Supplemental Digital Content is available in the text.
Keywords: brain, inflammation, long-term study, nebulized surfactant, respiratory distress syndrome
Objectives:
We have setup for the first time a long-term (72 hr) respiratory distress syndrome model in spontaneously breathing surfactant-deficient newborn piglets to investigate the continuous positive airway pressure failure rate with nebulized poractant alfa compared with that with the intubation surfactant extubation technique or continuous positive airway pressure only.
Design:
Prospective randomized animal study.
Setting:
Biocruces-Bizkaia Health Research Institute Animal Facility.
Subjects-Interventions:
Eighteen newborn piglets (n = 6/group) with surfactant-deficient respiratory distress syndrome were randomized to three continuous positive airway pressure–ventilated groups: 1) nebulized surfactant (poractant alfa 400 mg/kg) via a customized investigational eFlow-Neos vibrating membrane nebulizer system, 2) bolus administration using the Intubation Surfactant Extubation method (200 mg/kg), or 3) continuous positive airway pressure alone.
Measurements and Main Results:
Pulmonary and hemodynamic variables were assessed at 6-hour intervals for 72 hours. Lung and brain histological analyses were performed. After bronchoalveolar lavages, piglets developed respiratory distress syndrome. Over the follow-up, both surfactant-treated groups had significantly better pulmonary outcomes than the continuous positive airway pressure alone group. Furthermore, unlike in the continuous positive airway pressure group, there were no cases of respiratory failure in either of the surfactant-treated groups.
Conclusions:
In newborn piglets with respiratory distress syndrome, the nebulization of 400 mg/kg of poractant alfa using a customized investigational eFlow-Neos nebulizer was found to be safe and effective in reducing the risk of respiratory failure in the 72 hours after treatment.
In recent years, the way surfactant is administered in neonatal ICUs has changed considerably, from standard bolus therapy (intubation-mechanical ventilation) to the intubation surfactant extubation (InSurE) method, the less invasive surfactant administration method, or investigational approaches like surfactant nebulization (1–3). The use of nebulized surfactant seems to be the least invasive approach, fully avoiding the risks associated with instillation of a surfactant bolus into the trachea in neonates with respiratory distress syndrome (RDS). Although preclinical and clinical studies using nebulized surfactant have not been always successful (studies having differed in nebulizer type and surfactant dosage), overall, the treatment appears to be safe and well tolerated (3–14).
A new device based on vibrating membrane technology (eFlow-Neos Nebulizer; Pari Pharma, Starnberg, Germany) has been designed for use in infants and is able to deliver high doses of surfactant, while surfactant characteristics remain unchanged after nebulization (15–17). Only one clinical trial has evaluated the efficacy of using this nebulizer (200 mg/kg; poractant-alfa) and found only less need for intubation in the first 3 days compared to that with nasal continuous positive airway pressure (nCPAP) treatment (3). Considering the known surfactant losses during nebulization, it is reasonable to also explore the efficacy of higher doses (18).
The aim of this study was to assess, for the first time over the critical period of 72 hours after surfactant treatment, the efficacy and safety of poractant alfa 400 mg/kg nebulization (18), using the eFlow-Neos nebulizer (Pari Pharma), as a noninvasive method of administering surfactant for the treatment of neonatal RDS. We hypothesized that the combination of nCPAP and nebulized surfactant would reduce the nCPAP failure rate as measured by the need for intubation and mechanical ventilation in spontaneously breathing newborn piglets, with bronchoalveolar lavage (BAL)–induced RDS. Furthermore, we assessed the impact of nebulized surfactant on gas exchange, hemodynamic parameters, oxygen metabolism, and lung and brain injury scores.
MATERIAL AND METHODS
Animal Preparation
The experimental protocol meets Spanish and European regulations for the protection of experimental animals (UE2010/63 and RD53/2013) and was approved by the Ethics Committee for Animal Welfare of Biocruces Bizkaia Health Research Institute (OEBA-CET-2016-003). First, 2–4-day-old newborn piglets were sedated (ketamine-diazepam-atropine intramuscular) and anesthetized with sevofluorane (19). A cuffed endotracheal tube (ET) was connected to a positive pressure ventilator with the following settings: Fio2 equals to 0.21–0.28, respiratory frequency equals to 28 breaths/min, positive end-expiratory pressure (PEEP) equals to 3 cm H2O, and positive inspiratory pressure (PIP) equals to 9–11 cm H2O to maintain a tidal volume (VT) equals to 8–10 mL/kg.
An arterial catheter was inserted into the carotid artery to monitor mean arterial blood pressure (MABP) and heart rate (HR) and to obtain blood samples for gas analysis. In addition, a 5F dual-lumen catheter was inserted into the jugular vein for administering fluids for hydration, parenteral nutrition, and collection of venous blood samples. The animals received prophylactic antibiotics (amoxicillin-clavulanate 100 mg/kg/d IV).
