Abstract
The mechanisms underlying the female-bias in autoimmunity are poorly understood. The contribution of genetic and epigenetics factors from the inactive X chromosome (Xi) are beginning to emerge as critical mediators of autoimmunity in females. Here, we ask how epigenetic features of the Xi change during disease development in B cells from the NZB/W F1 spontaneous mouse model of lupus, which is female-biased. We find that Xist RNA becomes increasingly mislocalized from the Xi with disease onset. While NZB/W F1 naïve B cells have H3K27me3 foci on the Xi, which are missing from healthy C57BL/6 and BALB/c mice, these foci are progressively lost in stimulated B cells during disease. Using single-molecule RNA FISH, we show that the X-linked gene Tlr7 is biallelically expressed in ~20% of NZB/W F1 B cells, and that the amount of biallelic expression does not change with disease. We also present sex-specific gene expression profiles for diseased NZB/W F1 B cells, and find female-specific upregulation of 20 genes, including the autoimmunity-related genes Cxcl13, Msr1, Igj, and Prdm1. Together, these studies provide important insight into the loss of epigenetic modifications from the Xi and changes with gene expression in a mouse model of female-biased SLE.
Keywords: Xist RNA, X-chromosome inactivation, H3K27me3, B cells, female-biased autoimmunity, NZB/W F1 mice, sex differences with gene expression
1. INTRODUCTION
Autoimmunity is one of the top diseases that exhibit a strong female bias, yet a mechanistic understanding of this sex bias is unknown. The autoimmune diseases with the greatest female bias include Hashimoto’s thyroiditis, Sjögren’s Syndrome, systemic lupus erythematosus (SLE), and Grave’s disease, where 85–95% of patients are women [1,2]. The sex hormones and sex chromosome content both contribute towards the observed sex differences with autoimmunity [3]. Intriguingly, XX women and individuals with multiple X chromosomes (XXX and XXY) have a higher likelihood of developing SLE and Sjögren’s Syndrome [4–7], suggesting that the presence of supernumerary X chromosomes may somehow play a role in female-biased autoimmunity.
Some spontaneous mouse models of lupus also exhibit a female bias, such as the classic F1 hybrid NZB × NZW strain (NZB/W F1) [8]. The NZB/W F1 model shares similarities with human SLE patients, where mice develop elevated serum antinuclear antibodies to dsDNA, immune complex deposition in the kidney, glomerulonephritis, and have a shortened lifespan [8–13]. NZB/W F1 mice begin exhibiting symptoms of lupus disease around 5–6 months of age and survive until 1 year of age. Notably, 100% of female animals acquire these phenotypes compared to less than 40% in males [11–13]. Although sex hormones are important contributors for disease progression in NZB/W F1 mice [12,14–16], there is some evidence that the genetics of the X chromosome also play a role. Bone marrow transplantation experiments where female NZB/W F1 cells are transplanted into irradiated male NZB/W F1 animals result in 100% of male mice acquiring lupus symptoms, which supports a hormone-independent role for the X chromosome in the development of lupus disease [13]. However, how the X chromosome contributes and whether there are X-linked gene expression changes that predispose these mice for disease development is unknown.
The X chromosome is home to many genes that are essential for immune cell function. Overexpression of the X-linked immunity genes TLR7, CXCR3, CD40L, OGT, FOXP3, BTK, and IL2RG has been observed in female (but not male) lupus patients [17–23]. In humans, duplication of the X-linked gene CD40L results in a two-fold increase in CD40L expression and is associated with multi-lineage autoimmune disease [24]. Transgenic mouse models with increased copy numbers of Tlr7 and Cd40l also develop lupus-like disease [25–27]. The BSXB/Yaa mouse model contains a duplication of the Tlr7 gene on the Y chromosome, and 100% of male animals develop elevated dsDNA antibodies and glomerulonephritis [28,29]. Reduction of Tlr7 expression in these mice eliminates the lupus-like phenotypes, while transgenic overexpression of Tlr7 in wildtype C57BL/6 mice produces phenotypes with similar or increased severity as the BSXB/Yaa mice [20]. Thus, it is essential that X-linked gene expression is properly regulated in female somatic cells to ensure the appropriate dose of X-linked immune-related genes.
Females have two X chromosomes compared to XY males, and utilize X-Chromosome Inactivation (XCI) to prevent dosage imbalance of X-linked genes. XCI occurs during female embryonic development, where one of the two X chromosomes is randomly selected for transcriptional silencing in every cell [30,31]. XCI is initiated and maintained by the long noncoding RNA Xist, which recruits the heterochromatic complexes PRC1 and PRC2 to the inactive X (Xi) for deposition of heterochromatic H3K27me3 and H2AK119ubiquitin (H2AK119Ub) modifications [32–36]. Xist RNA and heterochromatic histone modifications are enriched on the Xi, can be visualized cytologically [37], and remain associated on the Xi with each cell division [38]. Deletion of Xist in hematopoietic stem cells results in loss of Xist RNA and heterochromatic mark enrichment on the Xi, overexpression of about 200 X-linked genes, and a female-specific lethal blood cancer, likely resulting from partial reactivation of the Xi [39].
Mammalian lymphocytes cells have a unique and dynamic form of XCI maintenance [40–42]. Naïve lymphocytes lack the canonical localization of Xist RNA transcripts and heterochromatin modifications at the Xi, and some of these epigenetic modifications return to the Xi in activated lymphocytes. We have proposed that this unusual form of XCI maintenance could predispose certain genes on the Xi to become reactivated in female lymphocytes [40,41,43]. We and others have observed occasional biallelic expression of the immunity-related X-linked genes CD40L and CXCR3 in a subset of human T cells and also biallelic expression of TLR7 in B cells [40], including increased TLR7 transcripts and protein levels [44]. Together, this supports the hypothesis of a relaxed chromatin state for the Xi in female lymphocytes, where increased X-linked gene expression may contribute or predispose individuals to develop autoimmune disease. We recently reported that T cells from SLE patients and NZB/W F1 mice at late stages of disease have mislocalized nuclear patterns of Xist RNA [45]. However, the impact of Xist RNA mislocalization on heterochromatin mark enrichment at the Xi and expression of X-linked genes in T cells was not determined. Because B cells are responsible for autoantibody production and immune complex deposition in the NZB/W F1 mouse model, altered expression from the X chromosome in these cells could exacerbate disease or potentially predispose female mice to develop disease. Here, we examined both Xist RNA localization patterns and H3K27me3 enrichment at the Xi in naïve and in vitro stimulated splenic B cells from NZB/W F1 mice during disease progression. Using RNA samples from male and female NZB/W F1 mice at late-stage disease, we also profiled sex-specific expression patterns in activated B cells to investigate the genetic basis for the female-biased autoimmunity in NZB/W F1 mice.
2. Materials and Methods
2.1. Mice
NZB x NZW F1 (NZB/W F1) animals were generated by breeding New Zealand White (NZW/LacJ, Stock No: 001058, The Jackson Laboratory) with New Zealand Black (NZB/BINJ, Stock No: 000684, The Jackson Laboratory) animals. Wild-type (WT) female mice were of various genetic backgrounds (Balb/cJ, 129S1, or C57BL/6). WT and NZB/W F1 animals were between 3–12 months of age, depending on the disease stage. All animal experiments were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC), and euthanasia was performed using carbon dioxide directly prior to spleen isolation.
2.2. Disease assessment of NZB/W F1 mice
Disease onset in NZB/W F1 mice was assessed in two ways. First, bi-weekly proteinuria dipstick tests were used (Siemens, Ref: 2191) to monitor for increases in proteinuria levels (data not shown). Second, levels of dsDNA autoantibodies from sera were also monitored using sandwich ELISA detection. Briefly, calf thymus dsDNA capture antibody (Alpha Diagnostic International, 10 μg/mL, Catalog No: DNAD25-N-1) was coated on an Immulon high binding plate (ThermoFisher, Part No: 3455) and blocked in 1% BSA in 1X PBS. Serial dilutions of anti-dsDNA standard (Alpha Diagnostic International) were used to generate a standard curve where the highest concentration was 5 μg/mL. Sera were diluted as necessary to fall within the linear range of the standard curve. For Ig detection, goat Anti-Mouse IgG (H+L) conjugated with HRP (Southern Biotech, Catalog No: 1038–05) was diluted 1:4000 in 1% BSA in 1X PBS. The plate was developed with TMB (ThermoFisher, Catalog No: 2023), stopped with TMB stop solution (KPL, Catalog No: 150201), and read at 450 nm. Disease was classified into 3 stages (pre-disease, early-stage disease, and late-stage disease) based on serum concentrations of dsDNA (shown in Supplemental Figure 1) at the time of euthanasia. Histopathological analyses of glomerulonephritis were used to confirm disease status, where formalin-fixed kidney sections were evaluated by the Penn Vet Comparative Pathology Core.
2.3. Splenic B cell isolation and stimulation
Splenic B cells were isolated from wildtype and NZB/W F1 mice over multiple independent experiments. Splenic B cells were isolated and stimulated as previously described [40,41]. Briefly, mature follicular (FO) CD23+ B cells were isolated using MACS purification (Miltenyi Biotec). B cells were cultured using RPMI medium and stimulated for 24 hours with 1uM CpG (InvivoGen), then harvested for slide preparation.
2.4. RNA fluorescence in situ hybridization (FISH) and Immunofluorescence (IF)
Sequential RNA FISH and IF for splenic B cells was performed as previously described [40,41]. For Xist RNA FISH, Cy3-labeled oligonucleotides complementary to repeat regions of murine Xist RNA were synthesized by IDT. Hybridization and visualization of Xist RNA FISH was performed using established protocols [40,41]. For H3K27me3 IF, histone H3 trimethyl Lys27 antibody was used at 1:100 dilution (Active Motif, Catalog No: 39155). All nuclei were counterstained using DAPI Vectashield (Vector Labs). After obtaining images with a Nikon Eclipse, nuclei were categorized into four types of Xist RNA localization patterns, as described previously [40].
