Supplemental Digital Content is available in the text.
Keywords: consensus, insurance, Japan, pacemaker, stroke
Abstract
Background:
Current expert consensus recommends remote monitoring for cardiac implantable electronic devices, with at least annual in-office follow-up. We studied safety and resource consumption of exclusive remote follow-up (RFU) in pacemaker patients for 2 years.
Methods:
In Japan, consecutive pacemaker patients committed to remote monitoring were randomized to either RFU or conventional in-office follow-up (conventional follow-up) at twice yearly intervals. RFU patients were only seen if indicated by remote monitoring. All returned to hospital after 2 years. The primary end point was a composite of death, stroke, or cardiovascular events requiring surgery, and the primary hypothesis was noninferiority with 5% margin.
Results:
Of 1274 randomized patients (50.4% female, age 77±10 years), 558 (RFU) and 550 (Conventional follow-up) patients reached either the primary end point or 24 months follow-up. The primary end point occurred in 10.9% and 11.8%, respectively (P=0.0012 for noninferiority). The median (interquartile range) number of in-office follow-ups was 0.50 (0.50–0.63) in RFU and 2.01 (1.93–2.05) in conventional follow-up per patient-year (P<0.001). Insurance claims for follow-ups and directly related diagnostic procedures were 18 800 Yen (16 500–20 700 Yen) in RFU and 21 400 Yen (16 700–25 900 Yen) in conventional follow-up (P<0.001). Only 1.4% of remote follow-ups triggered an unscheduled in-office follow-up, and only 1.5% of scheduled in-office follow-ups were considered actionable.
Conclusions:
Replacing periodic in-office follow-ups with remote follow-ups for 2 years in pacemaker patients committed to remote monitoring does not increase the occurrence of major cardiovascular events and reduces resource consumption.
Registration:
URL: https://clinicaltrials.gov; Unique identifier: NCT01523704.
What Is Known?
Patients with implanted pacemakers are typically seen for device checks in hospital twice per year.
Expert opinion suggest that this can be reduced to once per year, if the patients are followed by remote monitoring.
What the Study Adds?
Replacing periodic in-office follow-ups with remote follow-up and monitoring for 2 years in pacemaker patients does not increase the occurrence of major cardiovascular events.
This strategy reduces resource consumption.
Recent consensus recommendations assign a Class 1A recommendation for the use of remote monitoring (RM) for postimplant management of patients receiving cardiac implantable electronic devices.1,2 These recommendations are mostly based on results of implantable cardioverter defibrillators and cardiac resynchronization therapy devices given the scarcity of data for outcomes of remote management of pacemakers, although these represent the majority of cardiac implantable electronic devices.3–6 This discrepancy may be responsible for (and result of) the lower rate of RM implementation in pacemakers worldwide than in implantable cardioverter defibrillators and cardiac resynchronization therapy devices.
Even with active RM, in-office evaluations are required at least yearly because of the lack of data on safety of longer intervals.2 Thus, we conducted a prospective randomized trial with scheduled in-clinic evaluations reduced to once in 2 years, in pacemaker patients committed to RM in the Japanese healthcare setting. We assessed safety and further hypothesized that overall in-office evaluations and follow-up costs would be reduced by the remote management plan.
Methods
The prospective, multicenter At-Home Study (Comparison of the Safety and Efficacy of the Management of Pacemaker Patients Followed Via Home Monitoring Versus Conventional In-Office Follow-Ups) was a noninferiority, open-label, parallel group randomized controlled trial comparing 2 follow-up schemes: remote follow-up (RFU) or conventional in-office follow-up (CFU) in 6-month intervals, both combined with daily automatic Home Monitoring (Biotronik SE & Co. KG, Berlin, Germany), for 2 years.
The study was done in 85 Japanese academic and nonacademic hospitals. It followed ICH Good Clinical Practice guidelines and the Declaration of Helsinki, including approval of the study protocol by appropriate national and local ethics committees. Patients provided written informed consent. The study is registered at clinicaltrials.gov (NCT01523704). The data that support study findings are available from the sponsor via the corresponding author upon reasonable request.
Patient Selection
Consenting patients were enrolled if they were at least 20 years old, had a pacemaker indication according to Japanese guidelines, had received (within 45 days) or were about to receive a Biotronik pacemaker with RM capabilities, were willing and able to comply to study procedures including daily automatic RM surveillance, were geographically stable and likely to return for in-office evaluations over a follow-up period of 27 months.
