Abstract
Stakeholder-informed strategies addressing cardiovascular disease (CVD) burden among people living with HIV (PWH) are needed within healthcare settings. This study provides an assessment of how human-centered design (HCD) guided the adaptation of a nurse-led intervention to reduce CVD risk among PWH. Using a HCD approach, research staff guided two multidisciplinary “design teams” in Ohio and North Carolina, with each having five HCD meetings. We conducted acceptability and feasibility testing. Six core recommendations were produced by two design teams of key stakeholders and further developed after the acceptability and feasibility testing to produce a final list of 14 actionable areas of adaptation. Acceptability and feasibility testing revealed areas for adaptation, e.g. patient preferences for communication and the benefit of additional staff to support patient follow-up. In conclusion, along with acceptability and feasibility testing, HCD led to the production of 14 key recommendations to enhance the effectiveness and scalability of an integrated HIV/CVD intervention.
Keywords: Human-centered design, CVD-HIV integration, nurse-led intervention, CVD prevention
Globally, life expectancy for people with HIV (PWH) has increased dramatically largely owing to the effectiveness of combination antiretroviral therapy; HIV is now considered a chronic disease (1). Despite decreased AIDS-related morbidity and mortality among PWHs, the growing burden of non-communicable diseases (NCD), particularly cardiovascular disease (CVD), threatens to limit their overall quality of life (2,3). Evidence-informed strategies which focus on integrated NCD care models within HIV care platforms are a promising approach to counter this growing burden of co-morbid NCDs (4,5). Such strategies, however, require significant stakeholder engagement and analysis prior to the development of integrative models of care.
Enhancing Stakeholder Engagement for Intervention Tailoring Through a Human-Centered Design Approach
Over the past decade human-centered design (HCD), a staple within the engineering field, has become an increasingly valued method to engage stakeholders in innovating solutions and new models of healthcare delivery (6,7). For example, working with business, social and governmental sectors, IDEO, a leading design and consulting company, has been a forerunner in applying a human-centered approach to innovate and problem-solve (8). Human-centered design is an interactive approach which uses an iterative process termed “design thinking” that includes empathic discovery, rapid creation and reflective evaluation to ensure outcomes are driven by the unique preferences and circumstances of the people they serve (9). The goal of design thinking is to develop interventions that are desirable, feasible, and viable for stakeholders through continuous attention to the stakeholder environment and their unique context (9–11). From a human-centered perspective, innovation is not sustainable unless the intervention process: 1) includes key stakeholders; 2) places empathy (i.e. ability to understand another person’s perspectives, emotions, needs etc.) at the core; and 3) is interdisciplinary (6,12). While methods and sequence may vary based on the type or maturity of the project, HCD generally begins with foundational work to develop empathy among the design team (9). This initial step is followed by idea generation and iterative cycles of solution “prototyping” to prompt participant feedback and ensure an intervention is addressing stakeholder needs before pilot testing (13,14). The use of HCD has not yet been studied as a framework to engage stakeholders in the development and adaptation of CVD prevention interventions for PWH within United States (US) HIV specialty clinics.
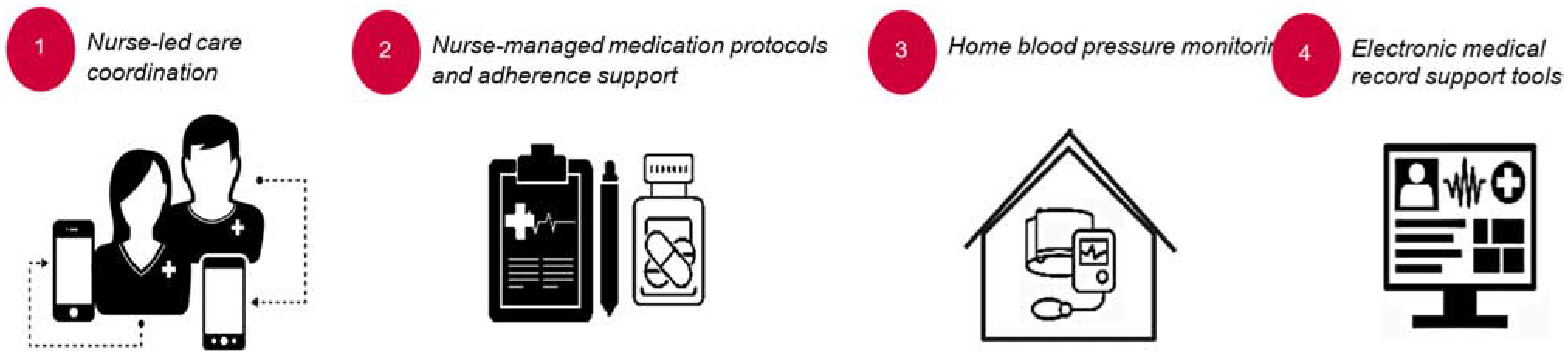
This study describes formative intervention tailoring for the EXTRA-CVD trial – a mixed-methods implementation research trial of a nurse-led intervention to improve control of hypertension and hyperlipidemia among individuals with well-controlled HIV infection. Research methods of EXTRA-CVD have been reported elsewhere (2). Core components of the EXTRA-CVD intervention include: 1) nurse-led CVD care coordination; 2) nurse-facilitated CVD medication protocols and adherence support; 3) home blood pressure monitoring; and 4) electronic medical record support tools (see Figure 1). Briefly, this on-going randomized clinical trial is being implemented within three federally-funded academic medical centers providing HIV specialty care for a racially and ethnically diverse population of PWH. The trial aims included a baseline assessment of CVD preventive care at study sites which informed the HCD process.
Figure 1: Core components of the EXTRA-CVD clinical trial.
