ABSTRACT
RNA molecules of all species contain modified nucleotides and particularly m5U residues. The vertebrate mitochondrial small subunit rRNA contains m5U nucleotide in a unique site. In this work we found an enzyme, TRMT2B, responsible for the formation of this nucleotide and m5U residues in a number of mitochondrial tRNA species. Inactivation of the Trmt2B gene leads to a reduction of the activity of respiratory chain complexes I, III and IV, containing the subunits synthesized by the mitochondrial protein synthesis apparatus. Comparative sequence analysis of m5U-specific RNA methyltransferases revealed an unusual evolutionary pathway of TRMT2B formation which includes consecutive substrate specificity switches from the large subunit rRNA to tRNA and then to the small subunit rRNA.
KEYWORDS: RNA methyltransferase, ribosome, rRNA, mitochondria, tRNA
Introduction
RNA molecules of all living organisms contain nearly two hundred kinds of modified nucleotides, the majority being methylated[1]. An emerging field of epitranscriptomics [2–4] addresses the significance of RNA modifications in normal and pathological [5–7] organism functioning. Mapping of modified residues within the predominant types of functional RNA molecules of the model species has been essentially completed [8,9], see [10–13] for reviews. The major quest to match existing modification sites with possible RNA modification enzyme candidates has already been finished for E. coli [14] and yeast [10] rRNA methyltransferases. At the same time, the annotation of mammalian RNA modification machinery is still underway [10,11].
Methylated nucleotides within the ribosomal RNA molecules are clustered in the ribosomal functional centres [11,15,16], mammalian ribosomes being no exception. In the course of evolution, some modification sites were preserved most likely from the last universal common ancestor, while other sites are lineage specific. Multiple events of convergent and divergent evolution were proposed for the enzymes involved in methylation of ribosomal RNA and other RNA types involved in translation, such as transfer RNA (see [11] for discussion). In a number of cases RNA methyltransferases were proposed to acquire a new RNA target, e.g. bacterial RlmN was shown to modify both 23S rRNA [17] and tRNA [18]. Mammalian methyltransferase METTL16, which is a likely homolog of bacterial 23S rRNA methyltransferase RlmF [19] modifies U6 snRNA and pre-mRNAs [20]. Dual specificity towards mitochondrial 16S rRNA [21] and tRNA [22] arose in the evolution of the TRMT61B enzyme.
Vertebrate mitochondrial ribosomal RNA molecules possess a number of distinct methylated residues [4,13,23–25] largely reminiscent of those found in other types of ribosomes [11]. Only two residues, m1A947 [26] of 16S rRNA and m5U429 [24] of 12S rRNA (human numbering) could only be found in the ribosomes of vertebrate mitochondria. An enzyme responsible for the modification of the former, TRMT61B [22], is a likely product of ancestral tRNA-specific methyltransferase gene duplication. An enzyme responsible for the formation of the m5U429 nucleotide of 12S rRNA was unknown, while TRMT2B was recently suggested to be responsible for the formation of m5U54 in human mitochondrial tRNAs [27].
In this work we pursued an idea that an enzyme capable of methylating 12S rRNA nucleotide U429 evolved from an ancestor methyltransferase responsible for the generation of m5U residue in other types of RNA. This idea lead us to the characterization of mouse protein TRMT2B as a dual specificity RNA methyltransferase responsible for the generation of m5U425 (mouse numbering, equivalent to human m5U429) residue of the mitochondrial 12S rRNA (Fig. 1A) and m5U54 residue in a number of mitochondrial tRNAs (Fig. 2A).
Figure 1.
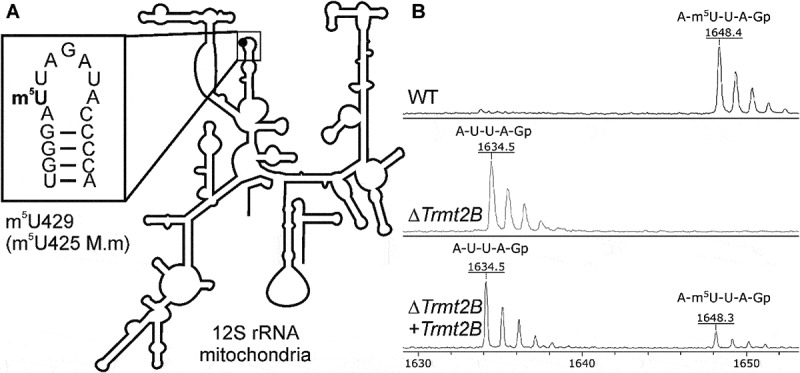
TRMT2B forms methylated m5U425 residue of mouse mitochondrial 12S rRNA. (A) Secondary structure of mouse mitochondrial 12S rRNA [65]. Inset the closeup of the loop of the rRNA helix 24 containing the methylated residue. (B) Mass spectra of the 12S rRNA fragment with U425 nucleotide digested by RNase T1. The upper panel corresponds to wild type cells, the middle panel corresponds to Trmt2B knockout cells, while the lower panel corresponds to the complementation of Trmt2B knockout cells by a reintroduced Trmt2B gene. Mass of 1648 Da corresponds to fully methylated fragment, 1634 Da – fragment without methyl group.
Figure 2.
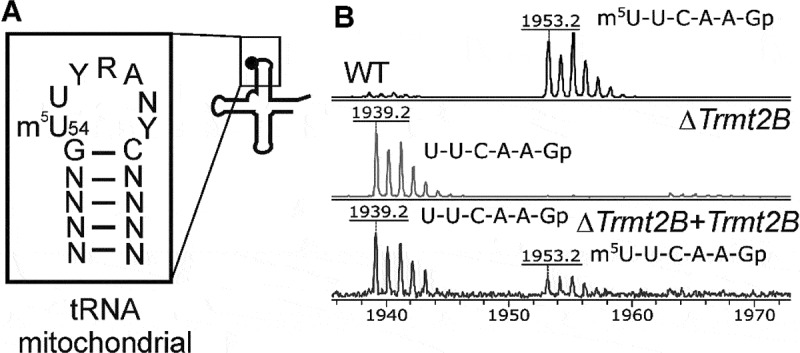
TRMT2B forms methylated m5U54 residue of mouse mitochondrial tRNAIle. (A) Secondary structure of mouse mitochondrial tRNAIle [41]. Inset the closeup of the T-loop consensus of mouse mitochondrial tRNA species containing the methylated residue m5U54. (B) Mass spectra of mouse mitochondrial tRNAIle fragment with U54 nucleotide digested by RNase T1. The upper panel corresponds to wild type cells, the middle panel corresponds to Trmt2B knockout cells, while the lower panel corresponds to the complementation of Trmt2B knockout cells bya reintroduced Trmt2B gene. Mass of 1953 Da corresponds to a fully methylated fragment, 1939 Da – a fragment without a methyl group.
