Abstract
Face attractiveness can influence memory for previously seen faces. This effect has been shown to differ for young and older perceivers. Two parallel studies examined the moderation of both the age of the face and the age of the perceiver on the relationship between facial attractiveness and face memory. Study 1 comprised 29 young and 31 older participants; Study 2 comprised 25 young and 24 older participants. In both studies, participants completed an incidental face encoding and a surprise old/new recognition test with young and older faces that varied in face attractiveness. Face attractiveness affected memory for young but not older faces. In addition, young but not older perceivers showed a linear effect of facial attractiveness on memory for young faces, while both young and older perceivers showed a quadratic effect on memory for young faces. These findings extend previous work by demonstrating that the effect of facial attractiveness on face memory is a function of both the age of the perceiver and the age of the face. Factors that could account for such moderations of face and perceiver age on the associations between face attractiveness and face memory are discussed (e.g., age differences in social goals and face similarity/distinctiveness).
Keywords: Face Attractiveness, Face Memory, Face Age, Perceiver Age
1. Introduction
Attractiveness is a salient facial feature that plays an important role in social perception and interpersonal interactions (Hugenberg & Wilson, 2013). For example, attractiveness is positively related to mate selection (e.g., in dating paradigms; Li et al., 2013) and results in a “beautiful-is-good” halo effect (Dion, Berscheid, & Walster, 1972) in other social contexts. Highly attractive compared to less attractive faces are more likely to be evaluated as more positive on dimensions such as intelligence (Zebrowitz & Rhodes, 2004), competence (Shahani-Denning, 2003), success (Eagly, Ashmore, Makhijani, & Longo, 1991), and favorable personality characteristics (Dion et al., 1972). These positive evaluations are associated with a broad array of advantages. For example, individuals who are more attractive compared to those who are less attractive have a greater chance of being hired (Desrumaux, De Bosscher, & Léoni, 2009; Dipboye, Fromkin, & Wiback, 1975; Gilmore, Beehr, & Love, 1986; Luxen & Van De Vijver, 2006), of receiving a higher income (Frieze, Olson, & Russell, 1991), and are more likely receive help and support from others (Benson, Karabenick, & Lerner, 1976).
Accurate face memory is important for successful social interactions and socioemotional well-being (Sommer, Hildebrandt, & Schacht, 2014). Past research on how memory for faces is affected by face attractiveness has produced mixed results. While some studies found better memory for attractive compared to unattractive faces (Cross, Cross, & Daly, 1971; Tsukiura & Cabeza, 2011; Zhang et al., 2011), other studies found that less attractive faces were better remembered (Light, Hollander, & Kayra-Stuart, 1981; Wiese, Altmann, & Schweinberger, 2014). In addition, some studies reported a nonlinear relationship, expressing that more and less attractive faces, compared to moderately attractive faces, were better remembered (Fleishman, Buckley, Klosinsky, Smith, & Tuck, 1976; Shepherd & Ellis, 1973). This mixed evidence regarding the link between face attractiveness and face memory may have resulted from methodological differences related to the face stimuli used and the participants tested across studies.
Interestingly, most prior research on the link between face attractiveness and face memory was conducted with young adult participants and used young adult faces. However, there is evidence that attractiveness evaluation depends on both perceiver age and face age (Ebner, 2008; Ebner et al., 2018; Foos & Clark, 2011; Lin, Lendry, & Ebner, 2016). Furthermore, there appears to be an age-of-perceiver moderation on the effect of face attractiveness on social cognition (e.g., impression formation). In particular, the attractiveness halo effect was weaker in older compared to younger perceivers, suggesting that facial attractiveness is less relevant for older adults when both age groups evaluated faces on dimensions like trustworthiness, health, competence, and likeability (Lin et al., 2016; Zebrowitz & Franklin, Jr, 2014). Based on these findings, it is plausible that the effect of face attractiveness on face memory varies as a function of perceiver and face age. Along this line, we recently demonstrated that both young and older adults had better memory for more and less attractive faces, compared to moderately attractive faces (i.e., quadratic effect), while only young but not older adults had additionally enhanced memory for more attractive faces (i.e., linear effect; Lin et al., 2016). While this previous study used both young and older faces as experimental stimuli, the analyses reported did not differentiate between faces of different ages (i.e., young vs. older faces), despite evidence of age of face as a developmentally relevant factor in face processing (Ebner, 2008; Ebner et al., 2018; Rhodes & Anastasi, 2012).
Thus, one possibility is that linear and quadratic effects of attractiveness reflect different processes that influence face memory. For example, Lin et al. (2016) observed that both young and older adults showed better memory for less and more attractive faces compared to moderately attractive faces. One possibility is that both low and high face attractiveness elicits attention, for example, because of distinctiveness or emotional arousal. This idea is supported by previous studies which show that memory was enhanced for distinctive (Gallo, Cotel, Moore, & Schacter, 2007; Schacter, Israel, & Racine, 1999) and emotional information (Budson et al., 2006; Fung & Carstensen, 2003; Kensinger, Allard, & Krendl, 2014) in both young and older adults.
In contrast, the linear association between face attractiveness and face memory for young but not older adults may reflect age differences in goal-directed processes (Lin et al., 2016). Attending to attractive faces may be particularly salient in the context of activities such as making new friends and developing romantic relationships, which are primary social goals in young adulthood (Erikson 1966; Fredrickson & Carstensen, 1990; Zimmer-Gembeck, 2002). For example, young adults compared to other age groups reported the largest number of friends (Gillespie, Lever, Frederick, & Royce, 2015), indicating the importance of making friends in young adulthood. Furthermore, reward network activity was greater when young adults evaluated attractive compared to unattractive faces (Aharon et al., 2001; Chatterjee, Thomas, Smith, & Aguirre, 2009; Cloutier, Heatherton, Whalen, & Kelley, 2008; Liang, Zebrowitz, & Zhang, 2010; O’Doherty et al., 2003; Winston, O’Doherty, Kilner, Perrett, & Dolan, 2007), suggesting greater rewarding value associated with attractive than unattractive faces for young adults. In contrast, as people age, forming new friendships and finding a partner are typically not primary social goals (Fredrickson & Carstensen, 1990; Lindau et al., 2007). Rather, older adults increasingly focus on fostering current close and emotionally significant relationships. Thus, face attractiveness likely becomes a less relevant feature with increasing age. Consistent with the idea that the relation between attractiveness and memory vary with age of the perceiver, Lin et al. (2016) observed enhanced memory for more attractive faces in young but not older adults. However, the encoding task in this previous study required participants to form associations between faces and personality traits. Therefore, it is impossible to exclude effects of personality trait ratings (e.g., valence, age-typicality) on face memory in this prior work. In contrast, the present project adopted a face old/new recognition test, in which only faces were presented during both incidental encoding and a surprise recognition memory test.
If age differences in social goals modulate the effects of face attractiveness on face memory, the age of a face may play a crucial role in how face attractiveness is processed, possibly in interaction with perceiver age, and with consequential effects on face memory. That is, face attractiveness may be particularly prominent when interacting with young adults (e.g., looking for a romantic partner, hiring a new employee) but less so when interacting with older adults (e.g., maintaining a close friendship, consulting an expert in a topic). Some evidence supporting this idea comes from a study in which participants of various ages were more likely to select young compared to older adults as dating targets (Kurzban & Weeden, 2005). However, this study did not report explicit comparisons between young and older adult participants. Based on the rationale that different-aged individuals are associated with different social goals, we propose that face attractiveness is a factor that is more likely to influence memory for young than older faces. Unlike previous work that did not consider age of face as a factor (Lin et al., 2016), the present study allowed for the examination of potential interaction effects between the age of perceiver and the age of the face and their relationship to face attractiveness and face memory.
