ABSTRACT
RNA methylation, catalysed by a set of RNA methyltransferases (RNMTs), modulates RNA structures, properties, and biological functions. RNMTs are increasingly documented to be dysregulated in various human diseases, particularly developmental disorders and cancer. However, the genomic and transcriptomic alterations of RNMTs, as well as their functional roles in human cancer, are limited. In this study, we utilized an unbiased approach to examine copy number alterations and mutation rates of 58 RNMTs in more than 10,000 clinical samples across 32 human cancer types. We also investigated these alterations and RNMT expression level as they related to clinical features such as tumour subtype, grade, and survival in a large cohort of tumour samples, focusing on breast cancer. Loss-of-function analysis was performed to examine RNMT candidates with important roles in growth and viability of breast cancer cells. We identified a subset of RNMTs, notably TRMT12, NSUN2, TARBP1, and FTSJ3, that were amplified or mutated in a subset of human cancers. Several RNMTs were significantly associated with breast cancer aggressiveness and poor prognosis. Loss-of-function analysis indicated FTSJ3, a 2ʹ-O-Me methyltransferase, as a candidate RNMT with functional roles in promoting cancer growth and survival. A subset of RNMTs, like FTSJ3, represents promising novel targets for anticancer drug discovery. Our findings provide a framework for further study of the functional consequences of RNMT alterations in human cancer and for developing therapies that target cancer-promoting RNMTs in the future.
KEYWORDS: RNA methyltransferase, cancer genomics, copy number alteration, mutation, amplification, FTSJ3, breast cancer
Introduction
RNA modifications, collectively termed the epitranscriptome, change the structural and chemical properties of RNA molecules, regulating their fundamental biological functions [1]. Most of the known RNA modifications are methylations, which add single or multiple methyl groups to one of four canonical RNA bases, the ribose moiety of the sugar backbone of RNA, or the 5ʹ Cap of mRNA transcripts [2]. RNA methylation can be categorized based on the nature of the RNA molecule modified (e.g., mRNA, tRNA, and rRNA) and the position of the methylation site [e.g. N6-methyladenosine (m6A) and 2ʹ-O-methylation (2ʹ-O-Me)] [1–3]. Different positions of RNA methylations correspond to distinct chemical properties. The m6A methylation destabilizes pairing with uracil (U) by changing the energetics of the A-U pair through steric hindrance [4]. 2′-O-Me enhances hydrophobicity, protects against nucleolytic attack, and stabilizes RNA helices [5]. RNA methylation plays important roles in RNA metabolism, processing, stability, nuclear export, translation efficiency, and others [1–3,6]. For example, m6A is the most prevalently modified nucleotide in mRNA, and it is required for diverse cellular and physiological processes by regulating mRNA metabolism and gene expression [7–9]. 2′-O-Me is predominantly found in rRNA and tRNA of bacteria and eukaryotes, as well as in the 5ʹ mRNA Cap of higher eukaryotes [10,11]. Recent studies have suggested that 2ʹ-O-Me is also present at internal sites in mRNA [11,12]. Generally, 2′-O-Me of rRNA regulates ribosome biogenesis and protein translation, while 2ʹ-O-Me of tRNA affects its accurate and efficient decoding ability [11,13,14]. Furthermore, 2ʹ-O-Me at the 5ʹ cap or internal sites of mRNA has a critical role in the innate immune response against RNA viral pathogens, as most viral RNAs lack this methylation [15]. Thus, accumulated studies demonstrated that functional roles of various RNA methylations are diverse and depend on the location and type of RNA molecule being modified.
RNA methylation is catalysed by a set of enzymes called RNA methyltransferases (RNMTs). RNMTs are S-adenosyl-methionine (SAM)-dependent methyltransferases that transfer a methyl group from the cofactor SAM to a substrate [16,17]. More than 50 human RNMTs have been identified [16]. Based on the structure of their catalytic domain, RNMTs can be divided into two general families. The majority of RNMTs belong to the ‘Rossmann-fold’ methyltransferases, along with DNA methyltransferases (DNMTs), protein arginine methyltransferases, and DOT1L (DOT1-like histone lysine methyltransferase) [16,17]. The second family of RNMTs is the SPOUT (SpoU-TrmD) methyltransferase, which is characterized by a catalytic domain with an unusual α/β fold forming a deep trefoil knot in the structure [18]. Additionally, based on their RNA molecule targets, RNMTs can be grouped as mRNA, tRNA, and rRNA RNMTs, among others. Notably, some of these enzymes may be able to methylate more than one type of RNA substrates, such as NSUN2 (NOP2/Sun RNA methyltransferase 2) that can catalyse the methylation of cytosine to 5-methylcytosine (m5C) of tRNAs, mRNAs, and non-coding RNAs [19–21].
Given the various functional roles of RNA methylation, mutation and dysregulation of several RNMTs have been directly linked to human diseases, especially developmental disorders and cancer [6,22]. Mutations of FTSJ1 (FtsJ RNA 2ʹ-O-methyltransferase 1) and NSUN2 cause autosomal-recessive intellectual disability [23–25]. Defective mutation of mitochondrial rRNA methyltransferase MRM2 (also known as FTSJ2) leads to disorders of mitochondrial respiration and MELAS (mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes)-like clinical syndrome [26]. Additionally, RNMTs belong to the superfamily of RNA binding proteins (RBPs) [27,28]. Several recent studies have examined various aspects of genomic aberrations of RBPs in human cancer [29–33]. For example, Neelamraju et al. determined the mutational landscape of ∼1,300 experimentally confirmed RBPs in ∼6,000 cancer genomes [30]. They identified 281 RBP genes to be enriched for mutations in at least one cancer type, five of which are RNMT including FTSJ3 (FtsJ RNA 2ʹ-O-methyltransferase 3) and NSUN2. By using the OncodriveFM approach (a bioinformatics approach to compute driver genes or gene modules), they identified 228 RBP candidate drivers with the majority of them specific to cancer types [30,34]. Among 228 RBPs, two are RNMTs: NSUN2 in melanoma and uterine corpus endometrial carcinoma (UCEC), and ALKBH8 (alkB homolog 8, tRNA methyltransferase) in stomach adenocarcinoma [30,34]. Another study performed genomic analysis of 1,542 RBPs in ∼7,000 clinical specimens across 15 TCGA cancer types [29]. They revealed 76 potential driver RBPs that displayed copy number alterations. Two of 76 are RNMTs: TARBP1 [TAR (HIV-1) RNA binding protein 1] in liver cancer and METTL1 (methyltransferase like 1) in lung adenocarcinoma [29]. However, no previous studies have collectively examined the genomic and transcriptomic alterations of RNMTs as well as their functional roles in human cancer.
In this study, we hypothesized that RNMTs with recurrent genetic alterations might play important roles in cancer progression and can serve as novel therapeutic targets for cancer treatment. We utilized an unbiased approach to examine genetic alterations of 58 RNMTs in more than 10,000 clinical samples across 32 cancer types. We also investigated these alterations and RNMT expression levels as they related to clinical features such as tumour subtype, grade, and survival in a large cohort of tumour samples, focusing on breast cancer. Furthermore, loss-of-function analysis was performed to examine RNMT candidates with important roles in growth and viability of breast cancer cells.
Results
Genetic alterations of RNMTS across 32 human tumour types
To understand the biological importance of RNA methylation in cancer progression and development, it is vital to determine the somatic copy number alteration (CNA) and mutation profiles of RNMTs in different types of human cancer. Based on the current ChromoHub database (http://apps.thesgc.org), 58 RNMTs have been shown or are predicted to be involved in methylation of various types of RNAs at different positions (Table 1 and Supplementary Figure S1) [16,35–74]. We first performed CNA and mutation analyses in more than 10,000 tumour samples across 32 cancer types from the Pan-Cancer Atlas of The Cancer Genome Atlas (TCGA) via cBioPortal (Supplementary Table S1) [75,76]. The copy number for each RNMT was generated by the copy number analysis algorithm GISTIC (Genomic Identification of Significant Targets in Cancer) and categorized according to copy number level per gene. The five categories of gene copy number are high-level amplification, low-level gain, diploid, shallow deletion (possibly heterozygous deletion), and deep deletion (possibly a homozygous deletion) [77]. In the TCGA Pan-Cancer cohort, we found that TRMT12 was the most frequently high-level amplified (6.25%) followed by NSUN2, TARBP1, TFB2M, METTL1, and TRMT1L, all between 2–3% (Fig. 1A and Supplementary Table S2). Two RNMT genes, ELP3 and TRMT9B, exhibited homozygous deletions in more than 2% of Pan-Cancer samples (Fig. 1B and Supplementary Table S3).
Table 1.