Results
Searching for possible mammalian m5U RNA methyltransferases
Two possible mechanisms are documented for m5U formation in RNA. The major pathway involves Rossman fold S-adenosyl-L-methionine (SAM) dependent methyltransferases [28], while an alternative reaction makes use of N5,N10-methylenetetrahydrofolate (MTHF) as a cofactor to introduce an m5U modification into tRNA of Gram-positive bacteria [29,30] or 23S rRNA of mycoplasma [31]. A homology search [32] for MTHF dependent RNA modification enzymes among mice or human proteins revealed only homologs of yeast MTO1 [33] involved in the formation of a hypermodified τm5s2U residue in the anticodon of mitochondrial tRNAs [34] and thus unlikely to be involved in formation of m5U residues due to the significant difference in the chemical reaction mechanisms.
A search for possible Rossman fold m5U RNA methyltransferases in the set of mice or human proteins revealed two possible candidates for making methylated residue in the mitochondrial 12S rRNA, TRMT2A and TRMT2B. The former, TRMT2A, was recently described as a protein with nuclear localization likely to be responsible for the formation of m5U54 residue within cytoplasmic tRNAs [35]. In contrast to TRMT2A, TRMT2B is likely to be localized in mitochondria [36,37]. Thus, we considered TRMT2B protein as a primary candidate for the putative methyltransferase forming m5U425 12S rRNA residue in the mouse mitochondrial ribosome.
TRMT2B methylates mitochondrial 12S rRNA
A model cell line for analysis of the Trmt2B gene’s function was chosen on the basis of Trmt2B gene expression among a number of mouse cell lines, such as NIH 3T3, NS0, Hepa1-6 and Aml12 using RT qPCR (SI Appendix, Fig. S1). An NS0 mouse myeloma cell line was used for further experimental analysis due to the highest Trmt2B gene expression and the ease of producing large amounts of biomass.
To investigate if TRMT2B methylates 12S rRNA we inactivated the Trmt2b gene in the NS0 mouse myeloma cell line using CRISPR/Cas9 system [38] to introduce double strand break in the first coding exon and selected clones with frameshift mutations generated due to the non-homologous end joining (NHEJ). As a result we obtained two independent cell lines with different sets of frameshift mutations in the Trmt2b gene (Fig. S2). To be sure that the phenotype of the knockout cell lines was not a result of the unlikely event of an off-target genome cleavage, we also checked 5 off-target sites (SI Appendix, Table S1) with the highest score according to Benchling CRISPR guide RNA designing tool (https://benchling.com) and found out there were no differences between WT and KO cell lines in possible off-target cleavage sites (Fig. S3).
To check whether mitochondrial 12S rRNA remained methylated at the U425 site upon Trmt2b inactivation, we prepared the total RNA from the wild type and KO cells and used it for affinity purification of the 12S rRNA fragment. To this end, we designed a 5ʹ-biotinylated oligodeoxyribonucleotide complementary to region 423–460 nucleotides of the 12S rRNA (SI Appendix, Table S2), hybridized it with the total RNA sample and cleaved the excess unprotected RNA with RNase T1. The biotinylated DNA/RNA duplex was bound to streptavidin sepharose and later denatured to release the 12S rRNA fragment containing the site of modification. The fragment was cleaved by a guanylate-specific RNase T1 and the resulting short oligonucleotides were analysed by MALDI mass spectrometry (Fig. 1B). The mass peak corresponding to the fragment containing U425 from the ΔTrmt2B cell line (Fig. 1B, middle panel, 1634 Da peak) appeared to be 14 Da lighter than the one from WT cell line (Fig. 1B, upper panel, 1648 Da peak), which indicates a lack of methylation due to Trmt2B gene inactivation.
To complement the inactivation of the Trmt2B gene in the knockout cell line, we cloned Trmt2B cDNA to the vector [39] capable of Sleeping beauty transposase [40]-driven incorporation into the genome. Complementation of the Trmt2B gene knockout cells resulted in a partial restoration of the 12S rRNA residue m5U425 modification (Fig. 1B, lower panel). Incompleteness of the complementation in our view might be explained either by suboptimal functionality of the TRMT2B protein produced from the artificial construct or by expression of the construct in only a subset of cells in a population.
TRMT2B methylates several mitochondrial tRNAs
Due to structural similarity of TRMT2B with tRNA specific methyltransferases of the TrmA/Trm2 family we hypothesized that TRMT2B may be capable to methylate the T-loop of mitochondrial tRNAs (Fig. 2A). To test this hypothesis we isolated mitochondrial tRNAIle from the wild type, Trmt2B knockout cell lines and the cell line where Trmt2B knockout was partially complemented by reintroduction of Trmt2B gene (Fig. 2B). As a result we saw that methylation of m5U54 of the mitochondrial tRNAIle observed in the wild type cells (Fig. 2B, upper panel, 1953 Da peak) disappeared upon Trmt2B gene inactivation (Fig. 2B, middle panel, 1939 Da peak). Complementation of the Trmt2B gene knockout cells resulted in a partial restoration of the tRNAIle modification (Fig. 2B, lower panel).
Next, we addressed the question whether TRMT2B is responsible for modification of other mitochondrial tRNA T-loops. Sequences of mitochondrial tRNAs are less conserved than those of cytoplasmic or bacterial tRNAs [41]. Only four of them, namely tRNAIle, tRNAGln, tRNALeu(tm5UAA) and tRNASer(UGA) have a ‘canonical’ UUC patch at the 5ʹ-side of the T-loop, while other tRNAs diverge from the T-loop consensus in both the length and composition up to a complete lack of the U54 nucleotide. We managed to check for the m5U54 modification in 10 out of 12 mitochondrial tRNAs that contain the U54 nucleotide (Table 1, Fig. S4). We also analysed possible T-loop methylation of tRNAGly that lacks U54 to make sure that TRMT2B does not methylate other nucleotides of the T-loop. Other mitochondrial tRNAs either lacked U54 or were not amenable to the mass-spectrometry based analysis method we used.
Table 1.
m5U methylation of mito-tRNA T-loop by TRMT2B protein.
| tRNA amino acid | Т-loop | m5U methylation by TRMT2B | |
|---|---|---|---|
| T-loop starts with UUC | Ile | UUCAAGC | + |
| Gln | UUCAAUU | + | |
| Leu(tm5UAA) | UUCAAAU | + | |
| Ser(UGA) | UUCGAUU | partial | |
| Т-loop starts with UU, but the third nucleotide is not С | Tyr | UUUAAAU | + |
| Asn | UUUAAUU | + | |
| Met | UUUAAAU | + | |
| Ser(GCU) | UUUAAAAA | - | |
| Glu | UUGAAUG | not analysed | |
| Т-loop starts with U, but the second nucleotide is not U | Thr | UCUUC | - |
| Asp | UCAAUAA | - | |
| Arg | UGAUGU | not analysed | |
| Leu(UAG) | UGCAAAU | ||
| Т-loop doesn’t start with U | Gly | AUAAAC | - |
| Ala, Cys, His, Lys, Phe, Pro, Trp, Val | not analysed |
As a result we demonstrated that most of the mitochondrial tRNAs whose T-loop starts with UU are methylated by TRMT2B, while those whose T-loop starts with UC or lack U54 are not (Table 1, Fig. S4). Exceptions are serine specific mitochondrial tRNAs. Partial methylation was observed for tRNASer(UGA), while tRNASer(GCU) was shown to lack m5U54 methylation. As a control, we also analysed m5U methylation of cytoplasmic tRNASec and tRNAGly and found that TRMT2B is not responsible for methylation of those tRNAs.
