Abstract
Objective:
Describe the use of three-dimensional(3D) patent ductus arteriosus(PDA) modeling to better define ductal anatomy to improve pre-procedural planning for ductal stent placement.
Background:
Ductal stenting is an alternative to surgical shunting in patients with ductal dependent pulmonary blood flow. Ductal anatomy is often complex with extreme tortuosity and risk of pulmonary artery isolation, thus increasing procedural risks.
Methods:
CT angiograms were segmented to produce 3D PDA models. Ductal morphology was characterized with attention to access approach, degree of pulmonary artery offset/risk of isolation and ductal tortuosity. 3D models were retrospectively compared with biplane angiography.
Results:
3D modeling was performed in 12 patients with adequate image quality for complete analysis in 11; median (interquartile range) age/weight 17 days (8-20 days) and 3.1kg (2.4-3.9kg). The PDA was reverse oriented in 9 with average length of 17.2±2.5 mm and high tortuosity (mean tortuosity index 52, range 3-108). From 3D modeling, two patients were excluded from ductal stenting--extreme ductal tortuosity and threatened pulmonary artery discontinuity, respectively. Ductal stenting was successful in the remaining 9 with no major procedural complications. 3D modeling predicted a successful access approach based on the aortic orientation of the ductus in all patients (5 carotid, 2 axillary, 2 femoral). When comparing 2D angiography with 3D models, angiography consistently underestimated ductal length (−3.2mm±1.6mm) and tortuosity (−14.8±7.2).
Conclusions:
3D modeling prior to ductal stent placement for ductal dependent pulmonary blood flow is useful in procedural planning, specifically for eligibility, access approach, and accurate ductal measurements. Further studies are needed to determine if 3D planning improves procedural outcomes.
Keywords: Congenital Heart Disease, Pediatrics, Imaging, Electron Beam CT/Multidetector CT, Pediatric Intervention, Stent, Bare Metal
Introduction
Ductal stenting is increasingly used as an alternative to surgical shunts in neonates with congenital heart disease and ductal dependent pulmonary blood flow. Two recent large, multicenter studies identified several advantages to ductal stent (DS) placement over surgical Blalock-Taussig shunts including the avoidance of cardiopulmonary bypass, reduced procedure-related complications, and shorter intensive care unit length of stay (1, 2). However, patients with ductal dependent pulmonary blood flow often have complex ductal anatomy including reverse orientation, tortuosity and offset of the pulmonary arteries from the ductus placing them at risk for isolation. These features contribute to the technical difficulty of the procedure and a reported procedural failure rate of 16% in one multi-center study (2). Traditional two-dimensional imaging, including echocardiography, is often inadequate in conveying detailed anatomic features critical for procedural success such as ductal tortuosity and length, aortic orientation to adjacent vessels, and ductal insertion to the pulmonary arteries.
Given the limitations of two-dimensional imaging, three-dimensional (3D) imaging is becoming more widely used in pre-catheterization planning and interventional guidance for complex, congenital heart disease (CHD) as it provides an enhanced understanding of the relevant spatial-structural relationships (3). No prior reports have documented the 3D anatomic features of ductal anatomy in patients undergoing DS placement, and the potential value of pre-procedural 3D planning to enhance procedural success.
In 2017, our center initiated a 3D imaging protocol prior to DS interventions in patients with a complex, reverse oriented patent ductus arteriosus (PDA). Here we report our initial experience with CT angiography (CTA) combined with 3D modeling and printing of ductal anatomy. These studies have advanced our understanding of the complex ductal anatomy and are used for appropriate patient selection, and for procedural planning, with specific attention to procedural eligibility and access approach.
Materials and Methods
Patient demographics and procedural details
We performed a retrospective analysis of all patients with pulmonary dependent ductal flow referred for consideration of DS at our institution from December 1, 2015 to December 1, 2018. In July 2017 coincident with a programmatic expansion of 3D imaging, we implemented a procedural protocol for ductal stenting that included CTA with 3D modeling for all patients determined to have reverse oriented PDAs by transthoracic echocardiogram or anatomy at risk for procedural failure – pulmonary atresia or other single ventricle physiology as described by Udink, et. al. (4). Otherwise, the pre-procedural care for the patients remained unchanged; specifically, the decision regarding timing to stop prostaglandin infusions prior to the procedure varied by interventionalist preference and clinical status of the patient – typical 0-4 hours prior. The study was approved by the Duke University Medical Center Institutional Review Board with waiver of informed consent.