Thus, going beyond previous work, both theoretically and methodologically, the present project tested the following research hypotheses in two independent studies: As face attractiveness is a more salient feature for processing young than older faces, we expected an effect of face attractiveness on face memory for young but not older faces in both adult age groups (Hypothesis 1; significant effects of face attractiveness on memory for young but not older faces for both young and older perceivers). Furthermore, as face attractiveness is more relevant to younger than older adults’ social goals, we expected a linear effect of face attractiveness on memory for young faces in young but not older perceivers (Hypothesis 2; better memory for more attractive compared to moderately attractive or less attractive young faces in young perceivers). In contrast, based on the previous work (Lin et al., 2016), we expected a quadratic effect of face attractiveness on face memory for young faces in both perceiver age groups (Hypothesis 3; better memory for more and less attractive compared to moderately attractive young faces in both young and older perceivers).
2. Materials and Methods
2.1. Participants
Study 1 comprised 29 young (M = 25.1 yrs., SD = 3.5, 20–31 yrs., 51.7% female) and 31 older (M = 68.4 yrs., SD = 2.7, 65–74 yrs., 58.1% female) participants. Participants were recruited through newspaper ads. Two young and two additional older participants were excluded because their face recognition responses were not successfully recorded. The local ethics committee in Stockholm, Sweden, approved the study protocol. We obtained informed consent from all participants before the start of the study. Young and older participants did not differ in years of education (Young Participants: M = 14.8 yrs., SD = 2.2; Older Participants: M = 14.4 yrs., SD = 3.6; F[1, 55] = .26, p = .61) or the Mini Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975; cut-off score < 27; Young Participants: M = 29.29, SD = 0.71; Older Participants: M = 28.93., SD = .94; F[1, 55] = 2.49, p = 0.12). All participants were in good health, with no known history of stroke, heart disease, or primary degenerative neurological disorders, and were right-handed. They all had normal or corrected-to-normal vision. Table 1 presents descriptive information and age-group differences in cognitive and affective measures in Study 1. Young compared to older participants showed better fluid cognitive abilities such as processing speed, episodic memory, and working memory. In contrast, older participants outperformed young participants on vocabulary, a measure of crystallized cognitive abilities. Young and older participants did not differ in negative affect (i.e., anxiety, depression).
Table 1.
Means (standard deviations)/percent and age differences in health, sensory, cognitive, and affective measures in Study 1 and Study 2
| Construct | Measure | Young Participants | Older Participants | F/χ2-value | Effect Size |
|---|---|---|---|---|---|
| Study 1 | |||||
| Cognitive | |||||
| Verbal Fluency | Verbal Fluency Task | 14.73 (4.96) | 16.37 (7.04) | 1.03 | 0.27 |
| Processing Speed | Letter Comparison Task | 11.11 (2.10) | 8.35 (1.87) | 27.99 | 1.39 |
| Episodic Memory | Free Word Recall Task | 10.04 (2.36) | 7.33 (1.84) | 23.73 | 1.27 |
| Working Memory | 2-Back Digit Task | 8.38 (1.41) | 6.33 (1.95) | 20.02 | 1.20 |
| Vocabulary | Swedish Synonym Task | 22.50 (3.69) | 26.17 (2.55) | 19.64 | 1.16 |
| Affective | |||||
| Anxiety | State-Trait Anxiety Inventory | 30.07 (5.23) | 28.9 (6.85) | 0.52 | 0.19 |
| Depression | Geriatric Depression Scale | 1.39 (1.66) | 1.57 (2.56) | 0.09 | 0.08 |
| Study 2 | |||||
| General Health | Single-Item | 4.36 (0.70) | 4.21 (.71) | 0.56 | 0.21 |
| Sensory | |||||
| Hearing Difficulty | Single-Item | 0.00% | 58.30% | 20.42 | 33.60 |
| Contrast Sensitivity | MARS Letter Contrast Sensitivity Test | 1.68 (0.15) | 1.56 (.14) | 7.5 | 1.24 |
| Vision | Rosenbaum Pocket Vision Screener | 22.40 (5.02) | 52.08 (50.43) | 8.58 | 1.32 |
| Cognitive | |||||
| Processing Speed | Digital-Symbol Substitute Test | 67.48 (11.96) | 45.46 (7.86) | 57.5 | 2.17 |
| Affective | |||||
| Positive Affect | Positive Affect and Negative Affect | 2.99 (0.57) | 4.03 (1.47) | 10.77 | 0.94 |
| Negative Affect | Schedule | 1.28 (0.48) | 1.19 (0.38) | 0.55 | 0.21 |
Note. In Study 1, Verbal Fluency Task (Lezak, 1995), higher score indicated better word fluency; Letter Comparison Task (Salthouse & Babcock, 1991), higher score indicated faster processing speed; Free Word Recall Task (i.e., recall a list of 16 words after 120s retention), higher score indicated better episodic memory; 2-Back Digit Task (Kirchner, 1958), higher score indicated better working memory; Swedish Synonym Task (Dureman, 1960), higher score indicated larger vocabulary; State-Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), higher score indicated more anxiety; Geriatric Depression Scale (Brink et al., 1982; Gottfries, 1997 for Swedish version), higher score indicated more depression. In Study 2, General Health (In general (i.e., over the past year), how would you rate your health and physical well-being?), higher score indicated better health condition, scale ranged from 1 = Poor to 5 = Excellent; Hearing Difficulty (Do you have any hearing difficulties?), 0 = No, 1 = Yes; MARS Letter Contrast Sensitivity Test (Arditi, 2005), higher score indicated better contrast sensitivity; Rosenbaum Pocket Vision Screener (Rosenbaum, Granham-FieldSurgical Co Inc, New York, NY), higher score indicated better vision; Digital-Symbol Substitute Test (Wechsler, 1981), higher score indicated faster processing speed; Positive Affect and Negative Affect Schedule (Watson, Clark, & Tellegen, 1988), higher score indicated more positive and more negative mood, respectively. Cohen’s d was calculated for all measures, except hearing difficulty, to indicate the effect size of group difference. Instead, the odds ratio was calculated as effect size of group difference in hearing difficulty. In addition, we presumed one young participant having hearing difficulty to make the calculation of this odds ratio possible. Bold print indicates significant effects at p < 0.05.
Study 2 comprised 25 young (M = 22.2 yrs., SD = 2.9, 19–29 yrs., 60% female) and 24 older (M = 73.9 yrs., SD = 7.8, 63–92 yrs., 71% female) participants. Young participants were recruited through flyers on the Yale University campus. Older participants were recruited from the local community and senior citizen centers, with a mean of 16.9 years of education (SD = 1.6). Table 1 presents descriptive information and age-group differences in health, sensory, cognitive, and affective measures in Study 2. Young compared to older participants showed better sensory abilities (i.e., hearing, vision) and faster processing speed. In contrast, older compared to young participants showed higher positive affect, while the age groups did not differ in negative affect. The study protocol was approved by the Institutional Review Board (IRB) at Yale University; all participants were consented prior to enrollment.
While young participants in Study 1 were older than young participants in Study 2 (t(47) = 3.51, p = .001), for older participants chronological age did not differ between the two studies (t(55) = .006, p = .995). Further, older participants in Study 1 had fewer years of education than older participants in Study 2 (t(52) = −3.01, p = .004). None of the other measures were the same for the two studies and thus could not be directly compared. However, as summarized in Table 1, the samples in the two studies were overall comparable on sensory, cognitive, and affective functioning.
