Abstract
Antibodies play a crucial role in virus control. The production of antibodies requires virus-specific B cells to encounter viral antigens in lymph nodes, become activated, interact with different immune cells, proliferate and enter specific differentiation programmes. Each step occurs in distinct lymph node niches, requiring a coordinated migration of B cells between different subcompartments. The development of multiphoton intravital microscopy has enabled researchers to begin to elucidate the precise cellular and molecular events by which lymph nodes coordinate humoral responses. This Review discusses recent studies that clarify how viruses interfere with antibody responses, highlighting how these mechanisms relate to our topological and temporal understanding of B cell activation within secondary lymphoid organs.
B cells play a crucial role in host protection against viral infections. Although B cells have innate antiviral functions1,2 (BOX 1), their main protective activity can arguably be ascribed to antigen-induced antibody production. The role of antibodies in antiviral defence was formally shown by the passive immunization of immunodeficient animals and subsequent protection from viral challenge (reviewed in REF. 3). However, this role is perhaps best epitomized by the protection that maternal antibodies confer to neonates4. Only a minor fraction of antiviral antibodies elicited after infection has direct antiviral activity in vitro; these antibodies are referred to as ‘neutralizing antibodies’. Neutralizing activity requires the antibodies to be of fairly high affinity and/or avidity for exposed structures on the surface of the virus, such as viral glycoproteins of enveloped viruses or the protein shell of nonenveloped viruses5. Neutralizing antibodies render virions noninfectious by directly interfering with receptor binding and cell entry, and are crucial for preventing reinfection and for the protection induced by currently available vaccines5,6. Antibodies can elicit an antiviral response regardless of whether they are neutralizing or non-neutralizing, as the nonvariant crystallizable fragment (Fc) domain can bind to Fc receptors on various immune cells and induce different effector functions7. As such, viruses have developed countermeasures to avoid elimination by antibodies. It is outside the scope of this Review to discuss the structural features of the virion surface that prevent immune recognition or that enable viral escape from emerging neutralizing antibody responses. However, it must be stated that these are important mechanisms that allow certain viruses to persist within infected hosts. For example, the large spacing between envelope spikes on a virion as well as extensive envelope glycosylation render HIV a weak B cell immunogen8. Other mechanisms adopted by viruses to evade antibody responses that fall outside the scope of this Review include mutational escape8–13, cell-to-cell transmission14 (viruses become unavailable to secreted antibodies) and the induction of B cell anergy or exhaustion15,16. In this Review, we discuss B cell activation and differentiation into antibody-secreting cells and examine the strategies that viruses utilize to interfere with this process.
Innate antiviral functions of B cells.
B cells have several innate antiviral functions related to lymphoid tissue organization and remodelling during infection that do not depend on their antigen-driven differentiation into antibody-secreting cells. Antibody-independent innate antiviral B cell functions rely on B cell-derived cytokines, particularly lymphotoxin α1β2 (LTα1β2) and tumour necrosis factor (TNF). B cell-derived cytokines can promote T cell responses, regulate lymphoid organogenesis and downmodulate the immune response. For example, B cell-derived LTα1β2 maintains a protective lymph node subcapsular sinus macrophage phenotype that prevents fatal viral infection independent of adaptive immunity1. Furthermore, LTα1β2 and TNF provision by B cells is crucial to support adaptive immunity as it promotes secondary lymphoid tissue organization in steady state conditions, as well as remodelling of these organs upon viral infection2. In addition, IL-10 produced by B cells can downregulate the immune response. For a detailed discussion of the antibody-independent functions of B cells, we refer readers to REF. 2.
The dynamics of B cell activation
B cells differentiate into antibody-secreting cells in secondary lymphoid organs; therefore, we begin by describing the structure and function of these organs.
The central role of lymph nodes during viral infection
Viruses typically enter an adult vertebrate organism by breaching the skin or mucosal membranes, and are usually contained at the site of entry by the innate immune system. However, some viruses can evade this first line of defence and threaten to disseminate systemically by entering lymph vessels that guide interstitial fluid from peripheral tissues to the venous circulation. Before reaching the blood, however, the tissue-derived lymph must first encounter one or more draining lymph nodes. These lymphoid organs are thought to represent a second line of defence by acting as filter stations that prevent lymph-borne viruses from entering the blood and spreading systemically17–19. Moreover, lymph nodes are the staging grounds for antiviral adaptive humoral and cellular immune responses because they provide a specialized environment for the presentation of virus-derived antigens to naive B and T cells20,21. This central function of lymph nodes is evident from the observation that lymph-node-deficient mice have defective antiviral immune responses22. Accordingly, investigations into the mechanisms whereby viruses interfere with immune responses in vivo can be highly informative when conducted within these highly organized organs. Naive lymphocytes gain access to lymph nodes via high endothelial venules (HEVs) in the T cell area of the lymph node cortex21 (FIG. 1). They typically spend less than 1 day in the lymph node, constantly migrating while searching for cognate antigens before they return to the blood by exiting via draining lymph sinuses located in the medulla21. Viral antigens can reach lymph nodes via the afferent lymph after first being processed by dendritic cells (DCs), which collect antigenic material in peripheral sites, before entering the draining lymphatics and migrating into the T cell zone23. Although antigen-bearing DCs primarily encounter T cells in this area, they can also contact and present antigens to newly homed B cells that are transitioning from their site of entry, the HEVs, to nearby B follicles24. DC-mediated antigen transport and T cell activation have been thoroughly investigated in the past few years25; however, we still have an incomplete understanding of how lymph-borne infectious viral particles that directly enter and replicate within lymph nodes are handled by different lymph node cell populations to stimulate or interfere with humoral immune responses.
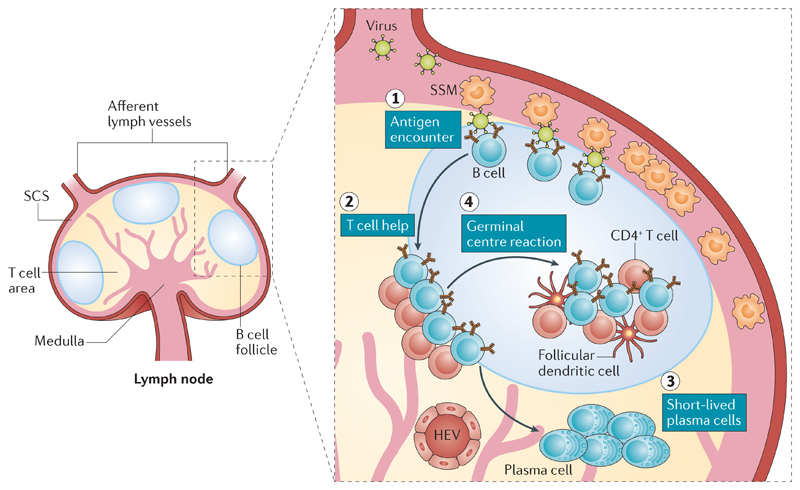
Figure 1. Spatiotemporal dynamics of B cell activation.