2.5. Tlr7 Single-molecule FISH (smFISH)
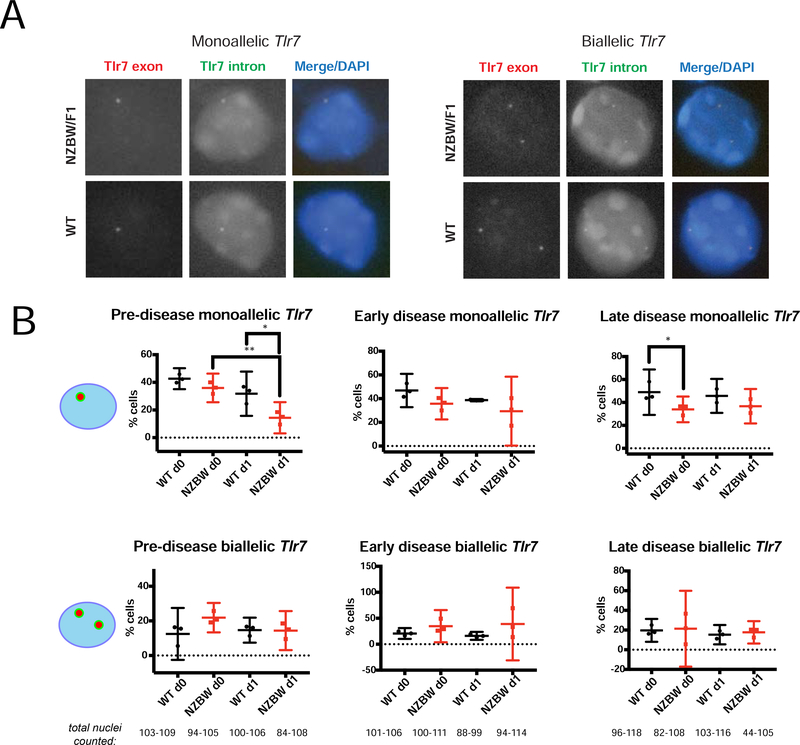
Visualization of exonic and intronic Tlr7 mRNA was performed using custom assay probe sets created by Stellaris® (LGC Biosearch Technologies). Tlr7 exonic probes were conjugated to Quasar 570 Dye (Cy3), and Tlr7 intronic probes were conjugated to Fluorescein Dye (FITC). Hybridization and visualization were performed as previously described [40]. Briefly, slides were incubated overnight with Stellaris® hybridization buffer and both intronic/exonic Tlr7 probes, and were washed according to established protocols. Nuclei were counterstained with DAPI and imaged using a Nikon Eclipse microscope. Cells were categorized as monoallelic or biallelic based on the distribution of intronic and exonic foci. Monoallelic Tlr7 nuclei contain a single focus with one co-localized intronic and exonic pinpoint. Biallelic Tlr7 nuclei contain two foci, each with a single co-localized intronic and exonic pinpoint.
2.6. RNA-sequencing methods
TRIzol (Invitrogen) was used to isolate RNA from CD23+ B cells stimulated for 1 day with CpG from three female and three male NZB/W F1 late-stage disease mice. RNA quality was assessed using the Agilent 4200 TapeStation system. Libraries were constructed using a TruSeq Stranded mRNA Library Preparation Kit (Illumina). Libraries were pooled and run on an Illumina NextSeq 500 sequencer (75bp-single end reads) with an average read depth of 101-million fragments per sample. Samples were run on the same flow cell by the Center for Host-Microbial Interactions at the University of Pennsylvania.
2.7. Bioinformatic Analysis
RNA-sequencing (RNA-seq) analysis was conducted using the statistical computing environment R (v3.6.0), RStudio (v1.2.1335), and the Bioconductor suite of packages [46]. Raw reads were mapped to the mouse reference genome (Ensembl, release GRCm38.p6) using Kallisto [47]. Tximport [48] was used to import the transcript-level data into R and summarized to the gene level. DESeq2 was used to normalize the data and correct for library size, and filtering was carried out to remove lowly expressed genes (cpm > 1). DESeq2 was used to identify differentially expressed genes with a false discovery rate [FDR] < 0.05. Comparisons of gene-specific expression were identified using the function plotCounts, which normalizes for library size and log2 transformed (labeled as normalized counts on graphs). Heatmaps were generated using heatmap.2 and gplots packages. Graphs were created in GraphPad Prism version 8.1.2. Data have been deposited in the Gene Expression Omnibus (GEO) database (data deposition and accession number currently underway).
2.8. Statistical Analysis
Data were graphed and visualized using Prism 7 software (GraphPad). Statistical significance was tested in Prism using unpaired t tests such that *p<0.05, **p<0.01, ***p<0.005, and ****p<0.0001. For each analysis, results are compiled from cells isolated during multiple independent experiments, where at least 3 independent replicates were used. The actual numbers of animals used for each experiment are shown in the figure legends.
3.0. Results
3.1. Xist RNA becomes mislocalized from the Xi in female NZB/W F1 splenic B cells at late-stage lupus disease
Lupus-like phenotypes in NZB × NZW F1 (NZB/W F1) mice exhibit a strong female-bias, yet the relationship between epigenetic status of the Xi to disease onset in NZB/W F1 mice is unknown. To determine whether XCI maintenance might be altered in NZB/W F1 female mice, we characterized the epigenetic features of the Xi in NZB/W F1 mice during disease development. For this study, we selected three categories of disease development: pre-disease, early-stage disease, and late-stage disease, using 4–7 animals for each group. We monitored disease onset in NZB/W F1 animals by following proteinuria content in urine and dsDNA autoantibodies in sera (Supplemental Fig. 1A). We confirmed the development of lupus-like disease in late-stage animals using kidney sections from NZB/W F1 females, which had evidence of glomerulonephritis (Supplemental Fig. 1B). To distinguish between early and late-stage disease, we measured serum concentration of dsDNA autoantibodies at the time of euthanasia for collection of splenic B cells. For this study, pre-disease animals resembled wild-type (WT) age-matched female animals (< 5–10 μg/mL dsDNA). Early-stage disease NZB/W F1 mice ranged from 5–11 months of age and had lower concentrations of dsDNA autoantibodies (200–2000 μg/mL dsDNA) and late-stage disease mice ranged from 9–11 months and had the highest concentrations of dsDNA autoantibodies (200–3000 μg/mL dsDNA) (Supplemental Fig. 1A).
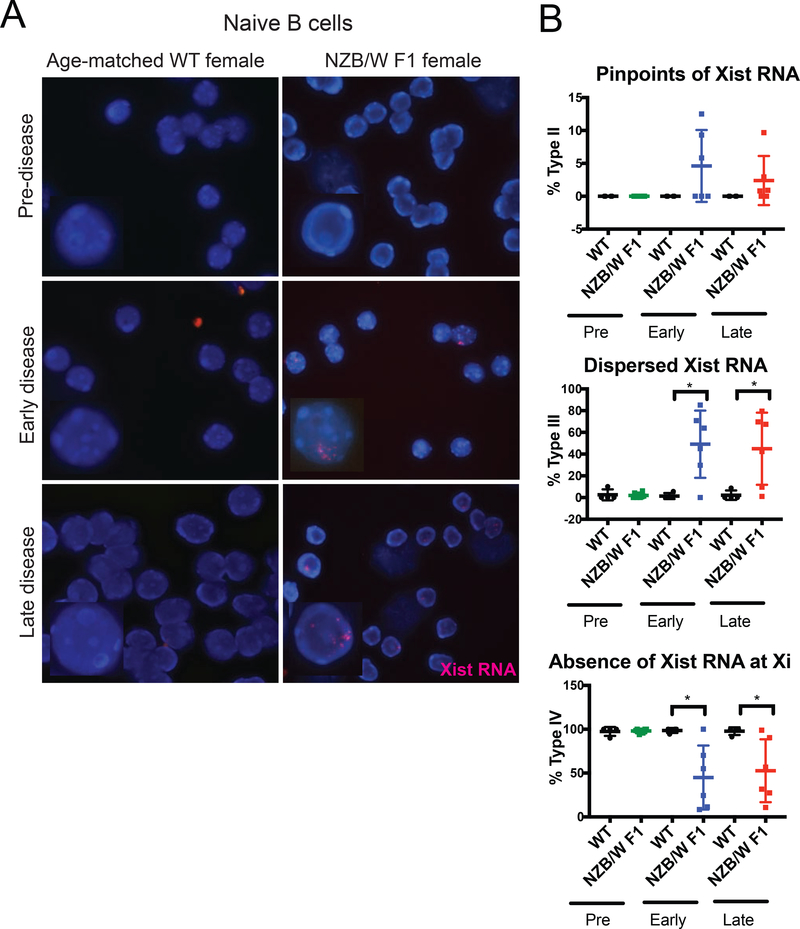
We first examined the localization of Xist RNA transcripts in nuclei from naïve follicular B cells of NZB/W F1 female mice and of age-matched healthy controls using Xist RNA FISH. We excluded NZB/W F1 males from these analyses as males have a single X chromosome, do not undergo XCI, and thus do not express Xist RNA (Supplemental Fig. 2A, 2B). The Xist RNA localization patterns in mammalian lymphocytes can be grouped into four categories [40,41]. Type I cells have dense Xist RNA present only at the Xi, Type II cells have more diffuse Xist RNA signals within a nuclear territory encompassing the Xi, Type III have Xist RNA localized throughout the nucleus, and Type IV cells lack detectable Xist RNA signals. Naïve B cells mostly lack detectable localization of Xist RNA transcripts at the Xi (Types I, II) [40,41]. Pre-disease NZB/W F1 naïve B cells, similar to age-matched wildtype controls, lacked detectible Xist RNA signals (Types I, II, III) (Fig. 1A, Supplemental Fig. 3). Interestingly, in early and late-stage disease, we observed pinpoints of Xist RNA (Type II) and dispersed Xist RNA (Type III) in naïve B cells (Figs. 1A, 1B). Age-matched wildtype naïve B cells lacked Type II and Type III Xist RNA signals (Fig. 1A, 1B, Supplemental Fig. 3). We also observed a decrease in the number of nuclei lacking detectable Xist RNA pinpoints (Type IV) in NZB/W F1 females (Fig. 1B). Interestingly, we did not observe changes in the pattern of Xist RNA when disease became more severe, as naïve B cells from early and late stages of disease were indistinguishable based on Xist RNA localization (Supplemental Fig. 3). Moreover, the appearance of Xist RNA signals in naïve B cells occurred with the onset of lupus-like disease, not before, suggesting that XCI maintenance is affected by disease and may not be causal.
