Abstract
Bacterial infection commonly complicates inflammatory airway diseases such as chronic obstructive pulmonary disease (COPD). The mechanisms of increased infection susceptibility and how use of the commonly prescribed therapy inhaled corticosteroids (ICS) accentuates pneumonia risk in COPD is poorly understood. Here, using analysis of samples from patients with COPD, we show that ICS use is associated with lung microbiota disruption leading to proliferation of Streptococcal genera, an effect that could be recapitulated in ICS-treated mice. To study mechanisms underlying this effect, we utilized human and mouse models of Streptococcal expansion with Streptococcus pneumoniae, an important pathogen in COPD, to demonstrate that ICS impairs pulmonary clearance of bacteria through suppression of the anti-microbial peptide cathelicidin. ICS impairment of pulmonary immunity was dependent on suppression of cathelicidin as ICS had no effect on bacterial loads in mice lacking cathelicidin (Camp-/-) and exogenous cathelicidin prevented ICS-mediated expansion of Streptococci within the microbiota and improved bacterial clearance. Suppression of pulmonary immunity by ICS was mediated by augmentation of the protease cathepsin D. Collectively, these data suggest a central role for cathepsin D/cathelicidin in suppression of anti-bacterial host-defence by ICS in COPD. Therapeutic restoration of cathelicidin to boost antibacterial immunity and beneficially modulate the lung microbiota might be an effective strategy in COPD.
Introduction
Bacterial infection is a major complication of chronic obstructive pulmonary disease (COPD), contributing to airway colonisation, exacerbations and pneumonia(1–3). These sequelae of events are responsible for a large burden of morbidity and mortality associated with the disease(1, 4–7). Anti-bacterial immunity is likely to be impaired in COPD and recent studies have raised concern that commonly used therapies, inhaled corticosteroids (ICS), can further weaken host-defence increasing pneumonia risk.(8–10) Mechanisms of susceptibility to bacterial infection in COPD and how ICS use accentuates this risk remain poorly understood.
Historically, our insight into roles of bacteria in COPD was based on studies using classical microbial culture techniques(11, 12). Modern understanding of the importance of bacteria in COPD has been completely revised by culture-independent techniques that have shed light on the existence of a respiratory tract microbiota containing complex communities that are altered in disease states(13–15). Specific factors that lead to alterations in microbiota composition in COPD have not been elucidated and knowledge of how underlying disease, therapies and microbiota interact to promote infection is limited.
The critical immune effectors that that are compromized in COPD and impaired by ICS are poorly defined. Anti-microbial peptides (AMPs) and surfactant proteins are major sentinels of pulmonary innate immunity which have, in some studies, been shown to be reduced in the airways of COPD patients or smokers(16–20) and could thus represent a disease-specific impaired anti-bacterial mechanism. AMPs can act as critical regulators of the microbiota at other mucosal surfaces (21–23). ICS are broad anti-inflammatory agents and are capable of suppressing the production of host-defence proteins(24–27) and therefore could promote bacterial infection through lung microbiota disruption.
Here, we demonstrated that ICS alter the resident lower respiratory microbiota promoting proliferation of Streptococcal genera and impair pulmonary bacterial control in human and mouse models of Streptococcus pneumoniae infection, effects that occur through suppression of the AMP cathelicidin. We identified a mechanism for impairment of cathelicidin responses by ICS in COPD through augmentation of the protease cathepsin D. Deficient cathelicidin responses are a component of lung anti-bacterial host-defence that is impaired by ICS use and may contribute to the increased pneumonia risk associated with use of these agents in COPD.
Results
Inhaled corticosteroids alter the resident lung microbiota promoting proliferation of Streptococcal genera
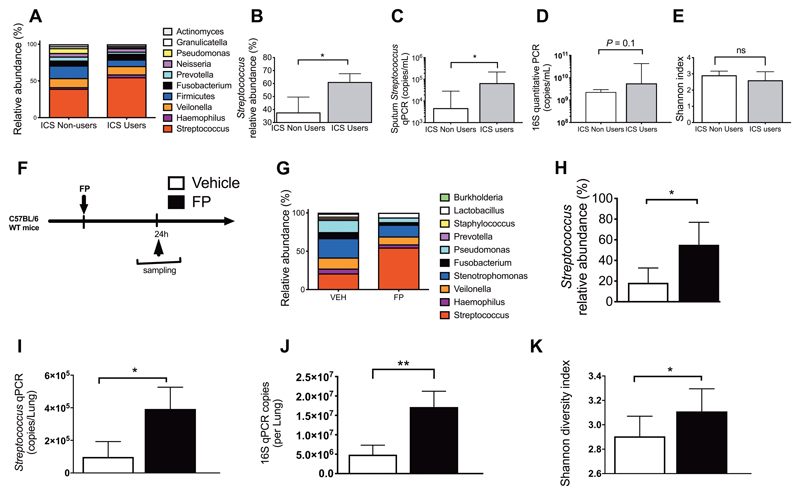
Clinical studies demonstrate that ICS use increases pneumonia risk in COPD(8, 9) but underlying mechanisms have not been elucidated. Because of its immunosuppressive effects, ICS could theoretically promote pneumonia by inducing bacterial proliferation within the existing lung microbiota. We therefore evaluated ICS effects on lung microbiota composition using 16S rRNA sequencing. We initially analyzed sputum samples from 23 patients with COPD during clinical stability, stratified according to non-use (n=13) or current use of ICS (n=10). Clinical characteristics of these patients are shown in Table 1. ICS-users showed a significant increase in relative abundance of Streptococcal genera compared to ICS non-users (P<0.05; Fig 1A and B). We confirmed these findings using quantitative (q)PCR specific for Streptococcus (P<0.05;Fig. 1C). Since ICS-users were significantly older than non-users in this cohort (P=0.013; Table 1), we also evaluated whether age might be a confounder for the increased Streptococcal load observed but we found no correlation between age and Streptococcus qPCR copies (fig. s1). We observed no difference in overall bacterial loads measured by 16S qPCR in ICS users versus non-users (P=0.1;Fig. 1D) and no difference in bacterial diversity (Fig. 1E). Given that cause and effect cannot be inferred from a cross-sectional human study, we evaluated whether experimental intranasal administration of the ICS fluticasone propionate (FP) in mice (Fig. 1F) at a dose previously shown to induce lung glucocorticoid receptor activation(27) caused a similar increase in Streptococci. 16S rRNA sequencing demonstrated that FP increased relative abundance of Streptococcal genera at 24 hours (P<0.05; Fig. 1G and H) and these findings were again confirmed using Streptococcus qPCR(Fig. 1I). FP also increased lung 16S bacterial loads (P<0.01; Fig. 1J) and bacterial diversity (P<0.05; Fig. 1K). These results indicate that ICS treatment promotes expansion of a genus that contains bacteria that are a major cause of infection in patients with COPD(1, 2, 28) and that these effects could be recapitulated in mice.
Table 1. Clinical characteristics of COPD patients included in analyses in figure 1 and 3c.
Data expressed as n(%) or median (IQR) and compared by Fisher’s exact test or Mann Whitney U test. Abbreviations: BODE =score comprising parameters of body Mass index, airflow obstruction, dyspnea and exercise; FEV1 = forced expiratory volume in 1 second; GOLD = Global Initiative for chronic obstructive lung disease; ICS = inhaled corticosteroid
| ICS Users (n=10) | ICS Non Users (n=13) | p value | |
|---|---|---|---|
| Age | 74 (69-78) | 68 (61-69) | 0.013 |
| Male sex | 7 (70%) | 9 (69.2%) | 1.0 |
| GOLD stage I/II/III/IV | 2 (1.8-2) | 2 (1.5-2.5) | 0.91 |
| FEV1 % predicted | 65 (53.0-80.3) | 67 (55.5-78.5) | 0.77 |
| BODE index | 4 (1.0-5.3) | 1 (0.5-3.0) | 0.11 |
| Long term oxygen therapy | 1 (10.0%) | 0 (0%) | 0.43 |
| Body Mass Index | 26.5 (22.9-28.7) | 23.8 (21.9-26.9) | 0.50 |
| Current Smoking | 1 (10%) | 3 (23.1%) | 0.60 |
| Comorbidities | |||
| Diabetes Mellitus | 0 (0%) | 1 (7.7%) | 1.0 |
| Hypertension | 1 (10.0%) | 0 (0%) | 0.43 |
| Ischemic Heart Disease | 2 (20.0%) | 0 (0%) | 0.18 |
| Osteoporosis | 2 (20.0%) | 3 (23.1%) | 1.0 |
| Number of exacerbations in preceding year | 1 (0-3) | 1 (0-1) | 0.53 |
| Treatments | |||
| Fluticasone propionate | 5 (50%) | n/a | - |
| Budesonide | 4 (40%) | n/a | - |
| Beclomethasone dipropionate | 1 (10%) | n/a | - |
| Muscarinic antagonist | 6 (60.0%) | 3 (23.1%) | 0.10 |
| Short or Long-acting Beta2 agonist | 9 (90.0%) | 9 (69.2%) | 0.33 |
Figure 1. Inhaled corticosteroids alter the lower respiratory tract microbiota inducing proliferation of Streptococcus genera.
(A-E) Evaluation of the lung microbiota in sputum samples from patients with COPD (n=10 ICS users and n=13 ICS non-users). (a) Relative abundance of the top ten operational taxonomic units (OTUs) in ICS users and non-users. (B) Relative abundance of Streptococcus (C) Streptococcus qPCR copies (D) total bacterial loads assessed by 16S qPCR and (E) Shannon diversity index in sputum samples from ICS users versus non-users. (F) Experimental outline. C57BL/6 mice were treated intranasally with 20ug fluticasone propionate or vehicle control. Lung tissue was harvested at 24 hours post-administration and lung microbiota was evaluated by 16S rRNA sequencing. (G) Relative abundance of the top ten operational taxonomic units (OTUs) in FP and vehicle treated mice. (H) Relative abundance of Streptococcus (I) Streptococcus qPCR copies (J) total bacterial loads assessed by 16S qPCR and (K) Shannon diversity index in FP and vehicle control treated mice. In (f)-(k) experiments comprise n=6-8 mice/group. Data shown as median (+/- IQR) and analyzed using Mann Whitney U test. n.s. non-significant *p<0.05 **p<0.01 ***p<0.001.
