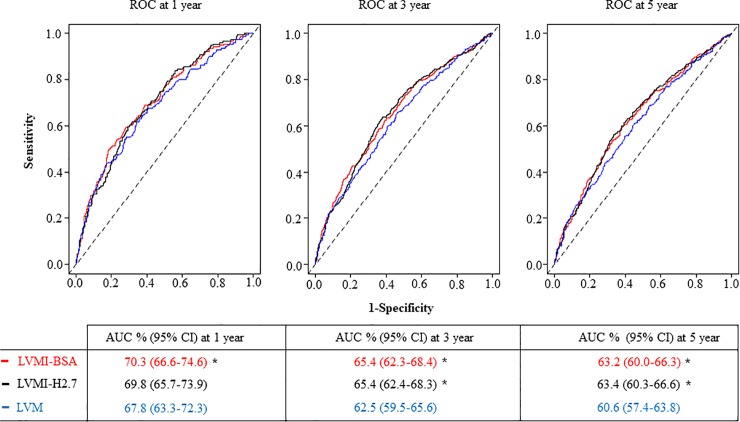
We analyzed the risk of LVM and its indexations for adverse clinical outcomes (Table 3). Increased LVM, LVMI-BSA, and LVMI-H2.7 were significantly associated with all clinical outcomes on univariate analysis. We performed multivariate analysis after adjusting for the effects of confounders and found that increased LVM and its indexations were independently associated with an increased risk of composite, renal, and cardiovascular outcomes (Table 3 and S1 Table). On the other hand, LVM and its indexations were not associated with all-cause mortality, according to multivariate analysis. We compared the AUCs with time-dependent ROC analysis, to identify the relative predictive ability of LVM and its indexations for composite outcome as the follow-up time increased (Fig 1). The AUCs of LVMI-BSA and LVMI-H2.7 for composite outcome were not different, and both were statistically greater than that of LVM, which had become more evident with the increase of follow-up time.
Table 3. Hazard ratios of left ventricular mass and its several indexations for adverse clinical outcomes.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Composite outcome | ||||
| LVM (g) | 1.007 (1.005–1.008) | <0.001 | 1.004 (1.002–1.005) | <0.001 |
| LVMI-BSA (g/m2) | 1.019 (1.015–1.022)* | <0.001 | 1.011 (1.006–1.016)* | <0.001 |
| LVMI-H2.7 (g/m2.7) | 1.042 (1.033–1.051)* | <0.001 | 1.023 (1.012–1.035)* | <0.001 |
| Renal outcome | ||||
| LVM (g) | 1.009 (1.007–1.011)* | <0.001 | 1.006 (1.003–1.009)* | <0.001 |
| LVMI-BSA (g/m2) | 1.022 (1.017–1.026)* | <0.001 | 1.015 (1.009–1.020)* | <0.001 |
| LVMI-H2.7 (g/m2.7) | 1.049 (1.039–1.059)* | <0.001 | 1.032 (1.019–1.045)* | <0.001 |
| CV outcome | ||||
| LVM (g) | 1.006 (1.003–1.008) | <0.001 | 1.005 (1.000–1.009) | 0.037 |
| LVMI-BSA (g/m2) | 1.014 (1.009–1.019) | <0.001 | 1.010 (1.002–1.017) | 0.012 |
| LVMI-H2.7 (g/m2.7) | 1.031 (1.020–1.043) | <0.001 | 1.023 (1.006–1.040) | 0.007 |
| All-cause mortality | ||||
| LVM (g) | 1.006 (1.002–1.009) | 0.001 | 1.001 (0.995–1.006) | 0.765 |
| LVMI-BSA (g/m2) | 1.014 (1.007–1.021) | <0.001 | 0.999 (0.990–1.009) | 0.905 |
| LVMI-H2.7 (g/m2.7) | 1.026 (1.010–1.042) | 0.001 | 0.996 (0.975–1.018) | 0.710 |
LVM, left ventricular mass; LVMI, left ventricular mass index; BSA, body surface area; H, height; W, weight; CV, cardiovascular. HR and its CI were calculated using Cox proportional hazard regression analysis. In multivariate analysis, covariates were age, sex, current smoking, causes of chronic kidney disease, systolic blood pressure ≥ 127 mmHg, diastolic blood pressure ≥ 77 mmHg, blood urea nitrogen≥ 8.6 mmol/l, estimated glomerular filtration rate ≥ 46.3 ml/min/1.73m2, bilirubin ≥ 10.3 μmol/l, albumin ≥ 42 g/l, cholesterol ≥ 4.4 mmol/l, hemoglobin ≥ 12.8 g/dl, body mass index, fasting glucose, urine protein creatinine ratio, and high sensitive C-reactive protein.