List of 58 human RNMT proteins and their methylated RNA type and position.
| RNMT | Gene Location | Full Name | RNA Type | Methylation Position | PubMed ID |
|---|---|---|---|---|---|
| ALKBH8 | 11q22.3 | ALKB Homolog 8 | rRNA/tRNA | mcm5U | 20,123,966 |
| BCDIN3D | 12q13.12 | BCDIN3 Domain Containing RNA Methyltransferase | tRNA/small RNAs (pre-miRNA) | 23,063,121 | |
| BUD23 | 7q11.23 | BUD23 rRNA Methyltransferase and Ribosome Maturation Factor | rRNA | m7G | 18,332,120 |
| CDK5RAP1 | 20q11.21 | CDK5 Regulatory Subunit Associated Protein | tRNA | ms2 | 22,422,838 |
| CDKAL1 | 6p22.3 | CDK5 Regulatory Subunity Associated Protein 1 like 1 | tRNA | ms2 | 21,841,312 |
| CMTR1 | 6p21.2 | Cap Methyltransferase 1 | mRNA | 2ʹOm | 20,713,356 |
| CMTR2 | 16q22.2 | Cap Methyltransferase 2 | mRNA | 2ʹOm | 21,310,715 |
| DIMT1 | 5q12.1 | DIMT1 rRNA Methyltransferase and Ribosome Maturation Factor | rRNA | m6A | 25,851,604 |
| ELP3 | 8p21.1 | Elongator Acetyltransferase Complex Subunit 3 | tRNA | m5C | 15,769,872 |
| EMG1 | 12p13.31 | EMG1 N1-Specific Pseudouridine Methyltransferase | rRNA | m1 | 20,047,967 |
| FBL | 19q13.2 | Fibrillarin | rRNA | 2ʹOm | 8,431,947 |
| FBLL1 | 5q34 | Fibrillarin like 1 | rRNA | 2ʹOm | 26,566,070 |
| FTSJ1 | Xp11.23 | FtsJ RNA 2ʹ-O-Methyltransferase 1 | tRNA | 2ʹOm | 11,927,565 |
| FTSJ3 | 17q23.3 | FtsJ RNA 2ʹ-O-Methyltransferase 3 | rRNA/mRNA | 2ʹOm | 30,626,973 |
| HENMT1 | 1p13.3 | HEN Methyltransferase Homolog 1 | small RNAs (piRNA) | 2ʹOm | 18,029,764 |
| MEPCE | 7q22.1 | Methlphosphate Capping Enzyme | small RNAs (snRNA) | 29,425,494 | |
| METTL1 | 12q14.1 | Methyltransferase like 1 | tRNA/mRNA | m7G | 31,031,084 |
| METTL14 | 4q26 | Methyltransferase like 14 | mRNA | m6A | 24,316,715 |
| METTL3 | 14q11.2 | Methyltransferase like 3 | mRNA | m6A | 24,316,715 |
| METTL4 | 18p11.32 | Methyltransferase like 4 | 26,566,070 | ||
| MRM1 | 17q12 | Mitochndrial rRNA Methyltransferase 1 | rRNA | 2ʹOm | 25,074,936 |
| MRM2 | 7p22.3 | Mitochndrial rRNA Methyltransferase 2 | rRNA | 2ʹOm | 25,074,936 |
| MRM3 | 17p13.3 | Mitochondrial rRNA Methyltransferase 3 | rRNA | 2ʹOm | 25,074,936 |
| NOP2 | 12p13.31 | NOP2 Nucleolar Protein | rRNA | m5C | 23,913,415 |
| NSUN2 | 5p15.31 | NOP2/Sun RNA Methyltransferase 2 | tRNA | m5C | 17,071,714 |
| NSUN3 | 3q11.2 | NOP2/Sun RNA Methyltransferase 3 | tRNA | m5C | 27,497,299 |
| NSUN4 | 1p33 | NOP2/Sun RNA Methyltransferase 4 | rRNA | m5C | 24,516,400 |
| NSUN5 | 7q11.23 | NOP2/Sun RNA Methyltransferase 5 | rRNA | m5C | 23,913,415 |
| NSUN5P1 | 7q11.23 | NSUN5 Pseudogene 1 | rRNA | 26,566,070 | |
| NSUN5P2 | 7q11.23 | NSUN5 Pseudogene 2 | rRNA | 26,566,070 | |
| NSUN6 | 10p12.31 | NOP2/Sun RNA Methyltransferase 6 | tRNA | 26,566,070 | |
| NSUN7 | 4p14 | NOP2/Sun RNA Methyltransferase Family Member 7 | 26,774,474 | ||
| RNMT | 18p11.21 | RNA Guanine-7 Methyltransferase | mRNA | m7G | 27422871 |
| RSAD1 | 17q21.33 | Radical S-adenosyl Methionine Domain Containing 1 | 26566070 | ||
| SPOUT1 | 9q34.11 | SPOUT Domain Containing Methyltransferase 1 | |||
| TARBP1 | 1q42.2 | TAR (HIV-1) RNA Binding Protein1 | tRNA | 2ʹOm | 31019095 |
| TFB1M | 6q25.3 | Transcription Facotr B1, Mitochondrial | rRNA | m6A | 17031457 |
| TFB2M | 1q44 | Transcription Facotr B2, Mitochondrial | rRNA | m6A | 17031457 |
| TGS1 | 8q12.1 | Trimethylguaosine Synthase 1 | small RNAs (snoRNA/snRNA) | m7G | 11983179 |
| THUMPD2 | 2p22.1 | THUMP Domain Containing 2 | tRNA | 26566070 | |
| THUMPD3 | 3p25.3 | THUMP Domain Containing 3 | tRNA | 26566070 | |
| TRDMT1 | 10p13 | tRNA Aspartic Acid Mthyltransferase 1 | tRNA | m5C | 16424344 |
| TRMT1 | 19p13.13 | tRNA Methyltransferase 1 | tRNA | mmG | 28784718 |
| TRMT10A | 4q23 | tRNA Methyltransferase 10A | tRNA | m1A | 31292261 |
| TRMT10B | 9p13.2 | tRNA Methyltransferase 10B | tRNA | m1A | 31292261 |
| TRMT10C | 3q12.3 | tRNA Methyltransferase 10C | tRNA | m1A | 29880640 |
| TRMT11 | 6q22.32 | tRNA Methyltransferase 11 Homolog | tRNA | 2ʹOm | 15899842 |
| TRMT112 | 11q13.1 | tRNA Methyltransferase Subunit 11-2 | rRNA | m7G | 22493060 |
| TRMT12 | 8q24.13 | tRNA Methyltransferase 11 Homolog | tRNA | 26566070 | |
| TRMT1L | 1q25.3 | tRNA Methyltransferase like 1 | tRNA | 26566070 | |
| TRMT2A | 22q11.21 | tRNA Methyltransferase 2 Homolog A | tRNA | m5U | 31361898 |
| TRMT2B | Xq22.1 | tRNA Methyltransferase 2 Homolog B | tRNA | m5C | 31361898 |
| TRMT44 | 4p16.1 | tRNA Methyltransferase 44 Homolog | tRNA | 2ʹOm | 26566070 |
| TRMT5 | 14q23.1 | tRNA Methyltransferase 5 | tRNA | m1A | 26189817 |
| TRMT61A | 14q32 | tRNA Methyltransferase 61A | tRNA | m1A | 30131402 |
| TRMT61B | 2p23.2 | tRNA Methyltransferase 61B | tRNA | m1A | 29107537 |
| TRMT9B | 8p22 | tRNA Methyltransferase 9B | tRNA | 23381944 | |
| TYW3 | 1p31.1 | tRNA-yW Synthesizing Protein 3 | tRNA | 27932585 |
Figure 1.
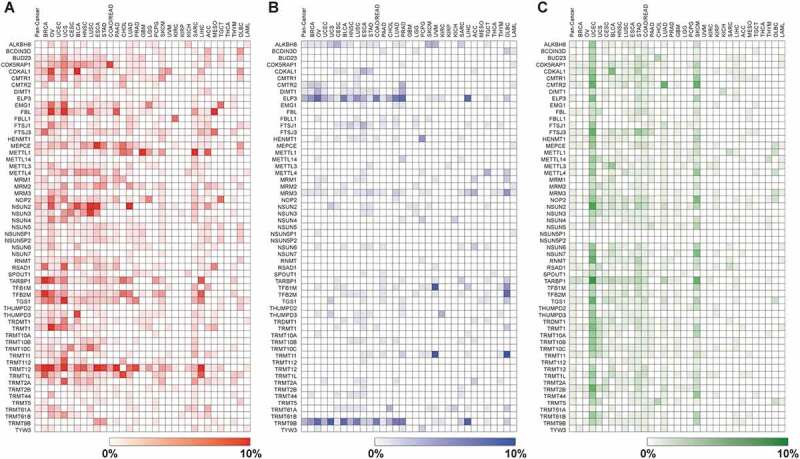
Heatmap showing the frequencies of (A) RNMT amplification (red), (B) deep deletion (blue) and (C) mutations (green) across all 32 TCGA tumour types. Heatmap was generated using Morpheus software from the Broad Institute (https://software.broadinstitute.org/morpheus/) .
Next, we performed copy number analysis of each RNMT in 32 TCGA individual tumour types and uncovered a considerable variation of CNA across different tumour types (Fig. 1A,B). We found that 26 RNMT genes were high-level amplified by more than 5% in at least one individual tumour type (Fig. 1A and Supplementary Table S2). Notably, TRMT12 was amplified in 26.22% of ovarian cancer (OV), NSUN2 in 12.11% of lung squamous cell carcinoma (LUSC), and FTSJ3 in 6.26% of breast cancer (BRCA) (Fig. 1A and Supplementary Table S2). Five RNMTs exhibited deep deletion of more than 5% in at least one individual tumour type, and the highest percentage of deep deletion was TRMT11 (10.42%) in diffuse large B-cell lymphoma (DLBC) (Fig. 1B and Supplementary Table S3).
For somatic mutation, eight RNMTs, including TARBP1, FTSJ3, and NSUN2, were mutated in more than 1% of the TCGA Pan-Cancer cohort (Fig. 1C and Supplementary Table S4). TARBP1 demonstrated the highest rate (1.86%) of mutation in the Pan-Cancer cohort, with 179 missense, 20 nonsense, 13 splice, 10 frame-shift, and 7 fusion mutations (Fig. 1C and Supplementary Figure S2). The highest rate of mutation in individual tumour type was TARBP1 in 8.12% of UCEC samples (Supplementary Table S4). Taken together among 58 RNMTs, several RNMT genes including TRMT12, NSUN2, FTSJ3, and TARBP1 had relatively higher frequencies of genetic alterations in a spectrum of human tumours.
