Abstract
The primary somatosensory cortex (SI) receives input from the contralateral forelimb and projects to homotopic sites in the opposite SI. Since homotopic sites in SI are linked by a callosal pathway, we proposed that repetitive intracortical microstimulation (ICMSr) of neurons in layer V of SI forelimb cortex would increase spike firing in the opposite SI cortex thereby strengthening the callosal pathway sufficiently to allow normally ineffective stimuli from the ipsilateral forelimb to excite cells in the ipsilateral SI. The forelimb representation in SI in one hemisphere was mapped using mechanical and electrical stimulation of the contralateral forelimb, a homotopic site was similarly identified in the opposite SI, the presence of ipsilateral peripheral input was tested in both homotopic sites, and ICMS was used to establish an interhemispheric connection between the two homotopic recording sites. The major findings are: (1) each homotopic forelimb site in SI initially received short latency input only from the contralateral forelimb; (2) homotopic sites in layer V in each SI were interconnected by a callosal pathway; (3) ICMSr delivered to layer V of the homotopic SI in one hemisphere generally increased evoked response spike firing in layer V in the opposite homotopic site; (4) increased spike firing was often followed by the expression of a longer latency normally ineffective input from the ipsilateral forelimb; (5) these longer latency ipsilateral responses are consistent with a delay time sufficient to account for travel across the callosal pathway; (6) increased spike firing and the resulting ipsilateral peripheral input were also corroborated using in-vivo intracellular recording; and (7) inactivation of the stimulating site in SI by lidocaine injection or local surface cooling abolished the ipsilateral response, suggesting that the ipsilateral response was very likely relayed across the callosal pathway. These results suggest that repetitive microstimulation can do more than expand receptive fields in the territory adjacent to the stimulating electrode but in addition can also alter receptive fields in homotopic sites in the opposite SI to bring about the expression of previously ineffective input from the ipsilateral forelimb.
Keywords: Interhemispheric Connectivity, Rodent Somatosensory Cortex, Forelimb, Forepaw Barrel Subfield (FBS), Callosal Connections, Cortical Reorganization, Layer V, Intracortical Microstimulation, ICMS
1. Introduction
The primary somatosensory cortex (SI) in cat was first described as receiving peripheral input exclusively from the contralateral body surface (Mountcastle et al., 1957), but subsequent studies in monkey (Conti et al., 1986; Manzoni et al., 1989; Ogawa et al., 1989), cat (Manzoni et al., 1980), and rodent (Pidoux and Verley, 1979; Shuler et al., 2001) demonstrated that SI also receives bilateral peripheral input from midline structures that include head (Dreyer et al., 1975), face (Schwarz and Fredrickson, 1971), and axial trunk (Manzoni et al., 1980; Innocenti, 1986; Conti et al., 1986), which are served by dense callosal connections. In contrast, it was reported that SI receives contralateral input only from the limbs that were largely devoid of callosal connections (Decosta-Fortune et al., 2015; Manzoni et al., 1980; Wise and Jones, 1976; Yorke and Caviness, 1975). However, later findings in monkey (Iwamura et al., 1994; Iwamura et al., 1996; Lipton et al., 2006; Taoka et al., 2000; Tommerdahl et al., 2006), cat (Innocenti et al., 1973), and flying fox (Calford and Tweedale, 1990) showed that SI also receives bilateral input from the limbs. In rat SI, bilateral input from the hindlimb (Angel and Lemon, 1975; Armstrong-James and George, 1988; Chapin and Lin, 1984; Pluto et al., 2005) and vibrissae (Pidoux and Verley, 1979; Shuler et al., 2001) has been reported. In these cases, SI neurons have receptive-field centers located, in large part, on homologous contralateral-and-ipsilateral skin surfaces; however, ipsilateral inputs (defined as input from the same side of the body surface as the recording electrode) had a delayed evoked response latency and smaller receptive fields compared to contralateral inputs (defined as input from the opposite side of the body surface as the recording electrode) (Armstrong-James and George, 1988; Pidoux and Verley, 1979; Tutunculer et al., 2006). In contrast, despite reports of callosal connections between homotopic forelimb representations in SI (Decosta-Fortune et al., 2015; Welker, 1971), evidence has been lacking for the existence of bilateral input to SI neurons in rodent forelimb cortex (Angel and Lemon, 1975; Shin et al., 1997), although Tutunculer and colleagues reported evidence to the contrary (Tutunculer et al., 2006).
Ipsilateral input from homologous sites in the periphery has been shown to modify evoked responses in contralateral SI in monkey (Calford and Tweedale, 1990; Korvenoja et al., 1995; Lipton et al., 2006; Tommerdahl et al., 2006), cat (Favorov et al., 2006; Innocenti et al., 1973), and rat (Armstrong-James and George, 1988; Pidoux and Verley, 1979; Shin et al., 1997; Shuler et al., 2001). For example, conditioning stimuli applied to the ipsilateral hindpaw in rat reduced or modified the response of hindlimb neurons in SI to a test stimulus applied to the contralateral hindlimb (Armstrong-James and George, 1988), and simultaneous bilateral stimulation of forelimb digits in monkey was accompanied by a reduction in response in SI to contralateral stimulation alone (Tommerdahl et al., 2006). Inactivation of forelimb receptive fields in flying fox and monkey is followed by an immediate bilateral expansion of receptive fields in SI (Calford and Tweedale, 1990) or conversely, inactivation of an ipsilateral homologous digit in rat suppressed contralateral homologous input in SI (Shin et al., 1997). Viewed collectively, these findings provide compelling evidence that ipsilateral input plays a modulatory role on contralateral SI.
Ipsilateral input to SI is very likely mediated through transcallosal connections between homotopic sites in SI cortices (Innocenti et al., 1973; Iwamura et al., 1994; Pidoux and Verley, 1979; Shin et al., 1997) whereby the corpus callosum provides a conduit for integrating both contralateral and ipsilateral input (Koralek et al., 1990; Olavarria et al., 1984; Shuler et al., 2001; White and DeAmicis, 1977). Evidence from several studies suggests that ipsilateral input in SI is reduced or abolished following inactivation of the opposite homotopic SI using polarizing currents (Innocenti et al., 1973), ablation (Pidoux and Verley, 1979; Iwamura et al., 1994), pharmacological application (Shin et al., 1997; Shuler et al., 2001), or cooling (Shin et al., 1997); although this finding is not without exception (Armstrong-James and George, 1988). Further support for transcallosal mediation of ipsilateral peripheral input is provided by the general finding that evoked response latencies in ipsilateral SI in rat are approximately 8–11 ms longer than are evoked response latencies recorded in contralateral SI following stimulation of the contralateral periphery (Armstrong-James and George, 1988; Shuler et al., 2001; Wiest et al., 2005). This delay may be accounted for by an approximate 7–10 ms evoked response latency for a signal to travel across the interhemispheric pathway to a homotopic site in opposite SI (Ramshur et al., 2019).
The present study arose from our previous experiences in mapping the forelimb representation in SI in Sprague-Dawley rats, where we had not encountered bilateral forelimb receptive fields using both mechanical and electrical peripheral stimulation. This said, the presence of an interhemispheric callosal pathway between homotopic forelimb cortices in rat SI raised the question of why SI forelimb cortical neurons responded only to input from the contralateral forelimb and not to the ipsilateral forelimb despite the presence of a callosal connection that could, in principle, relay ipsilateral input to the ipsilateral SI. The goals of the present study were to: a) determine whether ICMSr of one homotopic SI forelimb site could strengthen the callosal connection thereby leading to an increase in evoked cell response in the opposite SI, and b) determine whether the increase in evoked spiking would permit normally ineffective stimuli from the ipsilateral forelimb to excite cells in the ipsilateral SI. Our results suggest that increased spiking following ICMSr leads to expression of ipsilateral peripheral input in the ipsilateral SI, and the expression depends on having an intact callosal pathway between homotopic sites.
In preparing this manuscript, we discovered a paper that reported bilateral forelimb receptive fields in SI in rat (Tutunculer et al., 2006), and in our Discussion, we assess their findings in relationship to the present study.
2. Results
Experiments were carried out in a total of 27 adult Sprague-Dawley rats. In each experiment, sites in the wrist, forearm, or forepaw representation were identified by physiologically mapping receptive fields in layer V of SI in each hemisphere to identify homotopic sites that received input from similar areas of the periphery. An interhemispheric pathway was then identified using intracortical microstimulation (ICMS), and once established, repetitive ICMS (ICMSr) was delivered to one of the sites (stimulation site) in an attempt to increase spike firing in the opposite homotopic site (recording site). A strengthening of spike firing was defined as an increase (1.5 ×) in the number of evoked spikes following a series of repetitive stimulations compared to the number of spikes observed during an initial series of baseline stimulations, immediately after establishing the interhemispheric pathway. Prior to ICMSr and at 30-min intervals after the beginning of stimulation, neurons at the recording site were examined for the presence of input from the ipsilateral forelimb, a condition that was not observed before initiation of ICMSr even using stimulating currents 3 × greater than the current required to evoke a response in the contralateral SI. ICMSr (1.5 × threshold, mean current 43 μA, range = 28–71 μA) was delivered to the stimulating site in layer V for periods ranging from 1.0–5.0 hr and evoked responses to consecutive stimulations (range = 20–50) were collected from similar recording depths in layer V in the opposite hemisphere. In 10 rats, in-vivo intracellular recording was used to examine cell firing and ipsilateral forelimb input and/or interhemispheric latency; in 3 rats, the stimulation site was inactivated to determine if the previously ineffective ipsilateral input derived from the opposite homotopic SI. Two additional rats served as unstimulated controls.
2.1. Interhemispheric pathway strengthening
ICMSr increased the number of evoked spikes (mean evoked response increase = 178%; range = 59%–585%) in homotopic sites in 12 rats, and in 11 of these rats, pathway strengthening was followed by the expression of input from the ipsilateral forelimb. In 2 rats, ICMSr did not strengthen the connection (23%), and in one of these, ICMS reduced the spike firing (37%); in neither case were ipsilateral responses found. An example of response strengthening in one rat is shown in Fig. 1. In this rat, ICMS was delivered over a 3-hr period to a physiologically identified site in layer V in the wrist representation and evoked responses were recorded in layer V from a homotopic wrist site in the contralateral SI. Responses to 25 consecutive stimulations were collected at the beginning of stimulation (baseline) and compared to a similar number of stimulations measured 3 hr after ICMSr; these results are shown in Fig. 1A and Fig. 1B, respectively (inset shows single trace). Raster plots (Figs. 1C, 1D) and corresponding peri-stimulus time histograms (PSTH) (Figs. 1E, 1F) reflect an increase (2.34 ×) in number of evoked spikes recorded immediately after 3 hr of ICMSr compared to baseline.
Fig. 1 –
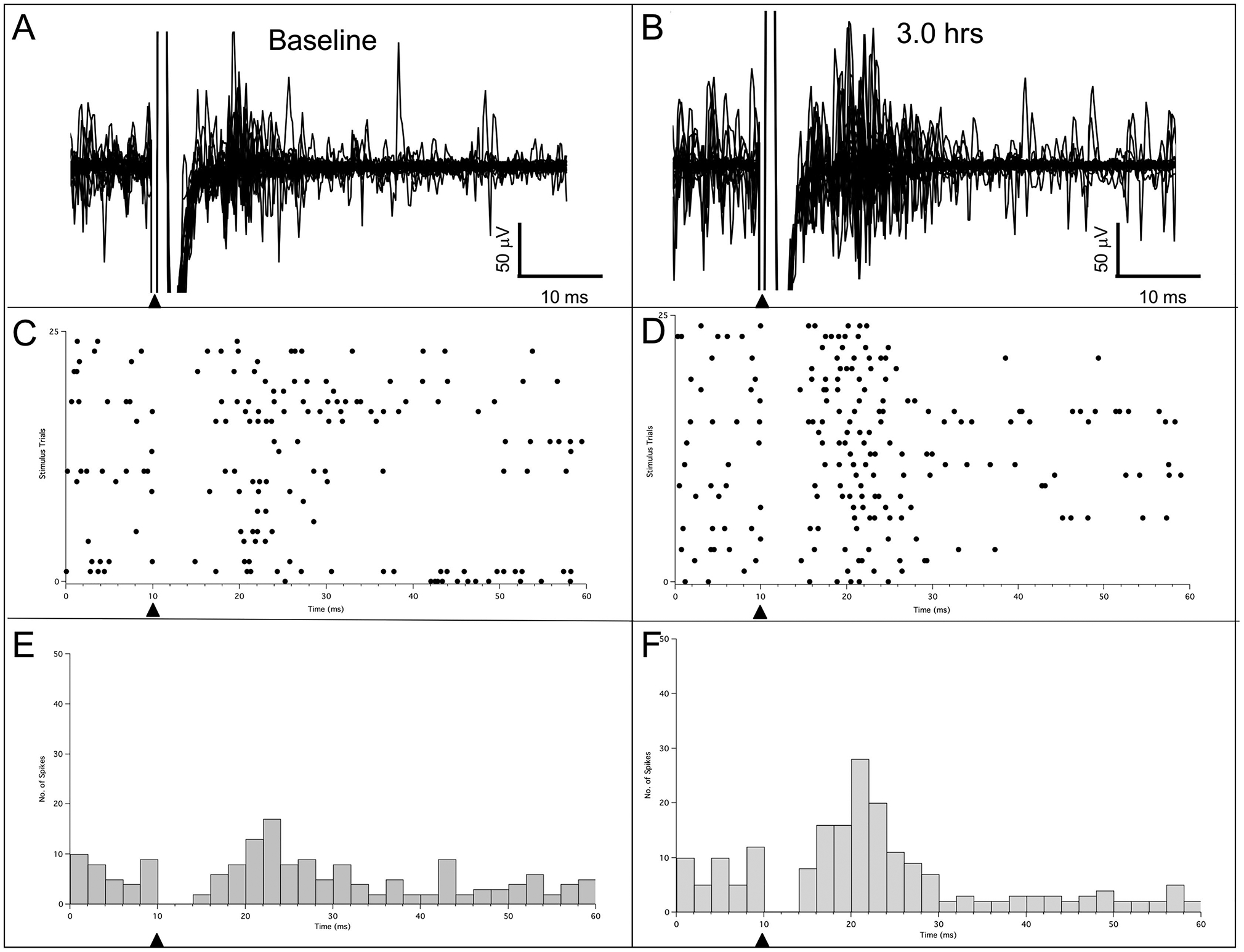
Representative example of evoked responses recorded in rat SI following 25 consecutive stimulations at a homotopic site in the wrist representation in the opposite SI at baseline and after 3 hr of ICMSr. (A) A total of 25 consecutive combined buffer traces obtained at the beginning of ICMS, which served as the initial baseline. (B) A total of 25 consecutive traces recorded at 3 hr after ICMSr. (C, D) Raster plots for baseline and 3 hr, respectively. (E, F) Post-stimulus histograms for baseline and 3 hr, respectively. Solid triangles mark the time of stimulus onset.
