Abstract
Pharmacological manipulations of constitutive nitric oxide (cNO) levels have been shown to have variable effects on Na absorption in vivo and in vitro in different tissues. Species differences, untoward in vivo effects (e.g. ENS, blood flow) and pharmacological non-specificity may account for these confounding observations. Thus, to directly and specifically determine the effect of cNO on brush border membrane Na/H exchange (NHE3) and Na-dependent glucose co-transport (SGLT-1), we inhibited cNO synthase (NOS3) with its siRNA in rat small intestinal epithelial cells (IEC-18) in vitro. As expected, intracellular cNO levels were reduced in siRNA NOS3 transfected cells. In these cells, SGLT-1 was significantly reduced compared to control. In contrast, NHE3 was significantly increased in siRNA NOS3 transfected cells. To determine if SGLT-1 changes were secondary to altered Na/K-ATPase, its activity was measured and found to be increased in NOS3 silenced cells. The mechanism of inhibition of SGLT-1 was secondary to diminished affinity of the co-transporter for glucose in NOS3 silenced cells. In contrast, the mechanism of stimulation of NHE3 is by increasing BBM exchanger numbers in siRNA NOS3 cells while the affinity was unaffected. Western blot studies of immunoreactive BBM proteins also confirmed the kinetic studies. All these data indicates that direct and specific inhibition of NOS3 with its siRNA inhibits SGLT-1 while stimulating NHE3 in the BBM. Thus, cNO uniquely and compensatorily regulates BBM NHE3 and SGLT-1 to maintain cellular Na homeostasis and these unique alterations by cNO are mediated by its intracellular 2nd messenger cGMP.
1. Introduction
In the mammalian small intestine and colon coupled NaCl absorption is mediated by the dual operation of Na/H and 3 exchange on the brush border membrane (BBM) of absorptive intestinal villus or colonic surface cells [1]. Nine isoforms of Na/H exchange (NHE) have been demonstrated to date [2,3]. Depending on the species and the segment of the intestine, NHE2 or NHE3 appears to be the predominant isoform involved in NaCl absorption [3,4]. NHE3 is the more predominant isoform in the small intestine as compared to the colon in several species including the rabbit distal small intestine [5]. Illustrating the physiological significance of the NHE3 isoform, its knock out mouse demonstrates many appropriate phenotypic changes including hypotension, acidosis and diarrhea while the NHE2 knock out mouse demonstrates no change in phenotype [6]. Many agents and conditions have been reported to alter NHE3 by altering its transcription, phosphorylation, trafficking from cytosol to the BBM [7,8].
Na-glucose co-transport is essential for the assimilation of sugar and Na in the normal intestine [9]. In intact cells Na/K-ATPase on the basolateral membrane (BLM) generates the favorable intracellular Na-gradient to facilitate Na-nutrient co-transporter (SGLT-1) [7]. SGLT-1 is present in villus, but not crypt cells in the normal rabbit intestine [10]. While SGLT-1 is also present in the cytosol, it must be present on the BBM of villus cells to be functional [10]. SGLT-1 is affected in diseases and by several agents [10-12]. The significance of SGLT-1 is illustrated by the fact that in recent SGLT-1 knock out mice, it has been shown that these mice require a glucose/galactose free diet to survive [13].
The effect of nitric oxide (NO) on SGLT-1 was previously not known. Regulation of small intestinal coupled NaCl absorption by NO was poorly understood. In a variety of studies using pharmacological manipulation, NO has been suggested to have no effect on rat small intestinal and colonic electrolyte transport, while other studies have suggested that inhibition of NO in the rat small intestine promotes secretion [14-17]. Species-specific differences in addition to pharmacological non-specificity may be an issue. For example, NO donating compounds have been shown to be secretory in rat and guinea pig ileum, however, pro-absorptive in dog, mouse, and rabbit ileum [14,15,18-21]. The inability of pharmacological agents to produce constitutive vs. inducible NO levels consistently in these studies may explain the discrepancies as these two different levels of NO may very well produce opposite effects. For example, in studies where inhibition of coupled NaCl absorption was observed (guinea pig and the rat intestine and colon), pathophysiological levels of NO may have been generated, whereas in studies where stimulation of coupled NaCl absorption was observed (mouse, dog, and rabbit small intestine) lesser levels of NO may have been achieved [21]. Thus, a better strategy would be to study physiological or constitutive levels of NO in the normal mammalian intestine to determine whether and what its role is in the regulation of NaCl absorption.