Lung Injury and Study Design
Surfactant-deficient lung injury was achieved by repetitive saline lavage (30 mL/kg; 37°C; Fio2:1) (19, 20). Lavage was repeated at 5-minute intervals until Pao2 less than 100 mm Hg was obtained with a PIP not exceeding 25 cm H2O. After 30 minutes of stabilization, all piglets received a bolus dose of caffeine citrate 20 mg/kg IV (Peyona 20 mg/mL; Chiesi Farmaceutici, Parma, Italy) before extubation. Tightly fitting short binasal prongs were placed in all animals. Once spontaneous breathing was established, piglets with BAL-induced RDS were randomly assigned using a sealed-envelope system to one of the following groups:
nCPAP group (n = 6): once breathing spontaneously, piglets had the ET removed and were maintained on nCPAP alone, without surfactant treatment.
nCPAP-InSurE group (n = 6): once breathing spontaneously, piglets were not extubated and received poractant alfa 200 mg/kg (Curosurf, Chiesi Farmaceutici, Parma, Italy) through the ET. Immediately after that, the ET was removed and they were maintained on nCPAP.
nCPAP-NebSurf group: once breathing spontaneously, piglets had the ET removed and received 400 mg/kg of nebulized surfactant (NebSurf) using an eFlow-Neos nebulizer (Pari Pharma) placed between the prongs and the connection to the nCPAP circuit. Following surfactant nebulization, the nebulizer was removed, and animals were maintained on nCPAP.
The nCPAP level was set at 5 cm H2O with a flow of 3 L/min for 72 hours.
Physiologic Measurements
The following physiologic parameters were measured or calculated at baseline (immediately after the induction of anesthesia, after surgery, upon intubation), immediately after inducing RDS by BAL, 30 minutes later (after a 30-min period of stabilization, animals having been under mechanical ventilation) and, following extubation, during nCPAP every 6 hours until the end of experiment, at 72 hours.
Arterial pH, Pao2, Paco2, base excess and Pao2/Fio2 and arterial oxygen content (Cao2) and
intrapulmonary-shunt (Qs/Qt); HR, and MABP.
Further, airway flow, mean airway pressure, and VT monitored with a flow sensor connected to the ET, whereas values of lung mechanics were measured with a computerized system (M1014A, Philips Medical System, Eindhoven, The Netherlands) during the intervals in which animals were intubated. The analyzer reported values for dynamic compliance (Cdyn), VT, and airway resistance at baseline, after the BAL-induced surfactant depletion, and after 30 minutes of stabilization of BAL-induced RDS. After 30 minutes of stabilization, all animals were extubated. During nCPAP, lung mechanics cannot be measured. At the end of the experiment (72-hr of follow-up), all animals were reintubated, connected to mechanical ventilation (using the same settings as at baseline), and lung mechanics were measured.
Reintubation-Extubation Criteria
Reintubation was performed if animals showed any of the following signs of nCPAP failure:
a rapid rise in Fio2 requirement (10%) over 1–2 hours; Fio2 greater than 0.50 with Pao2 less than 80 mm Hg or
respiratory acidosis (pH < 7.25 or Paco2 > 60 mm Hg) or
recurrent apneic episodes (>3 episodes/hr or >1 apneic episode/hr requiring stimulation).
Extubation was performed when all the following criteria were met:
good respiratory drive with spontaneous breaths over the ventilator and
mean airway pressure less than or equal to 8 cm H2O and
Fio2 less than 0.50 with Pao2 greater than 80–100 mm Hg and
pH greater than 7.25 with Paco2 less than 60 mm Hg.
Lung Tissue Analysis
Postmortem, the lungs were removed and perfused with saline. The left lung was isolated, occluded, and stored at –80°C until use in biochemical analysis, whereas the right lung was fixed (4% formalin) for histological analysis (19).
Frozen lung samples were used for measurements of interleukin (IL)–8, IL-1B, and tumor necrosis factor-α concentration using specific enzyme-linked immunosorbent assay kits (Abnova, Tapei City, Taiwan). Enzyme activities, catalase (19, 21), and superoxide dismutase were tested (Cayman Chemical, Ann Arbor, MI), whereas protein concentrations were determined by the Bradford method (22).
Formalin-fixed tissue was sectioned (5-µm thick), stained, and analyzed with light microscopy. Lung injury was scored by a pathologist blinded to treatment allocation. Pathologic signs of lung injury (atelectasis, alveolar and interstitial inflammation, alveolar and interstitial hemorrhage, edema, and necrosis) were each scored on a 0- to four-point scale: 0 corresponding to no injury; 1, 2, and 3 to injury to 25%, 50%, and 75% of the field; and 4 to injury across the field (20, 23). Additionally, all seven injury scores were summed to obtain a mean total lung injury score for each group, ranging from 0 to 28, values higher than 12 corresponding to quite severe lung injury (19).
Brain-Specific Protein Analysis
For assessing brain-specific protein levels, cerebrospinal fluid samples were taken at the end of the experiment and frozen at –80°C until assay. Enzyme immunoassays were used for measuring neuron-specific enolase (NSE) and S100 (DRG Instruments, Marburg, Germany).
Brain Tissue Analysis
The brain was fixed (4% formalin) and divided into cortex, inner regions (striatum-thalamus-hippocampus), and cerebellum and brain stem. A total of 20 fields were analyzed, and pathologic features of brain injury (necrosis, inflammation, hemorrhage, edema and infarction) were each scored on a zero- to three-point scale: 0 corresponding to no injury; and 1, 2, and 3 to injury to mild, moderate, and severe injury across the field. The presence of greater than 5 necrotic cells/field was considered to indicate neuronal necrosis (score range: 0–20) (24).
Statistical Analysis
Values are expressed as mean ± sem. Results were assessed using Levene’s test and the Kolmogorov-Smirnoff test to determine whether the assumption of homogeneity of variances between treatments was met and whether the data were normally distributed, respectively (JMP8; Statistical Discovery, SAS, NC). Data related to gas exchange, hemodynamic parameters, oxygen metabolism, and lung mechanics were analyzed using one-and two-way analysis of variance by group and time of repeated measures. Biochemical results and lung and brain injury scores were analyzed using the nonparametric Wilcoxon test. A p value of less than 0.05 was considered significant.