FP impairs bacterial control in models of Streptococcal expansion with S. pneumoniae
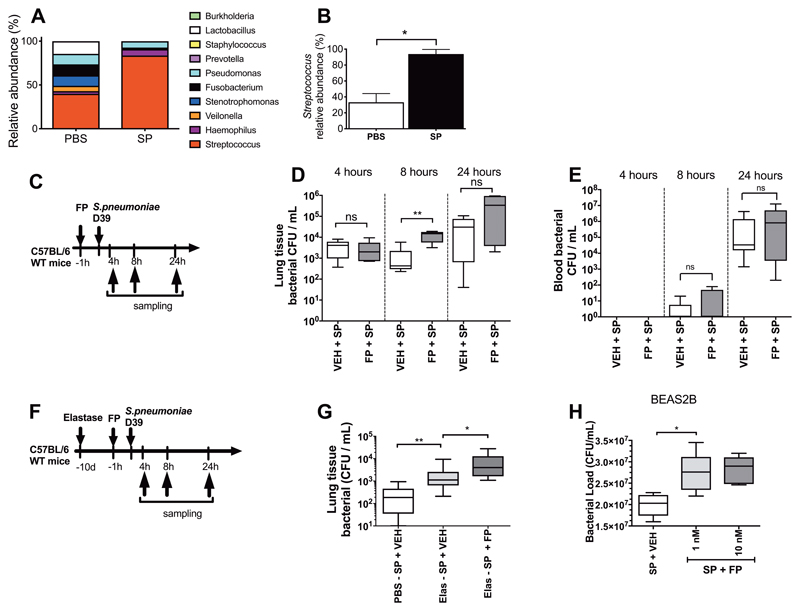
Having observed that ICS administration induces microbiota disruption in human and mouse models, we next sought to establish models of Streptococcal expansion with the COPD-relevant pathogen S. pneumoniae to understand how defects in pulmonary immunity associated with ICS use facilitate Streptococcal expansion. First, we demonstrated that intranasal S. pneumoniae infection in mice modelled Streptococcal lung expansion by confirming an increase in relative abundance of Streptococcus assessed by 16S rRNA sequencing compared to PBS-treated control mice (P<0.05; Fig. 2A and B). Then, we sought to investigate if FP promoted S. pneumoniae expansion. FP administration prior to S. pneumoniae challenge (Fig. 2C) increased lung bacterial loads assessed by quantitative culture (P<0.01; Fig. 2D). Despite increasing lung bacterial loads, FP had no effect on blood bacterial loads(Fig. 2E), suggesting that FP impairs local control of S. pneumoniae in the lungs without impacting upon systemic anti-bacterial defences.
Figure 2. Fluticasone propionate impairs pulmonary clearance of Streptococcus pneumoniae.
(A-B) Lung microbiota was evaluated by 16S rRNA sequencing in lung tissue from mice challenged with S. pneumoniae (SP) and PBS treated controls at 8 hours post-challenge. (A) Relative abundance of the top ten operational taxonomic units (OTUs) (B) Relative abundance of Streptococcus. (C) Experimental outline. C57BL/6 mice were treated with 20ug FP or vehicle DMSO control and additionally infected with S. pneumoniae D39. Bacterial loads in (D) lung tissue and (E) blood were measured at the indicated timepoints post-infection by quantitative culture. (F) Experimental outline. C57BL/6 mice were treated intranasally with a single dose of elastase or PBS as control. Ten days later, mice were treated intranasally with fluticasone propionate (20μg) or vehicle DMSO control and challenged with S. pneumoniae D39. (G) Bacterial loads were measured at 8 hours post-infection. (H) BEAS-2B cells were treated with 1 or 10nM fluticasone propionate, stimulated with S. pneumoniae D39 and bacterial loads were measured in cell supernatants by quantitative culture at 24 hours post-infection. Bacterial load data are displayed as box and whisker plots showing median (line within box), IQR (box) and minimum to maximum (whiskers). Experiments comprise n=6-8 mice/group, representative of at least two independent experiments. Data analyzed using Mann Whitney U test or one-way ANOVA with Bonferroni post-test. n.s. non-significant *p<0.05 **p<0.01 ***p<0.001.
These findings confirmed that FP could suppress pulmonary immunity and impair bacterial control in the healthy lung. However, in clinical practice ICS treatment is given in the context of chronic lung inflammation. We therefore next investigated whether FP treatment promoted S. pneumoniae expansion in this context and thus established a mouse model of elastase-induced emphysema combined with S. pneumoniae infection (Fig. 2F). We have previously reported that a similar model of rhinovirus-exacerbated elastase-induced emphysema recapitulated many features of human virus-associated exacerbation(29). Elastase-treated mice infected with S. pneumoniae had increased lung bacterial loads compared to mice given PBS prior to S. pneumoniae infection with further augmentation of bacterial loads observed with FP administration (P<0.01 and P<0.05 respectively; Fig. 2G). Consistent with effects of ICS administration in mouse models, we also observed that FP augmented bacterial loads in S.pneumoniae-infected human airway epithelial cell cultures (P<0.05; Fig. 2H). These observations confirmed that ICS administration can impair pulmonary clearance of S. pneumoniae, a species member of the Streptococcus genus and a frequent cause of pneumonia in COPD(3).
Airway cathelicidin concentrations are reduced in COPD and suppressed by ICS during bacterial infection
We next sought to investigate mechanisms whereby ICS promote pneumonia in COPD. We focused on AMPs because studies have shown reduced expression in the airways of COPD patients and smokers(16–19). Additionally, our data indicated rapid effects of FP in murine models, suggesting that the drug interferes with an innate component of the immune response.
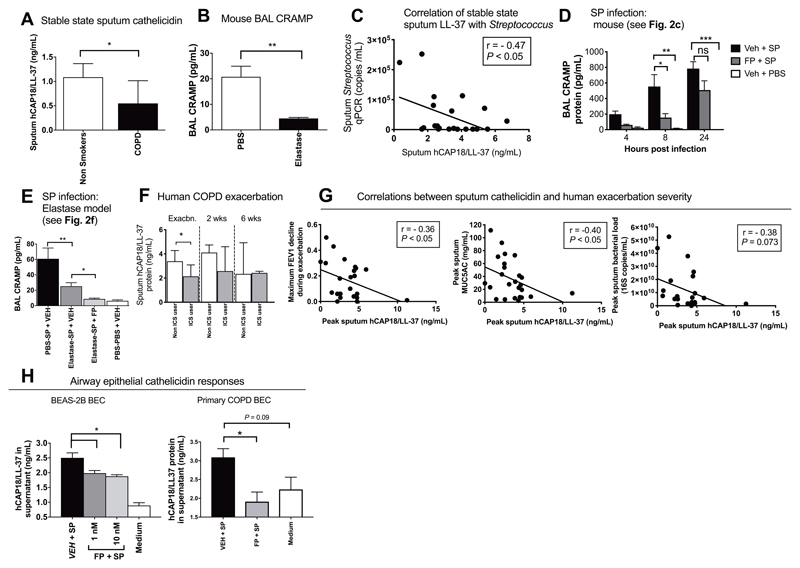
We initially studied expression of AMPs in baseline sputum samples from a cohort of patients with mild to moderate severity COPD (GOLD stage 0-2)(clinical details shown in table s1). We found that the human hCAP18/LL-37 protein was reduced in COPD patients (n=37) versus healthy non-smokers (n=19) (P<0.05; Fig. 3A) but no differences were observed in concentrations of other AMPs/surfactant proteins including α-defensin1-3, secretory leucocyte protease inhibitor (SLPI), surfactant protein-D or mannose-binding lectin 2 (fig. S2a-d). We additionally observed no differences in bronchoalveolar lavage(BAL) hCAP18/LL-37 protein concentrations in a sub-group of COPD patients (n=15) versus healthy non-smokers (n=10)(P=0.11; fig S3)(Clinical details shown in Table S2). Consistent with reduced airway cathelicidin in human COPD, we observed that experimental induction of COPD-like disease using elastase administration in mice also led to reduced baseline concentrations of the ortholog cathelicidin-related anti-microbial peptide (CRAMP) compared to PBS-treated control mice (P<0.01; Fig. 3B).
Figure 3. Cathelicidin responses to bacterial infection are impaired by inhaled corticosteroid and negatively correlate with COPD exacerbation severity.