3D rendering
Three dimensional visualization of volumetric images, such as CTA, on a 2D display typically employs either a volume rendering or surface rendering approach. Volume rendering uses a transparency map in addition to a color or grayscale map to assign appearance properties to each voxel based on the Hounsfield units. Through some variation of volume ray casting, the voxel appearances are projected to the viewing plane based on a summation of the voxels intersected by a collection of rays diverging from the view point. Surface rendering is an indirect volume rendering technique which first creates a surface within the volumetric data (representing the boundary of an object). Surface rendering involves the creation of a segmentation mask identifying the voxels in the object of interest (5, 6).
We chose to use a surface based approach since this representation facilitates 3D printing. However, volume rendering is a viable alternative to visualize and quantify the ductal anatomy.
Segmentation and 3D Modeling
The CTA was segmented by setting a threshold value above which the voxels were likely either contrast enhanced blood or bone. The thresholding was performed in Materialise Mimics 21.0 (Materialise Leuven, Belgium), which has tools to create line profiles through 2D slices to help identify the edges of the arteries. There are also tools for editing and splitting including region growing, morphological and Boolean operations. The surface representing the boundary of the segmentation mask, within the volumetric image, can then be created by interpolating between the voxels to find the surface at the threshold value. In practice it can be advantageous to use a combination of both surface and voxel based operations to define the region of interest.
Materialise 3-matic 13.0 (Materialise Leuven, Belgium) was used to further trim the surface to include only the regions of interest (aorta, ductus arteriosus, and pulmonary arteries) and prepare the model for 3D printing. A hollowing operation was performed to create the vessel walls (in a printable thickness 0.7 – 1.0 mm) from the blood pool model. Unique identifier labels were incorporated into the model design for future identification.
The surface must define a solid volume in order to be 3D printed. 3-matic was used to check for and fix bad edges, non-manifold surfaces, surface intersections, and identify separate shells or solid volumes. The solid model can then be exported as a stl (stereolithography) file for 3D printing or exported as a 3D PDF (Adobe Inc., San Jose, California). Assurance of accuracy of the model to original anatomy and quality control were performed by overlaying the stl file surface contours with orthogonal CTA views in Mimics 21.0.
The physical models were manufactured with a Stratasys Polyjet J750 (Stratasys Ltd. Eden Prairie, MN) 3D printer. The polyjet printer has multiple nozzles that allows for the use of a combination of rigid (Vero) and flexible (Tango or Agilus30) materials in addition to the soluble support material which encases the PDA model. Throughout the remaining manuscript this process of acquisition, segmentation, modeling and printing will be referred to as “3D planning.”
Ductal measurements
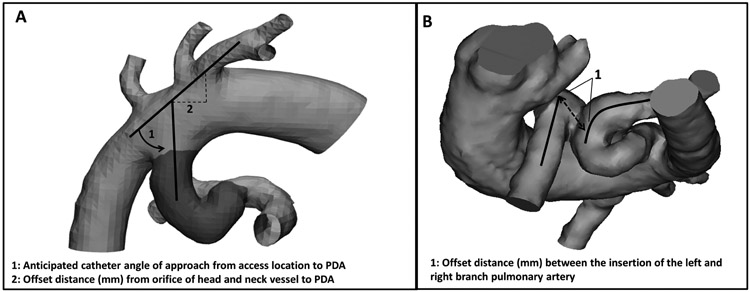
We used the 3D models to determine a total ductal length and ductal tortuosity index (DTI) for each patient. For PDA angle of orientation to adjacent vessels and branch pulmonary artery offset, the interventionalists made qualitative assessments for each patient. Retrospectively, quantitative measurements were obtained to more accurately describe the anatomic features. DTI was derived as an adaptation from previously described vertebral tortuosity index ((total ductal length/straight length – 1) x 100) (7). Total ductal length was defined as the length along the curvature of the patent ductus arteriosus from aortic origin to pulmonary insertion. Straight ductal length was defined as the shortest straight line distance from aortic origin to pulmonary insertion. Using Materialise Mimics 21.0, total ductal length was measured through the center of the vessel (Centerline function). To quantitatively inform access approach, aortic orientation of the PDA to adjacent vessels was measured as catheter angulation required to pass from adjacent vessel lumen to PDA lumen, as well as, offset distance from adjacent vessel orifice to PDA orifice (Fig. 1A). To quantitatively inform risk of pulmonary artery discontinuity, branch pulmonary artery offset was measured as the straight length distance along the main pulmonary artery from center lumen of the left pulmonary artery orifice to center lumen of the right pulmonary artery orifice (Fig. 1B). Pre-procedural ductal measurements from 3D planning were compared with procedural angiograms. Angiographic ductal measurements were performed using Phillips Xcelera software in angulated antero-posterior and lateral views using auto-calibration and catheter calibration.