FIGURE 1. NZB/W F1 females acquire dispersed Xist RNA signals in naïve B cells during lupus disease development.
(A) Xist RNA FISH images for nuclei from naïve CD23+ B cells in WT-age matched controls (n = 4) and NZB/W F1 females (n = 6), for each disease category. Disease was staged using criteria described in Supplemental Figure 1. Xist RNA is shown in red, DAPI nuclear counterstain in blue. Representative cells collected from multiple independent experiments are shown. (B) Quantification of Xist RNA localization patterns in NZB/W F1 and wildtype naïve B cells for the three disease categories. The percentage of cells with Xist RNA clustered at the Xi (top), Xist RNA dispersed throughout the nuclei (middle), and with no detectable Xist RNA signals (bottom) are shown. The mean across replicates and standard deviation of the mean is shown. Significance was determined by unpaired t test. *p<0.05. Total nuclei counts for each animal are shown in Supplemental Figure 3.
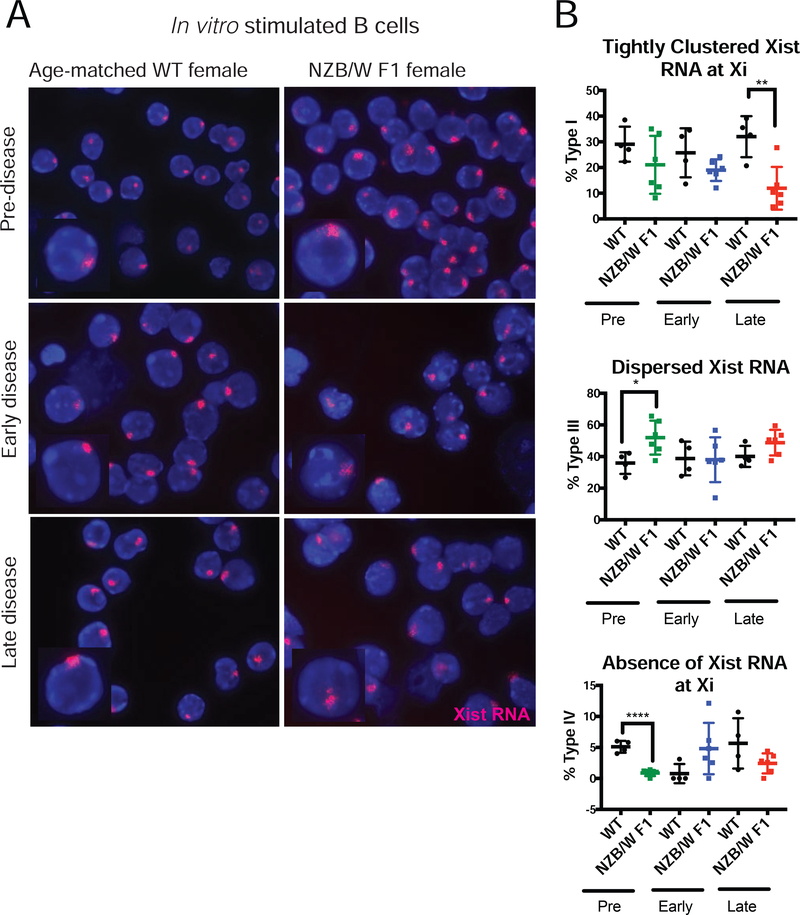
Lupus is characterized by hyperactive and autoreactive B cells that produce autoantibodies [49]. Therefore, we examined the Xist RNA localization patterns for in vitro stimulated B cells from NZB/W F1 mice. We observed a significant decrease in the amount of Xist RNA transcripts clustered at the Xi (Type I) during the late-stage of lupus disease in stimulated B cells (Figs. 2A, 2B, Supplemental Fig. 3). The average percentages for Type I cells in early-stage NZB/W F1 samples were lower compared to age-matched wildtype samples, but these differences were not statistically significant. Interestingly, we also found that pre-disease NZB/W F1 stimulated B cells have more Type III patterns and fewer Type IV cells compared to age-matched wildtype samples (Figs. 2A, 2B, Supplemental Fig. 3), suggesting that Xist RNA is mislocalized in stimulated B cells from NZB/W F1 mice. In healthy control wildtype animals, Xist RNA localization patterns in stimulated B cells were not affected by age (Figs. 2A, 2B, Supplemental Fig. 3). Taken together, we identified significant differences in Xist RNA localization patterns in both naïve and stimulated B cells from NZB/W F1 females, indicating that XCI maintenance is likely impaired by disease development.
FIGURE 2. Late-stage disease NZB/W F1 stimulated B cells lose Xist RNA localization at the Xi.
(A) Xist RNA FISH images for nuclei from CD23+ B cells stimulated in vitro for 24 hours with CpG, from WT-age matched controls (n = 4) and NZB/W F1 (n = 6) females, for each of the three disease categories (Supplementary Figure 1). Xist RNA is red, DAPI nuclear counterstain in blue. Representative cells collected from multiple independent experiments are shown. (B) Quantification of Xist RNA localization patterns in NZB/W F1 and wildtype stimulated B cells for the three disease categories. The percentage of cells with Xist RNA tightly clustered at the Xi (top), Xist RNA dispersed throughout the nuclei (middle), and with no detectable Xist RNA signals (bottom) are shown. The mean across replicates and standard deviation of the mean is shown. Significance was determined by unpaired t test. *p<0.05, **p<0.01, ****p<0.0001. Total nuclei counts for each animal are shown in Supplemental Figure 3.
3.2. H3K27me3 enrichment on Xi is progressively lost in NZB/W F1 B cells during disease development
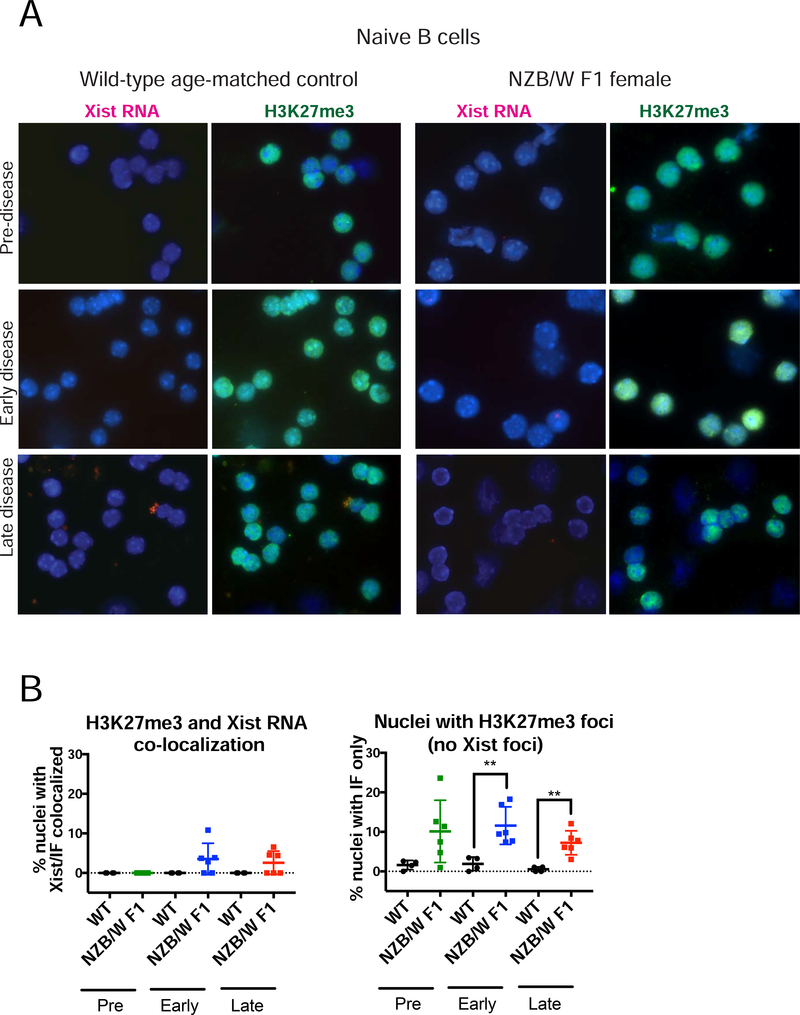
Xist RNA recruits the PRC1 and PRC2 heterochromatic complexes to form the Xi during XCI initiation, resulting in cytologically visible enrichment of H3K27me3 at the Xi [34,50]. Using sequential Xist RNA FISH and immunofluorescence (IF) detection, we previously found that Xist RNA and H3K27me3 appear together at the Xi during B cell stimulation [41]. Because Xist RNA localization at the Xi was perturbed in NZB/W F1 female naïve and stimulated B cells, we investigated whether H3K27me3 enrichment was also affected. We performed sequential Xist RNA FISH followed by IF for H3K27me3 on B cells isolated from the three groups of NZB/W F1 mice and age-matched wildtype controls. For naïve B cells, we observed that some NZB/W F1 samples exhibited co-localization of Xist RNA and H3K27me3 foci (Fig. 3A, 3B left). In addition, we found that low percentages of NZB/W F1 naïve B cells from all three stages had detectable H3K27me3 foci that lacked Xist RNA signal (Type IV) (Fig 3A, 3B right). Wild type age-matched naïve B cell samples lacked co-localization of Xist RNA and H3K27me3 foci, and very few cells had H3K27me3 foci alone (Fig. 3).
FIGURE 3. NZB/W F1 naïve B cells have more H3K27me3 foci compared to wildtype (C57BL/6; BALB/c) cells.
(A) Sequential Xist RNA FISH (left, red) followed by immunofluorescence (right, green) for H3K27me3 for naïve CD23+ B cells from wildtype age-matched mice (n = 4) and NZB/W F1 B cells (n = 6) for each disease category (Supplementary Figure 1). Arrowheads denote H3K27me3 foci; DAPI nuclear counterstain is shown in blue. Representative cells collected from multiple independent experiments are shown. (B) Quantification of co-localization of Xist RNA signals and H3K27me3 foci (left) and nuclei containing just H3K27me3 foci (right). (Left) The percentage of cells with detectable Xist RNA clusters overlapping with a focus of H3K27me3 was determined for each animal. (Right) The percentage of cells containing just a focus of H3K27me3 without any detectable clustering of Xist RNA is shown. The mean across replicates and standard deviation of the mean is shown. Significance was determined by unpaired t test. **p<0.01. Total nuclei counted: Pre-disease = 539, Pre-WT = 342, Early-disease = 432, Early-WT = 358, Late-disease = 511, Late-WT = 342.