Molecular profiling of RNMT genes in different subtypes of breast cancer
Breast cancer is the leading cause of cancer diagnoses in women, with more than twice the number of new cases than any other individual cancer type [78]. Breast cancer has been classified into five molecular subtypes with distinct risks and underlying biology; these five subtypes are luminal A, luminal B, epidermal growth factor receptor 2–enriched (HER2+), basal-like, and normal-like breast cancers [79,80]. Both luminal A and luminal B breast cancers are oestrogen receptor positive, but luminal B cancers have poorer outcomes [81]. Furthermore, basal-like breast cancer usually occurs in young women and is a highly aggressive subtype associated with very poor prognosis [82]. Well-known breast cancer genes such as BRCA1 and MYC were mutated in 2.53% and amplified in 15.05% of TCGA breast cancer samples, respectively [75,76]. Here, we examined the genetic alteration and expression profiling of each RNMT as they relate to molecular subtypes and other clinical features of breast cancer. In TCGA breast cancer, seven RNMTs, including TRMT12, TARBP1, and FTSJ3, had high-level amplification in more than 5% of samples (Supplementary Table S2). Next, we analysed copy number, mutation and mRNA expression of RNMTs independently across five subtypes of breast cancer samples. The frequencies of high-level amplification, low-level gain, diploid, heterozygous deletion, homozygous deletion, and somatic mutation of 58 RNMT genes in five TCGA breast cancer subtypes are shown in Supplementary Table S5. Remarkably, we found that TRMT12 exhibited high-level amplification in 27.49% of TCGA basal-like breast cancers. FTSJ3 exhibited high-level amplification in 5.61% of luminal A, 11.17% of luminal B, and 12.82% of HER2+ breast cancer subtypes. FTSJ3 also had the highest frequency of mutation (4.09%) in basal-like breast cancer (Supplementary Table S5). When we applied a binomial test to compare mutation rate of RNMTs with the median mutation rate of all genes (0.6%) in basal-like breast cancer, FTSJ3 was the only RNMT with statistical significance [p < 0.001 and false discovery rate (FDR) = 0.002].
Next, we analysed the correlation between copy number and mRNA level of 58 RNMTs from TCGA breast cancer specimens. As shown in Supplementary Table S6, all RNMT genes had positive correlations between DNA copy number and mRNA expression, and 23 of them had a Spearman correlation coefficient (r) greater than 0.5, with top three being ELP3, FTSJ3, and TRMT12. Similar results were also observed in other tumour types, suggesting that CNA may be a major factor in the dysregulation of RNMT mRNA in human cancer, particularly breast cancer. To determine how expression of each RNMT is associated with breast cancer subtypes, we assessed mRNA expression of each RNMT in breast cancer samples with molecular subtype data available. We found that the expression levels of three RNMTs (FTSJ3, TRMT12, and TGS1) were significantly higher (p < 0.001, mean Z score difference > 1.0) in luminal B compared to luminal A subtypes (Fig. 2A and Supplementary Table S7). Notably, FTSJ3 and HER2 genes are located within the same chromosome at 17q23.3 and 17q12 respectively, and luminal B breast cancer exhibited higher frequency of HER2 gain/amplification (67.51%) compared to that of luminal A breast cancer (22.65%). FTSJ3 was also highly expressed in HER2+ breast cancer subtype. Furthermore, 13 RNMTs, including NSUN2, FBL, and NOP2, were expressed significantly higher in basal-like compared to luminal subtype breast cancer (Fig. 2A and Supplementary Table S7).
Figure 2.
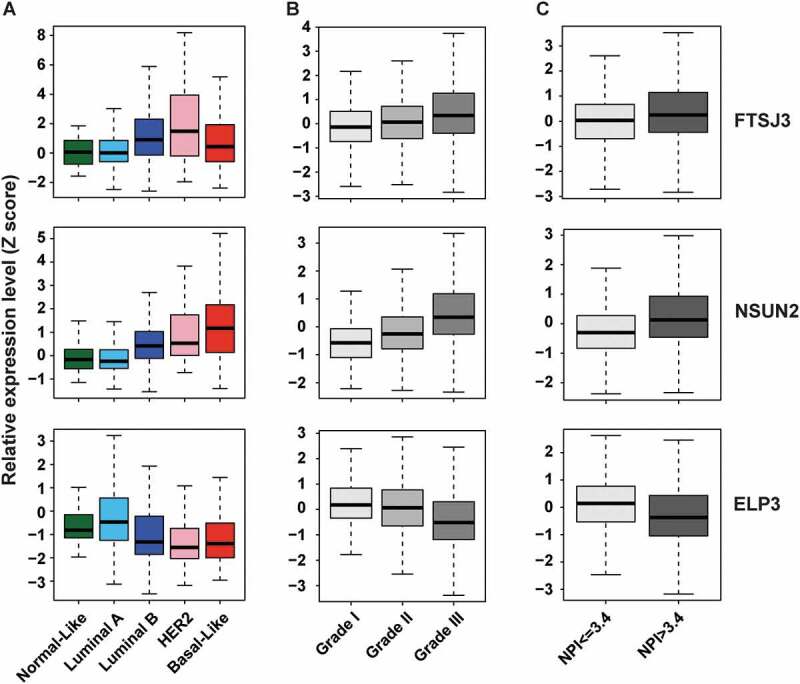
Expression levels of three RNMTs (FTSJ3, NSUN2, and ELP3) in breast cancer samples with different subtypes, grades, and NPIs. (A) Expression levels of three RNMTs across five subtypes of TCGA breast cancer samples. (B) Expression levels of three RNMTs in three grades of METABRIC breast cancer samples. (C) Expression levels of three RNMTs in METABRIC patients with poor prognosis (NPI > 3.4) and good prognosis (NPI ≤ 3.4) scores. High expressions of FTSJ3 and NSUN2, but lower expression of ELP3, were significantly (p < 0.001) associated with higher grade and NPI score of METABRIC breast cancers.
To validate our findings from the TCGA breast cancer dataset regarding RNMT genetic alterations, we conducted an independent analysis using two additional breast cancer datasets: the METABRIC dataset with 2509 primary samples and the MBC (Metastatic Breast Cancer) with 237 samples [83]. In the METABRIC dataset, we found that seven RNMTs, including TRMT12, TARBP1, and FTSJ3, had high-level amplification in more than 5% of breast cancer samples. The frequencies of high-level amplification, low-level gain, diploid, heterozygous deletions, and homozygous deletions of 58 RNMT genes in five METABRIC breast cancer subtypes are shown in Supplementary Table S8. Particularly, TRMT12 was found to be amplified in 40.19% of basal-like, TARBP1 in 30.29% of luminal A, and FTSJ3 in 17.05% luminal B of METABRIC breast cancers. We also analysed the frequencies of RNMT amplification, deep deletion and mutation in metastatic samples of breast cancer (MBC cohort). As shown in Supplementary Table S9, TRMT12 and FTSJ3 were the most amplified RNMTs, in 21.52% and 19.83% of metastatic breast cancer samples respectively. In summary, genomic profiling of 58 RNMTs in breast cancer revealed amplification of several RNMTs, notably TRMT12 and FTSJ3, with higher frequencies in primary and metastatic breast cancer. Moreover, different subtypes of breast cancer had different patterns of copy number and expression of each RNMT.
RNMT gene expression correlations with clinical breast cancer features
Since the METABRIC cohort contains approximately 2,000 breast cancer samples with histologic grade and long-term clinical follow-up data, we assessed RNMT copy number and expression by clinical features and disease-free survival in this cohort. Gene expression data of 52 RNMTs was available, while data for six RNMT genes, including TRMT12, was not available in the METABRIC cohort. We first examined expression levels of each RNMT gene at different grades of METABRIC breast cancer samples. The means of Z-score and p-value for each RNMT gene across grades 1–3 are shown in Supplementary Table S10. We found that ten RNMTs, including FTSJ3, NSUN2, NOP2, and FBL, were significantly highly expressed, while two genes (ELP3 and BCDIN3D) were lower expressed in higher-grade breast cancers (T-test: Grade 3 vs 1 + 2; p < 0.001 and means difference > 0.3; Fig. 2B and Supplementary Table S10). The Nottingham Positivity Index (NPI), one of the primary prognostic tools used in assessing breast cancer aggressiveness in Europe, was also available in the METABRIC cohort [84,85]. Thus, we compared expression levels of RNMTs between patients with high NPI (>3.4) versus those with low NPI (≤ 3.4). As shown in Fig. 2C and Supplementary Table S10, eight RNMTs, including FTSJ3 and NSUN2, were significantly over-expressed, while two RNMTs: ELP3 and BCDIN3D, were under-expressed in samples with higher NPI (p < 0.001 and means Z-score difference > 0.3) compared with that in lower NPI samples.
Next, we analysed the relationship between RNMT copy number, mRNA expression, and disease-free survival of METABRIC breast cancer patients. We found that copy number increases (Amp and Gain) of seven RNMTs (e.g. TRMT12 and FTSJ3) were significantly associated with shorter disease-free survival of breast cancer patients (p < 0.001) (Fig. 3A, Supplementary Figure S3 and Table S11). We also found that higher mRNA levels of four RNMTs (NSUN2, FTSJ1, NOP2, and CDK5RAP1) and lower expression of three RNMTs (ELP3, ALKBH8, and DIMT1) were significantly associated with shorter disease-free survival in METABRIC breast cancer patients (p < 0.001) (Fig. 3A and Supplementary Table S11). Higher expression of FTSJ3 was moderately associated with shorter survival in METABRIC breast cancer patients (Fig. 3A). Combined with genetic profiling of RNMTs in breast cancer, our results indicate several RNMTs that might contribute to breast cancer development and progression.