Fig. 1.
Three-dimensional models demonstrating the angle and offset measurements of patent ductus arteriosus to adjacent vessels (A), as well as, offset measurements of the branch pulmonary arteries (B).
Definitions and Statistical analysis
The primary study outcome was quantitative description of the ductus arteriosus and adjacent vessels as described above. Secondary outcomes included standard procedural metrics (i.e. fluoroscopy time, contrast, and radiation), procedural complications and hospital complications. Major procedural complications were defined as complications significantly affecting the immediate procedural outcome; including an inability to place a ductal stent, unplanned procedural intervention to maintain or re-establish pulmonary blood flow, pericardiocentesis, pleurocentesis, or need for mechanical support. Minor procedural complications were defined as any complication that did not affect the final placement of ductal stents or establishment/maintenance of pulmonary blood flow. Hospital complications were defined as occurring after the procedure and within the incident hospitalization, but significantly affecting recovery – including unplanned surgical intervention, unplanned catheter re-intervention, mechanical support, or death.
Baseline descriptive statistics were calculated for the patient population with comparative statistics between ductal measurements using Student’s t-test. All data was analyzed using STATA version 15.1 (StataCorp LLC, College Station, TX).
Results
Patient Description
Characteristics of the 12 patients included in our study cohort are presented in Table 1. The majority of patients had anatomy at risk for procedural failure with pulmonary atresia or other single ventricle physiology. Median (interquartile range) age and weight were 17 days (8-20) and 3.1kg (2.4-3.9).
Table 1:
Characteristics of patients with three-dimensional ductal imaging considered for ductal stent placement
| Patient Characteristics | N=12 |
|---|---|
| Male | 7 (58.3%) |
| Race/Ethnicity | |
| White | 4 (33.3%) |
| Black | 4 (33.3%) |
| Hispanic | 1 (8.4%) |
| Other | 3 (25.0%) |
| Gestational age (avg), wks | 37.7 (37.3-38.1) |
| Age (median), d | 17 (8-20) |
| Weight (avg), kg | 3.1 (2.4-3.9) |
| Cardiac anatomy | |
| PA-VSD/TOF-PA | 5 (41.7%) |
| Single Ventricle | 3 (25.0%) |
| TOF-PS | 3 (25.0%) |
| Severe PS | 1 (8.3%) |
| PDA | |
| 1 | 11 (91.7%) |
| 2 | 1 (8.3%) |
Ductal anatomy
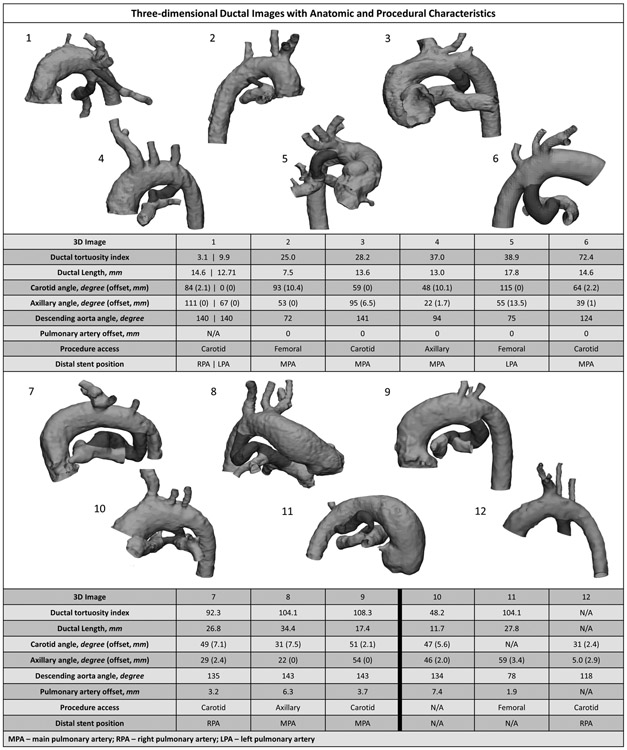