2.2. Selection of Face Stimuli and Face Attractiveness Ratings
The face stimuli used in this project were selected from the FACES database (Ebner, Riediger, & Lindenberger, 2010), a standardized and validated database that comprises digital, front-view color photographs of faces from young, middle-aged, and older adults. For the Face Encoding and Recognition Task (described below), we selected young (age range: 18–31 yrs.) and older (age range: 69–80 yrs.) faces with neutral expressions for a total of 96 face stimuli, with equal numbers of male and female faces in each age group. We created two sets of face stimuli for the task (see details below). Each set consisted of 12 faces per age-by-gender group. We counterbalanced use of a set as target vs. distractor faces across participants.
Attractiveness ratings used in this study were taken from an independent data collection (reported in Ebner et al., 2010, 2018). In particular, 52 young (M = 25.9 yrs., 20–31 yrs., 52% female), 51 middle-aged (M = 50.0 yrs., 44–55 yrs., 51% female), and 51 older (M = 73.6 yrs., 70–81 yrs., 47% female) participants rated all face images from the FACES database on face attractiveness (How attractive is this person?; response options: 0 = Not at all attractive; 100 = Very attractive) and other dimensions (e.g., distinctiveness, perceived age, etc.). Not all participants in this previous study rated all faces (given dropouts, session duration limitations, and to reduce participant burden). The total number of rating data from young and older raters for the 96 faces used in the present project resulted in 8380 observations for the present analyses1. An independent t-test showed that attractiveness ratings from young raters (M = 36.93, SD = 26.79, Range: 0 – 100) were lower than attractiveness ratings from older raters (M = 49.03, SD = 25.34, Range: 0 – 100; t(8378) = −21.24, p < 0.001; Cohen’s d = 0.46). Also, young faces (M = 53.09, SD = 25.33, Range: 0 – 100) were rated as more attractive than older faces (M = 32.93, SD = 24.24, Range: 0 – 100; t(8378) = 37.21, p < 0.001; Cohen’s d = 0.81).
Face attractiveness ratings were highly consistent across raters as measured by the intra-class coefficient (ICC; Shrout & Fleiss, 1979; Total sample: ICC = 0.992; Young Participants: ICC = 0.990; Older Participants: ICC = 0.992). However, there was considerable interindividual difference between raters in the use of the range of the rating scale (M = 79.23, SD = 19.46, Range: 25 – 100) and in the mean of face attractiveness ratings (M = 43.97, SD = 16.61, Range: 10.36 – 84.54). Therefore, we transformed the original attractiveness ratings of each face into z-scores for each rater. We averaged these z-scores for each face across young and older raters, respectively. We used this age-group specific averaged z-score as a measure of attractiveness for the face stimuli in all subsequent analyses. The mean attractiveness for young faces was 0.50 (SD = 0.55; Range: −0.64 – 1.81) for young participants and 0.48 (SD = 0.36; Range: −0.21 – 1.37) for older participants. The mean attractiveness for older faces was −0.51 (SD = 0.31; Range: −0.97 – 0.55) for young participants and −0.48 (SD = 0.36; Range: −1.27 – 0.40) for older participants. The two sets of faces selected for the face encoding and recognition task were not different from each other on face attractiveness (Young Participants: F[1, 94] = 0.02, p = 0.89, η2p < 0.001); Older Participants: F[1, 94] = 0.03, p = 0.87; η2p < 0.001). Based on the ratings, we evenly categorized face stimuli in each set into four attractiveness levels (i.e., unattractive, somewhat unattractive, somewhat attractive, attractive). Older faces were rated as less attractive than young faces, and thus the categorization procedure was conducted separately for young and older faces. Consequentially, in each set of faces, there were six young and six older faces of each of the four attractiveness levels.
2.3. Face Encoding and Recognition Task
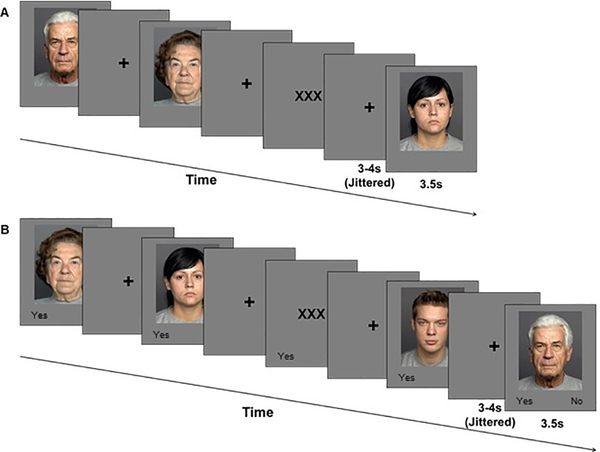
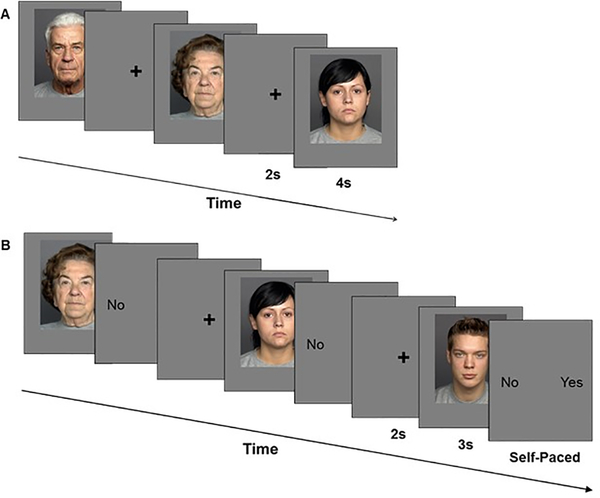
This task consisted of an encoding phase and a recognition phase (Panel A and B in Figures 1 and 2, respectively). The task was generally comparable for Study 1 and Study 2, with some modifications as described.
Figure 1.
Trial event timing for (A) encoding and (B) recognition phase of the Face Encoding and Recognition Task in Study 1.
Figure 2.
Trial event timing for (A) encoding and (B) recognition phase of the Face Encoding and Recognition Task in Study 2.
In Study 1, we pseudo-randomly intermixed 48 face trials from the target set with 24 low-level baseline trials (three Xs) during the encoding phase (Figure 1A). No more than two faces of the same age group or gender and no more than two low-level baseline trials followed in a sequence. Participants were instructed to view faces and baseline trials as if they were watching television at home (incidental encoding). The encoding session lasted 9 minutes.
A surprise old/new face recognition phase followed a retention interval of approximately eight to ten minutes (Figure 1B). During the recognition phase, face stimuli from the target and the distractor sets were pseudo-randomly intermixed with 48 low-level baseline trials. The position of target faces in the recognition list was controlled for based on their relative position in the encoding list by splitting the list into quarters. The first quarter of the recognition list comprised an equal number of target faces from each quarter of the encoding list. The same scheme applied to the creation of the second, third, and fourth quarter of the recognition list. Distractor faces were then evenly randomly intermixed in each quarter of the recognition list. No more than two faces of the same age or gender, no more than three faces, and no more than two low-level baseline trials followed in sequence. The response options Yes vs. No were presented below each face. Participants were instructed to use these responses to make the seen/not seen judgments as accurately and quickly as possible, while the face was presented on the screen. Each encoding and recognition trials were presented for 3500ms, followed by a fixation cross. The duration of the fixation cross was jittered (3000, 3250, 3500, 3750, or 4000ms). We used E-prime to present the experimental protocol (Schneider, Eschman, & Zuccolotto, 2002).
