Abstract
When breast cancer is detected and treated early, the chances of survival are very high. However, women in many settings face complex barriers to early detection, including social, economic, geographic and other inter-related factors, which can limit her access to timely, affordable, and effective breast health care services. Previously, the Breast Health Global Initiative (BHGI) developed resource-stratified guidelines for early detection and diagnosis of breast cancer. In this consensus paper from the 6th BHGI Global Summit held in October 2018, we describe “phases” of early detection program development, beginning with management strategies required for the diagnosis of clinically detectable disease based on awareness education and technical training, history and physical examination and accurate tissue diagnosis. We address core issues, including finance and governance, which pertain to successful planning, implementation and the iterative process of program improvement and are needed for a breast cancer early detection program to succeed in any resource setting. We present examples of implementation, process, and clinical outcome metrics that assist in program implementation monitoring. Country case examples are presented to highlight the challenges and opportunities of implementing successful breast cancer early detection programs, as we consider the complex interplay of barriers and facilitators to achieving early detection for breast cancer in real world settings.
Keywords: Phased implementation, breast cancer, resource stratification, breast cancer early detection, metrics
Precis
Women in many settings face complex barriers to early detection, including social, economic, geographic and other inter-related factors, which can limit her access to timely, affordable, and effective breast health care services. Here, we present “phases” of early detection program development, beginning with management strategies required for the diagnosis of clinically detectable disease, and address core issues pertaining to successful planning, implementation and the iterative process of program improvement, needed for a breast cancer early detection program to succeed in any resource setting.
Introduction
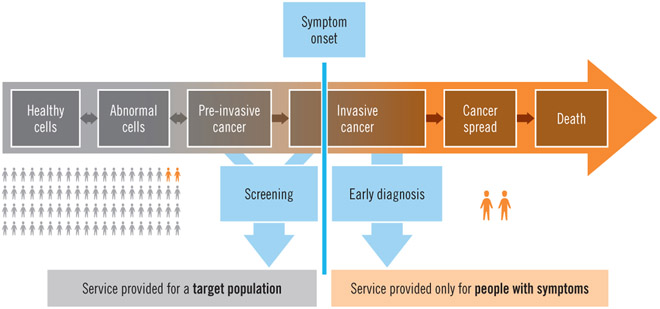
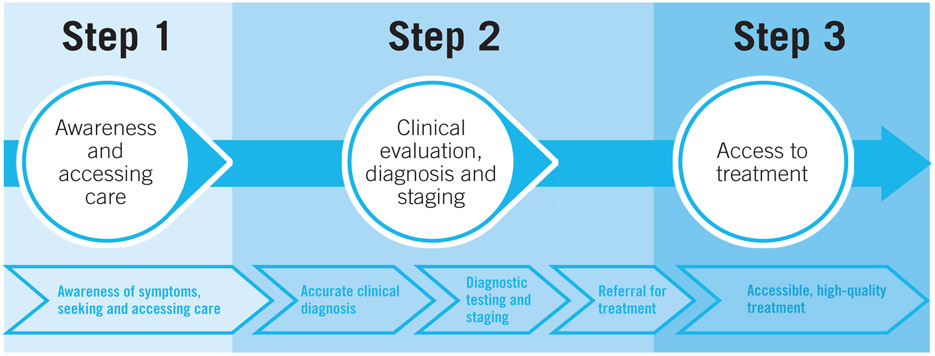
The World Health Organisation has defined two distinct but related strategies to promote the early detection of cancer, early diagnosis, that is, the recognition of symptomatic cancer at an early stage; and screening, that is the identification of asymptomatic disease in a target population of apparently healthy individuals1 (Figure 1). In low income and middle income countries (LMICs), a large proportion of women with breast cancer present or are ultimately diagnosed with later-stage (locally advanced or metastatic) disease.2 In such settings, efforts to promote early diagnosis are a necessary prerequisite to population-based screening, as early diagnosis will improve outcomes for all breast cancer patients whereas less than half of breast cancers are screen-diagnosed even in the most effective screening programs. As such, early diagnosis efforts should initially be prioritized over opportunistic or organized, population-based screening, until both infrastructure and organizational requirements for screening are in place to consider this additional activity. Health planners, policymakers, and other stakeholders including clinicians, educators, community members and advocates should be aware of the health system requirements as well as overall costs of these approaches to breast cancer early detection, in order to make effective investments, plans, and policies.
Figure 1.
Distinguishing screening from early diagnosis according to symptom onset (WHO Guide to Cancer Early Diagnosis 2017)
Resource-stratified guidelines for early detection of breast cancer were developed as a framework by the Breast Health Global Initiative (BHGI).3, 4 Here we expand on this work to develop a more nuanced framework for health planners and policymakers. We describe “phases” of early detection program development, beginning with management strategies required for the diagnosis of clinically detectable disease, based on history and physical examination. In general, each of the phases requires continuous evaluation and improvement to establish and maintain quality; however, phased implemenation is based on the premise that there are both prerequisites and a specific order to the implementation and scale up of certain interventions in order to advance high-quality breast health care. Phases can be implemented sequentially (in series) or in an overlapping fashion (in parallel) depending on the specific environment in which implementation is taking place. Figure 2 presents an overview of this approach, which is descibed in more detail in the subsequent sections.
Figure 2. Overview of Implementation Phases for Early diagnosis and Detection Pathways.
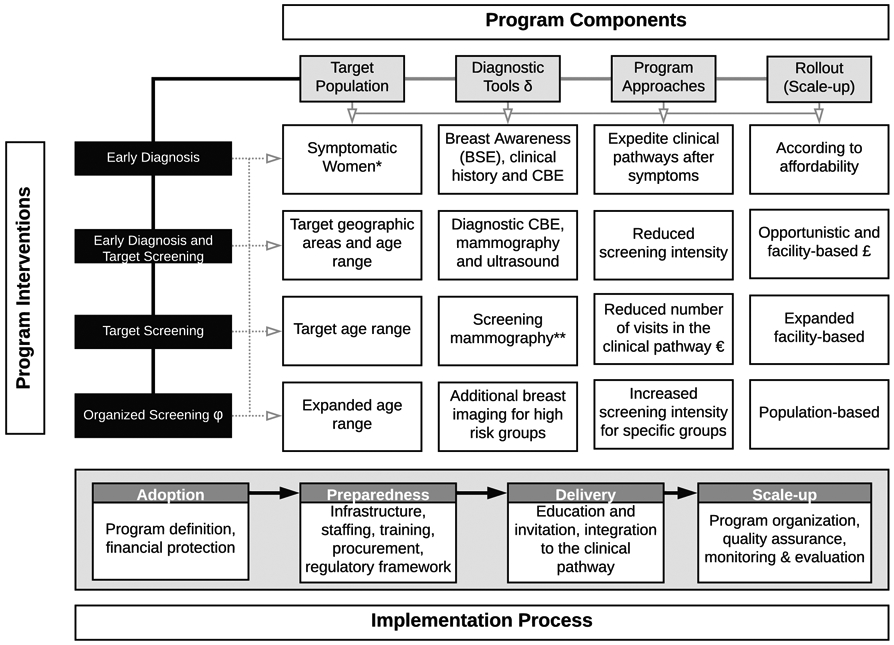
(δ) Pathology services as the basis for breast cancer diagnosis. (*) Regardless of age or domicile. (**) Some middle-income countries introduce clinical breast examination (CBE) combined with mammography to reduce mammography intensity or as stand-alone test for expanded age groups £) Systematic screening offered to women attending health services for any reason, including response to media campaigns promoting breast cancer early detection €) Definition of number of visits in the clinical pathway (one to three): screening, complementary studies, diagnosis. (ϕ) Organized screening as opposed to opportunistic screening. As early detection programs are successfully implemented, early diagnosis services need to be continually supported for all women.