Figure 2 depicts the anatomy for all 12 subjects and demonstrates the significant anatomic variability as well as ductal tortuosity. In one patient (12 in Fig. 2) the CTA contrast phase was mistimed and imaging was inadequate for complete anatomic evaluation. In the remaining 11 subjects 3D planning provided excellent anatomic detail. The ductus was reverse oriented in 9 patients with an average length by CTA of 17.2 ± 2.5 mm and a narrowest diameter of 2.7mm (range 2.3-3.1 mm). The mean tortuosity index was 51.9 ± 12.4mm (range 3-108), indicating an average total ductal length 52% greater than the point-to-point length from the aorta to the PA insertion. In 5 of 11 subjects with interpretable imaging, there was offset of the pulmonary arteries with two (8 and 10 in Fig. 2) judged to be at-risk for pulmonary artery isolation due to pulmonary artery offset lengths of 6.3 and 7.4 mm respectively.
Fig. 2.
Three-dimensional patent ductus arteriosus images with corresponding anatomic measurements and procedural information from ductal stent placement
Outcomes with 3D Planning
Based on 3D planning, two patients were judged to be poor candidates for ductal stenting. In the first (Patient 10, Fig. 2, see also Fig. 1B) there was felt to be risk of pulmonary artery isolation due to significant offset of the branch pulmonary arteries (7.4mm). The anatomy precluded stenting into the at risk pulmonary artery to prevent isolation, therefore a surgical shunt with pulmonary arterioplasty was preferred. In the second (Patient 11, Fig. 2), there was extreme ductal tortuosity (tortuosity index = 108) with no apparently superior access approach due to lack of directly adjacent aortic insertions of the head and neck vessels. In this patient with severe pulmonary stenosis, right ventricular outflow tract stenting was pursued instead due to the noted anatomic challenges. The remaining nine subjects underwent successful ductal stenting with no major intra-procedural complications (Table 2). In one patient considered at high risk for pulmonary artery isolation (offset = 6.3 mm) the ductal stent was extended into the at risk pulmonary artery. Access location was determined in all nine subjects based on the aortic orientation of the ductus. In patients with carotid access (n=5), the average PDA angle of orientation and offset relative to the carotid artery was 48.3 ±10.1 degrees and 2.3 ± 0.9 mm. For axillary access (n=2) the average PDA angle and offset was 22 ± 0 degrees and 0.9 ± 0.9 mm, while femoral access (n=2) PDA angle averaged 73.5 ±1.5 degrees. In all cases the ductus was crossed from the chosen access approach and no patient required additional access. When comparing 3D imaging with intra-procedural angiography, angiography consistently underestimated ductal length (−3.2mm ± 1.6mm; p=0.38) and ductal tortuosity (−14.8 ± 7.2mm; p=0.13). A description of stents placed is provided in the Supplemental Table.