The structure of a lymph node, showing the subcapsular sinus (SCS), T cell area and B cell follicle (left-hand side). Viruses drained by afferent lymph (right-hand side) are captured and retained by SCS macrophages (SSMs), which shuttle the virus across their surface towards naive B cells in the underlying follicle (step 1). Upon encounter with the antigen, naive B cells undergo early activation and proliferation and relocalize to the B cell–T cell boundary to search for T cell help (step 2). Interaction with cognate CD4+ T cells leads antigen-specific B cells either to differentiate into short-lived plasma cells secreting low-affinity antibodies (step 3) or to localize back to the follicle and enter a germinal centre reaction (step 4). During germinal centre reactions, antigen-specific B cells engage in interactions with T follicular helper cells and antigens (retained by follicular dendritic cells) and undergo an affinity maturation process, which ultimately results in the production of high-affinity neutralizing antibodies. HEV, high endothelial venule.
Blood-borne viruses are filtered in the spleen, where they are captured by specialized populations of macrophages and DCs26. The anatomical organization of the splenic white pulp resembles that of the lymph node, particularly with regard to the compartmentalization of B cell follicles and T cell areas26. We describe below the spatiotemporal dynamics of B cell activation in lymph nodes, although similar events have also been described as occurring in the spleen26.
B cell activation as a dynamic multistep process
In order to mount a humoral immune response, B cells must encounter antigens, interact with T helper (TH) cells and DCs, proliferate and differentiate into high-affinity plasma cells and memory B cells. Each of these steps takes place in distinct areas of the lymph nodes, thus requiring a rapid, coordinated migration of B cells from niche to niche27 (FIG. 1). Early investigations into the initiation of humoral immune responses in lymph nodes were based on static imaging techniques such as immunohistochemistry and electron microscopy21. In recent years, the advent of multiphoton intravital microscopy has taken the field to a whole new level, enabling the dynamic visualization of B cells within secondary lymphoid tissue in vivo26,28,29. Through the use of multiphoton intravital microscopy, several ground-breaking studies have shed new light on the way that B cells encounter antigens and become activated in lymph nodes19,26,28,29 (FIG. 1). Although none of these studies used live, intact pathogens, they all identified the lymph node subcapsular sinus (SCS) as a particularly relevant anatomic region for early B cell–antigen encounters (FIG. 1). The SCS receives unfiltered lymph from afferent lymph vessels, and the SCS lumen and floor are sparsely populated with macrophages (SCS macrophages), which are anchored to a layer of extracellular matrix30,31. Although small soluble antigens (molecules with a dynamic radius of less than ~5.5 nm, corresponding to a molecular mass of less than ~70 kD) readily diffuse through the SCS, reaching B cell follicles32, larger particulate antigens (for example, intact viruses) do not. Notably, ~200-nanometre-sized structures such as synthetic microspheres or inactivated viral particles are captured and retained by SCS macrophages, which can then translocate these particulate antigens across the SCS floor and display them to underlying follicular B cells17,33,34. In contrast to noncognate follicular B cells, antigen-specific follicular B cells make prolonged contacts with SCS macrophages, acquire antigens, upregulate CC-chemokine receptor 7 (CCR7) and the G-protein-coupled receptor 183 (GPR183; also known as EBI2) and migrate to the B cell–T cell boundary within the cortex, where they recruit CD4+ T cell help, which is likely necessary for maximal activation29,35–40 (FIG. 1). Following activation at the B cell–T cell boundary, B cells undergo extensive proliferation in the outer B cell follicle. Within a few days after antigen encounter, some B cells differentiate into plasmablasts and migrate to extrafollicular sites, whereas others coalesce into tight clusters in the centre of the follicle, giving rise to germinal centres41 (FIG. 1). Germinal centres are microanatomical structures where B cells undergo somatic hypermutation of the antigen-binding variable regions of immunoglobulin genes; mutated B cells are then selected on the basis of their affinity for antigens, so that B cells with a higher-affinity B cell receptor (BCR) progressively outcompete those endowed with lower-affinity BCRs42–44. Mature germinal centres are compartmentalized into two zones: the dark zone contains a tight cluster of highly proliferating B cells and is thought to be the site where somatic hypermutation induces clonal variants with differential affinities for antigens42–44; the light zone is less dense and more diverse as it contains other cells in addition to B cells, such as T follicular helper cells and follicular DCs42–44. The follicular DCs that reside within the light zone are fibroblastic stromal cells that are capable of long-term retention of intact antigens45. The light zone is the compartment where the selection of high-affinity clonal variants is thought to occur42–44; T follicular helper (TFH) cells play a crucial role in promoting selection within the light zone by sensing the density of peptide–MHC complexes on the surface of competing B cells46. A central property of the germinal centre reaction is its dynamic nature: germinal centre B cells constantly migrate between the dark zone and the light zone as they undergo iterative cycles of somatic hypermutation and selection42. The end result of this process, and one of the defining features of the humoral immune response, is the progressive increase in the affinity of antibodies over time42.
Multiphoton intravital microscopy
A fluorescence imaging technique that enables the study of cellular interactions in real time within living organisms. It uses infrared lasers that facilitate deep-tissue imaging while limiting phototoxicity and photobleaching.
Clodronate-encapsulated liposomes
Liposomes that contain the drug dichloromethylene diphosphonate. These liposomes are ingested by macrophages, resulting in cell death.
Latency
The ability of certain viruses to establish a reversible dormant state in infected cells with minimal production of viral proteins and absence of progeny virus production.
Polyclonal hypergammaglobulinaemia
Increased levels of nonspecific immunoglobulins in serum that typically occur during chronic viral infections and autoimmune diseases.
Pinocytosis
Also known as fluid-phase endocytosis. A process of engulfment of extracellular fluid and its solutes. It can be mediated by an actin-dependent mechanism that can engulf large volumes (macropinocytosis) or by other mechanisms that result in engulfment of smaller volumes (micropinocytosis).
Lymphopenia
The condition of having an abnormally low level of lymphocytes in the blood.
As mentioned above, almost all of the studies that helped to establish the paradigm for the spatiotemporal dynamics of B cell activation in secondary lymphoid organs used inactivated virus or viral components as antigens. Attempts to extend these findings to a replication-competent virus, which induces early, potent neutralizing antibody responses in mice (vesicular stomatitis virus (VSV)), showed that at least the initial B cell activation events are indistinguishable from those induced by nonreplicating antigens47. A perhaps more interesting research question pertains to the visualization of the spatiotemporal dynamics of B cell activation in response to viruses that fail to induce or that are known to interfere with neutralizing antibody responses.