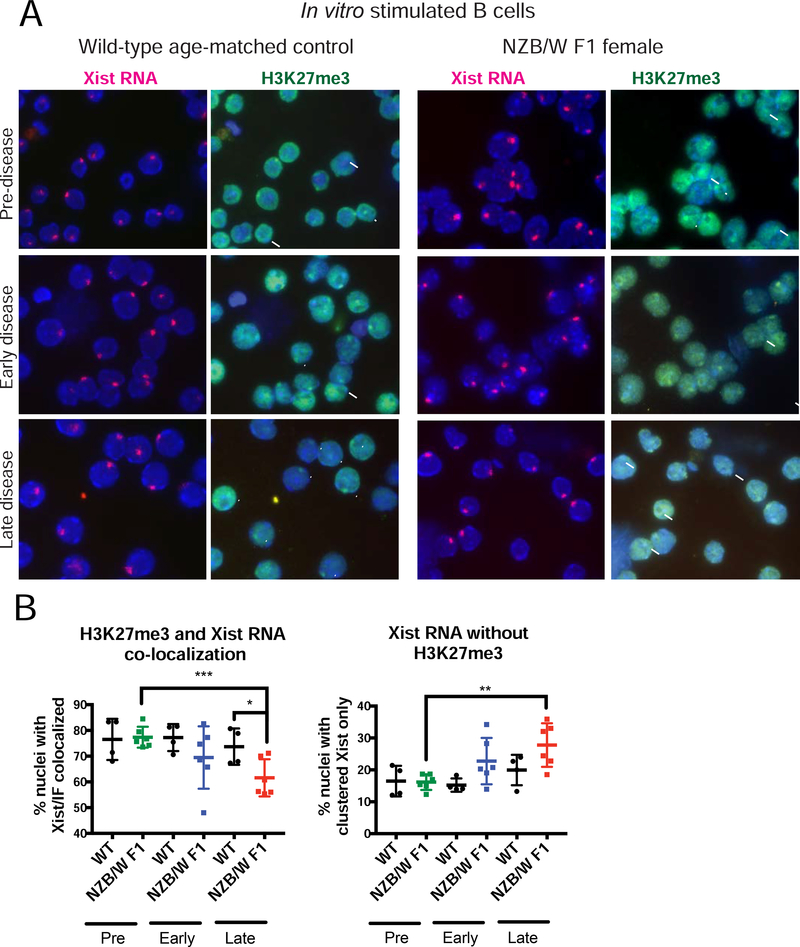
Next, we repeated this sequential Xist RNA FISH/IF analysis for in vitro stimulated B cells. For NZB/W F1 samples, Xist RNA and H3K27me3 co-localization frequencies decreased relative to wildtype controls when lupus-like disease was present (Fig. 4A, 4B). Stimulated B cells from animals with late-stage disease had the lowest levels of co-localization of Xist RNA and H3K27me3 (Fig 4B left). Unlike naïve B cells, which had detectible H3K27me3 foci, stimulated B cells from NZB/W F1 with disease had significant reductions of H3K27me3 foci (Fig. 4A), and we found significant increases in the numbers of nuclei with just Xist RNA signal (Fig. 4B right). Taken together, the reduced levels of Xist RNA and H3K27me3 enrichment at the Xi suggest that XCI maintenance might be impaired in B cells from NZB/W F1 diseased mice.
FIGURE 4. Stimulated B cells from diseased NZB/W F1 mice lose Xist RNA and H3K27me3 co-localization at the Xi.
(A) Sequential Xist RNA FISH (red) followed by IF for H3K27me3 (green) for in vitro stimulated B cells from wildtype age-matched mice (n = 4) and NZB/W F1 B cells (n = 6) for each disease category (Supplementary Figure 1). Arrowheads denote H3K27me3 foci that overlaps with Xist RNA signal; Arrows denote nuclei that contain clustered Xist RNA at the Xi but lack co-localized H3K27me3 enrichment. DAPI nuclear counterstain is shown in blue. Representative cells collected from multiple independent experiments are shown. (B) Quantification of co-localization of Xist RNA signals and foci of H3K27me3. (Left) The percentage of cells with detectable Xist RNA clusters overlapping with a focus of H3K27me3. (Right) The percentage of cells containing Xist RNA signals that lacked detectable H3K27me3 focus. The mean across replicates and standard deviation of the mean is shown. Significance was determined by unpaired t test. *p<0.05, **p<0.01. *p<0.05, **p<0.01. Total nuclei counted: Pre-disease = 559, Pre-WT = 238, Early-disease = 486, Early-WT = 281, Late-disease = 543, Late-WT = 284.
3.3. The X-linked gene Tlr7 does not have increased levels of escape from XCI in female NZB/W F1 B cells
Because Xist RNA and H3K27me3 enrichment were impaired in stimulated B cells, we hypothesized that immunity-related genes may be affected in NZB/W F1 mice. To address this, we took a candidate approach and focused on the X-linked gene Tlr7, which is expressed in B cells and has a dose-dependent association with autoimmune phenotypes. To determine B cells from NZB/W F1 mice exhibit increased levels of biallelic expression of Tlr7, we performed single molecule RNA FISH for Tlr7 using different colored intronic (FITC) and exonic (Cy3) probe sets, on both naïve and stimulated B cells from NZB/W F1 and wild-type age-matched mice from the three disease groups. We quantified the number of co-localized intronic and exonic pinpoints to assess whether each nucleus was monoallelic (one foci of intron/exon co-localization) or biallelic (two foci of intron/exon co-localization), indicating escape from XCI (Fig. 5A). Pre-disease NZB/W F1 stimulated B cells had significantly less monoallelic Tlr7 expression compared to age-matched controls, but similar percentages of biallelic Tlr7-expressing cells (Fig. 5B left). Late-stage disease NZB/W F1 naïve B cells also exhibited reduced monoallelic Tlr7 percentages, yet we did not see any differences in biallelic Tlr7 expression within this disease group (Fig. 5B right). Looking across the three disease categories (pre, early, late-stage disease), we did not observe any significant difference in Tlr7 biallelic expression between NZB/W F1 and wildtype B cells (Figure 5B). We conclude that Tlr7 escapes from XCI in naïve and stimulated B cells from NZB/W F1 mice, yet the frequency of escape does not change with autoimmune disease.
FIGURE 5. Percentages of biallelic-expressing Tlr7 B cells in NZB/W F1 mice do not change with disease.
(A) Single molecule RNA FISH for Tlr7 using probes specific for exonic regions (Cy3, red) and intronic regions (FITC, green). Representative nuclei showing examples of mono-allelic (left) and biallelic Tlr7 expression from NZB/W F1 or wildtype (C57Bl/6 or BALB/c) age-matched female B cells. (B) Quantification of monoallelic (top) and biallelic (bottom) smFISH signals in single nuclei from WT age-matched (n = 3) and NZB/W F1 (n = 3) female B cells (naïve and 24-hour CpG-stimulated), for the three disease categories. Only co-localization of red (exon) and green (intron) pinpoints were counted. The total number nuclei counted for wildtype and NZB/W F1 samples is shown below the bottom row of graphs. The mean across replicates and standard deviation of the mean is shown; significance was determined by unpaired t test. *p<0.05, **p<0.01.