Patients were excluded if they had a life expectancy shorter than 27 months, were likely to undergo heart transplant within 27 months, or were participating in another cardiology study.
Pacemakers and the RM System Studied
Single- or dual-chamber pacemaker from the Biotronik “Evia” family were used, with embedded Home Monitoring (HM) technology as described in the literature.7,8 In brief, a patient device named CardioMessenger, typically located in the patient’s bedroom, receives data from pacemakers wirelessly in 24-hour intervals without active participation of the patient. The CardioMessenger relays the data automatically via mobile network to the manufacturer’s central repository, the Home Monitoring Service Center.
Transmitted HM data include heart rate and rhythm statistics, records of mode switch episodes during atrial tachyarrhythmia, lead parameters including pacing thresholds, battery status, patient activity levels, and intracardiac electrograms. Healthcare providers at hospitals and clinical centers can review all transmitted data on a secure website at any time. Furthermore, they receive automated alert notifications by email if prespecified criteria are met.
Randomization and Follow-Up
Daily automatic HM was enabled after enrollment in all patients as recommended by the HRS experts.2 Three months later, patients were randomized 1:1 to RFU or CFU by a centralized, concealed randomization process stratified by site. Neither investigators nor patients were masked to treatment allocation. Device programming, HM alert settings, reactions to alerts and how to handle HM data if no alerts were received were at the discretion of the investigator.
In RFU, no in-clinic evaluation was scheduled for 2 years following randomization. Instead, remote follow-up sessions consisting of an analysis of the accumulated HM data were scheduled and conducted by the attending physician at 6, 12, and 18 months after randomization. These required no patient participation since data were automatically relayed by HM. The physicians sent letters to inform patients about remote follow-up findings and to schedule an in-office evaluation if HM data were indicative. In CFU, patients underwent standard in-office evaluations at 6, 12, and 18 months after randomization. In both study groups, the final in-office evaluation was performed at 24 months after randomization (27 months after enrollment). In either group, additional unscheduled in-office follow-ups could be initiated by patients or by physicians, based on symptoms, HM findings, or during hospital admissions. The physician or a technician/nurse at hospitals could review all transmitted data on the Home Monitoring Service Center website at any time. Furthermore, they received automated alert notifications for ventricular or supraventricular tachyarrhythmia episodes or intermittent or permanent capture or sensing failure. Each in-office follow-up was classified by investigators as actionable if clinically significant changes of the pacemaker settings or drug therapy were introduced.
Adverse events were assessed in both groups by screening hospital files.
Study End Points and Hypotheses
The primary end point was defined as a composite of death, stroke, or cardiovascular surgical procedure. An independent Clinical Event Committee consisting of 3 physicians, blinded to randomization assignment and investigational site, adjudicated primary end points by reviewing documented adverse events.
The primary hypothesis was that RFU is not inferior to CFU in freedom from primary end points, using a 5% noninferiority margin. Secondary hypotheses were that the numbers of in-office follow-up visits per year and costs to medical insurance would be reduced in RFU. Since a purpose of follow-up is battery management, we tested battery longevity in both arms. Further, we recorded all patient’s travel and waiting time and means of transport at the randomization and termination visits. Costs were calculated as the sum of insurance claims for in-office or/and remote follow-up (both types of follow-ups are reimbursed in Japan) and for diagnostic procedures performed associated with follow-ups, for example, 12-lead ECG, chest X-ray, or biochemical test.
Statistical Analysis
It was estimated that 477 patients per study group are needed to support the primary hypothesis with a power of 80% and α level of 0.05.9 To compensate for drop-out during to the long study period, 30% were added to the enrollment target, resulting in 682 per group. When it was clear that the drop-out was in fact lower, the sponsor concluded enrollment by October 31, 2013.