Viral subversion of B cell responses
We discuss below the known mechanisms of viral subversion of B cell responses and attempt to emphasize how these relate to our topological and temporal understanding of B cell dynamics within secondary lymphoid organs (FIG. 1; TABLE 1). We realize that this effort might leave out some known mechanisms of viral manipulation of B cells; therefore, we refer readers to recently published reviews15,16,48 for a thorough discussion of the molecular means utilized by pathogens to target B cell functions irrespective of when and where they occur during the natural course of infection.
Table 1. Viral subversion of B cell responses.
| B cell activation step | Virus | Effect on B cell function | Molecular mechanism | Effect on Ab production | Refs |
|---|---|---|---|---|---|
| Ag encounter and early B cell activation | Influenza virus | Ag-specific B cell infection and killing | BCR–haemagglutinin interaction | Suppression of primary Ab responses | 52 |
| Vaccinia virus and influenza virus | Disruption of the lining of SCS macrophages | TLR9-mediated and MyD88-mediated signalling | Suppression of secondary Ab responses | 51 | |
| HIV | Inhibition of B cell proliferation | gp120–α4β7 integrin interaction: induction of TGFβ and FcRL4 | ND | 53 | |
| Measles virus | ND | Suppression of Ab secretion | 54,55 | ||
| CMV | ND | ND | 56 | ||
| EBV | Inhibition of BCR signalling | LMP2A sequesters LYN and SYK kinases | ND | 61 | |
| HCV | Inhibition of BCR signalling and polyclonal expansion | E2–CD81 interaction leads to downregulation of CD21 and upregulation of anti-apoptotic genes | Low levels of polyclonal Ab | 62,63 | |
| B cell relocalization | LCMV | Recruitment of inflammatory monocytes and B cell apoptosis | Sustained type I interferon and CCL2 production | Lack of early neutralizing Ab | 47 |
| Interaction with T helper cells | HIV | Polyclonal hyper-gammaglobulinaemia | ND | Hypersecretion of total IgG | 71 |
| LCMV | Nonspecific B cells present viral Ag | Hypersecretion of nonspecific Ab | 70,73,75 | ||
| MHV-68 | ND | Hypersecretion of nonspecific Ab | 69 | ||
| Adenovirus | ND | Nonspecific Ab responses | 72 | ||
| LDV | ND | Hypersecretion of nonspecific Ab | 70,72,73 | ||
| LCMV | Decreased help from TFH cells | NK-mediated suppression of TFH cells | Lack of specific Ab | 76,77 | |
| Pichinde virus | ND | 77 | |||
| Differentiation into plasma cells | LCMV | Differentiation into short-lived plasma cells and B cell apoptosis | Sustained type I interferon production, induction of TNF and IL-10 | Lack of early neutralizing Ab | 79 |
| Germinal centres | HIV | Inhibition of CD40L-dependent immunoglobulin class switching | Nef-mediated induction of IκBα and SOCS proteins | Nonspecific Ab responses | 85,86 |
| HIV | Polyclonal hypermutation | gp120 binding to MCLR induces AID expression | Production of class-switched Ab | 87 | |
| HCV | E2–CD81 interaction induces AID expression and hypermutation | Production of low-affinity antibodies | 88–90 | ||
| BTV | FDC infection and killing | ND | Lack of neutralizing Ab | 91 | |
| CD8+ T cell response | Measles virus | CD8+ T cell-mediated B cell killing | ND | Suppression of Ab secretion | 92 |
| LCMV | Sustained type I interferon production | Lack of early neutralizing Ab | 74,94 | ||
| HBV | B cells present viral Ag on MHC class I | ND | 98 | ||
| LCMV | Generalized immunopathology and disruption of follicular architecture | ND | Lack of early neutralizing Ab | 99–102 |
Ab, antibody; Ag, antigen; AID, activation-induced cytidine deaminase; BCR, B cell receptor; BTV, bluetongue virus; CCL2, CC-chemokine ligand 2; CD40L, CD40 ligand; CMV, cytomegalovirus; EBV, Epstein–Barr virus; FcRL4, Fc receptor-like protein 4; FDC, follicular dendritic cell; HBV, hepatitis B virus; HCV, hepatitis C virus; IκBα, NF-κB inhibitor-α; LCMV, lymphocytic choriomeningitis virus; LDV, lactate dehydrogenase elevating virus; LMP2A, latent membrane protein 2A; MCLR, mannose C-type lectin receptor; MHV-68, murine gammaherpesvirus-68; MyD88, myeloid differentiation primary response protein; ND, no data; NK, natural killer; SCS, subcapsular sinus; SOCS, suppressor of cytokine signalling; TFH, T follicular helper; TGFβ, transforming growth factor-β; TLR9, Toll-like receptor 9; TNF, tumour necrosis factor.
In the subcapsular sinus: antigen encounter and early B cell activation
As mentioned above, SCS macrophages act as ‘flypaper’ cells, efficiently trapping lymph-borne viruses and translocating them across the SCS floor for presentation to underlying B cells17,19,33,34 (FIG. 1). The mechanism by which SCS macrophages capture viruses remains ill-defined, although, as far as VSV is concerned, it does not seem to involve antibodies, complement or glycosylation of the viral glycoproteins17. It was reported that binding of inactivated influenza virus to SCS macrophages requires mannose-binding lectin49; however, it remains to be determined whether other viruses are susceptible to opsonization by mannose-binding lectin or any other molecules. In addition, the efficiency by which different viruses are captured by SCS macrophages and the effect that this may have on the subsequent antiviral humoral immune response remains unclear. Relevant to this, initial attempts to understand the importance of SCS macrophage-mediated antigen presentation to B cells have been hampered by experimental limitations; SCS macrophages are often depleted from mice by subcutaneous injection of clodronate-encapsulated liposomes, which possess an intrinsic adjuvant effect that promotes B cell responses50. A recent study showed that viral or bacterial infection can disrupt the organization of SCS macrophages, as the SCS lining becomes less densely populated with macrophages and the cells move within the B cell follicle51. This behaviour was suggested to reduce the capacity of SCS macrophages to retain and present antigens in a subsequent secondary infection, resulting in diminished B cell responses51.
One potential consequence of the initial B cell encounter with viruses is B cell infection and eventual B cell death because of direct viral cytopathicity. Viruses can infect B cells via the BCR or specific cellular receptors. Influenza A virus is an example of a virus that can utilize the BCR as an entry receptor (FIG. 2a; TABLE 1). A recent study that used transgenic B cells that express an influenza virus (strain A/WSN/33) haemagglutinin-specific BCR showed that the influenza A virus infects virus-specific B cells, and this results in both disruption of antibody secretion and B cell death52 (FIG. 2a). The capacity of a virus to destroy the initial wave of rare, antigen-specific B cells that are capable of thwarting the infection is an efficient way of maximizing transmission to other susceptible hosts52. Although this mechanism was shown to occur in the lungs, it could potentially occur in other organs and during infections by other viruses.
