Abstract
Background:
HIV-negative individuals with in utero HIV exposure represent an emerging population, exceeding 18 million people worldwide. Long-term clinical outcomes among HIV-exposed uninfected (HEU) individuals into adolescence and young adulthood remain unknown.
Setting:
U.S. academic health system.
Methods:
In this observational cohort study, we leveraged a patient data registry to identify 50 HEU adolescents and young adults. We also identified 141 HIV-unexposed controls that were matched to HEU subjects up to 3:1 on age of last encounter (±2 years), birthdate (±5 years), sex, race/ethnicity, and zip code. All subjects were born since 1/1/1990 with medical records available into adolescence and young adulthood. Primary outcomes were most recent BMI z-score and presence of reactive airway disease (RAD). Records were manually reviewed to extract health information.
Results:
50 HEU adolescents and young adults (18±3 years, 54% men) and 141 matched controls (19±3 years, 54% men) were compared. HEU individuals had a higher BMI z-score (1.12±1.08 vs. 0.73±1.09, P=0.03) as well as an increased prevalence of obesity (42% vs. 22%, P=0.009) compared to controls. HEU subjects also had a higher prevalence of RAD versus controls (40% vs. 23%, P=0.03). These differences persisted upon adjusting for demographic, socioeconomic, maternal, and birth-related factors. Maternal prenatal CD4+ T cell count was inversely associated with BMI z-score among HEU adolescents (r=−0.47, P=0.01).
Conclusion:
HEU adolescents and young adults exhibited a heightened prevalence of obesity and RAD compared to HIV-unexposed controls. Additional studies are needed to optimize care for the expanding population of HEU individuals transitioning to adulthood.
Keywords: HIV-exposed uninfected, in utero, adolescent, young adult, metabolic disease
Introduction
Worldwide, over 1 million babies are born to mothers with HIV each year.1 With the global scale-up of prenatal antiretroviral therapy (ART), up to 98% of these infants may be HIV-exposed but uninfected (HEU).2 Notably, growing evidence suggests that HIV-negative individuals with in utero HIV exposure may be at heightened risk of adverse health outcomes.1 In studies focused on infants and young children, HEU individuals have been shown to exhibit stunted growth,3–5 altered fuel substrate utilization,6 immune activation,7,8 a Th2-shifted immune response,9 and increased infectious morbidity and mortality.10,11 These findings have been posited to reflect, in part, the influence of the intrauterine environment on fetal programming.12
While understanding the health consequences of in utero HIV exposure has been an area of active investigation, long-term clinical outcomes among HEU individuals into adolescence and adulthood remain largely unknown. With an estimated 18 million HEU individuals currently below 15 years of age,1 there is a pressing need to delineate clinically relevant phenotypes among HEU adolescents and young adults compared to HIV-unexposed controls. Evidence of health complications among this expanding cohort has important implications for a large population at a critical time of emergence into adulthood, and may signify the need for the development of novel prevention and treatment strategies.
In the current study, we assessed for the first time metabolic and immune-related comorbidities among HEU adolescents and young adults as compared to rigorously matched HIV-unexposed uninfected controls from the same U.S. healthcare system. Specifically, we examined differences between groups in obesity and reactive airway disease (RAD), which were selected a priori as clinically relevant surrogates for potential metabolic and immune dysregulation, respectively. Within the HEU group, we also related maternal prenatal characteristics to long-term health outcomes so as to elucidate possible mechanisms of disease. We hypothesized that HEU adolescents and young adults would exhibit a higher BMI z-score and a greater prevalence of RAD compared to well-matched HIV-unexposed controls. While obesity13,14 and RAD15 are known consequences of other pathologic intrauterine environments such as maternal obesity and gestational diabetes, a predisposition toward these endpoints has not been previously demonstrated in the context of maternal HIV. Our data reveal novel sequelae of in utero HIV exposure among HIV-negative individuals, suggesting the importance of close clinical follow-up and additional mechanistic studies.
Methods
Subjects
We utilized the Research Patient Data Registry (RPDR) at a large U.S. academic health center (Partners HealthCare, Boston, MA) to develop an observational cohort of HEU adolescents and young adults in addition to well-matched controls (Figure 1). All subjects were required to have been born since 1/1/1990 and to have medical records available beyond 13 years of age. As such, individuals were born between 1990 and 2005, and all available medical records since birth were reviewed. HEU subjects, comprising the exposed group, were defined as individuals without known HIV infection who were born to mothers with HIV diagnosed prior to or during pregnancy. In contrast, unexposed controls were defined as individuals who did not carry a diagnosis of HIV infection and whose mothers were not known to have HIV during pregnancy. This study was approved by the Institutional Review Board of Massachusetts General Hospital.
Figure 1: Flow of Study Participants.