Here, we detail how a HCD approach guided activities to adapt a nurse-led intervention to reduce the risk of CVD among PWH. In particular, we:
Outline the human-centered design process used to adapt the intervention before the trial begins;
Detail the final design team recommendations for adapting the EXTRA-CVD intervention;
Review and discuss the acceptability and feasibility testing phases of the HCD process;
Discuss how the final list of recommended adaptations were then integrated into the clinical trial phase; and
Summarize stakeholder feedback on the HCD process.
We aim to provide a comprehensive description of our approach for using HCD for stakeholder-informed intervention adaptation and development.
METHODS
Study Setting
This study was conducted at two hospitals in northeast Ohio (University Hospitals Cleveland and the MetroHealth System) and Duke Health in Durham, North Carolina. For logistical purposes, the two northeast Ohio hospitals were combined into one site for the design team experience. Thus, we organized two design teams (one in North Carolina and one in Ohio), which reduced the logistical difficulties of convening a unified group that spanned a large geographical distance. Having one design team in each geographical location also enabled in-person participation by all team members.
Conceptual Approach
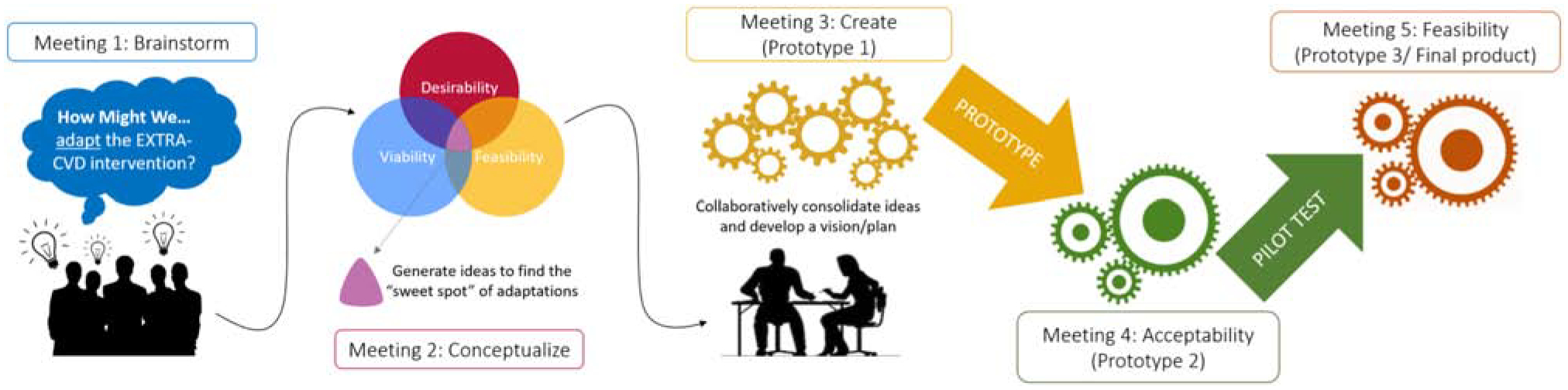
Applying a modified version of the IDEO approach to HCD, we used a participatory, iterative design process with two intervention “design teams” representing key stakeholders from each clinical site as described below. For the purposes of this study, the design process involved three key meetings, brainstorming (i.e. meeting #1), conceptualization (#2), and creation (#3), and two additional iteration meetings (#4 and #5) to refine the intervention for acceptability and feasibility before the trial began – for a total of five sessions for this iterative design process. Acceptability testing occurred between meetings 3 & 4; while the feasibility testing occurred between meetings 4 & 5. (See Figure 2)
Figure 2: EXTRA-CVD Design Process.
Additionally, to organize and integrate the initial recommendations from meeting 3 (creation), the Adaptome model was used to map them to the appropriate adaptation level defined by the model. Developed by Chambers and Norton (15), the Adaptome model was conceptualized as a potential data repository platform for capturing and organizing intervention adaptations over time in order to better chronicle the effect of the adaptations on outcomes related to implementation, service delivery, and health (16). The Adaptome model uses a five category taxonomy to depict the sources of intervention adaptation: Service Setting Adaptations; Target Audience Adaptations; Mode of Delivery Adaptations; Cultural Adaptations; and Core Components (15).
Participants
Each design team comprised 10 members and included study investigators as well as key stakeholders directly impacted by the intervention such as PWH with hypertension and high cholesterol, HIV specialists, cardiologists, primary care physicians, nurse practitioners, registered nurses, dieticians, social workers, information technology representatives, and pharmacists. Design members were recruited from the local clinics (i.e. the three study locations) who were willing and able to attend the design sessions. Based on site needs and logistics, the Ohio design team was the first to begin and complete the design process. This staggering of the two design teams, with roughly a month in-between meetings at each site, allowed for the refinement of the HCD process in response to feedback from Ohio design team participants.
Acceptability testing participants were comprised of 3 PWHs from the Ohio site. For the feasibility testing, there were 3 PWHs and 5 healthcare workers (2 physicians, 1 nurse practitioner, and 1 registered nurse) at the Ohio site. The feasibility testing for the North Carolina site included 1 PWH and 1 healthcare worker (physician assistant).
EXTRA-CVD Design Process
Design team activities targeted three core phases of HCD defined by IDEO: brainstorming, conceptualization, and creation. The first three meetings were each approximately four hours in duration. The final two meetings were each three hours in duration, and were scheduled following semi-structured intervention acceptability interviews and pilot test feasibility results, respectively. Two facilitators (JS and CR for the Ohio site; LO and KG for the North Carolina site) led each design team, with at least one facilitator from each site having participated in IDEO’s HCD online training course.