RESULTS
The 18 newborn piglets studied were similar in age (4 ± 1 d) and size (2.1 ± 0.1 kg). Multiple BALs (mean of 13–17) were needed to induce lung injury (Pao2 < 100 mm Hg), no significant differences being observed between groups in number of BALs required or amount of BAL recovered (>92% in all groups). The mean surfactant nebulization time was: 47 ± 6 minutes and nebulizer output was: 0.23 ± 0.05 mL/min.
Pulmonary Assessment
Gas Exchange and Lung Mechanics.
All animals had similar pH, Pao2/Fio2, Paco2, Cao2, and Cdyn parameters at baseline, after BAL, and after 30 minutes of stabilization (Fig. 1 and Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F431), with no significant differences between groups. BAL produced an abrupt decrease in Pao2/Fio2 (Fig. 1A), Cdyn (Fig. 1B), pH, and Cao2 (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F431), along with a significant increase in Paco2 (Fig. 1C).
Figure 1.
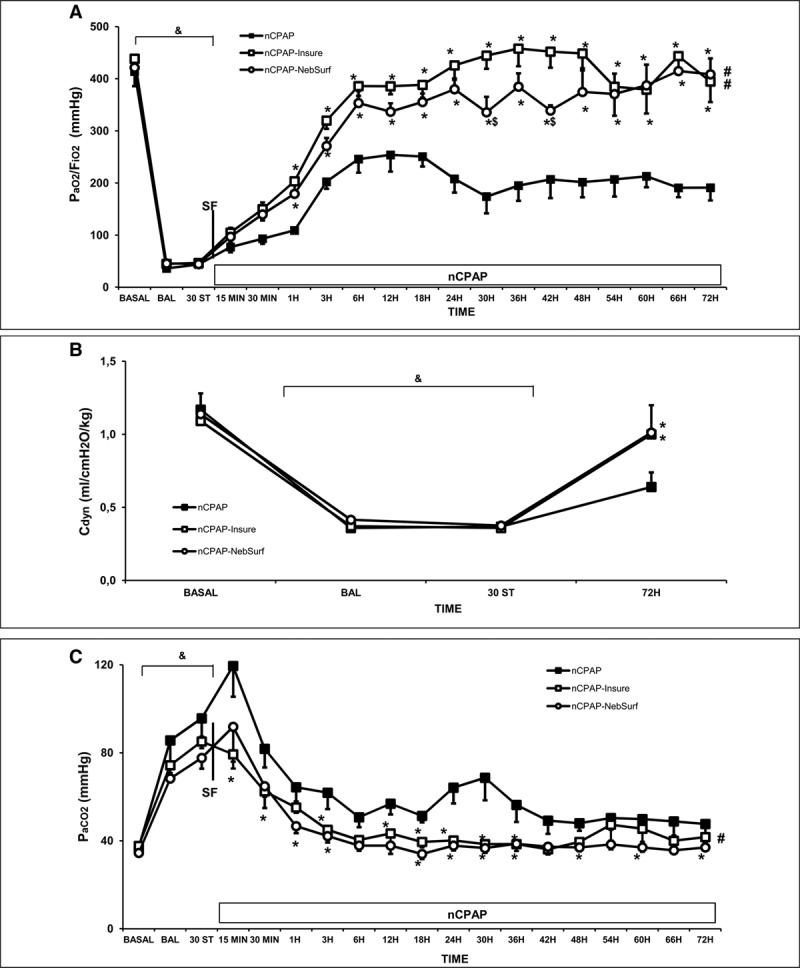
Changes in Pao2/Fio2 ratio, dynamic compliance (Cdyn), and Paco2 in newborn piglets with surfactant-deficient lung injury treated with nasal continuous positive airway pressure (nCPAP) without or with surfactant treatment, using the intubation surfactant extubation (InSurE) method or nebulized surfactant (NebSurf) over the 72-hr experimental period. Pao2/Fio2 (A), Cdyn (B), and Paco2 (C) values in the nCPAP (black square), nCPAP-InSurE (white square), and nCPAP-NebSurf (white circle) groups. (&)p < 0.05 versus basal point, (*)p < 0.05 versus nCPAP group, ($)p < 0.05 versus nCPAP-InSurE group (one-way analysis of variance); (#)p < 0.05 versus nCPAP group (two-way analysis of variance). Values are mean ± sem. BAL = bronchoalveolar lavage, BASAL = baseline point, ST = stabilization
Pao2/Fio2 ratio, Paco2, and pH improved more rapidly in the nCPAP-InSurE than the nCPAP group (Fig. 1 and Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F431). The administration of 400 mg/kg of nebulized surfactant produced significant improvements in these parameters, in line with the changes in the InSurE group. Although half of the animals in the nCPAP group required endotracheal intubation and mechanical ventilation due to recurrent apneic episodes, none in nCPAP-InSurE or nCPAP-NebSurf groups required intubation. None of intubated animals were extubated as they did not meet all the extubation criteria.
The respiratory rate (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F431) was maintained higher in the nCPAP group than nCPAP-InSurE and nCPAP-NebSurf groups, to avoid hypercapnia. Furthermore, more apnea episodes were recorded during the first 24 hours in the group given nCPAP alone than the surfactant-treated groups.