To illustrate the variety of effects of ICMSr, 4 additional examples are shown in Fig. 2. In the cases illustrated in Figs. 2A and 2B, the wrist representation was physiologically identified in layer V in each hemisphere, the interhemispheric pathway was delineated, and ICMSr was delivered to one of the hemispheres for 3 hrs. In both rats, ICMSr led to an increase in evoked responses in the contralateral SI and the resulting raster plots and PSTHs are shown. In the plots shown in Fig. 2A, the duration of the evoked response is longer following ICMS at the initial baseline stimulation and at 3 hr post-repetitive ICMS. This pattern can be compared to the example shown in Fig. 2B, where the duration of response is narrow for both baseline and post-stimulation periods. Note in this example, that ICMSr at 3 hr produced a robust response, but maintained a narrow response pattern.
Fig. 2 –
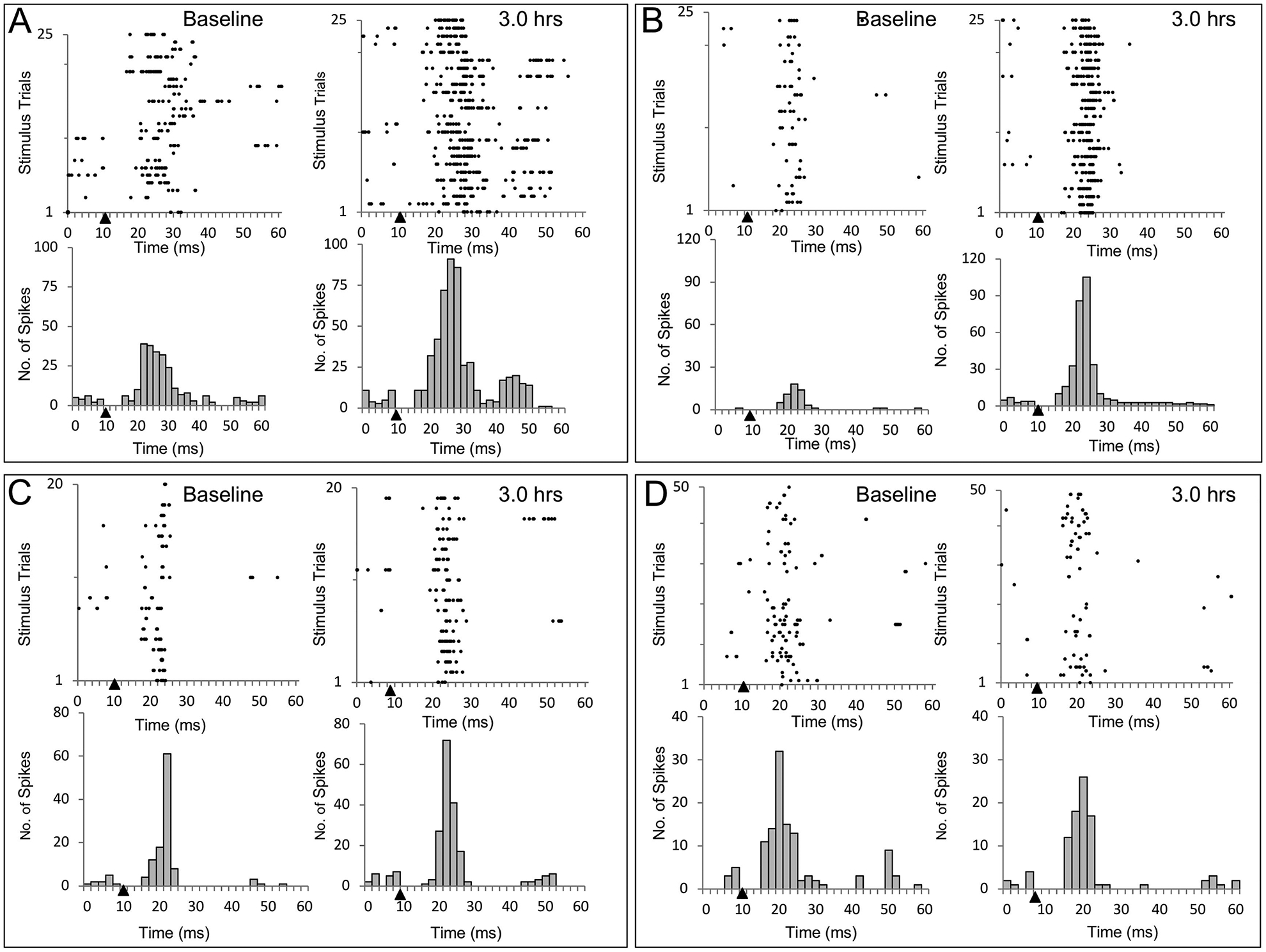
Evoked responses recorded from 4 different experiments showing three examples of spike facilitation and one case of a reduction in firing after ICMSr. (A, B, C) Increased spike activity was observed in each of these cases. (A) In this example, ICMSr evoked a strong multiunit response during baseline recording that resulted in similarly increased prolonged responsiveness 3 hr after ICMSr. In (B), both baseline and evoked responses had narrow responses, but after ICMSr, the evoked response was quite prominent. In both (A) and (B), stimulation and evoked responses were derived from layer V of the wrist representation in SI. (C) In this example taken from homotopic locations in digit 3 of the forepaw representation, a modest firing increase (58%) was observed after ICMSr. (D) In this rat, a modest decrease (18%) in firing from the wrist representation, which did not lead to an increase in spike activity or the appearance of ipsilateral input. Solid triangles mark the time of stimulus onset.
In the example shown in Fig. 2C, sites in the digit three (D3) representation in the forepaw barrel subfield (FBS) in each hemisphere in SI cortices were identified in layer V, and ICMSr delivered to one of the sites evoked a response in the same digit representation in the opposite hemisphere. Raster plots and histograms in Fig. 2C show the results of 20 consecutive stimulations; a resultant increase in spike activity in the opposite SI was observed at the end of 3 hr of ICMSr. The number of spikes detected within the evoked response window of 4–30 ms following stimulation increased modestly from 103 spikes to 163 spikes with a 58% increase that barely reached the criterion for pathway strengthening.
In one rat, ICMSr delivered for 3 hr failed to strengthen the interhemispheric pathway, but rather produced a slight decrease in excitability. In this rat, ICMSr was delivered to a physiologically defined wrist representation site in SI at an approximate depth of 1,300 μm; responses were recorded from a homotopic site in the opposite SI at a similar depth. Fifty consecutive stimulations were used to generate a baseline and the same number of stimulations was used to assess excitability after 3 hr. Inspection of raster and histogram plots in Fig. 2D showed a 18% decrease in number of spikes recorded 3 hr post-ICMS compared to baseline.
2.1.1. Time course for strengthening of interhemispheric pathway
To assess the time course for strengthening, responses to ICMSr were measured at the beginning of ICMS and at 30-min increments until the end of the experiment lasting between 3 and 5 hr. Spike detection was performed using an amplitude voltage threshold (Vthr). Strengthening of the interhemispheric pathway was met when the evoked number of spikes reached or exceeded 1.5 × the baseline number of spikes.
An example illustrating the time course for strengthening between homotopic sites is shown for one rat in Fig. 3. In this example, ICMSr was delivered for 5 hr to a physiologically identified wrist representation in layer V, and responses to 25 consecutive stimulations were recorded from a homotopic wrist representation site in layer V at a nearly identical depth in opposite SI (photomicrograph in Fig. 3). Raster plots and peri-stimulus histograms (2-ms bins) were generated throughout the 5 hr of ICMSr in 30-min increments. Collectively, these time files illustrate a nearly steady increase in neural activity in response to ICMSr over the 5 hr. The number of spikes detected increased from a baseline of 52 spikes to 320 spikes (515% increase) after ICMSr for 3 hr and 356 spikes (585% increase) after 5 hr. In this rat, the strengthening criterion (1.5 × baseline) was met within the first 30 min of stimulation where a total of 183 spikes were counted, equating to a 252% increase over baseline, and the increase spike number was maintained throughout the 5 hr of ICMSr. A positive correlation between the number of spikes detected and the length of ICMSr was found (R2 = 0.85). The overall increase in evoked response firing activity measured at 5 hr was 6.85 × baseline.
Fig. 3 –
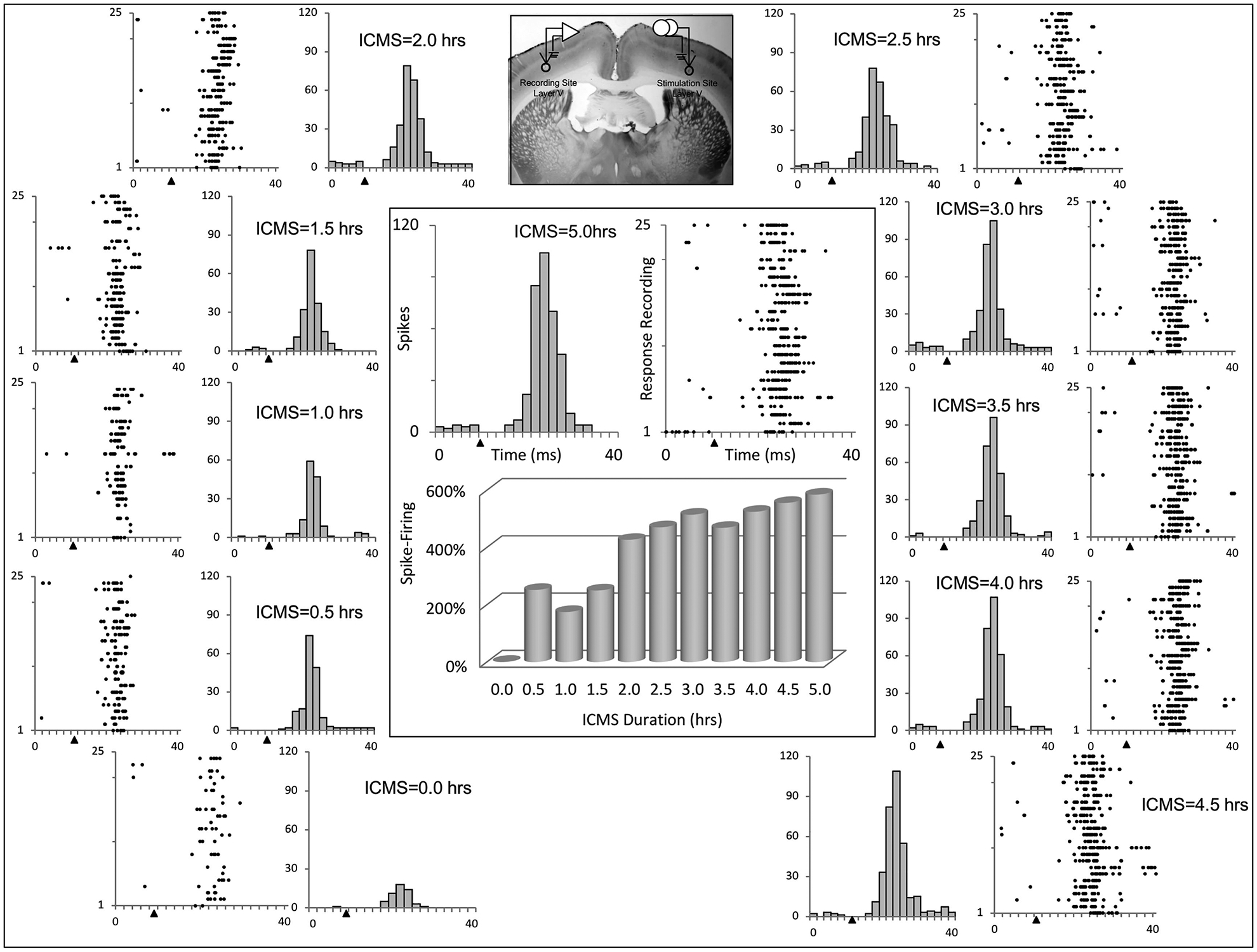
Raster plots and histograms of evoked responses examined at baseline and at 30-min intervals throughout a 5-hr window of ICMSr are illustrated for 1 rat. Bar graph at center shows the % change in spike number over the duration of ICMSr. Note the progression in spike activity observed over the window for ICMSr. The inset at top shows location of stimulating and recording sites in the wrist representation in layer V of SI. Solid triangles mark the time of stimulus onset.