Figure 2. Viral interference with early B cell activation.
The encounter of B cells with viruses can lead to B cell death or functional inhibition. a | Influenza virus can infect antigen-specific B cells via a haemagglutinin (HA)-specific B cell receptor (BCR) and induce their apoptosis. b | HIV binding to B cells (left) via viral envelope glycoprotein gp120–cellular α4β7 integrin interaction leads to overexpression of transforming growth factor-β1 (TGFβ1) and the inhibitory receptor Fc receptor-like protein 4 (FcRL4) that hinder B cell proliferation. Epstein–Barr virus (EBV)-derived latent membrane protein 2A (LMP2A) sequesters the tyrosine-protein kinases LYN and SYK (middle), thereby inhibiting BCR signalling. The interaction of hepatitis C virus (HCV)-derived E2 glycoprotein with CD81 on B cells (right) leads to polyclonal expansion of B cells and inhibition of antigen-specific BCR signalling. CR2, complement receptor type 2.
Viruses can also interact with specific cellular receptors on naive B cells and interfere with their normal differentiation programme (FIG. 2b). For example, the HIV-1 envelope protein gp120 can bind to the α4β7 integrin on naive B cells, inducing signalling that causes an abortive proliferative response through an increased expression of the immunosuppressive cytokine transforming growth factor-β and the inhibitory receptor Fc receptor-like protein 4 (REF. 53) (FIG. 2b). Similarly, infection of naive B cells with measles virus or cytomegalovirus has long been known to suppress both proliferation and differentiation into immunoglobulin-secreting cells54–56. Epstein–Barr virus (EBV) is thought to infect all B cells regardless of their antigen specificity or activation status and drive them to differentiate into resting memory B cells, the reservoir of latent EBV57 (TABLE 1). During latency, EBV does not express most viral genes; therefore, EBV remains invisible to the immune system58. One of the viral proteins consistently identified in latently infected B cells in vivo is latent membrane protein 2A (LMP2A)59,60. LMP2A provides a constitutive positive signal into the infected cell and, by sequestering the signalling molecules LYN and SYK, prevents normal BCR signal transduction61 (FIG. 2b). Signalling through the BCR is known to induce the lytic cycle of EBV. As such, LMP2A keeps a state of viral latency by averting BCR-mediated induction of lytic EBV replication and consequent immune recognition59. The hepatitis C virus (HCV) protein E2 was shown to bind CD81 on B cells and induce the downregulation of the complement receptor CD21, potentially reducing the ability of B cells to capture opsonized antigens and consequently decreasing antigen-specific B cell responses62 (FIG. 2b). In addition, the E2–CD81 interaction was shown to activate naive B cells63,64, induce them to proliferate63 and protect them from FAS-mediated apoptosis62 (FIG. 2b); the resulting polyclonal expansion of naive B cells may dilute the antiviral humoral response and contribute to the establishment of chronic HCV infection.
B cell relocalization
Following antigen encounter, naive B cells in the follicles undergo an initial activation process, which involves the upregulation of early activation markers and a first burst of proliferation19,26,28,29. However, in order to reach full activation, B cells need to closely interact with cognate CD4+ T cells at the B cell–T cell boundary. Therefore, a few hours after antigen encounter, B cells move to the outer border of the follicle, towards the T cell area, a process that is facilitated by the upregulation of the chemokine receptor CCR7 and the G protein-coupled receptor EBI2 (REFS 35,65). This step occurs even in viral infections that are not associated with an early, potent neutralizing antibody response, such as those caused by lymphocytic choriomeningitis virus (LCMV) in mice47 (FIG. 3). In the interfollicular and T cell areas of lymph nodes that drain LCMV infection sites, virus-specific B cells were shown to engage in long-term interactions with a population of CD11b+Ly6Chi inflammatory monocytes47 that are recruited to the lymph node in a type-I-interferon-dependent and CC-chemokine receptor 2 (CCR2)-dependent fashion47,66 (FIG. 3). The interaction of virus-specific B cells with inflammatory monocytes is responsible for the confinement of B cells to the interfollicular and T cell area of lymph nodes and for the induction of B cell apoptosis with the consequent inhibition of antibody production47 (FIG. 3). Accordingly, the experimental removal of inflammatory monocytes or inhibition of their lymph node recruitment (via type I interferon or CCR2 blockade) leads to the enhanced survival of LCMV-specific B cells and recovery of virus-neutralizing antibody responses47. B cell inhibition is mediated by nitric oxide produced by inflammatory monocytes, as monocytes deficient for nitric oxide production cannot impair B cell responses47 (FIG. 3). Moreover, lymph node recruitment of CCR2+ inflammatory monocytes in response to vaccination has been shown to negatively correlate with the development of effective antibody responses67,68, suggesting that strategies aimed at preventing inflammatory monocyte accumulation within secondary lymphoid organs enhance vaccine immunity67,68. The relative capacity of different viruses to induce the lymph node recruitment of inflammatory monocytes and the role that these cells may play in suppressing antiviral humoral responses are yet to be fully elucidated. For example, although CD11b+Ly6Chi monocytes are recruited to VSV-infected lymph nodes early on, they disappear within48–72 hours after infection, and depletion of these cells does not result in higher VSV-specific B cell responses47 Ongoing research efforts aim to understand whether the identity of inflammatory monocytes recruited to VSV-infected or LCMV-infected lymph nodes differ, whether their suppressive capacity depends on their ability to persist or to localize in proximity to activated B cells, and whether their inhibitory role is limited to lymph nodes or also extends to the spleen.
Figure 3. Inflammatory monocytes hinder antiviral B cell responses.
Upon lymphocytic choriomeningitis virus (LCMV) infection (left), antigen-specific B cells encounter the virus, upregulate early activation markers and relocalize from the centre of the follicle to the interfollicular area (IFA) and T cell area of the lymph node. Viral replication induces a type-I-interferon-dependent CC-chemokine ligand 2 (CCL2) gradient (middle), which attracts CC-chemokine receptor 2 (CCR2)-positive inflammatory monocytes to the virus-draining lymph node. Inflammatory monocytes localize to the IFAs, where they interact with LCMV-specific activated B cells. Following this interaction (right), LCMV-specific B cell responses are inhibited owing to apoptosis induced in B cells through a nitric oxide (NO)-dependent mechanism. SSM, subscapular sinus macrophage.