* meant results using time-dependent Cox hazard regression analysis.
Fig 1. Time-dependent receiver operator characteristics curve of left ventricular mass and its indexations for composite outcome.
AUC, area under the curve; CI, confidence interval; LVMI-BSA, left ventricular mass index by body surface area; LVMI-H2.7, left ventricular mass index by height to the 2.7 power; LVM, left ventricular mass; CV, cardiovascular. * meant P <0.05 when compared to LVM. The P-values of the comparison between AUCs of LVMI-BSA and LVMI-H2.7 were all above 0.05.
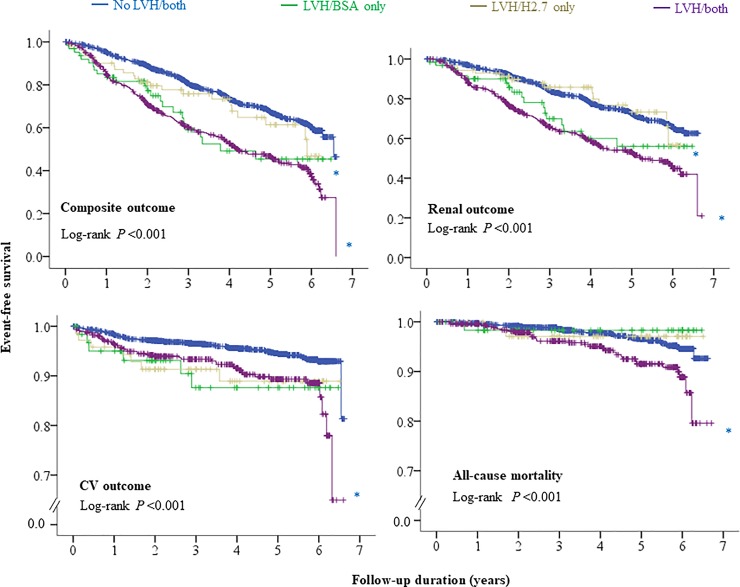
We compared the risk of the four LVH groups for adverse clinical outcomes. We confirmed that the LVH/BSA-only and LVH/both groups showed independent risks for composite and renal outcomes, while no risk was observed in the LVH/H2.7-only group, when compared to the no LVH/both group, according to multivariate Cox proportional hazard regression analysis (Table 4 and S2 Table). Kaplan-Meier’s survival curve analysis (Fig 2) revealed that the LVH/BSA-only and LVH/both groups showed significantly lower event-free survival for composite and renal outcomes, while the LVH/H2.7 only group showed similar event-free survival for composite and renal outcomes, compared to no LVH/both group. The LVH/both group showed significantly lower event-free survival for cardiovascular outcomes and all-cause mortality, compared to the no LVH/both group. However, this was not confirmed with multivariate analysis (Table 4 and S2 Table).
Table 4. Hazard ratio of left ventricular hypertrophy (LVH) groups defined by left ventricular mass index by body surface area or height to the 2.7 power for adverse clinical outcomes.