Figure 3.
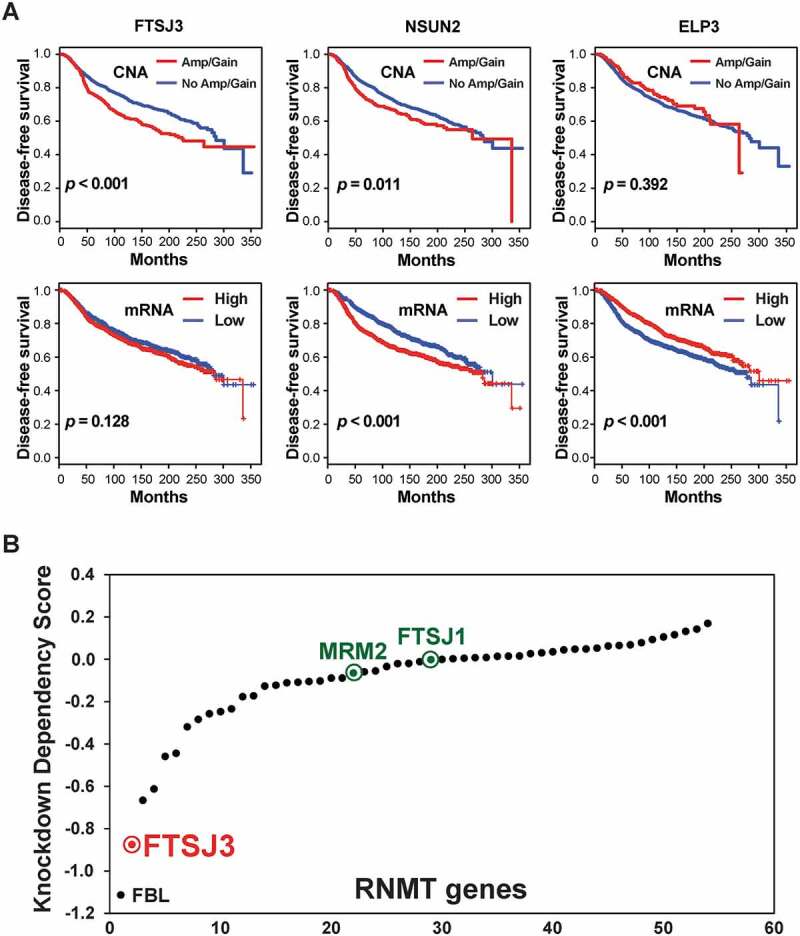
RNMTs associate with disease-free survival of breast cancer and contribute to growth and viability of tumour cells in vitro. (A) Kaplan-Meier plots of disease-free survival associated with copy number and mRNA expression levels of three RNMTs (FTSJ3, NSUN2, and ELP3) in METABRIC breast cancers. (B) Scatter plot showing mean of each RNMT dependency score in genome-scale loss-of-function screens of 712 tumour lines.
Loss-of-function analysis of RNMTS for cancer dependency
The availability of genome-wide loss-of-function screening in a large panel of tumour lines presents new opportunities for understanding cancer vulnerabilities [86,87]. Based on our genotranscriptomic meta-analysis, we hypothesized that several of these dysregulated RNMTs may act as genetic vulnerabilities in various cancers and represent potential therapeutic targets. To investigate potential RNMT dependencies in various tumours, particularly breast cancer, we analysed genome-wide shRNA screen data in 712 tumour lines [86,88]. Accordingly, we ranked average dependency (DEMETER2) scores of 54 RNMTs that were examined in genome-wide shRNA screens of 712 tumour lines [86,88,89]. Examining the impact of the shRNA knockdown of 54 RNMTs revealed that FBL, FTSJ3, NOP2, and EMG1 had the lowest cancer dependency scores among all RNMT genes assessed (Fig. 3B). The lower the score, the more critical that gene is to tumour cell growth and survival. Previous studies revealed that knockdown of FBL inhibits cell proliferation in vitro and reduces the frequency of tumour formation and the tumour volumes in vivo [90,91]. The second hit is FTSJ3, which together with its homologues FTSJ1 and MRM2 (also known as FTSJ2) are orthologues of bacterial FtsJ/RrmJ, a highly conserved 2ʹ-O- methyltransferase [11,37,54,92]. However, FTSJ1 and MRM2/FTSJ2 had no impact on the shRNA in vivo screen of most tumour cells (Fig. 3B). Given that FTSJ3 is commonly amplified and overexpressed in breast cancer, we performed a more detailed analysis of FTSJ3 shRNA screen data in breast cancer cell lines. Among 81 breast cancer lines, 18 lines have FTSJ3 DEMETR2 scores of less than −1.0. Altogether, this data places FTSJ3 as a candidate RNMT with functional roles in promoting cancer growth and progression.
Knockdown of FTSJ3 decreases cancer cell proliferation in breast cancer cells
In order to find the most suitable cell line models for further work that recapitulates FTSJ3 amplification and over-expression observed in primary tumours, we examined the copy number and expression of FTSJ3 in 13 breast cancer lines. Inspection of comparative genomic hybridization (CGH) array data that was previously published by us and others revealed FTSJ3 high-level amplification in SUM52, MCF7, and ZR75-1 luminal subtype breast cancer lines [93–96]. Notably, these three lines were established from pleural and ascetic effusions of patients with metastatic breast cancers, and their cell line data were consistent with that in clinical samples observed in MBC cohort [97–99]. Next, we assessed FTSJ3 levels in breast cancer cell lines by qRT-PCR and western blotting assays. Indeed, we revealed that mRNA and protein expression of FTSJ3 were dramatically higher in a subset of breast cancer cell lines, remarkably SUM52, MCF7, and ZR75-1 (Fig. 4A).
Figure 4.
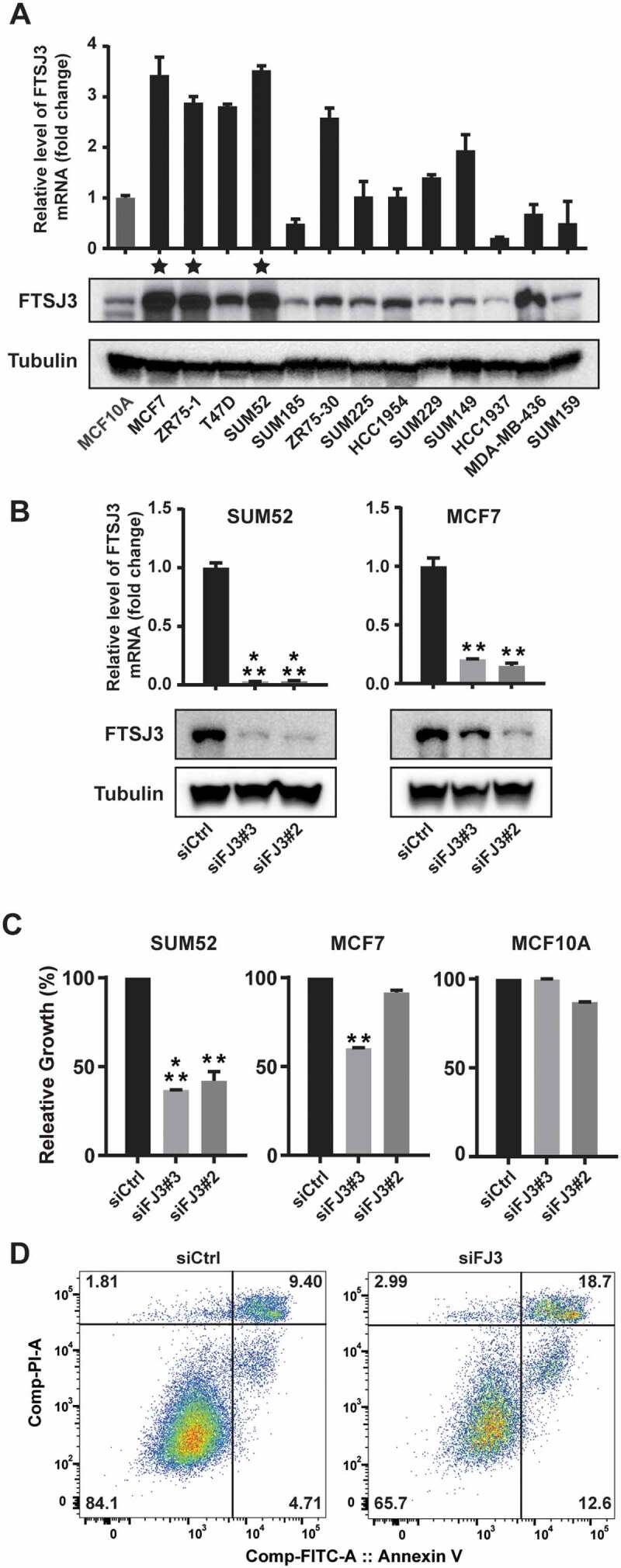
Knockdown of FTSJ3 inhibits cell proliferation and survival in breast cancer cell lines. (A) Expression levels of FTSJ3, measured by qRT-PCR and western blot assays, in a panel of 13 breast cancer cell lines plus MCF10A. mRNA expression levels in the immortalized but nontumorigenic breast epithelial cell line MCF10A cells were arbitrarily set as 1. Three stars indicate the cell lines with FTSJ3 gene amplification. (B) Knockdowns of FTSJ3 in SUM52 and MCF7 cells with two different siRNAs were confirmed by qRT-PCR and western blot assays. (FJ3 = FTSJ3). (C) Bar graph shows relative cell growth after knocking down FTSJ3 in SUM52 and MCF7 breast cancer cells (** p < 0.01, *** p < 0.001). Data are expressed as mean ± SD. (D) FTSJ3 depletion results in cell death 2 days after siRNA transfection in SUM52 cells, measured by flow cytometry and double staining with Annexin V-FITC and PI. These experiments were repeated independently three times with similar results.