Pharmacological, in vivo inhibition of NO production with l-NAME in rabbits selectively inhibited SGLT-1, but not Na-amino acid co-transport [22]. In contrast, this stimulated coupled NaCl absorption by stimulating villus cell BBM NHE3, but not Cl/HCO3 exchange [16,23]. Clearly, this observation is a culmination of alterations in multiple tissues as well as the subsequent effect on other physiological parameters such as blood flow, motility and enteric nervous system to name a few. Pharmacological in vitro inhibition of cNO production in intestinal epithelial cells (IEC-18 cells) also inhibited BBM SGLT-1 and stimulated NHE3 [24]. While illustrative, pharmacological manipulations are potentially fraught with non-specific alterations. Nevertheless, these observations suggested that NHE3 and SGLT-1 may be compensatorily regulated by NO to regulate Na absorption in the small intestine. Given, the physiological importance of this hypothesis, it is essential to very specifically and directly inhibit NOS3 with its siRNA to determine the effect of cNO on BBM NHE3 and SGLT-1 as was done in this study.
2. Materials and methods
2.1. Cell culture, RNA interference and treatments
Rat IEC-18 cells were grown in high glucose DMEM supplemented with 2 U/ml of insulin, 0.5 mM β-hydroxybutyrate and 10% fetal calf serum and incubated at 37 °C with 10% CO2 in a humidified atmosphere. Silencer predesigned negative (scrambled siRNA) control (catalog no. 4635; Ambion) and constitutive nitric oxide synthase (cNOS/eNOS/NOS3) specific (ID s128117; Ambion) siRNAs were used for siRNA transfections as described previously [25]. Cells grown as 7 days post transfected monolayers in 24 well plates were used for all the transport studies. To activate Protein Kinase G (PKG) pathway siNOS3 transfected IEC-18 cells were treated with either a cGMP (cyclic GMP) analog 8-Bromoguanosine 3′,5′-cyclic monophosphate sodium salt (8-Bro) (Sigma-Aldrich) (100 μM) or vehicle alone for 24 h prior to the uptake experiment.
2.2. Measurement of NO
Total (nitrate+nitrite) NO levels were measured in IEC-18 cells by Griess reaction colorimetric assay (Item No. 780001, Cayman chemicals, USA) according to the manufacturer's instruction. Briefly, 7 days post transfected monolayers of negative and siNOS3 IEC-18 cells were washed three times with PBS to remove any trace of media and lysed with phosphate buffered saline (PBS) by sonication. The cell lysate was then centrifuged and the clear supernatant was used for the Griess assay to measure total (nitate+nitrite) NO. Samples were finally read at 540 nm and total NO concentrations were determined with the nitrate standard curve.
2.3. Na-glucose co-transport uptake studies in IEC-18 cells
Uptake studies for Na-glucose co-transport were performed using of 3H-O-methyl glucose (OMG) as previously described with few modifications [26]. Briefly, cells were washed and incubated with Na-free buffer containing 130 mM TMACl, 4.7 mM KCl, 1 mM MgSO4, 1.25 mM CaCl2, 20 mM HEPES (pH 7.4 at 37 °C) for 10 min. Uptake was initiated by incubating the cells for 2 min in a Tris-HEPES (pH 7.4) reaction medium containing 130 mM NaCl, 10 μCi of 3H-O-methyl glucose (OMG) and 100 μM OMG in the presence or absence of 1 mM phlorizin (SGLT-1 specific inhibitor) and 10 mM D-glucose. The reaction was stopped and the cells were washed twice with ice cold Na-free buffer containing 25 mM D-glucose. The cells were then incubated with 1 N NaOH for 20 min at 70 °C, to digest the cells, before addition of 4 mL of scintillation fluid (Ecoscint; National Diagnostics). Radioactivity was determined in a Beckman 6500 Beta scintillation counter. SGLT-1 specific uptake was calculated by subtracting uptake with and without phlorizin. To determine the kinetic parameters of Na-glucose co-transport, experiments were performed with increasing extracellular OMG concentrations at time point of 30 s followed by analysis of the uptake numbers with GraphPad Prism 6.
2.4. Determination of Na/H exchange activity in IEC-18 cells
Na/H exchange was initiated in a reaction medium containing Na-free buffer with 10 μCi of 22Na and 1 mM NaCl in the presence or absence of 50 μM EIPA as previously described with few modifications [27]. To begin with, the cells were incubated for 10 min in acid load buffer (70 mM TMACl, 50 mM NH4Cl, 5 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 5 mM glucose, 15 mM Tris-HEPES (pH 7.4) and washed with wash buffer containing 120 mM TMACl and 15 mM Tris-HEPES (pH 7.4). 22Na uptake reaction was arrested at 2 min and the cells were washed twice with ice cold phosphate buffered saline and processed as described above. To determine the kinetic parameters of Na/H exchange, uptake experiments were performed with increasing concentration of extracellular NaCl at a fixed time of 30 s. The uptake numbers obtained from the experiments were used to derive kinetic parameters of Na/H exchange, with GraphPad Prism 6.
2.5. Effect of NOS3 on Na/K-ATPase activity in IEC-18 cells
Na/K-ATPase was measured from control and NOS3 transfected IEC-18 cell homogenates as previously described [22,28]. Enzyme specific activity was expressed as nanomoles of Pi liberated per milligram protein per minute.
