Abstract
Tumor-treating fields (TTFields) are alternating electrical fields of intermediate frequency and low intensity that can slow or inhibit tumor growth by disrupting mitosis division of cancerous cells through cell cycle proteins. In this work, for the first time, an in-house fabricated cyclo-olefin polymer made microfluidic bioreactors are integrated with Cr/Au interdigitated electrodes to test TTFields on yeast cells with fluorescent protein:Nop56 gene. A small gap between electrodes (50 μm) allows small voltages (<150 mV) to be applied on the cells; hence, uninsulated gold electrodes are used in the non-faradaic region without causing any electrochemical reaction at the electrode-medium interface. Electrochemical modeling as well as impedance characterization and analysis of the electrodes are done using four different cell nutrient media. The experiments with yeast cells are done with 150 mV, 150 kHz and 30 mV, 200 kHz sinusoidal signals to generate electrical field magnitudes of 6.58 V/cm and 1.33 V/cm, respectively. In the high electrical field experiment, the cells go through electroporation. In the experiment with the low electrical field magnitude for TTFields, the cells have prolonged mitosis from typical 80–90 min to 200–300 min. Our results confirm the validity of the electrochemical model and the importance of applying a correct magnitude of the electrical field. Compared to the so far reported alternatives with insulated electrodes, the here developed thermoplastic microfluidic bioreactors with uninsulated electrodes provide a new, versatile, and durable platform for in vitro cell studies toward the improvement of anti-cancer therapies including personalized treatment.
I. INTRODUCTION
Tumor-treating fields (TTFields) are alternating electrical fields of intermediate frequency (∼100–500 kHz) and low intensity (1–3 V/cm) that are proposed as a cancer treatment modality.1 The mechanism of TTFields is to slow or inhibit tumor growth by disrupting mitosis division of the cancerous cells through cell cycle proteins.2,3 Their effects may be on the misalignment of proteins with large dipole moments such as tubulin dimers and septin under the influence of electrical fields.4–6 The hourglass shape of the dividing cell during telophase can cause highly non-uniform electrical fields inside the cell with higher field intensities close to the narrow furrow region.1 Such field inhomogeneity results in dielectrophoretic (DEP) forces7 possibly leading to irregular aggregation of polarizable particles, thereby disrupting cell division.5,8 Studies point to the disruption of microtubule polymerization and the prevention of proper chromosome segregation during mitosis.9 Septin, which serves as a scaffold for the actin myosin ring closing the cytokinetic furrow, may fail to localize to the cell mid-zone under these electrical fields. This, in turn, may lead to ectopic blebbing during telophase and abnormal mitosis.4 TTFields can also inhibit cell migration10 and DNA damage repair.11
The promising results of TTFields have been demonstrated on glioblastoma multiforme (GBM), one of the most common and aggressive human brain cancers. They are applied on GBM patients and were found to prolong patient life.12 The TTField study was also conducted on lung cancer cells of mice and rabbits and showed a significant decrease in metastases.13 Some researchers have combined chemotherapy (paclitaxel, doxorubicin, cyclophosphamide, dacarbazine, and temozolomide) with the TTField treatment on GBM and have recorded positive results on patients. Chemotherapy at doses below the therapeutic threshold combined with the TTField increased the regression of cancer as a result of cell cycle arrest.14–17 Pemetrexed, cisplatin, and paclitaxel drugs intended for lung cancer were administered along with TTFields and showed improvements in the prognosis of this disease.18,19 Similarly, combined treatment was tested on pancreatic cancer using gemcitabine, irinotecan, 5FU, paclitaxel, and nab-paclitaxel, and the results suggested an antimitotic effect on cancer cells.20,21 Its application on ovarian cancer, malignant pleural mesothelioma, has also been classified as safe and effective.22,23 The general conclusion of the application of TTFields with chemotherapy is that systemic toxicity does not increase, and survival is prolonged. The TTField therapy is approved by the U.S. Food and Drug Administration for the treatment of GBM.1
While TTFields are applied clinically on patients, they have also been studied in vitro using several preclinical laboratory research systems. These systems include the application of sinusoidal voltages through wires with insulation of ethylene tetrafluoroethylene5 and polyvinyl chloride10 with thicknesses of 0.125 mm and 0.17 mm, respectively. They are used with cell dishes, requiring high voltages (range 300–800 V). Electrodes are also insulated with very high dielectric constant ceramic (εr > 5000) to remedy the problem of high voltage usage. However, these materials ask for special design and manufacturing capabilities and cause heat generation, which requires a refrigerated incubator.24 A Polydimethylsiloxane (PDMS) microfluidic cell culture platform with embedded insulated electrodes is also reported for TTFields.25 The electric field to the cells is applied through 100 μm wide PDMS and 500 μm wide cell culture medium. This PDMS device eliminates the disadvantages of classical culture dishes. It offers reduced usage of reagents, lower costs, and the capability of observing cells in real time under the electrical field and thus better mimics the in vivo situation. This microfluidic device shows that TTFields led to reduced proliferation of breast cancer cells, while leaving normal human endothelial cells largely unaffected.25 However, because PDMS can absorb small drug molecules and may cause cross-contamination, precautions must be taken if these devices are used to test TTFields with the chemotherapy treatment using different drugs. Their usage of insulated electrodes in the form of cured silver flake–PDMS mixture inside microfluidic channels also complicates the fabrication process. Furthermore, the results of microfluidic devices would be more decisive if they are capable of running multiple experiments (i.e., control group) at the same time on the same chip.
For these reasons, for the first time in this work, we engineered a thermoplastic microfluidic device with integrated electrodes to investigate the effects of TTFields on cells in a highly controlled microenvironment. Thermoplastic devices offer better surface properties and stability and could be commercialized more easily compared to their PDMS based counterparts.26 Our fully saturated, highly stable, and rigid cyclo-olefin polymer (COP) microfluidic device (bioreactor) with Cr/Au electrodes allows two experiments at the same time under continuous flow. Thermal evaporation, lithography, hot-embossing, and thermo-compression bonding methods are used to produce these devices. These bioreactors allow single cell tracking under the fluorescence microscope with the help of C-shaped trapping regions. We can monitor real time single cell behavior and see whether electroporation, morphological, or mutational changes occur unlike the traditional studies with Petri-dishes using flow cytometry to count the number of cells. This is a good example of a platform for in-depth cell cycle analysis under TTFields to identify parameters such as frequency and field strength that can be optimized to limit different types of cancer cell proliferation while minimizing detrimental effects to neighboring normal cells of various types present in tissues. Compared to previously reported insulated alternatives, the usage of uninsulated electrodes with a small electrode gap of 50 μm allows the generation of TTFields by applying very small voltages in the range of 30 mV.