Next, we further assessed the functional role of FTSJ3 by knocking down FTSJ3 using siRNA oligos in breast cancer cell lines with high FTSJ3 expression (SUM52: luminal B subtype; and MCF7: luminal A subtype) and in immortalized breast epithelial cells (MCF10A). To perform siRNA knockdown experiments, we obtained three siRNAs targeting different regions of FTSJ3, and all three siRNAs attenuated the expression of FTSJ3 in breast cancer cells. As shown in Fig. 4B, qRT-PCR and western blot assays revealed that two selected potent siRNAs significantly decreased the expression of FTSJ3 at mRNA and protein levels. FTSJ3 knockdown dramatically slowed SUM52 and MCF7 cell growth compared with that in the negative control cells (Fig. 4C). Conversely, in breast epithelial cells (MCF10A), no significant inhibitory effects were observed (Fig. 4C). We also found that FTSJ3 depletion in SUM52 cells led to marked induction of apoptosis, as determined by Annexin V staining (Fig. 4D). Additionally, we knocked down FTSJ3 in two non-breast cancer cell lines (HepG2: liver cancer and KYSE150: oesophageal cancer) in which FTSJ3 is highly expressed (Supplementary Figure S4A). FTSJ3 knockdown significantly slowed HepG2 and KYSE150 cell growth compared to that of the negative control cells (Supplementary Figure S4). Together, this data demonstrates that targeting FTSJ3 can attenuate the growth of FTSJ3 amplified/over-expressed cancer cells.
Discussion
In this study, we performed integrated genotranscriptomic and clinicopathological analyses of 58 RNMTs in a large cohort of primary tumours and cell lines. We identified that a subset of RNMT genes, including TRMT12, NSUN2, TARBP1, and FTSJ3, has high frequencies of genomic amplification and/or mutation in a spectrum of human cancers, particularly in breast cancer. Different subtypes of breast cancer had different patterns of copy number and expression of each RNMT. We revealed that expressions of several RNMTs, including NSUN2 and FTSJ3, were associated with aggressive subtype and poor prognosis of breast cancer patients. Loss-of-function analysis indicated FTSJ3, a 2ʹ-O-Me methyltransferase, as a candidate RNMT with functional roles in promoting breast cancer growth and survival.
Based on their RNA molecule targets, RNMTs could be grouped into mRNA, tRNA, rRNA, small RNA methyltransferases. In this study, we found that mRNA methyltransferase group has the highest frequency of mutation (ANOVA test, p < 0.001), with most mutations in UCEC (5.00%, p < 0.001) and SKCM (3.03%, p < 0.005). Among 58 RNMTs, the most mutated RNMT in TCGA Pan-cancer is TARBP1, which is also amplified more than 5% in five TCGA tumour types (BRCA 10.09%, OV 6.99%, LIHC 6.81%, CHOL 5.56%, and UCS 5.36%), suggesting it likely has oncogenic potential. TARBP1 belongs to the SPOUT methyltransferase family, and it facilitates the activation of HIV gene expression [100,101]. Previous immunohistochemistry assays revealed that over-expression of TARBP1 proteins is significantly associated with advanced grade, advanced clinical stage, and shorter overall survival of lung cancer [102]. TARBP1 is also over-expressed in skin and liver cancers [103,104]. Despite the known functions of TARBP1 in regulating HIV transcription and innate immune responses, the functional consequences of TARBP1 amplification and mutation in cancer are worth further investigation [105].
A finding of particular interest from our current study is that FTSJ3 is amplified in a subset of human cancer, particularly in breast cancer, and promotes cancer growth and survival. The FTSJ3 protein, together with its two close homologues FTSJ1 and MRM2/FTSJ2, contains a conserved 2ʹ-O-methyltransferases domain: FtsJ/RrmJ [24,37,54,92]. The bacterial FtsJ/RrmJ protein is responsible for 2′-O-Me of 23S rRNA U2552 (Um2552), which is located in the Peptidyl Transferase Centre (PTC) [106,107]. Deletion of FtsJ/RrmJ in E.coli results in a severe growth defect with cold sensitivity and accumulation of the 45S precursor [108]. In addition, FTSJ3 is a homologue of yeast Spb1 that contains an Spb1 C-terminal domain and a nuclear localization signal in the Spb1 domain [37]. Indeed, based on COMPARTMENTS (subcellular localization database) and SubCellBarCode database, the three human FTSJ proteins have different subcellular localizations [109,110]. FTSJ1 is the primarily cytosolic protein that methylates the 2ʹ-O-ribose of nucleotides at positions 32 and 34 of the tRNA anticodon loop [111]. FTSJ2/MRM2 is a mitochondrial protein that modifies U1369 at the PTC of human mitochondrial 16S rRNA, the equivalent position (U2791) in the yeast mitochondrial 21S rRNA [54,112]. FTSJ3 is a nuclear protein that contains a C-terminal SPB1 domain in addition to the N-terminal FtsJ/RrmJ enzymatic domain. The yeast methyltransferase, Spb1, methylates both U2921 and G2922 residues in the late 27S pre-rRNA precursor [113]. Markedly, about 70% of 2′-O-Me sites are conserved from yeast to human, particularly those located within functional regions of rRNAs such as PTC [114]. Yeast U2921 (U2552 in E. coli) and G2922 are conserved to U4498 and G4499 in humans. Genetic studies in yeast have demonstrated that 2′-O-Me is important for the translational capacity of ribosomes [13]. Furthermore, previous studies have revealed that human FTSJ3 is involved in pre-rRNA processing via its interaction with NIP7, a transacting factor required for ribosome biogenesis [115,116]. Thus, FTSJ proteins are highly conserved methyltransferases from bacteria to human, indicating their functional importance in many aspects of RNA processing including ribosome biogenesis and translation in normal and pathological conditions.
Recent studies demonstrated that the rRNA 2′-O-Me pattern modification is associated with changes in translational control during tumorigenesis [90,91]. For example, FBL catalyzes the 2′-O-Me of rRNAs at specific positions guided by small nucleolar RNAs (snoRNAs) from the C/D box family, which carry a sequence complementary to the target rRNA. In cellular models of cancer, forced FBL up- or down-regulation modulated tumour progression [90,91]. In breast cancer, FBL over-expression alters rRNA 2′-O-Me patterns, triggers changes in translational fidelity, and promotes translation of subsets of mRNAs (e. g. MYC) involved in tumorigenesis and cell survival [90]. Here, analysis of the genome-wide loss-of-function screen in a large panel of tumour cell lines also revealed FBL as the top hit of RNMTs for cancer cell survival. The second RNMT hit from loss-of-function screening in tumour cell lines is FTSJ3. Furthermore, our siRNA knockdown assays reveal that depletion of FTSJ3 induces apoptosis and inhibits breast cancer growth and survival in vitro. Thus, we speculate that modulation of the rRNA 2′-O-Me pattern at specific positions induced by changes in FTSJ3 expression, at least partly, might favour cancer cell growth and progression.
Interestingly, recent studies also revealed that FTSJ3 and yeast Spb1 are involved in 2ʹ-O-Me of internal sites of mRNA [11,12]. Ringeard et al. identified FTSJ3 as the interacting partner of TAR RNA-binding protein (TRBP also known as TARBP2), and TARBP2 plays significant roles in many biological and pathological conditions, including viral expression of HIV-1, microsatellite instability, cancer stem cell properties, and tumour progression [37,117–119]. Furthermore, they found that FTSJ3 catalyzes 2ʹ-O-Me of HIV RNAs and leads to the inhibition of innate immune sensing and response [37]. A recent study deposited in BioRxiv claimed that yeast Sbp1 has 2′-O-MTase activity and is responsible for methylation of thousands of sites in yeast mRNA, suggesting that human FTSJ3 may have the same function [120]. More interestingly, gene ontology (GO) enrichment analysis of Spb1-methylated yeast mRNAs identified that GO terms related to ribosomes, ribosome biogenesis, and translation were exclusively over-represented [120]. It is largely assumed that cancer cells become addicted to ribosomes and translation owing to their enhanced need for protein production to sustain their unrestricted growth. Thus, even though the exact role of FTSJ3 in cancer remains to be established and better characterized, our results suggest that FTSJ3 is a critical 2ʹ-O methyltransferase that targets rRNA and mRNA in ribosome biogenesis and translation, subsequently contributing to cancer cell proliferation and survival.
In summary, integrated genomic, transcriptomic, and clinicopathological data in a large cohort of primary tumours and cell lines identified a subset of RNMTs, especially TRMT12, NSUN2, TARBP1, and FTSJ3, that was amplified or mutated in a subset of human cancer. Several RNMTs were significantly associated with breast cancer aggressiveness and poor prognosis. Loss-of-function analysis revealed that FTSJ3 had important roles in promoting breast cancer cell growth and survival. To date, DNMT and protein methyltransferase inhibitors have been successfully identified and several inhibitors have been approved or tested for cancer treatment, suggesting that the RNMTs represent a new avenue for anticancer drug discovery [121]. Our findings provide a framework for further study of the functional consequences of RNMT alterations in human cancer and for developing therapies that target RNMTs in the future.