(A) Stable state sputum hCAP18/LL-37 concentrations were measured in 37 subjects with COPD (GOLD stage 0-II) and 19 healthy control subjects by ELISA. (B) Cathelicidin-related anti-microbial peptide (CRAMP) concentrations were measured in mice at 10 days following intranasal treatment with 1.2 units of porcine pancreatic elastase or PBS control. (C) Correlation between sputum hCAP18/LL-37 and Streptococcal qPCR copies in 23 subjects with COPD. (D) C57BL/6 mice were treated intranasally with fluticasone propionate (20μg) or vehicle DMSO control and challenged intranasally with S. pneumoniae D39. CRAMP concentrations in BAL were measured by ELISA at the indicated timepoints. (E) C57BL/6 mice were treated intranasally with porcine pancreatic elastase or PBS control. Ten days later, mice were treated intranasally with fluticasone propionate, challenged with S.pneumoniae D39 and CRAMP concentrations in BAL measured at 8 hours post-infection. (F) Subjects with COPD (n=27) were monitored prospectively and sputum samples taken during exacerbation. Sputum hCAP18/LL-37 concentrations were measured by ELISA at the indicated timepoints. (G) Correlation between sputum hCAP18/LL-37 and FEV1 decline, sputum MUC5AC concentrations and sputum bacterial loads. (H, left) BEAS-2B cells were treated with 1 or 10nM fluticasone propionate, stimulated with S. pneumoniae D39 and hCAP18/LL-37 concentrations in cell supernatants were measured at 8 hours by ELISA. (h, right) Primary bronchial epithelial cells from 6 subjects with COPD were cultured, treated with 10nM FP, stimulated ex vivo with S. pneumoniae and hCAP18/LL-37 concentrations in cell supernatants were measured at 8 hours. In panels (B), (D), (E) and (H) data shown as mean (+/- sem) and analyzed by one-way ANOVA with Bonferroni’s post-test. For human sputum analyses in (A) and (F) data are shown as median (IQR) and analyzed by Mann Whitney U test. In (C) and (G), correlation analysis used was nonparametric (Spearman’s correlation). Animal experiments comprise n=5-8 mice/group, representative of at least two independent experiments. BEAS2B experiments comprise n=4 independent experiments. n.s. non-significant *p<0.05 **p<0.01 ***p<0.001
To determine whether cathelicidin expression is related to bacterial control in COPD airways, we examined relationships between sputum hCAP18/LL-37 concentrations and presence of Streptococcus assessed by qPCR in stable-state samples from the COPD subjects used to evaluate the microbiome (n=23, clinical characteristics are shown in Table 1). There was a significant negative correlation between sputum hCAP18/LL37 and Streptococcus qPCR copies (P<0.05; Fig. 3C).
Having observed that stable-state airway cathelicidin concentrations are reduced in COPD, we next determined whether ICS suppress this AMP. FP administration suppressed early CRAMP induction by S. pneumoniae in mice at 8 hours post-infection (P<0.05; Fig. 3D) but had no effect on S. pneumoniae induction of other AMPs β-defensin 2, mannose-binding lectin 2, lactoferrin or SLPI (fig. S4a-e). FP also suppressed neutrophils at 24 hours post-infection (P<0.01; fig. S4f). In the elastase COPD mouse model, induction of CRAMP by S. pneumoniae was deficient in elastase- versus PBS-treated mice and further impaired by FP administration (P<0.01 and P<0.05 respectively; Fig. 3E). Elastase-treated mice infected with S. pneumoniae (fig. S5A) also had deficient induction of pro-inflammatory cytokines IL-6 (P<0.05; fig S5B), TNF (P<0.001; fig S5c) and IL-1β in BAL (P<0.05; fig. S5D) compared to PBS-treated mice. Conversely, elastase-treated mice had increased cellular airway inflammation (P<0.001; BAL total cells, fig. S5E) in response to S. pneumoniae infection. FP administration suppressed IL-6 concentrations (P<0.05; fig S5B) and BAL total (P<0.001; fig S5E) and neutrophil cell counts (P<0.05; fig S5F) but had no effect on TNF or IL-1β concentrations (fig. S5C and D).
To confirm the clinical relevance of our findings, we measured sputum cathelicidin concentrations in a cohort of patients reporting COPD exacerbations (30)(with pathogen detection at exacerbation as follows: virus alone n=14, bacteria alone n=4; virus/bacteria co-infection n=4; no pathogen identified n=5). Patients were stratified according to current use (n=11) or non-use (n=16) of ICS with samples assessed during exacerbation (at onset and 2 weeks) and at resolution (6 weeks). Clinical characteristics of exacerbating patients are shown in Table S3. In keeping with findings in animal models, ICS users had suppressed sputum supernatant concentrations of hCAP18/LL-37 versus ICS non-users at exacerbation onset (P<0.01; Fig. 3F) but not at 2 and 6 weeks post-exacerbation. To further investigate the clinical importance of cathelicidin during exacerbation, we examined relationships between sputum hCAP18/LL-37 and markers of exacerbation severity. Sputum hCAP18/LL-37 correlated negatively with peak acute FEV1 decline from baseline and additionally with sputum concentrations of the mucin glycoprotein MUC5AC (P<0.05 respectively; Fig. 3G). There was no correlation between sputum hCAP18/LL-37 and 16S qPCR bacterial loads during exacerbation (P= 0.073; Fig. 3G).
Combined, these data confirm that ICS use impairs cathelicidin production in vivo and additionally suggest that cathelicidin might be an important determinant of bacterial clearance and clinical severity during exacerbations.
ICS exert inhibitory effects on cathelicidin responses at the bronchial epithelium
We next sought to understand how ICS use dampens lung Streptococcal defences. Cathelicidin can be produced by the bronchial epithelium(31) and also by airway inflammatory cells, particularly neutrophils(32). Given our observations that FP-mediated impairment in bacterial clearance occurred at 8 hours (P<0.01; Fig. 2D), an earlier timepoint than airway neutrophil recruitment occurred in S. pneumoniae-infected mice (24 hours, fig. S4F) and since FP could induce microbiota disruption in unchallenged mice, where neutrophil recruitment does not occur, we reasoned that FP was not acting through effects on neutrophil-produced cathelicidin in our models. We hypothesised that ICS mediates its inhibitory effects on early cathelicidin production by the pulmonary epithelium. BEAS2B bronchial epithelial cells treated with FP prior to S. pneumoniae infection had reduced hCAP18/LL-37 production compared to non-FP treated controls infected with S. pneumoniae (P<0.05; Fig. 3H). Western blot analysis confirmed that cathelicidin is secreted in the uncleaved form (18kDa) in airway epithelial cell culture supernatants (Fig S6A), as previously reported(33). We observed no induction of hCAP18/LL-37 by heat-killed S. pneumoniae or individual agonists for the major pneumococcal pattern recognition receptors(PRR) TLR-2, TLR-9 or NOD-2 in epithelial cell cultures, indicating that viable pneumococci are required to stimulate cathelicidin production in this experimental system (Fig. S6B). FP had no effect on the induction of surfactant protein-D, β defensin-2 or mannose-binding lectin-2 (Fig S7A to C). Other AMPs evaluated including α-defensin1-3, SLPI and elafin were not detectable in cell supernatants.
In keeping with the observed effect in an airway epithelial cell line, FP also impaired ex vivo production of hCAP18/LL-37 by primary bronchial epithelial cells from patients with COPD (P<0.05; Fig. 3H)(clinical characteristics are shown table S4). This confirms that ICS can impair cathelicidin responses in cells taken directly from COPD patients and indicates that these effects are likely to be important clinically.
Impairment of pulmonary immunity by FP is dependent on suppression of cathelicidin
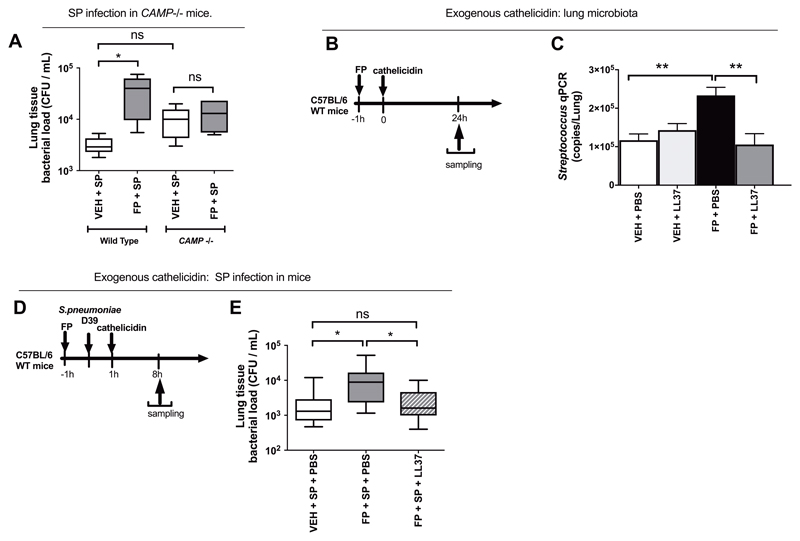
To confirm the functional importance of FP-mediated suppression of cathelicidin, we examined whether FP had effects on lung bacterial control in mice with gene-targeted deletion of cathelicidin (Camp-/-). In contrast to wild-type mice, FP administration had no effect on bacterial loads in Camp-/- mice, thereby confirming that FP suppression of cathelicidin plays a major role in effects on bacterial control (Fig. 4A). We additionally found that exogenous replacement of cathelicidin using recombinant protein administration (Fig. 4B) restored the disrupted lung microbiota associated with FP administration by reversing FP-mediated increases in Streptococcus bacterial load in mice (P<0.01; Fig. 4C). Exogenous cathelicidin administration in FP-treated S. pneumoniae-infected mice (Fig. 4D) also significantly reduced bacterial loads (P<0.05; Fig. 4E) without affecting suppression of BAL neutrophil chemokines CXCL2/MIP-2 or CXCL1/KC (Fig. S8A to C) or pro-inflammatory cytokines IL-6, TNF or IL1β, (Fig. S8D to F). These experiments confirmed that cathelicidin is both necessary and sufficient for ICS to mediate effects on lung bacterial control in vivo.
Figure 4. Impairment of pulmonary immunity by fluticasone propionate is dependent on cathelicidin.
(A) Wild type or CAMP -/- C57BL/6 mice were treated with 20ug fluticasone propionate or vehicle DMSO control and challenged intranasally with S. pneumoniae D39. Lung bacterial loads were measured by quantitative culture at 8h post-infection (B) Experimental outline. C57BL/6 mice were treated intranasally with 20ug fluticasone propionate or vehicle control and additionally with 10 ug recombinant LL-37. Lung tissue was harvested at 24h post-administration. (C) Lung Streptococcus was measured by qPCR (D) Experimental outline. C57BL/6 mice were treated intranasally with 20ug fluticasone propionate or vehicle DMSO control, challenged with S. pneumoniae D39 and additionally treated with 10 ug recombinant LL-37. Lung tissue was harvested at 8h post-administration. (E) Lung bacterial loads measured by quantitative culture. Data in (C) shown as mean (+/- S.E.M). Bacterial load data displayed as box and whisker plots showing median (line within box), IQR (box) and minimum to maximum (whiskers). Animal experiments comprise n=5-10 mice/group, representative of at least two independent experiments. Data analyzed by one-way ANOVA with Bonferroni post-test. n.s. non-significant *p<0.05 **p<0.01.