Table 2:
Procedural characteristics in patients who underwent ductal stent placement
| Procedural Characteristics | Adequate 3D Model N=9 (64.3%) |
Inadequate/No 3D Model N=5 (35.7%) |
|---|---|---|
| Procedure Access | ||
| Femoral | 2 (22.2%) | 3 (60.0%) |
| Carotid | 5 (55.6%) | 1 (20.0%) |
| Axillary | 2 (22.2%) | 1 (20.0%) |
| Median number of stents | 1 (1-2) | 1 (0-1) |
| Procedural success | 9 (100.0%) | 4 (80.0%) |
| Procedural complications | ||
| Major* | 0 (0.0%) | 1 (20.0%) |
| Minor | 1 (11.1%) | 0 (0.0%) |
| None | 8 (88.9%) | 4 (80.0%) |
| Hospital complications | ||
| Major* | 0 (0.0%) | 2 (40.0%) |
| None | 9 (100.0%) | 3 (60.0%) |
| Fluoro time (avg), min^ | 32.0 (±6.5) | 17.3 (±4.4) |
| Total contrast (avg), mL/kg | 6.0 (±0.7) | 4.3 (±0.7) |
| Total radiation (avg), mGy | 67.1 (±15.6) | 44.4 (±26.0) |
Major procedural complication: Inability to place stent due to ductal spasm and extreme tortuosity. Minor procedure complication: distal stent migration. Major hospital complications: Unplanned surgery for modified Blalock-Taussig shunt and central shunt with left pulmonary arterioplasty
Three patients without 3D models had a normal ductal orientation, simplifying the procedure and likely reducing average fluoroscopic time and radiation doses.
Outcomes without 3D Planning
Although the numbers are small and significantly confounded, we nonetheless sought to evaluate potential improvements in procedural outcomes by comparing outcomes with the subset of patients undergoing ductal stenting at our institution without 3D planning (n=4) or with inadequate 3D imaging (n=1). In these five subjects, ductal stenting was successful in three (60%). The two unsuccessful cases included one patient with a very tortuous PDA where we were unable to pass a wire across the PDA from femoral arterial approach, and one case where the CTA was inadequate to delineate the pulmonary anatomy due to mistiming of the CTA contrast phase. In this latter case, we elected to proceed and successfully stented the ductus. However, we failed to recognize intervening ductal tissue at the origin of the left pulmonary artery. The patient developed isolation of this vessel leading to complete occlusion and required surgical shunting, stent removal with ductal ligation, and pulmonary arterioplasty.
Discussion
We report a single center retrospective study describing the use of pre-procedural 3D imaging to inform procedural planning for DS placement in patients with ductal dependent pulmonary blood flow. In our opinion, 3D planning has potential to improve procedural success and reduced adverse events by helping to determine the optimal access approach, and by clearly defining pertinent anatomy – in particular, excess ductal tortuosity and pulmonary artery offset.
Ductal stent placement for neonates with ductal dependent pulmonary blood flow has been shown to be an appropriate alternative to surgical shunting, but remains a high-risk interventional procedure with high procedure failure rates (1, 2, 8-12). Mortality and major complication rates have ranged from 9-17% in larger studies (1, 2, 4, 10). Notably, in our cohort there were no major complications in the patients who had adequate pre-procedural CT angiography and 3D planning but we did have several complications in patients with inadequate imaging. Both Sartoro et al. and Udink ten Cate et al. describe complications related to isolated branch pulmonary stenosis (4, 9). We found that patients at risk for threatened discontinuity of the pulmonary arteries may have the potential for identification prior to the procedure based on the pulmonary artery offset. After 3D planning, one patient in our series was found to have unfavorable anatomy for DS and underwent a surgical shunt with pulmonary arterioplasty, and a second patient with severe pulmonary artery offset had successful stenting across the at risk pulmonary artery to prevent pulmonary artery isolation. Conspicuously, one patient with inadequate 3D imaging had unrecognized left pulmonary artery intervening ductal tissue that led to left pulmonary artery isolation following successful ductal stent placement. The risk of threatened discontinuity based on anatomic features at this time remains subjective and the current study, as an initial retrospective description, cannot quantitatively associate the degree of pulmonary artery offset with risk of discontinuity. However, our experience and reported anatomic quantifications may provide an opportunity for future research and, at minimum, consideration of alternate interventional approaches to attempt avoiding these complications. This need is echoed by results of a larger study from Qureshi et. al. who found a more tortuous ductus and pulmonary artery jailing was associated with higher risk of overall and unintended re-intervention (13). Based on operator experience and center preference, alternate interventional considerations may include surgical repair or variations in stenting approach – including intentional jailing of a branch pulmonary artery.