Regarding Cdyn, while values recovered to or close to baseline values in almost all surfactant-treated animals (80%–100% in nCPAP-InSurE and nCPAP-NebSurf groups), Cdyn recovery was observed in only around 50%–60% of animals receiving nCPAP alone (Fig. 1B). No significant differences were observed between groups in VT or resistance parameters (data not shown).
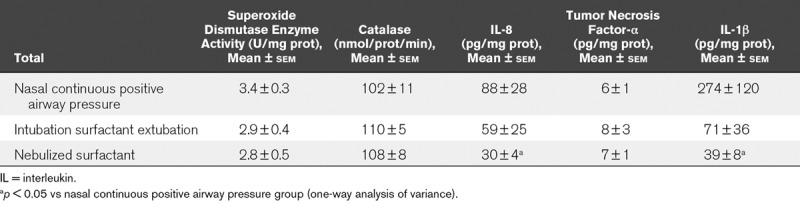
Lung Inflammatory Markers.
Table 1 shows the antioxidant enzyme activity and concentrations of the acute-phase cytokines IL-8, IL1-β, and tumor necrosis factor-α measured in lung homogenate. After 72 hours of nCPAP ventilation, there were no significant differences in lung antioxidant enzyme activity between groups. The nebulization of surfactant 400 mg/kg was associated with lower IL-8 and IL-1B lung cytokine levels than those in the nCPAP group, differences compared with the InSurE group not reaching significance.
TABLE 1.
Lung Biochemical Analysis in Surfactant-Deficient Newborn Piglets Treated With Nasal Continuous Positive Airway Pressure Without or With Surfactant Treatment, Using the Intubation Surfactant Extubation Method or Nebulized Surfactant After 72 Hours of Treatment
Lung Injury.
At 72 hours, total lung injury scores were lower in both InSurE and surfactant nebulization groups than the nCPAP alone group. Furthermore, both types of surfactant treatment were associated with significantly less atelectasis, edema, alveolar inflammation, and alveolar-interstitial hemorrhage (Table 2 and Fig. 2A–C).
TABLE 2.
Total Lung Injury Scores in Surfactant-Deficient Newborn Piglets Treated With Nasal Continuous Positive Airway Pressure Without or With Surfactant Treatment, Using the Intubation Surfactant Extubation Method or Different Doses of Nebulized Surfactant After 72 Hours of Experimental Period
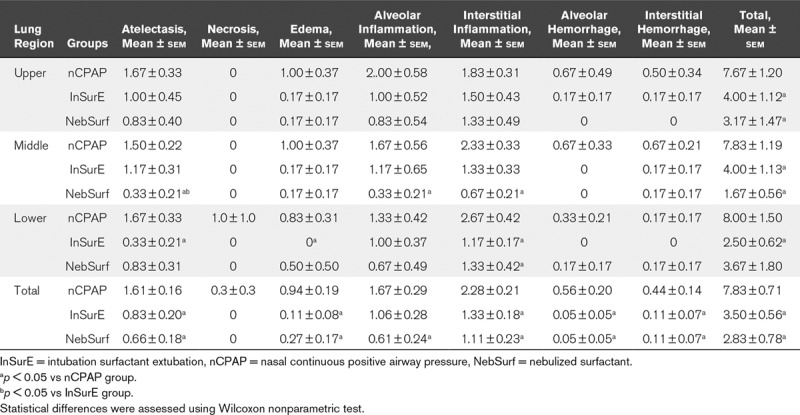
Figure 2.
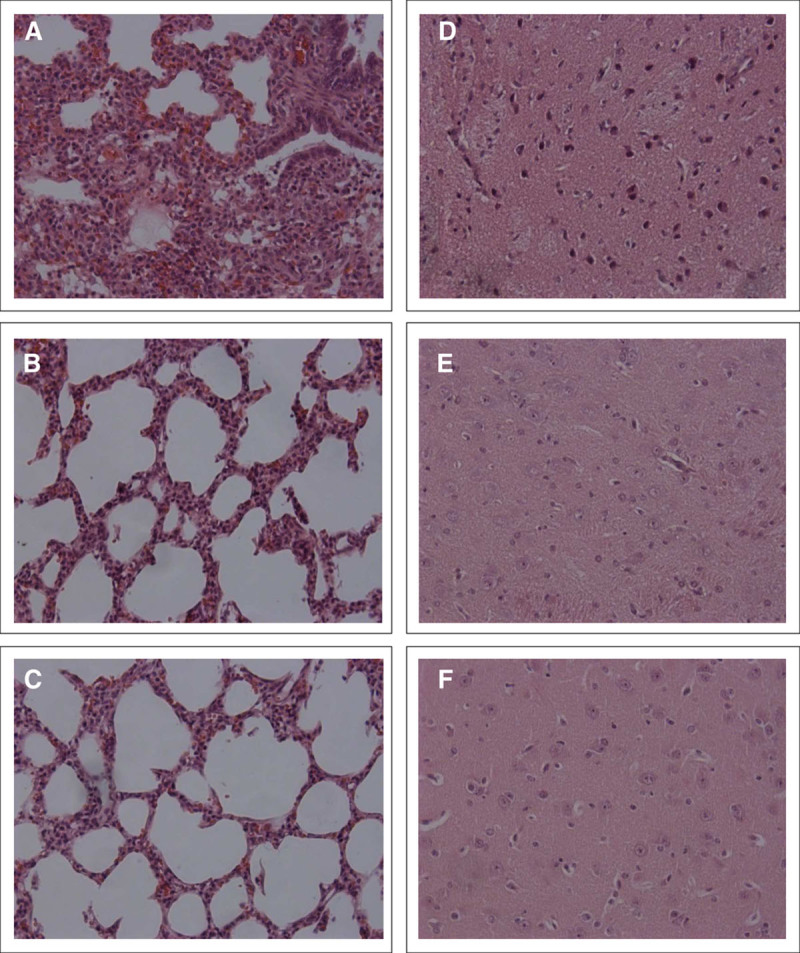
Photomicrographs (200× magnification) of representative lung sections from nasal continuous positive airway pressure (nCPAP) (A) nCPAP-intubation surfactant extubation (InSurE) (B), and nCPAP-nebulized surfactant (NebSurf) (C) groups and representative brain section from nCPAP (D), nCPAP-InSurE (E), and nCPAP-NebSurf (F) groups. Panels were obtained from the middle region of the lung and the striatum region of the brain.
