Abstract
Background
Cancer diagnostics and surgery have been disrupted by the response of health care services to the coronavirus disease 2019 (COVID-19) pandemic. Progression of cancers during delay will impact on patients' long-term survival.
Patients and methods
We generated per-day hazard ratios of cancer progression from observational studies and applied these to age-specific, stage-specific cancer survival for England 2013–2017. We modelled per-patient delay of 3 and 6 months and periods of disruption of 1 and 2 years. Using health care resource costing, we contextualise attributable lives saved and life-years gained (LYGs) from cancer surgery to equivalent volumes of COVID-19 hospitalisations.
Results
Per year, 94 912 resections for major cancers result in 80 406 long-term survivors and 1 717 051 LYGs. Per-patient delay of 3/6 months would cause attributable death of 4755/10 760 of these individuals with loss of 92 214/208 275 life-years, respectively. For cancer surgery, average LYGs per patient are 18.1 under standard conditions and 17.1/15.9 with a delay of 3/6 months (an average loss of 0.97/2.19 LYGs per patient), respectively. Taking into account health care resource units (HCRUs), surgery results on average per patient in 2.25 resource-adjusted life-years gained (RALYGs) under standard conditions and 2.12/1.97 RALYGs following delay of 3/6 months. For 94 912 hospital COVID-19 admissions, there are 482 022 LYGs requiring 1 052 949 HCRUs. Hospitalisation of community-acquired COVID-19 patients yields on average per patient 5.08 LYG and 0.46 RALYGs.
Conclusions
Modest delays in surgery for cancer incur significant impact on survival. Delay of 3/6 months in surgery for incident cancers would mitigate 19%/43% of LYGs, respectively, by hospitalisation of an equivalent volume of admissions for community-acquired COVID-19. This rises to 26%/59%, respectively, when considering RALYGs. To avoid a downstream public health crisis of avoidable cancer deaths, cancer diagnostic and surgical pathways must be maintained at normal throughput, with rapid attention to any backlog already accrued.
Key words: COVID-19, delay, diagnostics, oncology, survival
Highlights
-
•
Lockdown and re-deployment due to the COVID-19 pandemic have caused significant disruption to cancer diagnosis and management.
-
•
A 3-month delay to surgery across all stage 1–3 cancers is estimated to cause >4700 attributable deaths per year in England.
-
•
The impact on life-years lost of 3–6-month delay to surgery for stage 1–3 disease varies widely between tumour types.
-
•
Strategic prioritisation of patients for diagnostics and surgery has potential to mitigate deaths attributable to delays.
-
•
The resource-adjusted benefit in avoiding delay in cancer management compares favourably with admission for COVID-19 infection.
Introduction
Following the first case reports in Hubei province, China, in late 2019, a pandemic of coronavirus disease 2019 (COVID-19) was declared by the World Health Organization in March 2020. Although COVID-19 causes minimal or mild illness in most, a small but appreciable proportion of individuals require oxygen therapy and often admission to an intensive care unit (ICU). The ensuing unprecedented pressure on hospital wards and ICUs has necessitated rapid re-deployment of staff and capacity towards the management of COVID-19 cases with deprioritisation of non-emergency clinical services, including diagnostics and elective specialist surgery. Concurrently, lockdown of the population has impacted dramatically on presentation and referral of symptomatic patients from primary into secondary care.1
For patients with cancer, delay of surgery has the real potential to increase the likelihood of metastatic disease, with some patients' tumours progressing from being curable (with near-normal life expectancy) to noncurable (with limited life expectancy).2 The situation has been further exacerbated by recent safety concerns regarding aerosol generation from endoscopy, cystoscopy and surgery.3 , 4
Current projections indicate that COVID-19-related disruption may well last for ≥18 months, until there is either long-term effective containment in the population or large-scale vaccination. To inform health care prioritisation and resource allocation, we have examined the impact on cancer outcomes of different periods of delay of cancer surgery with disruption extending over variable periods, comparing resource-weighted outcomes with hospital management of COVID-19 patients.