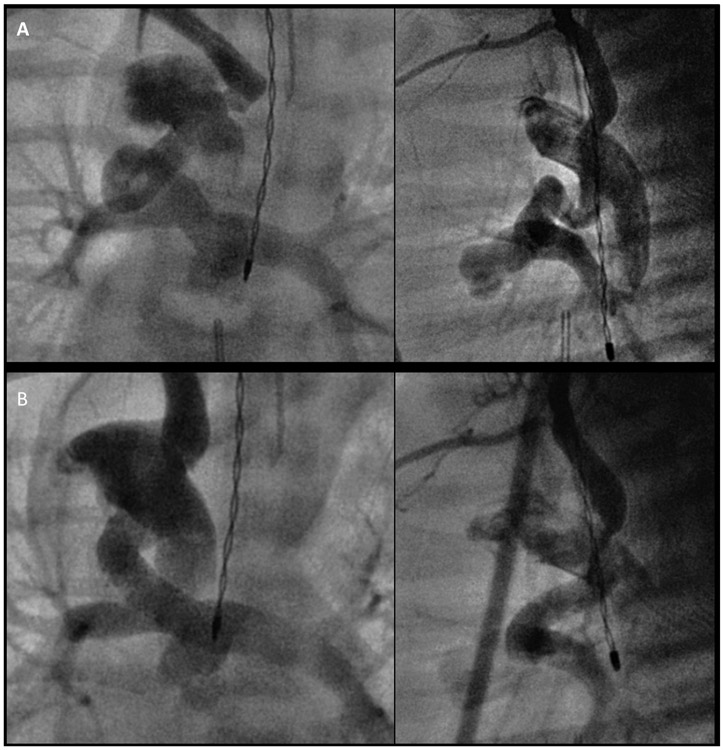
Albeit small, we report the first use of 3D planning to describe the complex anatomic relationships pertinent to DS placement. In addition to the anatomic knowledge gained by visual inspection and tactile manipulation of 3D models, the use of 3D planning prior to DS stenting allows for quantification of pertinent anatomic relationships – ductal tortuosity, total ductal length, aortic orientation of the ductus to adjacent vessels, and offset of the origin of the branch pulmonary arteries. Although exploratory at this time, the quantification of ductal tortuosity may give a better appreciation of the extent to which the ductus may elongate and change configuration with the placement of wires and stents (Fig. 3). For example, a ductus with a tortuosity index of 100 would be expected to significantly change conformation with wire and stent placement, while a ductus with a lower tortuosity index of 20 would not. Furthermore, understanding the degree to which angiography can underestimate total ductal length in a tortuous PDA may inform stent length selection and potentially reduce both the number of stents placed and the procedure time. Quantification of the angle of orientation and degree of offset of the aortic PDA insertion to adjacent vessels allows the interventionalist select an access approach that minimizes catheter manipulation. In a similar manner, quantifying the degree of branch pulmonary offset may hold future promise for a more accurate assessment of the risk of pulmonary isolation. By continuing to quantify anatomic relationships with 3D planning, future studies may be able to determine angulation and length measurements that best predict procedural success and/or complications.
Fig. 3.
Biplane fluoroscopic angiograms demonstrating the change in position and apparent straightening of a tortuous patent ductus arteriosus before (A) and after (B) stent placement.
The use of 3D imaging for procedural planning and execution has been described for several trans-catheter interventions including atrial septal device closure, atrial appendage device closure, aortic and pulmonary valve replacement, and aortic arch interventions (3, 14-19). As with other applications, improved spatial and anatomic understanding through 3D imaging has the potential to improve success rates and reduce procedural complications in ductal stenting. We found that angiography alone consistently underestimated ductal length and tortuosity. Previous studies have found increased ductal tortuosity to be a relative contraindication and predictor of procedure failure (4, 9-11). In our experience the 3D planning allowed us to successfully place ductal stents in patients we would not have previously considered, in part by allowing us to (1) identify a vascular approach that yields the most direct access to the PDA and (2) to more accurately estimate the total ductal length allowing for more informed stent selection. It should be noted that the 3D printed model has the benefit of physically testing pre-procedural placement of sheaths and wires to simulate varying access approaches, which may be desired in select cases. However, for many cases in which the vascular approach is evident, we found the 3D print may provide minimal additional benefit to the computerized 3D model.