Intrapulmonary Shunt and Hemodynamic Assessment
BAL produced an abrupt increase in Qs/Qt (Fig. 3A). Surfactant administration using the InSurE technique or nebulization was associated with rapid improvements in Qs/Qt during the first 6–12 hours after the procedure. These improvements were sustained over time, the values remaining significantly lower than those in the nCPAP group.
Figure 3.
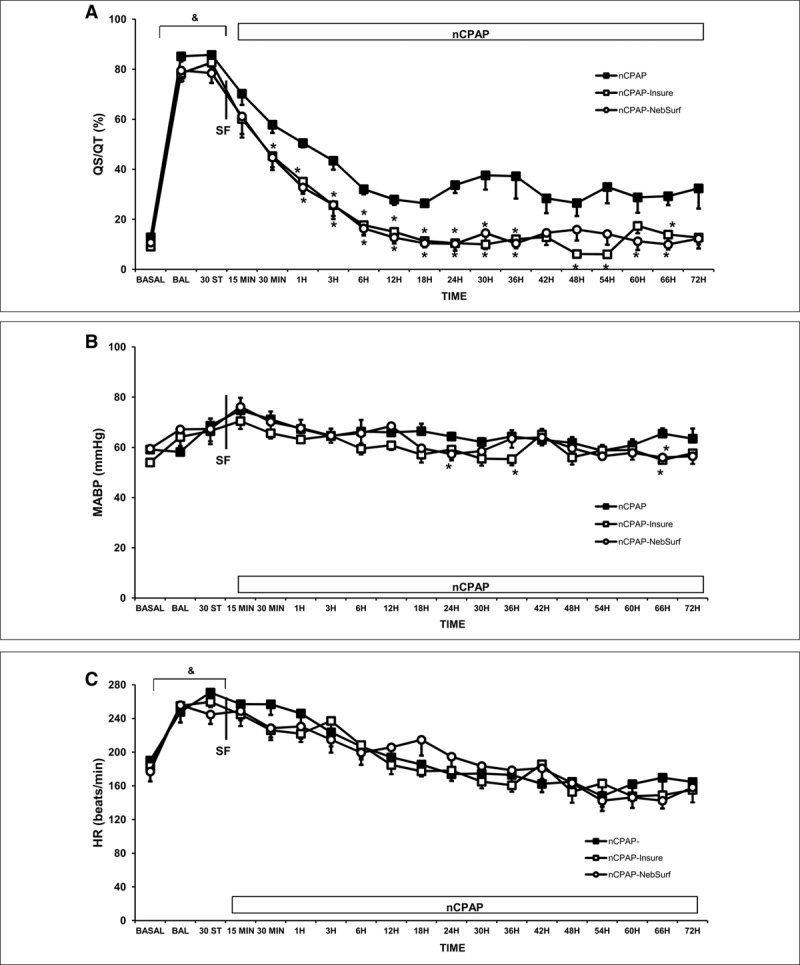
Changes in intrapulmonary shunt (Qs/Qt), arterial blood pressure (MABP), heart rate (HR), and carotid blood flow (D) in newborn piglets with surfactant deficient lung injury treated with nasal continuous positive airway pressure (nCPAP) without or with surfactant treatment, using the intubation surfactant extubation (InSurE) method or nebulized surfactant (NebSurf) over the 72-hr experimental period. Mean Qs/Qt (A) MABP (B) and mean HR (C) values in the nCPAP (black square), nCPAP-InSurE (white square), and nCPAP-NebSurf (white circle) groups. (&) p < 0.05 versus basal point, (*) p < 0.05 versus nCPAP group (one-way analysis of variance). Values are mean ± sem. BAL = bronchoalveolar lavage, BASAL = baseline point, ST = stabilization.
No significant changes in MABP were observed after BAL (Fig. 3B), while the HR rose (Fig. 3C). Only transient differences were observed in MABP between groups (values remaining in the physiologically normal range) throughout the follow-up.
Cerebral Assessment
There were no significant differences in the concentration of S100 and NSE proteins in cerebrospinal fluid between the nCPAP (S100: 2.2 ± 0.7 µg/L; NSE: 5.3 ± 1.0 µg/L) and surfactant-treated groups (InSurE:S100: 2.1 ± 0.7 µg/L; NSE: 3.5 ± 0.8 µg/L; NebSurf:S100: 2.0 ± 0.4 µg/L; NSE: 3.5 ± 0.8 µg/L). All groups studied obtained low brain injury scores, with similar hemorrhage, inflammation, and infarction scores in all regions studied (Fig. 2 D–F and Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/CCM/F432).