Ten percent of patients in both groups were assumed to experience a primary end point and the noninferiority margin was defined with 5%. The primary end point rate was calculated from all patients who either experienced an end point or remained in the study until the regular termination (per-protocol), and noninferiority was tested according to Farrington-Manning. All patients of the analysis cohort were analyzed as randomized. In addition to the per-protocol analysis, we estimated the end point rate and its CI at 24 months with the Kaplan-Meier method to correctly consider drop-out (intention-to-treat). Continuous variables are shown as mean±SD and/or median and interquartile range (IQR) and compared using the 2-sided t test or Mann-Whitney U test as appropriate. Categorical variables are given as numbers and percentages and compared with Fisher exact test. A 2-sided P<0.05 was considered significant. Multiple tests were corrected with the Bonferroni-Holm method. Rates of follow-ups and costs per patient-year were calculated patient-individually in all patients with data, and their distributions were statistically compared with the Mann-Whitney U test. Reduction of in-office follow-up burden and of costs was estimated by comparing the quotients of the population total divided by the cumulative study duration. The battery status at study termination was taken from the last HM data transmission in the study period, if this message was at least 24 months after enrollment. Syncopes, fractures, and falls were identified from the adverse event reporting. All deaths were adjudicated by the Clinical Event Committee based on a copy of the death certificate submitted by the participating physicians. Statistical analyses were conducted with the SAS software package 9.4 (SAS Institute, Inc, NC) and with R 3.3 statistical software (R Development Core Team, Vienna, Austria).
Results
Patients
From January 2012 to October 2013, 1327 patients were enrolled, slightly less than planned because of an organizational issue. At the 3-month follow-up, 1274 patients were randomized, 636 to RFU and 638 to CFU (Figure 1). Follow-up ended in February 2016. Eighty-five Japanese sites took part in the study (see Data Supplement).
Figure 1.
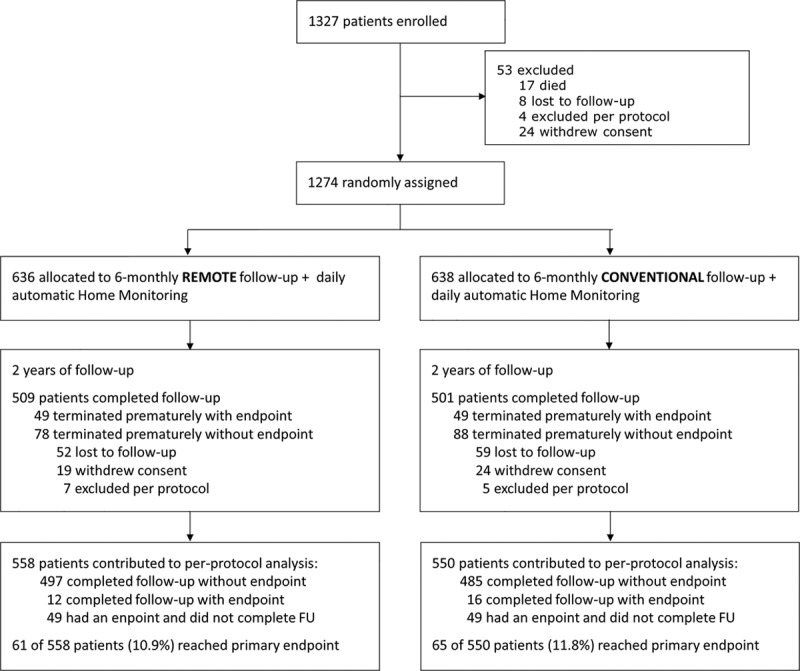
Trial flowchart. FU indicates follow-up.
Patient baseline characteristics were well balanced between study groups (Table 1). The mean age was 77±10 years. Both sexes were evenly distributed. Rhythm disturbances were sick sinus syndrome (45.2%), atrioventricular block (51.5%) including pacemaker-dependent patients, and atrial fibrillation (33.9%). Major comorbidities were hypertension (61.1%), heart failure (25.0%), and diabetes mellitus (20.5%).
Table 1.
Patient Characteristics at Enrollment

Follow-Up Period
After randomization, median (IQR) follow-up was 728 days (700–735) in RFU and 728 days (690–736) in CFU (P=0.40). Cumulative follow-up was 1324 (RFU) versus 1296 patient-years (CFU). In all patients, follow-up was done according to randomization. Regular study termination at 24 months post-randomization was achieved in 1009 patients (79.3% of the randomized cohort), 509 in RFU (80.0%), and 500 in CFU (78.4%). In patients who did not experience a primary end point, the reasons for premature termination in RFU and CFU were loss to follow-up (8.2% versus 9.2%), withdrawal of consent (3.0% versus 3.8%), and exclusion per protocol (1.1% versus 0.8%; all P >0.2; Figure 1).