Lack of TRMT2B influences activity of ETC complexes I, III and IV
To analyse an influence of Trmt2B gene inactivation on the function of mitochondria we performed a number of assays. The amount of mitochondrial DNA in a cell is a widely used proxy for the mitochondrial content [42]. The number of mitochondria in the wild type cells and cells with inactivated Trmt2b gene was compared by assessment of the amount of mitochondrial DNA with the help of qPCR (Fig. S5A) and found to be the same. Likewise to check whether inactivation of the Trmt2B gene would result in the decrease in the 12S rRNA abundance we used RT qPCR (Fig. S5B) and found no significant difference.
The role of the TRMT2B methyltransferase in the efficiency of mitochondrial protein synthesis was assessed via [35S]methionine incorporation into the proteins synthesized by the parental NS0 cells and Trmt2b knockout cells, while protein synthesis by the cytoplasmic ribosomes is inhibited by cycloheximide. No significant difference in the radioactive methionine inclusion into proteins synthesized by a mitochondrial protein synthesis apparatus was revealed (Fig. S5C).
While the yield of proteins made with the help of mitochondrial translation machinery was not affected by inactivation of the Trmt2B gene, the activity of the synthesized proteins might be compromised, e.g. if the fidelity of protein synthesis or the folding of nascent proteins is affected. The mammalian mitochondrial genome encodes 13 proteins, all of them being subunits of electron transfer chain (ETC) complexes. To this end we examined ETC complexes’ activity by measuring oxygen consumption in permeabilized cells while being sequentially supplied with appropriate substrates and, later, inhibitors of specific respiratory chain complexes. This way we revealed a small, but statistically significant decrease in the activity of ETC complexes I, III and IV, the ones that contain subunits synthesized in mitochondria. At the same time, the activity of complex II, which is composed entirely of subunits encoded by a nuclear genome was found not to depend on the Trmt2B gene’s inactivation (Fig. 3).
Figure 3.
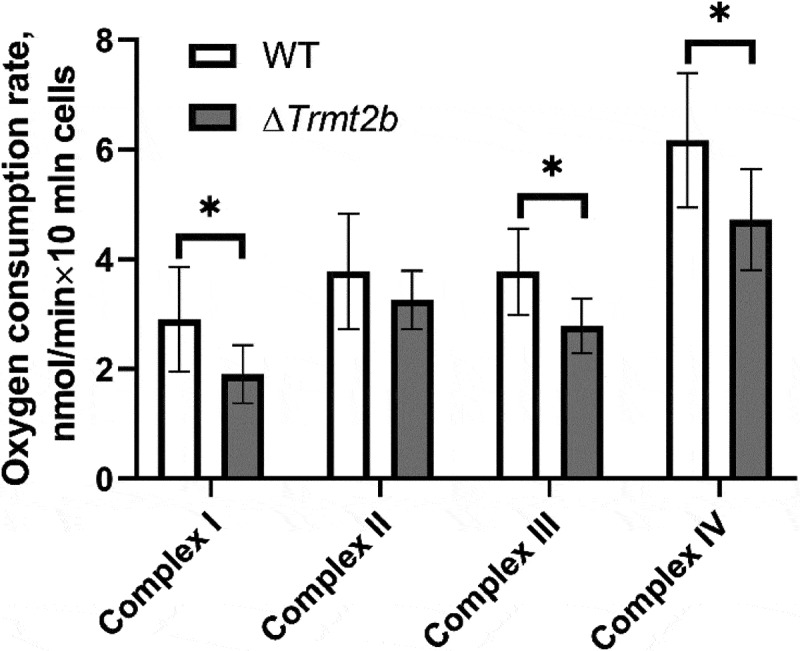
TRMT2B inactivation leads to statistically significant reduction of the respiratory chain complexes I, III and IV activity. Shown are oxygen consumption rates of the permeabilized wild type (open bars) and Trmt2B knockout (grey bars) cells. Substrates and inhibitors of the complexes I-IV were added sequentially, see materials and methods.
Discussion
The mitochondrial ribosome most likely evolved from the ribosome of a bacterial endosymbiont of the predecessor of eukaryotic cells [43]. In the process of evolution, the mitochondrial ribosome underwent a significant reduction of its RNA components concomitant with a swelling of ribosomal proteins [44,45]. Likely, because of that, a set of modified nucleotides of the mitochondrial ribosome, particularly mammalian mitoribosome [4,11,13,23,24], resembles a subset of those in a bacterial ribosome (Table 2). Unique for the mitochondrial rRNA are modified nucleotides m1A947 [26] of the 16S rRNA and m5U429 [24] of the 12S rRNA (human numbering), both modified with dual-specific rRNA/tRNA methyltransferases. Curiously, in our analysis of tRNA T-loop modification we observed a lack of m1A58 modification in mouse mitochondrial tRNALeu(tm5UAA), which was previously reported to be methylated by TRMT61B in human cells [21]. This apparent discrepancy is explained by the lack of Trmt61B gene functionality in mice [46] which is remarkably accompanied by the substitution of its target nucleotide m1A947 of the mitochondrial 16S rRNA to uridine.
Table 2.
Enzymes responsible for the modification of mammalian mitochondrial rRNA.
| 12S rRNA | Enzyme/reference | Bacterial homolog |
|---|---|---|
| m5U429(m5U425)* | TRMT2B (this study) | no |
| m4C839 | METTL15[50] and Laptev et al, in revision | RsmH[47] |
| m5C841 | NSUN4[60] | RsmF**[48] |
| m26A936, m26A937 | TFB1M[51] | KsgA[54] |
| 16S rRNA | ||
| m1A947 | TRMT61B[22] | no |
| Gm1145 | MRM1[52] | RlmB[59] |
| Um1369 | MRM2[52] | RlmE[55,56] |
| Gm1370 | MRM3[52] | no |
*Human numbering, mouse numbering is provided in parenthesis.
