Abstract
Nitrogen mustard (NM) is a highly reactive bifunctional alkylating agent that induces inflammation, edema and blistering in skin. An important mechanism mediating the action of NM and related mustards is oxidative stress. In these studies a modified murine patch-test model was used to analyze DNA damage and the antioxidant/stress response following NM exposure in isolated epidermis. NM (20 μmol) was applied to glass microfiber filters affixed to a shaved dorsal region of skin of CD-1 mice. NM caused structural damage to the stratum corneum as reflected by increases in transepidermal water loss and skin hydration. This was coordinate with edema, mast cell degranulation and epidermal hyperplasia. Within 3 h of NM exposure, a 4-fold increase in phosphorylated histone H2AX, a marker of DNA double-stranded breaks, and a 25-fold increase in phosphorylated p53, a DNA damage marker, were observed in the epidermis. This was associated with a 40% increase in 8-oxo-2'-deoxyguanosine modified DNA in the epidermis and a 4-fold increase in 4-hydroxynonenal modified epidermal proteins. At 12 h post NM, there was a 3-75 fold increase in epidermal expression of antioxidant/stress proteins including heme oxygenase-1, thioredoxin reductase, superoxide dismutase, glutathione reductase, heat shock protein 27 and cyclooxygenase 2. These data indicate that NM induces early oxidative epidermal injury in mouse skin leading to an antioxidant/stress response. Agents that enhance this response may be useful in mitigating mustard-induced skin injury.
Keywords: oxidative stress, vesicants, mouse epidermis, DNA damage
Introduction
Sulfur mustard (SM, bis(2-chloroethyl)sulfide) and nitrogen mustard (NM, bis(2-chloroethyl) methylamine) are bifunctional alkylating agents initially synthesized for chemical warfare (Graham and Schoneboom, 2013; Kehe and Szinicz, 2005; Malaviya et al., 2019). Acting as vesicants and largely targeting the skin, eyes and lung, these agents are thought to function by modifying small molecular weight metabolites including amino acids and sulfhydryl containing antioxidants such as glutathione, as well as macromolecules including nucleic acids, lipids and proteins (Kehe et al., 2009; Naghii, 2002; Otter and D'Orazio, 2019; Zheng et al., 2013).This results in damage to many subcellular components in target tissues causing alterations in their structure and function. For example, while mustard-induced DNA damage disrupts nuclear function, mitochondrial damage following mustard exposure has been linked to apoptosis and cell death (Jain et al., 2014a; Weinberger et al., 2011).
In the skin, the extent of injury caused by mustards is related to the dose and time following exposure (Dachir et al., 2012; Ghabili et al., 2010; Otter and D'Orazio, 2019). In human skin, mustards initially induce inflammation including erythema, pruritus and edema of delayed onset; the formation of large fluid filled blisters follows. Healing following mustard exposure is prolonged and often associated with dermatitis, psoriasis, changes in pigmentation, and scarring (Ghabili et al., 2010; Graham and Schoneboom, 2013). Our laboratory has developed a cutaneous murine patch model of skin damage induced by NM that resembles the response of human skin to mustards (Composto et al., 2016). NM was used as a surrogate for SM as the two chemicals induce comparable responses in skin. In this model, localized epidermal and dermal injury occur within 24 h of NM exposure, which progresses to an eschar within 48-72 h. This is associated with a marked inflammatory cell infiltration and thickening of the skin which is followed by extensive epidermal hyperplasia during wound healing.
Earlier studies have suggested that the actions of mustards in tissues are initiated by oxidative stress (Kumar et al., 2015; Laskin et al., 2010; Naghii, 2002). A hallmark of oxidative stress is the generation of excessive amounts of highly cytotoxic reactive oxygen species (ROS) including superoxide anion, hydrogen peroxide and hydroxyl radicals (Paromov et al., 2007). In the presence of nitric oxide, superoxide anion can form peroxynitrite, a potent oxidizing agent (Cals-Grierson and Ormerod, 2004; Jourd'heuil et al., 2001). Evidence for the formation of ROS and nitric oxide in skin following mustard exposure include diminished cellular antioxidant protective mechanisms, in particular, depletion of cellular glutathione, a thiol tripeptide that detoxifies ROS and nitric oxide (Beigi Harchegani et al., 2019; Rothmiller et al., 2018). There are also increases in the formation of protein carbonyls and DNA oxidation products including adducts such as 8-hydroxy-2'-deoxyguanosine; epidermal cells also accumulate reactive lipid peroxidation products such as malondialdehyde and 4-hydroxynonenal (Ayala et al., 2014; Zheng et al., 2014)
Important in protecting cells from oxidative stress is induction of an ‘adaptive response’ whereby antioxidants and stress response genes are upregulated (Rahal et al., 2014; Thorpe et al., 2004). In keratinocytes, adaptive response genes include various glutathione S-transferases, a class of glutathione-metabolizing enzymes that detoxify electrophilic compounds, and ROS scavengers such as catalase, superoxide dismutase and thioredoxin reductase (Jan et al., 2014; Laskin et al., 2010). In the present studies, we characterized NM-induced epidermal injury using our mouse skin patch test model, and the adaptive response to oxidative stress. These studies are novel as we have analyzed the adaptive response in isolated epidermis following in vivo injury. Our findings of a marked increase in the expression of antioxidants in the epidermis suggests that the development of antioxidants or agents that further enhance the expression of adaptive response genes may be an effective strategy in mitigating mustard-induced skin injury.