3.4. Late-stage disease NZB/W F1 B cells have sex-specific gene expression profiles
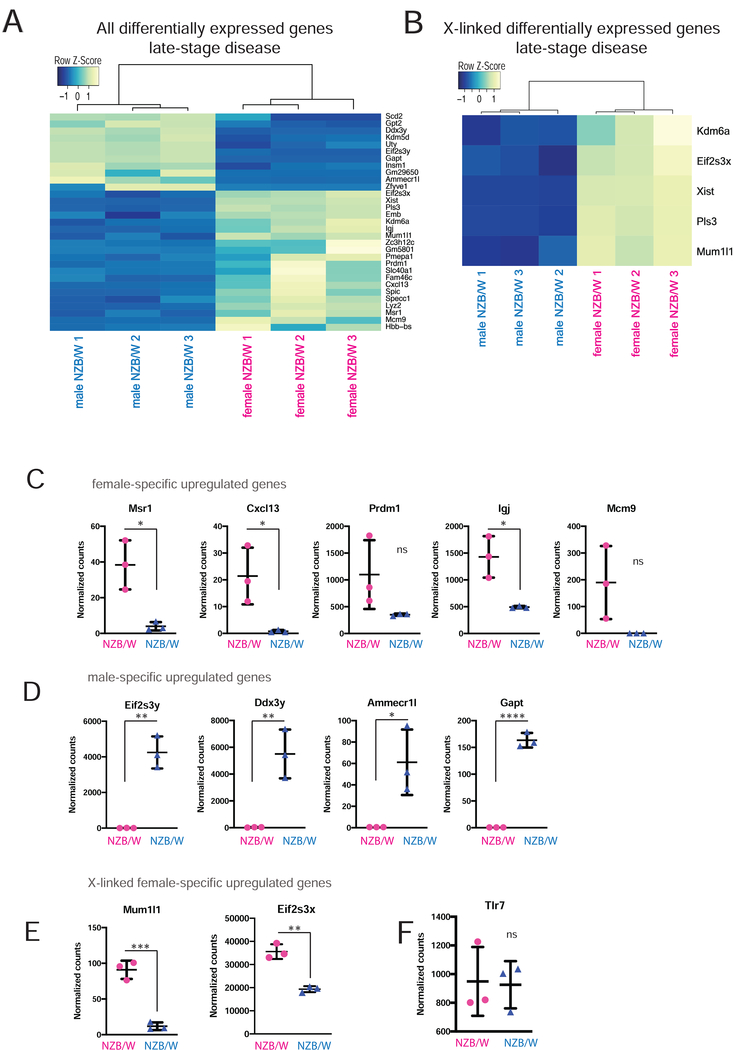
To determine whether the altered Xist RNA and H3K27me3 enrichment patterns affected X-linked genes besides Tlr7, we determined the gene expression profiles of stimulated B cells from late-stage disease female (XaXi) compared to male (XaY) NZBW F1 mice disease using RNAseq. By comparing the genes that are upregulated in female samples relative to male samples, we can indirectly assess XCI maintenance, as female-specific increased expression could arise from either the Xi, Xa, or both chromosomes. Both groups (n = 3 for each sex) of mice displayed similar levels of double-stranded DNA antibodies (Supplemental Figure 1A), and therefore likely have similar states of lupus-like disease. Surprisingly, principle component analysis revealed that female NZB/W F1 samples had largely heterogeneous global gene expression patterns, while male NZB/W F1 samples clustered together (Supplemental Figure 4), perhaps reflecting disease heterogeneity typical of SLE patients [51]. We observed significant sex-specific gene expression differences for 31 genes, with female-specific upregulation of 20 genes and downregulation of 11 genes (Fig. 6A). The most highly expressed gene in female B cells, after Xist, was Mcm9, which is a homologous DNA repair factor that localizes to sites of DNA damage for repair of double strand breaks [52]. Other upregulated genes include some that are important for various immunological processes such as blood cell development (Hbb-bs, Spi-C) and response to bacterial pathogens (Lyz2). Notably, there were four upregulated genes, Cxcl13, Msr1, Igj, and Prdm1, that have previously been implicated in autoimmunity (Fig. 6C). Cxcl13 is a B cell specific chemokine that interacts with Cxcr5 and sometimes Cxcr3 (in humans) to recruit B cells for organization and formation of germinal centers [53–55]. Msr1 regulates soluble autoantigen concentrations and plays a role in peripheral tolerance [56]. Surprisingly, Cxcl13 and Msr1 are not typically expressed at high levels in B cells, suggesting that mis-regulation of these genes could contribute to the disease phenotype of NZB/W F1 female mice. In contrast, Prdm1 and Igj are highly expressed in B cells, particularly in plasma B cells. Prdm1 (also known as Blimp-1) is required for both plasma cell formation and Ig secretion, and induction of Prdm1 leads to upregulation of Igj in plasma cells [57]. Prdm1 is a susceptibility locus for SLE, and is upregulated in NZB/W F1 mice and plasma cells from human SLE patients [58–60]. Female-specific upregulation of these genes likely contributes to the observed sex differences in the NZB/W F1 model. In support, the most highly downregulated gene in female B cells was Gapt, which negatively regulates B cell proliferation [61] (Fig. 6A).
FIGURE 6. Sex-specific gene expression profiling of diseased NZB/W F1 B cells reveal female-specific upregulation of autoimmunity-associated genes.
RNA from in vitro stimulated B cells from late-stage disease male and female NZB/W F1 animals was isolated for RNAseq analysis. (A) Heatmap for all differentially expressed genes (genome-wide) in B cells from female and male NZB/W F1 with late stage disease. (B) Heatmap for differentially expressed X-linked genes in female and male samples. (C) Graphs of normalized counts for some female-specific upregulated genes. (D) Graphs of normalized counts for some male-specific upregulated genes. (E) Normalized read counts of two X-linked genes. (F) Normalized counts for the X-linked gene Tlr7. Pink circles indicate female samples, blue triangles indicate male samples.
We identified 5 X-linked genes that were upregulated in female NZB/W F1 B cells compared to male samples (Fig. 6B). As expected, this list includes Xist, which is exclusively expressed in female cells. Two of these genes are known to escape XCI (Eif2s3x, Kdm6a/Utx), thus it is not surprising that female B cells exhibit increased expression compared to male cells. Indeed, the Y-linked homologs of these two XY gene pairs (Eif2s3y and Uty) and two additional Y-linked genes Kdm5d, Ddx3y were upregulated in male NZB/W F1 B cells (Fig. 6A, 6D). The gene Mum1l1 is subject to XCI [62], and was significantly upregulated in female compared to male samples (Fig. 6E). Pls3 exhibits variable escape from XCI in human fibroblasts [63,64], thus this gene may be subject to XCI in B cells. The X-linked gene Tlr7 did not exhibit sex differences with expression (Fig. 6F). Although we found 5 X-linked genes that were significantly upregulated in female cells, it is possible that other X-linked genes were reactivated from the Xi, but could not be detected here because our RNA samples were heterogenous for XCI (which is random) and also not allele-specific. Most XCI escape genes are expressed at levels (10 – 75%) less than the amounts from the Xa [65], potentially precluding detection by our study.
Because female NZB/W F1 stimulated B cells had dispersed patterns of Xist RNA during late-stage disease, we asked whether female mice had altered gene expression levels for factors known to localize RNA to the Xi. We determined the sex-specific expression profiles for Yy1, hnRNP-U, and Ciz1, which are required for Xist RNA localization in lymphocytes [40,41,66]. We found that there was no significant difference in expression of these genes between male and female B cells (Supplementary Fig. 5). Female B cells exhibited variability among replicate samples for these three genes, whereas male replicates were consistent. We also investigated expression profiles for ~ 30 additional factors that interact with Xist RNA (termed the ‘Xist Interactome’), and observed female-specific variability among replicates (data not shown). In sum, B cells from NZB/W F1 mice with lupus-like disease had sex-specific gene expression profiles that may contribute towards the strong female bias of this mouse model.
4.0. DISCUSSION
The molecular mechanisms involving the sex chromosomes that underlie female-biased autoimmunity are not well understood. Here, we investigated how the epigenetic features of the Xi change in B cells during disease progression in the NZB/W F1 mouse model, where 100% of female animals develop lupus-like disease. We found that enrichment of the epigenetic features Xist RNA and H3K27me3 decreases with the onset of lupus-like disease in NZB/W F1 B cells. Using RNAseq, we generated the first sex-specific gene expression profiles of stimulated B cells from disease NZB/W F1 mice, and discovered female-specific upregulation of some autoimmunity-related genes.
In our study, we observed that naïve B cells from NZB/W F1 females at early and late stages of lupus-like disease have nuclear Xist RNA signals and H3K27me3 foci (Figs. 1, 3), which are missing from wildtype age-matched animals. In this study and other work from our group, we showed that wildtype animals are characterized by Xist RNA signals emerging at the Xi during in vitro stimulation, and that Xist RNA appears concurrently with H3K27me3 foci in B cells [41]. Because SLE patients and mouse models of lupus are characterized by increased numbers of activated B cells [67,68], the presence of Xist RNA signals in splenic B cells from late-stage disease mice likely reflect circulating activated B cells. It is intriguing that we also observed higher levels of H3K27me3 foci, independent of Xist RNA signals, across the three disease categories of NZB/W F1 mice (Fig. 3B, right). One interpretation of this finding is that the chromatin of the Xi could be more compact in naïve B cells of NZB/W F1 animals compared to C57BL/6 and BALB/c mice, perhaps reflecting impairments with nuclear organization factors in these lupus-prone mice. It is possible that there is more heterochromatin and compaction genome-wide in B cells of diseased NZB/W F1 mice, with elevated deposition of H3K27me3 modifications across all chromosomes beside the X. It will be interesting to repeat these sequential RNA FISH/IF experiments to determine whether other heterochromatin modifications (such as H2AK119Ub and H4K20me1) are also enriched independently of Xist RNA on the Xi. The Xi is known to be more compact than the Xa in fibroblasts [69–71], yet whether this difference with compaction also occurs in lymphocytes and if autoimmunity affects compaction has not been examined.
Our investigations of in vitro stimulated B cells revealed that Type I Xist RNA patterns were significantly reduced in late-stage disease NZB/W F1 B cells (Fig. 2B). We recently reported that T cells from NZB/W F1 animals exhibited reduced Type I Xist RNA localization patterns for late-stage disease animals [45]. For this study we focused on B cells, and we were intrigued to determine whether altered Xist RNA localization and H3K27me3 enrichment would precede disease development, or occur concurrently with disease onset. The NZB/W F1 animals had less Xist RNA Type I cells compared to wildtype controls, although only late-stage disease samples were statistically significant (Fig. 2B, top). Thus, active lupus disease affects Xist RNA localization at the Xi in both T and B cells. We also observed that percentages of nuclei with Xist RNA and H3K27me3 co-localization at the Xi are similar between pre-disease NZB/W F1 and wildtype samples. However, as disease develops, NZB/W F1 B cells have reduced levels of co-localization compared to wildtype samples (Fig. 4B, left). Disease progression also resulted in loss of H3K27me3 foci in NZB/W F1 B cells, yet nuclei still contained dispersed Type II and III Xist RNA signals (Fig. 4B, right). These data suggest that Xist RNA localization at the Xi is necessary to retain H3K27me3 enrichment on this chromosome in NZB/W F1 B cells, and that dispersed Xist transcripts result in loss of H3K27me3 foci. Whether Xist RNA actively recruits PRC2 for continuous deposition of H3K27me3 marks across the Xi in stimulated B cells, similar to mechanisms of XCI initiation in differentiating embryonic stem cells [72,73], is unknown. It is intriguing to speculate that H2AK119Ub modifications may exhibit similar reductions in Xist RNA co-localization with disease progression in NZB/W F1 B cells, suggesting cooperative recruitment of PRC1 that could be mediated by Xist RNA. Additional experiments in stimulated B cells are necessary to investigate the molecular mechanisms of PRC1 and PRC2 recruitment dynamics to the Xi, and the role of Xist RNA in the cytological and molecular enrichment of these complexes in these cells.