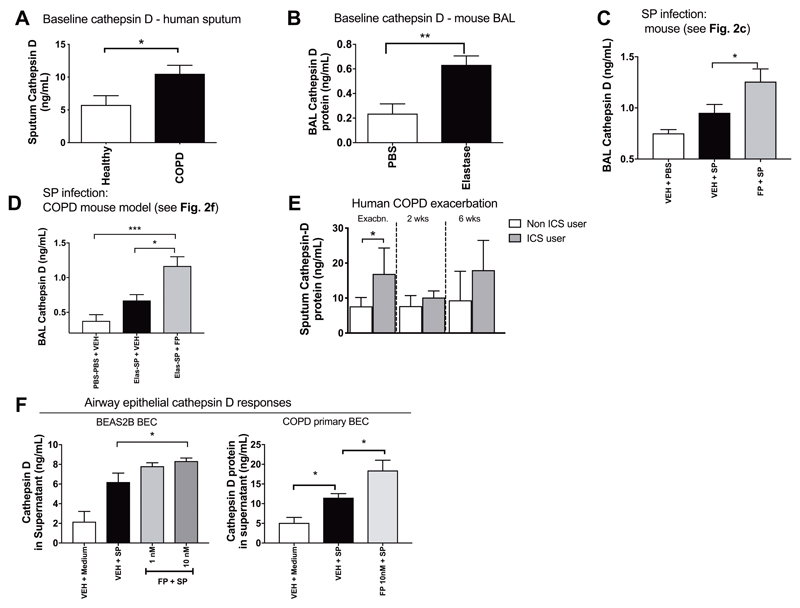
Airway concentrations of the protease Cathepsin D are enhanced in COPD and further augmented by ICS during bacterial infection
Next, we sought to understand how FP reduces airway cathelicidin. Previous studies have suggested that proteolytic cleavage by enzymes such as neutrophil elastase and cathepsin D may contribute to LL-37 degradation in the airway(34, 35). Given that airway concentrations of neutrophil elastase and cathepsin D are known to be augmented in COPD(36, 37), we hypothesized that elevations in these enzymes may drive the reduced cathelicidin observed in our models. We observed suppression rather than enhancement of neutrophil elastase by FP in the mouse S. pneumoniae infection model (P<0.05; Fig. S9), suggesting that neutrophil elastase-mediated cleavage of cathelicidin is unlikely to account for the reduced concentrations observed with ICS administration in COPD.
Cathepsin D concentrations were significantly increased in sputum from COPD subjects versus healthy subjects at clinical stability (P<0.05; Fig. 5A). Characteristics of patients included in this analysis are shown in Table S1. Mice treated with elastase to induce COPD-like disease also had increased BAL concentrations of cathepsin D (P<0.01; Fig. 5B). FP administration augmented BAL cathepsin D concentrations in S. pneumoniae-infected mice (P<0.05; Fig. 5C) and also increased BAL cathepsin D concentrations in elastase-treated S. pneumoniae-infected mice (P<0.05; Fig. 5D). To further investigate relationships between ICS treatment and cathepsin D during COPD exacerbations, we measured sputum concentrations from the COPD exacerbation cohort (see Table S3). ICS users had increased sputum supernatant cathepsin D concentrations versus ICS non-users at exacerbation onset (P<0.05; Fig. 5E).
Figure 5. Airway cathepsin D is increased in COPD and further enhanced by inhaled corticosteroid administration during bacterial infection.
(A) Stable state sputum cathepsin D concentrations were measured in 37 subjects with COPD (GOLD stage 0-II) and 19 healthy control subjects by ELISA. (B) Cathepsin D concentrations were measured in mice at 10 days following intranasal treatment with 1.2 units of porcine pancreatic elastase or PBS control. (C) C57BL/6 mice were treated intranasally with fluticasone propionate (20μg) or vehicle DMSO control and challenged with S. pneumoniae D39. Cathepsin D concentrations in BAL were measured by ELISA at 8 hours post-infection. (D) C57BL/6 mice were treated intranasally with porcine pancreatic elastase or PBS control. Ten days later, mice were treated intranasally with fluticasone propionate, challenged with S.pneumoniae D39 and cathepsin D concentrations in BAL measured at 8 hours post-infection. (E) Subjects with COPD (n=27) were monitored prospectively and sputum samples taken during exacerbation. Sputum cathepsin-D concentrations were measured by ELISA at the indicated timepoints. (F, left) BEAS-2B cells were treated with 1 or 10nM fluticasone propionate, stimulated with S. pneumoniae D39 and cathepsin D concentrations in cell supernatants were measured at 8 hours. (F, right) Primary airway epithelial cells from 6 subjects with COPD were cultured, treated with 10nM FP, stimulated ex vivo with S. pneumoniae and cathepsin D concentrations in cell supernatants were measured at 8 hours. In panels (B)-(D) and (F) data shown as mean (+/- sem) and analyzed by one-way ANOVA with Bonferroni’s post-test. For human sputum analyses in (A) and (E), data are shown as median (IQR) and analyzed by Mann Whitney U test. Animal experiments comprise n=6-8 mice/group, representative of at least two independent experiments. n.s. non-significant *p<0.05 **p<0.01 ***p<0.001.
Given our prior observations that FP exerts inhibitory effects on cathelicidin production by the bronchial epithelium, we evaluated FP effects on cathepsin D production by epithelial cell cultures following S. pneumoniae infection. FP administration augmented cathepsin D protein induction by S. pneumoniae in BEAS2B airway epithelial cells (P<0.05; Fig. 5F). Similar effects occurred in COPD cells with augmentation of ex vivo S. pneumoniae induction of cathepsin D by FP (P<0.05; Fig .5F). Further evidence that FP exerts inhibitory effects on cathelicidin through increased cathepsin-D mediated degradation rather than impairment of gene transcription was demonstrated by lack of an effect of FP administration on S. pneumoniae upregulation of CAMP mRNA in airway epithelial cells (fig S10). Combined, these observations indicate that cathepsin D, a known negative regulator of cathelicidin, is enhanced in COPD and augmented by ICS during bacterial lung infection.
Exogenous Cathepsin D protein administration attenuates S. pneumoniae induction of cathelicidin in airway epithelial cells
To further confirm that cathepsin D can degrade cathelicidin released by the airway epithelium in response to bacterial infection, we administered recombinant cathepsin D protein in S. pneumoniae treated airway epithelial cells. Cathepsin D administration had no effects in the absence of infection but significantly attenuated hCAP18/LL-37 induction in response to S. pneumoniae infection in BEAS2B airway epithelial cell cultures (P<0.05; fig S11).
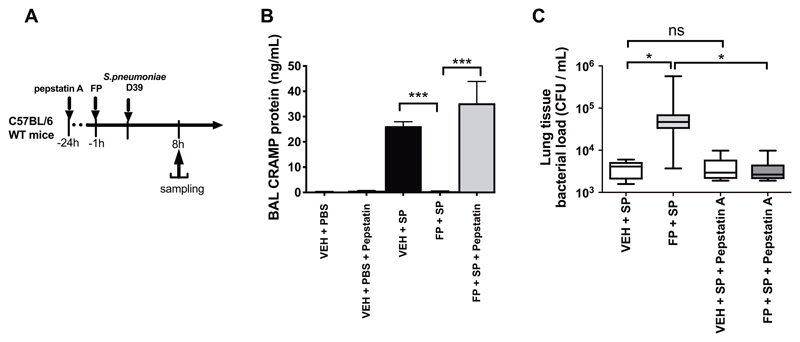
Inhibition of cathepsin D reverses suppressed cathelicidin and restores impaired bacterial control associated with FP administration
To confirm that cathepsin D contributes to impaired cathelicidin responses associated with ICS administration in COPD, we assessed the effect of inhibiting its action during S. pneumoniae infection using the lysosomal protease inhibitor pepstatin-A. Pepstatin-A administration prior to S. pneumoniae infection in mice (Fig. 6A) reversed FP-suppression of BAL CRAMP protein at 8 hours post-infection (P<0.001; Fig. 6B) with a concomitant reduction in FP-mediated increases in bacterial loads (P<0.05;Fig. 6C). These observations indicate that FP regulates cathelicidin through effects on cathepsin D and suggest that enhanced cathepsin D plays a mechanistic role in the suppression of pulmonary immunity associated with ICS use in COPD.
Figure 6. Inhibition of cathepsin D reverses FP-mediated suppression of cathelicidin and restores lung bacterial control.
(A) Experimental outline. C57BL/6 mice were treated with 60mg/kg intraperitoneal pepstatin-A, 24 hours prior to intranasal treatment with 20μg fluticasone propionate or vehicle control and challenge with S. pneumoniae D39. (B) CRAMP concentrations in BAL were measured by ELISA at 8h post-infection. (C) Lung bacterial loads were measured by quantitative culture at 8 hours post-infection. Data in (B) shown as mean (+/- S.E.M). Bacterial load data in (C) displayed as box and whisker plots showing median (line within box), IQR (box) and minimum to maximum (whiskers). Animal experiments comprise n=8-10 mice/group, representative of at least two independent experiments. Data analyzed by one-way ANOVA with Bonferroni post-test. n.s. non-significant *p<0.05 **p<0.01 ***p<0.001.
Discussion
The underlying mechanisms involved in susceptibility to bacterial infection in COPD and how the disease, treatment and microbiota interact to promote exacerbations and pneumonia represents a crucial research question in the field. Our study fits with an emerging conceptual framework whereby ICS use contributes to impaired anti-bacterial host-defence in COPD through deleterious effects on innate immunity. Here, we provide mechanistic insight into how patients with COPD treated with ICS are at increased risk of bacterial infection. Using a combination of mouse and human COPD models, we identify that ICS-mediated suppression of cathelicidin drives expansion of Streptococci within the microbiota and that cathelicidin is necessary and sufficient for ICS impairment of bacterial control. Our studies indicate a mechanism for impairment of cathelicidins by ICS through augmentation of the protease cathepsin D. Using human studies, we additionally demonstrate that lower cathelicidin concentrations are associated with increased clinical severity during COPD exacerbations.