We apply TTFields on Saccharomyces cerevisiae (S. cerevisiae), which is a model organism for fundamental studies and used to predict the behavior of higher order cells.27,28 S. cerevisiae cells are unicellular organisms and mostly utilized in cellular and molecular biology research due to their short proliferation time.29,30 In the culture environment, the growth and division of yeast cells can be managed effectively. At least 30% of yeast genes are homologs of human disease genes and 47% of essential yeast genes can be replaced by their orthologous human genes. The above-mentioned advantages make yeast cells favorable for many biological studies.30,31 Here, the luminescence of red fluorescent protein (RFP) tagged Nop56 protein is observed to record the behavior of yeast cells. Nop56 is an essential evolutionary conserved nucleolar protein and found as a member of the box C/D snoRNP complexes. It joins the ribosome biogenesis and pre-RNA processing.32,33 It has a role in DNA replication and it shortens the duration of the G1 phase of the cell cycle.33,34
II. MATERIALS AND METHODS
A. Materials
Saccharomyces cerevisiae cells [EY0987 genetic background Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 (S288C)] with RFP tagged Nop56 gene is kindly delivered by Peter Arvidson from Harvard University, HHMI. The COP substrates with Zeonor 10-0672-0349-1.0-05 product code and polyethylene tubing with BB31695-PE/p product code are purchased from Microfluidic ChipShop Company (Jena, Germany) and Scientific Commodities Inc. (SCI, Lake Havasu City, AZ, USA), respectively. All the chemicals and reagents for medium preparation are purchased from Sigma-Aldrich (Taufkirchen, Germany).
B. Overview of the microfluidic device and platform
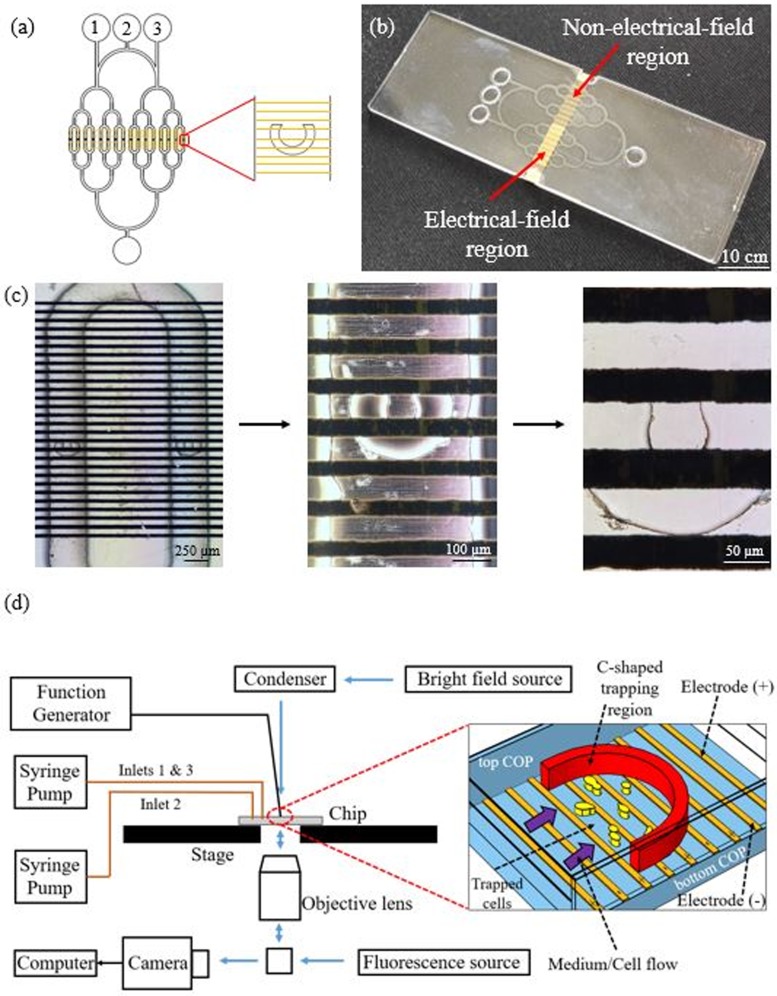
Figure 1 shows the design, fabricated device, and the experimental setup schematic of the system. The in-house fabricated microfluidic device includes two individual sections, each having eight chambers with C-shaped regions. These regions are placed along the channels to trap and hold the yeast cells sent to the device [Fig. 1(a)]. The design enables the cultivation of two different strains in one type of nutrient medium by sending corresponding strains through inlets 1 and 3 and the nutrient medium through inlet 2. The cultivation of one strain in two different nutrient media is also possible by sending the strain using inlet 2 and the two different nutrient media through inlet 1 and 3. Hence, there is no need to change the connections of the syringes to the inlet channels to introduce both cell cultures and nutrient flows, which can cause undesired contamination between channels and gas bubbles.
FIG. 1.
(a) Design of the microfluidic device with cell and nutrient inlets of 1, 2, and 3. (b) Fabricated device of COP. (c) Electrode images within a chamber at 4×, 20×, and 40× magnification. (d) Experimental setup schematic.
In this work, the same strain type and the same medium are used in two different sections of the chip. Inlet 2 is used as a dedicated line to introduce yeast cells to the two different sections of the chip. The same nutrient medium is supplied to both of these sections through inlets 1 and 3. However, the electrode design enables us to perform two different experiments on the same chip at the same time. Electrodes are designed so that only one section of the device includes interdigitated electrodes with 50 μm gap covering the whole eight chambers in the C-trap regions of this section. The other section does not have any interdigitated electrodes. It only contains lines of the same electrode for routing purposes so that this electrode can reach the other section to form the interdigitated electrodes there [Fig. 2(d)]. Because these lines are part of the same electrode and have the same electrical potential value, there cannot be any electrical field between these electrode lines. Therefore, the cells in the eight chambers on this side do not experience any electrical field and are used as the control group. The electric field can be applied only to the cells in the eight chambers on the other side of the chip.
FIG. 2.
(a) Mold created via electrochemical wet etching. (b) Hot-embossing procedure of the drilled COP substrate. (c) Hot-embossed COP substrate. (d) Cr/Au electrodes deposited on the COP substrate. (e) Thermo-compression bonding step.