In Study 2 (Figure 2), we used no low-level baseline trials, and applied the same counterbalancing scheme as in Study 1. Therefore, the incidental encoding phase (instructing participants to view faces as if watching television) comprised 48 trials and the surprise yes/no recognition phase comprised 96 trials. The face presentation duration was 4000ms during encoding (Figure 2A) and 3000ms during recognition (Figure 2B). The duration of the fixation cross was 2000ms (not jittered). For recognition trials, the face stimuli disappeared after 3000ms, and the response options appeared on the screen, prompting participants to make the old/new judgments (response options Yes vs. No; self-paced). We presented the encoding phase with Gaze Tracker (Eye Response Technologies, Inc., Charlottesville, VA) and the recognition phase with E-prime (Schneider et al., 2002). The retention interval between the encoding and the recognition phase in Study 2 was comparable to that in Study 1.
2.4. Procedure
Study 1 started with informed consent followed by two sessions. In the first session, as summarized in Table 1, participants completed several paper-pencil questionnaires and worked on various cognitive tests on the computer (see Table 1 for details about questionnaires and computer tests). During the second session, which followed approximately one week later, participants worked on the Face Encoding and Recognition Task while undergoing functional magnetic resonance imaging (not reported here). At the end of the study, we debriefed and financially compensated participants for their study participation.
Study 2 also started with informed consent, followed by one test session in which participants first completed the encoding phase of the Face Encoding and Recognition Task. During the retention interval, participants responded to a short questionnaire about their demographics and physical health. Also, participants completed the Digit-Symbol-Substitution Test as a measure of processing speed (Wechsler, 1981). After completion of the recognition phase, participants completed short measures to assess sensory and affective functioning (Table 1). At the end of the study, we debriefed and financially compensated participants for their study participation2.
3. Results
The data had a nested structure (i.e., face trials nested within perceivers). Therefore, we used multilevel logistic regression (Hox, 2010) to determine the effect of face attractiveness on face memory for young and older faces in young and older perceivers. Our data analysis focused on target face trials. The outcome variable ‘correct memory for target faces’ was dichotomous and referred to the selection of “Yes” for a target face (i.e., correctly indicating a previously presented target face as seen/old). We estimated both linear and quadratic effects of face attractiveness on face memory, and going beyond Lin et al. (2016), we also considered their interaction with both perceiver age and face age. Following Aiken and West’s (1991) approach, we centered face attractiveness to make sure the linear and quadratic factors of face attractiveness were orthogonal. To make sure that the effects we were interested in did not simply reflect participants’ overall memory performance and response bias, we calculated the sensitivity (d’) and decision criterion (C) for each participant and added those two variables as covariates in the model.
We also conducted parallel analyses on the distractor faces to separately assess the effect of face attractiveness on identifying novel faces. In these analyses, the outcome variable ‘correct rejection of distractor faces’ was selection of “No” for a distractor face (i.e., correctly indicating that the distractor face had not been seen before). The results of analyses for distractor face trials are reported in the supplementary material.
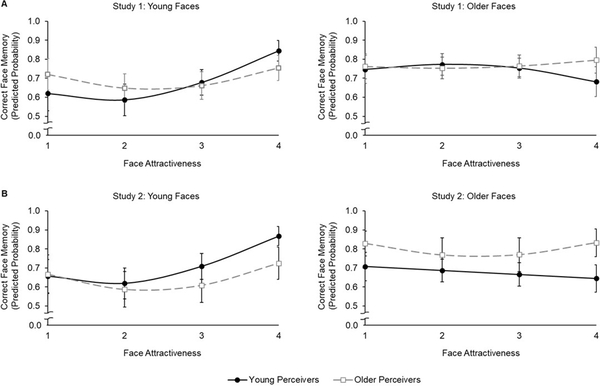
Study 1 (see Figure 3A)
Figure 3.
Predicted probability of correct face memory (dichotomous variable; 0 = Not correct; 1 = Correct) as a function of face attractiveness (1 = Unattractive, 2 = Somewhat Unattractive, 3 = Somewhat Attractive, 4 = Attractive) in young (black solid line) and older (grey dashed line) perceivers for young and older faces in Study 1 (A) and Study 2 (B), respectively. Error bars represent 95% confidence intervals. In both studies, effects of face attractiveness were only observed in memory for young (the left panel) but not older (the right panel) faces. Regarding memory for young faces, young perceivers (black solid line) showed a quadratic plus a positive linear effect of face attractiveness on face memory. In contrast, older perceivers (grey dashed line) showed only a quadratic effect of face attractiveness on face memory.
In Study 1, the linear effect of face attractiveness on memory for target faces was significant (B = 0.12, z = 3.12, p = 0.002, odds ratio = 1.13). The two-way interaction between face age and the linear trend of face attractiveness (B = −0.14, z = −3.04, p = 0.002, odds ratio = 0.87) and the three-way interaction between perceiver age, face age, and the linear trend of face attractiveness (B = 0.14, z = 3.08, p = 0.002, odds ratio = 1.15) were significant. This suggested a moderation by perceiver age and face age on the linear effect of face attractiveness on memory for target faces. In addition, the quadratic effect of face attractiveness on memory for target faces was significant (B = 0.11, z = 2.44, p = 0.015, odds ratio = 1.12). Furthermore, the two-way interaction of face age and the quadratic trend of face attractiveness (B = −0.15, z = −4.08, p < 0.001, odds ratio = 0.86) was significant, suggesting a moderation by face age on the quadratic effect of face attractiveness on memory for target faces. The three-way interaction between perceiver age, face age, and the quadratic trend of face attractiveness (B = 0.07, z = 1.88, p = 0.06, odds ratio = 1.07) did not meet our significance threshold but reached marginal significance.
To allow interpretation of the moderation of face age, we conducted follow-up analyses to estimate the linear and the quadratic effects of face attractiveness and their interactions with perceiver age for young and older faces separately. As shown in Figure 3A, all significant effects relevant to face attractiveness on memory for target faces held for young but not older faces. These results supported Hypothesis 1 in that face attractiveness affected memory for young but not older faces in both perceiver age groups. In particular, for young faces, the linear effect of face attractiveness was significant (B = 0.26, z = 4.24, p < 0.001, odds ratio = 1.29). This effect was further qualified by a significant moderation of perceiver age (B = −.19, z = −3.16, p = 0.001, odds ratio = 0.83) with a significant positive linear effect of face attractiveness on memory for young faces in young (B = 0.45, z = 4.72, p < 0.001, odds ratio = 1.57) but not older (B = 0.07, z = 0.86, p = 0.39, odds ratio = 1.07) perceivers. These findings supported Hypothesis 2 that the more attractive compared to either moderately attractive or less attractive young faces were better remembered by young perceivers (with this effect not present in older perceivers). In addition, the quadratic effect of face attractiveness was significant (B = 0.26, z = 4.43, p < 0.001, odds ratio = 1.30). Confirming Hypothesis 3, this quadratic effect of face attractiveness on memory for target faces was not moderated by perceiver age (B = −0.04, z = −0.70, p = 0.49, odds ratio = 0.96). That is, more and less attractive compared to moderately attractive young faces were better remembered by both young and older perceivers.