Methods
Data sources
Number and age-specific 5-year net survival of cancer patients that had potentially curative surgical resections for nonhematological malignancies between 2013 and 2017 were obtained from the Public Health England National Cancer Registration Service.5 As well as cancer stage at diagnosis for each cancer type, breast tumour receptor data allowed subtyping of these cancers as ER+ HER2−, HER+ (any), ER− HER2− and other. Estimates for nosocomial infection rates, median duration of hospital stay for each cancer type, staffing of theatres, ICU and surgical wards were based on information from three large UK surgical oncology centres. Patterns of administration of adjuvant systemic anticancer therapy (SACT) were based on oncologist-reviewed standard practice guidance.6 ICU COVID-19 mortality, distribution by age and duration of stay and proportion referred into ICU were obtained from ICNARC (Intensive Care National Audit and Research Centre) and data from hospitalised UK cases.7 , 8 Because of lack of UK data, data from Wuhan were used as the basis for the age distribution of community infection, age-specific likelihoods of admission from community to hospital and case fatality rates for non-ICU COVID-19 patients9 , 10 (see supplementary Table S1, available at Annals of Oncology online).
Analysis
Impact of COVID-associated delay on cancer outcomes
We used published data from studies examining the impact on overall survival from delay in cancer surgery to estimate per-day hazard ratios (HRs) associated with delay for different cancers (the ‘Fatality HR’).11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 We had sufficient data to generate Fatality HRs for three tumour types and assigned these to other tumours, based on comparability of 5-year survival as low (>90%), moderate (50%–90%) or high (<50%) progressiveness tumours.5 Because we were unable to identify any suitable observational data for tumours of high progressiveness (e.g. oesophageal, gastric), we applied the Fatality HR from tumours of moderate progressiveness; this is likely to be a conservative assumption (supplementary Table S2, available at Annals of Oncology online).
By accounting for COVID-related postsurgical mortality and changes in SACT, we adjusted 5-year net survival figures for each cancer for surgical patients under ‘standard’ care to estimate ‘current’ 5-year net survival. To model outcomes of surgery ‘post-delay’, we apply to standard 5-year net survival the Fatality HR relating to the specified number of days of delay, again including COVID-related postsurgical mortality. Based on estimates from a UK surgical oncology centre, supported by the literature, we applied a current per-day rate of nosocomial infection of 5%. Assuming improvement in cold protocols, we modelled reduction in this rate over time. We estimated COVID-associated surgical mortality based on per-day rate of nosocomial infection, operation-specific duration of postsurgical admission and age-specific mortality from infection. We estimated COVID-19-associated mortality for SACT administration, based on per-day rate of nosocomial infection, the frequency of SACT scheduling, increased risk associated with immunosuppression and age-specific mortality from infection. We assumed, where standard of care, that SACT offers a uniform survival benefit (5% in stage 1, 7.5% in stage 2 and 10% in stage 3) and administration would only continue where this benefit exceeds COVID-related mortality.
We used mean life expectancies per 10-year age group to calculate life-years gained (LYGs), averaged per patient. We examined reduction in overall survival and LYGs, comparing surgery under standard care, current conditions and postdelay, by cancer type and by age and stage. Using 2013–2017 surgical workload data, we calculated, across all adult cancers examined, the total number of deaths and life-years lost attributable to delay. To address possible scenarios, we considered per-patient delay of up to 6 months, and 1- and 2-year periods of disruption.
COVID-19 outcome
To compare life-years associated with timely cancer surgery with those afforded by hospitalisation of COVID-19 patients, we modelled a volume of community-ascertained COVID-19 infection, resulting in an equivalent volume of hospital admissions to cancer surgeries (see supplementary Table S1, available at Annals of Oncology online).
Resource
We analysed health care resource units (HCRUs) focused specifically on frontline medical and nursing staff, where one HCRU is one 12-hour shift of direct nursing or medical care. We upweighted for shifts from health care workers of high salary (senior doctors) and/or of current scarcity (anaesthetists, ICU nurses). We calculated HCRUs per patient using estimated staffing ratios for theatres, ICU and ward care and operation-specific data for theatre hours, ICU stay and ward days from oncology centres.
Details of assumptions and parameter estimates are detailed in Table 1 and supplementary Table S1, available at Annals of Oncology online. Analyses were performed using STATA (version 15; StataCorp, College Station, TX) and transcribed to Excel (Microsoft, Redmond, WA), to provide a full visibility of parametrisation, model outputs and opportunity for the reader to customise parameters (supplementary Materials, available at Annals of Oncology online).
Table 1.