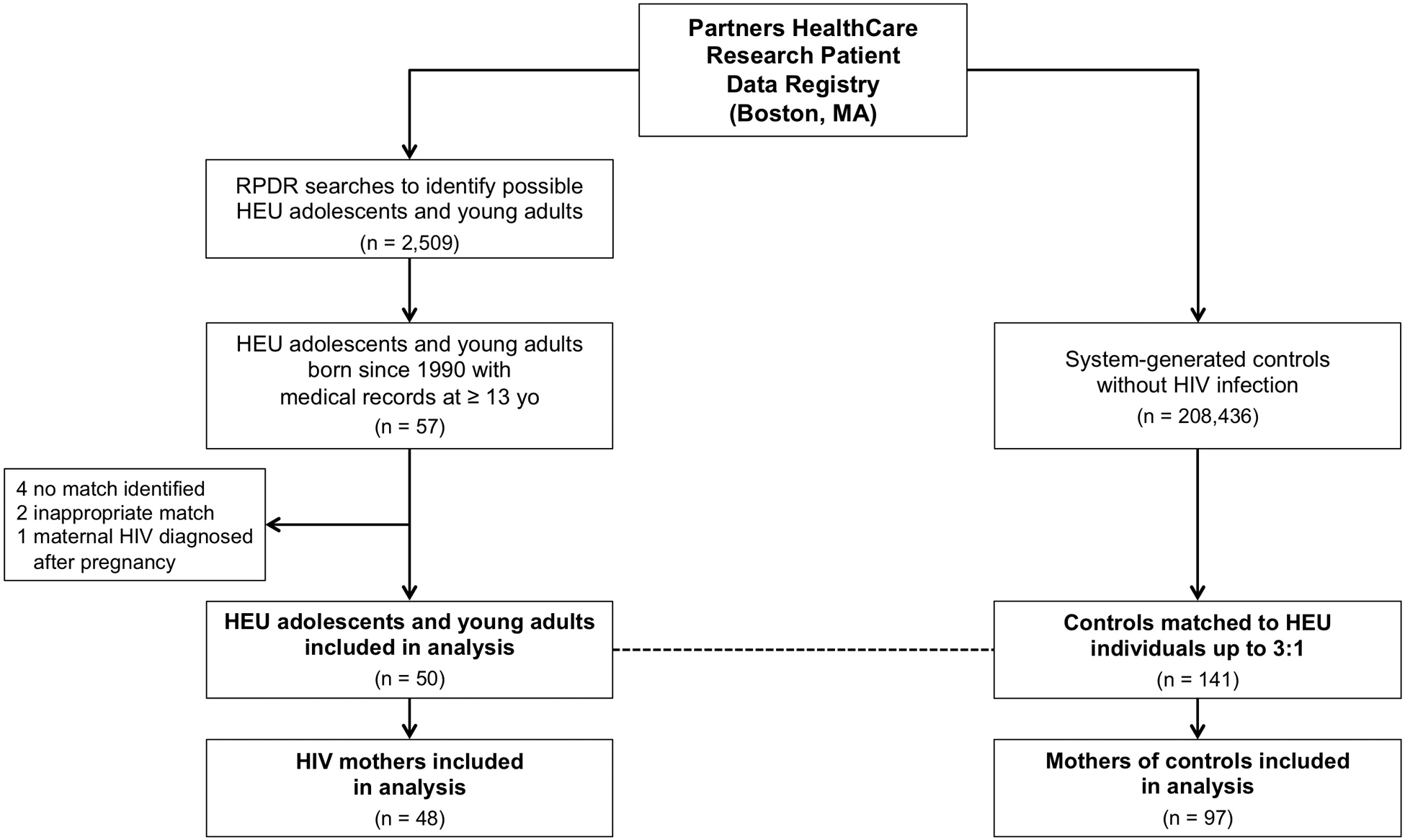
50 HEU individuals and 141 HIV-unexposed controls were included in this analysis. Controls were randomly matched to HEU participants up to 3:1 on age of last encounter (± 2 years), birthdate (± 5 years), sex, race/ethnicity, and zip code. A total of 42 HEU subjects had 3 matched controls, whereas 7 subjects had 2 matched controls and 1 subject had 1 matched control.
HEU subjects were identified through RPDR searches that detected women with HIV who underwent pregnancy, labor, or delivery prior to 2005 based on ICD-9 coding as well as their HIV-negative offspring (n = 2,509). Among mothers resulting from these searches, we confirmed HIV status and assessed the timing of HIV infection with respect to pregnancy. Adolescents born to mothers with HIV during pregnancy, who themselves were HIV-negative, were included within our HEU group.
From the same patient data registry, we obtained 208,436 system-generated controls without a diagnosis of HIV infection based on ICD-9 coding. From this pool, controls were randomly matched up to 3:1 to HEU adolescents on age of last encounter (± 2 years), birthdate (± 5 years), sex, race/ethnicity, and five-digit zip code (SAS 9.4).16 We chose to match on these variables since they comprise important biological and environmental determinants of health that could be readily ascertained from the RPDR on all potential subjects. Upon completing our matching algorithm, a total of 50 HEU adolescents were matched to 141 controls. Among controls, we located maternal medical records to confirm HIV status and to extract health information. In some instances, the mother’s medical record could not be located if she was not seen within the Partners HealthCare system, or if her identifying information could not be reliably ascertained from the young adult’s medical record. Each control was verified to have no evidence of HIV infection or in utero HIV exposure based on medical history and available HIV testing.
Study Procedures
Prior to our analysis, we selected BMI z-score and RAD as our primary outcomes of interest as surrogates for metabolic and immune health, respectively. Adolescent and maternal charts were comprehensively reviewed between 7/1/2018 and 5/1/2019 by trained study staff to extract relevant health information. Sex- and age-adjusted BMI z-score was calculated from the Centers for Disease Control and Prevention (CDC) growth charts using the most recent paired weight and height obtained beyond 12 years of age.17 Individuals were classified as obese if their BMI was greater than or equal to the 95th percentile adjusted for age and sex or greater than or equal to 30 kg/m2. RAD was defined as a clinical report of ‘RAD’ or ‘asthma’ in the medical record at greater than or equal to 12 years of age. Albuterol inhaler use beyond 12 years of age also was collected as evidence of asthma-like symptoms during adolescence and young adulthood. BMI data were available in 96% of HEU individuals and 94% of controls, whereas RAD data were available in 96% of HEU subjects and 98% of controls.
In addition to our primary outcomes, we collected data on biological, behavioral, and socioeconomic factors of interest including birth weight, gestational age, and mode of delivery. We also collected maternal data pertaining to pregnancy such as age at delivery, earliest BMI, and pregnancy complications. For mothers with prenatal HIV infection, we extracted from the medical record a detailed HIV-related history including disease duration at delivery, third-trimester CD4+ T cell count, third-trimester HIV viral load, and ART regimen. Lastly, we recorded data on contemporary maternal characteristics that may reflect the household environment to which each adolescent was exposed including earliest BMI between 35 and 50 years of age, highest educational degree, and any history of tobacco use. Median household income was estimated based on current adolescent zip code according to U.S. census data.18
Statistical Analysis
Assuming a sample of 50 HEU subjects with 5% incomplete matching and 5% missing data, 3:1 matching of controls to HEU subjects would yield 80% power to detect an absolute difference in BMI z-score of 0.47 standard deviation and a risk difference in RAD of 19% between groups (α=0·05, two-sided). These effect sizes were considered clinically significant, whereas increasing the matching ratio beyond 3:1 would not meaningfully decrease the effect size that the study was powered to detect.
We compared differences between groups using a two-tailed independent samples t-test for continuous variables that were normally distributed, Wilcoxon rank-sum test for continuous variables that were not normally distributed, and chi-square test for categorical variables. Outcomes that were significantly different between groups were further evaluated in multivariable logistic regression models adjusting for biological factors of interest, including those found to significantly differ between groups. We additionally performed two sensitivity analyses pertaining to our adjusted models. First, we noted that some subjects were not included in our multivariable models due to missing data, which were assumed to be missing at random. As such, we retested our models utilizing multiple imputation of missing data with 1200 iterations, discarding the first 200 iterations. Second, we recognized that infant feeding practices were not routinely documented in the medical record. Nonetheless, breastfeeding is expected to be lower in infants born to mothers with HIV in the U.S.,19 and has been associated with lower odds of obesity20 and asthma.21 Accordingly, we performed a quantitative bias analysis to adjust for breastfeeding as an unmeasured potential confounder.22 Using the range of data in the literature, we chose a scenario of extreme bias, assuming that the prevalence of breastfeeding was 0.01% in HEU individuals and 80% in controls,23 and that the odds ratio relating breastfeeding to obesity or asthma was 0.74 as previously published.20,21
Lastly, in exploratory analyses, we sought to identify maternal prenatal factors that were associated with long-term health outcomes among HEU adolescents and young adults. Specifically, prenatal characteristics among mothers with HIV were related to outcomes of interest among HEU offspring using linear regression for continuous outcomes and odds ratios (OR) with 95% confidence intervals for categorical outcomes. Significant associations were further examined in multivariable models that controlled for biologically relevant prenatal factors.