The X-linked gene Tlr7 is an important intracellular nucleic acid sensor for RNA-containing immune complexes and Tlr7 overexpression resulting from gene duplication results in systemic autoimmunity in mice [28,29]. Recent work has demonstrated that B cells from human females and males with Klinefelter syndrome (XXY) have biallelic expression of TLR7 from the Xi in approximately 39% of cells, and that female cells had more TLR7 protein compared to male cells [44]. Thus, in this study, we investigated the possibility that Tlr7 also escapes from XCI in NZB/W F1 females and whether there is increased biallelic expression of Tlr7 with disease progression. We discovered that both WT and NZB/W F1 female B cells have similar levels of biallelic Tlr7 expressing cells (~20%), and that these levels do not change with disease (Fig 5B). We did observe that pre-disease NZB/W F1 mice has significantly less Tlr7 monoallelic-expressing cells (Fig. 5B, top row), yet this reduction did not influence the percentage of biallelic cells. Importantly, we did not observe differences with steady-state levels of Tlr7 transcripts, either by qRT-PCR (data not shown) or using RNAseq (Fig. 6F), which confirms that disease does not increase the levels of Tlr7 RNA. This result supports the idea that there are multiple pathways that initiate and propagate autoimmunity, and that Tlr7 overexpression is not a requirement for female-biased lupus in this mouse model.
Our work is the first to determine sex-specific gene expression profiles of disease-stage B cells from NZB/W F1 mice. We compared male to female RNAseq datasets, and were surprised by the amount of variation among female samples. We can eliminate disease severity as the cause for this variability because all female animals had elevated sera dsDNA concentrations and evidence of proteinuria. It is possible that the variability among female RNAseq gene expression profiles reflects disease heterogeneity with lupus symptoms, which is common for female SLE patients. Averaging the female and male samples, we found that both autosomal and sex-linked genes exhibited expression differences with expression based on sex. Importantly, we discovered a subset of autoimmunity-related genes that are specifically upregulated in female B cells: Msr1, Cxcl13, Prdm1, Igj, and also the DNA repair protein Mcm9 (Fig. 6C). Additional work is necessary to determine how these genes become upregulated in female mice, and their contribution to disease onset or progression.
We also found that just 5 X-linked genes that were significantly upregulated in female diseased NZB/W F1 B cells compared to males (Fig 6B). Given the large number of genes on the X chromosome (~1000 genes) and our observed disruptions in Xist RNA and H3K27me3 localization on the X, we were surprised that there were so few X-linked genes that were upregulated specifically in female B cells. It is possible that our results are an underestimate of the actual number of affected X-linked genes. One explanation for this underestimate could be that we were unable to detect reactivation from the Xi if the level of gene expression was low, as we were making comparisons between the sexes and have a cutoff for significance of differentially expressed genes. Another possibility is that there could be heterogeneity among individual cells for numbers of reactivated genes from the Xi, as recently observed in fibroblast samples [74,75]. In support, we observed female-specific variability in our RNAseq data between replicates (Supplemental Fig. 4), which may reflect both autosomal and X-chromosome transcriptional differences. We predict that Type IV Xist RNA cells, which lack detectable Xist RNA signals, could have more reactivated/escape genes on the Xi compared to Type I or II Xist localization patterns. Because there is a mixture of Xist RNA localization patterns in stimulated B cells, it is difficult to determine the transcriptional status of the Xi for each type of Xist localization pattern. A combination of single-cell FISH and transcriptional profiling of stimulated B cells is a possible approach, and future studies investigating the transcriptional status of the Xi for each particular pattern of Xist RNA localization are underway.
Supplementary Material
Supplemental Figure 5. Sex-specific expression profiles of three Xist RNA localization factors that function in lymphocytes: Yy1, Hnrnpu, Ciz1. Female B cells of NZB/W F1 mice exhibit variability with expression levels of these genes. Pink circles indicate female samples, blue triangles indicate male samples.
Supplemental Figure 1. Parameters used for classification of three disease stages (pre-disease, early-stage disease, late-stage disease) in male and female NZB/W F1 animals. (A) Sera concentrations (ug/mL) of dsDNA auto-antibodies from NZB/W F1 and wildtype mice used in this study. (B) Representative histopathological analysis of kidney sections from late-stage disease NZB/W F1 female and WT age-matched control female mice. Black arrowheads in wiltype (WT) denote healthy glomeruli (top left); arrowheads in NZB/W F1 sections indicate features of disease: glomerulonephropathy (bottom left), tubular proteinosis (top and middle right), and glomerular crescent (bottom right).
Supplemental Figure 2. Xist RNA FISH analyses in NZB/W F1 males from three disease categories (n = 4 animals for each group). (A) Representative fields of Xist RNA FISH in nuclei from naïve and 24-hour CpG-stimulated CD23+ B cells in NZB/W F1 males. Disease was staged using criteria described in Supplemental Figure 1. Xist RNA is red, DAPI nuclear 593 counterstain in blue. Data are representative of cells collected in multiple independent experiments. (B) Quantification of Types I/II (top) and IV (bottom) Xist RNA localization patterns.
Supplemental Figure 3. Quantification of Xist RNA localization patterns (Types I, II, III, IV) in NZB/W F1 and age-matched wildtype (C57Bl/6, BALB/c) female B cells, for each disease stage (pre-disease, early-stage disease, late-stage disease). Total nuclei counted are shown above each sample. (A) NZB/W F1 females. (B) Wild-type age-matched females.
Supplemental Figure 4. Principle component analysis (PCA) of RNAseq profiles of male and female stimulated B cells from diseased NZB/W F1 mice. Female samples in red; male samples in turquoise.
Highlights.
Xist RNA and H3K27me3 epigenetic modifications are lost from the inactive X (Xi) in B cells of NZB/W F1 mice
Diseased B cells of NZB/W F1 mice exhibit sex differences with gene expression
Female B cells have significant upregulation of some autoimmunity-related genes
NZB/W F1 B cells do not exhibit changes with biallelic expression of Tlr7, an X-linked gene whose overexpression results in lupus-like phenotypes.
ACKNOWLEDGEMENTS
We would like to thank the members of the Anguera lab for critical discussions and comments on this manuscript; M. May, M. Cancro, T. Laufer, and B. Freedman for helpful discussions; A. Martin for initial investigations of Xist RNA patterns in splenic B cells and assistance with mouse colony organization; L. Buza and the Penn Vet Comparative Pathology Core for the kidney pathology assessments. This work was supported by DOD grant LR170055: W81XWH-18-1-06-F31 and (IS) 055428-training grant T32 AI; (MCA) AI134834-R01 and NIH grant (CMS). GM123604
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cooper GS, Stroehla BC, The epidemiology of autoimmune diseases, 2 (2003) 119–125. [DOI] [PubMed] [Google Scholar]
- [2].Jacobson DL, Gange SJ, Rose NR, Graham NMH, Epidemiology and Estimated Population Burden of Selected Autoimmune Diseases in the United States, Clin. Immunol. Immunopathol 84 (1997) 223–243. [DOI] [PubMed] [Google Scholar]
- [3].Rubtsova K, Marrack P, V Rubtsov A, Sexual dimorphism in autoimmunity, J. Clin. Invest 125 (2015) 2187–2193. doi: 10.1172/JCI78082.Evidence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Harris VM, Sharma R, Cavett J, Kurien BT, Liu K, Koelsch KA, Rasmussen A, Radfar L, Lewis D, Stone DU, Kaufman CE, Li S, Segal B, Wallace DJ, Weisman MH, Venuturupalli S, Kelly JA, Alarcon-Riquelme ME, Pons-Estel B, Jonsson R, Lu X, Gottenberg JE, Anaya JM, Cunninghame-Graham DS, Huang AJW, Brennan MT, Hughes P, Alevizos I, Miceli-Richard C, Keystone EC, Bykerk VP, Hirschfield G, Xie G, Ng WF, Nordmark G, Bucher SM, Eriksson P, Omdal R, Rhodus NL, Rischmueller M, Rohrer M, Wahren-Herlenius M, Witte T, Mariette X, Lessard CJ, Harley JB, Sivils KL, Scofield RH, Klinefelter’s syndrome (47,XXY) is in excess among men with Sjögren’s syndrome, Clin. Immunol 168 (2016) 25–29. doi: 10.1016/j.clim.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, Reveille JD, Alarcón GS, Vilá LM, Reid J, Harris B, Li S, Kelly JA, Harley JB, Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: Support for the notion of a gene-dose effect from the X chromosome, Arthritis Rheum. 58 (2008) 2511–2517. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Slae M, Heshin-bekenstein M, Simckes A, Heimer G, Engelhard D, Eisenstein EM, Female polysomy-X and systemic lupus erythematosus, Semin. Arthritis Rheum 43 (2014) 508–512. doi: 10.1016/j.semarthrit.2013.07.014. [DOI] [PubMed] [Google Scholar]