We address some of the core feasibility issues including those regarding finance and governance, that are key to effective planning and implementation of effective breast cancer early detection programs, as well as an iterative process of program improvement, necessary for success in any resource setting. We also present examples of implementation, process, and clinical outcome metrics that allow measurement of program feasability, implementation adoption, and success, among others.. Country case examples are presented to highlight the challenges and opportunities, and we consider the complex interplay of barriers and facilitators to achieving early detection for breast cancer in real world settings.
Implementation phases:
I. Early Diagnosis: management of clinically detectable disease
A primary challenge to the successful implementation of any breast cancer program is the ability to manage clinically detectable disease; and to do so in an equitable manner for the target population, that is, for all adult women with signs and/or symptoms suggestive of breast cancer. A significant proportion of breast cancer in LMICs is diagnosed at an advanced stage (AJCC stage III or IV), ranging from 30–50% in Latin America to 75% in Sub-Saharan Africa.5, 6 The great majority of these advanced cancers are initially detected by the patient herself based on changes that she appreciates as a lump, thickening or other progressive change. 7, 8 Once she presents to the healthcare system with signs and/or symptoms in the breast, diagnostic services need to be available such that a prompt and accurate diagnosis (benign versus malignant) can be provided.
The capacity to effectively diagnose and treat clinically detectable breast cancer begins with clinical breast assessment (CBA) by taking a medical history and performing a focused physical examination including clinical breast exam (CBE). CBE is followed by diagnostic imaging, and tissue sampling with pathologic evaluation, the so-called “triple test” of breast diagnosis.9 As detailed in previous BHGI publications10,4 and explored further in this series [treatment consensus paper], prompt diagnosis followed by surgery (at least a quality modified radical mastectomy) and systemic therapy (chemotherapy and endocrine therapy as appropriate) must be affordable for patients and accessible in a timely manner. Availability of medication for pain and symptom management is also imperative 11 . Only after these essential diagnostic and treatment modalities are available should more advanced imaging and management options, such as breast conserving surgery, radiotherapy, or additional targeted systemic therapy, be considered.12
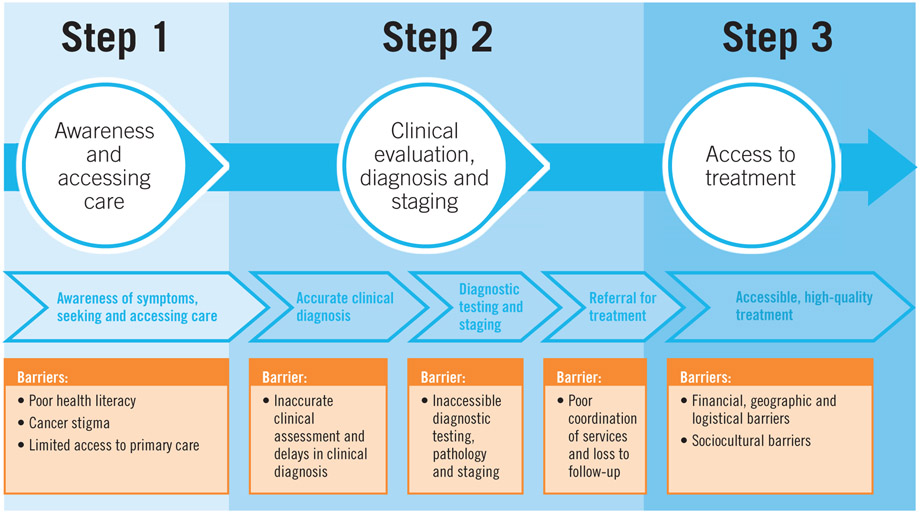
Delays to breast cancer treatment of greater than three months have been associated with more advanced disease stage at diagnosis and poorer survival.7, 13 At the same time, education of primary care providers to recognize the early signs and symptoms of breast cancer is necessary for prompt referral through the healthcare system. Barriers to care should be identified and addressed. These are complex and multifactorial, including structural, sociocultural, personal and financial factors that can influence a woman’s opportunities to seek and receive care.14 Even when a patient seeks care soon after the onset of symptoms (i.e., “early presentation”), this does not always translate to early diagnosis. For example, if the provider she first sees (or even subsequently sees) does not have the appropriate training or knowledge to recognize an early breast cancer, does not know where or how to refer for necessary diagnostic intervention(s), and/or the health system is fragmented in a way that prevents the patient from making her way through the entire care pathway, diagnostic delay will result. Figure 3 provides an overview of interventions or strategies to overcome common barriers to early diagnosis.
Figure 3a.
Common Barriers to Early Diagnosis
Once high-quality, accessible services are in place to diagnose and treat clinically apparent disease, early detection in the form of screening programs can then be considered in addition to continuing to ensure effective early-diagnosis for all women. If a screening program, however well intentioned, is introduced into a healthcare system that is not equipped to refer, diagnose and treat the abnormalities it detects, the program will not succeed and may be counterproductive if it reinforces pre-existing beliefs that cancer cannot be cured, thereby perpetuating a cycle of late presentation.
II. Early Diagnosis: Management of image-detected disease
Breast imaging, if available, is used to evaluate women with breast symptoms or suspicious clinical findings. Ultrasound is portable, valuable in the assessment of breast masses, and has uses beyond breast imaging, making it more widely available than mammography in LMICs.15, 16 However, ultrasound is highly operator dependent. If used for screening, rather than for the assessment of palpable disease, it has the potential for a high false negative rate.17
In contrast, mammography has a high specificity.18 However, mammography has reduced sensitivity in women with high breast density. Mammogram machines are expensive, and their only application is in breast imaging, limiting their accessibility in LMICs. For mammography to be effective, whether for screening or diagnostic purposes, the health system must support training for radiologists and radiographers with ongoing quality control, patient tracking and effective communication for patient follow-up, and provider feedback, all associated with significant and ongoing operating costs.
Diagnostic (or “targeted”) ultrasound is indicated as the sole imaging test to evaluate women less than 30 years of age, with focal breast signs and symptoms, and is seen as equivalent to mammography in women 30–39 years at an average risk for breast cancer, based on family history.19 Ultrasound can distinguish between cysts, probably benign masses, and suspicious masses. This imaging modality is less affected by breast density than is mammography, which makes it the preferred imaging tool in younger women who more commonly have dense breast tissue. While mammography shows similar diagnostic accuracy to ultrasound in women 30–39 years, ultrasound is preferred because it has potential to identify treatable causes of symptoms (e.g. a cyst), and does not use radiation. Ultrasound is also useful in women 40 years and older, either as the only available imaging modality in cases where mammography is not available, or as an adjunct to mammography for diagnostic work-up including evaluation of the axilla.
Diagnostic mammography is indicated for women 40 years and older with breast signs and symptoms, and has the added benefit of simultaneously screening for breast cancer unrelated to the presenting symptom.19 Mammography and ultrasound are used in combination to characterize masses as likely benign or suspicious. Ultrasound is indicated for findings seen on the mammogram with “probably benign” features to determine if a benign-appearing cyst versus mass is present. “Probably benign” masses can be followed by repeated imaging studies at intervals (typically 6 months). Ultrasound is also indicated to further evaluate mammographic findings that are suspicious, and to determine if an ultrasound-guided biopsy can be performed. If mammography is not available, then ultrasound should be performed. Medical imaging, while reassuring, is not perfect. Women with a negative imaging work-up (i.e. no findings or benign findings only) should be followed clinically. In some cases when a clinician has concerns regarding apparent discordance between the physical examination and imaging findings, a surgical biopsy should be performed.