2.6. Western blot analyses
Western blot analyses of IEC-18 BBM were performed according to standard protocols as described previously [25]. Solubilized BBM proteins were separated (custom made 8% poly acrylamide gel) and transferred to BioTrace PVDF membrane. For immunoreactive protein determination, membranes were probed with anti-NOS3 antibodies raised in rabbit (SC-654, Santacruz Biotechnology, USA) to determine NOS3 levels, with anti-SGLT-1 antibodies raised in rabbit (ab14686, Abcam, USA) for SGLT-1, with anti-NHE3 antibodies raised in chicken (Invitrogen custom antibody services, USA) for NHE3, and with anti-Ezrin antibodies (ab231907, Abcam, USA) raised in rabbit for Ezrin. Horseradish peroxidase coupled goat anti-rabbit antibody for NOS3, SGLT-1 and Ezrin, and rabbit anti-chicken antibody for NHE3 were used and detected by chemiluminescence with ECL Detection Reagent (GE Healthcare). NOS3, SGLT-1 and NHE3 protein density was quantitated via a densitometric scanner FluorChem™ instrument (Alpha Innotech, San Leandro, CA).
2.7. Protein assay
Proteins were quantified with the DC™ protein assay kit (Lowry's method) according to manufacturer's protocols (Bio-Rad).
2.8. Statistics
Results presented represent means ± SE of experiments performed and calculated by the GraphPad Prism 6 (San Diego, CA). All uptakes were done in triplicate. Student's t-test was performed for statistical analysis.
3. Results
3.1. Effect of NOS3 siRNA in IEC-18 cells on intracellular NO levels and NOS3 protein expression
Silencing NOS3 with its siRNA significantly decreased intracellular NO levels in IEC-18 cells, approximately 3 fold (1.0 ± 0.08 μmol/mg protein in siRNA NOS3 IEC-18 cells and 3.0 ± 0.29 μmol/mg protein in control IEC-18 cells) as shown in Fig. 1a. Control cells treated with scrambled siRNA had no effect. Western blot analysis was performed to determine cellular NOS3 protein levels in IEC-18 cells. Immunoreactive NOS3 protein levels was significantly decreased in the cell lysate of NOS3 silenced IEC-18 cells as shown in Fig. 1bA. Densitometric quantitation shown in Fig. 1bB, confirmed these findings.
Fig. 1.
Effect of NOS3 silencing in IEC-18 cells. a) In IEC-18 cells transfected with NOS3 siRNA there was a significant decrease in intracellular NO levels compared to control; b) NOS3 siRNA transfected IEC-18 cells showed significant downregulation of NOS3 protein expression compared to control, A: a representative Western blot of separate experiments is shown. B: densitometric quantitation confirmed that in NOS3 siRNA transfected cells, cell lysate NOS3 was significantly decreased.
3.2. Effect of NOS3 on Na-glucose co-transport in IEC-18 cells
Na-dependent glucose co-transport, defined as 3H-OMG uptake in the absence of co-transporter inhibitor phlorizin minus the presence of it, was significantly diminished in NOS3 silenced in IEC-18 cells (Fig. 2: 1525 ± 93 pmol/mg protein•2 min in control and 895 ± 58 in NOS3 siRNA transfected IEC-18 cells; n = 5, p < 0.001). These data indicate that reduced intracellular NOS3 inhibits Na-glucose co-transport in IEC-18 cells.
Fig. 2.
Effect of NOS3 silencing on BBM SGLT-1 activity in IEC-18 cells. In IEC-18 cells transfected with NOS3 siRNA, there was a significant reduction in Na-dependent 3H-OMG uptake when compared with control IEC-18 cells.
3.3. Effect of NOS3 on Na/H exchange in IEC-18 cells
Na/H exchange, defined as 22Na uptake in the absence minus the presence of Na/H exchange inhibitor EIPA, was significantly increased in NOS3 silenced IEC-18 cells (Fig. 3: 4108 ± 59 pmol/mg protein•2 min in control IEC-18 cells; and 5213 ± 95 in NOS3 silenced IEC-18 cells; n = 4, p < 0.001). This data indicated that decreased intracellular NO levels stimulates Na/H exchange in contrast to the inhibition of Na-glucose co-transport in NOS3 silenced IEC-18 cells.
Fig. 3.
Effect of NOS3 silencing on BBM NHE3 activity in IEC-18 cells. In IEC-18 cells transfected with NOS3 siRNA, there was a significant increase in Na/H exchange activity when compared with control IEC-18 cells.
3.4. Effect of NOS3 on Na/K-ATPase activity in IEC-18 cells
Since altered intracellular Na levels maintained by basolateral membrane Na/K-ATPase can affect BBM Na transport, Na/K-ATPase activity was determined in cellular homogenates from control and NOS3 silenced IEC-18 cells. Na/K-ATPase levels were significantly increased in NOS3 silenced IEC-18 cells compare to control cells (Fig. 4: 24.5 ± 1.8 nmol Pi/mg protein•min in control and 35.6 ± 0.8 in siRNA NOS3 cells; n = 3, p < 0.01). These data indicate that the effect of cNO on Na-glucose co-transport and Na/H exchange activity is not secondary to altered Na-extruding capacity of the cells but rather at the level of the BBM transporters themselves.