- [7].Liu K, Kurien BT, Zimmerman SL, Kaufman KM, Taft DH, Kottyan LC, Lazaro S, Weaver CA, Ice JA, Adler AJ, Chodosh J, Radfar L, Rasmussen A, Stone DU, Lewis DM, Li S, Koelsch KA, Igoe A, Talsania M, Kumar J, Maier-moore JS, Harris VM, Gopalakrishnan R, Jonsson R, Lessard JA, Lu X, Gottenberg J, Anaya J, Cunninghame-graham DS, Huang AJW, Brennan MT, Hughes P, Illei GG, Miceli-richard C, Keystone EC, Bykerk VP, Hirschfield G, Xie G, Ng W, Nordmark G, Eriksson P, Omdal R, Rhodus NL, Rischmueller M, Rohrer M, Segal BM, Vyse TJ, Wahren-herlenius M, Guthridge JM, Witte T, Pons-estel B, Alarc ME, James JA, Lessard CJ, Kelly JA, Thompson SD, Gaffney PM, Montgomery CG, Edberg JC, Kimberly RP, Langefeld CL, Gilkeson GS, Kamen DL, Tsao BP, Mccune WJ, Salmon JE, Merrill JT, Weisman MH, Wallace DJ, Utset TO, Bottinger EP, Amos CI, Siminovitch KA, Mariette X, Sivils KL, Harley JB, Scofield RH, X Chromosome Dose and Sex Bias in Autoimmune Diseases Increased Prevalence of 47, XXX in Systemic Lupus Erythematosus and € gren ‘ s Syndrome Sj o, Arthritis Rheum. 68 (2016) 1290–1300. doi: 10.1002/art.39560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Helyer BJ, Howie JB, Renal Disease associated with Positive Lupus Erythematosus Tests in a Crossbred Strain of Mice, Nature. 197 (1963) 197–197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- [9].Seegal BC, Accinni L, Andres GA, Beiser SM, Christian CL, Erlanger BF, Hsu KC, Immunologic Studies of Autoimmine Disease in NZB/NZW F1 mice, JEM. 4259 (1969) 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lambert PH, Dixon FJ, Pathogenesis of the Glomerulonephritis of NZB/W mice, JEM. (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bupp G, Jørgensen TN, Kotzin BL, Identification of candidate genes that influence sex hormone-dependent disease phenotypes in mouse lupus, Genes Immun. (2008) 47–56. doi: 10.1038/sj.gene.6364447. [DOI] [PubMed] [Google Scholar]
- [12].Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK, Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice., J. Exp. Med 147 (1978) 1568–83. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].David A, Trigunaite A, MacLeod MK, Johnson AC, Marrack P, Jørgensen TN, Intrinsic autoimmune capacities of hematopoietic cells from female New Zealand hybrid mice, Genes Immun. 15 (2014) 153–161. doi: 10.1038/gene.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roubinian JR, Papoian R, Talal N, Androgenic hormones modulate autoantibody responses and improve survival in murine lupus, J. Clin. Invest 59 (1977) 1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roubinian J, Talal N, Siiteri PK, Sadakian JA, Sex hormone modulation of autoimmunity in NZB/NZW mice, Arthritis Rheum. (1979). [DOI] [PubMed] [Google Scholar]
- [16].Perry D, Sang A, Yin Y, Zheng Y, Morel L, Murine Models of Systemic Lupus Erythematosus, J. Biomed. Biotechnol 2011 (2011). doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hewagama A, Gorelik G, Patel D, Liyanarachchi P, Joseph McCune W, Somers E, Gonzalez-Rivera T, The Michigan Lupus Cohort, F. Strickland, B. Richardson, Overexpression of X-Linked genes in T cells from women with lupus, J. Autoimmun 41 (2013). doi: 10.1016/j.jaut.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B, Demethylation of CD40LG on the Inactive X in T Cells from Women with Lupus, J. Immunol. 179 (2007) 6352–6358. doi: 10.4049/jimmunol.179.9.6352. [DOI] [PubMed] [Google Scholar]
- [19].Bonelli M, Von Dalwigk K, Savitskaya A, Smolen JS, Scheinecker C, Foxp3 expression in CD4 + T cells of patients with systemic lupus erythematosus : a comparative phenotypic analysis, Ann. Rheum. Dis (2008) 664–671. doi: 10.1136/ard.2007.074690. [DOI] [PubMed] [Google Scholar]
- [20].Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S, Article Control of Toll-like Receptor 7 Expression Is Essential to Restrict Autoimmunity and Dendritic Cell Proliferation, (2007) 801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marshak-rothstein A, Toll-like receptors in systemic autoimmune disease, Nat. Rev. Immunol 6 (2006) 823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bradley SJ, Suarez-fueyo A, Moss DR, Kyttaris VC, T Cell Transcriptomes Describe Patient Subtypes in Systemic Lupus Erythematosus, PLoS One. 10 (2015) 1–19. doi: 10.1371/journal.pone.0141171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kong W, Deng W, Sun Y, Huang S, Zhang Z, Shi B, Increased expression of Bruton’s tyrosine kinase in peripheral blood is associated with lupus nephritis, Clin. Rheumatol 37 (2018) 43–49. doi: 10.1007/s10067-017-3717-3. [DOI] [PubMed] [Google Scholar]
- [24].Le Coz C, Trofa M, Syrett CM, Martin A, Jyonouchi H, Jyonouchi S, Anguera MC, Romberg N, CD40LG duplication-associated autoimmune disease is silenced by nonrandom X-chromosome inactivation, J Allergy Clin Immunol (2018). doi: 10.1016/j.jaci.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S, Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell – mediated autoimmunity, PNAS. 109 (2012) 16276–16281. doi: 10.1073/pnas.1209372109/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clegg CH, Rulffes JT, Haugen HS, Hoggatt IH, Aruffo A, Durham SK, Farr AG, Hollenbaugh D, Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice, Int. Immunol 9 (1997) 1111–1122. [DOI] [PubMed] [Google Scholar]
- [27].Pérez-melgosa M, Hollenbaugh D, Wilson CB, Hollenbaugh D, Cutting Edge: CD40 Ligand Is a Limiting Factor in the Humoral Response to T Cell-Dependent Antigens, J. Immunol 163 (1999) 1123–1127. [PubMed] [Google Scholar]
- [28].Pisitkun P, Deane Jonathan A., Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S, Autoreactive B Cell Responses to RNA-Related Antigens Due to TLR7 Gene Duplication, Science (80-. ). 312 (2006) 1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- [29].Subramanian S, Tus K, Li Q, Wang A, Tian X, Zhou J, Liang C, Bartov G, Mcdaniel LD, Zhou XJ, Schultz RA, Wakeland EK, A Tlr7 translocation accelerates systemic autoimmunity in murine lupus, PNAS. (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Payer B, Lee JT, X Chromosome Dosage Compensation : How Mammals Keep the Balance, Annu. Rev. Genet (2008). doi: 10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- [31].Lyon MF, Gene action in the X-chromosome of the mouse (mus musculus L.), Nature. 190 (1961) 372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- [32].Brown CJ, Lafreniere RG, Powers VE, Sebastio G, Ballabio a, Pettigrew a L., Ledbetter DH, Levy E, Craig IW, Willard HF, Localization of the X inactivation centre on the human X chromosome in Xq13., Nature. 349 (1991) 82–4. doi: 10.1038/349082a0. [DOI] [PubMed] [Google Scholar]
- [33].Brown CJ, Hendrich BD, Rupert JL, Xing Y, Lawrence J, Willard F, The Human X / ST Gene : Analysis of a 17 kb Inactive X-Specific RNA That Contains Conserved Repeats and Is Highly Localized within the Nucleus I ‘, Cell. 71 (1992). [DOI] [PubMed] [Google Scholar]
- [34].Plath K, Fang J, Mlynarczyk-evans SK, Cao R, Worringer KA, Wang H, De Cruz CC, Otte AP, Panning B, Role of Histone H3 Lysine 27 Methylation in X Inactivation, Science (80-. ). 300 (2003) 131–136. [DOI] [PubMed] [Google Scholar]
- [35].Zhao J, Sun BK, Erwin JA, Song J, Lee JT, Polycomb Proteins Targeted by a Short Repeat RNA to the Mouse X Chromosome, Science (80-. ). 322 (2008) 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sunwoo H, Wu JY, Lee JT, The Xist RNA-PRC2 complex at 20-nm resolution reveals a low Xist stoichiometry and suggests a hit-and-run mechanism in mouse cells, PNAS. (2015). doi: 10.1073/pnas.1503690112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chadwick BP, Willard HF, Barring gene expression after XIST: Maintaining facultative heterochromatin on the inactive X, Semin. Cell Dev. Biol 14 (2003) 359–367. doi: 10.1016/j.semcdb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- [38].Jonkers I, Monkhorst K, Rentmeester E, Grootegoed JA, Grosveld F, Gribnau J, Xist RNA Is Confined to the Nuclear Territory of the Silenced X Chromosome throughout the Cell Cycle, Mol. Cell. Biol 28 (2008) 5583–5594. doi: 10.1128/MCB.02269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yildirim E, Kirby JE, Brown DE, Mercier FE, Sadreyev RI, Scadden DT, Lee JT, Xist RNA is a potent suppressor of hematologic cancer in mice, Cell. 152 (2013) 727–742. doi: 10.1016/j.cell.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang J, Syrett CM, Kramer MC, Basu A, Atchison ML, Anguera MC, Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X, Proc. Natl. Acad. Sci (2016) 201520113. doi: 10.1073/pnas.1520113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Syrett CM, Sindhava V, Hodawadekar S, Myles A, Liang G, Zhang Y, Nandi S, Cancro M, Atchison M, Anguera MC, Loss of Xist RNA from the inactive X during B cell development is restored in a dynamic YY1-dependent two-step process in activated B cells, PLoS Genet. 13 (2017) 1–28. doi: 10.1371/journal.pgen.1007050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Syrett CM, Sindhava V, Sierra I, Dubin AH, Atchison M, Diversity of Epigenetic Features of the Inactive X-Chromosome in NK Cells, Dendritic Cells, and Macrophages, Front. Immunol 9 (2019) 1–10. doi: 10.3389/fimmu.2018.03087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Syrett CM, When the balance is broken : X-linked gene dosage from two X chromosomes and female-biased autoimmunity, J. Leukoc. Biol (2019) 1–14. doi: 10.1002/JLB.6RI0319-094R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, Pienkowski C, Chaumeil J, Mejía JE, Guéry J, TLR7 escapes X chromosome inactivation in immune cells, Sci. Immunol 8855 (2018) 1–11. [DOI] [PubMed] [Google Scholar]
- [45].Syrett CM, Atchison M, Syrett CM, Paneru B, Sandoval-heglund D, Wang J, Banerjee S, Sindhava V, Behrens EM, Atchison M, Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases Find the latest version : Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases, 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, Gottardo R, Hahne F, Hansen KD, Irizarry RA, Lawrence M, Love MI, Macdonald J, Obenchain V, Oleś AK, Pagès H, Reyes A, Shannon P, Smyth GK, Tenenbaum D, Waldron L, Morgan M, Orchestrating high-throughput genomic analysis with Bioconductor, Nat. Methods 12 (2015) 115–121. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bray NL, Pimentel H, Melsted P, Pachter L, Near-optimal probabilistic rna-seq quantification, Nat. Biotechnol 34 (2016) 4–8. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- [48].Soneson C, Love MI, Robinson MD, Differential analyses for RNA-seq : transcript-level estimates improve gene-level inferences, F1000 Res. 4 (2019) 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Marrack P, Kappler J, Kotzin BL, Autoimmune disease: why and where it occurs., Nat. Med 7 (2001) 899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- [50].Colognori D, Sunwoo H, Kriz AJ, Wang C, Lee JT, Xist Deletional Analysis Reveals an Interdependency between Xist RNA and Polycomb Complexes for Spreading along the Inactive X Article Xist Deletional Analysis Reveals an Interdependency between Xist RNA and Polycomb Complexes for Spreading along the Inactive X, Mol. Cell (2019) 1–17. doi: 10.1016/j.molcel.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Erythematosus L, Nagafuchi Y, Shoda H, Fujio K, Immune Profiling and Precision Medicine in Systemic Lupus Erythematosus, Cells. 8 (2019). doi: 10.3390/cells8020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Traver S, Coulombe P, Peiffer I, Kitzmann M, Latreille D, Me M, Traver S, Coulombe P, Peiffer I, Hutchins JRA, Kitzmann M, Latreille D, MCM9 Is Required for Mammalian DNA Mismatch Repair, Mol. Cell (2015) 831–839. doi: 10.1016/j.molcel.2015.07.010. [DOI] [PubMed] [Google Scholar]