Summary of sources for parameter estimates for the cancer surgical model (see supplementary Table S1, available at Annals of Oncology online, for full description)
| Component of model | Elements | Data source | Comment | Reference/specific values |
|---|---|---|---|---|
| Life-years lost due to delay in surgery | Proportion of patients surviving after surgery | 5-year survival rates for cancer surgery in England | Age, site and stage-specific 5-year cancer survival in individuals in whom major resection was performed | PHE National Cancer Registration and Analysis Service4 |
| Decrease in survival due to delay in treatment | Observational studies of increased death rate due to delay in treatment | Hazard ratio for increase in death rate for each day delay in treatment based on estimates from literature applied to standard survival rates; applied to tumours depending on tumour aggressiveness |
Cancer progressiveness based on 5-y survival: Low: >90% Moderate: 50%–90% High: <50% Per-day hazard ratio for fatality10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20: Low: 0.0030 Moderate: 0.0056 High: 0.0056 |
|
| COVID-related postsurgical mortality. SACT-related mortality | Nosocomial infection rate | Based on literature, estimate from clinical site data | 5% per day29 | |
| Mortality from COVID infection | Age-specific data from international series | 0–39 y: 0.2% 30–39 y: 0.2% 40–49 y: 0.4% 50–59 y: 1.3% 60–69 y: 3.6% 70–79 y: 8.0% ≥80 y: 14.8% |
||
| Survival benefit from SACT | Expert clinical interpretation of literature | Stage 1: 5% Stage 2: 7.5% Stage 3: 10%30 |
||
| Increase in COVID-related mortality due to SACT | Based on UK and international literature | Two-fold7,8 | ||
| Life expectancy after survival | General population mean life expectancies per 10-year age band | Expected remaining life-years in the treated group based on proportion who survive after treatment (with and without delay) | ONS Life Tables31 | |
| Health care resourcing | Duration of operation, ICU and inpatient ward stay | Data from UK surgical oncology centres | Calculated as health care resource units (HCRUs) of direct clinical care. 1 HCRU = one 12-h medical/nursing shift | |
| Staffing ratios in theatre, wards, ICU |
COVID-19, coronavirus disease 2019; ICU, intensive care unit; PHE, Public Health England; SACT, systemic anticancer therapy.
Results
Impact of surgical delay on survival for different cancers
The greatest rates of deaths arise following even modest delays to surgery in aggressive cancers, with >30% reduction in survival at 6 months and >17% reduction in survival at 3 months for patients with stage 2 or 3 cancers of the bladder, lung, oesophagus, ovary, liver, pancreas and stomach (Table 2 ; see also supplementary Table S3 and supplementary Materials, available at Annals of Oncology online). Accounting for nosocomial COVID-19 infection, for cancers with a relatively good overall prognosis, delay of surgery by 3 months had a minimal impact on survival: <1% for all stage 1 ER+ and HER2+ breast cancers, for example. In older patients (aged >70), for early stage colorectal, kidney and ER+ breast cancers, the current impact on survival of COVID-related mortality exceeded the impact of 3 or even 6 months' delay (Table 2; see also supplementary Table S3, available at Annals of Oncology online).
Table 2.
Reduction in 5-year net survival as a consequence of 6-month delay to surgery for 13 cancer types, by tumour stage and age of diagnosis
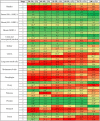 |
Reduction in survival above the median is represented in red, at the median in yellow and below the median in green. Survival analysis is based on per-day hazard ratios for disease fatality.
a Strata estimates of lower confidence whereby crude rather than net survival estimates were applied.
For a high proportion of solid cancers, survival at 5 years is generally considered to be equivalent to cure. Predicated on this assertion, we considered LYGs adjusting for resource [resource-adjusted life-years gained (RALYGs)]. Perhaps unsurprisingly, most benefit is afforded in younger age groups for operations that are shorter with no associated ICU requirement. For example, trans-urethral resection of stage 1 bladder cancers affords on average 23.4 RALYGs per patient aged 30–39, whereas cystectomy for stage 2 bladder cancer is only associated with 1.2 RALYGs in that age group (supplementary Table S4, available at Annals of Oncology online). In the context of prioritisation, avoidance of a 6-month delay restitutes on average 4.1 RALYGs in the former group, compared with 0.7 in the latter (Table 3 ; see also supplementary Table S5, available at Annals of Oncology online). Wide local excision for breast cancer has low resource requirement and therefore confers substantial RALYGs, even in good prognosis subtypes.