| Composite outcome | Renal outcome | CV outcome | All-cause mortality | |||||
|---|---|---|---|---|---|---|---|---|
| LVH groups | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| No LVH/Both (n = 1,372) | Ref. | Ref. | Ref. | Ref. | ||||
| LVH/BSA only (n = 56) | 1.908 (1.233–2.953) | 0.004 | 1.820 (1.109–2.986) | 0.018 | 2.484 (0.978–6.307) | 0.056 | 0.519 (0.070–3.866) | 0.522 |
| LVH/H2.7 only (n = 64) | 1.079 (0.680–1.712) | 0.747 | 0.835 (0.478–1.457) | 0.525 | 1.696 (0.694–4.145) | 0.247 | 0.766 (0.171–3.424) | 0.727 |
| LVH/both (n = 410) | 1.352 (1.123–1.626) | 0.001 | 1.414 (1.155–1.730) | 0.001 | 1.299 (0.839–2.011) | 0.240 | 1.133 (0.676–1.900) | 0.635 |
LVH, left ventricular hypertrophy; BSA, body surface area; H2.7, height to the 2.7 power; CV, cardiovascular; Ref, reference; HR, hazard ratio; CI, confidence interval. HR and its CI were analyzed using multivariate Cox proportional hazard regression analysis entering into age, sex, current smoking, causes of chronic kidney disease, systolic blood pressure ≥ 127 mmHg, diastolic blood pressure ≥ 77 mmHg, blood urea nitrogen≥ 8.6 mmol/l, estimated glomerular filtration rate ≥ 46.3 ml/min/1.73m2, bilirubin ≥ 10.3 μmol/l, albumin ≥ 42 g/l, cholesterol ≥ 4.4 mmol/l, hemoglobin ≥ 12.8 g/dl, body mass index, fasting glucose, urine protein creatinine ratio, and high sensitive C-reactive protein as covariates.
Fig 2. Kaplan Meier survival curve of left ventricular hypertrophy (LVH) groups defined by left ventricular mass index by body surface area (BSA) or height to the 2.7 power (H2.7) for adverse clinical outcomes.
*, †, and ‡ meant P < 0.01 when compared to No LVH/both, LVH/BSA only, and LVH/H2.7 only groups, respectively, by using log-rank test.
Discussion
Increased LVM can predict cardiovascular events and mortality in patients with [23–27] or without CKD [5, 28–34] LVM can increase physiologically in individuals with a large body size (11). Therefore, the need for indexing LVM has been suggested for better calculation of LVM by minimizing the effect of body size [10, 11, 13, 16, 35–38] However, whether calculation of LVM using several indexations results in improvement in predictions of adverse clinical outcomes needs investigation. This study compared the AUCs of LVM and its indexations for several clinical outcomes and found that LVM indexing with BSA or height2.7 significantly improved the predictive power for renal and cardiovascular events. We also analyzed the association between LVMs indexed with BSA and height2.7 and clinical outcomes. Both LVMI-BSA and LVMI-H2.7 independently predicted the development of renal and cardiovascular events. However, LVM and its indexations were not associated with all-cause mortality, which may be attributed to the overall low mortality in this population [39].
Several indexing methods exist, including height2.7 13, BSA1.5, and fat-free mass [11, 12, 35], among which, BSA and height2.7 indexations are the most studied [10, 11, 13, 16, 35–38]. Although it is obvious that the prevalence of LVH, as defined by LVMI-BSA and LVMI-H2.7 can vary [36–38] the impact of different classifications of LVH using different LVMIs on adverse clinical outcomes has been studied scarcely, with inconclusive results [10, 12, 16]. Moreover, the impact of LVM and its indexations on renal events has been rarely studied [40]. In this study, both LVMI-BSA and LVMI-H2.7 were independently associated with renal and cardiovascular events, and composite outcome. The AUCs of time-dependent ROC of LVMI-BSA and LVMI-H2.7 for composite outcome were comparable, which was largely attributed to the relationship with renal and cardiovascular events (S3 Table). Net reclassification improvement of LVMI-H2.7 over LVMI-BSA for composite outcome was not statistically significant (S4 Table). In the analysis for the diagnostic performance of LVMI-BSA and LVMI-H2.7 for LVH using the highest sex-specific quartiles for the respective LVMIs, the LVH/both group was undoubtedly independently associated with an increased risk of composite and renal events. Although the LVH/H2.7-only group was not associated with composite and renal events, the LVH/BSA only group showed a significantly high risk for composite and renal events, despite having very few patients. Therefore, we assumed that BSA may be as useful as height2.7 in indexing LVM in pre-dialysis CKD patients.