Study 2 (see Figure 3B)
The pattern of results in Study 2 largely replicated the findings in Study 1. In particular, the linear effect of face attractiveness on memory for target faces was significant (B = 0.12, z = 3.20, p = 0.001, odds ratio = 1.18). In addition, the two-way interaction of face age and the linear trend of face attractiveness (B = −0.16, z = −5.35, p < 0.001, odds ratio = 0.85) and the three-way interaction of perceiver age, face age, and face attractiveness (B = 0.08, z = 2.54, p = 0.011, odds ratio = 1.08) were significant. Consistent with Study 1, these findings suggested that the linear effect of face attractiveness on memory for target faces varied depending on perceiver’s age and age of the face. In addition, the quadratic effect of face attractiveness on memory for target faces was significant (B = 0.16, z = 4.16, p < 0.001, odds ratio = 1.18). Inconsistent with Study 1, however, this quadratic effect of face attractiveness on memory for target faces was neither moderated by the perceiver’s age nor by face age.
Consistent with Study 1, all significant effects relevant to face attractiveness on memory held for young but not older faces (Figure 3B). These results lend further support to Hypothesis 1, indicating that face attractiveness affected memory for young but not older faces in both perceiver age groups. For young faces, the linear effect of face attractiveness was significant (B = 0.28, z = 5.83, p < 0.001, odds ratio = 1.32). This effect was further qualified by a significant moderation of the perceiver’s age (B = −0.16, z = −3.44, p = 0.001, odds ratio = 0.85) with a significant positive linear effect on memory for target faces in young (B = 0.44, z = 6.13, p < 0.001, odds ratio = 1.55) but not older (B = 0.11, z = 1.80, p = 0.07, odds ratio = 1.12) perceivers. These findings replicated those in Study 1 and lend further support to Hypothesis 2. The quadratic effect of face attractiveness was also significant (B = 0.23, z = 4.19, p < 0.001, odds ratio = 1.26). However, this quadratic effect of face attractiveness was not moderated by perceiver age (B = - 0.02, z = −0.33, p = 0.74, odds ratio = .98), in line with Study 1 and in support of Hypothesis 3.
In summary, as depicted in Figure 3A (Study 1) and 3B (Study 2), regarding memory for young faces (on the left panel), both young (black solid line) and older (grey dashed line) perceivers showed better memory for more and less attractive compared to moderately attractive faces. Young perceivers (black solid line), in addition, showed a memory advantage for more attractive faces. In contrast, neither young nor older perceivers showed either linear or quadratic associations between face attractiveness and memory for older faces (on the right panel).
4. Discussion
This project examined the effects of attractiveness of young and older faces on face memory in young and older perceivers. Our findings, replicated in two independent studies, were largely consistent with our predictions and qualify previous findings on the link between face attractiveness and face memory. They illustrate the importance of an adult developmental perspective on face perception and face memory by demonstrating: (i) In line with Hypothesis 1, face attractiveness affected memory for young but not older faces in both young and older perceivers. (ii) In line with Hypothesis 2, the linear effect of face attractiveness on face memory for young faces was significant in young but not in older perceivers. (iii) In line with Hypothesis 3, both young and older perceivers showed a quadratic effect of face attractiveness on memory for young faces; moderately attractive faces were remembered less well than more or less attractive faces. Furthermore, these associations between face attractiveness and correct recognition of previously presented faces did not vary by overall memory performance as measured as d’ and C. In the following, we will discuss these novel findings regarding their theoretical implications and in relation to the literature.
Most previous studies of associations between face attractiveness and face memory considered exclusively young adult faces as experimental stimuli (Light et al., 1981; Shepherd & Ellis, 1973; Tsukiura & Cabeza, 2011; Wiese et al., 2014; Zhang et al., 2011). In contrast, the present study adopted an adult developmental perspective by also considering older adult faces. This design is in line with the notion in developmental/aging theory that the relevance of attractiveness as a facial feature and its impact on memory varies as a function of the age of the face (Ebner, 2008; Ebner et al., 2018). Indeed, we found that face attractiveness affected memory for young but not older faces. This may be because young compared to older faces are more likely the target of activities in which face attractiveness plays a critical role (e.g., mate selection, Buss & Barnes, 1986; hiring, Commisso & Finkelstein, 2012; Fruhen, Watkins, & Jones, 2015; Gilmore et al., 1986). In addition, previous studies have shown that various facial features (e.g., orbital region, mouth, skin texture), which are essential for face attractiveness evaluation, are affected by the normal aging process (Coleman & Grover, 2006; Ramanathan, Chellappa, & Biswas, 2009). Those age-related changes in facial features may result in greater similarity among older than young faces (i.e., reduced distinctiveness in older faces; Ebner, 2008, 2018). This age-related increase in similarity may reduce variability in face attractiveness levels among older compared to young faces and render face attractiveness a relatively less distinct facial feature in older faces. In line with this speculation, older compared to young face stimuli used in the present studies had a narrower range on face attractiveness ratings from both young and older raters and showed less variability in ratings from young raters (Ebner et al., 2018). As our findings suggested that face attractiveness may be less likely a factor that influences memory for older faces than young faces, an un-answered question for future studies to address is what facial feature (e.g., trustworthiness, life experience, etc) does affect individuals’ memory for older faces.
In addition to the moderation effect of face age, the present two-study project replicated previous work (Lin et al., 2016) that perceiver age moderates the effect of face attractiveness on face memory. In particular, both young and older perceivers showed better memory for more and less attractive compared to moderately attractive faces (quadratic effect), while only young but not older perceivers showed enhanced memory for more attractive compared to less attractive and moderately attractive faces (linear effect). We suggest that these perceiver age differences in the link between face attractiveness and face memory reflect reduced relevance of attractiveness as a facial feature in older compared to young perceivers. Face attractiveness constitutes a salient feature in young adulthood in social interactions (e.g., making new friends, developing romantic relationships and mate selection; Gillespie et al., 2015; Erikson 1966; Fredrickson & Carstensen, 1990), and the importance of attractiveness (further reinforced by movies, advertisements, and social media, disproportionately displaying as well as targeting young adults) may underlie the enhanced memory for more attractive faces in young adulthood. In contrast, as people age, face attractiveness may become a less relevant feature in social interactions since older adults are more likely to focus on fostering close and emotionally significant relationships (Fredrickson & Carstensen, 1990), social goals for which face attractiveness may be less relevant. This reduced relevance of attractive faces for social goals in older adults may explain the absence of a linear memory-enhancing effect of face attractiveness in older adulthood. Consistent with the idea that young adults are sensitive to face attractiveness, Tsukiura and Cabeza (2011) observed greater blood-oxygen-level-dependent (BOLD) activity in medial orbital frontal cortex (mOFC) and better memory for attractive than unattractive faces in young perceivers (the study did not include older adults). Enhanced mOFC activity may reflect the greater reward value associated with attractive compared to unattractive faces. The present results suggest that a comparison of mOFC activity in young and older perceivers when viewing young and older faces that vary in attractiveness would be informative.
As we did not experimentally manipulate social motivation in the present study, we can only speculate about the possibility that changes across adulthood in social motivation plays a role in the differences observed between young and older adults in the association between face attractiveness and face memory. For a direct examination of this mechanism, future research could ask participants to engage in encoding contexts in which processing face attractiveness is either task-relevant (e.g., pretending to be an editor of a fashion magazine who selects models for the magazine cover) or task-irrelevant (e.g., guessing the age of the person based on their face picture). If age differences in social goals underlie the differences in the association between face attractiveness and face memory between young and older adults, the linear effect of face attractiveness on face memory should also be present in older adults when they encode faces in a task in which face attractiveness is highly relevant.