**E.coli RsmF modifies the nucleotide m5C1407 of the 16S rRNA [48], which is proximal to C1404, equivalent to m5C841.
Now, when the search for mammalian mitochondrial rRNA methyltransferases has been completed with this work and the work of Powell and Minczuk [49] and identification of METTL15 as m4C-specific 12S rRNA methyltransferase (REF [50] and Laptev et al., under revision) we can compare the essentiality of all the enzymes from the list (Table 2). On the average, the more conserved mitochondrial rRNA methyltransferases are the more severe is the phenotype of their knockout. The universally conserved TFB1M [51] and MRM2 [52,53] proteins which belong to the families of bacterial KsgA [54] and RlmE [55,56] methyltransferases are important for mitochondrial ribosome assembly and translation [53,57]. The MRM3 protein responsible for the modification of nucleotide Gm1370 of the mitochondrial 16S rRNA A-loop, next to the Um1369 nucleotide modified by MRM2, is also important, since its deficiency results in a marked decrease of mitochondrial translation [52]. Modification of the 16S rRNA P-loop by MRM1 [52] appears to be less crucial [58], and likewise modification of the bacterial P-loop by its ortholog, RlmB [59]. Similarity in the significance of bacterial rRNA methyltransferases and their mitochondrial counterparts does not always hold true. E.g. a lack of bacterial RsmF protein responsible for the formation of the m5C1407 nucleotide of 16S rRNA does not have momentous consequences beyond moderate growth retardation, while inactivation of its mitochondrial ortholog NSUN4 is embryonically lethal due to mitochondrial ribosome instability and ETC complexes’ assembly failure [60]. In addition to its function as a modification enzyme, NSUN4 is known to form a stable complex with the mitochondrial ribosome assembly factor MTERF4, thus playing a role in both ribosome modification and assembly [61,62]. Not all mitochondrial rRNA methyltransferase knockouts resulted in spectacular phenotypes. E.g. a deficiency of TRMT61B [22] is likely to manifest itself in some specific and yet to be established conditions. Inactivation of TRMT2B, as we demonstrated in this work, resulted in statistically significant reduction in the activity of ETC complexes containing mitochondrially encoded subunits. However, the consequences of TRMT2B knockout are much milder than those of TFB1M or NSUN4 inactivation (see above). While, apparently, the mitochondrial protein synthesis yield was unreduced upon Trmt2b gene inactivation (Fig. S5C) in total agreement with the results of Minczuk group [49], the reduction in the activity of ETC complexes containing mitochondrially encoded subunits might hypothetically be explained by a reduction of protein synthesis fidelity. Unfortunately, an in vitro translation system is unavailable for mitochondria making us unable to directly test this possibility.
We also have to admit that we could not definitely state whether the observed phenotype is explained by the lack of 12S rRNA or tRNA modification. Methylation of the tRNA T-loop might be important for its stability, at least when combined with secondary tRNA mutations, decreasing its propensity to fold into a correct structure [63]. Curiously, bovine mitochondrial tRNAs lack U54 methylation [64]. Although it is not known whether bovine mitochondrial 12S rRNA carries m5U residue, the genome of Bos taurus contains a seemingly functional Trmt2B gene, hypothetically responsible only for 12S rRNA modification. Among human mitochondrial tRNAs, whose modification patterns are known only tRNALeu(tm5UAA) and tRNAPro(UGG), contain m5U54 residue[1]. These two species are the only among the known set containing a 7 nucleotide T-loop starting with UU, thus the rules determining whether tRNA would be a substrate for TRMT2B are likely to be the same for mouse and human mitochondria.
The dual specificity of the TRMT2B methyltransferase might be explained by a remarkable similarity between the structures of the 12S rRNA loop 24 (Fig. 1A) and the T-loop of tRNA (Fig. 2A). However, a possibility that modification of the 12S rRNA by TRMT2B is simply a coincidence explained by the structural similarity of the loops that are modified, in our view is unlikely. While the 16S nucleotide m1A947 modified by another dual specific rRNA/tRNA methyltransferase is not conserved, nucleotide m5U425 of the 12S rRNA is universally conserved and located in the highly conserved [65] loop 24, contacting mRNA [66] and forming a ridge between the P and E-site bound tRNA anticodon loops [67]. While the methyl group of the nucleotide m5U425 is not in direct contact with the ligands of the ribosome it is likely that this modification could stabilize the structure of the RNA loop 24 it belongs to. In line with this idea, cytoplasmic ribosomes contain pseudouridine [68], known to stabilize stacking [69], at the position equivalent to that of nucleotide m5U425 of mitochondrial 12S rRNA.
In an enzymatic system of m5U formation one could find examples of both convergent and divergent evolution (see [11] for discussion) including switches between Rossman fold SAM-dependent RNA methyltransferases and MTHF dependent enzymes [29–31]. Various blends of single and dual specificity enzymes acting on rRNA and tRNA are present even among the Rossman fold m5U forming RNA methyltransferases (Fig. 4A, Fig. S6). The ancestral m5U RNA methyltransferase is likely to be bacterial RlmD [70], an enzyme which modifies nucleotide m5U1939 of the 23S rRNA. In a number of bacterial lineages, RlmD-like enzymes acquired an ability to modify both m5U1939 and m5U747 of the 23S rRNA [71]. This new function is likely to require an insertion of two loops into the structure of the central domain of the parental RlmD-like protein [72] (Fig. 4B, Fig. S6). In proteobacteria, such as E. coli, most likely due to gene duplication and the following specialization, RlmC and TrmA enzymes have been evolved in addition to RlmD (Fig. 4). RlmC, an enzyme that lacks the TRAM domain of RlmD and RlmCD, but retains insertions in the central domain, is responsible for the modification of m5U747 of the 23S rRNA [73]. TrmA is the m5U54 tRNA methyltransferase [74] that lacks RlmC and RlmCD-specific insertions, but has a new loop in the Rossman fold methyltransferase domain (Fig. 4B, Fig. S6) [75]. Surprisingly, fungal Trm2 proteins responsible for m5U54 modification in tRNA [76] form a separate lineage close to bacterial rRNA-specific RlmCD methyltransferases rather than to bacterial TrmA tRNA-specific methyltransferases (Fig. 4). Enzymes of higher eukaryotes responsible for m5U formation are also likely to originate from the predecessor rRNA-specific methyltransferase (Fig. 4). They lack a TrmA-specific extension in the Rossman fold methyltransferase domain (Fig. 4, Fig. S6), but have insertions in the central domain reminiscent of bacterial RlmCD/RlmC. Unlike fungal Trm2 and bacterial RlmD and RlmCD, the ancestor of m5U specific methyltransferases of higher eukaryotes acquired an RRM domain instead of a TRAM domain (Fig. 4). It is likely that this enzyme was tRNA-specific since tRNAs are the common targets for all enzymes of this clade. Finally, in the evolution of vertebrates, TRMT2B methyltransferase, as we demonstrated in this work, became again a dual specificity RNA methyltransferase, this time capable of modifying not only mitochondrial tRNA, but the mitochondrial small subunit ribosomal 12S RNA, thus being the only group in the entire family acting on the small ribosomal subunit. Enzymes responsible for m5U modification switched the substrate several times from rRNA to tRNA and later to an unrelated site of another rRNA molecule.
Figure 4.