Primary End Point and Its Components
For the per-protocol analysis, 1108 of 1274 patients contributed who had either completed the 24-month follow-up or reached the primary end point, or both. The primary end point occurred in 61 of 558 contributing RFU patients (10.9% [95% CI, 8.8%–13.1%]) and in 65 of 550 contributing CFU patients (11.8% [95% CI, 9.5%–14.1%]). The primary hypothesis of noninferiority with 5% margin was met (P=0.0012). The intention-to-treat incidences of the primary end point at 24 months estimated with the Kaplan-Meier method are very close to the per-protocol result (Figure 2).
Figure 2.

Kaplan-Meier curves of incidence of primary end points, starting at randomization. CFU indicates conventional follow-up; and RFU, remote follow-up.
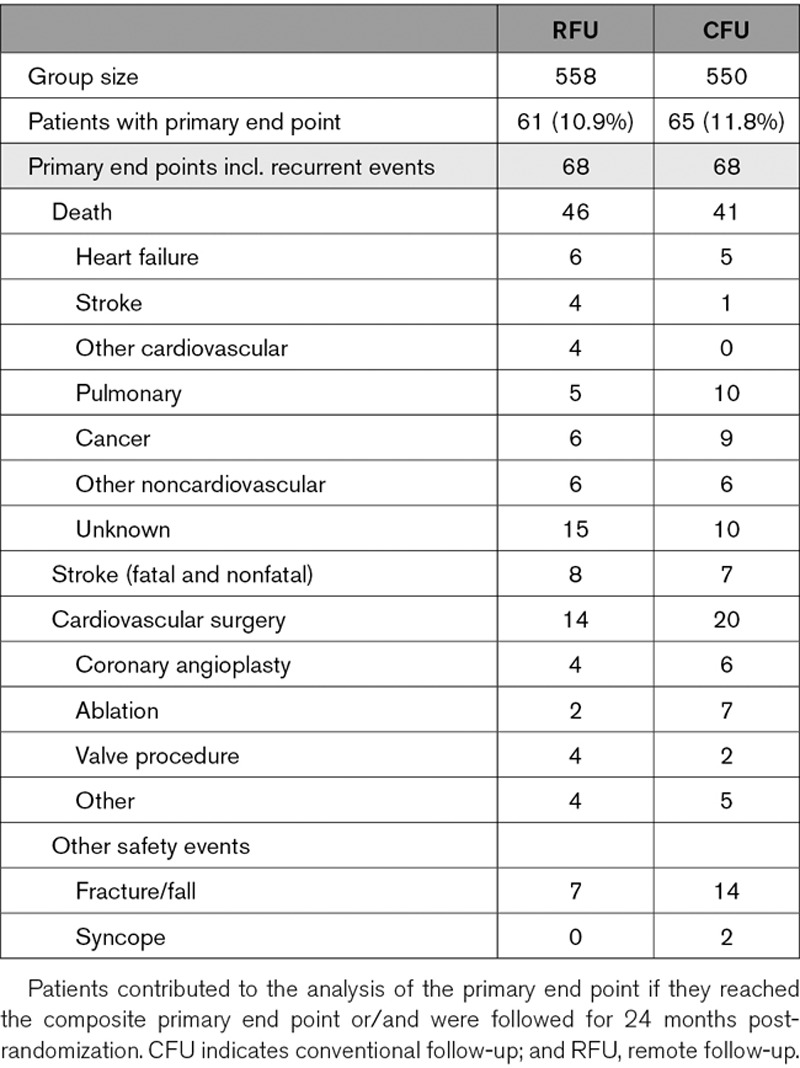
The occurrence of primary end point components is shown in Table 2. Among 136 events in 126 patients, the majority were deaths (64.0%) followed by cardiovascular surgery (25.0%) and stroke (11.0%). The majority of deaths were noncardiac, and none were device related. There were no significant differences between study groups. The incidence of stroke was as low as 0.006 per patient-year.
Table 2.
Primary End Point
In-Office and Remote Follow-Ups
The median (IQR) number of patient-individual in-office follow-ups per year in RFU and CFU were 0.50 (0.50–0.63) and 2.01 (1.93–2.05; P<0.001). Between randomization and 24-month follow-up, there were 201 in-office patient evaluations in RFU (all unscheduled) versus 1775 in CFU (sum of scheduled and unscheduled). Including the 24-month visit, 710 scheduled and unscheduled in-office follow-ups were performed in RFU (0.54 per patient-year) versus 2275 in CFU (1.76 per patient-year). This translates into a 69.5% reduction of in-office follow-ups in the population. The proportion of actionable in-office follow-ups was ≈1.5% for scheduled visits and ≈10% for additional unscheduled visits, irrespective of the study group (Table 3).