At the B cell–T cell border: interaction with T helper cells
B cell–T cell crosstalk is mediated by several co-stimulatory molecules (among which is the inter-action between CD40 on B cells and CD40 ligand (CD40L) on T cells) and is key for an efficient (class-switched, high-affinity IgG) B cell response29. Therefore, it is not surprising that viruses have evolved strategies to interfere with the activation and differentiation of CD4+ T cells into TH cells. Affected TH cells may provide too much or too little cellular help to virus-specific B cells. Examples of viruses that subvert TH cells to overactivate B cells include adenovirus, lactate dehydrogenase elevating virus, HIV-1, murine gammaherpesvirus 68 and LCMV69–73. These viruses were shown to induce a polyclonal hypergamma-globulinaemia that originates when virus-specific CD4+ TH cells recognize B cells that present viral antigens irrespective of their BCR specificity69,70. The mechanisms whereby B cells present viral MHC class II peptides could involve B cell infection69,74, BCR-independent Fc or complement receptor-mediated uptake of viral antigens or nonspecific pinocytosis in the presence of a high amount of antigens70. Regardless of the mechanism, nonspecific B cell activation may dilute and delay antiviral antibodies75. Importantly, partial CD4+ T cell depletion reduces polyclonal B cell activation and results in earlier and more potent development of neutralizing antibodies to LCMV75.
Viruses could also fail to induce or actively down-regulate the TFH cell response, thus devoiding B cells of the necessary cellular help to undergo class switching and somatic hypermutation. The relative capacity of different viruses to induce an efficient TFH cell response, which could depend in part on the differential induction of cytokines, including type I interferon, remains ill-defined. However, viruses that are known inducers of natural killer (NK) cell activation may cause NK-cell-mediated CD4+ TFH cell elimination76,77, as seen during infection with the arenaviruses LCMV and Pichinde virus76,77.
Outside B cell follicles: differentiation of B cells into plasma cells
Some viruses, such as LCMV in mice, induce an immediate activation and differentiation of the majority of naive antigen-specific B cells into short-lived IgM-producing B cells, thus preventing isotype switching and the formation of memory responses78. It was shown that reducing the ratio between antigens and virus-specific B cells or enhancing CD4+ T cell help during LCMV infection could reduce the differentiation of antiviral B cells into short-lived plasma cells, increase class switching and improve B cell survival78. More recently, Pinschewer and co-workers demonstrated that the differentiation of activated B cells into short-lived plasma cells during LCMV infection was attributable to an early type I interferon response79. Type I interferon was shown to act on several cell types, including DCs, T cells and Gr-1+ myeloid cells, to induce the production of several cytokines (including tumour necrosis factor and IL-10) that, in turn, lead to terminal B cell differentiation and subsequent antiviral B cell deletion79. In addition, high levels of type I interferon during HIV infection correlate with progression to AIDS80, and B cells isolated from patients with HIV viraemia show a propensity to terminal differentiation and cell death and display an upregulation of interferon-stimulated genes81. Moreover, type I interferon, even when administered at low doses, has been shown to have a deleterious effect on B cells82. Together, these observations potentially highlight a more general role of type I interferon in viral subversion of B cell responses. Regarding the downstream mediators of type I interferon that are responsible for B cell differentiation into short-lived plasma cells, it is worth noting that TNF, receptors of the TNF superfamily and IL-10 have been previously associated with B cell dysfunction in HIV-1 infection81,83,84.
In germinal centres: immunoglobulin class switching
Immunoglobulin class switching is crucial for antiviral immunity and requires an interaction between antigen-specific B cells and T cells via several molecules, including interactions between MHC class II molecules and T cell receptors, CD40 and CD40L and numerous cytokines. HIV-1-derived negative factor protein Nef was shown to penetrate B cells (possibly via long-range intercellular conduits85) and to suppress the immunoglobulin class switch86. The mechanism involves inhibition of the nuclear factor-κB pathway and induction of suppressors of cytokine signalling (SOCS) proteins, thereby attenuating CD40, IL-4 receptor and IL-10 receptor signalling and ultimately inhibiting class-switch recombination86 (FIG. 4). Thus, HIV-1 may evade virus-specific T cell-dependent class switching by hijacking negative feedback pathways in B cells86. HIV-1 is also thought to be capable of inducing CD40-independent class-switch recombination87. HIV-1-derived gp120 can bind to B cells that express the mannose C-type lectin receptor and induce the upregulation of activation-induced cytidine deaminase, the key enzyme involved in class-switch recombination87 (FIG. 4). This polyclonal chronic activation of B cells regardless of BCR specificity might impair protective humoral immunity by inducing immune exhaustion87. The relative contribution of these two nonmutually exclusive mechanisms to the overall humoral response to HIV in vivo has not yet been fully elucidated. Another virus that is known to interfere with class-switch recombination is HCV. By binding to the cellular receptor CD81 via the E2 protein, HCV was shown to induce hypermutation of the immunoglobulin heavy chain in B cells88,89, thus reducing the affinity and specificity of HCV-specific antibodies, enabling HCV to escape from immune surveillance90 (FIG. 4).
Figure 4. Viral subversion of B cell responses in germinal centres.
Some viruses can affect the process of immunoglobulin class switching and somatic hypermutation in the dark zone. For example, the hepatitis C virus (HCV) protein E2 can interact with CD81 on B cells to induce polyclonal nonspecific hypermutation, thus reducing the affinity of HCV-specific antibodies. Similarly, HIV glycoprotein gp120 can bind to mannose C-type lectin receptor (MR) and induces the B cell expression of activation-induced cytidine deaminase (AID), leading to polyclonal hypermutation. In addition, HIV-derived negative factor protein Nef can penetrate B cells and suppress immunoglobulin class switching via induction of IκBα (NF-κB inhibitor-α) and suppressors of cytokine signalling (SOCS) proteins, which block CD40 signalling. Viruses can also interfere with B cell activation in the light zone. For example, bluetongue virus (BTV) can infect and kill follicular dendritic cells (FDCs), thereby causing germinal centre (GC) disruption. CD40L, CD40 ligand.
In germinal centres: follicular dendritic cells
As mentioned above, in addition to B cells and TFH cells, germinal centres contain a population of fibroblastic stromal cells referred to as follicular DCs42. In germinal centres, follicular DCs enable the formation of tight B cell clusters, retain antigens for long periods of time and support germinal centre B cell survival42. Therefore, disruption of the follicular DC network can have a deleterious impact on humoral immune responses. Bluetongue virus (BTV), an arbovirus transmitted to ruminants through insect bites, has been shown to adopt such a strategy to evade humoral immune responses91 (FIG. 4). At early time points, BTV infects a number of cells, including lymphatic endothelial cells, macrophages and B cells91. Similar to Trojan horses, B cells carry the virus inside follicles, where it infects follicular DCs91. As a result, follicular DCs die, and B cells fail to differentiate into high-affinity, neutralizing-antibody-producing cells91. Follicular DC disruption is highly immunosuppressive during primary infection and during a secondary unrelated antigenic challenge91.