Fig. 4.
Effect of NOS3 silencing on Na/K-ATPase activity in IEC-18 cells. In IEC-18 cells transfected with NOS3 siRNA, there was a significant increase in Na/K-ATPase activity when compared with control IEC-18 cells.
3.5. Na-glucose co-transport kinetic studies in IEC-cells
To determine the mechanism of inhibition of Na-glucose co-transport in NOS3 silenced cells, kinetic studies were performed. The affinity (1/Km) for glucose was significantly increased in NOS3 silenced IEC-18 cells (Table 1). However, the maximal rate of uptake of glucose (Vmax) was not altered in IEC-18 cells transfected with NOS3 siRNA (Table 1). These kinetic parameters indicated that the mechanism of NOS3 mediated inhibition of Na-glucose co-transport activity is secondary to a decrease in the affinity of the co-transporters for glucose without a change in the number of co-transporters.
Table 1.
Na-glucose co-transport kinetic parameters in IEC-18 cells.
| vmax (nmol/mg protein•30sec) |
Km (mM) |
|
|---|---|---|
| Control | 5.3 ± 0.2 | 5.9 ± 0.3 |
| siNOS3 | 5.5 ± 0.5 | 9.3 ± 0.6* |
The maximal rate of uptake of glucose (Vmax) was not altered in NOS3 silenced IEC-18 cells; however, the affinity (1/Km) was significantly decreased between control and NOS3 siRNA transfected IEC-18 cells
p < 0.01, n = 3.
3.6. Kinetic studies for Na/H exchange in IEC-18 cells
To determine the mechanism of stimulation of N/H exchange in NOS3 silenced cells, kinetic studies were performed in IEC-18 cells. The maximal rate of uptake of Na was significantly increased in IEC-18 cells transfected with NOS3 siRNA compared with control IEC-18 cells (Table 2). In contrast, the affinity for Na was not altered in NOS3 silenced IEC-18 cells (Table 2). These data indicated that the mechanism of NHE3 stimulation when NOS3 is silenced is secondary to an increased in the number of exchangers rather than altered affinity of the exchangers for sodium.
Table 2.
Kinetic parameters of N/H exchange in IEC-18 cells.
| vmax (nmol/mg protein•30sec) |
Km (mM) |
|
|---|---|---|
| Control | 1.9 ± 0.1 | 4.4 ± 0.3 |
| siNOS3 | 3.60 ± 0.2* | 4.2 ± 0.3 |
The maximal rate of uptake of Na (Vmax) was significantly increased in NOS3 silenced IEC-18 cells; however, the affinity (1/Km) was unchanged between control and NOS3 silenced IEC-18 cells
p < 0.01, n = 3.
3.7. SGLT-1 molecular studies
Given that kinetic studies indicated that SGLT-1 inhibition in NOS3 silenced cells is likely not at the level of co-transporter numbers, Western blot analysis was performed to determine BBM SGLT-1 protein levels. Immunoreactive SGLT-1 protein levels are not altered in the BBM of NOS3 silenced IEC-18 cells as shown in Fig. 5A. Densitometric quantitation shown in Fig. 5B, confirmed these findings. The unaltered BBM SGLT-1 protein levels in NOS3 silenced IEC-18 cells, in conjunction kinetic studies above indicated that indeed the mechanism of inhibition of SGLT-1 is secondary to a decreased in the affinity of the co-transporters for glucose, not altered number of co-transporters.
Fig. 5.
Effect of NOS3 silencing on BBM SGLT-1 protein expression in IEC-18 cells. SGLT-1 protein expression on BBM was not altered in NOS3 silenced IEC-18 cells compared with control IEC-18 cells. A: a representative Western blot of separate experiments is shown. B: densitometric quantitation confirmed that in NOS3 siRNA transfected cells, BBM SGLT-1 was not altered.