Below, we provide details on each of the five meetings as well as the acceptability and feasibility testing, and interaction among all of these components. Following these details, descriptions of the data collection and analysis process are included. Table 1 provides a brief summary of the meetings, activities, duration for each meeting, and the output or results from each meeting. For the HCD component, an operations manual outlined design activities and ensured consistency in design processes across sites. This operations manual was supplemented by a facilitator’s manual with step-by-step details for conducting each design activity as well as IDEO’s Field Guide to Human-Centered Design for additional resources and details on each activity selected. Design team members were given a workbook which included goals for each meeting along with materials to support each week’s design activities. Following each meeting, participants received a one-page summary drafted by the facilitators to highlight their team’s progress and to prepare them for future design activities.
Table 1:
Brief summary of the EXTRA-CVD Design process
| Meeting | Activities | Duration | Output |
|---|---|---|---|
| Brainstorming (Meeting 1) |
|
4 Hours |
|
| Conceptualization (Meeting 2) |
|
4 Hours |
|
| Creation (Meeting 3) |
|
4 Hours |
|
| Acceptability Testing | HCD activities not applicable. Focus group discussions were conducted. | 1 hour |
|
| iteration Meeting 4 |
|
3 Hours |
|
| Feasibility Testing | HCD activities not applicable. Semi-structured interviews were conducted. | 1 hour |
|
| Iteration Meeting 5 |
|
3 Hours |
|
This meeting occurred in-person for the Duke site with the design team completing the listed HCD activities. The Ohio design team members individually completed “homework” online for this meeting.
Brainstorming
In this meeting, we introduced design team members to the research study team, the core components of the EXTRA-CVD intervention, results of the baseline assessment of site’s CVD prevention practices and stakeholder perceptions, and how the HCD approach would be used to adapt the EXTRA-CVD intervention. In line with traditional HCD approaches (9–11,17), prior to idea generation and crafting of solutions, we aimed to first empathize with EXTRA-CVD stakeholders: 1) PWH at risk for CVD, 2) nurses who would deliver CVD preventive care, and 3) clinic staff and providers who would be affected by CVD task-shifting. With empathy being a core component of HCD, this session began with IDEO’s activity “Empathy Mapping,” before moving to activities geared toward identifying and articulating adaptation ideas. These included IDEO’s “Frame your Design Challenge”, “Finding Themes”, and “Create Insight Statements” (9). At the end of this first meeting, design team members created a list of insights and themes, relevant to the intervention itself, to guide the adaptation process.
Conceptualization
Conceptualization gives participants the opportunity to extend the practice of empathy into visioning, i.e. developing concrete solutions or ideas for intervention adaptation. Design team members deliberated about what areas of the intervention could be improved in order to better support PWH in CVD preventive care, to help the nurse optimize outcomes and engagement, and to improve the intervention in a way that adds value to the clinic. During conceptualization, members also developed “core values” or a set of values-based criteria that served as a requisite for any team suggestions to be included in the group’s recommendations to the study team. Design team members evaluated the advantages and disadvantages of the ideas generated in meeting 1 (brainstorming) and discussed opportunities to make viable changes to the intervention components. Activities included “How Might We…”, “Bundling Ideas”, and “Design Principles”(9). Upon the conclusion of meeting 2, design team members produced a list of potential solutions for adapting the EXTRA-CVD intervention.
Creation
During this meeting, design team members summarized and better refined the solutions from the previous sessions and produced six core recommendations, 3 from each design team, to adapt the EXTRA-CVD intervention as needed (Prototype 1). The six core recommendations served as the foundation for future iterations. Human-centered design activities for this meeting included Storyboarding (9) in which members illustrated their concepts using graphics rather than strictly words, allowing themselves and their audience to visualize their solutions in a new way, followed by Create a Pitch (9) where members consolidated their recommendation into a brief descriptive and compelling statement.
Acceptability Testing
Following the creation meeting, we conducted focus group discussions with PWHs at both sites who planned to participate in the pilot testing to assess acceptability of the refined EXTRA-CVD intervention. We obtained supplemental feedback on the refined intervention from HIV community advisory board (CAB) members at both sites. Study investigators recruited eligible PWH from the study clinics for roughly 60 minutes of focus group discussion, with these sessions taking place on site at the clinic and using a structured question guide. The question guide included items examining general perceptions on CVD care and management for HIV positive patients, as well as thoughts on the facilitators and barriers to the EXTRA-CVD prototype for PWH with or at risk for CVD.
Iteration Meeting #4 (Post-Acceptability Phase)
Meeting 4 took place after the acceptability testing and focused on further refining the EXTRA-CVD intervention in response to insights gleaned from the acceptability interviews and feedback – ahead of the pilot testing. For the Ohio site, this meeting occurred online due to conflicts in schedules for participants. In lieu of meeting in person, design team members submitted potential activities or recommendations based on the findings of the acceptability testing. The North Carolina design team opted for an in-person meeting and participated in the How Might We and Bundling Ideas HCD activities. At the end of this meeting, both design teams expanded on the six core recommendations from the creation meetings with more specific recommendations (“Prototype 2”).