In this design, yeast cells can be kept in the C-shapes of the chambers, where the electric field can be applied to each cell. Electrodes within the device on the C-shaped region are displayed in Fig. 1(c) and every chamber within the device is considered as an independent microbioreactor, thus, each experiment is repeated for eight times. Figure 1(d) shows the general schematic of the microfluidic platform. The fabricated device is placed on the stage of the Nikon Ti-E inverted fluorescence microscope and tubings for nutrients and cell loading are connected to syringe pumps (New Era Pump Systems). The electric field within the device is created by a function generator (Agilent 33220A 20 MHz Function/Arbitrary Waveform Generator), and data acquisition is handled with the Nikon DS-Ri2 camera detector.
C. Device fabrication
The microfluidic device is made using two COP (75.5 × 25.5 × 1 mm3) substrates and is integrated with Cr/Au electrodes. The device design which meets the requirements of the experiments is drawn with AutoCAD. COMSOL Multiphysics 5.3a software is used to optimize the fluid dynamics within the device.35 The design is transferred to a film photomask. Then, a stainless steel mold is created by photolithography (with the mask) and electrochemical wet etching methods, respectively [Fig. 2(a)]. The details can be found in our previous work.36,37
A blank COP substrate is taken and inlet, outlet, and electrode holes are drilled on it using a computer numerical control (CNC) machine. Burrs around holes are cleaned via an ultrasonic bath. This is followed by a planarization step to obtain a smooth surface for proper bonding, which is needed because of the bumps formed around mechanically drilled regions. Hydraulic press machine with hot plates (Carver 3851 CE-0, USA) is set to 130 °C, and 35 bar pressure is applied to the COP substrate with drilled holes between two glasses for 10 min. All the pieces are left for cooling to 60 °C under pressure. After the planarization step, the mold [Fig. 2(a)] is used for hot-embossing of this COP substrate [Fig. 2(b)]. The mold and the substrate are placed in the hydraulic press machine, and 35 bar pressure is applied at 130 °C for 10 min. After 10 min, the system is left for cooling to 60 °C under pressure, and a hot-embossed piece is obtained [Fig. 2(c)]. With this step, the bottom substrate is ready for bonding.
Another blank COP substrate is taken to form the top substrate for bonding. Chromium (10 nm thick) and gold (50 nm thick) layers are deposited on this substrate using a high vacuum thermal evaporator (Nanovak, Ankara/Turkey). Electrodes are patterned on metal layers using a photolithography step (Photoresist PR 1828 and developer solution MF-319) with the electrode mask of the design. Wet gold and chromium etchants are used to etch unmasked regions of the metals. Acetone, isopropyl alcohol, and de-ionized water (DIW) are used to clean the photoresist, respectively, and the COP piece is dried via a N2 gun. This step forms interdigitated Cr/Au electrodes with 50 μm gap [Fig. 2(d)]. This is the only fabrication step applied on the top substrate before bonding.
Fabrication of the microfluidic device is finished by thermo-compression bonding of the hot-embossed bottom COP and electrode formed top COP substrates. The top substrate is flipped before bonding so that the electrodes are placed in between the substrates, touching the microfluidic channels. These two substrates are put into the hydraulic press machine at 125 °C. A pressure of 25 bar is applied for 45 min [Fig. 2(e)]. Cooling to room temperature finalizes the fabrication of the microfluidic device, shown in Fig. 1(b). The final height of the microfluidic channels is ∼9 μm, which is ideal to form monolayers of yeast cells, which have sizes of around 5 μm.
D. Medium preparation and system operation
For the impedance analysis part of this work, impedances of three different media that are commonly used in yeast cell research and DIW are characterized. The YPD medium includes 20 g/l glucose, 10 g/l peptone, and 20 g/l yeast extract. The medium for growth of Nop56:RFP tagged yeast cells includes 20 g/l glucose, 1.7 g/l yeast nitrogen base, 5 g/l ammonium sulfate, 0.1 g/l leucine, 0.02 g/l histidine, 0.03 g/l lysine, and 0.02 g/l uracil. The medium for growth of Rpl5:GFP tagged yeast cells includes 20 g/l glucose, 1.7 g/l yeast nitrogen base, 5 g/l ammonium sulfate, 0.02 g/l histidine, 0.1 g/l leucine, 0.02 g/l methionine, and 0.03 g/l lysine. Every medium (including DIW) is applied at a flow rate of 0.1 μl/min via syringe pumps connected to all the inlets of the device. The electrodes are connected to the impedance analyzer (Keysight E4990A, USA). Each syringe with the medium is connected to the same device one after the other, and a fresh medium is flown for at least an hour before impedance measurements are made to eliminate any contamination from the previous medium.
For the cell experiments, the devices are used without any treatment or internal coating of the channels after their fabrication. The tested yeast cells are non-adhesive, therefore, no cell stiction problem is experienced. Syringe pumps and function generators are connected to the microfluidic device on the microscope [Fig. 1(d)]. Syringe pump containing cells is connected to inlet 2 and the syringe pump of the medium is connected to the inlets 1 and 3. The nutrient medium is sent to the device at first. When the entire device is full of the medium, the cells are introduced until they are seen between the electrodes in the C-shaped regions. At this stage, the syringe pump carrying the cells is stopped without disconnecting it from its tubing. However, the medium continues to flow through the inlets 1 and 3. This way untrapped cells can be swept away and no new cell can come from the inlet side. After the cells are trapped and the medium flow is steady, the electric field is applied for 6 h (yeast cell proliferation time is 90 min), and the brightfield and fluorescence images are acquired at 15–20 min intervals up to the end of the experiments. One of the most important features of the present microfluidic device is that it can endure long durations of experiments (more than 40 h) without losing its mechanical integrity.
E. Impedance and electrical field analysis
The impedance analysis is very crucial to find the magnitude of the applied electric field. Because there is no room for a reference electrode in between the interdigitated electrode gap, the magnitude of the applied electric field has to be calculated from the electrode–solution circuit model and the applied voltage signals. That is why a separate impedance analysis is done for the fabricated chips with the cell medium between the electrodes using the same conditions as the cell experiments. The circuit model used in this work can be seen on the right side of Fig. 3, with two electrical double layer capacitances (CEDL) and the resistance of the bulk of the medium solution (Rsol) all in series. This model is valid for non-faradaic electrochemical cell conditions, which is the case in the cell experiments. The voltage values applied to the gold electrodes are kept below 150 mV in the non-faradaic region, a safe level that would not cause any electrochemical reaction. Depending on the type of ions in the electrolyte, electrochemical reactions can start at different electrochemical cell potentials. The electrochemical window for DIW is 1.2 V.38,39 The onset of faradaic conduction is reported to be around 0.9 V on platinum electrodes inside the phosphate buffered saline solution.40 Stimulation of neural cells with microelectrodes are done using voltage amplitudes of a few hundred mV, i.e., 450 mV but not exceeding 1 V.38
FIG. 3.