Materials and methods
Genomic and clinical data of cancer samples
Genetic and expression alteration data from 10,967 tumour samples spanning 32 tumour types in The Cancer Genome Atlas (TCGA) Pan-Cancer studies were obtained from the cBio Cancer Genomics Portal (http://www.cbioportal.org) [75,76,122–124]. In the cBioPortal, the copy number for each RNMT gene was generated by the GISTIC algorithm and categorized as copy number level per gene: ‘-2’ is a possible homozygous deletion, ‘-1’ is a heterozygous deletion, ‘0’ is diploid, ‘1’ indicates a low-level gain, and ‘2’ is a high-level amplification. The relative expression of an individual gene and the gene’s expression distribution in a reference population were analysed in mRNA expression data. The reference population consists of tumours that are diploid for the gene in question. The Z score represents the number of standard deviations the expression of a gene is from the reference population gene expression. Somatic mutation data were obtained by exome sequencing [75,76]. Breast cancer subtype and clinicopathologic information in the TCGA cohort was obtained from a previous publication and extracted via the cBioPortal [76,79]. Among the 1084 breast cancer samples, 981 had intrinsic subtype data available, including 36 normal-like, 499 luminal A, 197 luminal B, 78 HER2+, and 171 basal-like breast cancers [76,123]. A detailed description of the METABRIC dataset can be found in the original publication [83]. The CNAs and normalized expression data from the METABRIC database were downloaded with permission from the European Genome-phenome Archive (https://www.ebi.ac.uk/ega) under accession number EGAC00000000005 as well as from the cBio Cancer Genomics Portal [76]. In the METABRIC dataset, 1974 samples had subtype data available, including 199 normal-like, 718 luminal A, 488 luminal B, 240 HER2+, and 329 basal-like breast cancers [83]. The CNA and mutation data from 237 MBC (Metastatic Breast Cancer) samples were also obtained from the cBio Cancer Genomics Portal.
Semiquantitative PCR reactions
To assess gene expression at the mRNA level, RNA was prepared from human breast cancer cell lines and the MCF10A cell line by using an RNeasy Plus Mini Kit (QIAGEN) [122]. RNA was mixed with qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA) and then converted to cDNA through a reverse-transcription (RT) reaction. The cDNA was then used for real-time PCR reactions. Primer sets were obtained from Life Technologies (Carlsbad, CA, USA). A PUM1 primer set was used as a control. Semiquantitative RT-PCR was performed using the FastStart Universal SYBR Green Master (Roche Diagnostics, Indianapolis, IN, USA) as described earlier [122,123].
Immunoblotting and antibodies
Immunoblot assays were performed as previously described [122,123]. Whole-cell lysates were prepared by scraping cells from the dishes into cold RIPA lysis buffer. Centrifugation protein content was estimated by the Bradford method. A total of 20–50 μg of total cell lysate was resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. Antibodies used for the western blot in the study included anti-FTSJ3 (A304-199, Bethyl Laboratories, Montgomery, TX, USA) and anti-β-Tubulin (Sigma-Aldrich T8328, St. Louis, MO, USA).
Cell culture and growth assays
The SUM cell lines were obtained from Dr. Stephen P. Ethier, and the remaining cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). All cell lines were tested routinely and authenticated using cell morphology, proliferation rate, a panel of genetic markers, and contamination checks. To determine the effect of FTSJ3 overexpression on the growth of human cancer in vitro, FTSJ3 expression was knocked down using small interfering RNA (siRNA) in breast, liver, and oesophageal cancer lines as well as the control line, MCF10A. The FTSJ3 and negative control siRNAs were purchased from Sigma-Aldrich (St. Louis, MO, USA). For the transfection procedure, cells were seeded in appropriate cell culture plates and maintained overnight under standard conditions. Plate sizes, cell densities, and siRNA quantities were dependent on the cell line and the experimental setup; siRNA was transfected using the MISSION siRNA transfection reagent according to the manufacturer’s protocol (Sigma-Aldrich). Five days after siRNA transfection, CellTiter-blue cell viability assays (Promega) were performed according to the manufacturer’s guideline.
Apoptosis assays
FTSJ3 siRNA pools and negative control siRNA were used for apoptosis assays in SUM52 cells. Cells were collected and stained with FITC–Annexin V and propidium iodide (PI) according to the manufacturers’ instructions (FITC Annexin V Apoptosis Detection Kit I, BD Biosciences, San Jose, CA). Apoptotic cells were detected by flow cytometry using an LSR II (BD Biosciences; excitations at 488, 561, 640 nm), and data were analysed with FlowJo software. Cells were gated by FSC-A x SSC-A to exclude debris and then by FSC-H × FSC-A to exclude cell aggregates.
Statistical analysis
Statistical analyses were performed using R software (http://www.r-project.org) and Graphpad Prism software [122,123]. Statistical significance of the differences in mRNA expression level for each RNMT among different subtypes, grades, and NPI score of breast cancer samples was determined using ANOVA and Welch’s t-test as described earlier [122,123]. Spearman, Kendall, and Pearson correlation tests were used to correlate copy numbers and mRNA levels of each RNMT from TCGA breast cancer specimens. We used the ‘cor’ function in R for computation, specifying which type of test we wanted (Spearman, Kendall, or Pearson). Relationships between RNMT copy number, mRNA expression, and disease-free survival in METABRIC breast cancer were analysed by dividing samples into amp/gain (amplification plus gain) and no amp/gain, or high and low expression groups for each RNMT.
Funding Statement
This work was supported by the DOD Prostate Cancer Research Program [PC130259]; DMC Foundation [2018-3242]; DoD Breast Cancer Research Program (US) [BC161536].
Acknowledgments
This work was partially supported by grants from the Department of Defense (DoD) Breast Cancer Program BC161536, DoD Prostate Cancer Program PC130259, DMC Foundation and Molecular Therapeutics Program of Karmanos Cancer Institute to Dr. Zeng-Quan Yang; and by funding from Susan G. Komen GTDR14299438 and Wayne State University Graduate School Dean Mathur Fellowship to Morenci Manning. The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by NIH Cancer Center Support Grant No. P30CA022453 to the Karmanos Cancer Institute at Wayne State University. We thank Dr. Stephen P. Ethier for providing the SUM breast cancer cell lines. We are grateful to Dr. George S. Brush and Dr. Zhe Yang for input on this study. We thank Qianhui Huang, Hui Liu, and Era Cobani for technical contributions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Li S, Mason CE.. The pivotal regulatory landscape of RNA modifications. Annu Rev Genomics Hum Genet. 2014;15:127–150. [DOI] [PubMed] [Google Scholar]
- [2].Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Roundtree IA, Evans ME, Pan T, et al. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roost C, Lynch SR, Batista PJ, et al. Structure and thermodynamics of N6-methyladenosine in RNA: a spring-loaded base modification. J Am Chem Soc. 2015;137:2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kumar S, Mapa K, Maiti S. Understanding the effect of locked nucleic acid and 2ʹ-O-methyl modification on the hybridization thermodynamics of a miRNA-mRNA pair in the presence and absence of AfPiwi protein. Biochemistry. 2014;53:1607–1615. [DOI] [PubMed] [Google Scholar]
- [6].Kadumuri RV, Janga SC. Epitranscriptomic code and its alterations in human disease. Trends Mol Med. 2018;24:886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang X, Huang J, Zou T, et al. Human m(6)A writers: two subunits, 2 roles. RNA Biol. 2017;14:300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu J, Harada BT, He C. Regulation of gene expression by N(6)-methyladenosine in cancer. Trends Cell Biol. 2019;29:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Perry RP, Kelley DE. Existence of methylated messenger-rna in mouse L cells. Cell. 1974;1:37–42. [Google Scholar]
- [10].Boccaletto P, Machnicka MA, Purta E, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–D307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ayadi L, Galvanin A, Pichot F, et al. RNA ribose methylation (2ʹ-O-methylation): occurrence, biosynthesis and biological functions. Biochim Biophys Acta Gene Regul Mech. 2019;1862:253–269. [DOI] [PubMed] [Google Scholar]
- [12].Dai Q, Moshitch-Moshkovitz S, Han D, et al. Nm-seq maps 2ʹ-O-methylation sites in human mRNA with base precision. Nat Methods. 2017;14:695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Erales J, Marchand V, Panthu B, et al. Evidence for rRNA 2ʹ-O-methylation plasticity: control of intrinsic translational capabilities of human ribosomes. Proc Natl Acad Sci U S A. 2017;114:12934–12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ranjan N, Leidel SA. The epitranscriptome in translation regulation: mRNA and tRNA modifications as the two sides of the same coin? FEBS Lett. 2019;593:1483–1493. [DOI] [PubMed] [Google Scholar]
- [15].Hyde JL, Diamond MS. Innate immune restriction and antagonism of viral RNA lacking 2-O methylation. Virology. 2015;479–480:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schapira M. Structural chemistry of human RNA methyltransferases. ACS Chem Biol. 2016;11:575–582. [DOI] [PubMed] [Google Scholar]