In the T cell area: CD8+ T cell responses
Virus-specific CD8+ T cells can hinder humoral immune responses, either by directly killing antigen-specific B cells or by disrupting the follicular architecture of secondary lymphoid organs and eliminating antigen-presenting cells. The first mechanism can occur when infected B cells present viral antigens on MHC class I molecules and thus become the target of virus-specific cytotoxic T cells, as happens during measles virus infection92. Measles virus infects B cells by the viral haemagglutinin glycoprotein binding to the cellular receptor CD150 (REF. 93). As such, the measles virus can potentially target polyclonal nonspecific B cells, thus resulting in temporary immunological amnesia, which is thought to be the mechanism contributing to the immune suppression associated with this infection. A rapid oligoclonal expansion of virus-specific lymphocytes and bystander cells masks this depletion, explaining the short duration of measles-induced lymphopenia but the long duration of immune suppression92. Viruses can also infect virus-specific B cells by binding to the BCR, as described above for influenza virus. This is proposed to occur during LCMV infection in mice94, and it may explain why animals deficient in CD8+ T cells are able to develop an early, potent virus-neutralizing antibody response95. However, subsequent experiments were unable to reproduce the original findings94. Recently, this issue has been revisited in elegant experiments that took advantage of a new recombinant LCMV that expresses codon-improved Cre recombinase that was used to infect Confetti mice (a reporter mouse line used for fluorescent imaging)74. The investigators detected fluorescent protein-positive germinal centre B cells 40 days after LCMV infection, which led them to conclude that these virus-specific B cells had been previously infected with LCMV. However, it remains possible, although unlikely, that LCMV-specific B cells obtained Crecontaminated cytoplasmic material (perhaps from dying infected cells) through the BCR and thus underwent Cre-mediated recombination without being infected96. Indeed, noninfected B cells can also become targets of cytotoxic T lymphocytes by presenting exogenous viral antigens in association with MHC class I molecules in a process referred to as ‘cross-presentation’ (REF. 97). To our knowledge cross-presentation and consequent cytotoxic T lymphocyte-mediated killing of virus-specific B cells has also been shown for hepatitis B virus infection98.
Another pathogenic mechanism by which cytotoxic T lymphocytes have been proposed to hinder neutralizing antibody responses is the elimination of antigen-presenting cells and the disruption of the follicular architecture as well as the fibroblastic reticular cell network of secondary lymphoid organs99–102. This immunopathology has been described to occur upon LCMV infection and to thwart immune responses as early as day 6 after infection. It is notable that, in order to expand and differentiate into effector cells upon viral infection, naive CD8+ T cells require type I interferon receptor signalling103. Therefore, it is perhaps not surprising that blockade of type I interferon receptor signalling protects antiviral B cells by promoting effector CD8+ T cell dysfunction74.
Conclusions and future perspectives
Antibodies play a crucial yet heterogeneous role in the natural history of viral infections. On the one hand, they are required for host survival during potentially lethal acute viral infections; on the other hand they also contribute to sterilizing immunity to persistency-prone viruses. Therefore, it is not surprising that viruses that cause persistent infections have evolved strategies to avoid or interfere with the induction of antibodies. Although the advantage of these strategies to the virus is obvious, hosts might also benefit from an incomplete clearance of chronic infections, as low-level viral replication may help to maintain functional immune memory, as well as increase base levels of innate resistance to other infections.
New advances in photonics and imaging technologies are allowing us to visualize the spatiotemporal dynamics of B cell differentiation into antibody-secreting cells within living hosts. We have discussed in this Review how these technologies are beginning to help unravel the heterogeneity of B cell responses to different viral infections as well as the mechanisms used by viruses to interfere with humoral responses. However, with these new insights come many new questions. For example, what are the viral features that are responsible for the great variability in the mechanisms of viral evasion of B cell responses? Does it depend on distinct viral tropism? Is it contingent on the different innate sensing pathways triggered? In addition, type I interferon has emerged as a pleiotropic cytokine that inhibits antiviral humoral immune responses at multiple levels104 (FIG. 5). First, it induces the lymph node recruitment of inflammatory monocytes that inhibit antiviral B cells47. Second, it induces the expansion and differentiation of cytotoxic T lymphocytes that kill antiviral B cells74. Third, it indirectly induces the differentiation of antiviral B cells into short-lived plasma cells79. Do these effects occur during all viral infections that are known to trigger type I interferon responses? Or do they depend on the amount, duration and cellular origin of type I interferon? Finally, how are the inhibitory effects on B cells eventually overcome so that neutralizing antibodies are ultimately generated?
Figure 5. Type I interferon-mediated suppression of antibody responses.
Sustained type I interferon and subsequent CC-chemokine ligand 2 (CCL2) production leads to the recruitment of inflammatory monocytes to the virus-draining lymph node; inflammatory monocytes interact with and inhibit lymphocytic choriomeningitis virus (LCMV)-specific B cell responses by inducing apoptosis in a nitric oxide (NO)-dependent fashion. Type I interferon sustains expansion and differentiation of CD8+ T lymphocytes, which interact with LCMV-specific B cells and directly kill them upon secretion of cytolytic granules. Finally, type I interferon induces an inflammatory milieu (including tumour necrosis factor (TNF) and IL-10 secretion by different cell types) that triggers differentiation of activated LCMV-specific B cells into short-lived plasma cells, eventually resulting in B cell apoptosis. CTL, cytotoxic T lymphocyte; DC, dendritic cell.
Because an efficient antibody response requires the specific localization of B cells in unique niches and interaction with neighbouring cells and the local microenvironment, we predict that further technological development in our ability to link spatial information to transcriptional analysis at single-cell and genome-wide resolution could begin to answer these questions and allow the identification of new cellular and molecular mechanisms used by pathogens to evade immune control. This information might lead to new treatment for the termination of chronic viral infections and instruct the design of novel, rational vaccines.
Acknowledgements
The authors thank M. Silva for secretarial assistance, V. Cerundolo, F. V. Chisari, L. G. Guidotti, R. Pardi and Z. Shulman for critical reading of the manuscript and all the members of the Iannacone laboratory for helpful discussions. Work in the Iannacone laboratory discussed in this Review was supported by European Research Council grants 281648 and 725038 (to M.I.), Italian Association for Cancer Research (AIRC) grants 15350 and 9965 (to M.I.), Italian Ministry of Health grant GR-2011-02347925 (to M.I.), Italian Ministry of Education grant SIR-RBSI14BAO5 (to M.K.), Fondazione Regionale per la Ricerca Biomedica grant 2015–0010 (to M.I.), the European Molecular Biology Organization (EMBO) Young Investigator Program (M.I.) and a Career Development Award from the Giovanni Armenise-Harvard Foundation (to M.I.).