Feasibility Testing
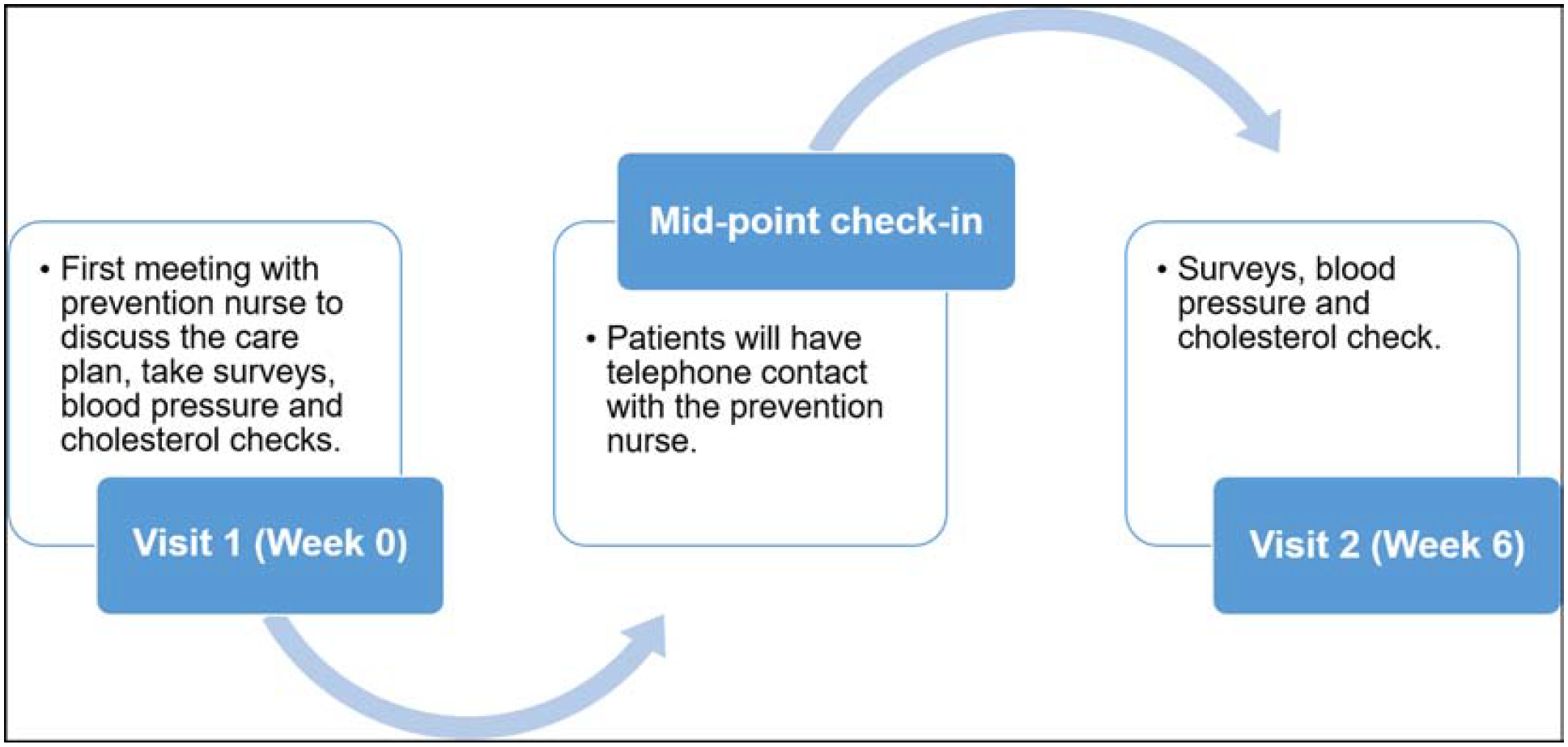
Following meeting 4, both sites conducted a six-week pilot of Prototype 2 with PWH and providers. Figure 3 provides a schematic of the pilot study steps. Following this phase, the same individuals who participated in the pilot testing then engaged in feasibility testing. We assessed feasibility through semi-structured interviews with PWH pilot participants and HIV providers. Interviews lasted roughly 60 minutes and focused on the participants’ perceptions and experience participating in the pilot testing in addition to their feedback on the suitability of the intervention being integrated within their clinic.
Figure 3. Pilot study steps.
Iteration Meeting #5 (Post-Feasibility Testing)
This session took place after the six-week pilot testing of the intervention and interviews with the PWH and HIV providers who participated in the pilot. Consequently, this session integrated the feedback from the feasibility interviews to refine the intervention as needed before going into the final trial phase. Similar to iteration meeting 4, How Might We and the Bundling Ideas activities were used in this session. At the end of this meeting, the design teams formulated a final list of recommendations – 39 in total from both sites – which stemmed from the six core recommendations produced in the creation meeting (“Prototype 3”). Additionally, this final session included a focus group discussion with all interested design team members on their participation in the HCD process.
Data Collection
During the first design team meeting, participants completed a brief demographic survey which included two open-ended questions on their experience using human-centered design and interest participating in the design team. The survey was administered in paper format and collected before participants began the design team process. At the final design team meeting, a focus group discussion was conducted to gather the perceptions of the design team members in HCD with questions focusing on thematic areas such as: 1) overall thoughts on participating in the intervention adaptation using a HCD approach; 2) reflections on specific experiences (i.e. activities) from the 5 sessions; and 3) general thoughts on the recommendations produced for the EXTRA-CVD intervention. Focus group sessions were audio recorded and transcribed. The acceptability and feasibility testing focus group discussions and semi-structured interviews were also audio recorded and then transcribed for analysis.
Data Analysis
All transcribed focused group discussions from both design teams in addition to the acceptability and feasibility testing were analyzed independently to identify salient themes or insights based on the prepared questions.
Analytical approach for recommendations
Along with using the Adaptome model to organize the initial six core recommendations, we developed scoring criteria, according to the categories of desirability, viability, and feasibility (9), to prioritize which of the specific 39 recommendations from the design process (Prototype 3) would ultimately be adapted for the trial phase of the EXTRA-CVD intervention. To better suit the needs of the intervention, the three lenses for innovation were interpreted accordingly: feasibility meant the recommendation was functionally possible in the foreseeable future to implement for the intervention; viability meant the recommendation was considered sustainable for the intervention or organizational model; and desirability meaning that the recommendation makes sense or is most appealing to the target population based on their needs, hopes, and fears (9). Using these three lenses of innovation, the design team facilitators scored the recommendations for each of the three lenses as follows: 3 points (most feasible, viable, or desirable); 2 points (somewhat feasible, viable, or desirable); 1 point (not feasible, viable, or desirable). Supplemental Table 1 provides a template for this scoring system. The four design team facilitators, two from both teams, scored the recommendations individually using this scoring system. Once all four had scored the recommendations, a total score for each recommendation was taken with the most points possible per a recommendation being 36 and the least points being 4 points. Recommendations with a total above 30 points were considered as a priority for integrating into the intervention for the trial period, and were selected as the final set of recommendations for adaptation (“Final Product”).