- [17].Petrossian TC, Clarke SG. Uncovering the human methyltransferasome. Mol Cell Proteomics. 2011;10:M110 000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krishnamohan A, Jackman JE. A family divided: distinct structural and mechanistic features of the SpoU-TrmD (SPOUT) methyltransferase superfamily. Biochemistry. 2019;58:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shinoda S, Kitagawa S, Nakagawa S, et al. Mammalian NSUN2 introduces 5-methylcytidines into mitochondrial tRNAs. Nucleic Acids Res. 2019;47:8734–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang X, Yang Y, Sun BF, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sajini AA, Choudhury NR, Wagner RE, et al. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat Commun. 2019;10:2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stojkovic V, Fujimori DG. Mutations in RNA methylating enzymes in disease. Curr Opin Chem Biol. 2017;41:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khan MA, Rafiq MA, Noor A, et al. Mutation in NSUN2, which encodes an RNA methyltransferase, causes autosomal-recessive intellectual disability. Am J Hum Genet. 2012;90:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jensen LR, Garrett L, Holter SM, et al. A mouse model for intellectual disability caused by mutations in the X-linked 2ʹOmethyltransferase Ftsj1 gene. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2083–2093. [DOI] [PubMed] [Google Scholar]
- [25].Freude K, Hoffmann K, Jensen LR, et al. Mutations in the FTSJ1 gene coding for a novel S-adenosylmethionine-binding protein cause nonsyndromic X-linked mental retardation. Am J Hum Genet. 2004;75:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garone C, D’Souza AR, Dallabona C, et al. Defective mitochondrial rRNA methyltransferase MRM2 causes MELAS-like clinical syndrome. Hum Mol Genet. 2017;26:4257–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Neelamraju Y, Hashemikhabir S, Janga SC. The human RBPome: from genes and proteins to human disease. J Proteomics. 2015;127:61–70. [DOI] [PubMed] [Google Scholar]
- [29].Wang ZL, Li B, Luo YX, et al. Comprehensive genomic characterization of RNA-binding proteins across human cancers. Cell Rep. 2018;22:286–298. [DOI] [PubMed] [Google Scholar]
- [30].Neelamraju Y, Gonzalez-Perez A, Bhat-Nakshatri P, et al. Mutational landscape of RNA-binding proteins in human cancers. RNA Biol. 2018;15:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kechavarzi B, Janga SC. Dissecting the expression landscape of RNA-binding proteins in human cancers. Genome Biol. 2014;15:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Correa BR, de Araujo PR, Qiao M, et al. Functional genomics analyses of RNA-binding proteins reveal the splicing regulator SNRPB as an oncogenic candidate in glioblastoma. Genome Biol. 2016;17:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang J, Liu Q, Shyr Y. Dysregulated transcription across diverse cancer types reveals the importance of RNA-binding protein in carcinogenesis. BMC Genomics. 2015;16(Suppl 7):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gonzalez-Perez A, Lopez-Bigas N. Functional impact bias reveals cancer drivers. Nucleic Acids Res. 2012;40:e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu L, Zhen XT, Denton E, et al. ChromoHub: a data hub for navigators of chromatin-mediated signalling. Bioinformatics. 2012;28:2205–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang LS, Liu C, Ma H, et al. Transcriptome-wide Mapping of Internal N(7)-Methylguanosine Methylome in Mammalian mRNA. Mol Cell. 2019;74:1304–1316 e1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ringeard M, Marchand V, Decroly E, et al. FTSJ3 is an RNA 2ʹ-O-methyltransferase recruited by HIV to avoid innate immune sensing. Nature. 2019;565:500–504. [DOI] [PubMed] [Google Scholar]
- [38].Howell NW, Jora M, Jepson BF, et al. Distinct substrate specificities of the human tRNA methyltransferases TRMT10A and TRMT10B. RNA. 2019;25:1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Freund I, Buhl DK, Boutin S, et al. 2ʹ-O-methylation within prokaryotic and eukaryotic tRNA inhibits innate immune activation by endosomal Toll-like receptors but does not affect recognition of whole organisms. RNA. 2019;25:869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Carter JM, Emmett W, Mozos IR, et al. FICC-Seq: a method for enzyme-specified profiling of methyl-5-uridine in cellular RNA. Nucleic Acids Res. 2019;47:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shelton SB, Shah NM, Abell NS, et al. Crosstalk between the RNA methylation and histone-binding activities of MePCE regulates P-TEFb activation of chromatin. Cell Rep. 2018;22:1374–1383. [DOI] [PubMed] [Google Scholar]
- [42].Schwartz S. m1A within cytoplasmic mRNAs at single nucleotide resolution: a reconciled transcriptome-wide map. RNA. 2018;24:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Oerum S, Roovers M, Rambo RP, et al. Structural insight into the human mitochondrial tRNA purine N1-methyltransferase and ribonuclease P complexes. J Biol Chem. 2018;293:12862–12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li X, Xiong X, Zhang M, et al. Base-resolution mapping reveals distinct m1A methlome in nuclear-and mitochondrial-encoded transcripts. Mol Cell. 2017;68:993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dewe JM, Fuller BL, Lentini JM, et al. TRMT1-catalyzed tRNA modifications are required for redox homeostasis to ensure proper cellular proliferation and oxidative stress survival. Mol Cell Biol. 2017;37:e00214-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Currie MA, Brown G, Wong A, et al. Structural and functional characterization of the TYW3/Taw3 class of SAM-dependent methyltransferases. RNA. 2017;23:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Varshney D, Petit AP, Bueren-Calabuig JA, et al. Molecular basis of RNA guanine-7 methyltranserase (RNMT) activation by RAM. Nucleic Acids Res. 2016;44:10423–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Haag S, Sloan KE, Ranjan N, et al. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. Embo J. 2016;35:2104–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Aquilo F, Li S, Balasubramaniyan N, et al. Deposition of 5-methylcytosine on enhancer RNAs enables the coactivation function of PGC-1 alpha. Cell Rep. 2016;14:479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zorbas C, Nicolas E, Wacheul L, et al. The human 18S rRNA base methyltransferases DIMT1L and WBSCR22-TRMT112 but not rRNA modification are required for ribosome biogenesis. Mol Biol Cell. 2015;26:2080–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Powell CA, Kopajtich R, D’Souza AR, et al. TRMT5 mutations cause a defect in post-transcriptional modification of mitochondrial tRNA associated with multiple respiratory-chain deficiencies. Am J Hum Genet. 2015;97:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Metodiev MD, Spahr H, Loguercio Polosa P, et al. NSUN4 is a dual function mitochondrial protein required for both methyltion of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10:e1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lee KW, Bogenhagen DF. Assignment of 2ʹ-O-methyltransferases to modification sites on the mammalian mitochondrial large subunit 16 S ribosomal RNA (rRNA). J Biol Chem. 2014;289:24936–24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sharma S, Yang J, Watzinger P, et al. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 2013;41:9062–9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Begley U, Sosa MS, Avivar-Valderas A, et al. A human tRNA methyltransferase 9-like protein prevents tumour growth by regulating LIN9 and HIF1-alpha. EMBO Mol Med. 2013;5:366–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Xhemalce B, Robson SC, Kouzarides T. Human RNA methyltransferase BCDIN3D regulates microRNA processing. Cell. 2012;151:278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Reiter V, Matschkal DM, Wagner M, et al. The CDK5 repressor CDK5RAP1 is a methythiotransferase acting on nuclear mitochondrial RNA. Nucleic Acids Res. 2012;40:6235–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Figaro S, Wacheul L, Schillewaert S, et al. Trm112 is required for Bud23-mediated methylation of the 18S rRNA at position G1575. Mol Cell Biol. 2012;32:2254–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Werner M, Purta E, Kaminska KH, et al. 2ʹ-O-ribose methylation of cp2 in human: function and evolution in a horizontally mobile family. Nucleic Acids Res. 2011;39:4756–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wei FY, Suzuki T, Watanabe S, et al. Deficit of tRNA(Lys) modification by Cdkal1causes the development of type 2 diabetes in mice. J Clin Investig. 2011;121:3598–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wurm JP, Meyer B, Bahr U, et al. The ribosome assembly factor Nep1 responsible for Bowen-Conradi syndrome is a pseudouridine-N1-specific methyltransferase. Nucleic Acids Res. 2010;38:2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Songe-Moller L, van den Born E, Leihne V, et al. Mammalian ALKBH8 possesses tRNA methyltransferase activity required for the biogenesis of multiple wobble uridine modifications implicated in translational decoding. Mol Cell Biol. 2010;30:1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Belanger F, Stepinski J, Darzynkiewicz E, et al. Characterization of hMTr1, a human Cap1 2ʹ-O-ribose methyltransferase. J Biol Chem. 2010;285:33037–33044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].White J, Li Z, Sardana R, et al. Bud23 methylates G1575 of 18S rRNA and is required for efficient nuclear export of pre-40S subunits. Mol Cell Biol. 2008;28:3151–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kirino Y, Mourelatos Z. 2ʹ-O-methyl modification in mouse piRNAs and its methylase. Nucleic Acids Symposium Series (Oxford). 2007;417–418:51. [DOI] [PubMed] [Google Scholar]
- [67].Goll MG, Kirpekar F, Maggert KA, et al. Methylation of tRNAAsp by the DNA methyltransferase homolong Dnmt2. Science. 2006;311:395–398. [DOI] [PubMed] [Google Scholar]
- [68].Cotney J, Shadel GS. Evidence for an early gene duplication event in the evolution of the mitochondrial transcription factor B family and maintenance of rRNA methyltransferase activity in human mtTFB1 and mtTFB2. J Mol Evol. 2006;63:707–717. [DOI] [PubMed] [Google Scholar]