Molecular culture-independent techniques have revealed the existence of a lower respiratory tract microbiome consisting of complex bacterial communities(13) which are altered in chronic respiratory diseases such as COPD(14, 15). The COPD microbiota varies according to the population studied but is broadly characterised by an outgrowth of Proteobacteria phylum and an increase in Streptococci and Staphylococci within Firmicutes phylum(13–15). Microbiota shifts including increases in Streptococcus occur during COPD exacerbations (38). Our data indicate that ICS may further accentuate expansion of a bacterial genus that is already increased within the COPD microbiota. A previous clinical trial similarly reported increased Firmicutes following 12 months ICS treatment in COPD(39) and another study showed that systemic corticosteroid administration in mice can perturb the intestinal microbiota with expansions in disease-relevant genera(40).
Within the Streptococcus genus, S. pneumoniae is commonly implicated in colonization, exacerbations and pneumonia in COPD(1, 2, 28). COPD is, additionally, a risk factor for severe pneumonia and invasive pneumococcal disease(41, 42). Using mouse models of Streptococcal expansion with S. pneumoniae, we demonstrate that ICS further impairs pulmonary bacterial clearance in vivo. Our findings confirm those of previous studies showing impaired clearance of Klebsiella pneumoniae(43) and Pseudomonas aeruginosa(25) with ICS administration in mice and advance our understanding by indicating that ICS-mediated effects on bacterial control also, importantly, occur with pneumococcal infection in COPD and are thus likely to be of relevance to the reported clinical pneumonia signal.
Mechanisms of susceptibility to bacterial infection in COPD are poorly understood and a number of contributory abnormalities in pulmonary defences have been postulated including altered PRRs expression(44–46), immune cell dysfunction(47, 48) and mucociliary impairment(49, 50). However, many of these defects are unaffected or corrected, rather than worsened, by corticosteroids(43, 47, 51–53) and therefore do not provide adequate explanation for the increased pneumonia risk shown in clinical studies for a range of ICS agents(8, 9, 54–56). We focused on AMPs and surfactant proteins, soluble molecules present in the airway that form an important first-line of defence against pathogens. These mediators have been shown to be reduced in airway samples from COPD patients or smokers(16–20) and are also susceptible to corticosteroid suppressive effects(24–27). We found that concentrations of the AMP cathelicidin were reduced in COPD airways, that FP administration impairs cathelicidin induction by S. pneumoniae in experimental models and that ICS users have reduced cathelicidin at time of COPD exacerbation. Therefore, in contrast to a range of other AMPs/surfactant proteins shown here to be unaffected by FP, cathelicidin is suppressed by ICS use in COPD. Studies have similarly reported corticosteroid impairment of cathelicidin induction by vitamin D3 in vitro(24) and to Pseudomonas aeruginosa infection in mice(25) and we again extend these prior observations to confirm that similar effects specifically occur in the context of S. pneumoniae infection in COPD.
Using loss and gain of function studies in mice, we show that impairment of pulmonary immunity by FP is dependent on cathelicidin suppression, thereby demonstrating a major mechanistic role in ICS-related pneumonia in COPD. There is interest in the potential to therapeutically manipulate the microbiome and influence lung immunity. We show here that an AMP applied exogenously to boost anti-bacterial immunity can beneficially modulate the lung microbiota and, theoretically, reduce risk of a therapy-associated infective complication. Recombinant LL-37 administration has been shown to alter gut microbiota in mice(21) and systemic cathelicidin concentrations correlate with microbiota composition in infants hospitalised with bronchiolitis(57). Combined with our findings, this highlights an emerging role for cathelicidins in microbiota regulation. Cathelicidins have wide ranging anti-bacterial effector functions including bacterial killing, modulation of PRR-mediated responses(58) and inflammatory cell chemotaxis(59). These functions could all theoretically affect microbiota composition. Cathelicidins also have host-defence roles against other pathogens including Klebsiella(60), Pseudomonas(61), respiratory syncytial virus(62) and influenza(63), suggesting that the importance of this peptide may extend beyond its role in S. pneumoniae infection, to be involved in immunity to numerous other COPD-relevant infections.
Airway regulation of cathelicidins in COPD is likely to be complex; our findings are consistent with studies showing reduced cathelicidin concentrations in sputum from patients with severe COPD (GOLD stage III-IV) versus healthy individuals(16) and reduced serum concentrations in frequent exacerbators versus healthy individuals(64) but contradictory studies report increased airway hCAP18/LL-37 in COPD(65, 66). Reasons for discrepancies between existing studies is likely to be multifactorial with a number of confounders potentially affecting airway cathelicidin including ICS, inflammatory profile, microbiota composition and vitamin D concentrations. Our finding that sputum hCAP18/LL-37 negatively correlates with severity measures provides additional evidence that cathelicidin responses may be important during COPD exacerbations. Combined with the observation that exogenous LL-37 improves bacterial control, this implicates a central role for cathelicidin during COPD bacterial infections and raises speculation that therapies aimed at boosting cathelicidin responses might provide an effective strategy. Administration of bioengineered cathelicidin-secreting probiotic bacteria has been shown to protect against intestinal bacterial colonization in animal studies(67) and it is plausible that similar approaches could be effective in treating infections associated with chronic lung diseases. In the current study, we also observed that, in addition to cathelicidin, FP administration impaired other anti-bacterial responses to S. pneumoniae in the mouse model of elastase-induced COPD including BAL IL-6 concentrations and neutrophils, effects which may also contribute to increased infection susceptibility. Our finding that exogenous cathelicidin administration restores bacterial clearance without affecting FP-suppression of these other responses gives support to the relative importance of cathelicidin. In contrast to the suppressive effects of ICS on epithelial cathelicidin production reported here, other studies have also shown that corticosteroids can increase some host-defence mediators including lactotransferrin(68), CCL20(69) and surfactant protein-A(70). However, our finding that no effect of FP on bacterial loads was observed in Camp-deficient mice and that exogenous cathelicidin blocked effects of FP on bacterial loads suggests that cathelicidin suppression is more important than effects on other mediators.
We identified a mechanism for impairment of cathelicidin responses by FP in COPD through augmentation of cathepsin D, a lysosomal protease shown to be increased in COPD(37) and can inactivate AMPs such as cathelicidin through proteolytic cleavage(34, 35). Cathepsin D is known to be a steroid-inducible gene and glucocorticoids can increase expression and activity of this protease in a range of tissues.(71–73) Administration of pepstatin-A, an inhibitor of cathepsin D activity, reconstituted suppressed cathelicidin responses and reversed impaired bacterial control associated with FP administration in mice. Pepstatin-A has also previously been shown to reduce dissemination and mortality in a Candida albicans infection mouse model,(74) and this compound is also under investigation as a therapy for other diseases such as breast cancer(75). In contrast to our findings in COPD, cathepsin D expression has been shown to be unchanged in asthma(76). This may explain why the risk of pneumonia associated with ICS use in clinical studies has been shown to occur exclusively in COPD but not asthma(77, 78), where cathepsin D concentrations may be lower and thus have lesser inhibitory effects on cathelicidin and subsequent anti-bacterial immunity. Our data supports further investigation into development of cathepsin D inhibitors as potential therapeutic agents in COPD.
Calverley et al reported that ICS-related pneumonia episodes may occur either following a protracted symptomatic exacerbation or ‘de novo’ (44.8% and 55.2% of pneumonic episodes respectively)(79). We have recently reported that ICS can suppress innate anti-viral immune responses to rhinovirus infection leading subsequently to impaired production of the AMP SLPI and increased bacterial loads(27). These effects provide a mechanism to explain secondary pneumonias that follow an initial virus-induced exacerbation but since >50% of ICS-related pneumonic episodes may occur de novo, distinct mechanisms other than effects on virus-induced secondary bacterial infection may be involved. In the current study, we utilised direct experimental models of bacterial infection and, in contrast to effects seen in virus infection, we found no suppressive effect of FP on SLPI in these models but instead found that cathelicidin was the major AMP involved. We speculate that two distinct mechanisms of ICS-induced pneumonia may occur depending on whether or not a preceding virus infection is involved. Our data adds to the increasingly recognised importance of anti-microbial peptides in COPD anti-bacterial host defence and further suggest that ICS impairment of these peptides could be important clinically.
This study has some limitations. The effects of ICS on the resident lung microbiota were evaluated in a cross-sectional human study combined with analyses following direct administration of fluticasone in mice. Definitive evidence that ICS therapy can alter the lower respiratory tract microbiota in man would require sequential analyses before and after initiation of fluticasone in patients. Additionally, the in vivo functional effects of ICS on cathelicidin and pneumococcal clearance in COPD were demonstrated in our study using a mouse model of elastase-induced disease. We have previously reported that this model recapitulates features of human COPD exacerbation when combined with rhinovirus infection (29). However, neither elastase models nor other models such as smoke exposure in mice can completely recapitulate the complexities and heterogeneity of human disease. Given that cigarette smoking has previously been shown to affect the airway microbiota (80, 81), animal studies using smoke exposure models are required to confirm our findings.
In conclusion, our study identifies a central role for cathelicidin in COPD anti-bacterial host-defence and demonstrates that suppression by ICS mediates microbiota dysregulation and impaired pulmonary immunity. Therapeutic restoration of cathelicidin responses to enhance antibacterial host-defence, either using recombinant protein administration or through inhibition of negative regulator cathepsin D, might beneficially modulate the lung microbiota and improve bacterial control, providing an effective strategy for treating COPD.