Variables with a normal distribution were expressed as mean ± standard deviation. Variables that were not normally distributed were denoted as median [interquartile range] or were logarithmically transformed to achieve normality. All available data were analyzed. A critical value of P < 0.05 was the pre-defined threshold for statistical significance. Statistical analyses were performed using JMP Pro 12.0.1 or SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Adolescent and Maternal Characteristics
A total of 50 HEU adolescents and young adults (18 ± 3 years old, 54% men) and 141 controls (19 ± 3 years old, 54% men) were analyzed. Demographic, behavioral, and birth characteristics were summarized in Table 1A. Groups were well balanced in terms of age, sex, race/ethnicity, and median household income as defined by current adolescent zip code. Furthermore, birth weight and gestational age did not differ between groups. HEU adolescents were more often born by Cesarean section than controls (40% vs. 22%, P = 0.03), a finding that likely reflects trends in obstetrical practices to prevent maternal to child HIV transmission.19
Table 1:
Characteristics of Mother-Adolescent Dyads
| HEU Adolescents (n = 50) | Controls (n = 141) | P-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age, years | 18 ± 3 | 19 ± 3 | 0.12 |
| Male, % | 54 | 54 | 0.99 |
| Race/Ethnicity, % | >0.99 | ||
| White | 26 | 26 | |
| Black | 40 | 40 | |
| Asian | 2 | 2 | |
| Hispanic | 30 | 31 | |
| Other | 2 | 1 | |
| Median household income, $ | 57,391 [51,875, 85,399] | 57,143 [51,875, 80,829] | 0.86 |
| Deceased mother, % | 9 | 4 | 0.24 |
| Behavioral Health | |||
| Ever tobacco use, % | 13 | 12 | 0.82 |
| Birth History | |||
| Birth weight, kg | 3.1 ± 0.6 | 3.3 ± 0.7 | 0.20 |
| Full term, % | 80 | 88 | 0.20 |
| C-section, % | 40 | 22 | 0.03 |
| Mothers of HEU Adolescents (n = 48) | Mothers of Controls (n = 97) | P-value | |
| General Prenatal History | |||
| Age at delivery, years | 30 ± 4 | 28 ± 7 | 0.11 |
| Earliest BMI during pregnancy, kg/m2 | 27.2 ± 4.7 | 27.6 ± 5.1 | 0.75 |
| Obesity during pregnancy, % | 25 | 31 | 0.60 |
| Diabetes during pregnancy, % | 0 | 5 | 0.14 |
| Preeclampsia, % | 0 | 4 | 0.23 |
| HIV-Specific Prenatal History | |||
| Duration of HIV, years | 4 [1, 7] | ||
| CD4 count, third trimester, cells/mm3 a | 402 [191, 621] | ||
| HIV viral load < 400 copies/mL, third trimester, % | 73 | ||
| ART during pregnancy, % | 93 | ||
| NRTI | 93 | ||
| NNRTI | 26 | ||
| PI | 48 | ||
| Contemporary Maternal Characteristics | |||
| Education, % | 0.60 | ||
| Less than high school degree | 20 | 18 | |
| High school degree | 56 | 49 | |
| College degree | 24 | 33 | |
| BMI, 35–50 years old, kg/m2 | 27.0 ± 6.8 | 31.6 ± 6.9 | 0.001 |
| BMI category, 35–50 years old, % | 0.003 | ||
| Underweight | 0 | 0 | |
| Normal weight | 45 | 22 | |
| Overweight | 29 | 18 | |
| Obese | 26 | 60 | |
| Ever tobacco use, % | 50 | 30 | 0.03 |
Variables that are normally distributed are expressed as mean ± SD, whereas variables that are not normally distributed are expressed as median [IQR]. Bold text denotes statistical significance with P < 0.05.
Abbreviations: C-section, Cesarean section; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The following number of study participants had data available (HEU, control): (A) adolescent age (50, 141); sex (50, 141); race/ethnicity (50, 141); median household income (50, 141); deceased mother (44, 116); ever tobacco use (45, 133); birth weight (34, 58); full term (45, 86); C-section (45, 82) (B) maternal age at delivery (48, 94); earliest BMI during pregnancy (32, 29); diabetes during pregnancy (41, 59); preeclampsia (40, 56); duration of HIV (47), third-trimester CD4 count (30), third-trimester HIV viral load (30); ART during pregnancy (44); education (41, 78); BMI at 35–50 years old (38, 68); ever tobacco use (44, 82).
One maternal CD4+ T cell count was removed prior to analysis as an outlier confirmed by the Dixon test.
Prenatal and contemporary maternal characteristics were compared in Table 1B. Mothers were of comparable age at the time of delivery. Mothers with HIV were infected for 4 [1, 7] years with third-trimester CD4+ T cell count 402 [191, 621] cells/mm3 and third-trimester HIV viral load undetectable in 73% of individuals. A total of 93% of mothers received prenatal ART. Maternal BMI and prevalence of diabetes during pregnancy were comparable between groups. In contrast, BMI between 35 and 50 years of age was lower among mothers with HIV versus mothers of controls (27.0 ± 6.8 vs. 31.6 ± 6.9 kg/m2, P = 0.001). Any history of tobacco use (50% vs. 30%, P = 0.03) was higher among mothers with HIV than mothers of controls.
Obesity Among HEU Adolescents versus Controls
HEU adolescents had a higher BMI z-score (1.12 ± 1.08 vs. 0.73 ± 1.09, P = 0.03) as well as a heightened prevalence of obesity (42% vs. 22%, P = 0.009) compared to controls (Figure 2). In utero HIV exposure conferred over four-fold increased odds of adolescent obesity upon adjusting for adolescent age and sex, median household income, mode of delivery, maternal BMI between 35 and 50 years of age, and maternal history of tobacco use (OR 4.6, 95% CI 1.4 to 17.0) (Supplemental Table 1). Subjects included in our multivariable model were representative of the overall study sample (Supplemental Table 2). Furthermore, multiple imputation of missing data confirmed our primary results (estimated OR 3.7, 90% coverage 3.1 to 4.6). In a quantitative bias analysis, we estimated that confounding by breastfeeding could potentially attenuate the odds ratio relating HEU status to obesity by approximately 20%.