It is also possible that the positive linear effect of face attractiveness on memory for young target faces reflects a more liberal decision criterion in the recognition of positive than negative stimuli (Grider & Malmberg, 2008; Phaf & Rotteveel, 2005).Consistent with this possibility, our parallel analyses for distractor faces found a negative linear effect of face attractiveness on correct rejection of young distractor faces. That is, the more attractive young distractor faces were, the less likely they were correctly rejected. This effect was present in young participants in both studies and in older participants in Study 2. However, in addition or alternative to a response bias, it is possible that more attractive compared to the less attractive faces share more similarity with each other and therefore it is harder to discriminate previously seen from novel faces. This interpretation is in line with evidence that one of the critical facial qualities associated with attractiveness is averageness (Foo, Simmons, & Rhodes, 2017; Langlois, Roggman, & Musselman, 1994; Rhodes & Tremewan, 1996). Future research will be needed to dissociate these alternative explanations by, for example, looking at how attractiveness affects the ability to perceptually differentiate between two faces, or controlling in a recognition memory study the level of similarity between old and new faces at different levels of attractiveness.
While we observed an age-differential pattern for the linear effect of face attractiveness on face memory, both young and older adults showed a quadratic effect of face attractiveness on face memory. This quadratic association between face attractiveness and face memory may reflect the impact of emotion on memory (Adelman & Estes, 2013; Sommer, Gläscher, Moritz, & Büchel, 2008). That is, emotional information is often better remembered than neutral information (Brown & Kulik, 1977; Kensiger & Corkin 2003). Consistent with our present findings, previous literature showed that the memory advantage of emotional over neutral information is found in older adults, despite well-documented age-related decline in memory overall (Budson et al., 2006; Fung & Carstensen, 2003; Kensinger, Allard, & Krendl, 2014; see Murphy & Isaacowitz, 2008 for a meta-analysis).
It is reasonable to assume that very attractive or unattractive faces are more emotionally arousing than moderately attractive faces, particularly for young adults. The anomalous face overgeneralization hypothesis posits that the evolutionary significance of sensitivity to bad gene carriers (i.e., individuals with anomalous facial qualities) makes individuals overgeneralize their response to anomalous faces to normal faces with low attractiveness (Zebrowitz et al., 2003; Zebrowitz & Rhodes, 2004). As we discussed above, mate selection is a critical social motivation for young adults. For them, unattractive faces may therefore trigger a particularly negative response, while attractive faces may elicit a particularly positive response. In line with this proposition is evidence of a quadratic association between face attractiveness of young faces and amygdala activity in young adults (Liang et al., 2010; Winston et al., 2007).
In older adults, in contrast, face attractiveness may not be a relevant dimension related central social goals (e.g., fostering close and emotionally significant relationships) but other facial features may play a more prominent role. For example, face trustworthiness may constitute a salient factor when older adults process faces of unfamiliar others. In line with evidence that socially relevant traits perceived from facial appearance are strongly interrelated (Dion, Berscheid, & Walster, 1972; see also Oosterhof & Todorov, 2008), more and less attractive faces are likely to be also perceived as more and less trustworthy, respectively. Thus, it is possible that the quadratic effect of young face attractiveness on memory in older adults observed in the present study was accounted for by variation in perceived face trustworthiness. However, we did not assess face trustworthiness explicitly in the present project and this data was not available for the face stimuli from previous research. Therefore, we were not able to test this possible explanation in the present study. Extended future research will be beneficial to determine the relative contribution of diverse facial features as well as their interrelations (Cortes, Laukka, Ebner, & Fischer, 2019) in their impact on memory in young and older adults.
It is also possible that the quadratic effect of face attractiveness on face memory was a reflection of varying distinctiveness of more vs. less attractive faces (Sarno & Alley, 1997; Wiese et al., 2014). That is, highly attractive faces as well as highly unattractive faces may possess facial features to make them deviate from the average face (Perrett et al., 1998; Said & Todorov, 2011). These deviations may result in greater visual salience and better memory for those faces. Face distinctiveness ratings were available from Ebner et al. (2018) for the faces we used in the present project. Thus, we conducted a post-hoc analysis to examine whether face distinctiveness accounted for the quadratic effect of face attractiveness on memory for young target faces. After adding face distinctiveness as predictor into the model, the quadratic effect of face attractiveness was still significant in both studies, suggesting that face distinctiveness did not account for the quadratic effect of face attractiveness on memory for young faces. However, distinctiveness ratings in Ebner et al. were solely based on self-report. Future research may benefit from use of more objective feature-based scores of face distinctiveness (e.g., distance between landmarks; Zebrowitz, Kikuchi, & Fellous, 2007).
Although further work is needed to identify the underlying variables accounting for age-related variations in face memory, a reasonable hypothesis is that, for young faces, the quadratic effect that both young and older perceivers show may reflect greater initial attention to more and less compared to moderately attractive faces, while the linear effect may reflect the greater salience of attractiveness for young adults (i.e., leading to extended processing such as refreshing, e.g., Johnson. 1992; Johnson, Raye, Mitchell, Greene, Cunningham, & Sanislow, 2005). The fact that attractiveness ratings of older faces did not affect face memory for either young or older perceivers suggests that, as noted above, older faces vary less in attractiveness, and/or that attractiveness ratings as defined in the current study are not capturing features most relevant for eliciting the processes that would contribute to memory for older faces.
In conclusion, in two independent studies, we extended evidence of a dissociation between a linear and a quadratic relationship between face attractiveness and face memory, when considering perceiver age and face age. Our findings provide clear evidence that the link between face attractiveness and face memory is variable and that adoption of an adult developmental perspective to this research is informative. The present research highlights the importance of considering changes across adulthood in social motivational processes in their impact on encoding and remembering faces and emphasizes the need to conceptualize socio-affective memory as a dynamic construct. In addition to this potential for theory development, our findings may have practical implications in contexts in which face attractiveness is likely to influence decision making (e.g., advertisement, hiring).
Supplementary Material
Acknowledgement
Study 1 was supported by the Swedish Research Council (2008-2356) and Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse awarded to HF, National Institute on Aging grant (R37AG009253) to MKJ, and German Research Foundation Research Grant (DFG EB436/1-1) to NCE and was conducted at the Karolinska Institute MR-Center, Huddinge Hospital, Stockholm, Sweden. Study 2 was supported by the National Institute on Aging grant (NIH R37AG009253) to MKJ and a German Research Foundation Research Grant (DFG EB 436/1-1) to NCE and was conducted at Yale University.
The authors wish to thank Sebastian Gluth for assistant in programming the task, Anna Rieckmann for data collection in Study 1, Michael Marsiske, Ronald Cohen, and Andreas Keil for constructive feedback regarding data analysis and manuscript writing, and Sevilay Yumusak for proofreading the manuscript.
Footnotes
The authors report no conflict of interest.
The data that support the findings of this study are openly available in Open Science Framework at https://osf.io/8xfzp/?view_only=8a10708dd3674828befddf9538e477ec.
One young rater chose 0 for 95% of the ratings (M = 0.69, SD = 3.25, Range: 0 – 25), indicating failure to understand or low compliance with instructions. We excluded ratings from this rater.
In Study 1, participants underwent functional magnetic resonance imaging during the face encoding and recognition phases (Ebner et al., 2012 for neuroimaging details). In Study 2, participants’ eye movements were recorded during the encoding phase (He, Ebner, & Johnson, 2011 for details about the eye-tracking set-up). Here, we do not report results from brain and eye-tracking data but focus on the behavioral data pertaining to the relation between face recognition memory and ratings of face attractiveness.