Materials And Methods
Study design
The primary objective of this study was to define the effects of inhaled corticosteroids on the lung microbiota and pulmonary immune responses to bacterial infection in COPD. All animal experiments were performed under the authority of the UK Home Office outlined in the Animals (Scientific Procedures) Act 1986 after ethical review by Imperial College London Animal Welfare and Ethical Review Body (project licence PPL 70/7234). The number of animals in each treatment group was determined by power calculations based on extensive previous experience with the model systems and is shown in the respective figure legends. For analyses from human subjects, sample sizes were opportunistic and carried out using historical samples from previously conducted studies. Authors were blinded for analyses of hCAP-18/LL-37 and other immune mediators. All studies were ethically approved and are detailed in the relevant section within the methods. No samples or animals were excluded from any analyses.
Streptococcus pneumoniae infection and treatment of mice
Female C57BL/6 mice of 6-8 weeks age, purchased from Charles River Laboratories, were used for animal studies. Camp-/- mice of 6-8 weeks age on a C57BL/6 background were purchased from Jackson Laboratories. Mice were housed in individually ventilated cages under specific pathogen-free conditions.
FP powder (Sigma-Aldrich) was resuspended in DMSO at a concentration of 357μg/mL, followed by dilution 1:1000 in PBS. Mice were lightly anaesthetized with isofluorane then treated intranasally with 50 μl FP solution (equating to 20μg dose) or vehicle DMSO diluted 1:1000 in PBS as control. One hour following FP administration, mice were intranasally infected with 50 μl containing 5 x 105 CFU S. pneumoniae D39 or PBS control. In experiments to evaluate the effect of ICS administration on the lung microbiota in mice, FP was administered intranasally, as detailed above, without S. pneumoniae infection. In separate experiments, one hour following S. pneumoniae infection, mice were additionally treated intranasally with 50μl PBS containing 10μg recombinant human LL-37(Bio-Techne), a similar dose to that used previously in animal infection studies(61, 62). In additional experiments, mice were treated intraperitoneally with 60mg/kg pepstatin-A, a dose previously shown to inhibit cathepsin D activity in mouse lung(86), 24 hours prior to S. pneumoniae infection.
For evaluation of the effect of FP in a mouse model of COPD bacterial infection, mice were treated intranasally with 1.2 units of porcine pancreatic elastase (Merck) to induce emphysematous lung changes(29) and additionally treated intranasally with 20 μg FP, one hour prior to infection with 5 x 105 CFU S. pneumoniae D39 or PBS control.
Mice were culled at 4, 8 or 24h post administration of fluticasone and/or S. pneumoniae infection. BAL fluid, whole lung and blood were taken and prepared for analyses, as previously described(87).
The St. Mary’s Hospital naturally-occurring human COPD exacerbation cohort
A cohort of 40 subjects was recruited to a prospective study investigating naturally-occurring exacerbations in patients with COPD between June 2011 and December 2013, as previously reported(30). All subjects were confirmed to have COPD by spirometry and all treatments were permitted. A full medication history was taken at recruitment and subjects were classified as current ICS users or non-users. Subjects gave informed written consent and the study was approved by the East London Research Ethics Committee (Protocol number 11/LO/0229). All subjects had an initial baseline visit during clinical stability for medical assessment, peak expiratory flow rate measurement, spirometry (forced expiratory volume in 1 second (FEV 1), forced vital capacity (FVC) and clinical sample collection, including spontaneous or induced sputum, taken as previously described(36, 88).
Subjects had repeat visits at three monthly intervals when clinically stable and were followed up for a minimum of 6 months. Subjects reported to the study team when they developed symptoms of an acute exacerbation defined using the East London cohort criteria(5). Subjects were reviewed by the study team within 48 hours of symptom onset. Sputum samples were collected at the onset of exacerbation, at 2 weeks during and 6 weeks after exacerbation. Viruses were detected in sputum using polymerase chain reaction as described previously (36).
Statistical analysis
Experiments in mouse models involved 5-10 animals per treatment condition and data is presented as mean+/-SEM, representative of at least two independent experiments. In vitro experiments in BEAS-2B cells were performed 3-5 times and data were analyzed using one-way ANOVA with significant differences between groups assessed by Bonferroni’s multiple comparison test. In vitro primary airway epithelial cell experiments were performed on cells from n=6 patients and data were analyzed using one one-way ANOVA with significant differences between groups assessed by Bonferroni’s multiple comparison test. Data from the human samples is shown as median +/- IQR and analyzed using the Mann-Whitney U test. Correlations between datasets were examined using Spearman's rank correlation coefficient. All statistics were performed using GraphPad Prism 6 software. Differences were considered significant when p<0.05.
Supplementary Material
One Sentence Summary.
Inhaled corticosteroids promote bacterial infection in chronic obstructive pulmonary disease by suppressing the anti-microbial peptide cathelicidin.
Acknowledgements
We thank staff within the Imperial Clinical Respiratory Research Unit for their assistance with recruitment and sampling during the human exacerbation studies.
Funding
This work was supported by a Wellcome Trust Clinical Research Training Fellowship to AS [grant number WT096382AIA], a pump priming grant from the British Lung Foundation to AS [grant number PPRG15-9], a research grant from the British Medical Association to AS [grant ref: HC Roscoe 2015 grant] a National Insitute for Health Research (NIHR) Senior Investigator Award to SLJ, the NIHR Clinical Lecturer funding scheme (PM & JF) and funding from the Imperial College and NIHR Biomedical Research Centre (BRC) scheme. T.B.C. is a Sir Henry Dale Fellow jointly funded by the Wellcome Trust and Royal Society [Grant Number 107660/Z/15]).
Footnotes
Author contributions:
AS designed, conducted and interpreted all animal experiments, with input from NG, NWB and SLJ. AS performed the statistical analysis and prepared the manuscript. JF, MBT, MAC, PM and SLJ were instrumental in the design, recruitment and sample processing from the human COPD exacerbation studies. LJF, SVK, PF and JAW were instrumental in bronchoscopy studies to obtain primary airway epithelial cells. AS performed the in vitro experiments in collaboration with MRE, EB, LJF and PF. AS, in collaboration with LC, ET and PLJ, conducted quantitative PCR/16S rRNA sequencing work. TBC, MM, WOC provided key reagents and contributed discussions throughout the work.
Competing interests relevant to manuscript: S.L.J. has personally received consultancy fees from Myelo Therapeutics GmbH, Concert Pharmaceuticals, Bayer, and Sanofi Pasteur, and Aviragen; he and his institution received consultancy fees from Synairgen, Novartis, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Centocor. S.L.J. is an inventor on patents on the use of inhaled interferons for treatment of exacerbations of airway diseases (Interferon-beta therapy for anti-virus therapy for respiratory diseases. International Patent Application No. PCT/GB05/50031 and Interferon-Lambda therapy for treatment of respiratory disease UK Patent application No. 6779645.9). M.A.C. was employed by Chiesi Pharmaceuticals from January 2015 to November 2017. The remaining authors declare no competing interests.
References
- 1.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002 Sep;57:759. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. The New England journal of medicine. 2002 Aug 15;347:465. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Junyent J, Garcia-Vidal C, Viasus D, Millat-Martinez P, Simonetti A, Santos MS, Ardanuy C, Dorca J, Carratala J. Clinical features, etiology and outcomes of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. PloS one. 2014;9:e105854. doi: 10.1371/journal.pone.0105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1998 May;157:1418. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002 Oct;57:847. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Annals of the American Thoracic Society. 2013 Apr;10:81. doi: 10.1513/AnnalsATS.201208-043OC. [DOI] [PubMed] [Google Scholar]
- 7.Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012 Nov;67:970. doi: 10.1136/thoraxjnl-2012-202103. [DOI] [PubMed] [Google Scholar]
- 8.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. The New England journal of medicine. 2007 Feb;356:775. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 9.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. American journal of respiratory and critical care medicine. 2008 Jan 1;177:19. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 10.Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2007 Jan 15;175:144. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 11.Rosell A, Monso E, Soler N, Torres F, Angrill J, Riise G, Zalacain R, Morera J, Torres A. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Archives of internal medicine. 2005 Apr 25;165:891. doi: 10.1001/archinte.165.8.891. [DOI] [PubMed] [Google Scholar]
- 12.Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2006 May 1;173:991. doi: 10.1164/rccm.200509-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, et al. Disordered microbial communities in asthmatic airways. PloS one. 2010 Jan 05;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW, Hogg JC. The lung tissue microbiome in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2012 May 15;185:1073. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pragman AA, Lyu T, Baller JA, Gould TJ, Kelly RF, Reilly CS, Isaacson RE, Wendt CH. The lung tissue microbiota of mild and moderate chronic obstructive pulmonary disease. Microbiome. 2018 Jan 9;6:7. doi: 10.1186/s40168-017-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golec M, Reichel C, Lemieszek M, Mackiewicz B, Buczkowski J, Sitkowska J, Skorska C, Dutkiewicz J, Milanowski J, Ziesche R. Cathelicidin LL-37 in bronchoalveolar lavage and epithelial lining fluids from COPD patients and healthy individuals. J Biol Regul Homeost Agents. 2012 Oct-Dec;26:617. [PubMed] [Google Scholar]