Figure 2: Obesity and Reactive Airway Disease Among HEU Adolescents versus Controls.
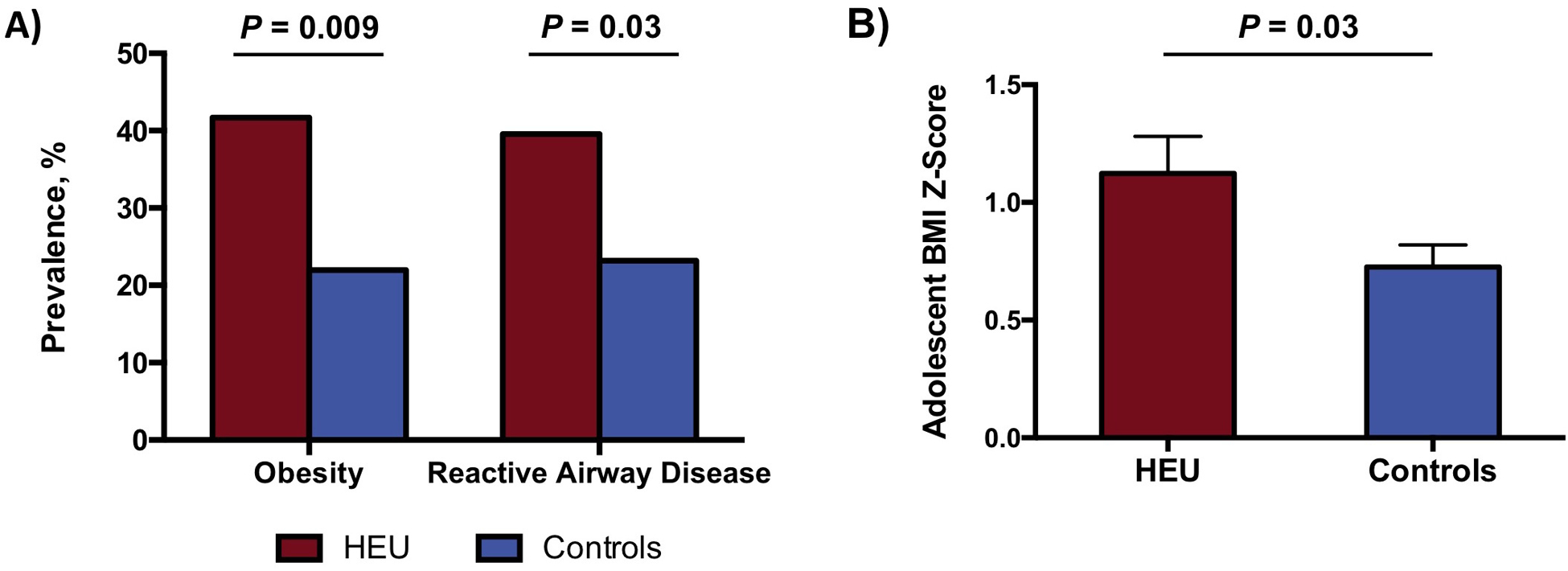
A) HEU adolescents and young adults had a higher prevalence of obesity (42% vs. 22%, P = 0.009) and reactive airway disease (40% vs. 23%, P = 0.03) compared to HIV-unexposed controls. P-values were determined by chi-square test. B) HEU adolescents and young adults had a higher sex- and age-adjusted BMI z-score versus HIV-unexposed controls (1.12 ± 1.08 vs. 0.73 ± 1.09, P = 0.03). Boxes and error bars correspond to mean and standard error of the mean, respectively, with P-value based on two-tailed independent samples t-test.
Maternal obesity between 35 and 50 years of age was associated with an increased frequency of adolescent obesity (P = 0.002) (Figure 3). Among controls, adolescents born to non-obese mothers had an obesity prevalence of 7%, whereas adolescents born to obese mothers had an obesity prevalence of 37%. Notably, within each maternal BMI category, HEU adolescents had a higher frequency of obesity relative to controls (P = 0.007). In contrast to an obesity prevalence of 7% among controls born to non-obese mothers, 35% of HEU adolescents born to non-obese mothers were obese (Figure 3).
Figure 3: Adolescent Obesity by Study Group and Maternal BMI Category.