There are three basic methods for tissue sampling of a mass or other abnormality detected by physical examination or imaging: namely, fine needle aspiration (FNA), core biopsy, and excisional biopsy,4 each with differing characteristics in terms of sensitivity, specificity, positive and negative predictive value, and have different training and health system requirements. It should be noted that excisional biopsy should not be done routinely as a (first) diagnostic procedure.
III. Population-based screening
There is limited evidence of efficacy for CBE as a population-based screening modality20 in settings where mammography is not routinely performed. Despite several studies demonstrating clinical downstaging,21 none have yet demonstrated improved breast cancer specific survival (in any time-frame) or a reduction in mortality. However, important caveats remain. If clinical down-staging can be achieved with screening by CBE, mortality might be reduced, assuming timely and high-quality diagnostic services coupled with effective treatment and follow-up care are readily available, accessible, and affordable. Although the WHO does not recommend population-based organized screening with CBE in any resource setting, in the absence of well-organized mammogram-based screening programs, CBE is considered a reasonable approach in a lower resource setting, provided it is evaluated in a research context.22 A recent cross-sectional study of women with newly diagnosed breast cancer in Peru8 showed that women who had undergone a previous CBE (unrelated to their current diagnosis) had shorter delays from symptom development to presentation, and were more likely to be diagnosed with earlier stage disease (AJCC Stage 0, I, II) compared with women who had never had a CBE. This also suggests that CBE as part of comprehensive breast health awareness may have value in improving the opportunities for early diagnosis of a (potential) future breast cancer.
A recent study from Brazil demonstrated that early detection policies introduced in 2004, which included raising public awareness and implementing screening with CBE and mammography, was not associated with a shift from late to early stage disease. It was estimated that in 2012, 2,500 breast cancer deaths could have been averted by effective mammographic screening. However, they estimated that if 50–80% of patients diagnosed at stage 3 or 4 in the previous five years had been downstaged to stage 2, 8000 deaths could have been prevented.23 The authors conclude that clinical downstaging would have greater impact that mammographic screening on breast cancer deaths in settings where women present with late-stage disease. This highlights the need for further research to understand and overcome barriers to early diagnosis of breast cancer.24
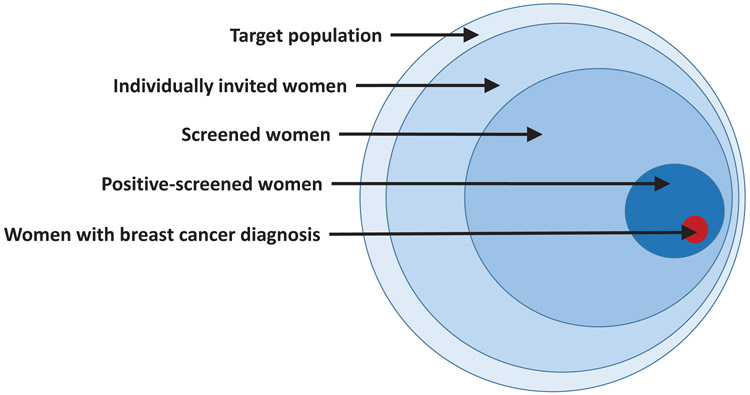
In contrast to screening with CBE, population-based screening with mammography has been associated with significant reductions of about 20% in breast cancer mortality from studies based on high income countries where such data are available. 20, 22, 25 However, for population-based screening with mammography to succeed in reducing mortality, many criteria must be met, including individual identification of every person in the target population, individual invitation, and individual follow-up throughout the whole clinical pathway to ensure access to the screening, diagnostic, and treatment procedures (Figure 4). In addition, a strong health system, sustainable financing, and number of systems requirements including quality control, feedback, monitoring and evaluation criteria must be in place.
Figure 4.
Paradigm of early detection via population-based screening
Irrespective of screening modality, the development of a population-based screening program should be considered within the framework of a national cancer control plan and within the national health financing strategy. The financial cost both at a national and societal level is considerable, beginning with the costs of mammography, and including supportive and programmatic costs as outlined above, and should be balanced against competing health priorities.26
Offering appropriate diagnostic and treatment services involves ensuring geographic access, which is determined by the available infrastructure and workforce. Some programs have used mobile units to improve access to screening and diagnosis; 27 however, evidence of the effectiveness of these interventions is currently limited.28
Situational analysis
Breast cancer survival varies widely across the globe, and is strongly associated with a country’s GDP as well as its public spending on health.29 Complex mechanisms underlie the contribution of human development —national income, life expectancy, education and/or fertility rates— in conjunction with the strength of the health system, to a woman’s likelihood of experiencing long-term survival from breast cancer.30 However, stage at diagnosis can be considered as a starting point, the first measurable factor that most directly influences survival.
A recent review of cancer control plans in 158 countries reported that that there were fewer breast cancer early detection programs in LMICs compared to HICs.31 Even in HICs, there are large cancer health disparities, including access to early detection, diagnosis, and treatment for women with breast cancer.32-35 Ineffective and redundant referral pathways cause system delays and are a major contributor to cancer disparities worldwide.36 Delays from symptom onset to diagnosis range from weeks to many months in sub-Saharan Africa. 37 Less than half of cancer plans recently surveyed elaborate the role of primary care physicians and referral pathways in early breast cancer diagnosis.31 Untrained health workers are more likely to misdiagnose cancer.38 Thus the importance of both system and patient-related delays cannot be underestimated.
Expanding cancer awareness or early diagnosis programs without planning concurrent expansion of diagnostic and treatment facilities results in dismal outcomes, by increasing the number of women presenting with disease at the health system, which lacks the capacity to diagnose and treat them effectively. This negates the benefits of early detection, builds mistrust of the public health system, increases reliance on unorthodox treatment methods, and forces patients to seek high-cost care in the private sector. An earlier review from Thailand found that despite an increase in the number of mammograms available, the majority were in private facilities demanding high out of pocket payment thereby overstretching the few in public facilities.39
Besides geographic, financial, and other structural factors, access to breast cancer early detection is also determined by the quantity and quality of human resources for health. However, human resource needs for breast cancer screening or early diagnosis depend upon national policies and guidelines. The availability of human resources for health depends on the capacity of the education system to produce various cadres of providers, and the ability of the health system to attract, motivate and retain them. 40 Policymakers must consider the future demand created by a growing and ageing population, combined with a rising incidence of breast and other cancers, as part of developing a long-term plan for the workforce. The prime focus for workforce staffing should be to strengthen diagnostic skills in primary care,41 and introduce essential diagnostic tools comprising CBE, breast imaging (mammography and ultrasound), and histopathological capacity. 42 Task-sharing and task-shifting approaches have been used successfully in different settings to surmount the workforce shortage for breast cancer early detection, including training, centralized services. Additional support can be supplied through digital or ‘e-health’ and telemedicine.40, 43-47
In general terms, all diagnostic tools are performer-dependent, thus prompting a trade-off between access and accuracy: the greater the level of performer training the greater the accuracy and the lower the access. 48, 49 No data on early diagnosis of symptomatic disease are available, but it supposes the need of strengthening diagnostic skills (not screening) throughout the health system 41 with a lower burden for diagnostic services (breast imaging and pathology), making this approach more suitable for low-income settings [WHO]. Indeed, data from high-income countries show an increasing demand and a shortage of trained workforce for breast imaging in both organized and opportunistic screening. 50, 51
The BCI2.5 Toolkit for Breast Cancer Situational Analyses
The Breast Cancer Initiative 2.5 (BCI2.5) 52 is a global campaign to reduce disparities in breast cancer outcomes and improve access to breast health care worldwide. It is not an institution itself, but a mechanism for collaboration, advocacy, and information dissemination to increase the effectiveness of independent and collective efforts while catalysing greater global investment and commitment to breast health care. Since 2014, collaborations under BCI2.5 have produced educational resources and reports to assist policymakers and health planners, “identifying bottlenecks in breast health care delivery, and determining appropriate interventions in specific settings”. The BCI2.5 self-assessment toolkit developed by BHGI can help countries conduct a comprehensive breast healthcare situational analysis 53
We present examples of five different scenarios to highlight the challenges faced and opportunities to achieve early detection for breast cancer in a target population. In an underserved community in the Appalachian Ohio, a breast cancer early detection programme allows a woman to undergo screening mammography and uses patient navigation to ensure that any abnormalities are followed up until resolution [Box 1]. In China [Box 2], The Eastern Michigan University Centre for Health Disparities received a grant to increase breast cancer awareness and early detection in six provinces in China. This program trained 2000 breast cancer survivors to be breast health ambassadors, as well as 800 health care providers, with support from multiple stakeholders to ensure treatment of positively screened patients. However, the impact of this program has not yet been evaluated. The Mexico case [Box 3] shows that despite specific efforts of the government, civil society, and academia, there continues to be challenges to achieving early detection for women with breast cancer. It is also a salient example of how efforts, however well-intentioned, to improve breast cancer early detection, can be ineffective and even wasteful. In Panama, low participation in a screening program prompted the Ministry of Health to conduct a situational analysis, and found that diagnostic delays occur in almost every step from referral to biopsy, and an implementation strategy to reduce delay is urgently required [Box 4]. Lastly, the Tanzania case [Box 5] provides a description of the BCI2.5-facilitated situational analysis and subsequent recommendations. Although this approach can provide a model for countries that similarly challenged with competing health priorities, it remains to be seen the impact and feasibility of their conclusions.