Table 3.
Estimated average life-years gained per unit of health care resource for cancer surgery for 13 cancer types, by tumour stage and age of diagnosis comparing current surgery with surgery after 6 months' delay based on 5-year net survival
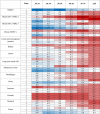 |
HCRUs, health care resource units; LYG, life-year gained.
a Strata estimates of lower confidence whereby crude rather than net survival estimates were applied. Values for LYG per HCRU above the median are represented in blue, at the median in white and below the median in red.
Impact of surgical delay on cancer survival combined across cancer types
Each year, 94 912 surgical resections for common invasive adult cancer types are performed in England, with 80 406 of those patients surviving their cancer at 5 years. A surgical delay of 3 months across all incident solid tumours over 1 year would incur 4755 excess deaths, escalating to 10 760 excess deaths for a 6-month delay. This includes at 6 months, attributable deaths of 2980 for colorectal cancer, 1439 for lung cancer and 804 for breast cancer (Figure 1 and supplementary Table S6, available at Annals of Oncology online).
Figure 1.
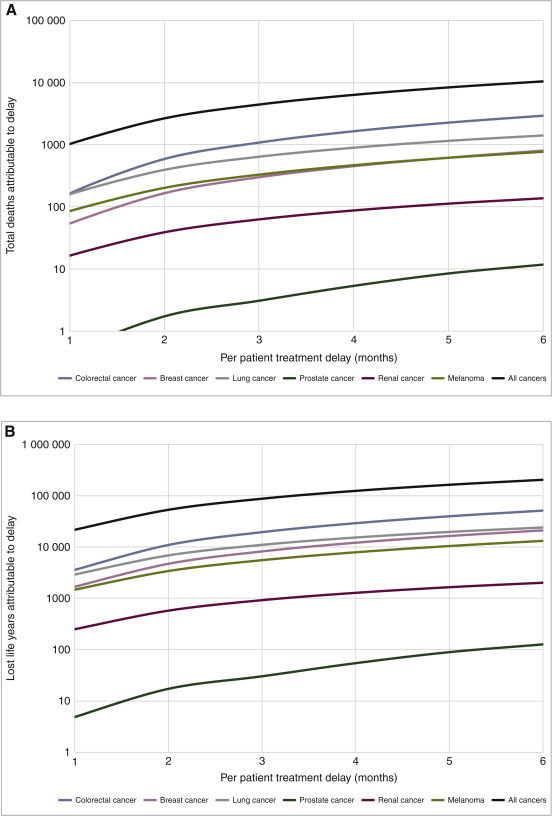
Impact from 6-month delay lasting 1 year for all solid cancers analysed and six common cancer types in England expressed in (A) attributable deaths and (B) life-years lost.
For a high proportion of solid cancers, 5-year survival is generally considered to be equivalent to cure. Predicated on this assertion, across all cancers a treatment delay of 3 months would lead to a reduction of 92 214 life-years, whereas a treatment delay of 6 months would lead to a reduction of 208 275 life-years (Table 3). Prior to the COVID-19 crisis, each year cancer surgery was directly responsible for 1 717 051 LYGs. This represents on average 18.1 LYG per patient, which reduces to 17.1 with 3 months' delay and to 15.9 with 6 months' delay. Cancer surgery per year requires 764 765 units of health care resource. Assuming this to be unchanged by delay, this affords on average 2.25 RALYGs per patient under standard conditions, reducing to 2.12 with 3 months' delay and 1.97 with 6 months' delay, an average loss of 0.12 and 0.27 RALYGs, respectively, per patient.
Resource comparison for outcomes afforded by cancer surgery and COVID-19 management
For contextualisation, we compare the impact of cancer surgery delay with hospital care for patients with community-acquired COVID-19 infection. COVID-19 ICU admission for those aged 40–49 yielded on average 27.5 LYGs and 0.8 RALYGs. Those aged >80 admitted to ICU benefit by on average 2.1 LYGs and 0.06 RALYGs. For non-ICU admission, average benefit is 9.3 LYGs and 1.5 RALYGs for those aged 40–49 and 1.4 LYGs and 0.2 RALYGs for those aged >80 years (supplementary Materials, available at Annals of Oncology online). These estimates are inherently conservative as they do not take into account the impact on life expectancy of the excess comorbidities associated with many hospitalised COVID-19 cases.