Table 3.
Number of In-Office and Remote Follow-Ups

When inclusive of remote follow-ups, RFU had 1.85 follow-ups per patient-year after randomization, versus 1.76 in CFU. Only 25 of a total of 1738 remote follow-ups (1.4%) indicated a need for in-office follow-up, the majority to verify lead function (n=14), for medical (n=6) or other reasons (n=5). Patients received letters about the findings of 1507 remote follow-ups (86.7%). In 14 of 1274 randomized patients (1.1%), a lead dislocation or infection was reported.
Of 304 additional follow-ups in both groups, 205 (67%; RFU 129, CFU 76) were conducted because the patient presented with symptoms or was in hospital for other reasons. Only 67 (22%; RFU 49, CFU 18) took place for events reported by HM. In 35 cases, this was for pacing threshold issues (RFU 31, CFU 4), most often related to the failure of the automatic threshold measurement. Additional follow-ups after medical events reported by HM (arrhythmia in all cases) were rare and evenly distributed between the groups (N=21, 7%; RFU 12, CFU 9). The 304 additional follow-ups occurred in 217 patients, which did not differ in indication for pacing, history of coronary artery disease, atrial fibrillation, heart failure, hypertension, diabetes mellitus, or renal failure (those with actionable follow-up were a minority (n=38) precluding meaningful statistical comparison).
Costs Connected to Follow-Up
The median (IQR) patient-individual follow-up costs per year were 18 800 Yen (16 500–20 700 Yen) in RFU and 21 400 Yen (16 700–25 900 Yen) in CFU (P<0.001; 100 Yen ≈1 US Dollar during the study). Total costs connected to pacemaker follow-up were reduced by 11.0% (Table 4). Follow-up reimbursement per year in RFU was slightly higher because of the slightly higher rate of total (remote and in-office) follow-ups, but the costs associated with additional diagnostic procedures were lower (Table 4).
Table 4.
Costs of Pacemaker Follow-Up

Further Results
Incidence of syncope, fractures, and falls were not increased in the RFU group (Table 2). The mean travelling time to a follow-up facility were 33±24 and 34±26 minutes in RFU and CFU, and waiting times were 59±45 and 59±50 (P=ns). Common ways of transportation were cars (63.8% of visits) and public transport (23.3%). Of all patients attending the randomization visit, 16.2% were still employed.
Of 1274 randomized patients, 1271 were registered at the HM system. Defined as the number of days with message divided by the study duration in the cumulated randomized period of these 1271 patients, the HM performance was 90.1%. Sixteen registered patients (1.3%) did not transmit any HM data. Median (IQR) of the patient-individual transmission success was 96.6% (89.6–99.0) in RFU and 95.5% (86.1%–98.6%) in CFU (P=0.03). The remaining battery capacity at study termination was 85.4±3.2% and 85.7±2.9% in RFU and CFU (P=0.21).
Discussion
In this large, randomized trial of pacemaker recipients, we found that 2 years of follow-up based completely on daily automatic RM was safe, and significantly reduced in-office visits and follow-up costs, compared with a regular 6-monthly in-office follow-up integrated with alert-based RM. The results suggest that scheduled in-office evaluations of pacemaker patients may be avoided for extended intervals when connectivity to automatic RM is maintained. The patients of the remote follow-up group complied with the strategy, as we observed no cross-over and similar numbers of withdrawal of consent in both groups. Patient satisfaction with the proprietary technology used in this study has been studied before and found to be excellent.10
Current recommendations for postimplant monitoring of cardiac implantable electronic device recipients advocate utilization of alert-based RM, integrated with at least yearly in person evaluation.1,2 This is built on the strength of data from recent randomized trials, which, however, have largely tested implantable cardioverter defibrillator and cardiac resynchronization therapy platforms. Further, they compared remote management to control without RM. In our trial, alert-based RM was used in both groups (in alignment with recommendations), and we assessed the value of additional periodic in-clinic follow-up compared with RFU for pacemaker management. Patient outcomes did not differ between those with and without scheduled 6 monthly in-office checks.
Historically, the purpose of in-office evaluation has been to ensure device safety and detect lead and generator problems. We show that the occurrence of these was very infrequent (in 1.1% of patients). In any case, when they do occur these are more reliably notified by RM.11 Further, we showed that regular in-office checks did not lead to a meaningful effect on the device battery. Notably, in our trial, no in-clinic evaluation was scheduled in RFU for 2 years, which is a significant extension to prior trials testing RM and to recent recommendations.1,2 Thus, our study provides compelling evidence to reduce the frequency of scheduled in-office evaluations to at least biennially when employing effective remote management.