Interhemispheric pathway strengthening was also found between homotopic forepaw representations; however, the pattern did not increase with time; these data are presented as raster plots and peri-stimulus time histograms in Fig. 4. In this rat, ICMSr (3 hr) was delivered to the ventral tip of digit three and responses to 20 consecutive stimulations were collected. An increase in spike activity is apparent when comparing baseline ICMS to that seen at 3 hr; however, a steady progression of spike firing was not observed throughout the course of ICMSr as commonly observed in the wrist representation, and the number of spikes detected and length of ICMSr were uncorrelated (R2 = 0.1403). The distribution of spike activity is summarized in the center plot in Fig. 4. Note that increased spike firing was seen at each time interval in which the largest increase was observed after 1.5 hr of ICMS but decreased over the next 2 hr before increasing again at 3 hr.
Fig. 4 –
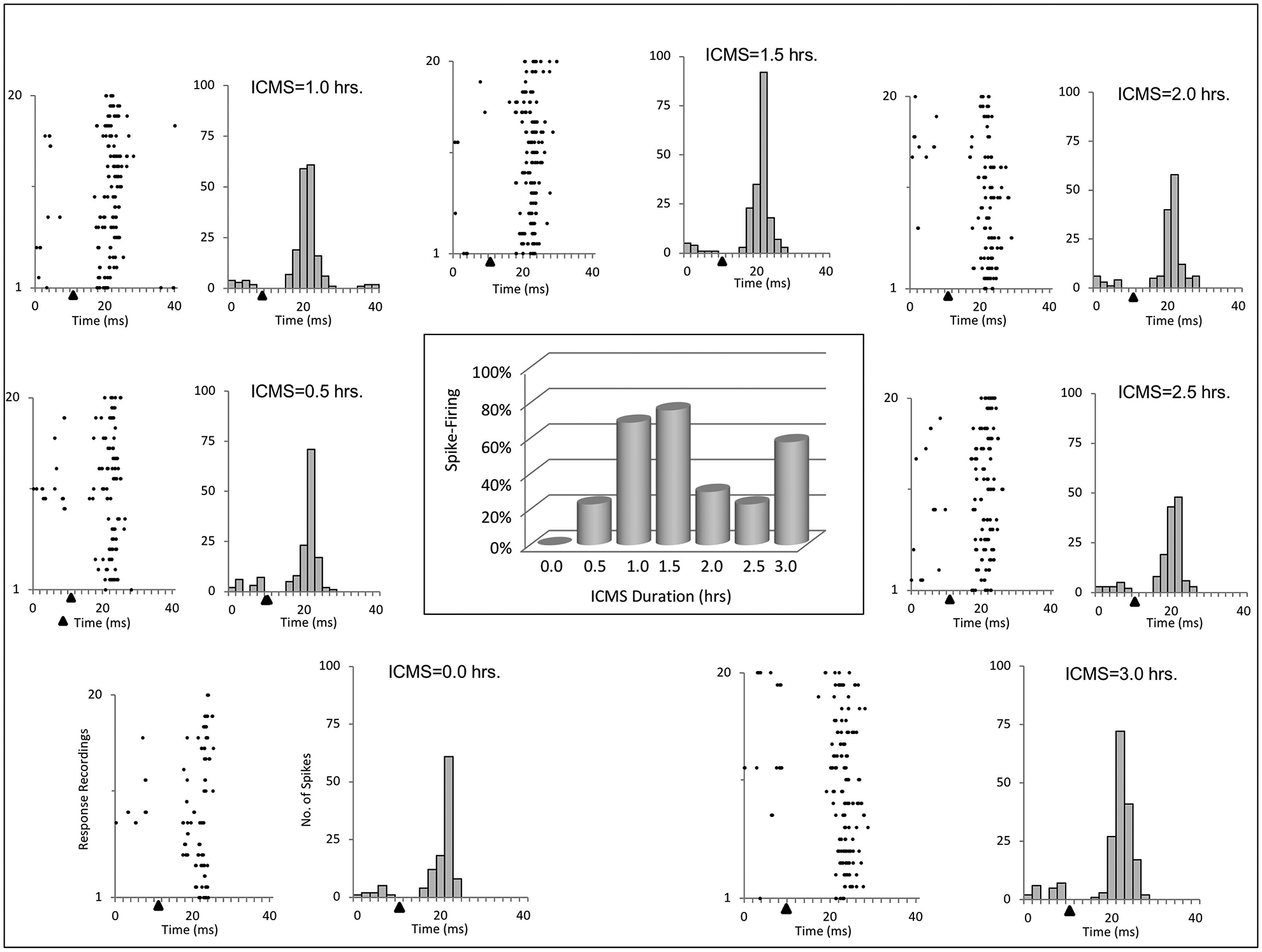
Raster plots and histograms of evoked responses recorded from homotopic sites in the forepaw representation over a 3-hr time course of ICMSr are shown for 1 rat. However in this example, the greater percent evoked response increases were observed at 1.0 and 1.5 hr after ICMSr and did not return to these levels during the remaining period of stimulation. This is shown in the bar graph (center) for the 3 hr duration time. Solid triangles mark the time of stimulus onset.
To determine the time period in which spike number was significantly different from baseline spike number, a repeated measures (one-way) analysis of variance (ANOVA) was performed on the number of spikes collected at 30-min intervals from those animals (n=6) that received ICMSr for at least 3 hr and showed an increase in spike firing of 1.5 × baseline. To assess changes in spike number, a 120-ms oscilloscope trace-window, triggered at time zero (0 ms) was followed 10 ms later by ICMS; the resulting evoked response was then examined at 3 time periods after trace onset (14–40 ms, 41–80 ms, and 81–120 ms), and these results are shown in Fig. 5. Significant changes in spike number were confined to the 14–40 ms time interval. Student-Newman-Keuls post-comparison test revealed the spike number was significantly greater following 2.5 hr of stimulation and remained so after 3.0 hr of ICMSr (p<0.05).
Fig. 5 –
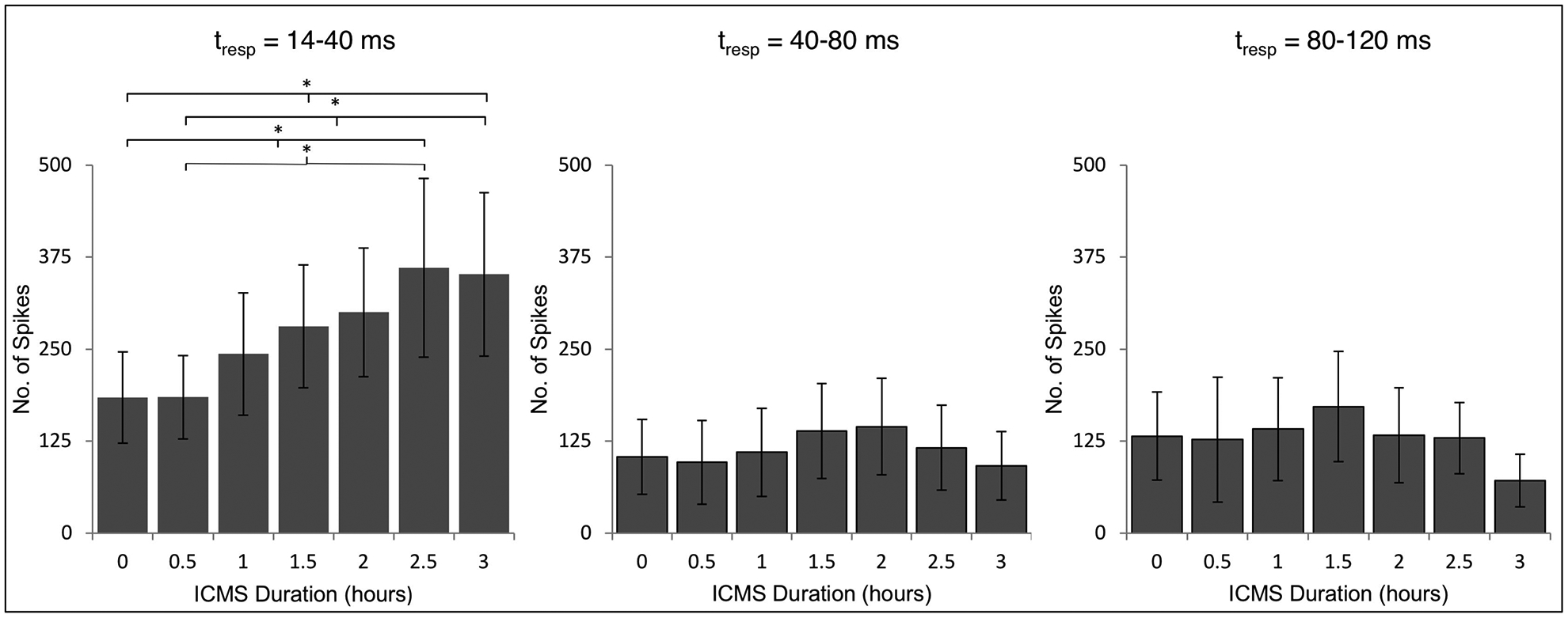
Time course for interhemispheric excitation from 6 rats that received ICMSr for a total of 3-hr. Graphs show number of spikes (mean ± standard deviation) for 3 time periods (tresp) in a 120-ms time period beginning 4 ms after stimulation (14 ms from onset of the oscilloscope trace). Baseline number of spikes is designated at ICMS duration time zero. Only after 2.5 hr of ICMSr was there a significant increase in spike number from that of baseline, and only in the time interval between 14 ms and 40 ms. (*p<0.05)
2.2. Ipsilateral input in SI using multiunit extracellular recording
Input to SI from the ipsilateral forelimb was examined, but not observed prior to strengthening of the interhemispheric pathway. In 11 rats, following strengthening of the interhemispheric pathway (wrist, n=8; forearm, n=2; forepaw, n=1), ipsilateral input was observed in SI. One additional rat also showed strengthening of the pathway (76%) after 3 hr of ICMSr, but no ipsilateral input was observed.
An example from one rat where ipsilateral input was observed in SI following ICMSr is shown in Fig. 6. In this example, baseline raw data, raster plot, and histogram are shown in Figs. 6A, 6B, 6C, respectively; similar data are shown in Figs. 6D, 6E, 6F following 3 hr of ICMSr. Inspection of the raster plot during baseline data collection revealed that most of the spikes occurred during a single trace. Following 3 hr of stimulation, a robust response was evoked with an onset latency of 16.2 ms and a response duration of 14.1 ms. The delayed evoked response onset is most likely accounted for by the added time for the input to travel across the interhemispheric pathway.