Electrical circuit model of the test setup used during cell experiments. CEDL is the electrical double layer capacitor formed between gold electrode and electrolyte. Rsol is the resistance of the bulk of the electrolyte solution. The voltage difference across this resistance forms the electric field the cells experience. Rs is the series resistance of the voltage source. It can also account for the series resistances of the ammeters used to measure the AC current flowing through the circuit. Csol, which models the capacitance of the bulk solution between electrodes, is not shown for clarity purposes.
Impedance comprises both real and imaginary values with the SI units of Ω.41 In the experiments for the impedance analysis, the frequency, f, of the excitation signal with a magnitude value of 10 mV and no DC value is swept from 20 Hz to 10 MHz. The measured impedance magnitude, │Z│ (Ω) and phase, θ (°) information of the electrodes in the microfluidic channel contacting the medium solution are recorded. The solution resistance (Rsol) values for each nutrient medium are calculated from these data and the mentioned electrical equivalent circuit. During the cell experiments, the electrical field applied on the cells using a function generator is calculated knowing this Rsol value, circuit model, and the applied voltage and frequency of the voltage signal. In the different experiments done with the same cell type and medium, sinewave voltage signals with peak to peak value Vpeak_to_peak of 150 mV and frequency of 150 kHz and Vpeak_to_peak of 30 mV and frequency of 200 kHz are applied.
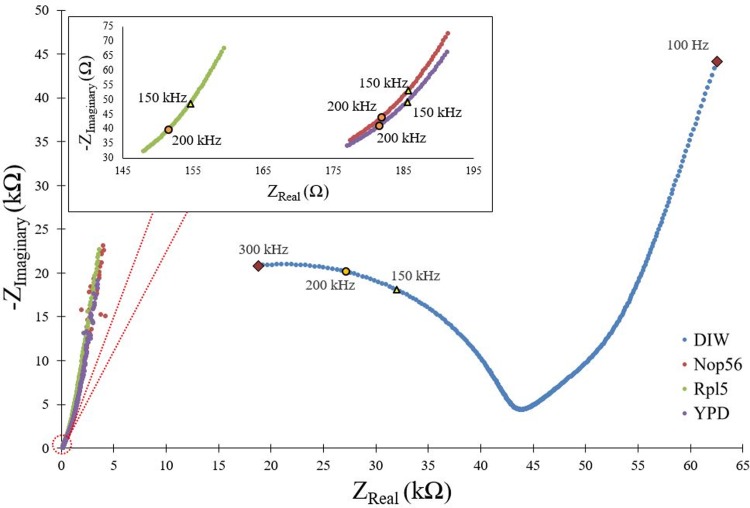
Every medium (including DIW) is sent through the microfluidic device at a flow rate of 0.1 μl/min because this is the flow rate used in the cell experiments during the nutrient feeding of the cells while the electrical field is applied. Figure 4 shows the Nyquist plot of all media between 100 Hz and 300 kHz frequency at this flow rate. Every point in the Nyquist plot shows the real and imaginary parts of the measured impedance for that specific frequency. The real and the imaginary values of the impedance are found from the magnitude and phase measurements of the impedance analyzer. θ is converted to radian from degree (1). The real and imaginary parts of the impedance are calculated according to (2). Angular frequency (ω) is also obtained by (3),
| (1) |
| (2) |
| (3) |
FIG. 4.
Nyquist plots of DIW, Nop56, Rp15, and YPD media for 0.1 μl/min flow rate. Real and imaginary impedance values are marked for the used frequencies of 150 and 200 kHz in the cell experiments.
Nyquist plots are shown here to get an insight on the real and imaginary values of the medium. However, bode plots of the impedance, which show the impedance magnitude and phase change with frequency, are more appropriate to extract the capacitance and resistance values of the electrical model shown in Fig. 3. According to this electrical model of the electrochemical cell, the CEDL value dominates at low frequencies (depending on the conductivity of the solution, typically below 10 kHz). These capacitances get smaller and become almost short-circuit at higher frequencies. Hence, at frequencies of around 1 MHz, the impedance of the cell is formed by Rsol and capacitor due to the bulk of the solution (Csol), which is parallel to this resistance (this capacitance is not shown in Fig. 3 for clarity purposes). Therefore, using intermediate frequency values from around 10 kHz to around MHz, but not larger than these, so that the solution capacitance does not get short-circuited, the real and imaginary plots of impedance vs frequency can be used to extract Rsol values. For these intermediate frequency values, ω vs Zmeasured_img/Zmeasured_real data can be plotted. A trend line can be fit to this graph with a slope of m1. Similarly, ω vs Zmeasured_img(1 + ω2m12) can be plotted, and a new slope, m2, can be found. Lastly, Rsol and Csol can be calculated as
| (4) |
| (5) |
After calculating the solution resistance, the electrical field applied on the cells during cell experiments can be determined. This can be done using the equivalent circuit model shown in Fig. 3, which points to a simple voltage division due to complex impedances. Here, the impedance of the bulk solution capacitor, Csol, is ignored because for the working frequencies of the cell experiments, in the range of a couple of hundred kHz, its impedance contribution can be ignored. The measured impedance values tell us the overall impedance of the electrochemical cell and the solution resistance value is calculated using these parameters. Furthermore, the magnitude and frequency of the applied voltage signal are known. The cells experience an electrical field only due to the voltage drop across the solution resistance. Therefore, we need to find the voltage drop across the solution resistance but not concerned with the voltage drops across the internal resistance of the signal generator and the CEDLs. This can be done using the following voltage division equations for complex impedances:
| (6) |
where Zmeasured is the complex impedance for the frequency used in the experiment, measured using the impedance analyzer as explained in the Methods section and marked in Fig. 4.