Box 1. Case-Study: Improving Access to Breast Cancer Screening in Appalachian Ohio:
Appalachia is a 13-state region defined by the Appalachian mountain range and designated by the Appalachian Regional Commission in 1969 in response to deficits in income, education and poverty. In Ohio, 32 counties are part of Appalachia. In addition to lower income, education and employment rates compared with national statistics this area suffers from disparities in access to care, including mammography services. Six of these counties do not have dedicated mammography services, and public transportation options to travel to nearby counties are limited. In response to this deficit, the Ohio State University Comprehensive Cancer Center’s Center for Cancer Health Equity (OSUCCC-CCHE) obtained grants from the Susan G. Komen Foundation (Columbus, OH) to initiate a continuum of care breast screening program. There are 3 integral pieces to this program – a mobile mammogram van (owned and operated by the Speilman Breast Center at the OSUCCC), community health workers (CHW), and a patient navigator (PN). This program employs CHWs, who are native Appalachians, to go into the community (in both community and on-on-one settings) and educate women on the need for regular breast screening. Once a woman is found who needs and wants screening, the CHW links the woman with a PN at the OSUCCC-CCHE. The PN establishes how the mammogram will be paid for (i.e., via insurance (including qualifying for Medicaid), Ohio Breast and Cervical Early Detection Program, Komen grant funds, or charity care), where the woman will get screened (mobile mammography scheduled in the respective county, or a local facility), when the woman can obtain her mammogram (i.e., an appointment is made), and what barriers need to be resolved for the woman to keep her appointment. The PN and CHW work as a team to assure the woman complete her scheduled appointment and receives any follow-up for any abnormality found and through treatment, if necessary. To date, 952 women in Appalachia have received a mammogram from this program, 73 women have had an abnormality detected, 70 have been followed through diagnostic resolution, and 6 women have been diagnosed with breast cancer (4 at an early stage).
Box 2. Case Study: China.
Breast cancer is the most common cancer among Chinese women. The five-year breast cancer specific survival has been increasing, but remains lower than in many high income countries. Currently, there is no population-based screening for breast cancer in China. The Chinese National Breast Cancer Screening Program, which began in 2005, was terminated due to lack of funding and concerns about the false-positive rate. The national guidelines released in 2018 recommends that women aged 40–69 should undergo mammographic screening every 1–2 years, annual clinical breast examination (CBE), and monthly breast self-examination (BSE); and for age 70 and older, to continue with monthly BSE and annual CBE. Nevertheless, opportunistic screening rates for mammograms, CBE, and BSE in China were only 21.7–33.8%. Therefore, effective strategies for improving breast awareness are needed, along with capacity-building to improve early detection. The Eastern Michigan University Center for Health Disparities Innovation and Studies received a grant from the Susan G. Komen Foundation to increase breast cancer awareness and early detection in China. Several components were instituted based on BHGI recommendations: 1. Training breast cancer survivors as “breast health ambassadors” to deliver breast health messages which improve health literacy and debunk myths regarding breast cancer, 2. Education of community-level healthcare providers to ensure early cancer signs and symptoms are correctly identified and referred to appropriate diagnostic services. As a result, the program produced ~2,000 breast health ambassadors in six provinces and more than 800 health care providers in Chengdu, GuangZhou, Inner Mongolia, and Xi’An, and 3. Multi-level collaboration involving the Political Union, All China Women Federation, hospitals/health systems, business partners, and municipal CDC offices to increase the infrastructure capacity for follow-up and treatment of positively screened patients. While the national population-based screening program is still developing, the ultimate success of cancer control for breast cancer will rely on measures to improve early diagnosis and treatment in the health system as well as the general public’s participation to increase awareness and promotion of early detection.
Box 3. Case Study: Mexico.
The first Mexican Official Normative (NOM-041) for the control of breast cancer was published in 2003 and updated in 2011. The NOM-041 recommends: 1) monthly breast self-exam (BSE) for women starting at age 20, 2) annual clinical breast exam (CBE) from 25 years onwards, and 3) screening mammogram every 2 years for women aged 40 to 69. Since 2007, treatment is covered by the government for all uninsured Mexican breast cancer patients. Despite these policies for early detection, the majority of women with BC are diagnosed with locally advanced or metastatic disease. The median time from symptom discovery to treatment initiation is 7 months and the longest delays occur between the patient’s first contact with the health services and diagnostic confirmation.
Since 2007, the main focus has been the promotion of screening mammography, despite the ongoing controversy in high-income countries regarding cost-effectiveness and overdiagnosis. Public investment in breast cancer preventive services (mainly mammography screening promotion) accounted for approximately USD 43.6 million in 2015. However, national screening coverage remains low, at approximately 23%, and only 15% of BC patients are detected through mammography screening, attributed to lack of available human resources for mammography interpretation. The required financial and human resources to increase BC screening above the minimal 70% level recommended by the World Health Organization are insurmountable for a middle-income country like Mexico, with many competing priorities.
The efforts to increase mammography screening coverage have resulted in an increased but disorganized offer of mammograms in private facilities and services, subcontracted by the government, without guarantee of study quality, patient follow-up or access to diagnosis and treatment of patients with abnormal results. Meanwhile, breast cancer patients that present symptomatically face long delays in diagnosis and treatment, due to a weak healthcare and referral system. The more important issues, such as strengthening of the first level of care to manage women with suspected breast cancer, ensuring the quality of diagnostic imaging tests and access to high-quality pathology services, and expedited referral routes to cancer care facilities are neglected. With the change of government administration at the end of 2018, there are plans to revise current BC early detection practices and shift priorities towards the strengthening early BC diagnosis programs.
Box 4. Case study: Panama.
Health care delivery in Panama is governed by the Ministry of Health (MINSA), which also provides health care services to those lacking health coverage under the Social Security System.
MINSA approved an opportunistic screening program for breast cancer, but participation rates were low such that most women presented at primary care facilities with symptomatic disease. Women with breast symptoms are subsequently referred to secondary level facilities for diagnostic workup, including diagnostic imagining, tissue biopsy and diagnostic pathology. Confirmed breast cancer cases are referred to the National Oncology Institute (NOI) in Panama City for molecular pathology and treatment, free for all patients.