Fig. 6 –
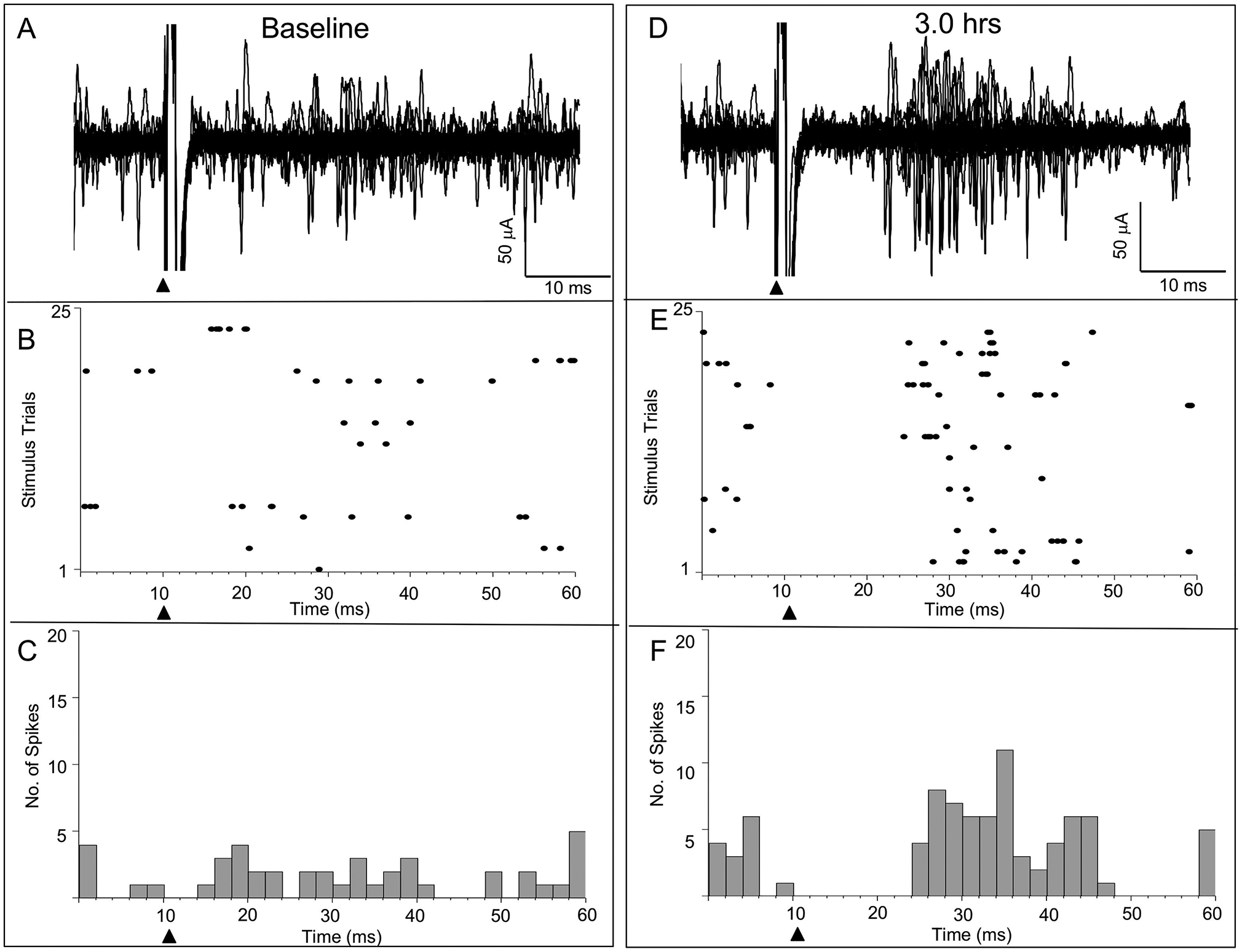
Ipsilateral input in facilitated SI following ICMSr – multiunit record. Baseline evoked response data for 13 consecutive stimulations is shown at left along with raster plot and histogram is illustrated for 1 rat. Similarly, raw data, raster plot, and histogram are shown after 3-hr of ICMSr delivered to the wrist representation in SI. Note the absence of evoked responses following stimulation of the ipsilateral wrist during baseline stimulation, and, in contrast, the appearance of a robust response to the same ipsilateral forelimb stimulation following 3-hr of ICMSr of the wrist representation in layer V of SI. Solid triangles mark the time of stimulus onset.
Two additional rats were included as controls where homotopic sites in the wrist representation were physiologically identified in SI. Neither rat exhibited pathway strengthening or ipsilateral input after 3 hr of stimulation (data not shown).
2.3. Ipsilateral input in SI using intracellular recording
In one experiment aimed at examining corticocortical connectivity between homotopic sites in the forepaw representation in layer V, after identifying homotopic sites in the digit 3 forepaw representation, the extracellular recording electrode was replaced by an intracellular electrode and the electrode was driven into layer V where it impaled a cell that was held for 4 hr, before the electrode was withdrawn from the cell. In this cell, stimulation in SI evoked an initial subthreshold response in the opposite SI, and this is shown in the left inset in Fig. 7A. However, within 30 min of ICMSr, the subthreshold response became elevated to a suprathreshold response, and these data are shown in Fig. 7A; a single trace is shown in the right inset in Figure 7A. Raster plot and peri-stimulus histogram for 25 consecutive stimulations are shown in Fig. 7B and 7C, respectively. The stimulation site in layer V is shown in the inset in Fig. 7C. Following 3 hr of ICMS, the neuron responded to input from the ipsilateral forelimb (digit 3), and this result is shown in Fig. 7D; the inset shows a single trace. While this cell was not tested for ipsilateral input prior to ICMSr, it is noteworthy that the evoked response is delayed as previously seen following ICMSr in the extracellular recording records. However, in 2 additional rats, ipsilateral input was not observed in 3 intracellular recorded SI neurons prior to the beginning of ICMSr. In each case, the cell was not held for a sufficient time to carry-out all parts of the experiment (data not shown). Raster plot and peri-stimulus histogram are shown in Fig. 7E and 7F, respectively. The inset in Fig. 7F shows the location of the recorded cell after 30-min injection with biocytin; the cell body is magnified in the upper left of the inset.
Fig. 7 –
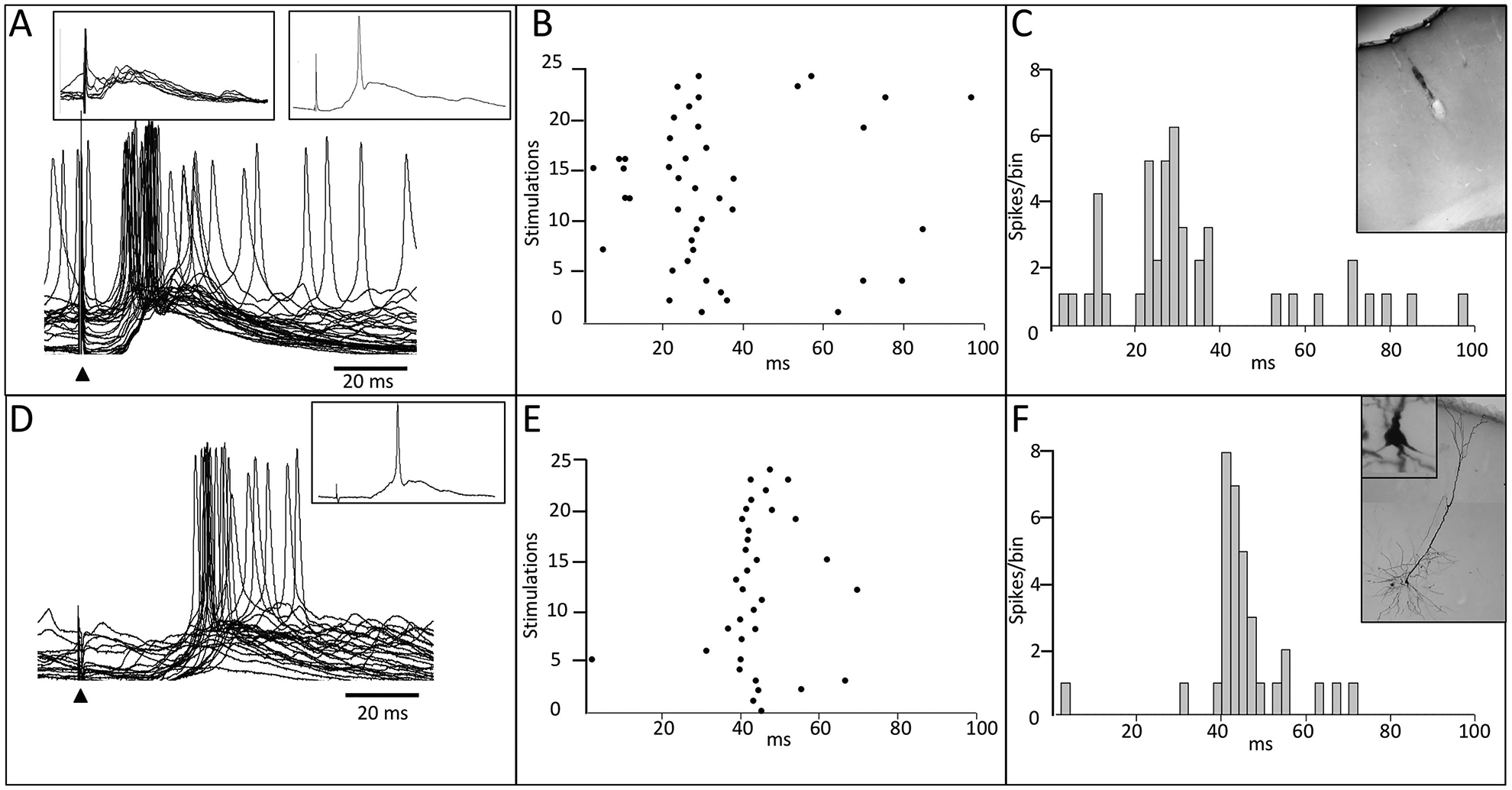
Ipsilateral input in facilitated SI following ICMSr – intracellular record. (A) Initial stimulation in the forepaw representation in layer V of SI evoked a post-synaptic response in a homotopic site in the opposite SI forepaw cortex; left inset in (A) shows a series of 10 consecutive stimulations, and the inset at right, shows a single response. After approximately 30 min of ICMSr, the subthreshold response became elevated to a suprathreshold firing level, and this is shown in the bottom trace in (A). Raster plot and histogram are shown in (B) and (C), respectively. Inset in (C) shows the location of the stimulation site in SI. At 3-hr of ICMSr, stimulation of the ipsilateral forepaw (digit 3) evoked a response and the raw data are shown in (D); single trace are shown in the inset in (D). Raster plot and histogram are shown in (E), and (F), respectively. The inset in (F) shows the labeled cell in layer V that was injected with biocytin. Solid triangles mark the time of stimulus onset.
The corticocortical evoked response latency for the neuron recorded in Fig. 7 was 7.78 ms. In 7 additional rats, evoked response latencies were measured between homotopic sites in layer V in 17 intracellularly recorded neurons. In these neurons, evoked responses had an average latency of 7.55 ms (range = 6.44–8.60 ms).
2.4. Source of ipsilateral input
In 3 rats, where pathway strengthening and ipsilateral input were found, inactivation (lidocaine or cooling) of the SI stimulation site abolished the ipsilateral input in the opposite homotopic SI forelimb, and these results are shown in Fig. 8. The upper three traces, Figs. 8A, 8B, 8C show baseline evoked responses recorded in ipsilateral SI of each rat following stimulations (25) of the ipsilateral forearm/wrist prior to the initiation of intercortical microstimulation; note the absence of evoked ipsilateral responses. In comparison, the middle traces, Figs. 8A-1, 8B-1, and 8C-1, show delayed evoked responses in the ipsilateral SI for each rat following periods of ICMSr. The stimulating site in SI was then inactivated by lidocaine injection into the depths of the cortex or local cooling of the brain surface. In each rat, evoked spikes disappeared, and these records are shown in the lower traces, Figs. 8A2, 8B2, 8C2. In one lidocaine injected rat shown in Fig. 8B-3, some evidence of recovery from lidocaine was observed 56 min after the injection.