INFORMED CONSENT
Design team members were recruited at the first meeting and consented through written form to participate in the research component (i.e. the brief demographic survey and focus group discussion). Team members were compensated for their participation at each meeting. Participants in the acceptability, feasibility and pilot testing were consented through written form prior to participation in the meetings. All procedures were approved by the University Hospitals Cleveland Medical Center IRB with reliant review agreements at MetroHealth and Duke.
RESULTS
Table 2 provides demographic characteristics of both design teams (N=20). Briefly, both teams were comprised mostly of men (65%), the majority had graduate degrees (80%), and were White or Caucasian (80%). Fifty-nine percent of the design team members reported not having any design experience prior to their participation. Design team members highlighted a number of reasons for their interest in the EXTRA-CVD design process: “gain something new”; “make the research and intervention more effective”; “contribute to improvements for our patients”; “participate in decision-oriented projects [and] provide some ‘give-back’ to long term survival [within the] HIV/AIDS community”.
Table 2:
Demographics of the Cleveland & North Carolina design teams
| OHIO | NORTH CAROLINA | |
|---|---|---|
| Category | N (%) | N (%) |
| Gender | ||
| Male | 7 (70%) | 6 (60%) |
| Age | ||
| <49 years | 5 (50%) | 6 (60%) |
| 49+ years | 5 (50%) | 4 (40%) |
| Ethnicity | ||
| White | 9 (90%) | 7 (70%) |
| Education | ||
| Bachelor degree or below | 3 (30%) | 2 (20%) |
| Graduate degree | 7 (70%) | 8 (80%) |
| Employment | ||
| Full-time | 9 (90%) | 7 (70%) |
NOTE: While not reported in this Table, 3 design team members did not report their past experience with HCD; hence, the 59% reported in the text represents 10 out of 17 design team members.
Core Recommendations for Intervention Adaptation
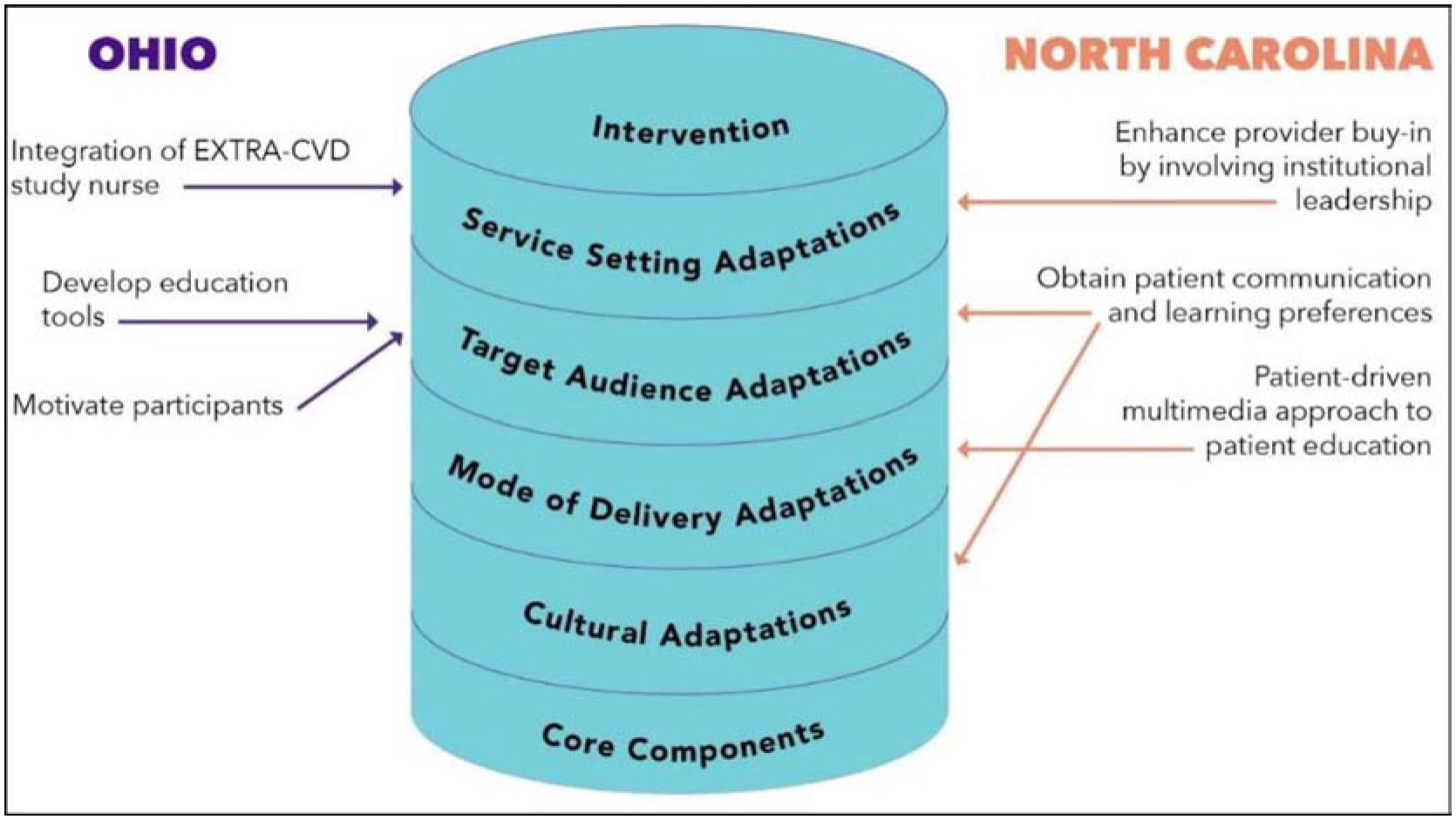
At the completion of the third design meeting (creation) and prior to prototyping, both teams formulated six core recommendations for adapting the EXTRA-CVD intervention to make it more context-appropriate. These recommendations were further developed into specific ideas in iteration meetings 4 and 5, based on feedback from the acceptability and feasibility testing, respectively. Below, we present the core recommendations from both sites generated in the creation meeting and share the outcome of the research team synthesizing the recommendations using the Adaptome model (as noted in Figure 4) to better inform the integration of the recommendations for the pilot study as well as the trial.