COVID-19 community-acquired infection of 683 083 individuals would result in 94 912 hospital admissions (i.e. the equivalent number to number of annual admissions for cancer surgery; Table 4). For these 94 912 admissions, 16 135 will require ICU (critical cases) and 78 777 will not require ICU (severe cases); 1 052 949 HCRUs are required in total and there are 15 587 deaths, 25 752 attributable lives saved and 482 022 attributable LYGs (8241 deaths/7894 attributable lives saved/223 227 LYGs for ICU admissions; 7346/17 858/258 795 for non-ICU). This represents on average 5.08 LYGs and 0.46 RALYGs per hospitalised COVID-19 patient.
Table 4.
Summary outcomes from delays in cancer surgery, with comparison to an equivalent number of admissions for community-acquired COVID-19 infection. Only major resections for common adult cancers are included
 |
Reference population: England.
COVID-19, coronavirus disease-2019; HCRUs, health care resource units; ICU, intensive care unit; LY, life-years; RALYs, resource-adjusted life-years.
It is therefore noteworthy that a delay of surgery by 6 months results in 208 275 lost life-years for an annual quota of surgical patients: this equates to 43% of the total 482 022 LYGs from hospitalisation of an equivalent number of community-acquired COVID-19 cases. This rises to 59% when adjusted for differences in resource (RALYGs).
Sensitivity analysis
The outcomes from the model were mostly sensitive to changes in the Fatality HR for the per-day delay: varying this by ±8% (1SD) caused the average LYGs with a 6-month delay to range from 15.7 to 16.1, and attributable LYs lost by 2.00–2.39. Sensitivity analysis for other parameters is shown in supplementary Table S2, available at Annals of Oncology online.
Discussion
We provide estimates derived from reported surgical outcomes to quantify the impact on survival of delay of cancer treatment, within the parameters of the assumptions of the model.
Implications for health care planning
For aggressive cancers, our analysis demonstrates that even short delays (3 months) have a significant impact on patient survival. However, even for cancers of comparatively favourable prognosis, a delay of 6 months will result in significant summed attributable deaths as many of these cancers are common. Delay will also result in tumours being more advanced, meaning not only is survival poorer, but also the upstaged cancers will be more costly to treat in terms of both surgery and/or chemotherapy. Furthermore, resource requirements (e.g. ICU stay) are dramatically higher for the many who will inevitably present as emergencies such as with obstruction, perforation or acute bleeding of the gastrointestinal tract.22
Critical to mitigating cancer deaths is recognition that delay or bottleneck may arise at any point in the linear patient journey from (i) self-presentation of the symptomatic patient to primary care, (ii) primary care review and referral into secondary care (iii) diagnostic investigation, and (iv) surgery (or radiotherapy) with curative intent. Alongside any ‘bulge’ in accumulated cases will be the normal stream of incident cancer presentations. In the face of prolonged stress, it will be challenging to provide extra capacity to address these bulges alongside standard demands. In the short term, to avoid knock-on delays, immediate diversion of supra-normal resource volumes are required to process the backlog of cases that will have accrued in the initial months of the pandemic, in which referrals, investigations and surgeries have been reduced by up to 80%.1 In the medium-to-long term (over the next 3–24 months), avoidance of delay in cancer surgery should be of the highest priority: urgent attention is required to ensure sufficient resourcing for standard capacity of all pathway elements in primary care, cancer diagnostic and surgical.
Delay in cancer surgery will have a highly deleterious health and economic impact. For the most part, the surgery will still be required (and may be more complex and costly) but results in rapid diminution of resultant LYGs and resource-adjusted life-years. Comparing equivalent-sized hospital populations adjusted for resource, the health impact of delaying cancer surgery for 6 months will approximate 60% of health gains of hospitalisations for community-acquired COVID-19 infection. We need to consider resourcing in the likely event of sizeable requirement for COVID-19 management for a sustained period, potentially up to 2 years. Although large facilities may be built/repurposed for COVID-19 management, these facilities are competing for the same fixed pool of health care workers that care for non-COVID-19 patients.