The current study is also unique for being the largest randomized trial, and with the longest follow-up, of remote management of pacemaker patients. The PREFER trial (Pacemaker Remote Follow-Up Evaluation and Review) evaluated a patient activated system.6 Early detection of significant events was >5 months, rendering this system ineffective for early detection and intervention. This technology has since been superseded by automatic continuous RM.7,12 The COMPAS study (Comparative Follow-Up Schedule With Home Monitoring) was the first to test such a system and indicated safety of remote management but in a smaller study population with a follow-up period of only 18 months and, importantly, with pacemaker-dependent patients excluded.4 Moreover, HM was deactivated in the control arm, and scheduled in clinic follow-up was left to implanters’ discretion. Thus, we are able to isolate the value of in-office evaluations during RM. In our test group, the reduction of pacing clinic visits was more effective (69.5% from 1.76 to 0.54 per patient-year) than in COMPAS (36.2% from 1.63 to 1.04 per patient-year).
Apart from facilitating efficient follow-up, RM promises improved patient care.13 Our study was not designed to assess the effect of RM on clinical outcome because both groups had RM. Mortality was similar in both study groups, and no death was attributed to a device issue. Syncope, fractures, and falls were not increased in the RFU group. Given the occurrence of few significant events in this general pacemaker population, demonstration of a clinical benefit would require powering a much larger trial. In COMPAS, less hospitalizations for atrial arrhythmias (4 versus 10) and less strokes (2 versus 8) were observed with remote management, though the trial may have been underpowered to assess this.4
Costs of remote monitoring are relevant, and the lack of reimbursement has been identified as one barrier to implementaion.14 The cost benefit of remote management of pacemakers has been a source of debate. This is because pacemaker systems have less system-related problems to troubleshoot compared with implantable cardioverter defibrillator/cardiac resynchronization therapy platforms, and pacemaker patients generally have few comorbidities and are considered to benefit less from alerts. In this regard, our cost analysis is illuminating. Although in-office visits were not scheduled for 2 years in RFU, remote follow-up was continued at 6 monthly intervals, following guidelines, and unscheduled visits (which had higher actionability) continued. Despite the fact that the overall number of remote and in-office follow-ups was larger in RFU (1.85 versus 1.76 per patient-year), remote management was associated with 11.0% reduced costs in RFU because of the reduced need for additional diagnostic procedures, such as routine ECG and laboratory tests, which followed in person evaluation. In health systems that do not reimburse for remote care, the majority, the cost benefit of remote management will be greater than those we have demonstrated here.15 Notably, our analysis underestimated total cost savings since it concentrated on payer costs and did not account for nursing and physician time, which is considerably reduced with remote management16,17 and patient costs (entailing time away from work, travel time, etc). Hence, our analysis represents a conservative estimate of cost reduction associated with RM.
Implications
The study confirms the low actionability (1.5%) of calendar-based pacemaker follow-ups, and that these may be replaced safely for 2 years by complete remote management and evaluation on basis of unscheduled visits. These occurred infrequently (0.15 visits per patient-year) between randomization and the 24-month follow-up but more often required significant adjustment of pacing or medical therapy or important in-person evaluation (9.0%). Whether the interval between scheduled visits may be safely extended further requires further investigation.
Strengths and Limitations
We used one RM platform exclusively. This maintained continuous RM with 90.1% daily transmission success over 2 years, matching rates observed in other (shorter) trials using the same system18 and associated with more efficacious notification ability.19 Whether our results are transferable to other RM platforms is uncertain. The importance of maintaining connectivity was observed in a cohort analysis, in which mortality varied significantly between those maintaining lesser compared with greater connectivity (3.0% versus 5.4%; P<0.001).20 In our study, all patients were seen at 3 months after implantation. Whether this is necessary remains to be investigated. Further, our study was conducted in a single country (Japan), but the result are fully compatible with COMPAS which was conducted in France.4
The number of patients lost to follow-up (112/1274; 8.9%) during 2 years was less than the 30% catered for during study design but is not insignificant. We confirmed that at least 67 patients (36/52 in RFU and 31/60 in CFU) were alive in the week before the scheduled study termination because their remote transmissions remained active. In view of the low mortality rate observed in this pacemaker trial, it is improbable that a significant number of end points (which were largely deaths) were overlooked. Moreover, the fact that the event rates and their CIs estimated by the Kaplan-Meier method match the per-protocol analysis indicates that no bias is introduced by the drop-out.