Our results were discordant with the study by Zoccali et al. (16). They analyzed 254 patients undergoing dialysis and reported that height2.7 provides better indexation of LVM than BSA for predicting overall and cardiovascular mortality and cardiovascular events. We assumed that difference in the volume status, based on the dialysis status of patients with CKD may be responsible for different results. Unlike pre-dialysis patients with CKD [41], dialysis patients tend to have a greater volume overload [15] and dialysis procedure affected much on volume status and echocardiographic measures [42]. In subgroup analysis according to CKD stages (S5 Table), LVMI-BSA was significantly associated with composite outcome in groups with stage 3a-b and stage 4–5, while LVMI-H.27 was associated with composite outcome only in group with stage 4–5. Although effect modification of CKD stages on the relationship between LVM indexations and composite outcome was marginal, which might be because subjects with CKD stage 1–2 had very low rates of adverse events, it is obvious that volume is more likely to be overloaded with the progression of CKD stage [43]. Therefore, the evident relationship between LVMI-H2.7 and composite outcome only in advanced CKD may be in line with the results from Zoccali et al. and the poorer performance of LVMI-BSA in the study by Zoccali et al. [16] may be attributed to the high volume status in dialysis patients, which was in line with the poorer performance of BSA in populations with obesity [35, 36].
This study had several strengths. First, this was the largest study to examine the clinical significance of LVM and its indexation on adverse clinical outcomes in pre-dialysis patients with CKD. The study was the first to demonstrate the association between LVM and its indexations and renal events. Second, missing rates of major variables were low. Third, the study results support the current ASE guideline, which uses BSA as a major indexation [1, 14]. The LVH is important cardiovascular risk factor in CKD patients and many studies have been done. The validation which LVM index is better could help researchers to use unified LVMI and facilitate to compare the studies. According to our results, we suggest to use the LVMI-BSA in the pre-dialysis CKD patients. The study also had several limitations. First, the cardiovascular events and all-cause mortality were too low, despite a moderate follow-up duration [39]. Therefore, the null association of LVM and its indexations with all-cause mortality needs to be re-evaluated, when a considerable number of events are developed. Second, we did not evaluate markers for the volume status. Although we had echocardiographic measures, cardiac geometry analysis showed that both eccentric and concentric stresses were increased with the progression of CKD stages (S6 Table). Therefore, the effect of volume overload in pre-dialysis patients with CKD could not be presented in this study. Third, the common ethnicity of the study’s participants limits the generalizability of the results.
In conclusion, LVM indexing improved the predictive ability of future adverse outcomes. BSA may be as useful as height2.7 in indexing LVM in pre-dialysis patients with CKD for predicting future adverse outcomes. Subsequent studies are needed to confirm our results.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The Know-CKD Investigator Group
Patient Recruitment. Seoul National University Hospital, Curie Ahn, MD, Kook-Hwan Oh, MD (PI), Hajeong Lee, MD, Seung Seok Han, MD, Hyunjin Ryu, MD, Eunjeong Kang, MD, Minjung Kang, MD, Youngok Ko, RN, Jeongok So, RN, and Aram Lee, RN. Seoul National University Bundang Hospital, Dong Wan Chae, MD (SubPI), Yong Jin Yi MD, Hyun Jin Cho, RN and Jung Eun Oh RN. Yonsei University, Severance Hospital, Kyu Hun Choi, MD (SubPI), Seung Hyeok Han, MD, Tae- Hyun Yoo, MD, and Mi Hyun Yu, RN. Kangbuk Samsung Hospital, Kyu-Beck Lee, MD and Young Youl Hyun, MD, Hyun Jung Kim, RN. The Catholic University of Korea, Seoul St. Mary’s Hospital, Yong-Soo Kim, MD and Sol Ji Kim, RN. Gachon University Gil Medical Center, Wookyung Chung, MD, Ji Yong Jung, MD and Kwon Eun Jin, RN. Nowon Eulji Medical Center, Eulji University. Su Ah Sung, MD, Sung Woo Lee, MD, Hyang Ki Min, MD, and Soon Bin Kwon, RN. Chonnam National University Hospital, Soo Wan Kim, MD, Seong Kwon Ma, MD, Eun Hui Bae, MD, Chang Seong Kim, MD, Hong Sang Choi, MD, Minah Kim, MD, Tae Ryom Oh, MD, Sang Heon Suh, MD, Su Hyun Song, MD, and Se Jeong Lee, RN. Inje University, Pusan Paik Hospital, Yeong Hoon Kim, MD, Sun Woo Kang, MD, Hoseok Koo, MD, Tae Hee Kim, MD and Yun Mi Kim. MD and Young Eun Oh, MSc. Pusan National University Hospital, Eun Young Seong, MD, Sang Heon Song, MD, Miyeun Han, MD, Hyo Jin Kim, MD, Seunghee Ji, RN, National Health Insurance Service Ilsan Hospital, Tae Ik Chang, MD, Ea Wha Kang, MD, Kyoung Sook Park, MD, Aei Kyung Choi, RN. Hallym University Dongtan Sacred Heart Hospital Ja-Ryong Koo, MD, Jang-Won Seo, MD, Sun Ryoung Choi, MD, Seon Ha Baek, MD and Myung Sun Kim, RN. Seoul National University Boramae Medical Center, Yun Kyu Oh, MD (SubPI), Jeong Mi Park, RN.