III. RESULTS AND DISCUSSION
This study is the first attempt toward the usage of uninsulated electrodes with a small electrode gap of 50 μm for the generation of TTFields by applying very small voltages in the range of 30 mV. The voltage values applied between gold electrodes of the chips are kept below 150 mV in the non-faradaic region so that no electrochemical reaction, i.e., electron exchange at the electrode surface and the release of electrochemical reaction byproducts into the medium, can occur between the gold electrodes and the solution. The validity of the non-faradaic electrical circuit model and the calculated magnitude of the electrical field the cells experience are also confirmed here as explained below. Cell experiments are conducted after impedance measurements and their analyses are accomplished for de-ionized water (DIW) and three different cell nutrient media in the microfluidic device. 150 mV and 150 kHz voltage signal is applied across the electrodes to generate 6.58 V/cm electrical field magnitude across the cells with the intention of causing electroporation. Furthermore, 30 mV and 200 kHz signal is applied to generate the 1.33 V/cm electrical field magnitude to find the effects of TTFields on the cells. Electroporation does not solely depend on the electric field strength but also on the number and duration of applied voltage pulses. Similar electroporation results can be obtained by using equivalent pulse parameters. Typically, a single or dozen pulses of μs durations are used in the orders of thousand V/cm electric field magnitudes for electroporation. Similar results can be achieved using ms or s pulse durations with electric field magnitudes of hundreds and tens of V/cm, respectively. Derived models can be used to find equivalent pulse parameters for the intended electric field magnitude to cause electroporation.42
A. Electrical field calculations
Nyquist graphs are compact forms to show the complex impedance values of the electrochemical cells. This format is chosen to show the differences between different media and to be able to pick the real and imaginary values of the impedances to be used in the electrical field calculations. As expected, the impedance of the nutrient media is much lower than DIW (Fig. 4). As the ions of the salt and amino acid in the medium are introduced into the water, the conductivity of the solution increases. Smaller differences among feeding media are also noticeable in the inset of Fig. 4. Although the YPD medium and the nutrient medium for Nop56:RFP tagged cells have very similar conductivity values, the medium for Rpl5:GFP tagged cells is more conductive than these. The only difference between these growth media for Rpl5 and Nop56 tagged cells is the existence of methionine in one and uracil in the other. The addition of methionine increases the conductivity of the solution. In Fig. 4, the imaginary and real values of the measured impedances for different media are marked for 150 and 200 kHz frequencies because these frequencies are used in the cell experiments and their values are needed in the calculation of the applied electrical field magnitude.
Rs is the internal resistance of the function generator, which is equal to 50 Ω. Since during the experiments, we also monitored the flowing AC current using a precise multimeter (U1252B, Keysight Technologies, USA) in series, it also adds 100 Ω value to this series resistance. That is why 150 Ω is inserted in (6). The real and imaginary values of Zmeasured are found according to the corresponding frequency. For the first experiment (V = 150 mV, f = 150 kHz), Zmeasured_real is 185.73 Ω and Zmeasured_ima is 53.08 Ω, whereas for the other experiment (V = 30 mV, f = 200 kHz) Zmeasured_real is 181.90 Ω and Zmeasured_ima is 43.86 Ω. ΔVapplied across the solution resistance is found from (6). Once this value is divided by the gap, d, between the electrodes, the magnitude of the applied electrical field, Eapplied is calculated as
| (7) |
Since there is a lack in the literature on the electrochemical characterization of cell growth media, we tested three different nutrient media that are frequently used in cell culture studies. The impedance differences between the growth media are calculated and found to be close to each other. Since RFP:Nop56 tagged yeasts are used in the cell experiments, the calculations for only that medium are considered below. After cell loading, the nutrient feeding rate is set to 0.1 μl/min, and the solution resistance of the medium for Nop56 :RFP cells at this rate is found as 149.26 Ω. These results reveal that for the high voltage experiment at 150 mV and 150 kHz, the magnitude of the applied electrical field is 6.58 V/cm and for the low voltage experiment (30 mV and 200 KHz), it is 1.33 V/cm.
In the above analysis, it is assumed that the electrical field is uniform in the cell trapping region between planar electrodes. This is confirmed by making 3D electrical field simulations in COMSOL that includes the effect of c-shape, even though simulation results point to high non-uniform electrical fields in the 10 μm vicinity of interdigitated planar electrodes. Our simple approximation predicts an average electrical field magnitude of 1.33 V/cm (6.7 mV/50 μm) for the applied 30 mV peak to peak voltage. 3D COMSOL simulations reveal 1.1 to 0.9 V/cm magnitude change in one axial direction of 30 μm length and 1.06–1.12 V/cm change in the orthogonal direction of 70 μm length in the central cell trapping region of the microfluidic channel. Even though, at the edge of the electrode, the electrical field can reach a maximum peak value of 5.2 V/cm, this drops to 1.12 V/cm by moving 10 μm away from the electrode edge. That results in a cell trapping region with only 21% change in the field uniformity. The results also reveal that (7) overestimates the electrical field magnitude in the trapping region by 19%.
B. Electrical field effect on yeast cells
This section summarizes the results of the cell experiments. The control experiments (cells not-electrical-field treated) show regular proliferation of the cells as expected. In the experiment, where a high electrical field of 6.58 V/cm is used, the electroporation of the cell membranes occurs, destroying the cells. Proper application of the TTFields with the electrical field magnitude of 1.33 V/cm causes a prolongation of the mitosis phase. The details are as follows.
1. Cells not treated by the electrical field
Figures 5(a) and 6(a) show the control cell (not-electrical-field treated) results of the experiments. A continuous increase in cell number and size is observed throughout the experiment as evident from cell count and perimeter of the cells plotted in Fig. 5(a). Morphological changes of the cells are also examined on single cell basis, and their increase in the dimension is noticeable on the right side of Fig. 6(a). These results are in accordance with the proliferation of healthy yeast cells. If a single cell is followed [Fig. 6(a)-right], its perimeter increases at the beginning and then suddenly drops to almost its half due to cell division and then both mother's and daughter's perimeters again monotonically increase. The daughter can also generate another daughter (second generation), so and so forth. The left side of Fig. 6(a) shows the whole fluorescence intensity generated by the cells. As usual with these types of yeast cells, there is a decline in their fluorescence intensity at the beginning because when cells enter the new confined microaerated environment, they are affected and their fluorescence intensity is slightly reduced. However, after a while, as the cells adapt to their environment, an increase in the fluorescence intensity is observed with cell proliferation. The time-lapse microphotographs for the control cells in Fig. 7 also show typical cell proliferation of the yeast cells.
FIG. 5.
Cell count and cell perimeter–time profiles of (a) not-electrical-field treated cells as the control group showing regular proliferation with increasing number and perimeter (b) 6.58 V/cm electrical-field treated cells showing the absence of proliferation with no cell count increase, electroporation happens and cell perimeter increases only in the first 150 min, and (c) 1.33 V/cm electrical-field treated cells showing delayed proliferation with constant cell size and cell count in the first 150 min and 250 min, respectively.
FIG. 6.