We did not record symptomatic bradycardia in a systematic fashion. Further, we did not assess patient satisfaction since both groups had RM enabled, and prior studies testing this fully automatic RM technology have indicated excellent patient satisfaction.10 Thus, in our study, patients of the remote follow-up group complied with the strategy. The 5% noninferiority margin may be considered too wide to be truly meaningful. However, our study size exceeds all earlier studies of remote monitoring in pacemaker patients (and most studies with other cardiac implantable electronic devices) and a lower noninferiority margin would have resulted in a prohibitive sample size.
Conclusions
Automatic RM may supplant the majority of routine in-office evaluations. It does not increase the occurrence of major cardiovascular events and provides efficient and cost-effective management of pacemaker recipients that is well accepted by patients. The demonstration of these benefits with biennially scheduled in-clinic evaluation supports an adjustment to current follow-up recommendations.1,2
Acknowledgments
We are thankful to Motoaki Kuroishi and Yosuke Honda for study management, Ulrich Gauger, PhD, for statistical analysis and Dejan Danilovic, PhD, for critical reading and editing of the manuscript.
Sources of Funding
This work was supported by Biotronik Japan, Inc (Tokyo, Japan). Dr Watanabe designed the protocol with the sponsor. The sponsor managed the trial and performed the analysis. We take full responsibility for the result and the decision to submit the article.
Disclosures
Dr Watanabe has received research grants from Abbott, Biotronik, Boston Sc., Daiichi-Sankyo, Medtronic and Nihon Koden and speaker fees from Boehringer Ingelheim, Daiichi-Sankyo, Eisai, and Pfizer. Drs Yamazaki, T. Yamamoto, Sato, Kasai, Yamakawa, Y. Ueda, K. Yamamoto, Tokunaga, Tanaka, and Asai have received research grants from Biotronik. Drs Goto, Hirooka, M. Ueda, Sugai, and Hiramatsu have received research grants and traveling support from Biotronik. Dr Arakawa has received research grants, travelling support and speakers feed from Biotronik. Dr Schrader is an employee of Biotronik. Dr Varma has received consultancy fees from Abbott, Biotronik, and Medtronic and lecture fees from Abbott and Biotronik. Dr Ando has received research grants from Biotronik, consultancy fees from Boston Sc., Japan Lifeline and Terumo, and speaker fees from Biotronik and Medtronic.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CFU
- conventional follow-up
- COMPAS
- comparative follow-up schedule with home monitoring
- CRT
- cardiac resynchronization therapy
- HM
- home monitoring
- IQR
- interquartile range
- PREFER
- Pacemaker Remote Follow-Up Evaluation and Review
- RFU
- remote follow-up
- RM
- remote monitoring
For Sources of Funding and Disclosures, see page 425.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.119.007734.
References
- 1.Dubner S, Auricchio A, Steinberg JS, Vardas P, Stone P, Brugada J, Piotrowicz R, Hayes DL, Kirchhof P, Breithardt G, et al. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs). Europace. 2012;14:278–293. doi: 10.1093/europace/eur303. doi: 10.1093/europace/eur303. [DOI] [PubMed] [Google Scholar]
- 2.Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, Galizio NO, Glotzer TV, Leahy RA, Love CJ, et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12:e69–100. doi: 10.1016/j.hrthm.2015.05.008. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Raatikainen MJP, Arnar DO, Merkely B, Nielsen JC, Hindricks G, Heidbuchel H, Camm J. A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace. 2017;19(suppl_2):ii1–ii90. doi: 10.1093/europace/eux258. doi: 10.1093/europace/eux258. [DOI] [PubMed] [Google Scholar]