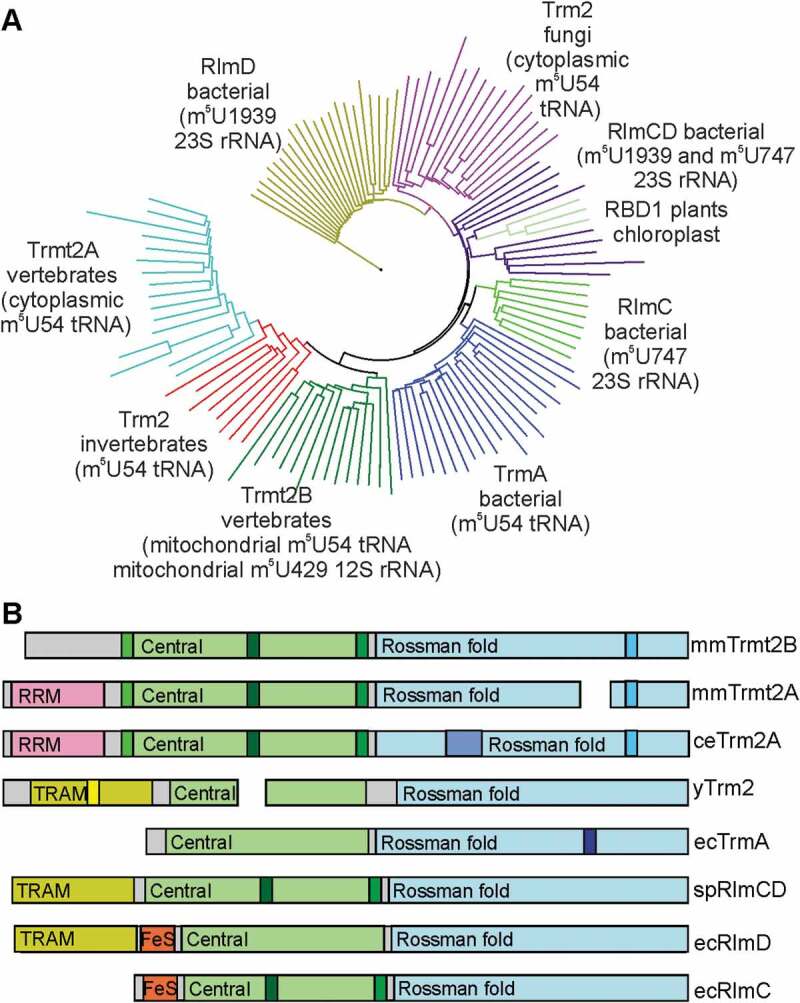
Evolution of m5U RNA methyltransferases. (A) Evolutionary tree of m5U RNA methyltransferases. Different clades are marked by different colours and labelled with the name of enzyme, group of organisms and substrate. (B) A scheme of domain structures and group-specific insertions. Enzyme names preceded by organism designations are shown on the right side of the scheme: Mus musculus (mm), Caenorhabditis elegans (ce), yeast Saccharomyces cerevisiae (y), Escherichia coli (ec) and Streptococcus pneumoniae (sp). Different colours correspond to common protein domains labelled accordingly, such as Central domain, Rossman fold domain, TRAM domain, RRM domain and iron-sulphur cluster (FeS). Group specific insertions are marked with different hues.
Materials and methods
Cell culture
Mouse NS0 cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% FBS, GlutaMAX and penicillin and streptomycin at 37°C, 5% CO2. Large cell culture volumes were grown in Thomson Optimum Growth 1.6 L flasks in a shaker-incubator at 110 rpm. NIH3T3, Hepa1-6 and Aml12 were cultured in tissue treated flasks in DMEM/F12 medium (Gibco) supplemented with 10% FBS, GlutaMAX and penicillin and streptomycin at 37°C, 5% CO2.
Trmt2b gene inactivation
The sequence of gRNA (5ʹ-GTGCTTCTTAGCTATCACGC-3ʹ) for Cas9 targeting the first coding exon of Trmt2b was generated using the Benchling CRISPR guide RNA designing tool (https://benchling.com). Two complementary oligodeoxyribonucleotides containing the gRNA sequence and BbsI ligation adapters were annealed and ligated into BbsI-digested pX458 vector [38] (Addgene #48138). NS0 cells were transfected with the pX458 plasmid containing the gRNA sequence using a Lipofectamine 3000 reagent (Thermo Scientific). 24 hours after transfection GFP-positive cells were sorted using BD FACS Aria III in 96-well plates containing 0.2 mL of RPMI medium per well. Individual clones were analysed by PCR amplification of ~250 bp Trmt2b fragment (5ʹ-TCAAGAGTCCTAAATGCACAACC-3ʹ and 5ʹ-CCAGGAGTCATCTCTACAATGC −3ʹ) and sequencing of the amplicon. For off-target analysis we chose 5 off-targets with the highest score according to the Benchling CRISPR guide RNA designing tool (https://benchling.com). Each off-target was analysed by PCR amplification of ~250 bp. Sequences of possible off-targets and primers for them are shown in the Table S1.
Ectopic expression of the Trmt2b gene
Total RNA was isolated from the NS0 cell line using a PureLink RNA Mini Kit (Thermo Scientific). TRMT2B cDNA was synthesized with reverse transcription by Maxima Reverse Transcriptase (Thermo Scientific) and a specific primer (5ʹ-TGTCTCCTATGGCAACCTGG-3ʹ) followed by PCR with Q5 High-Fidelity DNA Polymerase (NEB) using primers (5ʹ-GGCCTCTGAGGCCATGCACAACCC-AAGACTATTC-3ʹ and 5ʹ-GGCCTGACAGGCCTTACCGAGTGAAGAGGAGAAC-3ʹ). PCR product was ligated into a SmaI-digested and dephosphorylated pUC19 vector. TRMT2B ORF was then inserted into pSBtet-Neo [39] (Addgene #60509) by restriction free cloning [77] using the following primers for the first PCR: forward – 5ʹ-TCGAAAGGCCTCTGAGGCCATG-3ʹ, reverse – 5ʹ-GCTT-GGCCTGACAGGCCTTAC-3ʹ.
The Trmt2b KO cell line cell line was electroporated with plasmids coding for the TRMT2B and SB100x transposase [40] (pCMV(CAT)T7-SB100, Addgene #34879) in a mass ratio of 19:1 using a Neon Transfection System (Thermo Scientific) according to the manufacturer’s instructions. 24 hours after transfection, electroporation cells were subjected to G418 (1 mg/mL, Thermo Scientific). Selection was carried out for 2 weeks and then cells were cultivated in the medium without G418.
12S rRNA and mt-tRNAs isolation
Total RNA was isolated using an ExtractRNA reagent (Evrogen). The 12S rRNA fragment and tRNAs was isolated using 5ʹ-biotinylated oligodeoxyribonucleotides (Table S2) complementary to the region containing the nucleotide to be analysed. Total RNA (2 mg/mL, 6 mg) and the oligonucleotide (200 pmol/mL) were heated in a 6x SSC buffer for 5 min at 95°C and left in the dry block to anneal. In case of rRNA, after cooling to ~37°C, RNase T1 (Thermo Scientific) was added to the mixture to a final concentration of 1 unit/ul and incubated for 1 h at 37°C. Then the solution was mixed with Streptavidin Sepharose High performance (GE Healthcare) and incubated for 20 min at room temperature with rotation. The resin was washed 3 times with 3x SSC, 4 times with 1x SSC and 4 times with 0.1x SSC. The 12S rRNA fragment was eluted from the resin by adding elution buffer (0.1x SSC, 6M urea) and incubating for 5 min at 75°C. RNA from the eluate was precipitated with isopropanol overnight at −20°C. The precipitate was dissolved in 1x RNA Loading Dye (Thermo Scientific) and loaded on to 12% denaturing polyacrylamide gel. The gel was stained by ethidium bromide and the desired band was excised. The RNA band was fragmented and washed twice with a washing buffer (25 mM ammonium citrate, 50% acetonitrile) at room temperature for 10 min and dried with 100% acetonitrile.