- [69].Brzezicha B, Schmidt M, Makalowska I, et al. Identification of human tRNA: m5C methyltransferase catalysing intron-dependent m5Cformation in the first position of hte anticodon of the pre-tRNA Leu (CAA). Nucleic Acids Res. 2006;34:6034–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Purushothaman SK, Bujnicki JM, Grosjean H, et al. Trm11p and Trm112p are both required for the formation of 2-methylguanosine at position 10 in yeast tRNA. Mol Cell Biol. 2005;25:4359–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Huang B, Johansson MJ, Bystrom AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11:424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pintard L, Lecointe F, Bujnicki JM, et al. Trm7p catalyses the formation of two 2ʹ-O-methylriboses in yeast tRNA anticodon loop. European Molecular Biology Organization. 2002;21:1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mouaikel J, Verheggen C, Bertrand E, et al. Hypermethylation of the cap structure of both yeast snRNAs and snoRNAs requires a conserved methyltransferase that is localized to the nucleolus. Mol Cell. 2002;9:891–901. [DOI] [PubMed] [Google Scholar]
- [74].Tollervey D, Lehtonen H, Jansen R, et al. Temperature-sensitive mutations demonstrate roles for yeast fibrallarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell. 1993;72:443–457. [DOI] [PubMed] [Google Scholar]
- [75].Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mermel CH, Schumacher SE, Hill B, et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [79].N. Cancer Genome Atlas . Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- [81].Creighton CJ. The molecular profile of luminal B breast cancer. Biologics. 2012;6:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bertucci F, Finetti P, Birnbaum D. Basal breast cancer: a complex and deadly molecular subtype. Curr Mol Med. 2012;12:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Galea MH, Blamey RW, Elston CE, et al. The Nottingham prognostic index in primary breast cancer. Breast Cancer Res Treat. 1992;22:207–219. [DOI] [PubMed] [Google Scholar]
- [85].Blamey RW, Ellis IO, Pinder SE, et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990–1999. Eur J Cancer. 2007;43:1548–1555. [DOI] [PubMed] [Google Scholar]
- [86].Tsherniak A, Vazquez F, Montgomery PG, et al. Defining a cancer dependency map. Cell. 2017;170:564–576 e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].McDonald ER 3rd, de Weck A, Schlabach MR, et al. Project DRIVE: A compendium of cancer dependencies and synthetic lethal relationships uncovered by large-scale, deep RNAi screening. Cell. 2017;170:577–592 e510. [DOI] [PubMed] [Google Scholar]
- [88].Marcotte R, Sayad A, Brown KR, et al. Functional genomic landscape of human breast cancer drivers, vulnerabilities, and resistance. Cell. 2016;164:293–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].McFarland JM, Ho ZV, Kugener G, et al. Improved estimation of cancer dependencies from large-scale RNAi screens using model-based normalization and data integration. Nat Commun. 2018;9:4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Marcel V, Ghayad SE, Belin S, et al. p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell. 2013;24:318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Su H, Xu T, Ganapathy S, et al. Elevated snoRNA biogenesis is essential in breast cancer. Oncogene. 2014;33:1348–1358. [DOI] [PubMed] [Google Scholar]
- [92].Hodel AE, Gershon PD, Shi XN, et al. The 1.85 angstrom structure of vaccinia protein VP39: A bifunctional enzyme that participates in the modification of both mRNA ends. Cell. 1996;85:247–256. [DOI] [PubMed] [Google Scholar]
- [93].Ray ME, Yang ZQ, Albertson D, et al. Genomic and expression analysis of the 8p11-12 amplicon in human breast cancer cell lines. Cancer Res. 2004;64:40–47. [DOI] [PubMed] [Google Scholar]
- [94].Liu G, Bollig-Fischer A, Kreike B, et al. Genomic amplification and oncogenic properties of the GASC1 histone demethylase gene in breast cancer. Oncogene. 2009;28:4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Barretina J, Caponigro G, Stransky N, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lee AV, Oesterreich S, Davidson NE. MCF-7 cells–changing the course of breast cancer research and care for 45 years. J Natl Cancer Inst. 2015;107:djv073. [DOI] [PubMed] [Google Scholar]
- [98].Ethier SP, Kokeny KE, Ridings JW, et al. erbB family receptor expression and growth regulation in a newly isolated human breast cancer cell line. Cancer Res. 1996;56:899–907. [PubMed] [Google Scholar]
- [99].Engel LW, Young NA, Tralka TS, et al. Establishment and characterization of three new continuous cell lines derived from human breast carcinomas. Cancer Res. 1978;38:3352–3364. [PubMed] [Google Scholar]
- [100].Wu F, Garcia J, Sigman D, et al. tat regulates binding of the human immunodeficiency virus trans-activating region RNA loop-binding protein TRP-185. Genes Dev. 1991;5:2128–2140. [DOI] [PubMed] [Google Scholar]
- [101].Wu H, Min J, Zeng H, et al. Crystal structure of the methyltransferase domain of human TARBP1. Proteins. 2008;72:519–525. [DOI] [PubMed] [Google Scholar]
- [102].Ye J, Wang J, Zhang N, et al. Expression of TARBP1 protein in human non-small-cell lung cancer and its prognostic significance. Oncol Lett. 2018;15:7182–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sand M, Skrygan M, Georgas D, et al. Expression levels of the microRNA maturing microprocessor complex component DGCR8 and the RNA-induced silencing complex (RISC) components argonaute-1, argonaute-2, PACT, TARBP1, and TARBP2 in epithelial skin cancer. Mol Carcinog. 2012;51:916–922. [DOI] [PubMed] [Google Scholar]
- [104].Ye J, Wang J, Tan L, et al. Expression of protein TARBP1 in human hepatocellular carcinoma and its prognostic significance. Int J Clin Exp Pathol. 2015;8:9089–9096. [PMC free article] [PubMed] [Google Scholar]
- [105].Parent M, Yung TM, Rancourt A, et al. Poly(ADP-ribose) polymerase-1 is a negative regulator of HIV-1 transcription through competitive binding to TAR RNA with Tat.positive transcription elongation factor b (p-TEFb) complex. J Biol Chem. 2005;280:448–457. [DOI] [PubMed] [Google Scholar]
- [106].Hager J, Staker BL, Jakob U. Substrate binding analysis of the 23S rRNA methyltransferase RrmJ. J Bacteriol. 2004;186:6634–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hager J, Staker BL, Bugl H, et al. Active site in RrmJ, a heat shock-induced methyltransferase. J Biol Chem. 2002;277:41978–41986. [DOI] [PubMed] [Google Scholar]
- [108].Bugl H, Fauman EB, Staker BL, et al. RNA methylation under heat shock control. Mol Cell. 2000;6:349–360. [DOI] [PubMed] [Google Scholar]
- [109].Orre LM, Vesterlund M, Pan Y, et al. SubCellBarCode: proteome-wide mapping of protein localization and relocalization. Mol Cell. 2019;73:166–182 e167. [DOI] [PubMed] [Google Scholar]
- [110].Binder JX, Pletscher-Frankild S, Tsafou K, et al. COMPARTMENTS: unification and visualization of protein subcellular localization evidence. Database (Oxford). 2014;2014:bau012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Guy MP, Shaw M, Weiner CL, et al. Defects in tRNA anticodon Loop 2ʹ-O-Methylation are implicated in nonsyndromic X-Linked intellectual disability due to mutations in FTSJ1. Hum Mutat. 2015;36:1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Rorbach J, Boesch P, Gammage PA, et al. MRM2 and MRM3 are involved in biogenesis of the large subunit of the mitochondrial ribosome. Mol Biol Cell. 2014;25:2542–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lapeyre B, Purushothaman SK. Spb1p-directed formation of Gm2922 in the ribosome catalytic center occurs at a late processing stage. Mol Cell. 2004;16:663–669. [DOI] [PubMed] [Google Scholar]
- [114].Decatur WA, Fournier MJ. rRNA modifications and ribosome function. Trends Biochem Sci. 2002;27:344–351. [DOI] [PubMed] [Google Scholar]
- [115].Morello LG, Coltri PP, Quaresma AJ, et al. The human nucleolar protein FTSJ3 associates with NIP7 and functions in pre-rRNA processing. PLoS One. 2011;6:e29174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Simabuco FM, Morello LG, Aragao AZ, et al. Proteomic characterization of the human FTSJ3 preribosomal complexes. J Proteome Res. 2012;11:3112–3126. [DOI] [PubMed] [Google Scholar]
- [117].Fish L, Navickas A, Culbertson B, et al. Nuclear TARBP2 drives oncogenic dysregulation of RNA splicing and decay. Mol Cell. 2019;75:967–981 e969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Goodarzi H, Zhang S, Buss CG, et al. Metastasis-suppressor transcript destabilization through TARBP2 binding of mRNA hairpins. Nature. 2014;513:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Daniels SM, Gatignol A. The multiple functions of TRBP, at the hub of cell responses to viruses, stress, and cancer. Microbiol Mol Biol R. 2012;76:652–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bartoli KM, Schaening C, Carlile TM, Gilbert WV, et al. Conserved methyltransferase Spb1 targets mRNAs for regulated modification with 2′-O-Methyl Ribose. bioRxiv. 2018. [Google Scholar]
- [121].Boriack-Sjodin PA, Ribich S, Copeland RA. RNA-modifying proteins as anticancer drug targets. Nat Rev Drug Discov. 2018;17:435–453. [DOI] [PubMed] [Google Scholar]
- [122].Jiang Y, Liu L, Shan W, et al. An integrated genomic analysis of Tudor domain-containing proteins identifies PHD finger protein 20-like 1 (PHF20L1) as a candidate oncogene in breast cancer. Mol Oncol. 2016;10:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Liu H, Liu L, Holowatyj A, et al. Integrated genomic and functional analyses of histone demethylases identify oncogenic KDM2A isoform in breast cancer. Mol Carcinog. 2016;55:977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chu X, Guo X, Jiang Y, et al. Genotranscriptomic meta-analysis of the CHD family chromatin remodelers in human cancers - initial evidence of an oncogenic role for CHD7. Mol Oncol. 2017;11:1348–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