Footnotes
Author contributions
M.I. and M.K. both contributed to the research of data, and the discussion, writing, review and editing of this manuscript.
Reviewer information
Nature Reviews Immunology thanks J. Cyster, B. Ludewig and the other anonymous reviewer for their contribution to the peer review of this work.
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moseman EA, et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity. 2012;36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 3.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 4.Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345:1331–1335. doi: 10.1056/NEJMra012493. [DOI] [PubMed] [Google Scholar]
- 5.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [This is an excellent review on the heterogeneity of antiviral antibody responses discussed in the context of co-evolution of the host and viruses] [DOI] [PubMed] [Google Scholar]
- 6.Zinkernagel RM. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 7.Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton DR, Hangartner L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol. 2016;34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Hahn T, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, et al. Limited humoral immunity in hepatitis C virus infection. Gastroenterology. 1999;116:135–143. doi: 10.1016/s0016-5085(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 11.Ciurea A, et al. Viral persistence in vivo through selection of neutralizing antibody-escape variants. Proc Natl Acad Sci USA. 2000;97:2749–2754. doi: 10.1073/pnas.040558797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705–742. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 13.Escolano A, Dosenovic P, Nussenzweig MC. Progress toward active or passive HIV-1 vaccination. J Exp Med. 2016;214:3–16. doi: 10.1084/jem.20161765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong P, Agosto LM, Munro JB, Mothes W. Cell-to-cell transmission of viruses. Curr Opin Virol. 2013;3:44–50. doi: 10.1016/j.coviro.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moir S, Fauci AS. B-Cell exhaustion in HIV infection: the role of immune activation. Curr Opin HIV AIDS. 2014;9:472–477. doi: 10.1097/COH.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 16.Moir S, Fauci AS. B-Cell responses to HIV infection. Immunol Rev. 2017;275:33–48. doi: 10.1111/imr.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 18.Iannacone M, et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuka M, Iannacone M. The role of lymph node sinus macrophages in host defense. Ann NY Acad Sci. 2014;1319:38–46. doi: 10.1111/nyas.12387. [DOI] [PubMed] [Google Scholar]
- 20.von Andrian UH, Mackay CR. T-Cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 21.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 22.Karrer U, et al. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11−/−) mutant mice. J Exp Med. 1997;185:2157–2170. doi: 10.1084/jem.185.12.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi H, Egen JG, Huang AYC, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 25.Qi H, Kastenmuller W, Germain RN. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu Rev Cell Dev Biol. 2014;30:141–167. doi: 10.1146/annurev-cellbio-100913-013254. [DOI] [PubMed] [Google Scholar]
- 26.Heesters BA, van der Poel CE, Das A, Carroll MC. Antigen presentation to B cells. Trends Immunol. 2016;37:844–854. doi: 10.1016/j.it.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010;22:413–419. doi: 10.1093/intimm/dxq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 29.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 30.Clark SL. The reticulum of lymph nodes in mice studied with the electron microscope. Am J Anat. 1962;110:217–257. doi: 10.1002/aja.1001100303. [DOI] [PubMed] [Google Scholar]
- 31.Farr AG, Cho Y, de Bruyn PP. The structure of the sinus wall of the lymph node relative to its endocytic properties and transmural cell passage. Am J Anat. 1980;157:265–284. doi: 10.1002/aja.1001570304. [DOI] [PubMed] [Google Scholar]
- 32.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [References 17, 33 and 34 reveal a role for macrophages within the lymph node SCS in the presentation of large particulate antigens, immune complexes and inactivated viruses to follicular B cells] [DOI] [PubMed] [Google Scholar]
- 35.Cyster JG, Dang EV, Reboldi A, Yi T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol. 2014;14:731–743. doi: 10.1038/nri3755. [DOI] [PubMed] [Google Scholar]
- 36.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31:259–269. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannedouche S, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 40.Yi T, et al. Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity. 2012;37:535–548. doi: 10.1016/j.immuni.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coffey F, Alabyev B, Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009;30:599–609. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mesin L, Ersching J, Victora GD. Germinal center B cell dynamics. Immunity. 2016;45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bannard O, Cyster JG. Germinal centers: programmed for affinity maturation and antibody diversification. Curr Opin Immunol. 2017;45:21–30. doi: 10.1016/j.coi.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Heesters BA, et al. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38:1164–1175. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sammicheli S, et al. Inflammatory monocytes hinder antiviral B cell responses. Sci Immunol. 2016;1:eaah6789. doi: 10.1126/sciimmunol.aah6789. [This study utilizes multiphoton intravital microscopy to analyse the spatiotemporal dynamics of B cell activation upon viral infections. It identifies the type-I-interferon-dependent, CCR2-dependent lymph node recruitment of inflammatory monocytes as a critical inhibitor of antiviral B cell responses] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nothelfer K, Sansonetti PJ, Phalipon A. Pathogen manipulation of B cells: the best defence is a good offence. Nat Rev Microbiol. 2015;13:173–184. doi: 10.1038/nrmicro3415. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez SF, et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 2010;11:427–434. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tonti E, et al. Bisphosphonates target B cells to enhance humoral immune responses. Cell Rep. 2013;5:323–330. doi: 10.1016/j.celrep.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaya M, et al. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science. 2015;347:667–672. doi: 10.1126/science.aaa1300. [This study describes how viral infections disrupt the organization of lymph node SCS macrophages, thus thwarting antibody responses to subsequent antigenic challenge.] [DOI] [PubMed] [Google Scholar]
- 52.Dougan SK, et al. Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature. 2013;503:406–409. doi: 10.1038/nature12637. [This paper shows that influenza virus can infect haemagglutinin-specific B cells via the BCR, causing both disruption of antibody secretion and B cell death.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jelicic K, et al. The HIV-1 envelope protein gp120 impairs B cell proliferation by inducing TGF-β1 production and FcRL4 expression. Nat Immunol. 2013;14:1256–1265. doi: 10.1038/ni.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McChesney MB, Kehrl JH, Valsamakis A, Fauci AS, Oldstone MB. Measles virus infection of B lymphocytes permits cellular activation but blocks progression through the cell cycle. J Virol. 1987;61:3441–3447. doi: 10.1128/jvi.61.11.3441-3447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McChesney MB, Fujinami RS, Lampert PW, Oldstone MB. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986;163:1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 56.Rice GP, Schrier RD, Oldstone MB. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci USA. 1984;81:6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells. in vivo Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 58.Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3:801–812. doi: 10.1038/nri1201. [DOI] [PubMed] [Google Scholar]