MALDI MS analysis
For MALDI MS a fragmented gel band was dried from acetonitrile in air at room temperature and RNA was digested with 1 unit/ul RNase T1 or 20 ug/mL RNase A in 50 mM ammonium citrate for 3 hours at 37°C. A digestion mixture (0.5 ul) was mixed with 50 mg/mL 2,5-dihydroxybenzoic acid in 0.5% trifluoroacetic acid and 30% acetonitrile (1.5 ul) and was left to air dry at room temperature. MALDI mass spectrometry was performed in a reflector mode on an Ultraflex III BRUKER equipped with a UV laser (Nd, 335 nm) detecting positive ions.
qPCR
To compare the amount of different rRNAs in WT and KO cell lines, total RNA was isolated using PureLink RNA Mini Kit (Thermo Scientific). Total RNA was treated with DNase I (Thermo Scientific) followed by reverse transcription using Maxima First Strand cDNA Synthesis Kit (Thermo Scientific). SYBR Green PCR Master Mix (Thermo Scientific) was used to carry out real-time PCR reactions using a Biorad CFX96 Touch Real-Time PCR Detection System. Each sample was run in triplicate and samples from 4 different experiments were analysed in parallel. The primer sequences for analysis are the following:
12S rRNA – 5ʹ-CTCAAAGGACTTGGCGGTAC-3ʹ, 5ʹ-GTTTGCTGAAGATGGCGGTA-3ʹ; 16S rRNA – 5ʹ-TGAAATTTCGGTTGGGGTGA-3ʹ, 5ʹ-TCCCTAGGGTAACTTGGTCC-3ʹ; 18S rRNA – 5ʹ-GTAACCCGTTGAACCCCATT-3ʹ, 5ʹ-GGCCTCACTAAACCATCCAAT-3ʹ; Gapdh – 5ʹ-GGTCCCAGCTTAGGTTCATCAG-3ʹ, 5ʹ-GTCGTTGATGGCAACAATCTCCAC-3ʹ.
Relative changes in transcript levels were calculated using the ΔCt method. The mean expression level of Gapdh was used for normalization.
For comparison of mtDNA copy number, total DNA was isolated by a phenol-chloroform extraction method and precipitated by ethanol. qPCR reactions were performed as described before, except that the reverse transcription step was omitted. Each sample was run in triplicate and samples from 4 different experiments were analysed in parallel. Primers for 12S rRNA and 16S rRNA were the same as for the analysis of the rRNA amount. Relative changes in DNA levels were calculated using the ΔCt method. The concentration of a single copy locus of the nuclear DNA (primers – 5ʹ-CTTCCTTCTCTTACCTGCACGCC-3ʹ, 5ʹ-GGTTACCAATGTCAGCGACGAGG-3ʹ) was used for normalization.
Mitochondrial protein synthesis
Mitochondrial protein biosynthesis was analysed as described in [78] with minor modifications. Briefly, ~1 · 106 cells, cultured at 37°C and in a 5% CO2 atmosphere in the appropriate medium, were harvested by centrifugation at 200 × g for 5 min, rinsed with 1 ml of PBS and resuspended in 1 ml of methionine-free medium supplemented with 200 ug/ml cycloheximide. After 5 minute incubation at 37°C in 5% CO2, 5–10 uCi of EasyTag™ L-[35S]-labelled methionine (Perkin Elmer) was added, and incubation continued for 45 minutes. The labelling was stopped by addition of non-radioactive methionine (final concentration 80 mM) and puromycin (final concentration 4 ug/ml). Cells were harvested, washed with PBS twice, resuspended in 100–150 ul of PBS and lysed with ultrasound. Protein concentrations were measured by Bradford, equal amounts of total protein from different samples (30–50 ug) were applied to 15% – 20% gradient denaturing polyacrilamide gel. After electrophoresis at 25 mA for 5–6 hours, gels were stained with Coomassie G-250, dried and analysed by autoradiography with a Storm 865 scanner (GE Healthcare). Gels images were analysed using ImageJ software (NCBI)
Activity of respiratory complexes
NS0 cells were harvested at ~106 cells/ml, 107 cells were washed with PBS, resuspended in respiration medium (0.137 M NaCl, 5 mM KCl, 0.7 mM NaH2PO4, 25 mM Tris-HCl solution with pH 7.4), incubated with 20 ug/mL digitonin 5 min at 37°C and were placed in an Oxytherm (Hansatech, UK) chamber heated to 37°C. Stepwise control of the ETC-CI linked respiration was assessed using the protocol described in [79]. Briefly, substrates or their precursors and then inhibitors of ETC complexes I (glutamate, malate – rotenone), II (succinate – malonate), III (glycerol-3-phosphate – antimycin A) and IV (TMPD, ascorbate – KCN) were added to the chamber consequentially. The next substance was added to the chamber when the oxygen consumption rate was stable for 2 min. Activity of ETC complexes were calculated as the difference in oxygen consumption rates after addition of the substrate and inhibitor.
Funding Statement
This work was supported by the Russian Science Foundation [19-14-00043]; Russian Foundation for Basic Research [18-29-07002, 17-00-00366].
Acknowledgments
Authors are very thankful to Alex Lebedeff for improvement of the English of the manuscript. This work was supported by RFBR grant 17-00-00366 (P.S.) in part related to analysis of translation, RFBR grant 18-29-07002 (S.L.) in part related to respiratory chain activity measurements and RSF grant 19-14-00043 (P.S.) in all other parts. Stipend of participants was in part covered by Moscow State University Grant for Leading Scientific Schools “Depository of the Living Systems” in frame of the MSU Development Program.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author contributions
PS designed the project and performed bioinformatic analysis of putative RNA methyltransferases, IL and ES performed major part of experimental work except the parts listed below, SL performed mitochondrial translation assays, MS performed mass spectrometry analysis, MR performed cell sorting to create cell lines; AB, IL, PS, PK, and OD contributed to manuscript writing.
Supplementary material
The Supplemental data for this article can be accessed here.