3.8. NHE3 molecular studies
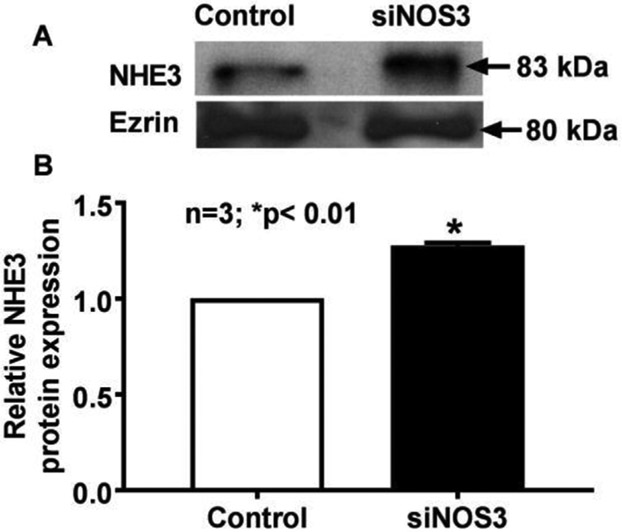
Unlike SGLT-1, kinetic studies indicated that NHE3 stimulation in NOS3 silenced cells is likely at the level of transporter numbers. Thus, Western blot analysis was performed to determine BBM NHE3 protein levels. BBM NHE3 protein levels were increased in NOS3 silenced IEC-18 cells as shown in Fig. 6A. Densitometric quantitation shown in Fig. 6B, confirmed these findings. The increase in BBM NHE3 protein, in conjunction kinetic studies demonstrated that the mechanism of stimulation of NHE3 is secondary to an increase in the number of BBM exchangers rather altered affinity of the exchangers in NOS3 silenced cells.
Fig. 6.
Effect of NOS3 silencing on BBM NHE3 protein expression in IEC-18 cells. Decreased intracellular cNO significantly increased BBM NHE3 protein expression in IEC-18 cells compared with control cells. A: a representative Western blot of separate experiments is shown. B: densitometric quantitation confirmed that in NOS3 silenced IEC-18 cells, BBM NHE3 was significantly increased.
3.9. Effect of addition of 8-bro-cGMP in NOS3 silenced cells on SGLT-1 and NHE3
Constitutive nitric oxide generally mediates its effects via intracellular cGMP. Thus, an observation that results from reduced cNO via diminished cGMP should potentially be reversible with the addition of cell membrane permeant cGMP, specifically 8-bro-cGMP. As shown in Fig. 7a, addition of 8-bro-cGMP to NOS3 silenced cells essentially reverses the inhibition of SGLT-1 (1131 ± 32 pmol/mg protein•2 min in control IEC-18 cells; 860 ± 40 in NOS3 silenced IEC-18 cells; and 1157 ± 19 in NOS3 silenced IEC-18 cells + 8-bro-cGMP; n = 4, p < 0.001). Similarly, as shown in Fig. 7b, upon the addition of 8-bro-cGMP, the stimulation of NHE3 in NOS3 silenced cells also reverses to baseline levels (4025 ± 57 pmol/mg protein•2 min in control IEC-18 cells; 5096 ± 88 in NOS3 silenced IEC-18 cells; and 4061 ± 14 in NOS3 silenced IEC-18 cells + 8-bro-cGMP; n = 4, p < 0.001). These data indicate that indeed the intracellular pathway of regulation of SGLT-1 and NHE3 is likely via cGMP in NOS3 silenced cells.
Fig. 7.
Effect of 8-bro-cGMP on SGLT-1 and NHE3 in NOS3 silenced in IEC-18 cells. A: Decreased intracellular cNO significantly diminished SGLT-1 in NOS3 silenced IEC-18. Addition of 8-bro-cGMP reversed the inhibition of SGLT-1 in NOS3 silenced IEC-18 cells. B: NOS3 silenced IEC-18 cells significantly increased NHE3 activity. Addition of 8-bro-cGMP reversed this stimulation of NHE3 in NOS3 silenced IEC-18 cells.
4. Discussion
This manuscript demonstrates that direct and specific inhibition of cellular constitutive nitric oxide has unique effects on intracellular Na homeostasis. Specifically, cNO nominally appears to promote BBM SGLT-1 while retarding Na/H exchange. Further, this compensatory regulation of the two primary Na absorptive pathways by cNO appears to be meditated via intracellular cGMP.
Nitric oxide (NO) has been demonstrated to regulate gastrointestinal functions in normal and pathophysiological states [29]. There are three NO synthases: neuronal or nNOS or NOS1; endothelial or eNOS or NOS3; and inducible or iNOS or NOS2. NOS1 and 3 are considered constitutive (cNO) and produce nanomolar quantities of NO [21,30]. On the other hand, NOS2 is considered inducible (iNO) and predominantly occurs during pathophysiological conditions and produces micromolar quantities of NO [31-33]. The small quantities of NO have generally been thought to be beneficial while larger quantities produced in pathophysiological states (e.g. Crohn's disease and ulcerative colitis) are thought to be deleterious [33-35]. The epithelial cells, endothelial cells, mesenteric neuron and leukocytes (neutrophils, mast cells and macrophages) have been demonstrated to produce NO [36]. Thus, attempts to manipulate NO in vivo with pharmacological agents may have different effects at multiple cell types.