Figure 4: Mapping site recommendations using the Adaptome model.
Ohio Design Team Core Recommendations
For the Ohio design team, the core recommendations were as follows: 1) educational tools for PWH (e.g. a digital CVD prevention resource library); 2) strategies to integrate the prevention nurse within the clinic setting (e.g. team lunch and study introduction with the prevention nurse and clinic staff); and 3) motivating PWHs through social support activities and other incentives (e.g. transport reimbursement or child care). For these recommendations and based on the local context for the Ohio sites, recommendation # 1 (educational tools) & #3 (motivating PWHs) best suited the Target Audience Adaptations of the Adaptome, while # 2 (integrating the intervention nurse) was most aligned with the Service Setting Adaptations categorization.
North Carolina Design Team Core Recommendations
Similarly, the North Carolina design team came up with the following recommendations: 1) incorporating a patient-driven multimedia approach for patient education (e.g. website orientation at first meeting); 2) enhancing provider buy-in by involving institutional leadership (e.g. clinic directors serving as champions of the study); and 3) instituting a component to identify patient communication/ learning preferences (e.g. an intake survey). Applying the Adaptome, recommendation # 1 (patient-driven multimedia approach) was most applicable to the Mode of Delivery Adaptations. Recommendation # 2 (enhancing provider buy-in) was mapped to Service Setting Adaptations. Given the local context, # 3 (patient communication preferences) was dually applicable for the Target Audience and Cultural Adaptations categorization.
Acceptability Testing
The acceptability testing highlighted key thematic areas. These key areas included: the role of nurses in hypertension and hyperlipidemia management; general patient education tools and materials; communication preferences between the patient and the nurse; the use of home blood pressure monitoring; study-related surveys and documentation; and the real-world relevance of the EXTRA-CVD intervention. Regarding the role of nurses, some PWHs valued the care coordination role of new prevention/intervention nurse on their care team, but also wondered if their primary HIV nurse was better suited in comparison. This concern varied by site according to the degree of care coordination already performed by nurses at the site. For the other thematic areas, the PWH mostly noted general reflections such as preferring scheduled phone calls as the method of communication and wanting more education or training on using the home blood pressure monitors.
Feasibility Testing
Feasibility testing was done with participants and healthcare workers who interacted with the prevention nurse in the pilot study. At the Ohio site, the participants were strongly supportive of the intervention and noted that the prevention nurse was friendly. In terms of patient communication, the majority mentioned that the phone communication worked well. In terms of educational materials, the participants felt that the book provided (Living a Healthy Life with HIV) was sufficient. Conversely, they also highlighted opportunities for improvement for the overall intervention. These included shorter surveys and enrollment day and too much focus on the home blood pressure monitoring results during the phone calls with the prevention nurse. Regarding the suitability of the intervention in general, the healthcare workers noted that a perceived benefit was the additional staff to support patient follow-up while patient barriers were mostly related to their psychosocial issues and limited financial resources.
At the North Carolina site, participants appreciated flexible communication (i.e. phone calls and emails) and increased home self-monitoring habits as a result of their participation in the study. In terms of opportunities for the intervention to improve, the participant mentioned that the surveys were lengthy and that too much attention was focused on blood pressure control. Feedback from one healthcare worker noted that having the prevention nurse reduced the responsibilities of the provider, allowing for more attention to the patient’s health. Perceived benefits of the intervention from the healthcare worker’s perspective included increased ownership and motivation of the PWH to check their blood pressure at home.
Final List of Recommendations
Recommendations proffered in iteration meetings 4 and 5 were based on, and categorized, by the initial recommendations produced in the creation sessions. At the completion of iteration meeting 5, the 39 recommendations were organized by the research team for final assessment and review. Using the feasibility, viability and desirability scoring system described above, we prioritized 14 recommendations for adapting the intervention (Final Product). Table 3 provides the list of 14 recommendations and the applicable source of adaptation from the Adaptome model. In general, the majority of the final 14 recommendations prioritized for the EXTRA-CVD trial were considered applicable to the Target Audience and Service Setting adaptations as referenced by the model.