References
- [1].Boccaletto P, Machnicka MA, Purta E, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhao BS, Roundtree IA, He C.. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peer E, Rechavi G, Dominissini D. Epitranscriptomics: regulation of mRNA metabolism through modifications. Curr Opin Chem Biol. 2017;41:93–98. [DOI] [PubMed] [Google Scholar]
- [4].Bohnsack MT, Sloan KE. The mitochondrial epitranscriptome: the roles of RNA modifications in mitochondrial translation and human disease. Cell Mol Life Sci. 2018;75:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stojković V, Fujimori DG. Mutations in RNA methylating enzymes in disease. Curr Opin Chem Biol. 2017;41:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Batista PJ. The RNA modification N6-methyladenosine and its implications in human disease. Genomics Proteomics Bioinformatics. 2017;15:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jonkhout N, Tran J, Smith MA, et al. The RNA modification landscape in human disease. RNA. 2017;23:1754–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Piekna-Przybylska D, Decatur WA, Fournier MJ. The 3D rRNA modification maps database: with interactive tools for ribosome analysis. Nucleic Acids Res. 2008;36:D178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang J, Sharma S, Watzinger P, et al. Mapping of complete set of ribose and base modifications of yeast rRNA by RP-HPLC and mung bean nuclease assay. PLoS One. 2016;11:e0168873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sharma S, Lafontaine DLJ. ‘View from a bridge’: a new perspective on eukaryotic rRNA base modification. Trends Biochem Sci. 2015;40:560–575. [DOI] [PubMed] [Google Scholar]
- [11].Sergiev PV, Aleksashin NA, Chugunova AA, et al. Structural and evolutionary insights into ribosomal RNA methylation. Nat Chem Biol. 2018;14:226–235. [DOI] [PubMed] [Google Scholar]
- [12].Meyer KD, Jaffrey SR. Rethinking m 6 A readers, writers, and erasers. Annu Rev Cell Dev Biol. 2017;33:319–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rorbach J, Minczuk M. The post-transcriptional life of mammalian mitochondrial RNA. Biochem J. 2012;444:357–373. [DOI] [PubMed] [Google Scholar]
- [14].Golovina AY, Dzama MM, Osterman IA, et al. The last rRNA methyltransferase of E. coli revealed: the yhiR gene encodes adenine-N6 methyltransferase specific for modification of A2030 of 23S ribosomal RNA. RNA. 2012;18:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brimacombe R, Mitchell P, Osswald M, et al. Clustering of modified nucleotides at the functional center of bacterial ribosomal RNA. Faseb J. 1993;7:161–167. [DOI] [PubMed] [Google Scholar]
- [16].Sergiev PV, Golovina AY, Prokhorova IV, et al. Modifications of ribosomal RNA: from enzymes to function [Internet]. In: Rodnina MV, Wintermeyer W, Green R, editors. Ribosomes: structure, Function, and dynamics. Vienna: Springer Vienna; 2011. p. 97–110. [cited 2019 May18]. Available from: 10.1007/978-3-7091-0215-2_9 [DOI] [Google Scholar]
- [17].Toh S-M, Xiong L, Bae T, et al. The methyltransferase YfgB/RlmN is responsible for modification of adenosine 2503 in 23S rRNA. RNA. 2008;14:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Benítez-Páez A, Villarroya M, Armengod M-E. The Escherichia coli RlmN methyltransferase is a dual-specificity enzyme that modifies both rRNA and tRNA and controls translational accuracy. RNA. 2012;18:1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sergiev PV, Serebryakova MV, Bogdanov AA, et al. The ybiN gene of Escherichia coli encodes adenine-N6 methyltransferase specific for modification of A1618 of 23 S ribosomal RNA, a methylated residue located close to the ribosomal exit tunnel. J Mol Biol. 2008;375:291–300. [DOI] [PubMed] [Google Scholar]
- [20].Pendleton KE, Chen B, Liu K, et al. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169:824–835.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chujo T, Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18:2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bar-Yaacov D, Frumkin I, Yashiro Y, et al. Mitochondrial 16S rRNA is methylated by tRNA methyltransferase TRMT61B in all vertebrates. PLoS Biol. 2016;14:e1002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baer R, Dubin DT. The 3ʹ-terminal sequence of the small subunit ribosomal RNA from hamster mitochondria. Nucleic Acids Res. 1980;8:4927–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baer RJ, Dubin DT. Methylated regions of hamster mitochondrial ribosomal RNA: structural and functional correlates. Nucleic Acids Res. 1981;9:323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rebelo-Guiomar P, Powell CA, Van Haute L, et al. The mammalian mitochondrial epitranscriptome. Biochim Biophys Acta Gene Regul Mech. 2019;1862:429–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bar-Yaacov D, Avital G, Levin L, et al. RNA-DNA differences in human mitochondria restore ancestral form of 16S ribosomal RNA. Genome Res. 2013;23:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].de Crécy-lagard V, Boccaletto P, Mangleburg CG, et al. Matching tRNA modifications in humans to their known and predicted enzymes. Nucleic Acids Res. 2019;47:2143–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bujnicki JM. Sequence-structure-function studies of tRNA:m5C methyltransferase Trm4p and its relationship to DNA:m5C and RNA:m5U methyltransferases. Nucleic Acids Res. 2004;32:2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Urbonavicius J. Identification of a novel gene encoding a flavin-dependent tRNA: m5Umethyltransferase in bacteria–evolutionary implications. Nucleic Acids Res. 2005;33:3955–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Delk AS, Nagle DP, Rabinowitz JC. Methylenetetrahydrofolate-dependent biosynthesis of ribothymidine in transfer RNA of Streptococcus faecalis. Evidence for reduction of the 1-carbon unit by FADH2. J Biol Chem. 1980;255:4387–4390. [PubMed] [Google Scholar]
- [31].Lartigue C, Lebaudy A, Blanchard A, et al. The flavoprotein Mcap0476 (RlmFO) catalyzes m5U1939 modification in Mycoplasma capricolum 23S rRNA. Nucleic Acids Res. 2014;42:8073–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. [DOI] [PubMed] [Google Scholar]
- [33].Li X, Li R, Lin X, et al. Isolation and characterization of the putative nuclear modifier gene MTO1 involved in the pathogenesis of deafness-associated mitochondrial 12 S rRNA A1555G mutation. J Biol Chem. 2002;277:27256–27264. [DOI] [PubMed] [Google Scholar]
- [34].Umeda N, Suzuki T, Yukawa M, et al. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs: implications for the molecular pathogenesis of human mitochondrial diseases. J Biol Chem. 2005;280:1613–1624. [DOI] [PubMed] [Google Scholar]
- [35].Chang Y-H, Nishimura S, Oishi H, et al. TRMT2A is a novel cell cycle regulator that suppresses cell proliferation. Biochem Biophys Res Commun. 2019;508:410–415. [DOI] [PubMed] [Google Scholar]
- [36].Smith AC, Robinson AJ. MitoMiner v3.1, an update on the mitochondrial proteomics database. Nucleic Acids Res. 2016;44:D1258–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ran FA, Hsu PD, Wright J, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kowarz E, Löscher D, Marschalek R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol J. 2015;10:647–653. [DOI] [PubMed] [Google Scholar]
- [40].Mátés L, Chuah MKL, Belay E, et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet. 2009;41:753–761. [DOI] [PubMed] [Google Scholar]