In this study specific inhibition of the native generation of cNO with the siRNA for NOS3 resulted in diminished intracellular cNO. Reduction of cNO resulted in the inhibition of one of the predominant BBM Na absorptive pathways, specifically Na-glucose co-transport, SGLT-1. This reduction in SGLT-1 activity was not secondary to altered BLM Na/K-ATPase which provides the favorable transcellular Na gradient for this secondarily active co-transporter. The mechanism of inhibition of SGLT-1 when cNO is reduced was secondary to a decrease in the affinity of the co-transporter for glucose without a change in the number of BBM co-transporters.
In contrast to SGLT-1, the other predominant BBM Na absorptive pathway, NHE3 was stimulated when cNO production was inhibited. The mechanism of this stimulation was secondary to an increase in the number of BBM exchangers without an alteration in the affinity of the transporter. Thus, two primary BBM Na absorptive pathways are uniquely regulated by cNO to likely maintain intracellular Na homeostasis. Further, these unique alterations by cNO are mediated by its intracellular 2nd messenger cGMP.
In mammalian small intestinal epithelial cells, Na homeostasis in normal physiological conditions is maintained by NHE3 and Na-dependent co-transporters like SGLT-1. The decreased SGLT-1 activity by the inhibition of NOS3 is compensated by the increased NHE3 activity to maintain Na-homeostasis, as seen in the present study. However, during pathophysiological condition such as in IBD, when there is an increase in iNOS (NOS2) [31-33] Na absorption mediated by SGLT-1 is significantly inhibited, while NHE3 remains unaffected [10,12,37]. Thus, resulting in net inhibition of NaCl absorption leading to diarrhea, the most common morbidity of IBD.
Inhibition of SGLT-1 by diminishment of the affinity of the co-transporter of SGLT-1 by cNO is undoubtedly posttranslational. This may occur at the level of altered phosphorylation or glycosylation of SGLT-1. Previous studies have shown that cGMP mediated inhibition of SGLT-1 activity was not due to any significant changes in phosphorylation of SGLT-1 protein. Thus, the posttranslational mechanism responsible for the altered affinity of SGLT-1 was thought to be secondary to altered glycosylation of SGLT-1 protein [38].
Stimulation of NHE3 when cNO is inhibited may be due to enhanced mRNA stability or NHE3 transcription, resulting in increased level of BBM NHE3 protein and therefore increased NHE3 transport activity. NO has been shown to mediate its effects on NHE3 at the level of its transcription and at the level of the BBM protein. NO can affect NHE3 transcription via protein kinase G which can affect the activity of transcription factors by direct cGMP-dependent phosphorylation, (e.g., CREB). The promoter sequence of rat NHE3 reveals the presence of a AP1/CREB transcription factor binding site in the region of nucleotides between nucleotides + 36 and + 44. Further, the NHE3 core promoter region between nucleotides −118 to +59 contains the essential transcription factor interacting sites of SpA, SpB, SpC and GATA as well as AP1/CREB [39]. Nitric oxide can also affect NHE3 protein trafficking through cGMP mediated effects on NHERF2 and Myristoyl anchor protein [40]. NO has also been shown to directly affect the activity of NHE3 through cGMP by altering its phosphorylation status [41].
The above mentioned molecular mechanisms of regulation require that cNO mediate its effects via cGMP. Indeed as shown on Fig. 7a and b, exogenous provision of cGMP, results in the reversal of inhibition of SGLT-1 as well as the stimulation of NHE3 in NOS3 silenced cells. The results of the current study, where we show NO mediated effects through cGMP on NHE3 is comparable to a previous study which also showed cGMP mediated regulation of altered NHE3 activity [42].
In summary, inhibition of the production of cNO with its siRNA results in the inhibition of BBM SGLT-1 and stimulation of NHE3. The mechanism of inhibition of SGLT-1 is secondary to altered affinity of the co-transporter while the mechanism of stimulation of NHE3 is secondary to increased BBM exchanger numbers. Intracellularly cNO upstream appears to mediate both of these changes through cGMP. In conclusion, this study clearly demonstrates that direct and specific inhibition of native cNO production in intestinal epithelial cells results in unique and likely compensatory alterations of the primary BBM Na-absorptive pathways to maintain Na homeostasis.
Acknowledgements
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases research grants DK108054 and DK067420 and Veteran's Affairs Merit Review grant BX003443 to U. Sundaram.
The authors thank Dr. Usha Murughiyan for editorial assistance with the manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.niox.2018.04.007.