Table 3:
List of 14 Recommendations adapted into the intervention
| Recommendation Description | Core Recommendation | Adaptome Model: source of intervention adaptation |
|---|---|---|
| Education library with links to videos and guides to support activities like meditation and stress reduction. | Develop Education Tools | Target Audience Adaptation |
| Create/distribute prevention RN business card. | Integrate Nurse | Service Setting Adaptation |
| Develop capacity for prevention RNs to communicate with participants by text messaging. Include this in prevention RN’s business card. | Integrate Nurse | Service Setting Adaptation |
| Develop study fliers to include prevention RN’s contact info and picture for posting around clinic. | Integrate Nurse | Service Setting Adaptation |
| Develop SOP for cases of provider non-response to prevention RN’s recommendations. Should consider potential for PCP or HIV provider to be non-responder. | Integrate Nurse | Service Setting Adaptation |
| Re-confirm learning and communication preferences listed on intake survey with participant and at each study visit. | Understand/Capture Patient Preferences | Target Audience & Cultural Adaptation |
| Orient all participants to website at the enrollment visit. | Motivate/Sustain Participants | Target Audience Adaptation |
| Clearly define nurse interventionist availability and appropriate indications for nurse contact. | Integrate Nurse | Service Setting Adaptation |
| Ensure three “take home points” for each patient for each visit. | Motivate/Sustain Participants | Target Audience Adaptation |
| Shorten and pre-populate surveys as much as possible. | Motivate/Sustain Participants | Target Audience Adaptation |
| Provide study-labeled nominal gifts at enrollment visit. | Motivate/Sustain Participants | Target Audience Adaptation |
| Embed a 10-yr ASCVD risk score calculator on the study website (or at least provide a link). | Multi-Media Approach to Education | Mode of Delivery Adaptation |
| Email website link to patients | Multi-Media Approach to Education | Mode of Delivery Adaptation |
| Provide a participant feedback section to the study website. | Multi-Media Approach to Education | Mode of Delivery Adaptation |
Design Team Reflections
In general, design team participants shared that while the human-centered design experience was somewhat ambiguous at first, by the end of the 5 sessions, they were more comfortable and appreciative of the process. Themes generated from both design team focus group discussions of the HCD experience included: “providing an opportunity to share unique perspective and be listened to;” “being able to identify problems and think of possible solutions;” and “emphasis on empathy enhances the clinical research experience.” Reflecting on the experience one participant stated “…my job is not done here just thinking of a brilliant idea, but it’s also how does this impact, and how can I modify it to make it work. So that’s kinda cool.” Design team members at both sites reported feeling satisfied and proud of the final list of recommendations they had produced. Additionally, team members mentioned that the foundational step through the empathic discovery was very useful as it helped them have a better sense of ‘whom’ (i.e. PWH and the prevention nurse) they were designing for and why their role as designers was important.
DISCUSSION
Using HCD approach in this study led to a tailored intervention adaptation that was both patient- and provider-driven. The iterative cycles of the design thinking process allowed for real-time intervention adaptation prior to trial implementation – a critical step and opportunity for enhancing the potential impact of the intervention on the study outcomes. The six core recommendations produced by the design teams focused primarily on incorporating more approaches to engage and motivate the patient, provide sustainable support for the intervention nurse, and instituting more effective strategies to enhance provider buy-in and patient communication preferences. For additional synthesis, the six core recommendations were mapped onto the intervention adaptation categories of the Adaptome model: service setting adaptations; target audience adaptations; cultural adaptations; and mode of delivery adaptations. Mapping these initial recommendations using the Adaptome model helped to better define them in terms of the type or source of adaption and potentially optimize opportunities for intervention delivery by providing a guide on how to catalogue and organize the proposed recommendations/ adaptations. Proposed as an approach for synthesizing knowledge about intervention adaptations (18), the Adaptome model is particularly useful as it catalogues potential or thematic areas to assess the overall implications of the recommended adaptations on future iterations (i.e. acceptability and feasibility testing) prior to the trial initiation. In addition to being rated as highly feasible, viable, and desirable by the research team, the final 14 recommendations that were selected to be immediately adapted for the trial implementation highlight potential ideas and strategies to optimize the delivery and scalability of the intervention within clinic settings. In this study, the final 14 recommendations fit within the confines of what stakeholders deemed acceptable and feasible, demonstrating that the final product was positively rated by both the acceptability and feasibility participants, as well as the research team members.
Human-centered design is a practical approach to stakeholder engagement, particularly as it ensures that outcomes are driven by the unique preferences and circumstances of the target population. HCD is unique from other participatory design approaches (quality improvement, community based participatory research, participatory action, etc.) for its use of active empathy by those engaging in the design and of rapid prototyping to encourage the generation, experimentation, and selection of ideas that often go unvoiced and unrecognized. Within the health care field, HCD offers the opportunity to develop innovative, patient-driven solutions to complex issues affecting clinical conditions and public health outcomes. Other studies using design thinking strategies have also noted the robustness of the approach to increase stakeholder engagement and buy-in (19). Similarly, the current study yielded a number of key ideas and strategies for adapting the EXTRA-CVD intervention and seemingly increased buy-in among stakeholders who participated in the HCD process.
Acceptability testing is a useful tool that may be applied at any stage of implementation and focuses primarily on gathering feedback from key stakeholders at particular stages of the intervention to assess a particular practice, technology, service, or intervention (20). Our acceptability testing revealed that an important perceived benefit of the intervention was the addition of the prevention nurse to support patient needs and ease the responsibilities of the HIV provider. Potential areas of improvement for the intervention pointed to minor changes in terms of the length of data-collection surveys and perceived over-emphasis on the home blood pressure monitoring results during calls. The findings from the feasibility testing yielded important feedback regarding patient communication preferences and the role of the prevention nurse. Taken together, the findings from the acceptability and feasibility testing highlight that, in general, there was strong interest and support of the intervention on the part of both PWH and healthcare workers.
Feedback from the design team participants also noted the benefit of incorporating a HCD approach as a pre-implementation step. Design team members noted that the benefits included the multidisciplinary nature of the teams and the refreshing take on approaching the design work from a “place of empathy”. Most of the design team members mentioned that while they had participated in other clinical trials, this experience was their first time approaching the process through the lens of empathic discovery. Overall, the team members were very eager about the individual and collective contributions they had made throughout the process and were eager to see the outcomes of their contributions to the trial.