DISCUSSION
We have been able to demonstrate in our spontaneously breathing newborn piglet model of surfactant-deficient lung injury, that the eFlow Neos nebulizer is safe and able to produce a clinically relevant improvement in oxygenation and lung function. Furthermore, we observed a reduction in the risk of nCPAP failure in the first 72 hours after surfactant treatment, with similar pulmonary, hemodynamic, and cerebral behavior and lung histological findings to that in the InSurE group but lower lung inflammation scores.
In view of the comorbidities (airway obstruction, hypoxia, bradycardia, blood pressure fluctuation) associated with the currently approved way of administering surfactant (intubation, surfactant bolus and mechanical ventilation), in neonatal ICUs, there has been a notable trend toward a greater use of noninvasive ventilation such as nCPAP, combined with InSurE or less invasive surfactant administration techniques (1, 2, 25). Nonetheless, all of these approaches require instrumentation of the airway and still carry the risk associated with instillation of a fluid bolus into the trachea.
Despite the promising findings of the first study using nebulized surfactant within an incubator (26), over the years, mixed results of surfactant nebulization during nCPAP have been observed, in terms of clinical response and surfactant distribution in human (3, 11–14) and animal (4–10) studies. The reasons for the discrepancies between studies are likely related differences in the nebulizers used, as well as in the surfactant dosage and composition, among other factors.
The eFlow-Neos nebulizer (Pari Pharma) has been designed to enhance the delivery of surfactant to the neonatal respiratory system and nebulize surfactant without denaturing the protein. First, this vibrating membrane nebulizer is portable and simple to use, avoids surfactant aerosol dilution, and reduces the residual volume in the device (27). Second, it has been previously shown to nebulize surfactant at an appropriate particle size (2.5–3.5 µm) capable of penetrating deep into the distal airways (15–17). Third, high lung delivery efficacies of greater than 14% have been observed “in-vitro”, and in animal models (15, 16, 28), these findings suggesting that, in our study, at least 48 mg/kg of nebulized surfactant reached the distal airways. Finally, after nebulization, surfactant was found to maintain its biological activity (17, 18). All the aforementioned studies suggest that it is a promising innovative approach for effective delivery of nebulized surfactant in neonates.
This longer term study has provided for the first time evidence that in a clinically relevant time frame, all pulmonary outcomes are better in spontaneously breathing newborn piglets with BAL-induced RDS treated with nebulized poractant alfa 400 mg/kg than inuntreated controls, the improvements being in line with those observed after surfactant administration using the InSurE method. Furthermore, surfactant nebulization and administration using the InSurE method were associated with a 50% lower risk of respiratory failure (requiring intubation and mechanical ventilation) in the first 72 hours after surfactant treatment than nCPAP alone. Although nCPAP is effective in the treatment of RDS, despite early use of nCPAP, reintubation and mechanical ventilation have been found to be needed due to treatment failure in approximately 25%–50% of neonates with RDS (29). In line with this, in our study, 50% of the animals treated with nCPAP alone had to be reintubated, whereas none of the animals treated with surfactant needed reintubation.
Our results are in consistent with findings of the randomized controlled trial of Minocchieri et al (3); in that, these authors showed that early postnatal surfactant nebulization may reduce the need for intubation in the first 3 days of life compared with nCPAP alone in premature infants with mild RDS. Unlike in our study, Minocchieri et al (3) did not observe a positive effect on intubation rate in premature infants with moderate RDS. The discrepancies between the studies could be explained by two factors, namely, differences in surfactant dosage (20 vs 400 mg/kg) and nCPAP interface (face mask vs binasal prongs) during surfactant nebulization. Furthermore, for a vibrating membrane nebulizer, the nebulizer should be placed adjacent to the patient airways to avoid dead space and dilution effects (27). In our study, the administration of a high dose of nebulized surfactant (400 mg/kg) and reduction of dead space (binasal prongs and correct nebulizer placement) (15) may have increased the surfactant lung deposition, making it possible to achieve improvements in the moderate RDS developed in our surfactant-depleted newborn piglets. Nonetheless, nebulization was associated with a transient increase in Paco2 in a third of the piglets (attributable to nebulizer placement requiring disconnection/connection of ventilation), although values recovered over the first 15–30 minutes of nebulization (3, 15). No other significant changes in MABP, HR, or arterial oxygen content were observed during the nebulization or at any other point during the experiment.
Although our study was not designed to evaluate surfactant distribution in the lung, significantly lower total lung injury scores were observed after surfactant administration using a eFlow-Neos nebulizer (Pari Pharma), and this may be related larger surfactant pool sizes and a different pattern of surfactant distribution (15, 16). A previous study using this nebulizer (30) concluded that at 1 hour after surfactant nebulization, a similar amount of surfactant was distributed to different lung lobes. Our lower total lung histological scores in upper and middle regions may suggest that those regions of the lung received the highest proportion of the nebulized surfactant, but this needs to be confirmed in studies specifically designed to assess the pulmonary distribution of nebulized surfactant in patients with RDS. A benefit of nebulized surfactant is also observed when the level of lung inflammatory factors is assessed, with lung IL1-B and IL-8 levels being significantly lower than those observed with nCPAP alone. Natural surfactants have been described to have anti-inflammatory properties (31). In our study, lung IL-8 and IL-1B levels fell when surfactant was administered using the InSurE method, but the differences did not reach significance. Nonetheless, when poractant alfa was nebulized, we observed a significant reduction in those inflammatory markers. It could be hypothesized that this effect is related to a better surfactant distribution in the lung or that the nebulization process in some way improves the biological activity of surfactant, minimizing the initiation and progression of lung inflammation.