Epidemiology and Biostatistics. Department of Preventive Medicine, Seoul National University College of Medicine, Byung-Joo Park, MD, Sue K. Park, MD, Choonghyun Ahn, MD and Kyungsik Kim, BSc. School of Medicine, Inha University Department of Prevention and Management, Inha University Hospital, Joongyub Lee, MD.
Data Coordinating Center. Medical Research Collaborating Center, Seoul National University Hospital and Seoul National University College of Medicine, Jayoun Kim, PhD, Dayeon Nam, RN, Soohee Kang, MSc, Juhee Lee, MSc, and Heejung Ahn, RN. Central Laboratory. Dong Hee Seo, MD, and Soyoung Kim, MD, LabGenomics, Korea. Biobank. Korea Biobank, Korea Centers for Disease Control and Prevention, Osong, Korea. Korea Center for Disease Control and Prevention. Ok Park, Il Yoel Kim, Sung Hyun Kang, and Kyoung Hwa Kim
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
KHO This study was supported by the Research Program funded by the Korea Center for Disease Control and Prevention (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, and 2019E320100).
References
- 1.Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28(7):727–54. 10.1016/j.echo.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 2.Aro AL, Chugh SS. Clinical Diagnosis of Electrical Versus Anatomic Left Ventricular Hypertrophy: Prognostic and Therapeutic Implications. Circ Arrhythm Electrophysiol. 2016;9(4):e003629 10.1161/CIRCEP.115.003629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103(19):2346–51. 10.1161/01.cir.103.19.2346 [DOI] [PubMed] [Google Scholar]
- 4.Okin PM, Roman MJ, Lee ET, Galloway JM, Howard BV, Devereux RB. Combined echocardiographic left ventricular hypertrophy and electrocardiographic ST depression improve prediction of mortality in American Indians: the Strong Heart Study. Hypertension. 2004;43(4):769–74. 10.1161/01.HYP.0000118585.73688.c6 [DOI] [PubMed] [Google Scholar]
- 5.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43(12):2207–15. 10.1016/j.jacc.2003.11.064 [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C. Left ventricular mass index as an outcome measure in clinical trials in dialysis patients: a word of caution. Am J Nephrol. 2011;33(4):370–2. 10.1159/000326239 [DOI] [PubMed] [Google Scholar]
- 7.Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36(2):286–90. 10.1038/ki.1989.192 [DOI] [PubMed] [Google Scholar]
- 8.Stack AG, Saran R. Clinical correlates and mortality impact of left ventricular hypertrophy among new ESRD patients in the United States. Am J Kidney Dis. 2002;40(6):1202–10. 10.1053/ajkd.2002.36881 [DOI] [PubMed] [Google Scholar]
- 9.Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK, et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J J Am Soc Nephrol. 2004;15(8):2186–94. [DOI] [PubMed] [Google Scholar]
- 10.Cuspidi C, Facchetti R, Bombelli M, Sala C, Grassi G, Mancia G. Differential value of left ventricular mass index and wall thickness in predicting cardiovascular prognosis: data from the PAMELA population. Am J Hypertens. 2014;27(8):1079–86. 10.1093/ajh/hpu019 [DOI] [PubMed] [Google Scholar]
- 11.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20(5):1251–60. 10.1016/0735-1097(92)90385-z [DOI] [PubMed] [Google Scholar]
- 12.Liao Y, Cooper RS, Durazo-Arvizu R, Mensah GA, Ghali JK. Prediction of mortality risk by different methods of indexation for left ventricular mass. J Am Coll Cardiol. 1997;29(3):641–7. 10.1016/s0735-1097(96)00552-9 [DOI] [PubMed] [Google Scholar]