LIMITATIONS
Although the current study incorporates a design thinking approach for intervention adaption, the utility and applicability of the recommended adaptations may not be generalizable to other studies focused on integrating CVD management into the HIV care platform. The use of a HCD approach, however, may prove to be more pragmatic for future studies and as a pre-implementation step. Additionally, while HCD proved to be useful in encouraging stakeholder engagement and investment in the intervention, it is worth noting that our sample of participants was restricted and did not include all of the key stakeholders with different backgrounds and expertise who may be impacted by the intervention. Nonetheless, the design teams did include a good mix of providers and patients along with available practitioners who would be engaged in the implementation of the intervention in some degree. Also, the mapping of recommendations onto the Adaptome Model was conducted by only one research study team member (AA) and then discussed with the full research study team to modify as needed. An alternative approach would be to use a more “consensus-driven” process such as the Delphi (21). Moreover, providing an opportunity for both investigators and HCD design team members to map final recommendations onto the Adaptome model and determine any new interpretations from this mapping may potentially enhance the practical usefulness of the model as a guide for categorizing adaptations. Finally, we recognize that there is an evolving literature regarding the taxonomy and classification of adaptation, including the recently published FRAME framework (22), which may ultimately prove to be beneficial.
CONCLUSIONS
A novel method for integrated HIV/NCD care models, HCD along with acceptability and feasibility testing offers a systematic, stakeholder-centered approach to adapt healthcare interventions. This innovative approach to stakeholder engagement focuses primarily on using an interactive, iterative process to engage and motivate stakeholders. Our use of the Adaptome model also provided an opportunity to further synthesize and optimize the adaptations for intervention implementation. More importantly, the current study produced 14 key recommendations which could potentially enhance the effectiveness and scalability of the EXTRA-CVD intervention. Overall, this study provides evidence of how stakeholder engagement can be applied as a pre-implementation step for adapting an intervention based on the needs and context of those impacted.
Supplementary Material
Funding:
This work is supported in part by the National Institutes of Health (U01HL142099, K23HL137611, and K23HL123341)
Alphabetical List of Abbreviations:
- CVD
Cardiovascular Disease
- HCD
Human-centered Design
- HIV
Human Immunodeficiency Virus
- NCD
Non-communicable diseases
- PWH
People living with HIV
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI /Disclosures: None to declare.
Conflict of interest
None.
References
- 1.El-Sadr WM, Goosby E. Building on the HIV platform: tackling the challenge of noncommunicable diseases among persons living with HIV. Aids 2018;32:S1–S3. [DOI] [PubMed] [Google Scholar]
- 2.Okeke NL, Webel AR, Bosworth HB et al. Rationale and design of a nurse-led intervention to extend the HIV treatment cascade for cardiovascular disease prevention trial (EXTRA-CVD). American heart journal 2019;216:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cioe PA, Crawford SL, Stein MD. Cardiovascular risk-factor knowledge and risk perception among HIV-infected adults. Journal of the Association of Nurses in AIDS Care 2014;25:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lerner AM, Eisinger RW, Fauci AS. Comorbidities in Persons With HIV: The Lingering Challenge. JAMA : the journal of the American Medical Association 2020;323:19–20. [DOI] [PubMed] [Google Scholar]
- 5.Vorkoper S, Kupfer LE, Anand N et al. Building on the HIV chronic care platform to address noncommunicable diseases in sub-Saharan Africa: a research agenda. AIDS (London, England) 2018;32:S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazzano AN, Martin J, Hicks E, Faughnan M, Murphy LJPo. Human-centred design in global health: A scoping review of applications and contexts. 2017;12:e0186744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragouzeos D, Gandrup J, Berrean B et al. “Am I OK?” using human centered design to empower rheumatoid arthritis patients through patient reported outcomes. 2018. [DOI] [PMC free article] [PubMed]
- 8.IDEO. IDEO at a Glance. 2020.
- 9.IDEO. The Field Guide to Human-centered Design: Design Kit: IDEO, 2015.
- 10.Matheson GO, Pacione C, Shultz RK, Klügl MJAjopm. Leveraging human-centered design in chronic disease prevention. 2015;48:472–479. [DOI] [PubMed] [Google Scholar]
- 11.Catalani C, Green E, Owiti P et al. A clinical decision support system for integrating tuberculosis and HIV care in Kenya: a human-centered design approach. 2014;9:e103205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giacomin JJTDJ. What is human centred design? 2014;17:606–623. [Google Scholar]
- 13.Chokshi SK, Mann DMJJhf. Innovating From Within: A Process Model for User-Centered Digital Development in Academic Medical Centers. 2018;5:e11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann D, Hess R, McGinn T et al. Adaptive design of a clinical decision support tool: What the impact on utilization rates means for future CDS research. Digital health 2019;5:2055207619827716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers DA, Norton WE. The adaptome: advancing the science of intervention adaptation. American journal of preventive medicine 2016;51:S124–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt CL, Chambers DA. Opportunities and challenges in conducting community-engaged dissemination/implementation research. Transl Behav Med 2017;7:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vechakul J, Shrimali BP, Sandhu JSJM, journal ch. Human-centered design as an approach for place-based innovation in public health: a case study from Oakland, California. 2015;19:2552–2559. [DOI] [PubMed] [Google Scholar]
- 18.Escoffery C, Lebow-Skelley E, Udelson H et al. A scoping study of frameworks for adapting public health evidence-based interventions. Transl Behav Med 2018;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chokshi SK, Belli HM, Troxel AB et al. Designing for implementation: user-centered development and pilot testing of a behavioral economic-inspired electronic health record clinical decision support module. Pilot and feasibility studies 2019;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proctor E, Silmere H, Raghavan R et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. 2011;38:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Practical assessment, research & evaluation 2007;12:1–8. [Google Scholar]
- 22.Stirman SW, Baumann AA, Miller CJ. The FRAME: an expanded framework for reporting adaptations and modifications to evidence-based interventions. Implementation Science 2019;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