Fluorescence intensity and single cell perimeter-time profiles of (a) not-electrical-field treated cells showing regular proliferation. Decline in the fluorescence intensity at the beginning is due to shock of a new confined microaerated environment. With adaptation to the environment, increase in the fluorescence intensity starts. Perimeter profiles show the increase in the perimeter of single cell with time, then suddenly drops to almost its half due to cell division and then both mother's and daughter's perimeters again monotonically increase. (b) 6.58 V/cm electrical-field treated cells showing the absence of proliferation. The perimeters of single cells increase in the first 150 min, then stay constant without dividing. Continuous decline in the fluorescence intensity points to the cell death, and (c) 1.33 V/cm electrical-field treated cells showing dimensional changes of the individual cells with delayed budding time and delayed increase in the fluorescence intensity as evidence of maintained Nop56 protein functionality.
FIG. 7.
Time-lapse microphotographs of yeast cells experiments. Cells in the control group show regular increase in number and perimeter. Cells treated with the 6.58 V/cm electrical field show blurring borders with time as evidence of undergoing electroporation. Cells treated with the 1.33 V/cm electrical field stays almost the same at the beginning of the experiments then show delayed mitosis division.
2. High voltage treated cells: Electroporation
The cell experiments are conducted using fabricated microfluidic devices under the application of the 150 mV and 150 kHz voltage signal. Figure 5(b) shows the results of the yeast cell experiment under the generated electrical field magnitude of 6.58 V/cm. The results point to no cell proliferation and the occurrence of electroporation of their membranes as observed by real time monitoring of the individual cells [Fig. 7(b)]. Although the cell perimeter increases in the first 150 min, their dimensions do not change from that point onward [Fig. 5(b)-right]. The cell count is also constant throughout the experiment, which means no division occurred [Fig. 5(b)-left]. This is also obvious from the perimeter results of the single cells [Fig. 6(b)-right]. The perimeters of single cells increase in the first 150 min, then they stay in that size without dividing. There is a continuous decline in the fluorescence intensity of these cells [Fig. 6(b)-left]. Compared to the control cells, the fluorescence intensity never recovers. The main reason is that the cells undergo electroporation. Electroporation leads to cell death with a concomitant decrease in the luminescence of the cells. The morphological changes of the single cells due to electroporation are obvious in the time-lapse microphotographs in Fig. 7.
The electrical field has effects on cell physiology directly by intracellular disorganization, DNA and RNA impairment, and inactivation of enzymes. Due to the polarization of cells, electromechanical compression on the cell membrane occurs and permeabilization increases. Moreover, increasing membrane potential causes a decrease in membrane thickness. When the critical breakdown voltage is exceeded, the disruption of the cell wall occurs. Consequently, an exchange between intracellular and extracellular macromolecules becomes possible.43–47 This phenomenon is known as electroporation and seen in the middle row of Fig. 7. While the control cells continue their normal life cycle, the electroporation detected in the cells under the high electrical field leads to cell death. Smooth envelop and a round border is noticeable on the untreated cells but blurring is observed in the cells undergoing electroporation. The blurring of images and flattened yeast border with the destruction of the cell membrane structure were reported in the literature.48
3. Low voltage treated cells: Prolongation of mitosis
A low electric field (1.33 V/cm), which is at the right range of TTFields, is applied to the yeast cells in the microfluidic device. In contrast to the high voltage experiment, delayed cell proliferation is observed. The cell size and cell count stay constant in the first 150 min and 250 min, respectively, and then they start to increase [Fig. 5(c)]. The single cell monitoring also confirms this delayed cell division and cell size increase [Fig. 6(c)-right]. The dimensional changes of the individual cells can be clearly seen by continuous real time imaging and the budding time of the daughter cells can be noticed. The differences in the cell proliferation between the electrical field treated and control cells are obvious when Figs. 5(a) and 6(a) are compared to Figs. 5(c) and 6(c), respectively. There is an initial decline in the fluorescence intensity of the cells, but it remains almost constant in the first 150 min [Fig. 6(c)-left]. A slight increase in the fluorescence intensity is noticed after this point onward, as the cells start to proliferate, and Nop56 protein maintains its function.
The most important feature of this experiment is the prolongation of mitosis division time with the effect of the electrical field. While the average proliferation time of yeast cells is 80–90 min, the proliferation time of yeast cells under the electrical field of 1.33 V/cm increases up to 200–300 min. Figure 7 also proves this delayed proliferation. A misalignment of the internal molecules, such as microtubules, during mitosis is likely to occur due to the application of the electrical field. Thus, the mitotic process is disrupted, and this results in an anti-proliferative effect on cells as reported in the literature.49 In our experiment, the mitosis initiated normally, but it took a longer time to complete. This is the outcome of the deceleration in mitosis under the electrical field stimulation.5,9,25,50
C. Highlights (pros and cons) of thermoplastic materials for batch fabrication of microdevices
Thermoplastic materials can be mass-produced. Most of them are optically transparent and biocompatible such as polystyrene and poly(methyl methacrylate). Since fluorescence tagged yeast cells are used in our experiments, a thermoplastic material with low auto-fluorescence is needed and COP served this purpose. The COP material has a high moisture barrier and this is favorable when working with cell cultures. As a result, cells consume more oxygen from water, instead of its absorption through the surface of COP.51 It is also chemically inert. The most commonly used material for developing microfluidic devices in research laboratories is PDMS, despite its several disadvantages. PDMS has high gas permeability and a porous body that can absorb molecules, which may cause contamination within the microfluidic devices. Furthermore, attention must be paid to cure PDMS structures properly, otherwise, uncured polymer chains can leach into solutions.
Thermoplastics may also have disadvantages such as limited operation temperature. While low melting temperature points of thermoplastics can be disadvantageous for certain applications, it is an advantage for bonding. The process step of bonding is the most difficult part in our fabrication due to non-uniformity formed by the deposited Cr/Au electrodes on the COP substrate. The thermo-compression bonding parameters were precisely optimized and leak-free microfluidic bioreactors were achieved. Another limitation of thermoplastic substrates is the problem of openings access holes for reservoirs and electrode contacts. These holes are formed only at the edges of the substrates not to disrupt the uniformity of the substrates, which is very critical for bonding. This puts limitations to electrode routing. Furthermore, pure thermoplastics are expensive and hard to find in the microscopy slide format. Despite the above-mentioned limitations, thermoplastics can enable batch fabrication of reproducible and reliable microfluidic devices. They offer a versatile platform for combined chemotherapy and TTField treatments on tumor cells.