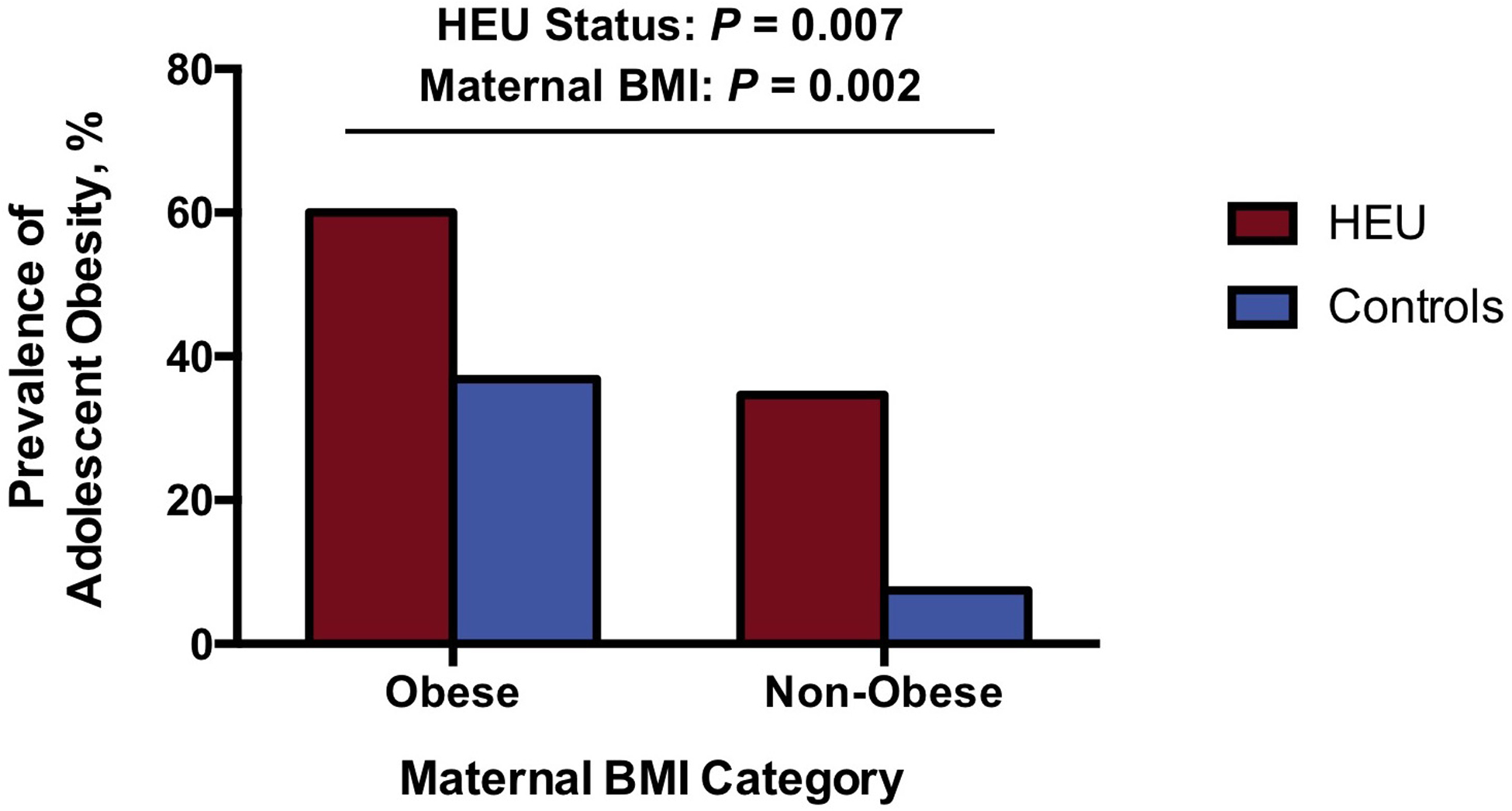
The prevalence of obesity in adolescents and young adults is shown by HEU status and maternal BMI category at 35 to 50 years of age. In utero HIV exposure (P = 0.007) and maternal obesity (P = 0.002) were independently associated with adolescent obesity in a multivariable model. The highest prevalence of obesity was among HEU individuals born to obese mothers. Notably, the prevalence of obesity among controls born to obese mothers approximated the prevalence of obesity among HEU individuals born to non-obese mothers.
Reactive Airway Disease Among HEU Adolescents versus Controls
HEU adolescents had a significantly higher prevalence of RAD compared to controls (40% vs. 23%, P = 0.03) (Figure 2). Additionally, as an index of ongoing asthma-like symptoms, the combination of RAD and albuterol inhaler use was more common among HEU adolescents versus controls (38% vs. 20%, P = 0.01). HEU status conferred four-fold increased odds of RAD in a multivariable model that adjusted for adolescent age and sex, median household income, mode of delivery, maternal BMI between 35 and 50 years of age, maternal history of tobacco use, and adolescent obesity (OR 4.0, 95% CI 1.2 to 15.0) (Supplemental Table 1). Notably, multiple imputation of missing data upheld our primary findings (estimated OR 2.3, 90% coverage 2.0 to 2.7). Additionally, in a quantitative bias analysis, we estimated that confounding by breastfeeding could potentially attenuate the odds ratio relating HEU status to RAD by approximately 20%.
Maternal Prenatal Factors Associated with Long-term Health Outcomes in HEU Adolescents
In an exploratory analysis, we sought to identify maternal prenatal characteristics that were associated with obesity and RAD among HEU adolescents and young adults (Figure 4). There was no evidence of associations between long-term HEU outcomes and maternal age at delivery, any history of tobacco use, HIV duration, HIV viral load, or ART class. On the other hand, there was an inverse correlation between log-transformed maternal third-trimester CD4+ T cell count and BMI z-score among HEU adolescents (r = −0.47, P = 0.01), which remained significant upon adjusting for maternal prenatal characteristics including age at delivery, any history of tobacco use, earliest BMI, HIV duration, and ART regimen. There was no evidence of an analogous relationship between maternal CD4+ T cell count and RAD within the HEU group.
Figure 4: Association of Prenatal Maternal Factors with Obesity in HEU Adolescents.
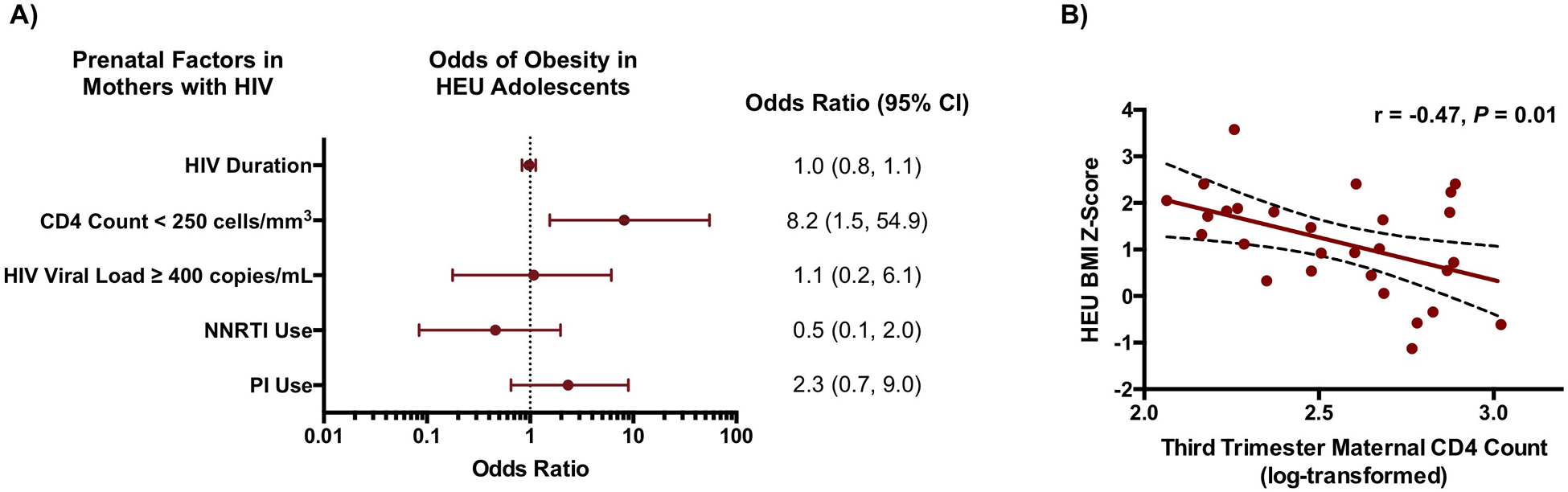
A) Relationships between prenatal factors in mothers with HIV infection and obesity among HEU adolescents and young adults were expressed as odds ratios (OR) with 95% confidence intervals. OR for HIV duration pertains to a one-year change. NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. B) Maternal CD4+ T cell count during pregnancy was inversely associated with BMI among HEU individuals in adolescence and young adulthood (r = −0.47, P = 0.01). The linear regression line is shown with the 95% confidence interval.