Given the increased lifetime risk of cancer associated with exposure to low-dose ionizing radiation, it is important to consider potential for additional radiation exposure combining CT angiography and catheterization (20, 21). In recent years, improvements in technology and protocols have significantly reduced the radiation exposure during a CT, now with effective doses of around 1 mSv (22, 23). Currently no benchmark radiation dose is available for DS placement; however, in children < 1 year of age, effective radiation doses from chest CT angiography are a fraction of those from diagnostic catheterizations with median doses of 0.76 mSv for CT compared to 13.4 mSv for catheterization (21, 24). Taken together, the risk from the small additional radiation dose of CT angiography in this patient population may be acceptable given the benefit to procedural planning and outcomes.
Several important limitations to the current study should be noted. The study is a retrospective analysis of our early experience with using 3D planning to assist in ductal stenting and is not powered to determine whether pre-procedural 3D planning leads to improved safety and/or efficacy of the procedure. Furthermore, the programmatic expansion of the 3D imaging program and availability of 3D planning resources affects patient selection and, thus, introduces potential bias in observed outcomes. As with all procedures, there is a learning curve resulting in an expected improvement in outcomes over time that may partially explain the procedural success rate in the study. There was not a sufficient number of patients to fully adjust for this temporal change. It was notable that throughout the study period all of the major adverse events in our analysis occurred in patients in which 3D planning was not performed, suggesting that adverse event rates could be affected by the use of pre-procedural 3D planning, independent of procedural proficiency gained over time. Finally, per our institutional protocol, the 3D planning included printing of a physical 3D model. It should be noted that the benefit of this additional step was not specifically addressed in this study and may come at an additional cost to the institution.
Conclusion
Three dimensional imaging is increasingly incorporated into pre-procedural planning for complex CHD. Routine use of 3D CTA planning prior to DS placement in patient with ductal dependent pulmonary blood flow is feasible and provides quantitative descriptions of ductal anatomy and adjacent vessels. Furthermore, information gained from 3D imaging has the potential to improve procedural success and reduce complications.
Supplementary Material
Acknowledgements
The authors would like to thank Chip Bobbert, Innovations Lab Director at Duke University, for his invaluable help and expertise in 3D printing. In addition, we would like to thank Joseph Davis, MD, Assistant Professor of Radiology within the Division of Pediatric Radiology at Duke University, for his instrumental role in obtaining high quality CTA images in this patient population.
Funding
Dr. Chamberlain was supported by the National Institute of General Medical Sciences and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number T32GM086330. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Glatz AC, et al. , Comparison Between Patent Ductus Arteriosus Stent and Modified Blalock-Taussig Shunt as Palliation for Infants With Ductal-Dependent Pulmonary Blood Flow: Insights From the Congenital Catheterization Research Collaborative. Circulation, 2018. 137(6): p. 589–601. [DOI] [PubMed] [Google Scholar]
- 2.Bentham JR, et al. , Duct Stenting Versus Modified Blalock-Taussig Shunt in Neonates With Duct-Dependent Pulmonary Blood Flow: Associations With Clinical Outcomes in a Multicenter National Study. Circulation, 2018. 137(6): p. 581–588. [DOI] [PubMed] [Google Scholar]
- 3.Goreczny S, et al. , Three-Dimensional Image Fusion of Precatheter CT and MRI Facilitates Stent Implantation in Congenital Heart Defects. Rev Esp Cardiol (Engl Ed), 2018. [DOI] [PubMed] [Google Scholar]
- 4.Udink Ten Cate FE, et al. , Stenting the arterial duct in neonates and infants with congenital heart disease and duct-dependent pulmonary blood flow: a multicenter experience of an evolving therapy over 18 years. Catheter Cardiovasc Interv, 2013. 82(3): p. E233–43. [DOI] [PubMed] [Google Scholar]
- 5.Hilton A and Illingworth J, Marching Triangles: Delaunay Implicit Surface Triangulation. 1997.