Three years ago, MINSA identified low levels of breast cancer knowledge at the primary level and weak referral pathways as two key areas for improving the early diagnosis of breast cancer. The country has since developed and implemented standardized trainings in each province to increase the capacity of primary level health care providers to identify early signs of breast cancer, and refer patients to the appropriate level of care, using PAHO’s virtual platform and blended learning. In 2018, the ministry invited BHGI and Susan G. Komen to visit the National Oncology Institute, and primary and secondary facilities in two regions of Panama, to assess barriers to effective early diagnosis.
The BHGI team identified the following opportunities to work with MINSA to further improve breast cancer early diagnosis: (1) continue developing a standardized and more efficient referral system, with emphasis in the diagnostic phase; (2) shift to greater use of needle biopsies for breast cancer diagnosis since the majority of biopsies performed at second level facilities are excisional and performed by surgeons which causes significant delays; (3) plan for the inevitable increased influx of patients to the NOI as screening rates grow.
MINSA has recently completed the new National Cancer Plan 2019–2029, as it continues strengthening early diagnosis. MINSA is currently working with BHGI in developing an implementation strategy based on the Exploration, Preparation, Implementation, Sustainment (EPIS) framework to support transition to increased rates of core needle biopsies, with the aim of reducing diagnostic delays.
Box 5. Case Study: Tanzania.
In Tanzania, inefficient clinical pathways for women with breast health concerns result in significant delays in detection, diagnosis (80% diagnosed at advanced stages) and treatment. In 2016, at the invitation of the Government of Tanzania, Susan G. Komen partnered with BHGI, WEMA (a Tanzanian women’s health organization) and the Ocean Road Cancer Institute to conduct a situational analysis of breast healthcare in Tanzania. Breast healthcare services were assessed using tools developed by BHGI/Breast Cancer Initiative (BCI)2.5, at the primary, district, regional, zonal and national levels, via questionnaires, in-person interviews, and site visits in Dar es Salaam, Mbeya, Moshi and Mwanza.
Findings include: (1) Protocols/guidelines for breast cancer early detection, diagnosis and treatment are not standardized. (2) Inefficient/hierarchical referral systems add delays, costs and increase rates of attrition. (3) Financial conditions—both institutional and individual—present significant barriers to care. (e.g., treatment is free with a confirmed diagnosis, but diagnostic fees are out-of-pocket). (4) Lack of trained specialized healthcare workforce, including pathologists, radiologists skilled in breast ultrasound, specialized breast surgeons and medical oncologists. (5) Frontline, primary and district level healthcare workers lack training in breast health education, clinical breast examination and the referral pathway. (6) Communication between facilities is poor and there is no feedback loop to relay diagnostic results.
Based on these findings, BHGI developed a resource-stratified, phased implementation framework, to integrate early detection programs with accurate diagnosis and timely, accessible and effective treatments, beginning with management of palpable disease. Prerequisites: situational analysis, referral/patient pathways, standardized guidelines, protocols and trained workforce. Phase 1: Systematic triage and diagnosis of palpable breast disease. Phase 2: Strengthening of resource-appropriate patient-centric care pathways (treatment planning and patient navigation) and reduction of access barriers. Phase 3: Scaling up of targeted education interventions for public and healthcare staff and clinical breast examination (CBE) to promote the downstaging of clinically detectable disease. Phase 4: Systematic upgrading of image-based diagnostic systems (ultrasound and mammography imaging used first for diagnostic work-up as a prerequisite to image-based screening). This partnership has resulted in new collaborations and continued engagement in the improvement of breast health care in Tanzania, including harmonization of the National Guidelines for Early Diagnosis and Referral for Treatment with the assessment findings, training of primary care providers and health systems and implementation research.
In all case studies the essential issue is the same; late stage at diagnosis is the main driver of poor survival, even in some higher income and ostensibly better resourced settings. Efforts to overcome these challenges need to be multi-pronged, and consider a variety of factors that can ultimately influence a woman’s opportunities for breast cancer early detection.
Metrics for early detection of breast cancer
Identifying a set of measures to monitor and evaluate a breast cancer early detection program is essential for program improvement and progress along a defined resource-stratified pathway. High-quality metrics should be appropriate for available resources and programs, feasible to measure, focus on program elements that can be acted upon and improved, and regularly reported to all relevant stakeholders. Ideally, metrics will have been previously demonstrated to be associated with reduced breast cancer mortality – however the evidence to identify such measures in LMICs is scarce.
While the ultimate goal of an early detection program is to reduce breast cancer mortality, several metrics can evaluate the program’s progress towards that long-term goal. At the ‘Enhanced’ and ‘Maximal’ resource levels where screening mammography is provided to a target population, performance indicators can be adapted from high-income settings and at a minimum include the proportion of the target population screened within the past 24 months. 54
Here we expand on metrics relevant to all resource levels. An essential metric is the proportion of cancers diagnosed at different stages, to allow monitoring of temporal trends in this stage distribution, given the clear link between stage at diagnosis and breast cancer survival. In a basic-resource setting where a new program is being implemented, provision of early detection services across health facilities is important to monitor short-term and ongoing progress on service availability and allow early challenges to be explored and addressed. Table 1 provides some additional example metrics that can be used to assess local, regional or national early detection programs, grouped by the lowest resource levels to which they are pertinent.
Table 1.
Examples of metrics to identify and track outcomes
| Metric | Resource level (basic, core, enhanced,) |
Metric type (process, implementation, health outcome) |
User of the metric |
|---|---|---|---|
| Community breast cancer awareness: % aware of breast cancer symptoms, % knowing where to go for a breast health concern |
Basic | Implementation metric, health outcome metric | System wide Community level/Regional Ministry |
| Provider breast cancer awareness: % providers trained to provide high-quality clinical breast examinations, % providers knowing proper care referrals for positive CBE |
Basic | Implementation metric, health outcome metric | System wide Medical education oversight? Health facility |
| Among women with suspected breast cancer, median days from symptom onset to first presentation at a health facility | Basic | Process metric | System wide -Community awareness -Access to care |
| Among women with suspected breast cancer, median days from first presentation at a health facility to diagnosis | Basic | Process metric | Health facility |
| Among patients diagnosed with breast cancer, median days from diagnosis to first treatment | Basic | Process metric | Health facility/network |
| % of patients with breast cancer diagnosed with early stage (stage I or II) disease | Basic | Health outcome metric | System wide Health facility/network |
| % of patients lost to follow up after initial presentation with a breast mass | Basic | Health outcome metric | Health facility/network |
| % of relevant health facilities offering early detection services (penetration) | Basic-CBE Core-Ultrasound Enhanced- Mammography |
Implementation metric | Health system/Ministry |
| Uptake (population coverage) of routine screening services | Basic/Core-CBE Enhanced/ Mammography | Implementation metric | System wide |
| Cost | All levels | Implementation metric | System wide |
Financing Early Detection of Breast Cancer
Financing of interventions for early detection of breast cancer is justified from a public health, economic and equity perspective. Numerous studies have documented the catastrophic health expenditures and economic hardships associated with late stage diagnosis of breast cancer patients in different world regions, regardless of resource level. 55-57 There also are considerable non-medical costs (transport, lodging, child care) that can account for up to 50% of total costs that must be considered to reduce the risk of impoverishment. 55
Effective prevention and early detection strategies can help reduce costs and achieve significant savings both to health systems and individuals, as cancers at earlier stages are less expensive to treat. An analysis of the total economic savings of a prevention/early detection/treatment strategy contrasted to a ‘treatment only’ approach was estimated at roughly 60% across all world regions. 58
Cost effectiveness analysis can help inform how resources should be allocated for ‘Best Buy’ interventions. The Disease Control Priorities (DCP3) identified a set of cost-effective and affordable interventions for most LMICs, including public education for target populations to raise awareness of the value of early detection, risk factors, and breast health awareness.59 The additional cost of the DCP3 ‘essential package’ of cost-effective cancer interventions would cost annually roughly $1.7, $1.8 and $5.7 extra per capita in low-income countries, lower-middle income countries, and upper middle-income countries, respectively. The 2017 WHO Global Action Plan for the Prevention and Control of Noncommunicable Diseases did not include population-based screening with mammography (every 2 years for women aged 50–69) or diagnosis and treatment of stage 1 and 2 breast cancer among the best buy interventions. 60 The Report of the WHO Commission on Macro-Economics and Health suggested that interventions that are not cost-effective (i.e. costing less than three times GDP per capita for each DALY averted) should be supported by the international community, if a country cannot afford to undertake them on its own. 61
The essential package funded in countries will depend on what is affordable. Governments may decide to offer subsidized care to a targeted population or they may initially cover fewer interventions and increase them over time, as resource envelopes rise, as has been done in several LMICs, including Mexico 62and Thailand. 63, 64
A combination of different sources of financing needs to be considered with an emphasis on domestic financing. Public financing remains key, particularly for ‘public goods’ that cannot be withheld from those who do not pay for them, such as public education. A case study in Malaysia found that the incidence of patients presenting with late stage breast cancer declined from 77 to 37% after a country-wide drive to increase awareness.65 With competing demands on health budgets, and even under the scenario of growing per capita incomes, prioritization of health interventions and mobilization of additional public funding will be essential. 66
Social health insurance represents the most equitable way to fund interventions that have a large ‘private good’ content, such as early detection, diagnosis and early treatment of breast cancer, incorporating progressively key interventions in benefit packages. There may also be some element of cost-sharing and cross-subsidization of out-of-pocket spending through supplementary private health insurance. It may be important to consider a tiered approach to increasing coverage, as social health insurance schemes mature, and adequate resources can be generated to make them financially sustainable.