- 17.Sallenave JM. The role of secretory leukocyte proteinase inhibitor and elafin (elastase-specific inhibitor/skin-derived antileukoprotease) as alarm antiproteinases in inflammatory lung disease. Respiratory research. 2000;1:87. doi: 10.1186/rr18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda Y, Takahashi H, Kuroki Y, Akino T, Abe S. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest. 1996 Apr;109:1006. doi: 10.1378/chest.109.4.1006. [DOI] [PubMed] [Google Scholar]
- 19.Pace E, Ferraro M, Minervini MI, Vitulo P, Pipitone L, Chiappara G, Siena L, Montalbano AM, Johnson M, Gjomarkaj M. Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PloS one. 2012;7:e33601. doi: 10.1371/journal.pone.0033601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amatngalim GD, Schrumpf JA, Henic A, Dronkers E, Verhoosel RM, Ordonez SR, Haagsman HP, Fuentes ME, Sridhar S, Aarbiou J, Janssen RAJ, et al. Antibacterial Defense of Human Airway Epithelial Cells from Chronic Obstructive Pulmonary Disease Patients Induced by Acute Exposure to Nontypeable Haemophilus influenzae: Modulation by Cigarette Smoke. Journal of innate immunity. 2017;9:359. doi: 10.1159/000455193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pound LD, Patrick C, Eberhard CE, Mottawea W, Wang GS, Abujamel T, Vandenbeek R, Stintzi A, Scott FW. Cathelicidin Antimicrobial Peptide: A Novel Regulator of Islet Function, Islet Regeneration, and Selected Gut Bacteria. Diabetes. 2015 Dec;64:4135. doi: 10.2337/db15-0788. [DOI] [PubMed] [Google Scholar]
- 22.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011 Oct 14;334:255. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nature immunology. 2010 Jan;11:76. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni NN, Gunnarsson HI, Yi Z, Gudmundsdottir S, Sigurjonsson OE, Agerberth B, Gudmundsson GH. Glucocorticoid dexamethasone down-regulates basal and vitamin D3 induced cathelicidin expression in human monocytes and bronchial epithelial cell line. Immunobiology. 2016 Feb;221:245. doi: 10.1016/j.imbio.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, Wang X, Yang X, Liu Z, Wu M, Li G. Budesonide suppresses pulmonary antibacterial host defense by down-regulating cathelicidin-related antimicrobial peptide in allergic inflammation mice and in lung epithelial cells. BMC Immunol. 2013 Feb 06;14:7. doi: 10.1186/1471-2172-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita T, Nagase T, Ohga E, Yamaguchi Y, Yoshizumi M, Ouchi Y. Molecular mechanisms underlying human beta-defensin-2 gene expression in a human airway cell line (LC2/ad) Respirology. 2002 Dec;7:305. doi: 10.1046/j.1440-1843.2002.00415.x. [DOI] [PubMed] [Google Scholar]
- 27.Singanayagam A, Glanville N, Girkin JL, Ching YM, Marcellini A, Porter JD, Toussaint M, Walton RP, Finney LJ, Aniscenko J, Zhu J, et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat Commun. 2018 Jun 8;9 doi: 10.1038/s41467-018-04574-1. 2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liapikou A, Polverino E, Ewig S, Cilloniz C, Marcos MA, Mensa J, Bello S, Martin-Loeches I, Menendez R, Torres A. Severity and outcomes of hospitalised community-acquired pneumonia in COPD patients. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2012 Apr;39:855. doi: 10.1183/09031936.00067111. [DOI] [PubMed] [Google Scholar]
- 29.Singanayagam A, Glanville N, Walton RP, Aniscenko J, Pearson RM, Pinkerton JW, Horvat JC, Hansbro PM, Bartlett NW, Johnston SL. A short-term mouse model that reproduces the immunopathological features of rhinovirus-induced exacerbation of COPD. Clinical science. 2015 Aug;129:245. doi: 10.1042/CS20140654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallia P, Webber J, Gill SK, Trujillo-Torralbo MB, Calderazzo MA, Finney L, Bakhsoliani E, Farne H, Singanayagam A, Footitt J, Hewitt R, et al. Role of airway glucose in bacterial infections in patients with chronic obstructive pulmonary disease. The Journal of allergy and clinical immunology. 2017 Dec 13; doi: 10.1016/j.jaci.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proceedings of the National Academy of Sciences of the United States of America. 1998 Aug 4;495:9541. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1977 Oct 1;90:2796. [PubMed] [Google Scholar]
- 33.Schrumpf JA, Amatngalim GD, Veldkamp JB, Verhoosel RM, Ninaber DK, Ordonez SR, van der Does AM, Haagsman HP, Hiemstra PS. Proinflammatory Cytokines Impair Vitamin D-Induced Host Defense in Cultured Airway Epithelial Cells. American journal of respiratory cell and molecular biology. 2017 Jun;56:749. doi: 10.1165/rcmb.2016-0289OC. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen OE, Gram L, Johnsen AH, Andersson E, Bangsboll S, Tjabringa GS, Hiemstra PS, Malm J, Egesten A, Borregaard N. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: a novel mechanism of generating antimicrobial peptides in vagina. The Journal of biological chemistry. 2003 Aug 01;278:28540. doi: 10.1074/jbc.M301608200. [DOI] [PubMed] [Google Scholar]
- 35.Bergsson G, Reeves EP, McNally P, Chotirmall SH, Greene CM, Greally P, Murphy P, O'Neill SJ, McElvaney NG. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J Immunol. 2009 Jul 01;183:543. doi: 10.4049/jimmunol.0803959. [DOI] [PubMed] [Google Scholar]
- 36.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. American journal of respiratory and critical care medicine. 2011 Mar 15;183:734. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohlmeier S, Nieminen P, Gao J, Kanerva T, Ronty M, Toljamo T, Bergmann U, Mazur W, Pulkkinen V. Lung tissue proteomics identifies elevated transglutaminase 2 levels in stable chronic obstructive pulmonary disease. American journal of Lung physiology cellular and molecular physiology. 2016 Jun 01;310:L1155. doi: 10.1152/ajplung.00021.2016. [DOI] [PubMed] [Google Scholar]
- 38.Jubinville E, Veillette M, Milot J, Maltais F, Comeau AM, Levesque RC, Duchaine C. Exacerbation induces a microbiota shift in sputa of COPD patients. PloS one. 2018;13:e0194355. doi: 10.1371/journal.pone.0194355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Contoli M, Pauletti A, Rossi MR, Spanevello A, Casolari P, Marcellini A, Forini G, Gnesini G, Marku B, Barnes N, Rizzi A, et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2017 Oct;50 doi: 10.1183/13993003.00451-2017. [DOI] [PubMed] [Google Scholar]
- 40.Huang EY, Inoue T, Leone VA, Dalal S, Touw K, Wang Y, Musch MW, Theriault B, Higuchi K, Donovan S, Gilbert J, et al. Using corticosteroids to reshape the gut microbiome: implications for inflammatory bowel diseases. Inflamm Bowel Dis. 2015 May;21:963. doi: 10.1097/MIB.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inghammar M, Engstrom G, Kahlmeter G, Ljungberg B, Lofdahl CG, Egesten A. Invasive pneumococcal disease in patients with an underlying pulmonary disorder. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013 Dec;19:148. doi: 10.1111/1469-0691.12182. [DOI] [PubMed] [Google Scholar]
- 42.Montull B, Menendez R, Torres A, Reyes S, Mendez R, Zalacain R, Capelastegui A, Rajas O, Borderias L, Martin-Villasclaras J, Bello S, et al. Predictors of Severe Sepsis among Patients Hospitalized for Community-Acquired Pneumonia. PloS one. 2016;11:e0145929. doi: 10.1371/journal.pone.0145929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson CM, Morrison RL, D'Souza A, Teng XS, Happel KI. Inhaled fluticasone propionate impairs pulmonary clearance of Klebsiella pneumoniae in mice. Respiratory research. 2012 May 31;13:40. doi: 10.1186/1465-9921-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respiratory research. 2005 Jul 08;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Scheele I, Larsson K, Dahlen B, Billing B, Skedinger M, Lantz AS, Palmberg L. Toll-like receptor expression in smokers with and without COPD. Respiratory medicine. 2011 Aug;105:1222. doi: 10.1016/j.rmed.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Baqir M, Chen CZ, Martin RJ, Thaikoottathil J, Case SR, Minor MN, Bowler R, Chu HW. Cigarette smoke decreases MARCO expression in macrophages: implication in Mycoplasma pneumoniae infection. Respiratory medicine. 2008 Nov;102:1604. doi: 10.1016/j.rmed.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Taylor AE, Finney-Hayward TK, Quint JK, Thomas CM, Tudhope SJ, Wedzicha JA, Barnes PJ, Donnelly LE. Defective macrophage phagocytosis of bacteria in COPD. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2010 May;35:1039. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 48.Sapey E, Stockley JA, Greenwood H, Ahmad A, Bayley D, Lord JM, Insall RH, Stockley R. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2011 May 1;183:1176. doi: 10.1164/rccm.201008-1285OC. [DOI] [PubMed] [Google Scholar]
- 49.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, Doerschuk CM, Alexis E, Anderson WH, Henderson AG, Barr RG, et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. The New England journal of medicine. 2017 Sep 07;377:911. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Cremoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. American journal of respiratory and critical care medicine. 1995 Mar;151:630. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 51.Muns G, Rubinstein I, Bergmann KC. Phagocytosis and oxidative burst of blood phagocytes in chronic obstructive airway disease. Scandinavian journal of infectious diseases. 1995;27:369. doi: 10.3109/00365549509032733. [DOI] [PubMed] [Google Scholar]
- 52.Mortaz E, Rad MV, Johnson M, Raats D, Nijkamp FP, Folkerts G. Salmeterol with fluticasone enhances the suppression of IL-8 release and increases the translocation of glucocorticoid receptor by human neutrophils stimulated with cigarette smoke. J Mol Med (Berl) 2008 Sep;86:1045. doi: 10.1007/s00109-008-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roca-Ferrer J, Mullol J, Perez M, Xaubet A, Molins L, de Haro J, Shelhamer J, Picado C. Effects of topical glucocorticoids on in vitro lactoferrin glandular secretion: comparison between human upper and lower airways. The Journal of allergy and clinical immunology. 2000 Dec;106:1053. doi: 10.1067/mai.2000.110476. [DOI] [PubMed] [Google Scholar]