Fig. 8 –
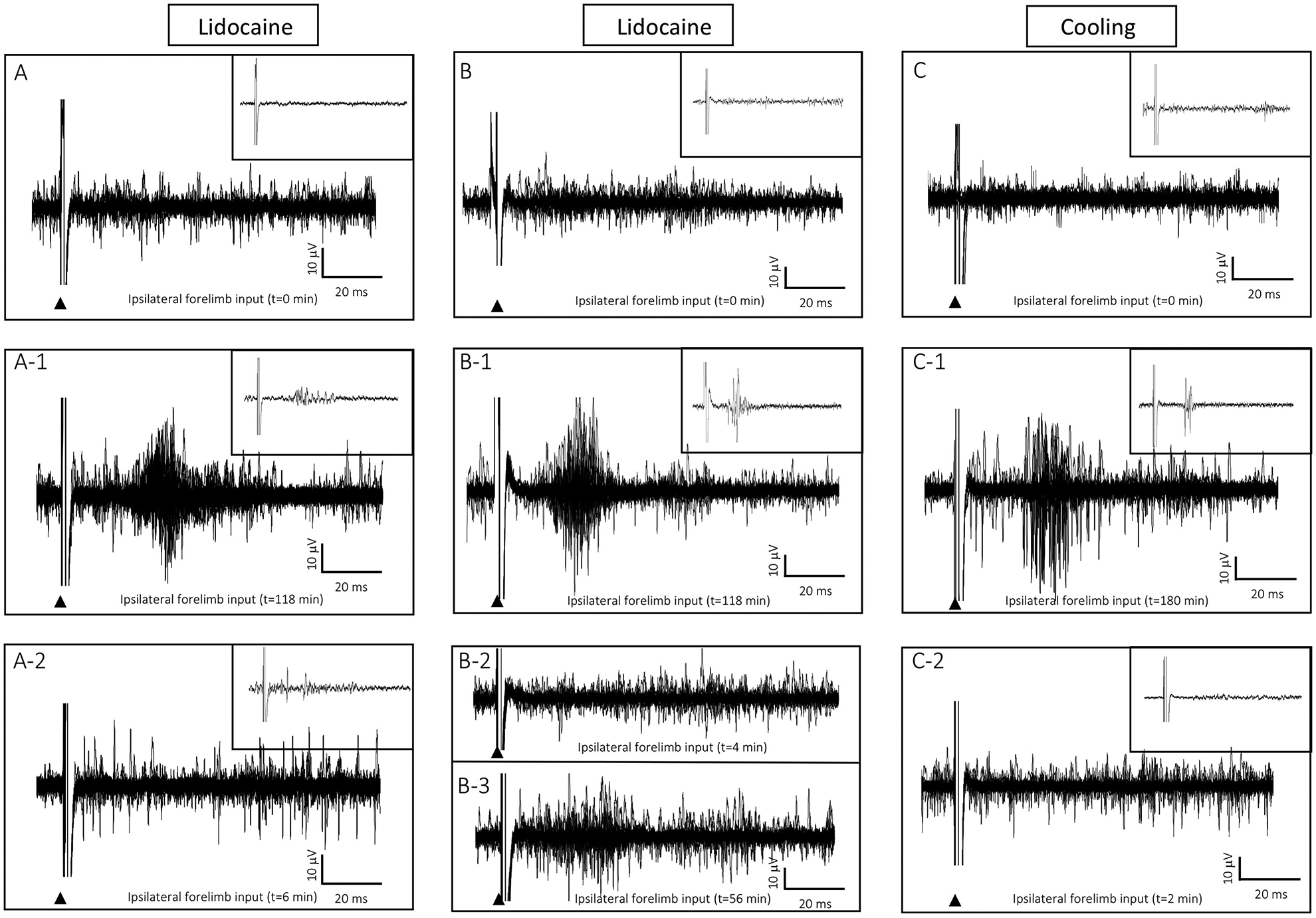
Inactivation (lidocaine injection or cooling) at the stimulation site in SI abolished ipsilateral forelimb responses in the homotopic site in the opposite SI. (A) Baseline recording in ipsilateral forelimb cortex following 25 consecutive stimulations of the ipsilateral forelimb did not evoke a response. (A-1) Following 118 min of ICMSr, stimulation of ipsilateral forelimb evoked a response in the ipsilateral SI. (A-2) Six minutes after lidocaine injection at the stimulation site in SI the ipsilateral evoked response in the opposite SI was abolished. (B–B2) Another example of lidocaine inactivation in a second rat. (B-3) In this rat, the ipsilateral response showed signs of returning 56-min after lidocaine inactivation. (C) Surface cooling over the stimulation site in SI also inactivated ipsilateral input forelimb input in ipsilateral SI; (C) baseline, C-1 ipsilateral forelimb response in ipsilateral SI, C-2 ipsilateral response is abolished 2-min after removal of the cooling probe.
3. Discussion
In our experience, neurons in forelimb SI representation receive peripheral input exclusively from the contralateral forelimb (Pearson et al., 1999; Waters et al., 1995). Homotopic sites in SI are linked by an interhemispheric pathway, which raised the question as to why these neurons do not also respond to input from the ipsilateral forelimb. The present study investigated whether neurons in layer V in the forelimb representation in SI could be induced to respond to previously ineffective input from the ipsilateral forelimb. We proposed that ICMSr delivered to the interhemispheric pathway would increase spike firing in the opposite SI strengthening this pathway sufficiently to allow normally ineffective stimuli from the ipsilateral forelimb to excite cells in the ipsilateral forelimb SI. The forelimb representation in SI in one hemisphere was mapped using mechanical and electrical stimulation of the contralateral forelimb, a homotopic site was similarly identified in the opposite SI, the presence of ipsilateral peripheral input in SI was tested in both homotopic sites, and ICMS was used to determine the existence of an interhemispheric connection between the two homotopic recording sites. The major findings of the present study are: (1) each forelimb site in SI initially received a short latency input only from the contralateral forelimb; (2) forelimb sites in layer V in each SI were interconnected by an interhemispheric pathway; (3) ICMSr delivered to layer V of SI forelimb cortex in one hemisphere generally resulted in increased spike firing in layer V in the opposite homotopic site; (4) this increased firing response was often followed by the new expression of ipsilateral input whose latency was longer than that of the contralateral input; (5) the longer latency ipsilateral responses are consistent with a delay time necessary for travel across the interhemispheric pathway; (6) the increased spike firing and the resulting ipsilateral responses were also corroborated using in-vivo intracellular recording; and (7) inactivation (lidocaine or cooling) of the stimulating site in SI, and hence the interhemispheric pathway, abolished the ipsilateral forelimb response in the opposite SI.
3.1. Ipsilateral receptive fields in rat
In this study, we used hand-held probes to deliver mechanical stimulation or electrical stimulation to the skin surface to evoke responses in the forelimb representation in layer V in SI in ketamine/xylazine-anesthetized rats. We have used similar techniques to map the FBS in layer IV in SI in rats anesthetized with Nembutal (Pearson et al., 1999; Waters et al., 1995).
In the data presented here and data from prior forelimb mapping studies in rats, despite using different anesthetics, we did not encounter bilateral forelimb receptive fields. Nonetheless, bilateral forelimb receptive fields have been reported in barrel field SI in rats anesthetized with Nembutal (Tutunculer et al., 2006). In their study, 2 arrays of electrodes were implanted in SI and 1 week later, under light Nembutal anesthesia, a fine-tipped metal stimulus probe was used to deliver 100 stimulations to a set of 10 standardized sites on both forelimbs while recordings were made from each electrode in the array. Using this technique, ipsilateral responses were reported in greater than 40% of the recorded neurons. Beside the absence of ipsilateral input in our present study before ICMSr, their experimental procedures differ from ours in a number of ways that include: site of recording, method of stimulation, anesthesia, and animal species. For example, in their Fig. 1, the recording electrodes appear deep in the cortex in presumptive layer VI adjacent to the white matter. If these locations are representative of their data set, then our recording sites are more superficial in layers Va and Vb. Interhemispheric connections may be considerably sparser in layer VI than compared to layers III and V, possibly suggesting an alternative pathway for ipsilateral input to reach the opposite SI (Decosta-Fortune et al., 2015). Additionally, they used a precisely controlled stimulator to deliver 100 consecutive stimulations to the skin surface at 10 separate sites while we used a mechanical (hand-held probe) or electrical probe to stimulate the skin surface for the examination of homotopic and surrounding sites. Inspection of their PSTHs from Fig. 2 revealed that the ipsilateral responses, in some cases, occurred in nearly every stimulus trial and the ipsilateral input was not limited to a homotopic site. While Nembutal was used in their study and ketamine/xylazine in the present study, we have previously used Nembutal to map the forepaw representation in SI barrel cortex in Sprague-Dawley rats (Waters et al., 1995). Although the goal of our previous study was to produce high-quality detailed maps of the forepaw representation in contralateral SI, stimulation of the ipsilateral forelimb failed to evoke responses in the ipsilateral SI. While it is hard to reconcile the differences between our study and theirs, other investigators using mechanical or electrical stimulation have described bilateral hindlimb input in rat SI (Angel and Lemon, 1975; Armstrong-James and George, 1988; Pluto et al., 2005). Armstrong-James and George (1988) used mechanical stimulation and reported bilateral input in homotopic regions of the hindlimb representation, noting that ipsilateral receptive fields were smaller than their contralateral counterpart and had a longer response latency. Angel and Lemon (1975) used electrical stimulation in the forelimb and hindlimb and reported bilateral evoked responses following stimulation of the hindlimb and concluded that the forelimb representation was restricted to only the contralateral SI.
3.2. Technical comments
In the present study, a microelectrode was inserted into the SI to map somatosensory input from a region of the forelimb. A second electrode was directed into the opposite SI to identify a homotopic region at a similar depth. To test for connectivity between the sites within the two hemispheres, one electrode was used to deliver single-pulse stimulation and the second electrode was used for recording evoked responses. Slight depth adjustments in the stimulating and recording electrodes were made to identify a location where a maximal response was evoked using minimal stimulation. Once these locations were identified, single pulses were delivered to establish a baseline evoked response. Single-pulse ICMSr was then continued for up to 5 hr during which evoked spike firing and the presence of ipsilateral input were evaluated.
Selection of stimulating and recording sites in layer V of SI forelimb barrel cortex was based, in part, on a previous study that used anatomical tracers and electroanatomy to examine projection patterns from physiologically identified sites in layer V in the forelimb and shoulder representations (Decosta-Fortune et al., 2015). In this study, we found that wrist, forearm, and shoulder representations projected predominately to homotopic sites in layer V in the opposite cortex, although some labeling was found in all cortical laminae in the contralateral homotopic SI. Because, we (Decosta-Fortune et al., 2015) and others (Akers and Killackey, 1978; Wise and Jones, 1976) have reported that layer V was strongly interconnected between the hemispheres, layer V was therefore chosen to study homotopic sites between these cortical layers. In contrast, little evidence was found to support a homotopic relationship between forepaw cortices; rather, injections of tracer made into the layer V within a column serving the forepaw representation terminated primarily in layer V in the opposite SI in dysgranular cortical regions outside, but adjacent to, the contralateral forepaw representation (Decosta-Fortune et al., 2015). However, in the present study, stimulation of a forepaw site in layer V evoked a subthreshold response in the forepaw representation in layer V in the opposite SI, suggesting that forepaw cortices are also connected by an interhemispheric pathway.
3.3. Evoked spike firing
We defined a change in the evoked response level when spike firing activity reached and/or exceeded a 1.5 × voltage amplitude spike threshold level over baseline firing measured prior to the beginning of ICMSr. While a significant increase (p ≤ 0.05) in spike activity was measured after 2.5 hours of ICMSr and remained, in 1 rat, for up to 5 hr, the criterion level was reached on average after 30 mins of ICMSr. Long-term changes of transcallosal excitatory connections have been associated with an increase in the number and efficacy of connections (Bogdanova and Sil’kis, 2002). It is very likely that the increase in evoked firing activity is due, in part, to the recruitment of additional neurons, some of which may have been responding at a subthreshold level during the initial baseline evaluation. This explanation appeared to hold true for the one example of the intracellularly recorded forepaw neuron which had a subthreshold evoked response during the initial evaluation but was subsequently brought to suprathreshold firing level during subsequent ICMSr (see Fig. 7).
We previously demonstrated that neurons in rodent forepaw barrel cortex receive short-latency suprathreshold input from a principal location on the forepaw and longer latency subthreshold input from a surrounding forepaw skin surface (Li and Waters, 1996); other investigators have reported similar findings in rodent vibrissa barrel cortex with the displacement of a primary whisker (suprathreshold input) and adjacent whiskers (subthreshold input) (Carvell and Simons, 1988; Moore and Nelson, 1998). Preexisting subthreshold input may become expressed after being raised to suprathreshold levels (Calford and Tweedale, 1990; Li and Waters, 1996; Moore and Nelson, 1998; Smits et al., 1991) which has been shown to occur following the release of GABAergic inhibition (Carvell and Simons, 1988; Li and Waters, 1996; Moore and Nelson, 1998; Smits et al., 1991). We injected a GABAA-receptor blocker into the FBS and reported that suppression of GABAergic inhibition was accompanied by enhanced responsiveness to stimulation from the surrounding receptive field on the digit surface which had previously provided subthreshold input (Li et al., 2002).
GABAA receptors receive excitatory inputs, in part, from callosal projecting neurons (Pluto et al., 2005). In rodent SI, these neurons are pyramidal cells located primarily in layers III and V (White and DeAmicis, 1977; Wise and Jones, 1976; Yorke and Caviness, 1975); layer V pyramidal neurons are reported to yield sub-and surprathreshold responses (Manns et al., 2004). Also, in rabbit SI, callosal projecting neurons at cortical depths corresponding to layers II/III – V exhibited sub- and suprathreshold receptive field characteristics (Swadlow and Hicks, 1996). Callosal projections terminate predominately in layers III and V of the opposite SI (Akers and Killackey, 1978; Koralek et al., 1990; Wise and Jones, 1976) on both excitatory pyramidal neurons and inhibitory interneurons (Pluto et al., 2005) providing direct excitation and indirect inhibition to target neurons. By modulating excitatory input, callosal connections can modulate inhibition (Clarey et al., 1996; Rema and Ebner, 2003; Shuler et al., 2001; Swadlow, 2003).
3.4. Repetitive stimulation
ICMSr applied in vivo to rat SI has been shown to induce representational plasticity (Dinse et al., 1993; Heusler et al., 2000; Kalarickal and Marshall, 2002; Recanzone et al., 1992). For example, Recanzone and colleagues identified receptive field input of neurons in layer IV in rat SI, delivered repetitive low frequency ICMS to that site for 2–6 hr, and reported receptive field expansion around the stimulation site to include adjacent cortical territory (Recanzone et al., 1992). Other investigators combined cross-correlation and ICMSr in SI and reported a functional coupling between the neurons in the expanded receptive field with neurons around the stimulating electrode (Dinse et al., 1993). We herein identified homotopic receptive field sites for the forelimb in layer V of SI in each hemisphere, used microstimulation to identify an interhemispheric connection, repetitively stimulated one of the sites, and increased firing activity in the homotopic site in the opposite SI. In 90% of these activated sites, the same receptive field recorded from neurons at the stimulation site (derived from the contralateral forelimb) became expressed by neurons in the homotopic site in the opposite SI. Whether the receptive field expansion in each of these studies resulted from unmasking of intracortical connections, the establishment of new functional connections, and/or activation of excitatory/inhibitory components within the ICMS-stimulated territory remains an open question.