Countries may also consider innovative financing options (e.g., tobacco, alcohol and sugar taxes; airline and mobile phone levies). External financing will play a critical role to: (i) lower costs of inputs in the spirit of “bending the curve” on the high costs of treatment; and (ii) support technical assistance and research 67 It is worth mentioning here that public-private partnerships or “PPPs” have been proposed as a solution to overcome some of the bottlenecks in the public health system. However, strong oversight, including accreditation, regulatory capacity and good governance mechanisms should be in place to ensure that such arrangements reach intended beneficiaries who present for care, without escalating costs for patients or governments.
Implementation Framework
Policies and governance
The BHGI stratified guidelines and phased implementation strategy provide a framework to define early detection policies according to level of resources, 3 upgrading from breast cancer early diagnosis alone to the addition of highly organized screening; however, implementing the same policy may differ across settings depending upon health system capacity and characteristics.
Therefore, the development and implementation of breast cancer early detection policies must rely on accurate situational analyses, including: assessment of the sociopolitical and economic context, workforce capacity, infrastructure, distribution of equipment and facilities (as determinants of geographic access), social structures, and funding. 68 Consideration should also be given to inclusion of social scientists (including gender scholars) and representatives from the target population in policy development, to ensure policies and interventions are acceptable, equitable and inclusive.
Basic components of breast cancer early detection policies comprise identifying the target population, defining the diagnostic tools, delineating the programmatic approaches, and elaborating the rollout and scale up process (Figure 4). For each component, different alternatives might be adopted, thus resulting in diverse strategies. In addition, mechanisms for financial protection should be established to minimize risk of incomplete diagnosis or treatment and impoverishment and inclusion of early detection interventions in essential health packages is critical, since treatment of early disease is less expensive, and will generate savings on medical costs. 69
Policy implementation should ensure sustainability. In limited resource settings the expansion of health services by governmental agencies is less likely to occur due to financial constraints and the presence of competing needs. 70 Medical societies, breast cancer survivors, and non-governmental organizations frequently step in to fill gaps in the delivery of breast cancer awareness and related health services for early detection in low-resource settings and underserved populations 68 via the provision of infrastructure, equipment, and staffing. However, if these services are not integrated into the existing health system and coordinated with the relevant ministries and institutions, these efforts may have limited effects. Further, there must be a strategy at the outset for transitioning NGO-supported initiatives to government-ownership. Hence, NGOs should play an active role in transitioning from the delivery of early detection services to mainstreaming these interventions in the health system with full governmental commitment to ensure sustainability.
Conclusion
All countries are challenged to meet the ambitious targets of the WHO Global Action Plan for the Prevention and Control of Non-communicable diseases (NCDs), and to achieve the related Sustainable Development Goals target, a one-third reduction in mortality from NCDs by the year 2030. 71 Breast cancer is the most common cancer in women globally –in all but 42 countries, where cervical cancer still predominates. Breast cancer survival is largely dependent on a woman’s access to timely, effective, and affordable care. Early detection is critical to breast cancer survival. When coupled with timely access to treatment, appropriate follow up, and survivorship care, there can be significant reductions in breast cancer mortality.
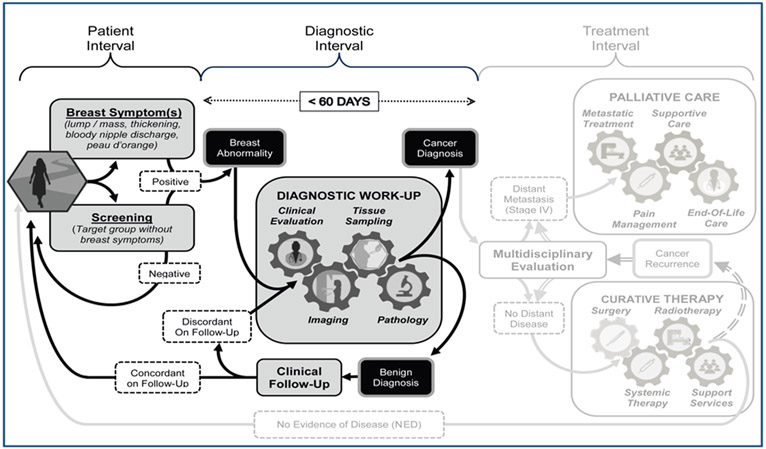
The complex interplay between barriers in access to early detection and examples of interventions to overcome these barriers are presented here, along with sample metrics and governance considerations that can be used as a practical framework to the phased approach to breast cancer early detection for a population in any setting. A robust health system is a prerequisite to provide the facilities for treatment of breast cancers that are diagnosed through the early detection programmes, whether with symptomatic breast cancer or through screening. While patient and health provider education may shorten the patient interval, in order to achieve a diagnostic interval target of less than 60 days requires coordination of the diagnostic pathway elements of clinical evaluation, imaging, tissue sampling and pathological assessment (Figure 5).
Figure 5.
Universal patient pathway for breast cancer management in three sequential intervals of care (Patient Interval, Diagnostic Interval, and Treatment Interval) highlighting the Patient and Diagnostic Intervals of care. The Patient Interval begins with the onset of clinical symptoms or an abnormal screening exam and extends to the time the patient presents for diagnostic work-up of a recognized or suspected breast abnormality. During the Diagnostic Interval, the identified breast abnormality undergoes a ‘triple test’ work-up based on clinical evaluation, imaging and tissue sampling to achieve a definitive benign or malignant diagnosis. The health system should endeavor to complete the diagnostic work-up within a 60 day (2 month) period, because worsened survival outcomes can result from diagnostic delays extending significantly beyond 3 months.
Figure 3b.