References
- [1].Knickelbein RG, Aronson PS, Dobbins JW, Membrane distribution of sodium-hydrogen and chloride-bicarbonate exchangers in crypt and villus cell membranes from rabbit ileum, J. Clin. Invest 82 (1988) 2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Doris PA, The sodium-hydrogen exchange system, in: Burnier M (Ed.), Sodium in Health and Disease, CRC Press, 2007, pp. 67–82. [Google Scholar]
- [3].Zachos NC, Tse M, Donowitz M, Molecular physiology of intestinal N+/H+ exchange, Annu. Rev. Physiol 67 (2005) 411–443. [DOI] [PubMed] [Google Scholar]
- [4].Kato A, Romero MF, Regulation of electroneutral NaCl absorption by the small intestine, Annu. Rev. Physiol 73 (2011) 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun C, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse C-M, NHE2 and NHE3 are human and rabbit intestinal brush-border proteins, Am. J. Physiol. Gastrointest. Liver Physiol 270 (1996) G29–G41. [DOI] [PubMed] [Google Scholar]
- [6].Ledoussal C, Woo AL, Miller ML, Shull GE, Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of NHE3-deficient mice, Am. J. Physiol. Gastrointest. Liver Physiol 281 (2001) G1385–G1396. [DOI] [PubMed] [Google Scholar]
- [7].Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X, NHERF family and NHE3 regulation, J. Physiol 567 (2005) 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Collazo R, Fan L, Hu MC, Zhao H, Wiederkehr MR, Moe OW, Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis, J. Biol. Chem 275 (2000) 31601–31608. [DOI] [PubMed] [Google Scholar]
- [9].Wright E, Hirayama B, Loo D, Active sugar transport in health and disease, J. Intern. Med 261 (2007) 32–43. [DOI] [PubMed] [Google Scholar]
- [10].Sundaram U, Wisel S, Rajendren V, West A, Mechanism of inhibition of Na +-glucose cotransport in the chronically inflamed rabbit ileum, Am. J. Physiol. Gastrointest. Liver Physiol 273 (1997) G913–G919. [DOI] [PubMed] [Google Scholar]
- [11].Wright EM, Martín M.n.G., Turk E, Intestinal absorption in health and disease—sugars, Best Pract. Res. Clin. Gastroenterol 17 (2003) 943–956. [DOI] [PubMed] [Google Scholar]
- [12].Sundaram U, Coon S, Wisel S, West AB, Corticosteroids reverse the inhibition of Na-glucose cotransport in the chronically inflamed rabbit ileum, Am. J. Physiol. Gastrointest. Liver Physiol 276 (1999) G211–G218. [DOI] [PubMed] [Google Scholar]
- [13].Powell DR, DaCosta CM, Gay J, Ding Z-M, Smith M, Greer J, Doree D, Jeter-Jones S, Mseeh F, Rodriguez LA, Improved glycemic control in mice lacking Sglt1 and Sglt2, Am. J. Physiol. Endocrinol. Metab 304 (2013) E117–E130. [DOI] [PubMed] [Google Scholar]
- [14].Barry MK, Aloisi JD, Pickering SP, Yeo CJ, Nitric oxide modulates water and electrolyte transport in the ileum, Ann. Surg 219 (1994) 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maher MM, Gontarek JD, Jimenez RE, Cahill PA, Yeo CJ, Endogenous nitric oxide promotes ileal absorption, J. Surg. Res 58 (1995) 687–692. [DOI] [PubMed] [Google Scholar]
- [16].Coon S, Sundaram U, Unique regulation of anion/HCO3-exchangers by constitutive nitric oxide in rabbit small intestine, Am. J. Physiol. Gastrointest. Liver Physiol 285 (2003) G1084–G1090. [DOI] [PubMed] [Google Scholar]
- [17].Kim JS, Choi KC, Jeong MH, Kim SW, Oh YW, Lee JU, Increased expression of sodium transporters in rats chronically inhibited of nitric oxide synthesis, J. Kor. Med. Sci 21 (2006) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].MacNaughton WK, Nitric oxide-donating compounds stimulate electrolyte transport in the Guinea pig intestine in vitro, Life Sciences 53 (1993) 585–593. [DOI] [PubMed] [Google Scholar]
- [19].Tamai H, Gaginella TS, Direct evidence for nitric oxide stimulation of electrolyte secretion in the rat colon, Free Radic. Res 19 (1993) 229–239. [DOI] [PubMed] [Google Scholar]
- [20].Rao R, Lopez Y, Riviere P, Pascaud X, Junien J, Porreca F, Nitric oxide modulates neuropeptide Y regulation of ion transport in mouse ileum, J. Pharmacol. Exp. Therapeut 278 (1996) 193–198. [PubMed] [Google Scholar]
- [21].Izzo AA, Mascolo N, Capasso F, Nitric oxide as a modulator of intestinal water and electrolyte transport, Dig. Dis. Sci 43 (1998) 1605–1620. [DOI] [PubMed] [Google Scholar]
- [22].Coon S, Kim J, Shao G, Sundaram U, Na-glucose and Na-neutral amino acid cotransport are uniquely regulated by constitutive nitric oxide in rabbit small intestinal villus cells, Am. J. Physiol. Gastrointest. Liver Physiol 289 (2005) G1030–G1035. [DOI] [PubMed] [Google Scholar]