Discussion
In one of the oldest HEU cohorts studied to date, we showed for the first time that HIV-negative adolescents and young adults with in utero HIV exposure had at least four-fold increased odds of obesity and RAD compared to well-matched HIV-unexposed controls. These differences persisted in multivariable models adjusting for demographic, socioeconomic, maternal, and early life factors. In addition, we found that lower maternal CD4+ T cell count during pregnancy was associated with higher BMI z-score among HEU individuals at least 12 years later. These findings underscore a potential biologic link between in utero HIV exposure and long-term health outcomes, and may suggest a role of maternal HIV infection in the fetal programming of lifelong susceptibility to disease.
The current study is among the first to indicate that HIV-negative individuals with in utero HIV exposure are at heightened risk of obesity later in life. Specifically, we found that a substantial 42% of HEU adolescents and young adults were obese, which was nearly twice the prevalence among matched controls. Relatedly, we demonstrated that BMI z-score was 0.40 units higher in HEU individuals versus controls. To contextualize this difference, expert consensus guidelines consider a change in BMI z-score of 0.20 to 0.25 to be clinically significant.24,25 In the general population, obesity during adolescence and young adulthood has been associated with high blood pressure, abnormal lipids, and insulin resistance.26 Studies also indicate that obesity early in life rarely remits spontaneously: in a large systematic review, 80% of obese adolescents continued to be obese as adults.27 Since most HEU individuals have not yet reached adulthood, our finding of increased obesity among this older cohort may foreshadow health complications for the expanding HEU population.
Our findings with regard to obesity add to growing evidence of metabolic derangements in the HEU population that begin in early life. In particular, HIV-negative infants with in utero HIV exposure have been shown to have lower pre-prandial insulin levels and altered fuel substrate utilization.6 Abnormalities in mitochondrial structure and function have also been described among HEU infants and children, even in the absence of ART exposure.28–30 Moreover, pooling data from the PHACS and NHANES cohorts, Jao et al. recently found that HEU youth (ages 6–18 years old) with obesity had higher rates of hypertension but lower rates of insulin resistance and dyslipidemia compared to matched obese controls.31 Taken together, these findings may indicate that in utero HIV exposure alters the metabolic program, predisposing not only to obesity but also to a distinct obesity phenotype among HEU individuals. Further studies are needed to elucidate the pathophysiology of elevated BMI in the HEU population so as to optimize prevention and treatment strategies for this group.
In addition to our findings pertaining to obesity, the current study is the first to suggest that HEU adolescents and young adults have an increased susceptibility to RAD compared to well-matched controls. Since our study was focused on long-term health outcomes, only individuals with a documented diagnosis of RAD or asthma beyond 12 years of age were classified as having RAD in this analysis. We further corroborated our findings by comparing the prevalence of asthma-like symptoms during adolescence or young adulthood between groups, defined as a documented diagnosis of RAD plus the use of an albuterol inhaler beyond 12 years of age. Importantly, the association of in utero HIV exposure with RAD remained significant upon controlling for obesity in a multivariable model, which implies that these two long-term health outcomes are independent of one another.
Our findings of increased RAD in HEU individuals are notable in the context of growing evidence of immune dysregulation among the HEU population. In particular, pro-inflammatory cytokines have been found to be elevated in HEU infants, independent of maternal cytokine levels.7,8 Remarkably, HEU children and adolescents also have been shown to exhibit abnormalities in T and B lymphocyte subsets despite many years since their last exposure to HIV viral antigens.32,33 Additionally, CD4+ T cells from HEU infants were found to exhibit a preferential Th2 response, mirroring the shifted Th1/Th2 ratio seen among individuals with HIV infection.9 The current study may extend evidence of immune dysregulation among the HEU population, demonstrating an increased prevalence of RAD in HEU adolescents and young adults. A caveat in this regard is that the pathogenesis of RAD may be multifactorial, although airway inflammation has been described to play a key role.34 Further immune phenotyping of HEU individuals during the transition to adulthood is a critical area for future study.
In our comparison of HEU individuals to the general population, we controlled for numerous biologically relevant factors through our experimental design and statistical analysis plan. By matching subjects on age, sex, race/ethnicity, and zip code, we achieved an even distribution between groups of key demographic factors that may modulate obesity and RAD risk.35 In particular, zip code serves as a crude surrogate for socioeconomic status as well as neighborhood characteristics. Indeed, previous studies have shown that zip code-based parameters including median household income have been associated with an individual’s likelihood of obesity and asthma.36–38
Beyond our matching strategy, we adjusted for multiple biologically relevant characteristics in multivariable models, among the most notable of which is maternal BMI. Maternal BMI reflects an aggregate of factors that may modulate obesity in offspring including lifestyle habits in the household as well as shared genetic susceptibility.39 Consistent with previous reports,39–41 we found that obesity in mothers at 35 to 50 years of age was associated with obesity in offspring during adolescence and young adulthood. However, even upon adjusting for maternal body habitus, HEU status remained independently associated with obesity later in life. Importantly, the prevalence of obesity among controls born to obese mothers approximated the prevalence of obesity among HEU individuals born to non-obese mothers. As such, maternal obesity and HEU status may represent “obesity risk equivalents” among adolescents and young adults.
The independent association of in utero HIV exposure with obesity and RAD may implicate a role for the intrauterine environment in modulating long-term health. For example, in utero exposure to maternal obesity or gestational diabetes has been linked to obesity,13,42 insulin resistance,43,44 asthma,15 and autoimmunity45 later in life. Mechanistic studies have further postulated that epigenetic modifications during fetal development may contribute to these adverse outcomes.42 Notably, HIV infection is characterized by chronic immune activation46,47 and metabolic derangements,48,49 which may confer a pathologic intrauterine state. Though obesity and RAD are known sequelae of fetal exposure to other maternal diseases,13,15,42 this is the first study to demonstrate such findings in the context of maternal HIV infection.