Potential interventions to strengthen early diagnosis
(WHO Guide to Cancer Early Diagnosis (2017)
Acknowledgments
Funding
The BHGI Global Summit was funded by grants from the Fred Hutchinson Cancer Research Center; Susan G. Komen (GSP18BHGI001); National Comprehensive Cancer Network; US National Institutes of Health (1R13CA224776–01A1); National Cancer Institute Center for Global Health; American Society of Clinical Oncology; American Society of Clinical Pathology; Journal of Global Oncology; National Breast Cancer Foundation, Inc.; pH Trust; Seattle Cancer Care Alliance; Union for International Cancer Control; University of Washington Department of Global Health. Support from unrestricted educational grants came from Cepheid; GE Healthcare; Novartis; Pfizer Inc.; and UE LifeSciences. Additional funding to cover publication costs was provided by GE Healthcare and Novartis.
The Susan G. Komen Leadership Grant (SAC170082) supported BOA, CD and AD.
Dr. Paskett is the Multiple Principal Investigator on a grant from Merck Foundation and has received funding for a study in the past from Merck, not related to the topic of this paper. She also has been a stockholder in Pfizer in the past three years.
Footnotes
Conflict of Interest Statement. The authors have no conflicts of interest to report with the exception of Dr. Pasket.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
References
- 1.(2017). WHO. Guide to cancer early diagnosis. World Health Organization. http://www.who.int/iris/handle/10665/254500. License: CC BY-NC-SA 3.0 IGO.
- 2.Yip CH. Challenges in the early detection of breast cancer in resource-poor settings. Breast Cancer Management. 2016. [Google Scholar]
- 3.Yip CH, Smith RA, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer. 2008;113: 2244–2256. [DOI] [PubMed] [Google Scholar]
- 4.Shyyan R, Sener SF, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: diagnosis resource allocation. Cancer. 2008;113: 2257–2268. [DOI] [PubMed] [Google Scholar]
- 5.Justo N, Wilking N, Jonsson B, Luciani S, Cazap E. A review of breast cancer care and outcomes in Latin America. Oncologist. 2013;18: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jedy-Agba E, McCormack V, Adebamowo C, Dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2016;4: e923–e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unger-Saldana K, Miranda A, Zarco-Espinosa G, Mainero-Ratchelous F, Bargallo-Rocha E, Miguel Lazaro-Leon J. Health system delay and its effect on clinical stage of breast cancer: Multicenter study. Cancer. 2015;121: 2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romanoff A, Constant TH, Johnson KM, et al. Association of Previous Clinical Breast Examination With Reduced Delays and Earlier-Stage Breast Cancer Diagnosis Among Women in Peru. JAMA Oncol. 2017;3: 1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetto J, Pommier R, Schmidt W, et al. Use of the “triple test” for palpable breast lesions yields high diagnostic accuracy and cost savings. Am J Surg. 1995;169: 519–522. [DOI] [PubMed] [Google Scholar]
- 10.Eniu A, Carlson RW, El Saghir NS, et al. Guideline implementation for breast healthcare in low- and middle-income countries: treatment resource allocation. Cancer. 2008;113: 2269–2281. [DOI] [PubMed] [Google Scholar]
- 11.Distelhorst SR, Cleary JF, Ganz PA, et al. Optimisation of the continuum of supportive and palliative care for patients with breast cancer in low-income and middle-income countries: executive summary of the Breast Health Global Initiative, 2014. Lancet Oncol. 2015;16: e137–147. [DOI] [PubMed] [Google Scholar]
- 12.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113: 2221–2243. [DOI] [PubMed] [Google Scholar]
- 13.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353: 1119–1126. [DOI] [PubMed] [Google Scholar]
- 14.Ginsburg O RA, Conteh L, Mutebi M, Paskett ED and Subramaniam S. . Breast Cancer Disparities Among Women in Low- and Middle-Income Countries. Current Breast Cancer Reports. 2018. [Google Scholar]
- 15.Tsu V SJ, Bishop A, Murray M, Weigl B, Lehman CD. Breast ultrasound following a positive clinical breast examination: Does it have a role in low- and middle-income countries? . J Glob Radiol. 2015;1. [Google Scholar]
- 16.Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited settings. Afr Health Sci. 2010;10: 89–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Scheel JR, Lee JM, Sprague BL, Lee CI, Lehman CD. Screening ultrasound as an adjunct to mammography in women with mammographically dense breasts. Am J Obstet Gynecol. 2015;212: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprague BL, Arao RF, Miglioretti DL, et al. National Performance Benchmarks for Modern Diagnostic Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey JA, Mahoney MC, Newell MS, et al. ACR Appropriateness Criteria Palpable Breast Masses. J Am Coll Radiol. 2016;13: e31–e42. [DOI] [PubMed] [Google Scholar]
- 20.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med. 2015;372: 2353–2358. [DOI] [PubMed] [Google Scholar]
- 21.Mittra I, Mishra GA, Singh S, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. Int J Cancer. 2010;126: 976–984. [DOI] [PubMed] [Google Scholar]
- 22.International Agency for Research on Cancer. Breast Cancer Screening; 2nd edition – IARC Handbooks on Cancer Prevention. Lyon: IARC; 2016. [Google Scholar]
- 23.Dos-Santos-Silva I, De Stavola BL, Renna NLJ, et al. Ethnoracial and social trends in breast cancer staging at diagnosis in Brazil, 2001–14: a case only analysis. Lancet Glob Health. 2019;7: e784–e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yip CH. Downstaging is more important than screening for asymptomatic breast cancer. Lancet Glob Health. 2019;7: e690–e691. [DOI] [PubMed] [Google Scholar]
- 25.(2014) WHO. WHO position paper on mammography screening. https://www.who.int/cancer/publications/mammography_screening/en/. [PubMed]
- 26.Shastri A, Shastri SS. Cancer screening and prevention in low-resource settings. Nat Rev Cancer. 2014;14: 822–829. [DOI] [PubMed] [Google Scholar]
- 27.Stanley E, Lewis MC, Irshad A, et al. Effectiveness of a Mobile Mammography Program. AJR Am J Roentgenol. 2017;209: 1426–1429. [DOI] [PubMed] [Google Scholar]
- 28.Cabanes A, Kapambwe S, Citonje-Msadabwe S, et al. Challenges, Opportunities, and Priorities for Advancing Breast Cancer Control in Zambia: A Consultative Meeting on Breast Cancer Control. Journal of Global Oncology. 2019: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu K LL, Tian W, Pan T, Ye J ,Zhang S The Outcome of Breast Cancer Is Associated with National Human Development Index and Health System Attainment. PLosONE. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaccarella S, Lortet-Tieulent J, Saracci R, et al. Reducing Social Inequalities in Cancer: Setting Priorities for Research. CA Cancer J Clin. 2018;68: 324–326. [DOI] [PubMed] [Google Scholar]
- 31.Romero Y, Trapani D, Johnson S, et al. National cancer control plans: a global analysis. Lancet Oncol. 2018;19: e546–e555. [DOI] [PubMed] [Google Scholar]
- 32.Ginsburg O, Badwe R, Boyle P, et al. Changing global policy to deliver safe, equitable, and affordable care for women’s cancers. Lancet. 2017;389: 871–880. [DOI] [PubMed] [Google Scholar]
- 33.Tin Tin S, Elwood JM, Brown C, et al. Ethnic disparities in breast cancer survival in New Zealand: which factors contribute? BMC Cancer. 2018;18: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones CE, Maben J, Lucas G, Davies EA, Jack RH, Ream E. Barriers to early diagnosis of symptomatic breast cancer: a qualitative study of Black African, Black Caribbean and White British women living in the UK. BMJ Open. 2015;5: e006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68: 297–316. [DOI] [PubMed] [Google Scholar]
- 36.Tapela NM, Peluso MJ, Kohler RE, et al. A Step Toward Timely Referral and Early Diagnosis of Cancer: Implementation and Impact on Knowledge of a Primary Care-Based Training Program in Botswana. Front Oncol. 2018;8: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espina C, McKenzie F, Dos-Santos-Silva I. Delayed presentation and diagnosis of breast cancer in African women: a systematic review. Ann Epidemiol. 2017;27: 659–671 e657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015;16: 1193–1224. [DOI] [PubMed] [Google Scholar]
- 39.Putthasri W et al. . Geographical distribution and utilization of mammography in Thailand ,Regional Health Forum.8.1(2004):84–91 International Health Policy Program. [Google Scholar]
- 40.Health Workforce 2030: A global strategy on human resources for health. https://www.who.int/hrh/documents/strategy_brochure2014/en/
- 41.Mandal R, Basu P. Cancer screening and early diagnosis in low and middle income countries : Current situation and future perspectives. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61: 1505–1512. [DOI] [PubMed] [Google Scholar]
- 42.Shyyan R, Masood S, Badwe RA, et al. Breast cancer in limited-resource countries: diagnosis and pathology. Breast J. 2006;12 Suppl 1: S27–37. [DOI] [PubMed] [Google Scholar]
- 43.Hyoju SK, Agrawal CS, Pokhrel PK, Agrawal S. Transfer of clinical breast examination skills to female community health volunteers in Nepal. Asian Pac J Cancer Prev. 2011;12: 3353–3356. [PubMed] [Google Scholar]
- 44.Torres-Mejia G, Smith RA, Carranza-Flores Mde L, et al. Radiographers supporting radiologists in the interpretation of screening mammography: a viable strategy to meet the shortage in the number of radiologists. BMC Cancer. 2015;15: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceber E, Turk M, Ciceklioglu M. The effects of an educational program on knowledge of breast cancer, early detection practices and health beliefs of nurses and midwives. J Clin Nurs. 2010;19: 2363–2371. [DOI] [PubMed] [Google Scholar]
- 46.Salazar AJ, Romero JA, Bernal OA, Moreno AP, Velasco SC, Diaz XA. Noninferiority and Equivalence Evaluation of Clinical Performance among Computed Radiography, Film, and Digitized Film for Telemammography Services. Int J Telemed Appl. 2016;2016: 3642960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organisation. Breast Cancer. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/.