- 4.Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A, Binet D, Daubert JC COMPAS Trial Investigators. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur Heart J. 2012;33:1105–1111. doi: 10.1093/eurheartj/ehr419. doi: 10.1093/eurheartj/ehr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halimi F, Clémenty J, Attuel P, Dessenne X, Amara W OEDIPE trial Investigators. Optimized post-operative surveillance of permanent pacemakers by home monitoring: the OEDIPE trial. Europace. 2008;10:1392–1399. doi: 10.1093/europace/eun250. doi: 10.1093/europace/eun250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crossley GH, Chen J, Choucair W, Cohen TJ, Gohn DC, Johnson WB, Kennedy EE, Mongeon LR, Serwer GA, Qiao H, et al. PREFER Study Investigators. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol. 2009;54:2012–2019. doi: 10.1016/j.jacc.2009.10.001. doi: 10.1016/j.jacc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Varma N, Ricci RP. Telemedicine and cardiac implants: what is the benefit? Eur Heart J. 2013;34:1885–1895. doi: 10.1093/eurheartj/ehs388. doi: 10.1093/eurheartj/ehs388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burri H, Senouf D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace. 2009;11:701–709. doi: 10.1093/europace/eup110. doi: 10.1093/europace/eup110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackwelder WC. “Proving the null hypothesis” in clinical trials. Control Clin Trials. 1982;3:345–353. doi: 10.1016/0197-2456(82)90024-1. doi: 10.1016/0197-2456(82)90024-1. [DOI] [PubMed] [Google Scholar]
- 10.Ricci RP, Morichelli L, Quarta L, Sassi A, Porfili A, Laudadio MT, Gargaro A, Santini M. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace. 2010;12:674–679. doi: 10.1093/europace/euq046. doi: 10.1093/europace/euq046. [DOI] [PubMed] [Google Scholar]
- 11.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 12.Burri H. Remote follow-up and continuous remote monitoring, distinguished. Europace. 2013;15(Suppl 1):i14–i16. doi: 10.1093/europace/eut071. doi: 10.1093/europace/eut071. [DOI] [PubMed] [Google Scholar]
- 13.Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, et al. IN-TIME study group. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–590. doi: 10.1016/S0140-6736(14)61176-4. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Madrid A, Lewalter T, Proclemer A, Pison L, Lip GY, Blomstrom-Lundqvist C Scientific Initiatives Committee, European Heart Rhythm Association. Remote monitoring of cardiac implantable electronic devices in Europe: results of the European Heart Rhythm Association survey. Europace. 2014;16:129–132. doi: 10.1093/europace/eut414. doi: 10.1093/europace/eut414. [DOI] [PubMed] [Google Scholar]
- 15.Heidbuchel H, Hindricks G, Broadhurst P, Van Erven L, Fernandez-Lozano I, Rivero-Ayerza M, Malinowski K, Marek A, Romero Garrido RF, Löscher S, et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): a provider perspective in five European countries on costs and net financial impact of follow-up with or without remote monitoring. Eur Heart J. 2015;36:158–169. doi: 10.1093/eurheartj/ehu339. doi: 10.1093/eurheartj/ehu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronin EM, Ching EA, Varma N, Martin DO, Wilkoff BL, Lindsay BD. Remote monitoring of cardiovascular devices: a time and activity analysis. Heart Rhythm. 2012;9:1947–1951. doi: 10.1016/j.hrthm.2012.08.002. doi: 10.1016/j.hrthm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Ricci RP, Vicentini A, D’Onofrio A, Sagone A, Rovaris G, Padeletti L, Morichelli L, Fusco A, De Vivo S, Lombardi L, et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: Results of the Health Economics Evaluation Registry for Remote Follow-up (TARIFF) study. Heart Rhythm. 2017;14:50–57. doi: 10.1016/j.hrthm.2016.09.008. doi: 10.1016/j.hrthm.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Varma N, Love CJ, Schweikert R, Moll P, Michalski J, Epstein AE TRUST Investigators. Automatic remote monitoring utilizing daily transmissions: transmission reliability and implantable cardioverter defibrillator battery longevity in the TRUST trial. Europace. 2018;20:622–628. doi: 10.1093/europace/eux059. doi: 10.1093/europace/eux059. [DOI] [PubMed] [Google Scholar]
- 19.de Ruvo E, Sciarra L, Martino AM, Rebecchi M, Iulianella RV, Sebastiani F, Fagagnini A, Borrelli A, Scarà A, Grieco D, et al. A prospective comparison of remote monitoring systems in implantable cardiac defibrillators: potential effects of frequency of transmissions. J Interv Card Electrophysiol. 2016;45:81–90. doi: 10.1007/s10840-015-0067-4. doi: 10.1007/s10840-015-0067-4. [DOI] [PubMed] [Google Scholar]
- 20.Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol. 2015;65:2601–2610. doi: 10.1016/j.jacc.2015.04.033. doi: 10.1016/j.jacc.2015.04.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