Further supporting a potential biologic link between the intrauterine environment and long-term health of HEU individuals, we showed a significant inverse association between maternal CD4+ T cell count during pregnancy and HEU BMI z-score in adolescence and young adulthood. This association persisted upon adjustment for additional prenatal maternal factors, including HIV-specific factors such as ART class. CD4+ T cell count is a clinical parameter used to monitor HIV infection with lower levels indicating more severe disease. This finding introduces the intriguing possibility that maternal immune dysregulation may be a key component of the HIV intrauterine environment that potentiates metabolic disease risk later in life. Relatedly, in an animal model of maternal inflammation, exposure to the bacterial cell-wall product lipopolysaccharide (LPS) during pregnancy led to increased adiposity and insulin resistance in adult offspring.50 Additional studies are needed to investigate the contribution of HIV-associated maternal immune dysfunction to long-term metabolic outcomes among HEU individuals.
This study comprises, to our knowledge, the oldest cohort of HEU individuals to be compared to general population controls with an average age of 18 years old. As another strength of this analysis, we ascertained both birth history and long-term health outcomes among study subjects, as well as prenatal and contemporary history among mothers of most participants. Furthermore, HEU subjects and controls were matched on numerous demographic factors, and evidence of heightened susceptibility to obesity and RAD remained robust in models adjusting for biologically relevant parameters. HEU and control subjects also originated from the same patient data registry, which served as an additional mechanism to promote balance between groups. Lastly, charts were manually reviewed to confirm HEU status and to extract health information, which has been shown to be more accurate than reliance on diagnostic codes.51
The sample size included in this study was relatively small, and thus our study may have been underpowered to detect relationships between certain covariates. Nonetheless, to our knowledge, our cohort reflects the largest group of HEU adolescents and young adults to be compared to HIV-unexposed controls. Furthermore, our review of over 2,500 medical records to develop our study cohort indicates that the current sample is likely to be representative of the regional population. Differing patterns of abnormalities may be seen among HEU adolescents and young adults in resource-limited settings, and studies are now needed in this regard. Moreover, while we did not observe relationships of prenatal ART class with obesity or RAD, the impact of maternal ART on long-term health outcomes among HEU individuals should be further evaluated in future work. Subjects included in our multivariable models, who had all data available, were comparable to those in our overall sample. Our findings also remained robust in sensitivity analyses involving multiple imputation of missing data. Lastly, we could not adjust for covariates that were not routinely recorded in the medical record such as individual-level household income and infant feeding practices, and thus there was a potential for residual confounding. Even so, our findings persisted in quantitative bias analyses that accounted for expected differences in breastfeeding practices between groups, utilizing conservative estimates.
In conclusion, we show for the first time that HIV-negative individuals with in utero HIV exposure had a significantly heightened prevalence of obesity and RAD, clinically relevant comorbidities, in adolescence and young adulthood compared to HIV-unexposed controls. This study suggests the need to prospectively evaluate the extent of metabolic and immune dysregulation among HEU adolescents and young adults as well as to further elucidate potential mechanisms of disease. Only by characterizing long-term disease burden among HIV-negative individuals with in utero HIV exposure can we optimize clinical care for this burgeoning global population.
Supplementary Material
Acknowledgements
We would like to thank the patients whose medical records made this study possible. Sources of funding for this work were NIH 1KL2TR002542-01 (L.T.F.) and NIH P30 DK040561 (S.K.G.). S.K.G. served as a consultant for Theratechnologies, and received grant support from Theratechnologies, Gilead, and Kowa Pharmaceuticals, all unrelated to this manuscript. T.L.S. received funding from Novo Nordisk for an investigator-initiated grant, also unrelated to the current project. L.T.F., C.S.P., I.Z., M.E.G., A.S., and H.L. have no conflicts of interest to disclose.
Footnotes
Meetings: This manuscript was presented in part at the Conference on Retroviruses and Opportunistic Infections (CROI) in Seattle, WA on March 4–7, 2019. This manuscript was also presented in part at the Endocrine Society Annual Meeting in New Orleans, LA on March 23–26, 2019 where it was selected for an Outstanding Abstract Award and was winner of the Presidential Poster Competition.
References
- 1.Slogrove AL, Becquet R, Chadwick EG, et al. Surviving and Thriving-Shifting the Public Health Response to HIV-Exposed Uninfected Children: Report of the 3rd HIV-Exposed Uninfected Child Workshop. Frontiers in pediatrics. 2018;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nesheim S, Taylor A, Lampe MA, et al. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics. October 2012;130(4):738–744. [DOI] [PubMed] [Google Scholar]
- 3.le Roux SM, Abrams EJ, Donald KA, et al. Growth trajectories of breastfed HIV-exposed uninfected and HIV-unexposed children under conditions of universal maternal antiretroviral therapy: a prospective study. The Lancet. Child & adolescent health April 2019;3(4):234–244. [DOI] [PubMed] [Google Scholar]
- 4.Sudfeld CR, Lei Q, Chinyanga Y, et al. Linear Growth Faltering Among HIV-Exposed Uninfected Children. J Acquir Immune Defic Syndr. October 1 2016;73(2):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filteau S, Baisley K, Chisenga M, Kasonka L, Gibson RS, Team CS. Provision of micronutrient-fortified food from 6 months of age does not permit HIV-exposed uninfected Zambian children to catch up in growth to HIV-unexposed children: a randomized controlled trial. J Acquir Immune Defic Syndr. February 1 2011;56(2):166–175. [DOI] [PubMed] [Google Scholar]
- 6.Jao J, Kirmse B, Yu C, et al. Lower Preprandial Insulin and Altered Fuel Use in HIV/Antiretroviral-Exposed Infants in Cameroon. J Clin Endocrinol Metab. September 2015;100(9):3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirajlal-Fargo S, Mussi-Pinhata MM, Weinberg A, et al. HIV-exposed-uninfected infants have increased inflammation and monocyte activation. AIDS. April 1 2019;33(5):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohman-Payne B, Gabriel B, Park S, et al. HIV-exposed uninfected infants: elevated cord blood Interleukin 8 (IL-8) is significantly associated with maternal HIV infection and systemic IL-8 in a Kenyan cohort. Clinical and translational medicine. September 10 2018;7(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunders MJ, van Hamme JL, Jansen MH, Boer K, Kootstra NA, Kuijpers TW. Fetal exposure to HIV-1 alters chemokine receptor expression by CD4+T cells and increases susceptibility to HIV-1. Sci Rep. October 24 2014;4:6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan AT, Bonawitz R, Gill CJ, et al. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS. September 24 2016;30(15):2351–2360. [DOI] [PubMed] [Google Scholar]
- 11.von Mollendorf C, von Gottberg A, Tempia S, et al. Increased risk for and mortality from invasive pneumococcal disease in HIV-exposed but uninfected infants aged <1 year in South Africa, 2009–2013. Clin Infect Dis. May 1 2015;60(9):1346–1356. [DOI] [PubMed] [Google Scholar]
- 12.Mofenson LM. New challenges in the elimination of pediatric HIV infection: the expanding population of HIV-exposed but uninfected children. Clin Infect Dis. May 1 2015;60(9):1357–1360. [DOI] [PubMed] [Google Scholar]
- 13.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman BL, Rizzo T, Green OC, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. December 1991;40 Suppl 2:121–125. [DOI] [PubMed] [Google Scholar]
- 15.Forno E, Young OM, Kumar R, Simhan H, Celedon JC. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics. August 2014;134(2):e535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandits G, Neuhaus J. Using SAS to Perform Individual Matching in Design of Case-Control Studies Paper presented at: SAS Global Forum 2010; Seattle, WA. [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC). 2000. CDC growth charts. http://www.cdc.gov/growthcharts/cdc_charts.htm. Accessed July 1, 2018.