Where the rate of nosocomial infection is high, for older groups in particular, surgery and/or SACT may in the short-term offer more risk than benefit (see supplementary Materials, available at Annals of Oncology online). Active focus is required to establish ‘cold’ sections of the health care system, with rigorous protocols for staff screening and shielding protocols. This will serve to minimise nosocomial acquisition and mortality from COVID-19, to protect staff and also to provide reassurance to the public regarding uptake of diagnostics and surgery for cancer.
Urgent review by professional bodies is required regarding best protection of their staffing groups, and guidance on surgical and diagnostic practice commensurate with risk/benefit of the respective procedure.3
Implications for prioritisation among cancer patients
Given an accrued backlog of cases and ongoing tight competition for resources, decisions regarding surgical prioritisation may be required for a number of years, with capacity varying geographically and temporally. Recognising its limitations regarding assumptions and parameters, we propose a model that provides a rational approach by which to evaluate across patients of different ages, tumour types and stages the benefit and resource implications of their cancer surgery. We highlight in our model those age-stage groups for which COVID-related mortality currently exceeds survival benefit for surgery and/or SACT. Although these and other groups for which benefit is marginal will be the most rationale to delay, they will nevertheless require monitoring and surgery downstream. Longitudinal planning, monitoring of progression, dynamic re-prioritisation and capacity planning will inevitably be highly challenging.
Broader and international relevance
Although we have used data for England, cancer survival is broadly similar across most economically developed countries, so the impact of delay per tumour is broadly applicable across Europe. However, variations in incidence of cancer, life expectancy and population age structure mean that predictions regarding total case numbers and LYGs and life-years lost are more difficult to extrapolate, even when scaling for relative size of reference population.
Although customised for surgical delay due to the COVID-19 pandemic, this model could readily be adapted to quantify the impact of surgical delay due to other causes.
Limitations
As with any model-based analysis, our predictions are predicated on the validity of assumptions and estimates used for parameterisation. While we have made use of observational data, our approach simplifies the complexity of cancer progression and is solely survival focused. For health care planning, a more elaborate model capturing stage shifting may offer additional utility. We base our analysis on survival data from 2013 to 2017; for some tumour types, standard of care and survival have evolved since this time. Our modelling of the benefit of SACT is simplistic as the scheduling, benefits and immunosuppressive consequences vary by chemotherapy regimen. While we have included in our model the impact of withholding SACT if nosocomial infection risk is high, we have not modelled additional reduction in survival from delays in administration of adjuvant therapy. Mortality from nosocomial COVID-19 infection during surgical admission or attendance for chemotherapy is based on a uniform per-day risk of infection: these are simplistic and will vary dramatically in time ans space. While our resourcing analysis deliberately focuses on the requirement for the direct medical and nursing staff who most limit health care provision, we acknowledge it does not capture other ‘costs’ incurred in hospital care, primary care and social care.
Our model of COVID-19 admissions is limited by availability of detailed individual-level UK data, in particular for non-CCU hospital admissions; this model is also conservative insofar as it disregards impact of comorbidities on life expectancy for hospitalised COVID-19 patients of comorbidities on life expectancy.
Further research
Within our current approach, we only estimate the effects of a specified period of per-patient delay. Contemporaneous data for NHS (National Health Service) activity offer the prospect of developing dynamic models to predict the impact of (i) differential prioritisation of patient groups, (ii) different patterns of re-presentation of ‘accumulated’ cases alongside incident cases, and (iii) varying release of bottlenecks in primary care, diagnostics and surgery. Evaluation is also important for the alternative management approaches being adopted, such as radiotherapy with curative intent where surgery is gold standard or a priori hormonal treatment for prostate and ER+ breast cancers. For any strategies involving deliberate delay to surgery, models including re-staging focused on the impact to surgery with curative intent; analyses are also required to quantify the impact on mortality of changes to life-extending chemotherapy and radiotherapy for patients with stage 4 disease.
Conclusion
Compared with COVID-19 management, cancer surgery is highly impactful in regard to LYGs per resource expended. Delays in diagnosis and surgery cause exponential burden of attributable mortality. The COVID-19 pandemic has placed unprecedented strain on health care provision. It is highly plausible that surges of population infection, lockdowns, resource competition, bottlenecks and back logs could recur over the next 2 years. Supra-normal capacity is required to manage backlogs of accumulated cancer cases alongside ongoing incident cases. To avoid a deferred public health crisis of unnecessary cancer deaths, urgent ringfencing of substantial resources is required.