- 59.Portis T, Longnecker R. Epstein-Barr virus LMP2A interferes with global transcription factor regulation when expressed during B-lymphocyte development. J Virol. 2003;77:105–114. doi: 10.1128/JVI.77.1.105-114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caldwell RG, Brown RC, Longnecker R. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J Virol. 2000;74:1101–1113. doi: 10.1128/jvi.74.3.1101-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller CL, et al. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z, et al. Hepatitis C virus protects human B lymphocytes from Fas-mediated apoptosis via E2-CD81 engagement. PLOS ONE. 2011;6:e18933. doi: 10.1371/journal.pone.0018933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosa D, et al. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102:18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliviero B, et al. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol. 2011;55:53–60. doi: 10.1016/j.jhep.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 65.Reif K, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 66.Norris BA, et al. Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity. 2013;38:309–321. doi: 10.1016/j.immuni.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell LA, Henderson AJ, Dow SW. Suppression of vaccine immunity by inflammatory monocytes. J Immunol. 2012;189:5612–5621. doi: 10.4049/jimmunol.1202151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitchell LA, Hansen RJ, Beaupre AJ, Gustafson DL, Dow SW. Optimized dosing of a CCR2 antagonist for amplification of vaccine immunity. Int Immunopharmacol. 2013;15:357–363. doi: 10.1016/j.intimp.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sangster MY, et al. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol. 2000;164:1820–1828. doi: 10.4049/jimmunol.164.4.1820. [DOI] [PubMed] [Google Scholar]
- 70.Hunziker L, et al. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol. 2003;4:343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- 71.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coutelier JP, Coulie PG, Wauters P, Heremans H, van der Logt JT. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J Virol. 1990;64:5383–5388. doi: 10.1128/jvi.64.11.5383-5388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coutelier JP, Johnston SJ, El Idrissi MEA El, Pfau CJ. Involvement of CD4+ cells in lymphocytic choriomeningitis virus-induced autoimmune anaemia and hypergammaglobulinaemia. J Autoimmun. 1994;7:589–599. doi: 10.1006/jaut.1994.1043. [DOI] [PubMed] [Google Scholar]
- 74.Moseman EA, Wu T, de La Torre JC, Schwartzberg PL, McGavern DB. Type I interferon suppresses virus-specific B cell responses by modulating CD8+ T cell differentiation. Sci Immunol. 2016;1:eaah3565. doi: 10.1126/sciimmunol.aah3565. [This study establishes that virus-induced type I interferon promotes the expansion and differentiation of cytotoxic T lymphocytes that kill antiviral B cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Recher M, et al. Deliberate removal of T cell help improves virus-neutralizing antibody production. Nat Immunol. 2004;5:934–942. doi: 10.1038/ni1102. [DOI] [PubMed] [Google Scholar]
- 76.Cook KD, Kline HC, Whitmire JK. NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. J Leukoc Biol. 2015;98:153–162. doi: 10.1189/jlb.4HI1214-594R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rydyznski C, et al. Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells. Nat Commun. 2015;6 doi: 10.1038/ncomms7375. 6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zellweger RM, Hangartner L, Weber J, Zinkernagel RM, Hengartner H. Parameters governing exhaustion of rare T cell-independent neutralizing IgM-producing B cells after LCMV infection. Eur J Immunol. 2006;36:3175–3185. doi: 10.1002/eji.200636087. [DOI] [PubMed] [Google Scholar]
- 79.Fallet B, et al. Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol. 2016;1:eaah6817. doi: 10.1126/sciimmunol.aah6817. [This paper describes how virus-induced type I interferon drives the deletion of antiviral B cells by supporting their differentiation into short-lived plasma cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mandl JN, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 81.Moir S, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–599. [PubMed] [Google Scholar]
- 82.Bosio E, Cluning CL, Beilharz MW. Low-dose orally administered type I interferon reduces splenic B cell numbers in mice. J Interferon Cytokine Res. 2001;21:721–728. doi: 10.1089/107999001753124453. [DOI] [PubMed] [Google Scholar]
- 83.Muller F, Aukrust P, Nordoy I, Froland SS. Possible role of interleukin-10 (IL-10) and CD40 ligand expression in the pathogenesis of hypergammaglobulinemia in human immunodeficiency virus infection: modulation of IL-10 and Ig production after intravenous Ig infusion. Blood. 1998;92:3721–3729. [PubMed] [Google Scholar]
- 84.Macchia D, et al. Membrane tumour necrosis factor-alpha is involved in the polyclonal B-cell activation induced by HIV-infected human T cells. Nature. 1993;363:464–466. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- 85.Xu W, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qiao X, et al. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol. 2006;7:302–310. doi: 10.1038/ni1302. [DOI] [PubMed] [Google Scholar]
- 87.He B, et al. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 88.Machida K, Cheng KTH, Pavio N, Sung VMH, Lai MMC. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–8089. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Machida K, et al. Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proc Natl Acad Sci USA. 2004;101:4262–4267. doi: 10.1073/pnas.0303971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Machida K, et al. Hepatitis C virus (HCV)-induced immunoglobulin hypermutation reduces the affinity and neutralizing activities of antibodies against HCV envelope protein. J Virol. 2008;82:6711–6720. doi: 10.1128/JVI.02582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melzi E, et al. Follicular dendritic cell disruption as a novel mechanism of virus-induced immunosuppression. Proc Natl Acad Sci USA. 2016;113:E6238–E6247. doi: 10.1073/pnas.1610012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Vries RD, et al. Measles immune suppression: lessons from the macaque model. PLOS Pathog. 2012;8:e1002885. doi: 10.1371/journal.ppat.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tatsuo H, Ono N, Yanagi Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J Virol. 2001;75:5842–5850. doi: 10.1128/JVI.75.13.5842-5850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Planz O, Seiler P, Hengartner H, Zinkernagel RM. Specific cytotoxic T cells eliminate B cells producing virus-neutralizing antibodies. Nature. 1996;382:726–729. doi: 10.1038/382726a0. [DOI] [PubMed] [Google Scholar]
- 95.Battegay M, et al. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- 96.Zomer A, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1:126–134. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 98.Barnaba V, Franco A, Alberti A, Benvenuto R, Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature. 1990;345:258–260. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- 99.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc Natl Acad Sci USA. 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roost H, et al. An acquired immune suppression in mice caused by infection with lymphocytic choriomeningitis virus. Eur J Immunol. 1988;18:511–518. doi: 10.1002/eji.1830180404. [DOI] [PubMed] [Google Scholar]
- 102.Scandella E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 103.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laidlaw BJ, Cyster JG. Interfer’n with antibody responses. Sci Immunol. 2016;1:eaaj1836. doi: 10.1126/sciimmunol.aaj1836. [DOI] [PubMed] [Google Scholar]