IV. CONCLUSION
In the present study, the integration of microfluidic device (bioreactor) and the electrical field was aimed and the effect of the electrical field magnitude on yeast cells was observed. The microfluidic device was fabricated with 16 bioreactors in parallel. Half of these bioreactors did not have electrical field application and were used in control experiments. Hot-embossing, photolithography, and thermo-compression bonding methods were used to produce the COP made thermoplastic microfluidic device including Cr/Au electrodes in the interdigitated form. The impedance measurements of four different cell nutrient media were accomplished at 0.1 μl/min flow rate. Electrochemical impedances of the bioreactors were evaluated by taking electrical double layer capacitances and solution resistance into consideration. 150 mV and 150 kHz corresponding to 6.58 V/cm and 30 mV and 200 kHz corresponding to 1.33 V/cm electrical fields were applied on yeast cells. In the experiment with intentional high electrical field application, the cells underwent electroporation, while in the other experiment with the right intensity range of TTFields, the cells had prolonged mitosis. Further usage of this microfluidic platform can reveal the optimal frequency and field strengths of different cell types under TTFields to selectively target the intended hazardous cells while minimizing their effect on healthy cells present in tissues. TTFields are expected to offer benefits for localized cancer types, having potential in extending patients' life and survival rate. Thus, as a promising anticancer treatment approach, they continue to be of scientific interest. Our microfluidic bioreactors integrated with uninsulated electrodes having very small gaps allow the application of very small voltages in the range of 30 mV. They provide a new, versatile, and durable platform for in vitro cell studies toward the improvement of anti-cancer therapies including personalized treatment.
ACKNOWLEDGMENTS
This work was supported by Bogazici University Research Fund through Project Nos. 13641D and 14261R.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Wenger C., Miranda P. C., Salvador R., Thielscher A., Bomzon Z., Giladi M., Mrugala M. M., and Korshoej A. R., IEEE Rev. Biomed. Eng. 11, 195 (2018). 10.1109/RBME.2017.2765282 [DOI] [PubMed] [Google Scholar]
- 2.Mun E. J., Babiker H. M., Weinberg U., Kirson E. D., and Von Hoff D. D., Clin. Cancer Res. 24, 266 (2018). 10.1158/1078-0432.CCR-17-1117 [DOI] [PubMed] [Google Scholar]
- 3.El Zakhem H., Lanoisellé J. L., Lebovka N. I., Nonus M., and Vorobiev E., J. Colloid Interface Sci. 300, 553 (2006). 10.1016/j.jcis.2006.04.055 [DOI] [PubMed] [Google Scholar]
- 4.Gera N., Yang A., Holtzman T. S., Lee S. X., Wong E. T., and Swanson K. D., PLoS One 10, 1 (2015). 10.1371/journal.pone.0125269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirson E. D., Gurvich Z., Schneiderman R., Dekel E., Itzhaki A., Wasserman Y., Schatzberger R., and Palti Y., Cancer Res. 64, 3288 (2004). 10.1158/0008-5472.CAN-04-0083 [DOI] [PubMed] [Google Scholar]
- 6.Kirson E. D., Dbalý V., Tovaryš F., Vymazal J., Soustiel J. F., Itzhaki A., Mordechovich D., Steinberg-Shapira S., Gurvich Z., Schneiderman R., Wasserman Y., Salzberg M., Ryffel B., Goldsher D., Dekel E., and Palti Y., Proc. Natl. Acad. Sci. U.S.A. 104, 10152 (2007). 10.1073/pnas.0702916104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pohl H. A., Dielectrophoresis: The Behavior of Neutral Matter in Nonuniform Electric Fields (Cambridge University Press, Cambridge, 1978). [Google Scholar]
- 8.Davies A. M., Weinberg U., and Palti Y., Ann. N.Y. Acad. Sci. 1291, 86 (2013). 10.1111/nyas.12112 [DOI] [PubMed] [Google Scholar]
- 9.Giladi M., Schneiderman R. S., Voloshin T., Porat Y., Munster M., Blat R., Sherbo S., Bomzon Z., Urman N., Itzhaki A., Cahal S., Shteingauz A., Chaudhry A., Kirson E. D., Weinberg U., and Palti Y., Sci. Rep. 5, 1 (2015). 10.1038/srep18046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim E. H., Song H. S., Yoo S. H., and Yoon M., Oncotarget 7, 65125 (2016). 10.18632/oncotarget.11372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karanam N. K., Srinivasan K., Ding L., Sishc B., Saha D., and Story M. D., Cell Death Dis. 8, e2711 (2017). 10.1038/cddis.2017.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrugala M. M., Engelhard H. H., Dinh Tran D., Kew Y., Cavaliere R., Villano J. L., Annenelie Bota D., Rudnick J., Love Sumrall A., Zhu J. J., and Butowski N., Semin. Oncol. 41, S4 (2014). 10.1053/j.seminoncol.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 13.Kirson E. D., Giladi M., Gurvich Z., Itzhaki A., Mordechovich D., Schneiderman R. S., Wasserman Y., Ryffel B., Goldsher D., and Palti Y., Clin. Exp. Metastasis 26, 633 (2009). 10.1007/s10585-009-9262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirson E. D., Schneiderman R. S., Dbal V., Tovary F., Vymazal J., Itzhaki A., Mordechovich D., Gurvich Z., Shmueli E., Goldsher D., Wasserman Y., and Palti Y., BMC Med. Phys. 9, 1 (2009). 10.1186/1756-6649-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta M., Wen P., Nishikawa R., Reardon D., and Peters K., Crit. Rev. Oncol. Hematol. 111, 60 (2017). 10.1016/j.critrevonc.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 16.Clark P. A., Gaal J. T., Strebe J. K., Pasch C. A., Deming D. A., Kuo J. S., and Robins H. I., J. Clin. Neurosci. 36, 120 (2017). 10.1016/j.jocn.2016.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bender E., Kozak K., Howard S., Hayes L., Bayouth J., and Robins H. I., J. Clin. Neurosci. 42, 172 (2017). 10.1016/j.jocn.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 18.Pless M., Droege C., von Moos R., Salzberg M., and Betticher D., Lung Cancer 81, 445 (2013). 10.1016/j.lungcan.2013.06.025 [DOI] [PubMed] [Google Scholar]