Reference
- Adelman JS, & Estes Z (2013). Emotion and memory: A recognition advantage for positive and negative words independent of arousal. Cognition, 129, 530–535. [DOI] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, & Breiter HC (2001). Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron, 32, 537–551. [DOI] [PubMed] [Google Scholar]
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Sage. [Google Scholar]
- Arditi A (2005). Improving the design of the letter contrast sensitivity test. Investigative Ophthalmology & Visual Science, 46, 2225–2229. [DOI] [PubMed] [Google Scholar]
- Benson PL, Karabenick SA, & Lerner RM (1976). Pretty pleases: The effects of physical attractiveness, race, and sex on receiving help. Journal of Experimental Social Psychology, 12, 409–415. [Google Scholar]
- Brink TL, Yesavage JA, Lum O, Heersema PH, Adey M, & Rose TL (1982). Screening tests for geriatric depression. Clinical Gerontologist, 1, 37–43. [DOI] [PubMed] [Google Scholar]
- Brown R, & Kulik J (1977). Flashbulb memories. Cognition, 5, 73–99. [Google Scholar]
- Budson AE, Todman RW, Chong H, Adams EH, Kensinger EA, Krangel TS, & Wright CI (2006). False recognition of emotional word lists in aging and Alzheimer disease. Cognitive and Behavioral Neurology, 19, 71–78. [DOI] [PubMed] [Google Scholar]
- Buss DM, & Barnes M (1986). Preferences in human mate selection. Journal of Personality and Social Psychology, 50, 559–570. [Google Scholar]
- Chatterjee A, Thomas A, Smith SE, & Aguirre GK (2009). The neural response to facial attractiveness. Neuropsychology, 23, 135–143. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, & Kelley WM (2008). Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience, 20, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commisso M, & Finkelstein L (2012). Physical attractiveness bias in employee termination. Journal of Applied Social Psychology, 42, 2968–2987. [Google Scholar]
- Cortes DS, Laukka P, Ebner NC, & Fischer H (2019). Age-related differences in evaluation of social attributes from computer-generated faces of varying intensity. Psychology and Aging. [DOI] [PubMed] [Google Scholar]
- Cross JF, Cross J, & Daly J (1971). Sex, race, age, and beauty as factors in recognition of faces. Perception & Psychophysics, 10, 393–396. [Google Scholar]
- Desrumaux P, De Bosscher S, & Léoni V (2009). Effects of facial attractiveness, gender, and competence of applicants on job recruitment. Swiss Journal of Psychology, 68, 33–42. [Google Scholar]
- Dion K, Berscheid E, & Walster E (1972). What is beautiful is good. Journal of Personality and Social Psychology, 24, 285–290. [DOI] [PubMed] [Google Scholar]
- Dipboye RL, Fromkin HL, & Wiback K (1975). Relative importance of applicant sex, attractiveness, and scholastic standing in evaluation of job applicant resumes. Journal of Applied Psychology, 60, 39–43. [Google Scholar]
- Dureman I (1960). SRB: 1. Stockholm: Psykologiförlaget. [Google Scholar]
- Eagly AH, Ashmore RD, Makhijani MG, & Longo LC (1991). What is beautiful is good, but…: A meta-analytic review of research on the physical attractiveness stereotype. Psychological Bulletin, 110, 109–128. [Google Scholar]
- Ebner NC (2008). Age of face matters: Age-group differences in ratings of young and old faces. Behavior Research Methods, 40, 130–136. [DOI] [PubMed] [Google Scholar]
- Ebner N, Luedicke J, Voelkle MC, Riediger M, Lin T, & Lindenberger U (2018). An adult developmental approach to perceived facial attractiveness and distinctiveness. Frontiers in Psychology, 9, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK, & Fischer H (2012). Neural mechanisms of reading facial emotions in young and older adults. Frontiers in Psychology. 3:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Riediger M, & Lindenberger U (2010). FACES – A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behavior Research Methods, 42, 351–362. [DOI] [PubMed] [Google Scholar]
- Erikson EH (1966). Eight ages of man. International Journal of Psychiatry, 2, 281–300. [PubMed] [Google Scholar]
- Fleishman JJ Buckley ML, Klosinsky MJ, Smith N, & Tuck B (1976). Judged attractiveness in recognition memory of women’s faces. Perceptual and Motor Skills, 43, 709–710. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Foos PW, and Clark MC (2011). Adult age and gender differences in perceptions of facial attractiveness: Beauty is in the eye of the older beholder. Journal of Genetic Psychology, 172, 162–175. [DOI] [PubMed] [Google Scholar]
- Foo YZ, Simmons LW, & Rhodes G (2017). Predictors of facial attractiveness and health in humans. Scientific Reports, 7, 39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, & Carstensen LL (1990). Choosing social partners: How old age and anticipated endings make people more selective. Psychology and Aging, 5, 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieze IH, Olson JE, & Russell J (1991). Attractiveness and Income for Men and Women in Management1. Journal of Applied Social Psychology, 21, 1039–1057. [Google Scholar]
- Fruhen LS, Watkins CD, & Jones BC (2015). Perceptions of facial dominance, trustworthiness and attractiveness predict managerial pay awards in experimental tasks. The Leadership Quarterly, 26, 1005–1016. [Google Scholar]
- Fung HH, & Carstensen LL (2003). Sending memorable messages to the old: age differences in preferences and memory for advertisements. Journal of Personality and Social Psychology, 85, 163–178. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Cotel SC, Moore CD, & Schacter DL (2007). Aging can spare recollection-based retrieval monitoring: The importance of event distinctiveness. Psychology and Aging, 22, 209–213. [DOI] [PubMed] [Google Scholar]
- Gillespie BJ, Lever J, Frederick D, & Royce T (2015). Close adult friendships, gender, and the life cycle. Journal of Social and Personal Relationships, 32, 709–736. [Google Scholar]
- Gilmore DC, Beehr TA, & Love KG (1986). Effects of applicant sex, applicant physical attractiveness, type of rater and type of job on interview decisions. Journal of Occupational Psychology, 59, 103–109. [Google Scholar]
- Gottfries CG, Noltorp S, & Nørgaard N (1997). Recognition and management of depression in the elderly. International Clinical Psychopharmachology, 12, S31–S36. [DOI] [PubMed] [Google Scholar]
- Grider RC, & Malmberg KJ (2008). Discriminating between changes in bias and changes in accuracy for recognition memory of emotional stimuli. Memory & Cognition, 36, 933–946. [DOI] [PubMed] [Google Scholar]
- He Y, Ebner NC, & Johnson MK (2011). What predicts the own-age bias in face recognition memory? Social Cognition, 29, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox JJ (2010). Multilevel analysis. Techniques and applications. 2nd Edition. New York: Routledge. [Google Scholar]
- Hugenberg K, & Wilson JP (2013). Faces are central to social cognition In Carlston DE (Eds), The Oxford Handbook of Social Cognition (pp. 167–193). New York: Oxford University Press. [Google Scholar]
- Johnson MK (1992). MEM: Mechanisms of recollection. Journal of Cognitive Neuroscience, 4, 268–280. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, & Sanislow CA (2005). Using fMRI to investigate a component process of reflection: Prefrontal correlates of refreshing a just-activated representation. Cognitive, Affective, & Behavioral Neuroscience, 5, 339–361. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Allard ER, & Krendl AC (2014). The effects of age on memory for socioemotional material: an affective neuroscience perspective. The Oxford Handbook of Emotion, Social Cognition, and Problem-Solving in Adulthood, 26–46. [Google Scholar]