Another important consideration is the effect of the use of new respiratory technology at birth on other organ systems, especially the brain (32), seeking to avoid ventilation-induced lung injury and its effect on systemic brain inflammation and injury, due to hemodynamic instability and a localized cerebral inflammatory response (33). In our study, neither of the surfactant therapies used was observed to have any clinically significant effects on brain injury score or brain-specific protein levels, confirming the safety of this new way of administering surfactant.
Limitations of this study include the use of newborn piglets (2–4 d) rather than premature animals. The use of noninvasive support such as nCPAP using premature lambs remains complicated, and the pulmonary outcomes after surfactant treatment in combination with nCPAP are difficult to interpret due to the high variability in response to nCPAP and surfactant (30). Surfactant washout lavage models have frequently been used in adult and juvenile animals to implement successful animal models in the context of RDS (19, 33, 34). The newborn piglet model was chosen because the brain maturation, lung volume, and birth weights resemble those of newborn infants. Although, in our 72-hour study, neither of the surfactant therapies used was observed to have effects on brain injury score or brain-specific protein levels, longer periods of time (weeks) may be needed to detect brain disease, such as periventricular leukomalacia.
CONCLUSIONS
Nebulization delivery of poractant alfa at a dose of 400 mg/kg using eFlow-Neos nebulizer (Pari Pharma), specifically designed for surfactant administration in neonates, is a safe and effective approach for relieving surfactant-deficiency in newborn piglets with RDS supported with nCPAP, improving the pulmonary outcomes, and reducing the risk of nCPAP failure in the first 72 hours after treatment, outcomes being similar to those with administration of surfactant using the InSurE method. Furthermore, there is immunologic evidence of less lung injury with the administration of nebulized surfactant. These findings will be verified by a randomized controlled trial in spontaneously breathing newborn infants currently ongoing (NCT03235986).
Supplementary Material
Footnotes
Drs. Rey-Santano and Mielgo contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by Carlos III Health Institute through the project PI18/00166 (cofinanced by the European Regional Development Fund “A way to make Europe”) and Chiesi Farmaceutici.
Drs. Rey-Santano’s, Mielgo’s, Gomez-Solaexe’s, and Loureiro’s institutions received funding from Carlos III Health Institute through the project PI18/00166 (cofinanced by the European Regional Development Fund “A way to make Europe”) and Chiesi Farmaceutici. Drs. Rey-Santano, Mielgo, Gomez-Solaetxe, Bianco, Salomone, and Loureiro disclosed off-label product use of eFlow Neos nebulizer. Drs. Bianco and Salomone disclosed that they are employees of Chiesi Pharmaceutici (manufacturer of poractant alfa sold under the brand name Curosurf).
REFERENCES
- 1.Verder H, Robertson B, Greisen G, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med 1994; 331:1051–1055 [DOI] [PubMed] [Google Scholar]
- 2.Kribs A, Roll C, Göpel W, et al. ; NINSAPP Trial Investigators: Nonintubated surfactant application vs conventional therapy in extremely preterm infants: A Randomized Clinical Trial. JAMA Pediatr 2015; 169:723–730 [DOI] [PubMed] [Google Scholar]
- 3.Minocchieri S, Berry CA, Pillow JJ; CureNeb Study Team: Nebulised surfactant to reduce severity of respiratory distress: A blinded, parallel, randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2019; 104:F313–F319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis JF, Ikegami M, Jobe AH, et al. Aerosolized surfactant treatment of preterm lambs. J Appl Physiol (1985) 1991; 70:869–876 [DOI] [PubMed] [Google Scholar]
- 5.Henry MD, Rebello CM, Ikegami M, et al. Ultrasonic nebulized in comparison with instilled surfactant treatment of preterm lambs. Am J Respir Crit Care Med 1996; 154:366–375 [DOI] [PubMed] [Google Scholar]
- 6.Ellyett KM, Broadbent RS, Fawcett ER, et al. Surfactant aerosol treatment of respiratory distress syndrome in the spontaneously breathing premature rabbit. Pediatr Res 1996; 39:953–957 [DOI] [PubMed] [Google Scholar]
- 7.Dijk PH, Heikamp A, Bambang Oetomo S. Surfactant nebulisation prevents the adverse effects of surfactant therapy on blood pressure and cerebral blood flow in rabbits with severe respiratory failure. Intensive Care Med 1997; 23:1077–1081 [DOI] [PubMed] [Google Scholar]
- 8.Fok TF, al-Essa M, Dolovich M, et al. Nebulisation of surfactants in an animal model of neonatal respiratory distress. Arch Dis Child Fetal Neonatal Ed 1998; 78:F3–F9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampland AL, Wolfson MR, Mazela J, et al. Aerosolized KL4 surfactant improves short-term survival and gas exchange in spontaneously breathing newborn pigs with hydrochloric acid-induced acute lung injury. Pediatr Pulmonol 2014; 49:482–489 [DOI] [PubMed] [Google Scholar]