- 18.United States Census Bureau. American Fact Finder. http://www.factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed May 5, 2019.
- 19.Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States. https://aidsinfo.nih.gov/contentfiles/lvguidelines/perinatalgl.pdf Accessed May 1, 2019. [Google Scholar]
- 20.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. December 13 2014;14:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. May 15 2014;179(10):1153–1167. [DOI] [PubMed] [Google Scholar]
- 22.Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. New York, NY: Springer; 2009. [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). Breastfeeding Report Card - United States, 2007. http://www.cdc.gov/breastfeeding/pdf/2007breastfeedingreportcard.pdf. Accessed May 1, 2019.
- 24.U. S. Preventive Services Task Force. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. June 20 2017;317(23):2417–2426. [DOI] [PubMed] [Google Scholar]
- 25.Wiegand S, Keller KM, Lob-Corzilius T, et al. Predicting weight loss and maintenance in overweight/obese pediatric patients. Horm Res Paediatr. 2014;82(6):380–387. [DOI] [PubMed] [Google Scholar]
- 26.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med. October 2015;373(14):1307–1317. [DOI] [PubMed] [Google Scholar]
- 27.Simmonds M, Burch J, Llewellyn A, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. June 2015;19(43):1–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jao J, Powis KM, Kirmse B, et al. Lower mitochondrial DNA and altered mitochondrial fuel metabolism in HIV-exposed uninfected infants in Cameroon. AIDS. November 28 2017;31(18):2475–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirier MC, Divi RL, Al-Harthi L, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. June 1 2003;33(2):175–183. [DOI] [PubMed] [Google Scholar]
- 30.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. August 15 2003;17(12):1769–1785. [DOI] [PubMed] [Google Scholar]
- 31.Jao J, Jacobson DL, Yu W, et al. A comparison of metabolic outcomes between obese HIV-exposed uninfected youth from the PHACS SMARTT Study and HIV-unexposed youth from the NHANES Study in the U.S. J Acquir Immune Defic Syndr. February 27 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto M, Pessoa SD, Ono E, et al. Low CD4+ T-cell levels and B-cell apoptosis in vertically HIV-exposed noninfected children and adolescents. Journal of tropical pediatrics. December 2010;56(6):427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigano A, Saresella M, Schenal M, et al. Immune activation and normal levels of endogenous antivirals are seen in healthy adolescents born of HIV-infected mothers. AIDS. January 11 2007;21(2):245–248. [DOI] [PubMed] [Google Scholar]
- 34.National Asthma Education and Prevention Program. Expert panel report III: Guidelines for the diagnosis and management of asthma. Bethesda, MD: 2007. [Google Scholar]
- 35.Ogden CL, Fryar CD, Hales CM, Carroll MD, Aoki Y, Freedman DS. Differences in Obesity Prevalence by Demographics and Urbanization in US Children and Adolescents, 2013–2016. JAMA. June 19 2018;319(23):2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez RP. Neighborhood risk factors for obesity. Obesity (Silver Spring). August 2007;15(8):2111–2119. [DOI] [PubMed] [Google Scholar]
- 37.Claudio L, Tulton L, Doucette J, Landrigan PJ. Socioeconomic factors and asthma hospitalization rates in New York City. The Journal of asthma : official journal of the Association for the Care of Asthma. June 1999;36(4):343–350. [DOI] [PubMed] [Google Scholar]
- 38.Lemke LD, Lamerato LE, Xu X, et al. Geospatial relationships of air pollution and acute asthma events across the Detroit-Windsor international border: study design and preliminary results. Journal of exposure science & environmental epidemiology. July 2014;24(4):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. September 25 1997;337(13):869–873. [DOI] [PubMed] [Google Scholar]
- 40.Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes Relat Metab Disord. April 2003;27(4):505–513. [DOI] [PubMed] [Google Scholar]
- 41.Burke V, Beilin LJ, Dunbar D. Family lifestyle and parental body mass index as predictors of body mass index in Australian children: a longitudinal study. Int J Obes Relat Metab Disord. February 2001;25(2):147–157. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, Dolinsky VW. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Critical reviews in clinical laboratory sciences. March 2018;55(2):71–101. [DOI] [PubMed] [Google Scholar]
- 43.Mingrone G, Manco M, Mora ME, et al. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care. September 2008;31(9):1872–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. December 2000;49(12):2208–2211. [DOI] [PubMed] [Google Scholar]
- 45.Lindell N, Carlsson A, Josefsson A, Samuelsson U. Maternal obesity as a risk factor for early childhood type 1 diabetes: a nationwide, prospective, population-based case-control study. Diabetologia. January 2018;61(1):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. July 1 2011;204(1):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified Coronary Atherosclerotic Plaque and Immune Activation in HIV-Infected Women. J Infect Dis. December 2013;208(11):1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. June 2012;205 Suppl 3:S383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peltenburg NC, Schoeman JC, Hou J, et al. Persistent metabolic changes in HIV-infected patients during the first year of combination antiretroviral therapy. Sci Rep. November 16 2018;8(1):16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsson C, Larsson BM, Jennische E, et al. Maternal endotoxemia results in obesity and insulin resistance in adult male offspring. Endocrinology. June 2001;142(6):2622–2630. [DOI] [PubMed] [Google Scholar]
- 51.Horsky J, Drucker EA, Ramelson HZ. Accuracy and Completeness of Clinical Coding Using ICD-10 for Ambulatory Visits. AMIA Annu Symp Proc. 2017;2017:912–920. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