- 13.Cuspidi C, Giudici V, Negri F, Meani S, Sala C, Zanchetti A, et al. Improving cardiovascular risk stratification in essential hypertensive patients by indexing left ventricular mass to height(2.7). J Hypertens. 2009;27(12):2465–71. 10.1097/HJH.0b013e32833105a6 [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 15.Hassan MO, Duarte R, Dix-Peek T, Vachiat A, Naidoo S, Dickens C, et al. Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin Nephrol. 2016;86 (2016)(13):131–5. 10.5414/CNP86S127 [DOI] [PubMed] [Google Scholar]
- 16.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, et al. Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol. 2001;12(12):2768–74. [DOI] [PubMed] [Google Scholar]
- 17.Lee SW, Kim JM, Lim HJ, Hwang YH, Kim SW, Chung W, et al. Serum hepcidin may be a novel uremic toxin, which might be related to erythropoietin resistance. Sci Rep. 2017;7(1):4260 10.1038/s41598-017-04664-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): design and methods. BMC Nephrol. 2014;15(80):1471–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 21.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71. [PubMed] [Google Scholar]
- 22.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Statistics in medicine. 2013;32(30):5381–97. 10.1002/sim.5958 [DOI] [PubMed] [Google Scholar]
- 23.Wang AY, Lam CW, Yu CM, Wang M, Chan IH, Lui SF, et al. Troponin T, left ventricular mass, and function are excellent predictors of cardiovascular congestion in peritoneal dialysis. Kidney Int. 2006;70(3):444–52. 10.1038/sj.ki.5001605 [DOI] [PubMed] [Google Scholar]
- 24.Chen SC, Chang JM, Liu WC, Chen YY, Chen LI, Huang JC, et al. The ratio of observed to predicted left ventricular mass is independently associated with increased cardiovascular events in patients with chronic kidney disease. Hypertens Res. 2012;35(8):832–8. 10.1038/hr.2012.40 [DOI] [PubMed] [Google Scholar]
- 25.Eckardt KU, Scherhag A, Macdougall IC, Tsakiris D, Clyne N, Locatelli F, et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J Am Soc Nephrol. 2009;20(12):2651–60. 10.1681/ASN.2009060631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Zhang DL, Guo W, Cui WY, Liu WH. Left ventricular mass index and aortic arch calcification score are independent mortality predictors of maintenance hemodialysis patients. Hemodial Int. 2012;16(4):504–11. 10.1111/j.1542-4758.2012.00700.x [DOI] [PubMed] [Google Scholar]
- 27.Badve SV, Palmer SC, Strippoli GF, Roberts MA, Teixeira-Pinto A, Boudville N, et al. The Validity of Left Ventricular Mass as a Surrogate End Point for All-Cause and Cardiovascular Mortality Outcomes in People With CKD: A Systematic Review and Meta-analysis. Am J Kidney Dis. 2016;68(4):554–63. 10.1053/j.ajkd.2016.03.418 [DOI] [PubMed] [Google Scholar]
- 28.Muiesan ML, Salvetti M, Paini A, Monteduro C, Galbassini G, Bonzi B, et al. Inappropriate left ventricular mass changes during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2007;49(5):1077–83. 10.1161/HYPERTENSIONAHA.107.087320 [DOI] [PubMed] [Google Scholar]
- 29.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29(6):741–7. 10.1093/eurheartj/ehm605 [DOI] [PubMed] [Google Scholar]
- 30.Eguchi K, Ishikawa J, Hoshide S, Ishikawa S, Pickering TG, Schwartz JE, et al. Differential impact of left ventricular mass and relative wall thickness on cardiovascular prognosis in diabetic and nondiabetic hypertensive subjects. Am Heart J. 2007;154(1):79 e9–15. [DOI] [PubMed] [Google Scholar]