- 54.Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014 Mar;10 doi: 10.1002/14651858.CD010115.pub2. CD010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, Wachtel A, Martinez FJ, Barnhart F, Sanford L, Lettis S, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. The Respiratory Lancet medicine. 2013 May;1:210. doi: 10.1016/S2213-2600(13)70040-7. [DOI] [PubMed] [Google Scholar]
- 56.Wedzicha JA, Singh D, Vestbo J, Paggiaro PL, Jones PW, Bonnet-Gonod F, Cohuet G, Corradi M, Vezzoli S, Petruzzelli S, Agusti A, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respiratory medicine. 2014 Aug;108:1153. doi: 10.1016/j.rmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa K, Mansbach JM, Ajami NJ, Petrosino JF, Freishtat RJ, Teach SJ, Piedra PA, Camargo CA., Jr Serum cathelicidin, nasopharyngeal microbiota, and disease severity among infants hospitalized with bronchiolitis. The Journal of allergy and clinical immunology. 2017 Apr;139:1383. doi: 10.1016/j.jaci.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, Powers JP, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006 Feb 15;176:2455. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 59.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000 Oct 02;192:1069. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovach MA, Ballinger MN, Newstead MW, Zeng X, Bhan U, Yu FS, Moore BB, Gallo RL, Standiford TJ. Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in Gram-negative bacterial pneumonia. J Immunol. 2012 Jul 01;189:304. doi: 10.4049/jimmunol.1103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beaumont PE, McHugh B, Gwyer Findlay E, Mackellar A, Mackenzie KJ, Gallo RL, Govan JR, Simpson AJ, Davidson DJ. Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PloS one. 2014;9:e99029. doi: 10.1371/journal.pone.0099029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Currie SM, Gwyer Findlay E, McFarlane AJ, Fitch PM, Bottcher B, Colegrav N, Paras A, Jozwik A, Chiu C, Schwarze J, Davidson DJ. Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans. J Immunol. 2016 Mar 15;196:2699. doi: 10.4049/jimmunol.1502478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, Davidson DJ, Donis RO. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PloS one. 2011;6:e25333. doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang YM, Guo YF, Zhang HS, Sun TY. Antimicrobial peptide LL-37 circulating levels in chronic obstructive pulmonary disease patients with high risk of frequent exacerbations. Journal of thoracic disease. 2015 Apr;7:740. doi: 10.3978/j.issn.2072-1439.2015.04.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Persson LJ, Aanerud M, Hardie JA, Miodini Nilsen R, Bakke PS, Eagan TM, Hiemstra PS. Antimicrobial peptide levels are linked to airway inflammation, bacterial colonisation and exacerbations in chronic obstructive pulmonary disease. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiolog. 2017 Mar;49 doi: 10.1183/13993003.01328-2016. [DOI] [PubMed] [Google Scholar]
- 66.Jiang Y, Xiao W, Zhang Y, Xing Y. Urokinase-type plasminogen activator system and human cationic antimicrobial protein 18 in serum and induced sputum of patients with chronic obstructive pulmonary disease. Respirology. 2010 Aug;15:939. doi: 10.1111/j.1440-1843.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhang L, Wu WK, Gallo RL, Fang EF, Hu W, Ling TK, Shen J, Chan RL, Lu L, Luo XM, Li MX, et al. Critical Role of Antimicrobial Peptide Cathelicidin for Controlling Helicobacter pylori Survival and Infection. J Immunol. 2016 Feb 15;196:1799. doi: 10.4049/jimmunol.1500021. [DOI] [PubMed] [Google Scholar]
- 68.van den Berge M, Jonker MR, Miller-Larsson A, Postma DS, Heijink IH. Effects of fluticasone propionate and budesonide on the expression of immune defense genes in bronchial epithelial cells. Pulmonary pharmacology & therapeutics. 2018 Jun;50:47. doi: 10.1016/j.pupt.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Zijlstra GJ, Fattahi F, Rozeveld D, Jonker MR, Kliphuis NM, van den Berge M, Hylkema MN, ten Hacken NH, van Oosterhout AJ, Heijink IH. Glucocorticoids induce the production of the chemoattractant CCL20 in airway epithelium. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiolog. 2014 Aug;44:361. doi: 10.1183/09031936.00209513. [DOI] [PubMed] [Google Scholar]
- 70.Liley HG, White RT, Benson BJ, Ballard PL. Glucocorticoids both stimulate and inhibit production of pulmonary surfactant protein A in fetal human lun. Proceedings of the National Academy of Sciences of the United States of America. 1988 Dec;85:9096. doi: 10.1073/pnas.85.23.9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Witek B, Ochwanowska E, Slewa A, Kolataj A. Effect of hydrocortisone on the activity of some lysosomal enzymes in mice. Neuro Endocrinol Lett. 2002 Apr;23:105. [PubMed] [Google Scholar]
- 72.Dardevet D, Sornet C, Taillandier D, Savary I, Attaix D, Grizard J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old Lack rats of regulation of the ubiquitin-proteasome proteolytic pathway in aging. The Journal of clinical investigation. 1995 Nov;96:2113. doi: 10.1172/JCI118264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong DH, Forsberg NE. Effects of dexamethasone on protein degradation and protease gene expression in rat L8 myotube cultures. Molecular and cellular endocrinology. 1995 Feb 27;108:199. doi: 10.1016/0303-7207(95)03476-n. [DOI] [PubMed] [Google Scholar]
- 74.Fallon K, Bausch K, Noonan J, Huguenel E, Tamburini P. Role of aspartic proteases in disseminated Candida albicans infection in mice. Infection and immunity. 1997 Feb;65:551. doi: 10.1128/iai.65.2.551-556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anantaraju HS, Battu MB, Viswanadha S, Sriram D, Yogeeswari P. Cathepsin D inhibitors as potential therapeutics for breast cancer treatment: Molecular docking and bioevaluation against triple-negative and triple-positive breast cancers. Mol Divers. 2016 May;20:521. doi: 10.1007/s11030-015-9645-8. [DOI] [PubMed] [Google Scholar]
- 76.Faiz A, Tjin G, Harkness L, Weckmann M, Bao S, Black JL, Oliver BG, Burgess JK. The expression and activity of cathepsins D, H and K in asthmatic airways. PloS one. 2013;8:e57245. doi: 10.1371/journal.pone.0057245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Byrne PM, Pedersen S, Carlsson LG, Radner F, Thoren A, Peterson S, Ernst P, Suissa S. Risks of pneumonia in patients with asthma taking inhaled corticosteroids. American journal of respiratory and critical care medicine. 2011 Mar 1;183:589. doi: 10.1164/rccm.201005-0694OC. [DOI] [PubMed] [Google Scholar]
- 78.Cazeiro C, Silva C, Mayer S, Mariany V, Wainwright CE, Zhang L. Inhaled Corticosteroids and Respiratory Infections in Children With Asthma: A Meta-analysis. Pediatrics. 2017 Mar;139 doi: 10.1542/peds.2016-3271. [DOI] [PubMed] [Google Scholar]
- 79.Calverley PM, Stockley RA, Seemungal TA, Hagan G, Willits LR, Riley JH, Wedzicha JA. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011 Mar;139:505. doi: 10.1378/chest.09-2992. [DOI] [PubMed] [Google Scholar]
- 80.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, Jablonski K, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. American journal of respiratory and critical care medicine. 2013 May 15;187:1067. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen P, Whelan FJ, Schenck LP, McGrath JJC, Vanderstocken G, Bowdish DME, Surette MG, Stampfli MR. Streptococcus pneumoniae Colonization Is Required To Alter the Nasal Microbiota in Cigarette Smoke-Exposed Mice. Infection and immunity. 2017 Oct;85 doi: 10.1128/IAI.00434-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng X, Chu F, Mirkin BL, Sudha T, Mousa SA, Rebbaa A. Role of the proteolytic hierarchy between cathepsin L, cathepsin D and caspase-3 in regulation of cellular susceptibility to apoptosis and autophagy. Biochim Biophys Acta. 2008 Dec;1783:2294. doi: 10.1016/j.bbamcr.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 83.Colino J, Snapper CM. Opposing signals from pathogen-associated molecular patterns and IL-10 are critical for optimal dendritic cell induction of in vivo humoral immunity to Streptococcus pneumoniae. J Immunol. 2003 Oct 1;171:3508. doi: 10.4049/jimmunol.171.7.3508. [DOI] [PubMed] [Google Scholar]
- 84.Ioannidis I, Ye F, McNally B, Willette M, Flano E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. Journal of virology. 2013 Mar;87:3261. doi: 10.1128/JVI.01956-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qiu HN, Wong CK, Chu IM, Hu S, Lam CW. Muramyl dipeptide mediated activation of human bronchial epithelial cells interacting with basophils: a novel mechanism of airway inflammation. Clinical and experimental immunology. 2013 Apr;172:81. doi: 10.1111/cei.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greenbaum LM, Sutherland JH. Host cathepsin D response to tumor in the normal and pepstatin-treated mouse. Cancer Res. 1983 Jun;43:2584. [PubMed] [Google Scholar]
- 87.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nature medicine. 2008 Feb;14:199. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pizzichini E, Pizzichini MM, Leigh R, Djukanovic R, Sterk PJ. Safety of sputum induction. The European respiratory journal Supplement. 2002 Sep;37:9s. doi: 10.1183/09031936.02.00000902. [DOI] [PubMed] [Google Scholar]
- 89.Footitt J, Mallia P, Durham AL, Ho WE, Trujillo-Torralbo MB, Telcian AG, Del Rosario A, Chang C, Peh HY, Kebadze T, Aniscenko J, et al. Oxidative and Nitrosative Stress and Histone Deacetylase-2 Activity in Exacerbations of COPD. Chest. 2016 Jan;149:62. doi: 10.1378/chest.14-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mallia P, Footitt J, Sotero R, Jepson A, Contoli M, Trujillo-Torralbo MB, Kebadze T, Aniscenko J, Oleszkiewicz G, Gray K, Message SD, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2012 Dec 1;186:1117. doi: 10.1164/rccm.201205-0806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cuthbertson L, Craven V, Bingle L, Cookson W, Everard ML, Moffatt MF. The impact of persistent bacterial bronchitis on the pulmonary microbiome of children. PloS one. 2017;12:e0190075. doi: 10.1371/journal.pone.0190075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bishop CJ, Aanensen DM, Jordan GE, Kilian M, Hanage WP, Spratt BG. Assigning strains to bacterial species via the internet. BMC Biol. 2009 Jan 26;7:3. doi: 10.1186/1741-7007-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.