- 6.Udupa JK, Hung HM, and Chuang KS, Surface and volume rendering in three-dimensional imaging: a comparison. J Digit Imaging, 1991. 4(3): p. 159–68. [DOI] [PubMed] [Google Scholar]
- 7.Morris SA, et al. , Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation, 2011. 124(4): p. 388–96. [DOI] [PubMed] [Google Scholar]
- 8.Rehman R, Marhisham MC, and Alwi M, Stenting the complex patent ductus arteriosus in tetralogy of Fallot with pulmonary atresia: challenges and outcomes. Future Cardiol, 2018. 14(1): p. 55–73. [DOI] [PubMed] [Google Scholar]
- 9.Santoro G, et al. , Arterial duct stenting in low-weight newborns with duct-dependent pulmonary circulation. Catheter Cardiovasc Interv, 2011. 78(5): p. 677–85. [DOI] [PubMed] [Google Scholar]
- 10.Santoro G, et al. , Ten-years, single-center experience with arterial duct stenting in duct-dependent pulmonary circulation: early results, learning-curve changes, and mid-term outcome. Catheter Cardiovasc Interv, 2015. 86(2): p. 249–57. [DOI] [PubMed] [Google Scholar]
- 11.Santoro G, et al. , Stenting of the arterial duct in newborns with duct-dependent pulmonary circulation. Heart, 2008. 94(7): p. 925–9. [DOI] [PubMed] [Google Scholar]
- 12.Sivakumar K, et al. , Longevity of neonatal ductal stenting for congenital heart diseases with duct-dependent pulmonary circulation. Congenit Heart Dis, 2012. 7(6): p. 526–33. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AM, et al. , Classification scheme for ductal morphology in cyanotic patients with ductal dependent pulmonary blood flow and association with outcomes of patent ductus arteriosus stenting. Catheter Cardiovasc Interv, 2019. 93(5): p. 933–943. [DOI] [PubMed] [Google Scholar]
- 14.Grant EK and Olivieri LJ, The Role of 3-D Heart Models in Planning and Executing Interventional Procedures. Can J Cardiol, 2017. 33(9): p. 1074–1081. [DOI] [PubMed] [Google Scholar]
- 15.Iriart X, et al. , Role of cardiac imaging and three-dimensional printing in percutaneous appendage closure. Arch Cardiovasc Dis, 2018. 111(6-7): p. 411–420. [DOI] [PubMed] [Google Scholar]
- 16.Lau I and Sun Z, Three-dimensional printing in congenital heart disease: A systematic review. J Med Radiat Sci, 2018. 65(3): p. 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenhagen P, et al. , Three-dimensional imaging of the aortic valve and aortic root with computed tomography: new standards in an era of transcatheter valve repair/implantation. Eur Heart J, 2009. 30(17): p. 2079–86. [DOI] [PubMed] [Google Scholar]
- 18.Valverde I, et al. , 3D printed models for planning endovascular stenting in transverse aortic arch hypoplasia. Catheter Cardiovasc Interv, 2015. 85(6): p. 1006–12. [DOI] [PubMed] [Google Scholar]
- 19.Goreczny S, et al. , Three-dimensional image fusion guidance of percutaneous pulmonary valve implantation to reduce radiation exposure and contrast dose: A comparison with traditional two-dimensional and three-dimensional rotational angiographic guidance. Neth Heart J, 2017. 25(2): p. 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brody AS, et al. , Radiation risk to children from computed tomography. Pediatrics, 2007. 120(3): p. 677–82. [DOI] [PubMed] [Google Scholar]
- 21.Cevallos PC, et al. , Radiation dose benchmarks in pediatric cardiac catheterization: A prospective multi-center C3PO-QI study. Catheter Cardiovasc Interv, 2017. 90(2): p. 269–280. [DOI] [PubMed] [Google Scholar]
- 22.Meinel FG, et al. , ECG-synchronized CT angiography in 324 consecutive pediatric patients: spectrum of indications and trends in radiation dose. Pediatr Cardiol, 2015. 36(3): p. 569–78. [DOI] [PubMed] [Google Scholar]
- 23.Rigsby CK, et al. , Radiation dose management for pediatric cardiac computed tomography: a report from the Image Gently ‘Have-A-Heart’ campaign. Pediatr Radiol, 2018. 48(1): p. 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson TG, et al. , Effective radiation dose in computed tomographic angiography of the chest and diagnostic cardiac catheterization in pediatric patients. Pediatr Cardiol, 2013. 34(3): p. 518–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.