- 31.Desai CS, Bartz TM, Gottdiener JS, Lloyd-Jones DM, Gardin JM. Usefulness of Left Ventricular Mass and Geometry for Determining 10-Year Prediction of Cardiovascular Disease in Adults Aged >65 Years (from the Cardiovascular Health Study). Am J Cardiol. 2016;118(5):684–90. 10.1016/j.amjcard.2016.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study). Am J Cardiol. 2008;102(9):1131–5. 10.1016/j.amjcard.2008.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai CL, Chien KL, Hsu HC, Su TC, Chen MF, Lee YT. Left ventricular mass and risk of cardiovascular events and all-cause death among ethnic Chinese—the Chin-Shan Community Cardiovascular Cohort study. Int J Cardiol. 2011;149(3):347–52. 10.1016/j.ijcard.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 34.Mostovaya IM, Bots ML, van den Dorpel MA, Goldschmeding R, den Hoedt CH, Kamp O, et al. Left ventricular mass in dialysis patients, determinants and relation with outcome. Results from the COnvective TRansport STudy (CONTRAST). PloS One. 2014;9(2):e84587 10.1371/journal.pone.0084587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hense HW, Gneiting B, Muscholl M, Broeckel U, Kuch B, Doering A, et al. The associations of body size and body composition with left ventricular mass: impacts for indexation in adults. J Am Coll Cardiol. 1998;32(2):451–7. 10.1016/s0735-1097(98)00240-x [DOI] [PubMed] [Google Scholar]
- 36.Gosse P, Jullien V, Jarnier P, Lemetayer P, Clementy J. Echocardiographic definition of left ventricular hypertrophy in the hypertensive: which method of indexation of left ventricular mass? J Hum Hypertens. 1999;13(8):505–9. 10.1038/sj.jhh.1000885 [DOI] [PubMed] [Google Scholar]
- 37.Ferrara LA, Vaccaro O, Cardoni O, Laurenzi M, Mancini M, Zanchetti A. Indexation criteria of ventricular mass and predictive role of blood pressure and body composition. Am J Hypertens. 2005;18(10):1282–7. 10.1016/j.amjhyper.2005.05.020 [DOI] [PubMed] [Google Scholar]
- 38.Cuspidi C, Meani S, Negri F, Giudici V, Valerio C, Sala C, et al. Indexation of left ventricular mass to body surface area and height to allometric power of 2.7: is the difference limited to obese hypertensives? J Hum Hypertens. 2009;23(11):728–34. 10.1038/jhh.2009.16 [DOI] [PubMed] [Google Scholar]
- 39.Orlandi PF, Huang J, Fukagawa M, Hoy W, Jha V, Oh KH, et al. A collaborative, individual-level analysis compared longitudinal outcomes across the International Network of Chronic Kidney Disease (iNETCKD) cohorts. Kidney Int. 2019;96(5):1217–33. 10.1016/j.kint.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 40.Chen SC, Chang JM, Yeh SM, Su HM, Chen HC. Association of uric acid and left ventricular mass index with renal outcomes in chronic kidney disease. Am J Hypertens. 2013;26(2):243–9. 10.1093/ajh/hps020 [DOI] [PubMed] [Google Scholar]
- 41.Thanakitcharu P, Jirajan B. Early detection of subclinical edema in chronic kidney disease patients by bioelectrical impedance analysis. J Med Assoc Thai. 2014;97 Suppl 11:S1–10. [PubMed] [Google Scholar]
- 42.Loutradis C, Sarafidis PA, Papadopoulos CE, Papagianni A, Zoccali C. The Ebb and Flow of Echocardiographic Cardiac Function Parameters in Relationship to Hemodialysis Treatment in Patients with ESRD. Journal of the American Society of Nephrology: JASN. 2018;29(5):1372–81. 10.1681/ASN.2017101102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yilmaz Z, Yildirim Y, Oto F, Aydin FY, Aydin E, Kadiroglu AK, et al. Evaluation of volume overload by bioelectrical impedance analysis, NT-proBNP and inferior vena cava diameter in patients with stage 3&4 and 5 chronic kidney disease. Renal failure. 2014;36(4):495–501. 10.3109/0886022X.2013.875815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.