- 48.Sano H, Goto R, Hamashima C. Does lack of resources impair access to breast and cervical cancer screening in Japan? PLoS One. 2017;12: e0180819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wing P, Langelier MH. Workforce shortages in breast imaging: impact on mammography utilization. AJR Am J Roentgenol. 2009;192: 370–378. [DOI] [PubMed] [Google Scholar]
- 50.Bender CE, Bansal S, Wolfman D, Parikh JR. 2018. ACR Commission on Human Resources Workforce Survey. J Am Coll Radiol. 2019. [DOI] [PubMed] [Google Scholar]
- 51.Farria DM, Schmidt ME, Monsees BS, et al. Professional and economic factors affecting access to mammography: a crisis today, or tomorrow? Results from a national survey. Cancer. 2005;104: 491–498. [DOI] [PubMed] [Google Scholar]
- 52.Breast Cancer Initiative 2.5. https://www.fredhutch.org/en/labs/phs/projects/breast-cancer-initiative_2-5.html
- 53.Duggan C, Cruz TA, Porto MRT, et al. Improving Breast Health Care in the State of Sergipe, Brazil: A Commentary. Journal of Global Oncology. 2018: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schleimer LE, Keating NL, Shulman LN, et al. Review of Quality Measures for Breast Cancer Care by Country Income Level. Journal of Global Oncology. 2018;4: 41s–41s. [Google Scholar]
- 55.Group AS. Policy and priorities for national cancer control planning in low- and middle-income countries: Lessons from the Association of Southeast Asian Nations (ASEAN) Costs in Oncology prospective cohort study. Eur J Cancer. 2017;74: 26–37. [DOI] [PubMed] [Google Scholar]
- 56.Subramanian S, Gakunga R, Kibachio J, et al. Cost and affordability of non-communicable disease screening, diagnosis and treatment in Kenya: Patient payments in the private and public sectors. PLoS One. 2018;13: e0190113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Neill KM, Mandigo M, Pyda J, et al. Out-of-pocket expenses incurred by patients obtaining free breast cancer care in Haiti: A pilot study. Surgery. 2015;158: 747–755. [DOI] [PubMed] [Google Scholar]
- 58.Knaul FM, Initiative HUGE. Closing the Cancer Divide. Harvard University Press, 2012. [Google Scholar]
- 59.Anderson B, Lipscomb J, Murillo R, Thomas D , . “Breast Cancer”. In: Disease Control Priorities (third edition): Volume 3, Cancer, edited by Gelband H, Jha P, Sankaranarayanan R, Horton S. Washington, DC: World Bank. . [PubMed] [Google Scholar]
- 60.World Health Organization. (2017). Tackling NCDs: ‘best buys’ and other recommended interventions for the prevention and control of noncommunicable diseases. World Health Organization. http://www.who.int/iris/handle/10665/259232. License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 61.WHO Commission on Macroeconomics and Health & World Health Organization. (2001). Macroeconomics and health : investing in health for economic development : executive summary / report of the Commission on Macroeconomics and Health. World Health Organization. http://www.who.int/iris/handle/10665/42463 [Google Scholar]
- 62.González-Robledo MC WR, Ornelas HA, Knaul FM. . Costs of breast cancer care in Mexico: analysis of two insurance coverage scenarios. Ecancermedicalscience. . 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukem S, Meng Q, Sriplung H, Tangcharoensathien V. Low Coverage and Disparities of Breast and Cervical Cancer Screening in Thai Women: Analysis of National Representative Household Surveys. Asian Pac J Cancer Prev. 2015;16: 8541–8551. [DOI] [PubMed] [Google Scholar]
- 64.Sruamsiri R, Ross-Degnan D, Lu CY, Chaiyakunapruk N, Wagner AK. Policies and programs to facilitate access to targeted cancer therapies in Thailand. PLoS One. 2015;10: e0119945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devi BC, Tang TS, Corbex M. Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: a pilot study of clinical downstaging in Sarawak, Malaysia. Ann Oncol. 2007;18: 1172–1176. [DOI] [PubMed] [Google Scholar]
- 66.Migowski A, Dias MBK, Nadanovsky P, Silva GAE, Sant’Ana DR, Stein AT. Guidelines for early detection of breast cancer in Brazil. III - Challenges for implementation. Cad Saude Publica. 2018;34: e00046317. [DOI] [PubMed] [Google Scholar]
- 67.Anderson B, Lipscomb J, Murillo R, Thomas D , . “Breast Cancer”. In: Disease Control Priorities (third edition): Volume 3, Cancer, edited by Gelband H, Jha P, Sankaranarayanan R, Horton S. Washington, DC: World Bank. [PubMed] [Google Scholar]
- 68.Anderson BO, Yip CH, Ramsey SD, et al. Breast cancer in limited-resource countries: health care systems and public policy. Breast J. 2006;12 Suppl 1: S54–69. [DOI] [PubMed] [Google Scholar]
- 69.Groot MT, Baltussen R, Uyl-de Groot CA, Anderson BO, Hortobagyi GN. Costs and health effects of breast cancer interventions in epidemiologically different regions of Africa, North America, and Asia. Breast J. 2006;12 Suppl 1: S81–90. [DOI] [PubMed] [Google Scholar]
- 70.Ortiz I, Durán-Valverde F, Pal K, Behrendt C, Acuña-Ulate A. Universal Social Protection Floors: Costing Estimates and Affordability in 57 Lower Income Countries Extension of Social Security – International Labour Office: ESS ─ Working Paper No. 58. Geneva: ILO; 2017. [Google Scholar]
- 71.collaborators NCDC. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet. 2018;392: 1072–1088. [DOI] [PubMed] [Google Scholar]