- Kirchner WK (1958). Age differences in short-term retention of rapidly changing information. Journal of Experimental Psychology, 55, 352–358. [DOI] [PubMed] [Google Scholar]
- Kurzban R, & Weeden J (2005). HurryDate: Mate preferences in action. Evolution and Human Behavior, 26, 227–244. [Google Scholar]
- Langlois JH, Roggman LA, & Musselman L (1994). What is average and what is not average about attractive faces?. Psychological Science, 5, 214–220. [Google Scholar]
- Lezak MD (1995). Neuropsychological Assessment 3rd Edition. New York: Oxford University Press, USA. [Google Scholar]
- Li NP, Yong JC, Tov W, Sng O, Fletcher GJ, Valentine KA, … & Balliet D (2013). Mate preferences do predict attraction and choices in the early stages of mate selection. Journal of Personality and Social Psychology, 105, 757–776. [DOI] [PubMed] [Google Scholar]
- Liang X, Zebrowitz LA, & Zhang Y (2010). Neural activation in the “reward circuit” shows a nonlinear response to facial attractiveness. Social Neuroscience, 5, 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light LL, Hollander S, & Kayar-Stuart F (1981). Why attractive people are harder to remember. Personality & Social Psychology Bulletin, 7, 269–276. [Google Scholar]
- Lin T, Lendry R, & Ebner NC (2016). Face likeability mediates the memory-enhancing effect of face attractiveness in young but not older adults. Memory, 24, 1396–1406. [DOI] [PubMed] [Google Scholar]
- Lindau ST, Schumm LP Laumann EO Levinson W, O’Muircheartaigh CA, & Waite LJ (2007). A study of sexuality and health among older adults in the United States. The New England Journal of Medicine, 357, 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxen MF, & Van De Vijver FJ (2006). Facial attractiveness, sexual selection, and personnel selection: When evolved preferences matter. Journal of Organizational Behavior, 27, 241–255. [Google Scholar]
- Murphy NA, & Isaacowitz DM (2008). Preferences for emotional information in older and younger adults: a meta-analysis of memory and attention tasks. Psychology and Aging, 23, 263–286. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, & Dolan RJ (2003). Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia, 41, 147–155. [DOI] [PubMed] [Google Scholar]
- Oosterhof NN, & Todorov A (2008). The functional basis of face evaluation. Proceedings of the National Academy of Sciences, 105, 11087–11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Lee KJ, Penton-Voak I, Rowland D, Yoshikawa S, Burt DM, Henzi SP, Castles DL, & Akamatsu S (1998). Effects of sexual dimorphism on facial attractiveness. Nature, 394, 884–887. [DOI] [PubMed] [Google Scholar]
- Phaf RH, & Rotteveel M (2005). Affective modulation of recognition bias. Emotion, 5, 309–318. [DOI] [PubMed] [Google Scholar]
- Rajaram S (1993). Remembering and knowing: Two means of access to the personal past. Memory & Cognition, 21, 89–102. [DOI] [PubMed] [Google Scholar]
- Rhodes MG, & Anastasi JS (2012). The own-age bias in face recognition: a meta-analytic and theoretical review. Psychological Bulletin, 138, 146–174. [DOI] [PubMed] [Google Scholar]
- Rhodes G, & Tremewan T (1996). Averageness, exaggeration, and facial attractiveness. Psychological Science, 7, 105–110. [Google Scholar]
- Said CP, Baron SG, & Todorov A (2009). Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience, 21, 519–528. [DOI] [PubMed] [Google Scholar]
- Said CP, & Todorov A (2011). A Statistical Model of Facial Attractiveness. Psychological Science, 22, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, & Babcock RL (1991). Decomposing adult age differences in working memory. Developmental Psychology, 27, 763–776. [Google Scholar]
- Sarno JA, & Alley TR (1997). Attractiveness and the memorability of faces: Only a matter of distinctiveness?. American Journal of Psychology, 110, 81–92. [Google Scholar]
- Schacter DL, Israel L, & Racine C (1999). Suppressing false recognition in younger and older adults: The distinctiveness heuristic. Journal of Memory and Language, 40, 1–24. [Google Scholar]
- Schneider W, Eschman A, & Zuccolotto A (2002). E-Prime reference guide. Pittsburgh, PA: Psychology Software Tools, Incorporated. [Google Scholar]
- Shahani-Denning C (2003). Physical attractiveness bias in hiring: What is beautiful is good. Hofstra Horizon, 14–17. [Google Scholar]
- Shepherd JW, & Ellis HD (1973). The effect of attractiveness on recognition memory for faces. American Journal of Psychology, 86, 627–633. [PubMed] [Google Scholar]
- Shrout PE, & Fleiss JL (1979). Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin, 86, 420–428. [DOI] [PubMed] [Google Scholar]
- Sommer T, Gläscher J, Moritz S, & Büchel C (2008). Emotional enhancement effect of memory: Removing the influence of cognitive factors. Learning & Memory, 15, 569–573. [DOI] [PubMed] [Google Scholar]
- Sommer W, Hildebrandt A, & Schacht A (2014). Face Perception In Encyclopedia of Quality of Life and Well-Being Research (pp. 2109–2112). Springer; Netherlands. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, and Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press. [Google Scholar]
- Todorov A, Baron SG, & Oosterhof NN (2008). Evaluating face trustworthiness: a model based approach. Social Cognitive and Affective Neuroscience, 3, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, & Cabeza R (2011). Remembering beauty: roles of orbitofrontal and hippocampal regions in successful memory encoding of attractive faces. Neuroimage, 54, 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). Manual for the Wechsler Adult Intelligence Scale—Revised. Psychological Corporation, New York. [Google Scholar]
- Wiese H, Altmann CS, & Schweinberger SR (2014). Effects of attractiveness on face memory separated from distinctiveness: Evidence from event-related brain potentials. Neuropsychologia, 56, 26–36. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, & Dolan RJ (2007). Brain systems for assessing facial attractiveness. Neuropsychologia, 45, 195–206. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Fellous JM, Mignault A, & Andreoletti C (2003). Trait impressions as overgeneralized responses to adaptively significant facial qualities: Evidence from connectionist modeling. Personality and Social Psychology Review, 7, 194–215. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA & Franklin RG Jr. (2014). The attractiveness halo effect and the baby face stereotype in older and younger adults: Similarities, own-age accentuation, and OA positivity effects. Journal of Experimental Aging Research, 40, 375–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz LA, Kikuchi M, & Fellous JM (2007). Are effects of emotion expression on trait impressions mediated by babyfaceness? Evidence from connectionist modeling. Personality and Social Psychology Bulletin, 33, 648–662. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, & Rhodes G (2004). Sensitivity to “bad genes” and the anomalous face overgeneralization effect: Cue validity, cue utilization, and accuracy in judging intelligence and health. Journal of Nonverbal Behavior, 28, 167–185. [Google Scholar]
- Zhang Y, Kong F, Chen H, Jackson T, Han L, Meng J, Yang Z, Gao J, & Najam ul Hasan A (2011). Identifying cognitive preferences for attractive female faces: An event‐related potential experiment using a study‐test paradigm. Journal of Neuroscience Research, 89, 1887–1893. [DOI] [PubMed] [Google Scholar]
- Zimmer-Gembeck MJ (2002). The development of romantic relationships and adaptations in the system of peer relationships. Journal of Adolescent Health, 31, 216–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.