- [53].Li EK, Chen DP, Lam CWK, Elevated Production of B Cell Chemokine CXCL13 is Correlated with Systemic Lupus Erythematosus Disease Activity, J. Clin. Immunol (2010) 45–52. doi: 10.1007/s10875-009-9325-5. [DOI] [PubMed] [Google Scholar]
- [54].Jenh C, Cox MA, Hipkin W, Lu T, Pugliese-sivo C, Gonsiorek W, Chou C, Narula SK, Zavodny PJ, Cxc T, Bca B-, Human B Cell-Attracting Chemokine 1 ( BCA-1; CXCL13 ) is an agonist for the human CXCR3 receptor, Cytokine. 1 (2001) 113–121. doi: 10.1006/cyto.2001.0923. [DOI] [PubMed] [Google Scholar]
- [55].Moreth K, Schaefer RM, Schaefer L, Moreth K, Brodbeck R, Babelova A, Gretz N, Spieker T, Zeng-brouwers J, Pfeilschifter J, The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis Find the latest version : The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine l, J. Clin. Invest 120 (2010) 4251–4272. doi: 10.1172/JCI42213.taining. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Haasken S, Auger JL, Taylor JJ, Hobday PM, Goudy BD, Titcombe PJ, Mueller DL, Binstadt BA, Goudy BD, Titcombe PJ, Mueller DL, Binstadt BA, Macrophage Scavenger Receptor 1 (Msr1, SR-A) Influences B Cell Autoimmunity by Regulating Soluble Autoantigen Concentration, J. Immunol. (2013) 1055–1062. doi: 10.4049/jimmunol.1201680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shapiro-shelef M, Lin K, Mcheyzer-williams LJ, Liao J, Mcheyzer-williams MG, Calame K, Blimp-1 Is Required for the Formation of Immunoglobulin Secreting Plasma Cells and Pre-Plasma Memory B Cells, Immunity. 19 (2003) 607–620. [DOI] [PubMed] [Google Scholar]
- [58].Luo J, Niu X, Liu H, Zhang M, Chen M, Deng S, Up-regulation of transcription factor Blimp1 in systemic lupus erythematosus, Mol. Immunol 56 (2013) 574–582. doi: 10.1016/j.molimm.2013.05.241. [DOI] [PubMed] [Google Scholar]
- [59].Panchanathan R, Liu H, Liu H, Fang C, Erickson LD, Pitha PM, Choubey D, Distinct Regulation of Murine Lupus Susceptibility Genes by the IRF5/Blimp-1 Axis, J. Immunol (2019). doi: 10.4049/jimmunol.1102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, Ortmann W, Kosoy R, Ferreira RC, Nordmark G, Gunnarsson I, Svenungsson E, Padyukov L, Sturfelt G, Jönsen A, Bengtsson AA, Rantapää-dahlqvist S, Baechler EC, Brown EE, Alarcón GS, Edberg JC, Ramsey-goldman R, G.M. Jr, Reveille JD, Vilá LM, Kimberly RP, Manzi S, Petri MA, Lee A, Gregersen PK, Seldin MF, Rönnblom L, Criswell LA, Syvänen A, Behrens TW, Graham RR, A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus, Nat. Genet 41 (2009) 1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liu Y, Zhang W, Mz B, Biol JL, Identification of a new transmembrane adaptor protein that constitutively binds Grb2 in B cells Abstract : Transmembrane adaptor proteins cou- ple antigen receptor engagement to downstream signaling cascades in lymphocytes. One example of ( LAT ), which, J. Leukoc. Biol (2008). doi: 10.1189/jlb.0208087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Balaton BP, Cotton AM, Brown CJ, Derivation of consensus inactivation status for X-linked genes from genome-wide studies, Biol. Sex Differ (2015) 1–11. doi: 10.1186/s13293-015-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Carrel L, Willard HF, X-inactivation profile reveals extensive variability in X-linked gene expression in females, Nature. 434 (2005). [DOI] [PubMed] [Google Scholar]
- [64].Cotton AM, Ge B, Light N, Adoue V, Pastinen T, Brown CJ, Analysis of expressed SNPs identifies variable extents of expression from the human inactive X chromosome, Genome Biol. 14 (2013). doi: 10.1186/gb-2013-14-11-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Berletch JB, Yang F, Xu J, Carrel L, Disteche CM, Genes that escape from X inactivation, Hum. Genet 130 (2011) 237–245. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ridings-Figueroa R, Stewart ER, Nesterova TB, Coker H, Pintacuda G, Godwin J, Wilson R, Haslam A, Lilley F, Ruigrok R, Bageghni SA, Albadrani G, Mansfield W, Roulson J-A, Brockdorff N, Ainscough JFX, Coverley D, The nuclear matrix protein CIZ1 facilitates localization of Xist RNA to the inactive X-chromosome territory, Genes Dev. (2017) 876–888. doi: 10.1101/gad.295907.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Grammer AC, Fischer R, Lee O, Zhang X, Lipsky PE, Flow cytometric assessment of the signaling status of human B lymphocytes from normal and autoimmune individuals, Arthritis Res. Ther 6 (2004) 28–38. doi: 10.1186/ar1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Izui S, Mcconahey PJ, Dixon FJ, Increased Spontaneous Polyclonal Activation of B Lymphocytes in Mice with Spontaneous Autoimmune Disease • Fast Publication ! 4 weeks from acceptance to publication Information about subscribing to The Journal of Immunology is online at : INCREASED S P O, J. Immunol 121 (1978) 2213–2219. [PubMed] [Google Scholar]
- [69].Smeets D, Markaki Y, Schmid VJ, Kraus F, Tattermusch A, Cerase A, Sterr M, Fiedler S, Demmerle J, Popken J, Leonhardt H, Brockdorff N, Cremer T, Schermelleh L, Cremer M, Three-dimensional super-resolution microscopy of the inactive X chromosome territory reveals a collapse of its active nuclear compartment harboring distinct Xist RNA foci, Epigenetics and Chromatin. 7 (2014) 1–27. doi: 10.1186/1756-8935-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Giorgetti L, Lajoie BR, Carter AC, Attia M, Zhan Y, Xu J, Chen CJ, Kaplan N, Chang HY, Heard E, Dekker J, Structural organization of the inactive X chromosome in the mouse, Nature. 535 (2016). doi: 10.1038/nature18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bonora G, Disteche CM, Lyon M, Disteche CM, Structural aspects of the inactive X chromosome, (2017). doi: 10.1098/rstb.2016.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pinter SF, Sadreyev RI, Yildirim E, Jeon Y, Ohsumi TK, Borowsky M, Lee JT, Spreading of X chromosome inactivation via a hierarchy of defined Polycomb stations, Genome Res. 22 (2012) 1864–1876. doi: 10.1101/gr.133751.111.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kohlmaier A, Savarese F, Lachner M, Martens J, Jenuwein T, Wutz A, A Chromosomal Memory Triggered by Xist Regulates Histone Methylation in X Inactivation, PLoS Biol. 2 (2004) 0991–1003. doi: 10.1371/journal.pbio.0020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Garieri M, Stamoulis G, Falconnet E, Ribaux P, Borel C, Santoni FA, Antonarakis S, Extensive cellular heterogeneity of X inactivation revealed by single-cell allele-specific expression in human fibroblasts., PNAS. (2018) 298984. doi: 10.1101/298984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tukiainen T, Villani A-C, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings BB, Castel SE, Karczewski KJ, Aguet F, Byrnes A, Gte. Consortium, Lappalainen T, Regev A, Ardlie KG, Hacohen N, MacArthur DG, Landscape of X chromosome inactivation across human tissues, Nature. 550 (2017) 244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 5. Sex-specific expression profiles of three Xist RNA localization factors that function in lymphocytes: Yy1, Hnrnpu, Ciz1. Female B cells of NZB/W F1 mice exhibit variability with expression levels of these genes. Pink circles indicate female samples, blue triangles indicate male samples.
Supplemental Figure 1. Parameters used for classification of three disease stages (pre-disease, early-stage disease, late-stage disease) in male and female NZB/W F1 animals. (A) Sera concentrations (ug/mL) of dsDNA auto-antibodies from NZB/W F1 and wildtype mice used in this study. (B) Representative histopathological analysis of kidney sections from late-stage disease NZB/W F1 female and WT age-matched control female mice. Black arrowheads in wiltype (WT) denote healthy glomeruli (top left); arrowheads in NZB/W F1 sections indicate features of disease: glomerulonephropathy (bottom left), tubular proteinosis (top and middle right), and glomerular crescent (bottom right).
Supplemental Figure 2. Xist RNA FISH analyses in NZB/W F1 males from three disease categories (n = 4 animals for each group). (A) Representative fields of Xist RNA FISH in nuclei from naïve and 24-hour CpG-stimulated CD23+ B cells in NZB/W F1 males. Disease was staged using criteria described in Supplemental Figure 1. Xist RNA is red, DAPI nuclear 593 counterstain in blue. Data are representative of cells collected in multiple independent experiments. (B) Quantification of Types I/II (top) and IV (bottom) Xist RNA localization patterns.
Supplemental Figure 3. Quantification of Xist RNA localization patterns (Types I, II, III, IV) in NZB/W F1 and age-matched wildtype (C57Bl/6, BALB/c) female B cells, for each disease stage (pre-disease, early-stage disease, late-stage disease). Total nuclei counted are shown above each sample. (A) NZB/W F1 females. (B) Wild-type age-matched females.
Supplemental Figure 4. Principle component analysis (PCA) of RNAseq profiles of male and female stimulated B cells from diseased NZB/W F1 mice. Female samples in red; male samples in turquoise.