- 19.Giladi M., Weinberg U., Schneiderman R. S., Porat Y., Munster M., Voloshin T., Blatt R., Cahal S., Itzhaki A., Onn A., Kirson E. D., and Palti Y., Semin. Oncol. 41, S35 (2014). 10.1053/j.seminoncol.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 20.Giladi M., Schneiderman R. S., Porat Y., Munster M., Itzhaki A., Mordechovich D., Cahal S., Kirson E. D., Weinberg U., and Palti Y., Pancreatology 14, 54 (2014). 10.1016/j.pan.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 21.Rivera F., Benavides M., Gallego J., Guillen-Ponce C., Lopez-Martin J., and Küng M., Pancreatology 19, 64 (2019). 10.1016/j.pan.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 22.Vergote I., von Moos R., Manso L., Van Nieuwenhuysen E., Concin N., and Sessa C., Gynecol. Oncol. 150, 471 (2018). 10.1016/j.ygyno.2018.07.018 [DOI] [PubMed] [Google Scholar]
- 23.Ceresoli G. L., Aerts J. G., Dziadziuszko R., Ramlau R., Cedres S., van Meerbeeck J. P., Mencoboni M., Planchard D., Chella A., Crinò L., Krzakowski M., Rüssel J., Maconi A., Gianoncelli L., and Grosso F., Lancet Oncol. 20, 1702 (2019). 10.1016/S1470-2045(19)30532-7 [DOI] [PubMed] [Google Scholar]
- 24.Porat Y., Giladi M., Schneiderman R. S., Blat R., Shteingauz A., Zeevi E., Munster M., Voloshin T., Kaynan N., Tal O., Kirson E. D., Weinberg U., and Palti Y., J. Vis. Exp. 2017, 1 (2017). 10.3791/55820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavesi A., Adriani G., Tay A., Warkiani M. E., Yeap W. H., Wong S. C., and Kamm R. D., Sci. Rep. 6, 1 (2016). 10.1038/srep26584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gencturk E., Mutlu S., and Ulgen K. O., Biomicrofluidics 11, 051502 (2017). 10.1063/1.4998604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonis P., Kersulis S., Stankevich V., Kaseta V., Lastauskiene E., and Stirke A., Bioelectrochemistry 115, 19 (2017). 10.1016/j.bioelechem.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 28.Valero A., Braschler T., Rauch A., Demierre N., Barral Y., and Renaud P., Lab Chip 11, 1754 (2011). 10.1039/c1lc00007a [DOI] [PubMed] [Google Scholar]
- 29.Sherman F., Encycl. Mol. Biol. Mol. Medicine 6, 302 (1997). [Google Scholar]
- 30.Meng D., Zhang P., Li S., Ho C. T., and Zhao H., J. Funct. Foods 38, 36 (2017). 10.1016/j.jff.2017.08.041 [DOI] [Google Scholar]
- 31.Lian J., Mishra S., and Zhao H., Metab. Eng. 50, 85 (2018). 10.1016/j.ymben.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 32.Gautier T., Bergès T., Tollervey D., and Hurt E., Mol. Cell. Biol. 17, 7088 (1997). 10.1128/MCB.17.12.7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogomolnaya L. M., Pathak R., Cham R., Guo J., Surovtseva Y. V., Jaeckel L., and Polymenis M., Curr. Genet. 45, 350 (2004). 10.1007/s00294-004-0497-5 [DOI] [PubMed] [Google Scholar]
- 34.Ertekin E., Gencturk E., Kasim M., and Ulgen K. O., OMICS 24, 96 (2019). 10.1089/omi.2019.0096 [DOI] [PubMed] [Google Scholar]
- 35.Gencturk E., Yurdakul E., Celik A. Y., Mutlu S., and Ulgen K. O., Biomed. Microdevices 22, 20 (2020). 10.1007/s10544-020-0474-x [DOI] [PubMed] [Google Scholar]
- 36.Puza S., Gencturk E., Odabasi I. E., Iseri E., Mutlu S., and Ulgen K. O., Biomed. Microdevices 19, 40 (2017). 10.1007/s10544-017-0182-3 [DOI] [PubMed] [Google Scholar]
- 37.Odabasi I. E., Gencturk E., Puza S., Mutlu S., and Ulgen K. O., Biomed. Microdev. 20(3), 57 (2018). 10.1007/s10544-018-0302-8 [DOI] [PubMed] [Google Scholar]
- 38.Shadmani A., Viswam V., Chen Y., Bounik R., Dragas J., Radivojevic M., Geissler S., Sitnikov S., Müllerand J., and Hierlemann A., IEEE Trans. Biomed. Eng. 66, 2481 (2019). 10.1109/TBME.2018.2890530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonmez B. G., Ertop O., and Mutlu S., J. Micromech. Microeng. 28, 115017 (2018). 10.1088/1361-6439/aae39e [DOI] [Google Scholar]
- 40.Jones M. H. and Scott J., IEEE Trans. Biomed. Circuits Syst 9, 441 (2015). 10.1109/TBCAS.2014.2333759 [DOI] [PubMed] [Google Scholar]
- 41.Callegaro L., Electrical Impedance: Principles, Measurement, and Applications (CRC Press, Boca Raton, FL, 2016). [Google Scholar]
- 42.Pucihar G., Krmelj J., Reberšek M., Napotnik T. B., and Miklavčič D., IEEE Trans. Biomed. Eng. 58, 3279 (2011). 10.1109/TBME.2011.2167232 [DOI] [PubMed] [Google Scholar]
- 43.Suga M., Goto A., and Hatakeyama T., J. Electrostat. 64, 796 (2006). 10.1016/j.elstat.2006.01.007 [DOI] [Google Scholar]
- 44.Guyot S., Ferret E., Boehm J. B., and Gervais P., Int. J. Food Microbiol. 113, 180 (2007). 10.1016/j.ijfoodmicro.2006.06.036 [DOI] [PubMed] [Google Scholar]
- 45.Longsine-Parker W., Wang H., Koo C., Kim J., Kim B., Jayaraman A., and Han A., Lab Chip 13, 2144 (2013). 10.1039/c3lc40877a [DOI] [PubMed] [Google Scholar]
- 46.Geng T. and Lu C., Lab Chip 13, 3803 (2013). 10.1039/C3LC50566A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ou Q. X., Nikolic-Jaric M., and Gänzle M., Bioelectrochemistry 115, 47 (2017). 10.1016/j.bioelechem.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 48.Huang K., Yu L., Liu D., Gai L., and Wang J., Food Res. Int. 54, 456 (2013). 10.1016/j.foodres.2013.07.046 [DOI] [Google Scholar]
- 49.Castellví Q., Ginestà M. M., Capellà G., and Ivorra A., Bioelectrochemistry 105, 16 (2015). 10.1016/j.bioelechem.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 50.Liang J., kee Mok A. W., Zhu Y., and Shi J., Bioelectrochemistry 94, 61 (2013). 10.1016/j.bioelechem.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 51.McCann R., Bagga K., Stalcup A., Vazquez M., and Brabazon D., Proc. SPIE 9351, 93511N (2015). 10.1117/12.2076916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.