Acknowledgements
This work uses data that have been provided by patients and collected by the NHS as part of their care and support. The data are collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of Public Health England (PHE).
Funding
CT, RSH and MEJ are supported by The Institute of Cancer Research. MEJ additionally received funding from Breast Cancer Now. BT and AG are supported by Cancer Research UK [grant number C61296/A27223]. CL is supported by and CT additionally receives funding from The Movember Foundation. RSH is supported by Cancer Research UK [grant number C1298/A8362] and Bobby Moore Fund for Cancer Research UK. AS is in receipt of an Academic Clinical Lectureship from National Institute for Health Research (NIHR) and Biomedical Research Centre (BRC) post-doctoral support. This is a summary of independent research supported by the NIHR BRC at the Royal Marsden NHS Foundation Trust and Institute of Cancer Research. The views expressed are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health. GL is supported by a Cancer Research UK Advanced Clinician Scientist Fellowship Award [grant number C18081/A18180] and is an Associate Director of the multi-institutional CanTest Collaborative funded by Cancer Research UK [grant number C8640/A23385].
Disclosure
The authors have declared no conflicts of interest.
Supplementary Data
References
- 1.Cancer referrals fell from 40,000 to 10,000 per week in April. In NHS Providers 14/05/20. 2020.
- 2.Kutikov A., Weinberg D.S., Edelman M.J. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172:756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Updated Intercollegiate General Surgery Guidance on COVID-19. Royal College of Surgeons England; 2020. [Google Scholar]
- 4.Endoscopy activity and COVID-19: British Society of Gastroenterology and Joint Advisory Group guidance – update 03.04.20. 2020. [Google Scholar]
- 5.National Cancer Registration and Analysis Service (NCRAS) Public Health England; 2018. [Google Scholar]
- 6.Cancer Treatment Information by Cancer Type. National Cancer Institute; 2020. [Google Scholar]
- 7.ICNARC report on COVID-19 in critical care (24/4/20). Intensive Care National Audit & Research Centre. 2020. [Google Scholar]
- 8.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) China CCDC; 2020. [PMC free article] [PubMed] [Google Scholar]
- 10.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) World Health Organisation; 2020. [Google Scholar]
- 11.Lee Y.H., Kung P.T., Wang Y.H. Effect of length of time from diagnosis to treatment on colorectal cancer survival: a population-based study. PLoS One. 2019;14:e0210465. doi: 10.1371/journal.pone.0210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards M.A., Westcombe A.M., Love S.B. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 13.Mano R., Vertosick E.A., Hakimi A.A. The effect of delaying nephrectomy on oncologic outcomes in patients with renal tumors greater than 4cm. Urol Oncol. 2016;34:239.e231–239.e238. doi: 10.1016/j.urolonc.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith E.C., Ziogas A., Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148:516–523. doi: 10.1001/jamasurg.2013.1680. [DOI] [PubMed] [Google Scholar]
- 15.Chu A.T., Holt S.K., Wright J.L. Delays in radical cystectomy for muscle-invasive bladder cancer. Cancer. 2019;125:2011–2017. doi: 10.1002/cncr.32048. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Ortiz R.F., Huang W.C., Mick R. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169:110–115. doi: 10.1016/S0022-5347(05)64047-5. discussion 115. [DOI] [PubMed] [Google Scholar]
- 17.May M., Nitzke T., Helke C. Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol. 2004;38:231–235. doi: 10.1080/00365590410029141. [DOI] [PubMed] [Google Scholar]
- 18.Bleicher R.J., Ruth K., Sigurdson E.R. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samson P., Patel A., Garrett T. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2015;99:1906–1913. doi: 10.1016/j.athoracsur.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C.-F.J., Wang H., Kumar A. Impact of timing of lobectomy on survival for clinical stage IA lung squamous cell carcinoma. Chest. 2017;152:1239–1250. doi: 10.1016/j.chest.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Richards M.A., Smith P., Ramirez A.J. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79:858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliss-Brookes L., McPhail S., Ives A. Routes to diagnosis for cancer – determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