- 10.Walther FJ, Hernández-Juviel JM, Waring AJ. Aerosol delivery of synthetic lung surfactant. PeerJ 2014; 2:e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorch G, Hartl H, Roth B, et al. Surfactant aerosol treatment of respiratory distress syndrome in spontaneously breathing premature infants. Pediatr Pulmonol 1997; 24:222–224 [DOI] [PubMed] [Google Scholar]
- 12.Arroe M, Pedersen-Bjergaard L, Alberstsen P, et al. Inhalation of aerosolized surfactant (Exosurf) to neonates treated with nasal continuous positive airway pressure. Prenat Neonatal Med 1998; 3:346–352 [Google Scholar]
- 13.Berggren E, Liljedahl M, Winbladh B, et al. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Paediatr 2000; 89:460–464 [DOI] [PubMed] [Google Scholar]
- 14.Finer NN, Merritt TA, Bernstein G, et al. An open label, pilot study of Aerosurf® combined with nCPAP to prevent RDS in preterm neonates. J Aerosol Med Pulm Drug Deliv 2010; 23:303–309 [DOI] [PubMed] [Google Scholar]
- 15.Linner R, Perez-de-Sa V, Cunha-Goncalves D. Lung deposition of nebulized surfactant in newborn piglets. Neonatology 2015; 107:277–282 [DOI] [PubMed] [Google Scholar]
- 16.Nord A, Linner R, Salomone F, et al. Lung deposition of nebulized surfactant in newborn piglets: Nasal CPAP vs Nasal IPPV. Pediatr Pulmonol 2020; 55:514–520 [DOI] [PubMed] [Google Scholar]
- 17.Minocchieri S, Knoch S, Schoel WM, et al. Nebulizing poractant alfa versus conventional instillation: Ultrastructural appearance and preservation of surface activity. Pediatr Pulmonol 2014; 49:348–356 [DOI] [PubMed] [Google Scholar]
- 18.Bianco F, Ricci F, Catozzi C, et al. From bench to bedside: In vitro and in vivo evaluation of a neonate-focused nebulized surfactant delivery strategy. Respir Res 2019; 20:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rey-Santano C, Mielgo VE, Gomez-Solaetxe MA, et al. Non-invasive ventilation and surfactant treatment as the primary mode of respiratory support in surfactant-deficient newborn piglets. Pediatr Res 2018; 83:904–914 [DOI] [PubMed] [Google Scholar]
- 20.Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand 1980; 24:231–236 [DOI] [PubMed] [Google Scholar]
- 21.Holmes RS, Masters CJ. On the tissue and subcellular distribution of multiple forms of catalase in the rat. Biochim Biophys Acta 1969; 191:488–490 [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–254 [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann AM, Roberts KD, Lampland AL, et al. Improved gas exchange and survival after KL-4 surfactant in newborn pigs with severe acute lung injury. Pediatr Pulmonol 2010; 45:782–788 [DOI] [PubMed] [Google Scholar]
- 24.Rey-Santano C, Mielgo VE, López-de-Heredia-y-Goya J, et al. Cerebral effect of intratracheal aerosolized surfactant versus bolus therapy in preterm lambs. Crit Care Med 2016; 44:e218–e226 [DOI] [PubMed] [Google Scholar]
- 25.Dargaville PA, Aiyappan A, De Paoli AG, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed 2013; 98:F122–F126 [DOI] [PubMed] [Google Scholar]
- 26.Robillard E, Alarie Y, Dagenais-Perusse P, et al. Microaerosol administration of synthetic β-γ-dipalmitoyl-L-α-lecithin in the respiratory distress syndrome: A preliminary report. Canad Med Ass J 1964; 90:55–57 [PMC free article] [PubMed] [Google Scholar]
- 27.Pillow JJ, Minocchieri S. Innovation in surfactant therapy II: Surfactant administration by aerosolization. Neonatology 2012; 101:337–344 [DOI] [PubMed] [Google Scholar]
- 28.Minocchieri S, Burren JM, Bachmann MA, et al. Development of the premature infant nose throat-model (PrINT-Model): An upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr Res 2008; 64:141–146 [DOI] [PubMed] [Google Scholar]
- 29.Dunn MS, Kaempf J, de Klerk A, et al. ; Vermont Oxford Network DRM Study Group: Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011; 128:e1069–e1076 [DOI] [PubMed] [Google Scholar]
- 30.Hütten MC, Kuypers E, Ophelders DR, et al. Nebulization of Poractant alfa via a vibrating membrane nebulizer in spontaneously breathing preterm lambs with binasal continuous positive pressure ventilation. Pediatr Res 2015; 78:664–669 [DOI] [PubMed] [Google Scholar]
- 31.Ikegami M, Whitsett JA, Martis PC, et al. Reversibility of lung inflammation caused by SP-B deficiency. Am J Physiol Lung Cell Mol Physiol 2005; 289:L962–L970 [DOI] [PubMed] [Google Scholar]
- 32.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull World Health Organ 2010; 88:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lampland AL, Meyers PA, Worwa CT, et al. Gas exchange and lung inflammation using nasal intermittent positive-pressure ventilation versus synchronized intermittent mandatory ventilation in piglets with saline lavage-induced lung injury: an observational study. Crit Care Med 2008; 36:183–187 [DOI] [PubMed] [Google Scholar]
- 34.Ricci F, Catozzi C, Murgia X, et al. Physiological, biochemical, and biophysical characterization of the lung-lavaged spontaneously-breathing rabbit as a model for respiratory distress syndrome. PLoS One 2017; 12:e0169190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