- [41].Juhling F, Morl M, Hartmann RK, et al. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Medeiros DM. Assessing mitochondria biogenesis. Methods. 2008;46:288–294. [DOI] [PubMed] [Google Scholar]
- [43].Sagan L. On the origin of mitosing cells. 1967. J NIH Res. 1993;5:65–72. [PubMed] [Google Scholar]
- [44].Ott M, Amunts A, Brown A. Organization and regulation of mitochondrial protein synthesis. Annu Rev Biochem. 2016;85:77–101. [DOI] [PubMed] [Google Scholar]
- [45].Greber BJ, Ban N. Structure and function of the mitochondrial ribosome. Annu Rev Biochem. 2016;85:103–132. [DOI] [PubMed] [Google Scholar]
- [46].Kawai J, Shinagawa A, Shibata K, et al. Functional annotation of a full-length mouse cDNA collection. Nature. 2001;409:685–690. [DOI] [PubMed] [Google Scholar]
- [47].Kimura S, Suzuki T. Fine-tuning of the ribosomal decoding center by conserved methyl-modifications in the Escherichia coli 16S rRNA. Nucleic Acids Res. 2010;38:1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Andersen NM, Douthwaite S. YebU is a m5C methyltransferase specific for 16 S rRNA nucleotide 1407. J Mol Biol. 2006;359:777–786. [DOI] [PubMed] [Google Scholar]
- [49].Powell CA, Minczuk M. TRMT2B is responsible for both tRNA and rRNA m5U-methylation in human mitochondria. RNA Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haute LV, Hendrick AG, D’Souza AR, et al. METTL15 introduces N4-methylcytidine into human mitochondrial 12S rRNA and is required for mitoribosome biogenesis. Nucleic Acids Res [Internet]. 2019;47:10267–10281. [cited 2019 October16]. Available from: https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkz735/5563944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Seidel-Rogol BL, McCulloch V, Shadel GS. Human mitochondrial transcription factor B1 methylates ribosomal RNA at a conserved stem-loop. Nat Genet. 2003;33:23–24. [DOI] [PubMed] [Google Scholar]
- [52].Lee K-W, Bogenhagen DF. Assignment of 2ʹ-O-methyltransferases to modification sites on the mammalian mitochondrial large subunit 16 S ribosomal RNA (rRNA). J Biol Chem. 2014;289:24936–24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rorbach J, Boesch P, Gammage PA, et al. MRM2 and MRM3 are involved in biogenesis of the large subunit of the mitochondrial ribosome. Mol Biol Cell. 2014;25:2542–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Poldermans B, Roza L, Van Knippenberg PH. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3ʹ end of 16 S ribosomal RNA of Escherichia coli. III. Purification and properties of the methylating enzyme and methylase-30 S interactions. J Biol Chem. 1979;254:9094–9100.383712 [Google Scholar]
- [55].Caldas T, Binet E, Bouloc P, et al. Translational defects of escherichia coli mutants deficient in the Um2552 23S ribosomal RNA methyltransferase RrmJ/FTSJ. Biochem Biophys Res Commun. 2000;271:714–718. [DOI] [PubMed] [Google Scholar]
- [56].Bügl H, Fauman EB, Staker BL, et al. RNA methylation under heat shock control. Mol Cell. 2000;6:349–360. [DOI] [PubMed] [Google Scholar]
- [57].McCulloch V, Shadel GS. Human mitochondrial transcription factor B1 interacts with the C-terminal activation region of h-mtTFA and stimulates transcription independently of its RNA methyltransferase activity. Mol Cell Biol. 2003;23:5816–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].De Silva D, Tu Y-T, Amunts A, et al. Mitochondrial ribosome assembly in health and disease. Cell Cycle. 2015;14:2226–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lövgren JM, Wikström PM. The rlmB gene is essential for formation of Gm2251 in 23S rRNA but not for ribosome maturation in Escherichia coli. J Bacteriol. 2001;183:6957–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Metodiev MD, Spåhr H, Loguercio Polosa P, et al. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10:e1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cámara Y, Asin-Cayuela J, Park CB, et al. MTERF4 regulates translation by targeting the methyltransferase NSUN4 to the mammalian mitochondrial ribosome. Cell Metab. 2011;13:527–539. [DOI] [PubMed] [Google Scholar]
- [62].Spahr H, Habermann B, Gustafsson CM, et al. Structure of the human MTERF4-NSUN4 protein complex that regulates mitochondrial ribosome biogenesis. Proc Nat Acad Sci. 2012;109:15253–15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Johansson MJO, Byström AS. Dual function of the tRNA(m(5)U54)methyltransferase in tRNA maturation. RNA. 2002;8:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Suzuki T, Suzuki T. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2014;42:7346–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sweeney BA, Petrov AI, Burkov B, et al.; The RNAcentral Consortium . RNAcentral: a hub of information for non-coding RNA sequences. Nucleic Acids Res. 2019;47:D221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Korostelev A, Trakhanov S, Laurberg M, et al. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. [DOI] [PubMed] [Google Scholar]
- [67].Schuwirth BS, Borovinskaya MA, Hau CW, et al. Structures of the bacterial ribosome at 3.5 Å resolution. Science. 2005;310:827–834. [DOI] [PubMed] [Google Scholar]
- [68].Maden EH, Wakeman JA. Pseudouridine distribution in mammalian 18 S ribosomal RNA. A major cluster in the central region of the molecule. Biochem J. 1988;249:459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Davis DR. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 1995;23:5020–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Auxilien S, Rasmussen A, Rose S, et al. Specificity shifts in the rRNA and tRNA nucleotide targets of archaeal and bacterial m5U methyltransferases. RNA. 2011;17:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Desmolaize B, Fabret C, Brégeon D, et al. A single methyltransferase YefA (RlmCD) catalyses both m5U747 and m5U1939 modifications in Bacillus subtilis 23S rRNA. Nucleic Acids Res. 2011;39:9368–9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jiang Y, Li F, Wu J, et al. Structural insights into substrate selectivity of ribosomal RNA methyltransferase RlmCD. PLoS One. 2017;12:e0185226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Madsen CT, Mengel-Jørgensen J, Kirpekar F, et al. Identifying the methyltransferases for m(5)U747 and m(5)U1939 in 23S rRNA using MALDI mass spectrometry. Nucleic Acids Res. 2003;31:4738–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kealey JT, Gu X, Santi DV. Enzymatic mechanism of tRNA (m5U54)methyltransferase. Biochimie. 1994;76:1133–1142. [DOI] [PubMed] [Google Scholar]
- [75].Alian A, Lee TT, Griner SL, et al. Structure of a TrmA-RNA complex: A consensus RNA fold contributes to substrate selectivity and catalysis in m5U methyltransferases. Proc Nat Acad Sci. 2008;105:6876–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Nordlund ME, Johansson JO, von Pawel-Rammingen U, et al. Identification of the TRM2 gene encoding the tRNA(m5U54)methyltransferase of Saccharomyces cerevisiae. RNA. 2000;6:844–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bond SR, Naus CC. RF-Cloning.org: an online tool for the design of restriction-free cloning projects. Nucleic Acids Res. 2012;40:W209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fernández-Silva P, Acín-Pérez R, Fernández-Vizarra E, et al. In vivo and in organello analyses of mitochondrial translation. Methods Cell Biol. 2007;80:571–588. [DOI] [PubMed] [Google Scholar]
- [79].Zhang J, Nuebel E, Wisidagama DRR, et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc. 2012;7:1068–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