Plasticity of callosal connections has been demonstrated in in vitro rat slice preparations showing that tetanic stimulation of the white matter enhances neuronal responsiveness (Lee, 1982; Bindman et al., 1988; Artola et al., 1990), and in vivo in rodent barrel cortex showing that the absence of interhemispheric activity down-regulates firing background and evoked responses in contralateral barrel cortex (Li et al., 2005). However, few reports are available where high frequency microstimulation induced long-term plasticity of transcallosal excitatory connections in vivo (Bogdanova and Sil’kis, 2002). On the other hand, the results presented here, provide a clear demonstration of plastic changes to transcallosal connections between homotopic SI layer V forelimb cortices in rat that leads to expression of ipsilateral responsiveness; however, our results are time-restricted and do not address the possible long-term effects of chronic ICMS.
Long-term effects have been reported following high-frequency stimulation of rabbit hippocampus (Bliss and Lomo, 1973) and cortex (Artola and Singer, 1987; Sakamoto et al., 1987). For example, long-term potentiation (LTP) following tetanic stimulation delivered to vertical and horizontal SI cortical synapses in rodent (Aroniadou-Anderjaska and Keller, 1995) is accompanied by a horizontal spread of ipsilateral potentiation in layer II/III (Lee et al., 1991), a vertical spread of ipsilateral potentiation from layer IV to layer II/III (Glazewski et al., 1998), and enhanced cortical response magnitude (Lee et al., 1991; Lee and Ebner, 1992). To induce LTP, levels of excitability need to be sufficiently elevated through an increase in N-methyl-d-aspartate (NMDA) receptor activation of excitatory cells or a diminished level of inhibition from a reduction in gamma-aminobutyric acid (GABA) receptor activation (Lee et al., 1991; Lee and Ebner, 1992). The threshold to induce LTP has been achieved, in studies using in vitro slice preparations, by reducing extracellular concentrations of Mg2+, which increased activation levels of NMDA receptors (Lee et al., 1991; Lee and Ebner, 1992), and by introducing low concentrations of a GABA blocker such as bicuculline, a GABAA blocker, to the recording site (Artola et al., 1990; Bindman et al., 1988) which disinhibited inhibition. Excitatory transcallosal connections can be induced by rhythmic stimulation (Sah and Nicoll, 1991) and provide a sufficient environment to study enhancement by elevating the activation levels of NMDA channels (Thomson, 1986). One important finding of the present study is that low frequency ICMSr of transcallosal connections between homotopic SI forelimb representations can increase responsiveness in contralateral SI as evidenced by a ≥ 50% increase in evoked response firing rate. Note that transcallosal connections have been generally reported as being scarce in cortical regions associated with the forelimb (Hayama and Ogawa, 1997; Henry and Catania, 2006; Manzoni et al., 1980; Wise and Jones, 1976); however, this was not without controversy (Decosta-Fortune et al., 2015).
While high frequency stimulation can induce long-term changes in rodent SI responsiveness, it has also been demonstrated that chronic microstimulation can induce long-term depression (LTD) of synaptic efficacy (Heusler et al., 2000) of both excitatory and inhibitory connections (Bogdanova and Sil’kis, 2002). For example, reduction in the density of excitatory perforated synapses and increase in density of inhibitory synapses in layer V of rodent sensorimotor cortex have been reported (Teskey et al., 2007). It has been suggested that LTD following chronic microstimulation occurs when the pathway being stimulated has both monosynaptic excitation and disynaptic inhibition and is facilitated by an increased excitation of inhibitory interneurons (Bogdanova and Sil’kis, 2002). In rodent, following a 24-hour period of chronic whisker stimulation, a dense increase in spineous GABAergic synapses was reported in the corresponding contralateral barrel cortex and thought to have mediated the observed depressed responsiveness (Quairiaux et al., 2007). In our study, low frequency stimulation led to a reduction (18% following 3.0 hours of ICMS) in contralateral SI evoked response in 1 rat. It is very likely that low frequency ICMS plays a similar role in regulating synaptic density as observed in both LTP and LTD.
3.5. Peripheral input reaches its ipsilateral SI via an interhemispheric pathway
Our findings reported here (1) confirm that it takes approximately 7–9 ms for a stimulus delivered to the peripheral forelimb to reach the mid-layers in the contralateral SI forelimb cortex, (2) a similar delay time is required for ICMS delivered to forelimb SI to evoke a response recorded in a homotopic site in the opposite SI, and (3) the ipsilateral evoked response in the ipsilateral SI is reflected by a combination of these two delay-times. The above-described delays in ipsilateral input have also been reported in rat for vibrissae (Pidoux and Verley, 1979; Shuler et al., 2001), hindlimb (Armstrong-James and George, 1988), and forelimb (Tutunculer et al., 2006).
To investigate the source of the enhanced ipsilateral forelimb input, we inactivated cells around the stimulating electrode by injection of lidocaine or local cooling and retested for ipsilateral input which was no longer present. Pidoux and Verley (1979) recorded ipsilateral vibrissae input in rat and mouse, ablated the contralateral SI, and reported that the ablation abolished the ipsilateral input. Other investigators have reported a similar finding of abolishment of ipsilateral input following contralateral SI inactivation (Shuler et al., 2001). The above findings are in contrast to the report by Armstrong-James and George (1988) where inactivation of the contralateral hindlimb SI had no effect on ipsilateral input. This study also failed to label neurons in contralateral hindlimb cortex following injection of retrograde tracer in the opposite hindlimb cortex. These latter findings can be contrasted to a previous report where injection of tracers into physiological identified regions in rat SI forelimb cortex labeled homotopic sites in the opposite SI; the exception was that injections made into forepaw SI primarily labeled regions in the opposite dysgranular SI adjacent to the forepaw representation, although some scattered labeling was also found in the FBS (Decosta-Fortune et al., 2015). We propose that ICMSr strengthens the interhemispheric pathway and brings about a change in evoked response firing levels in homotopic sites in the opposite SI possibly by elevating previously unresponsive neurons to suprathreshold firing level.
3.6. Implications for clinical rehabilitation strategies
It is well documented that cortical stimulation alters activity in targeted brain regions and has important clinical relevance in stroke recovery, reduction of motor tremor, and amelioration of chronic pain; when combined with behavioral intervention, cortical stimulation can bring about cortical reorganization that is often associated with recovery of function (Dancause and Nudo, 2011).
One of the most frequently posited mechanistic theories for phantom limb pain is neuronal network reorganization whereby deafferented limb cortex begins to respond to stimulus input from neighboring regions of the body; for example, neurons in a deafferented hand representation may begin to respond to stimuli from the face following limb amputation (Collins et al., 2018). Flor and colleagues reported a direct relationship between the amount of cortical reorganization and magnitude of phantom limb pain after limb amputation (Flor et al., 1995; Flor et al., 1998; Karl et al., 2001). One possible rehabilitation strategy suggested by our current findings, perhaps applicable for phantom limb pain, would be to use ICMSr to seed new receptive field input into the deafferented limb cortex at a critical time after limb amputation. Using a cortical repetitive stimulation protocol in forelimb amputated rats, as described here, we reported that ICMSr led to an increase in activity in the opposite deafferented cortex which was followed by the expression of previously unexpressed receptive fields from the intact forelimb (Ramshur et al., 2013). While our stimulation technique involved invasive stimulation, other noninvasive stimulation techniques such as direct transcortical stimulation (dTCS) (Medeiros et al., 2012; Nitsche et al., 2009) could be employed. The applicable time course after amputation, optimal stimulation parameters, duration of ICMSr, and an assessment whether the resulting reorganization would be adaptive or maladaptive would remain to be determined (Flor et al., 2006; Flor, 2008).
4. Experimental Procedures
4.1. Animals
A total of 27 Sprague-Dawley rats of either sex with a mean age of 11.22 wk (± 1.76 wk) and mean weight of 324.59 g (± 30.51 g) was used in this study. The experiments conformed to the Guide for the Care and Use of Laboratory Animals (Eight Edition) and were approved by the University of Tennessee Health Science Center (UTHSC) Institutional Animal Care and Use Committee (IACUC).
4.2. Animal preparation and receptive field mapping
Animal preparation and receptive field mapping have been previously described in detail (Waters et al., 1995) and are presented briefly here. Rats were anesthetized with ketamine/xylazine (87/13 mg/kg, i.m.) and supplemented hourly (10% of initial dose) or sooner if needed throughout the experiment to maintain areflexia. The animal was placed on a water-circulating heating pad to maintain body temperature (36.5–38.0°C), and the head was secured in a stereotaxic frame. The head was shaved, a local anesthetic (Carbocaine) was injected into the scalp, and a midsagittal incision was made in the skin to expose the underlying bone. A bilateral opening (2 mm posterior to Bregma, extending 4 mm anterior to Bregma, and 5 mm lateral) was made in the skull overlying SI in both hemispheres and the dura was removed from the brain surface. The brain surface was then covered with saline-soaked gauze to prevent drying. A recording chamber was constructed around the opening using dental cement, and the exposed cortices were bathed in warm silicone fluid (10,000 cs). A digital photograph of the cortical surface was taken and used to mark locations of electrode penetrations in the brain.
A carbon fiber electrode, attached to a Canberra-type microdrive, was inserted into layer V in SI to identify receptive fields in the forelimb representation. A second electrode was then inserted into layer V in the contralateral SI and used to identify a homotopic site having a receptive field similar to that of the opposite forelimb. Neuronal signals were fed into a preamplifier and subsequently amplified using a custom-built amplifier before being fed into an audio monitor and viewed on an oscilloscope. Receptive fields were initially measured using mechanical stimulation consisting of a hand-held blunt-tipped metal rod (00 gauge) attached to the end of a wooden dowel. A bipolar stimulating electrode fashioned from a pair of twisted silver wires (0.75 mm tip separation) was then placed bilaterally on the skin at the center of the receptive field and used to deliver current (1.5 × threshold, 1-ms pulse duration, 300 μm, 1 Hz) onto the skin surface to examine evoked response latency and contralateral and ipsilateral input. After initial testing, ipsilateral input to SI was examined at 30-min intervals throughout using ICMSr.
4.3. Microstimulation and corticocortical evoked response firing levels
Once the electrodes were inserted in homotopic sites in the two hemispheres, one electrode was used to deliver ICMS (cathodal pulse, 1.5 × threshold, 1-ms duration, 1 Hz), and the second electrode was used to record evoked responses. Slight adjustments in electrode depth were made to maximize the evoked responses using minimal current. Evoked responses to 20–50 consecutive stimulus pulses were recorded to establish baseline firing. ICMSr was then continued for periods between 1–5 hr, and increased spike activity, defined as an increase in number of evoked spikes (1.5 × baseline), was examined at 30-min intervals and at the end of stimulation to determine level of the evoked response.
4.4. Cortical inactivation by lidocaine and cooling
In two rats, a glass micropipette with a 20-μm opening was affixed to the tip of a 10-μL Hamilton syringe containing a 2% solution of lidocaine HCl. The pipette was then inserted into the previous stimulating site in SI, and 1 μL of lidocaine was pressure-injected into the SI. The pipette was then removed from the stimulating site in layer V, and 2 min later, the ipsilateral forelimb was stimulated again and evoked responses were re-examined in the ipsilateral SI. In one additional rat, inactivation was achieved by cooling the cortical surface over a physiologically identified stimulation site in SI by running cold water (~10°C) through a U-shaped metal tube which contacted a 1 mm2 area on the brain surface.
4.5. Histological processing
Selected stimulation and recording sites were identified by making electrolytic lesions in the brain using cathodal current (10 μA × 10 sec). Rats were administered a lethal dose of Nembutal (50 mg/kg, i.m.) and transcardially perfused with 0.9% saline followed by chilled 4% paraformaldehyde in 0.3 M sodium phosphate–buffered saline (NaPBS, pH 7.4, 21°C). Brains were removed, blocked, fixed in 4% paraformaldehyde at 4°C, and refrigerated overnight. The following day, tissue was sectioned in a coronal plane at 100-μm thickness using a vibratome. Sections were rinsed (3 × 10 min) with 0.01 M potassium phosphate–buffered saline (KPBS, pH 7.4, 21°C) and counterstained with cytochrome oxidase (CO). Tissue was then incubated in a diaminobenzidine (DAB)-sucrose-PBS mixture and placed in a warm water (38°C) bath until barrels were visible (Wong-Riley and Welt, 1980). Sections were rinsed (3 × 10 min) in buffer, mounted in distilled water on gelatin-coated glass slides, air dried overnight, and coverslipped.