- [23].Coon S, Shao G, Wisel S, Vulaupalli R, Sundaram U, Mechanism of regulation of rabbit intestinal villus cell brush border membrane Na/H exchange by nitric oxide, Am. J. Physiol. Gastrointest. Liver Physiol 292 (2007) G475–G481. [DOI] [PubMed] [Google Scholar]
- [24].Coon S, Kekuda R, Saha P, Talukder JR, Sundaram U, Constitutive nitric oxide differentially regulates Na-H and Na-glucose cotransport in intestinal epithelial cells, Am. J. Physiol. Gastrointest. Liver Physiol 294 (2008) G1369–G1375. [DOI] [PubMed] [Google Scholar]
- [25].Coon S, Kekuda R, Saha P, Sundaram U, Reciprocal regulation of the primary sodium absorptive pathways in rat intestinal epithelial cells, Am. J. Physiol. Cell Physiol 300 (2011) C496–C505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Turner JR, Black ED, NHE3-dependent cytoplasmic alkalinization is triggered by Na+-glucose cotransport in intestinal epithelia, Am. J. Physiol. Cell Physiol 281 (2001) C1533–C1541. [DOI] [PubMed] [Google Scholar]
- [27].Hodges K, Gill R, Ramaswamy K, Dudeja PK, Hecht G, Rapid activation of Na+/H+ exchange by EPEC is PKC mediated, Am. J. Physiol. Gastrointest. Liver Physiol 291 (2006) G959–G968. [DOI] [PubMed] [Google Scholar]
- [28].Forbush B, Assay of Na K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin, Anal. Biochem 128 (1983) 159–163. [DOI] [PubMed] [Google Scholar]
- [29].Whittle B, Nitric oxide in gastrointestinal physiology and pathology, in: Johnson L (Ed.), Physiology of the Gastrointestinal Tract, Raven, New York, 1994, pp. 267–294. [Google Scholar]
- [30].Miller MJS GT, Nitric oxide as a mediator of intestinal mucosal function, in: Gaginella F, Boca Raton TS (Eds.), Regulatory Mechanisms in Gastrointestinal Function, CRC, 1995, pp. 199–218. [Google Scholar]
- [31].Stuehr DJ, Griffith OW, Mammalian nitric oxide synthases, Adv. Enzymol. Relat. Area Mol. Biol 65 (1992) 287–346. [DOI] [PubMed] [Google Scholar]
- [32].Morris SM Jr., Billiar TR, New insights into the regulation of inducible nitric oxide synthesis, Am. J. Physiol 266 (1994) E829–E839. [DOI] [PubMed] [Google Scholar]
- [33].Kolios G, Valatas V, Ward SG, Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle, Immunology 113 (2004) 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dijkstra G, Moshage H, Jansen PL, Blockade of NF-κ B activation and donation of nitric oxide: new treatment options in inflammatory bowel disease? Scand. J. Gastroenterol 37 (2002) 37–41. [DOI] [PubMed] [Google Scholar]
- [35].Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB, Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease 1, 2, Free Radic. Biol. Med 33 (2002) 311–322. [DOI] [PubMed] [Google Scholar]
- [36].Nathan C, Nitric oxide as a secretory product of mammalian cells, Faseb. J 6 (1992) 3051–3064. [PubMed] [Google Scholar]
- [37].Coon S, Sundaram U, Mechanism of glucocorticoid-mediated reversal of inhibition of Cl(−)/HCO(−)(3) exchange during chronic ileitis, Am. J. Physiol. Gastrointest. Liver Physiol 278 (2000) G570–G577. [DOI] [PubMed] [Google Scholar]
- [38].Arthur S, Coon S, Kekuda R, Sundaram U, Regulation of sodium glucose co-transporter SGLT1 through altered glycosylation in the intestinal epithelial cells, Biochim. Biophys. Acta Biomembr 1838 (2014) 1208–1214. [DOI] [PubMed] [Google Scholar]
- [39].Kiela PR, LeSueur J, Collins JF, Ghishan FK, Transcriptional regulation of the rat NHE3 gene. Functional interactions between GATA-5 and Sp family transcription factors, J. Biol. Chem. 278 (2003) 5659–5668. [DOI] [PubMed] [Google Scholar]
- [40].Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, Tse CM, Yun C, de Jonge HR, Donowitz M, cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein, J. Biol. Chem 280 (2005) 16642–16650. [DOI] [PubMed] [Google Scholar]
- [41].Chen T, Kocinsky HS, Cha B, Murtazina R, Yang J, Tse CM, Singh V, Cole R, Aronson PS, de Jonge H, Sarker R, Donowitz M, Cyclic GMP kinase II (cGKII) inhibits NHE3 by altering its trafficking and phosphorylating NHE3 at three required sites: identification of a multifunctional phosphorylation site, J. Biol. Chem 290 (2015) 1952–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gill RK, Saksena S, Syed IA, Tyagi S, Alrefai WA, Malakooti J, Ramaswamy K, Dudeja PK, Regulation of NHE3 by nitric oxide in Caco-2 cells, Am. J. Physiol. Gastrointest. Liver Physiol 283 (2002) G747–G756. [DOI] [PubMed] [Google Scholar]