4.6. Data analysis
Evoked responses to cortical and peripheral stimulation were recorded, stored on tape, and exported for offline analysis using a custom spike detection algorithm developed in MATLAB or in IGOR Pro using NeuroMatic (Rothman and Silver, 2018). During recording, spike records were fed into an A/D converter (Instrutech) and visualized online using IGOR Pro 6.20 (Wavemetrics) and on an oscilloscope screen. Responses to consecutive cortical stimulations were collected before, during, and at the conclusion of ICMSr. Each recorded response trace was bandpass filtered with low and high cutoff frequencies set to 300 Hz and 1000 Hz, respectively (Quiroga et al., 2004; Rutishauser et al., 2006). In both MATLAB and NeuroMatic analyses, spike detection was performed by applying an amplitude threshold to each filtered response trace. In both analyses, a voltage threshold was set during 10 ms of baseline recording and the same threshold level was used for all measures throughout the experiment. Spike activity histograms and raster plots were generated for t = 0–120 ms in 2-ms time bins. Onset of the evoked response was defined as having occurred when the number of post-stimulus spikes within a 2-ms time bin reached 1.5 × the average number of pre-stimulus spikes.
When testing for ipsilateral input, each recorded response trace was examined for evoked responses following ipsilateral forelimb stimulation. In cases where spontaneous or bursting neuronal activity was observed in the ipsilateral forelimb cortex, emphasis was placed on response activity with an onset latency of ≥16 ms to set the detection threshold apart from the contralateral forelimb input that generally appeared with an onset latency between 7–10 ms.
To examine the time course of interhemispheric pathway strengthening, a repeated-measures ANOVA and Student-Newman-Keuls post-comparison tests were used to assess statistical significance (p<0.05) of the changes in the number of spikes detected throughout the microstimulation period; changes in response duration and latency were also assessed.
4.7. Intracellular recording
In 1 rat, the extracellular recording electrode was replaced by an intracellular electrode filled with 1 M K-Acetate and 2% biocytin and reinserted into SI and used to impale a cell at the approximate location of the previous extracellular electrode; the details of the intracellular technique have been previously described in detail (Arnold et al., 2001; Li et al., 2002). The receptive field of the impaled cell, interhemispheric connectivity, and peripheral input were examined as previously described. Spike activity and ipsilateral input were then tested. At the end of the recording, biocytin was injected into the cell (500-ms pulse, 1 nA intensity, 1 Hz). The rat was then perfused with 4% paraformaldehyde, the brain removed, and refrigerated overnight at 4°C in 4% paraformaldehyde. The next day, the cortex was sectioned in a coronal plane into 100-micron thick sections, washed (3 ×) in 0.1% PBS at 10-min intervals at room temperature. Sections were transferred to Avidin Biotin Complex (Vector) for 3–4 hr, rinsed 3 × in buffer and placed in 0.5% DAB and 0.01% hydrogen peroxide for 10–15 min to reveal the labeled cell. Tissue was rinsed for 10 min in buffer and incubated in CO in a DAB-sucrose-PBS mixture for 2–3 hr. Sections were incubated in this mixture until the barrel field was darker than the background tissue. Sections were then washed 3 × in 0.1% PBS, mounted in distilled water on gelatin-coated slides, air dried overnight, and coverslipped using Permount. These procedures have been previously described (Arnold et al., 2001; Li and Waters, 1996; Li et al., 2002).
Highlights:
We describe interhemispheric connections between homotopic forelimb sites in layer V of rat primary somatosensory cortex (SI).
We show that repetitive intracortical microstimulation (ICMSr) of the interhemispheric pathway increases spike firing in homotopic sites in the opposite SI.
We show that strengthening of the interhemispheric pathway allows normally ineffective stimuli from the ipsilateral forelimb to excite cells in the ipsilateral SI.
We show that the newly expressed ipsilateral input is dependent upon an intact interhemispheric pathway.
5. Acknowledgments
This research was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke; grant number NS-055236 to R.S.W. The authors thank Ms. M. Waters for editing the manuscript. We thank Ms. S. Vemulapalli for assisting with surgical preparation and tissue processing. We also thank Dr. A. Kulkarni for technical assistance with the Whole Slide Imaging system and Aperio software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Akers RM, Killackey HP, 1978. Organization of corticocortical connections in the parietal cortex of the rat. J Comp Neurol. 181, 513–37. https://www.ncbi.nlm.nih.gov/pubmed/690276 [DOI] [PubMed] [Google Scholar]
- Angel A, Lemon RN, 1975. Sensorimotor cortical representation in the rat and the role of the cortex in the production of sensory myoclonic jerks. J Physiol. 248, 465–88. https://www.ncbi.nlm.nih.gov/pubmed/1151793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, George MJ, 1988. Bilateral receptive fields of cells in rat Sm1 cortex. Exp Brain Res. 70, 155–65. https://www.ncbi.nlm.nih.gov/pubmed/3402562 [DOI] [PubMed] [Google Scholar]
- Arnold PB, Li CX, Waters RS, 2001. Thalamocortical arbors extend beyond single cortical barrels: an in vivo intracellular tracing study in rat. Exp Brain Res. 136, 152–68. https://www.ncbi.nlm.nih.gov/pubmed/11206278 [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Keller A, 1995. LTP in the barrel cortex of adult rats. Neuroreport. 6, 2297–300. https://www.ncbi.nlm.nih.gov/pubmed/8747140 [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W, 1987. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 330, 649–52. https://www.ncbi.nlm.nih.gov/pubmed/2446147 [DOI] [PubMed] [Google Scholar]
- Artola A, Brocher S, Singer W, 1990. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 347, 69–72. https://www.ncbi.nlm.nih.gov/pubmed/1975639 [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Murphy KP, Pockett S, 1988. Postsynaptic control of the induction of long-term changes in efficacy of transmission at neocortical synapses in slices of rat brain. J Neurophysiol. 60, 1053–65. https://www.ncbi.nlm.nih.gov/pubmed/2845015 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T, 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 232, 331–56. https://www.ncbi.nlm.nih.gov/pubmed/4727084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova OG, Sil’kis IG, 2002. Post-tetanic modification of the efficiency of excitatory transmission in neural networks including interhemispheric connections. Neurosci Behav Physiol. 32, 15–24. https://www.ncbi.nlm.nih.gov/pubmed/11838551 [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R, 1990. Interhemispheric transfer of plasticity in the cerebral cortex. Science. 249, 805–7. https://www.ncbi.nlm.nih.gov/pubmed/2389146 [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ, 1988. Membrane potential changes in rat SmI cortical neurons evoked by controlled stimulation of mystacial vibrissae. Brain Res. 448, 186–91. https://www.ncbi.nlm.nih.gov/pubmed/3390715 [DOI] [PubMed] [Google Scholar]
- Chapin JK, Lin CS, 1984. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol. 229, 199–213. https://www.ncbi.nlm.nih.gov/pubmed/6438190 [DOI] [PubMed] [Google Scholar]
- Clarey JC, Tweedale R, Calford MB, 1996. Interhemispheric modulation of somatosensory receptive fields: evidence for plasticity in primary somatosensory cortex. Cereb Cortex. 6, 196–206. https://www.ncbi.nlm.nih.gov/pubmed/8670650 [DOI] [PubMed] [Google Scholar]
- Collins KL, Russell HG, Schumacher PJ, Robinson-Freeman KE, O’Conor EC, Gibney KD, Yambem O, Dykes RW, Waters RS, Tsao JW, 2018. A review of current theories and treatments for phantom limb pain. J Clin Invest. 128, 2168–2176. https://www.ncbi.nlm.nih.gov/pubmed/29856366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, Fabri M, Manzoni T, 1986. Bilateral receptive fields and callosal connectivity of the body midline representation in the first somatosensory area of primates. Somatosens Res. 3, 273–89. https://www.ncbi.nlm.nih.gov/pubmed/3775151 [DOI] [PubMed] [Google Scholar]
- Dancause N, Nudo RJ, 2011. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 192, 273–95. https://www.ncbi.nlm.nih.gov/pubmed/21763529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosta-Fortune TM, Li CX, de Jongh Curry AL, Waters RS, 2015. Differential Pattern of Interhemispheric Connections Between Homotopic Layer V Regions in the Forelimb Representation in Rat Barrel Field Cortex. Anat Rec (Hoboken). 298, 1885–902. https://www.ncbi.nlm.nih.gov/pubmed/26332205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinse HR, Recanzone GH, Merzenich MM, 1993. Alterations in correlated activity parallel ICMS-induced representational plasticity. Neuroreport. 5, 173–6. https://www.ncbi.nlm.nih.gov/pubmed/8111006 [DOI] [PubMed] [Google Scholar]
- Dreyer DA, Loe PR, Metz CB, Whitsel BL, 1975. Representation of head and face in postcentral gyrus of the macaque. J Neurophysiol. 38, 714–33. https://www.ncbi.nlm.nih.gov/pubmed/1127463 [DOI] [PubMed] [Google Scholar]
- Favorov OV, Whitsel BL, Chiu JS, Tommerdahl M, 2006. Activation of cat SII cortex by flutter stimulation of contralateral vs. ipsilateral forepaws. Brain Res. 1071, 81–90. https://www.ncbi.nlm.nih.gov/pubmed/16412394 [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E, 1995. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 375, 482–4. https://www.ncbi.nlm.nih.gov/pubmed/7777055 [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Muhlnickel W, Pantev C, Wienbruch C, Taub E, 1998. Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees. Exp Brain Res. 119, 205–12. https://www.ncbi.nlm.nih.gov/pubmed/9535570 [DOI] [PubMed] [Google Scholar]
- Flor H, Nikolajsen L, Staehelin Jensen T, 2006. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 7, 873–81. https://www.ncbi.nlm.nih.gov/pubmed/17053811 [DOI] [PubMed] [Google Scholar]
- Flor H, 2008. Maladaptive plasticity, memory for pain and phantom limb pain: review and suggestions for new therapies. Expert Rev Neurother. 8, 809–18. https://www.ncbi.nlm.nih.gov/pubmed/18457537 [DOI] [PubMed] [Google Scholar]
- Glazewski S, Herman C, McKenna M, Chapman PF, Fox K, 1998. Long-term potentiation in vivo in layers II/III of rat barrel cortex. Neuropharmacology. 37, 581–92. https://www.ncbi.nlm.nih.gov/pubmed/9704999 [DOI] [PubMed] [Google Scholar]
- Hayama T, Ogawa H, 1997. Regional differences of callosal connections in the granular zones of the primary somatosensory cortex in rats. Brain Res Bull. 43, 341–7. https://www.ncbi.nlm.nih.gov/pubmed/9227846 [DOI] [PubMed] [Google Scholar]
- Henry EC, Catania KC, 2006. Cortical, callosal, and thalamic connections from primary somatosensory cortex in the naked mole-rat (Heterocephalus glaber), with special emphasis on the connectivity of the incisor representation. Anat Rec A Discov Mol Cell Evol Biol. 288, 626–45. https://www.ncbi.nlm.nih.gov/pubmed/16652365 [DOI] [PubMed] [Google Scholar]
- Heusler P, Cebulla B, Boehmer G, Dinse HR, 2000. A repetitive intracortical microstimulation pattern induces long-lasting synaptic depression in brain slices of the rat primary somatosensory cortex. Exp Brain Res. 135, 300–10. https://www.ncbi.nlm.nih.gov/pubmed/11146808 [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Manzoni T, Spidalieri G, 1973. Relevance of the callosal transfer in defining the peripheral reactivity of somesthetic cortical neurones. Arch Ital Biol. 111, 187–221. https://www.ncbi.nlm.nih.gov/pubmed/18843823 [PubMed] [Google Scholar]
- Iwamura Y, Iriki A, Tanaka M, 1994. Bilateral hand representation in the postcentral somatosensory cortex. Nature. 369, 554–6. https://www.ncbi.nlm.nih.gov/pubmed/8202155 [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Iriki A, Tanaka M, Toda T, 1996. Bilateral receptive field neurons in the postcentral gyrus: two hands meet the midline In Perception, memory and emotion: frontier in neuroscience. Vol., Ono T, McNaughton BL, Molotchnikoff S, Rolls ET, Nishijo H, ed.êds. Elsevier, Oxford, UK, pp. 33–44. [Google Scholar]
- Kalarickal GJ, Marshall JA, 2002. Rearrangement of receptive field topography after intracortical and peripheral stimulation: the role of plasticity in inhibitory pathways. Network. 13, 1–40. https://www.ncbi.nlm.nih.gov/pubmed/11873840 [PubMed] [Google Scholar]
- Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H, 2001. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci. 21, 3609–18. https://www.ncbi.nlm.nih.gov/pubmed/11331390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek KA, Olavarria J, Killackey HP, 1990. Areal and laminar organization of corticocortical projections in the rat somatosensory cortex. J Comp Neurol. 299, 133–50. https://www.ncbi.nlm.nih.gov/pubmed/2172324 [DOI] [PubMed] [Google Scholar]
- Korvenoja A, Wikstrom H, Huttunen J, Virtanan J, Laine P, Aronen HJ, Seppalainen AM, Ilmoniemi RJ, 1995. Activation of ipsilateral primary sensorimotor cortex by median nerve stimulation. Neuroreport. 6, 2589–93. https://www.ncbi.nlm.nih.gov/pubmed/8741769 [DOI] [PubMed] [Google Scholar]
- Lee SM, Weisskopf MG, Ebner FF, 1991. Horizontal long-term potentiation of responses in rat somatosensory cortex. Brain Res. 544, 303–10. https://www.ncbi.nlm.nih.gov/pubmed/1828185 [DOI] [PubMed] [Google Scholar]
- Lee SM, Ebner FF, 1992. Induction of high frequency activity in the somatosensory thalamus of rats in vivo results in long-term potentiation of responses in SI cortex. Exp Brain Res. 90, 253–61. https://www.ncbi.nlm.nih.gov/pubmed/1397139 [DOI] [PubMed] [Google Scholar]
- Li CX, Waters RS, 1996. In vivo intracellular recording and labeling of neurons in the forepaw barrel subfield (FBS) of rat somatosensory cortex: possible physiological and morphological substrates for reorganization. Neuroreport. 7, 2261–72. https://www.ncbi.nlm.nih.gov/pubmed/8951838 [DOI] [PubMed] [Google Scholar]
- Li CX, Callaway JC, Waters RS, 2002. Removal of GABAergic inhibition alters subthreshold input in neurons in forepaw barrel subfield (FBS) in rat first somatosensory cortex (SI) after digit stimulation. Exp Brain Res. 145, 411–28. https://www.ncbi.nlm.nih.gov/pubmed/12172653 [DOI] [PubMed] [Google Scholar]
- Li L, Rema V, Ebner FF, 2005. Chronic suppression of activity in barrel field cortex downregulates sensory responses in contralateral barrel field cortex. J Neurophysiol. 94, 3342–56. https://www.ncbi.nlm.nih.gov/pubmed/16014795 [DOI] [PubMed] [Google Scholar]
- Lipton ML, Fu KM, Branch CA, Schroeder CE, 2006. Ipsilateral hand input to area 3b revealed by converging hemodynamic and electrophysiological analyses in macaque monkeys. J Neurosci. 26, 180–5. https://www.ncbi.nlm.nih.gov/pubmed/16399685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Sakmann B, Brecht M, 2004. Sub- and suprathreshold receptive field properties of pyramidal neurones in layers 5A and 5B of rat somatosensory barrel cortex. J Physiol. 556, 601–22. https://www.ncbi.nlm.nih.gov/pubmed/14724202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni T, Barbaresi P, Bellardinelli E, Caminiti R, 1980. Callosal projections from the two body midlines. Exp Brain Res. 39, 1–9. https://www.ncbi.nlm.nih.gov/pubmed/7379877 [DOI] [PubMed] [Google Scholar]
- Manzoni T, Barbaresi P, Conti F, Fabri M, 1989. The callosal connections of the primary somatosensory cortex and the neural bases of midline fusion. Exp Brain Res. 76, 251–66. https://www.ncbi.nlm.nih.gov/pubmed/2670598 [DOI] [PubMed] [Google Scholar]
- Medeiros LF, de Souza IC, Vidor LP, de Souza A, Deitos A, Volz MS, Fregni F, Caumo W, Torres IL, 2012. Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry. 3, 110 https://www.ncbi.nlm.nih.gov/pubmed/23293607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Nelson SB, 1998. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol. 80, 2882–92. https://www.ncbi.nlm.nih.gov/pubmed/9862892 [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Davies PW, Berman AL, 1957. Response properties of neurons of cat’s somatic sensory cortex to peripheral stimuli. J Neurophysiol. 20, 374–407. https://www.ncbi.nlm.nih.gov/pubmed/13439409 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A, 2009. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol. 219, 14–9. https://www.ncbi.nlm.nih.gov/pubmed/19348793 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Ito S, Nomura T, 1989. Oral cavity representation at the frontal operculum of macaque monkeys. Neurosci Res. 6, 283–98. https://www.ncbi.nlm.nih.gov/pubmed/2725988 [DOI] [PubMed] [Google Scholar]
- Olavarria J, Van Sluyters RC, Killackey HP, 1984. Evidence for the complementary organization of callosal and thalamic connections within rat somatosensory cortex. Brain Res. 291, 364–8. https://www.ncbi.nlm.nih.gov/pubmed/6697197 [DOI] [PubMed] [Google Scholar]
- Pearson PP, Li CX, Waters RS, 1999. Effects of large-scale limb deafferentation on the morphological and physiological organization of the forepaw barrel subfield (FBS) in somatosensory cortex (SI) in adult and neonatal rats. Exp Brain Res. 128, 315–31. https://www.ncbi.nlm.nih.gov/pubmed/10501804 [DOI] [PubMed] [Google Scholar]
- Pidoux B, Verley R, 1979. Projections on the cortical somatic I barrel subfield from ipsilateral vibrissae in adult rodents. Electroencephalogr Clin Neurophysiol. 46, 715–26. https://www.ncbi.nlm.nih.gov/pubmed/87318 [DOI] [PubMed] [Google Scholar]
- Pluto CP, Chiaia NL, Rhoades RW, Lane RD, 2005. Reducing contralateral SI activity reveals hindlimb receptive fields in the SI forelimb-stump representation of neonatally amputated rats. J Neurophysiol. 94, 1727–32. https://www.ncbi.nlm.nih.gov/pubmed/15800076 [DOI] [PubMed] [Google Scholar]
- Quairiaux C, Armstrong-James M, Welker E, 2007. Modified sensory processing in the barrel cortex of the adult mouse after chronic whisker stimulation. J Neurophysiol. 97, 2130–47. https://www.ncbi.nlm.nih.gov/pubmed/17122325 [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Nadasdy Z, Ben-Shaul Y, 2004. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 16, 1661–87. https://www.ncbi.nlm.nih.gov/pubmed/15228749 [DOI] [PubMed] [Google Scholar]
- Ramshur JT, DeCosta-Fortune T, C-X. L, deJongh Curry AL, Waters RS, 2013. Functional modulation of deafferented forelimb barrel cortex in rat In Society for Neuroscience 42, Annual Meeting. Vol., ed.êds., San Diego, CA. [Google Scholar]
- Ramshur JT, Morshed BI, de Jongh Curry AL, Waters RS, 2019. Telemetry-controlled simultaneous stimulation-and-recording device (SRD) to study interhemispheric cortical circuits in rat primary somatosensory (SI) cortex. BMC Biomedical Engineering. 1, 19 10.1186/s42490-019-0019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Dinse HR, 1992. Expansion of the cortical representation of a specific skin field in primary somatosensory cortex by intracortical microstimulation. Cereb Cortex. 2, 181–96. https://www.ncbi.nlm.nih.gov/pubmed/1511220 [DOI] [PubMed] [Google Scholar]
- Rema V, Ebner FF, 2003. Lesions of mature barrel field cortex interfere with sensory processing and plasticity in connected areas of the contralateral hemisphere. J Neurosci. 23, 10378–87. https://www.ncbi.nlm.nih.gov/pubmed/14614097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JS, Silver RA, 2018. NeuroMatic: An Integrated Open-Source Software Toolkit for Acquisition, Analysis and Simulation of Electrophysiological Data. Front Neuroinform. 12, 14 https://www.ncbi.nlm.nih.gov/pubmed/29670519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Schuman EM, Mamelak AN, 2006. Online detection and sorting of extracellularly recorded action potentials in human medial temporal lobe recordings, in vivo. J Neurosci Methods. 154, 204–24. https://www.ncbi.nlm.nih.gov/pubmed/16488479 [DOI] [PubMed] [Google Scholar]
- Sah P, Nicoll RA, 1991. Mechanisms underlying potentiation of synaptic transmission in rat anterior cingulate cortex in vitro. J Physiol. 433, 615–30. https://www.ncbi.nlm.nih.gov/pubmed/1688165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Porter LL, Asanuma H, 1987. Long-lasting potentiation of synaptic potentials in the motor cortex produced by stimulation of the sensory cortex in the cat: a basis of motor learning. Brain Res. 413, 360–4. https://www.ncbi.nlm.nih.gov/pubmed/3607486 [DOI] [PubMed] [Google Scholar]
- Schwarz DW, Fredrickson JM, 1971. Tactile direction sensitivity of area 2 oral neurons in the rhesus monkey cortex. Brain Res. 27, 397–401. https://www.ncbi.nlm.nih.gov/pubmed/4994681 [DOI] [PubMed] [Google Scholar]
- Shin HC, Won CK, Jung SC, Oh S, Park S, Sohn JH, 1997. Interhemispheric modulation of sensory transmission in the primary somatosensory cortex of rats. Neurosci Lett. 230, 137–9. https://www.ncbi.nlm.nih.gov/pubmed/9259483 [DOI] [PubMed] [Google Scholar]
- Shuler MG, Krupa DJ, Nicolelis MA, 2001. Bilateral integration of whisker information in the primary somatosensory cortex of rats. J Neurosci. 21, 5251–61. https://www.ncbi.nlm.nih.gov/pubmed/11438600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits E, Gordon DC, Witte S, Rasmusson DD, Zarzecki P, 1991. Synaptic potentials evoked by convergent somatosensory and corticocortical inputs in raccoon somatosensory cortex: substrates for plasticity. J Neurophysiol. 66, 688–95. https://www.ncbi.nlm.nih.gov/pubmed/1753280 [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Hicks TP, 1996. Somatosensory cortical efferent neurons of the awake rabbit: latencies to activation via supra--and subthreshold receptive fields. J Neurophysiol. 75, 1753–9. https://www.ncbi.nlm.nih.gov/pubmed/8727411 [DOI] [PubMed] [Google Scholar]
- Swadlow HA, 2003. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex. 13, 25–32. https://www.ncbi.nlm.nih.gov/pubmed/12466212 [DOI] [PubMed] [Google Scholar]
- Taoka M, Toda T, Iriki A, Tanaka M, Iwamura Y, 2000. Bilateral receptive field neurons in the hindlimb region of the postcentral somatosensory cortex in awake macaque monkeys. Exp Brain Res. 134, 139–46. https://www.ncbi.nlm.nih.gov/pubmed/11037280 [DOI] [PubMed] [Google Scholar]
- Teskey GC, Young NA, van Rooyen F, Larson SE, Flynn C, Monfils MH, Kleim JA, Henry LC, Goertzen CD, 2007. Induction of neocortical long-term depression results in smaller movement representations, fewer excitatory perforated synapses, and more inhibitory synapses. Cereb Cortex. 17, 434–42. https://www.ncbi.nlm.nih.gov/pubmed/16547346 [DOI] [PubMed] [Google Scholar]
- Thomson AM, 1986. Comparison of responses to transmitter candidates at an N-methylaspartate receptor mediated synapse, in slices of rat cerebral cortex. Neuroscience. 17, 37–47. https://www.ncbi.nlm.nih.gov/pubmed/2870443 [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Simons SB, Chiu JS, Favorov O, Whitsel BL, 2006. Ipsilateral input modifies the primary somatosensory cortex response to contralateral skin flutter. J Neurosci. 26, 5970–7. https://www.ncbi.nlm.nih.gov/pubmed/16738239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutunculer B, Foffani G, Himes BT, Moxon KA, 2006. Structure of the excitatory receptive fields of infragranular forelimb neurons in the rat primary somatosensory cortex responding to touch. Cereb Cortex. 16, 791–810. https://www.ncbi.nlm.nih.gov/pubmed/16120794 [DOI] [PubMed] [Google Scholar]
- Waters RS, Li CX, McCandlish CA, 1995. Relationship between the organization of the forepaw barrel subfield and the representation of the forepaw in layer IV of rat somatosensory cortex. Exp Brain Res. 103, 183–97. https://www.ncbi.nlm.nih.gov/pubmed/7789426 [DOI] [PubMed] [Google Scholar]
- Welker C, 1971. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 26, 259–75. https://www.ncbi.nlm.nih.gov/pubmed/4100672 [PubMed] [Google Scholar]
- White EL, DeAmicis RA, 1977. Afferent and efferent projections of the region in mouse SmL cortex which contains the posteromedial barrel subfield. J Comp Neurol. 175, 455–82. https://www.ncbi.nlm.nih.gov/pubmed/915034 [DOI] [PubMed] [Google Scholar]
- Wiest MC, Bentley N, Nicolelis MA, 2005. Heterogeneous integration of bilateral whisker signals by neurons in primary somatosensory cortex of awake rats. J Neurophysiol. 93, 2966–73. https://www.ncbi.nlm.nih.gov/pubmed/15563555 [DOI] [PubMed] [Google Scholar]
- Wise SP, Jones EG, 1976. The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J Comp Neurol. 168, 313–43. https://www.ncbi.nlm.nih.gov/pubmed/950383 [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C, 1980. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci U S A. 77, 2333–7. https://www.ncbi.nlm.nih.gov/pubmed/6246540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorke CH Jr., Caviness VS Jr., 1975. Interhemispheric neocortical connections of the corpus callosum in the normal mouse: a study based on anterograde and retrograde methods. J Comp Neurol. 164, 233–45. https://www.ncbi.nlm.nih.gov/pubmed/1184784 [DOI] [PubMed] [Google Scholar]