MATERIALS AND METHODS
Chemicals and reagents
Unless otherwise indicated, NM and all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Animals and treatments
All animals received humane care in compliance with the Rutgers University guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. NM treatment of mouse skin was described previously (Composto et al., 2016). Briefly, female CD-1 mice (8 weeks of age, Charles River Laboratories, Wilmington, MA) were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (12 mg/kg) and randomly assigned to treatment groups. The dorsal skin of the mice was shaved with an animal clippers and washed with deionized water. One 24 mm glass microfiber filter disc (GE Healthcare, Buckinghampshire, UK) was placed on the lumbar region of the shaved skin and adhered with acetone (Fig. 1A). NM (120 μl, 20 μmol) in 20% deionized water/80% acetone (v/v) was applied to the filters, which were then covered with Parafilm (Pechiney, Menasha, WI). Acetone was used to enhance dermal penetration of NM through the stratum corneum into the epidermis (Composto et al., 2016). The filter disk model confines the injury to the NM exposure site. Control mice received the solvent without NM. After 6 min, the filter disc was removed and at appropriate times post-NM exposure, mice were euthanized, the skin removed, and the epidermis isolated as previously described with modifications (Frank et al., 1995). Briefly, mouse skin from treated sites was placed dermal side down on Parafilm on dry ice and frozen for ~3 sec. Using a scalpel blade, the epidermis was scraped off and immediately lysed in ice-cold RIPA buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid and 0.1% SDS) supplemented with protease inhibitors. After sonicating on ice, lysates were centrifuged (3,000 x g, 4°C) and clear supernatants stored at −80°C until analysis.
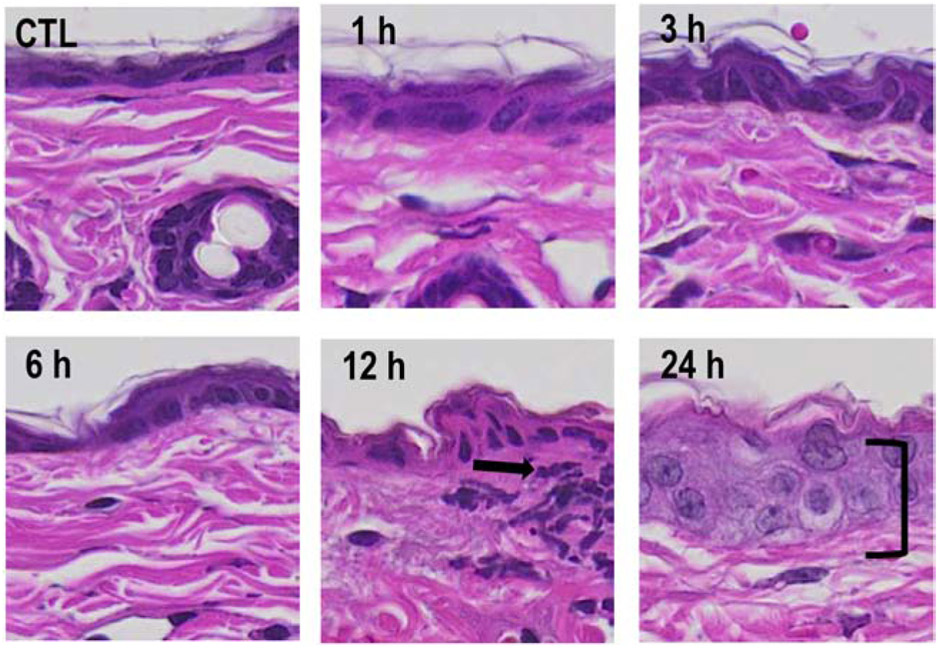
Fig. 1. Structural changes in mouse skin following exposure to NM.
Punch biopsies from control mouse skin (CTL) and mouse skin collected 1-24 h post-NM were stained with hematoxylin and eosin (H&E). One representative section from 4 mice/treatment group is shown (original magnification, x400). Black arrow, area of infiltrating immune cells; bracket, epidermal thickening.
Histology and immunohistochemistry
In separate experiments, 12 mm full thickness punch biopsies were collected from control and NM treated skin, trimmed and stored in ice cold phosphate buffered saline (PBS) containing 3% paraformaldehyde/2% sucrose. The tissue was subsequently embedded in paraffin and 6 μm sections prepared and stained with hematoxylin and eosin (H&E). To visualize metachromatic/basophilic granules, sections were stained with toluidine blue O. A VS120-L100 Olympus virtual slide microscope (Waltham, MA) was used to scan tissue sections. To quantify wound thickness, skin sections were divided into 4 equal parts and measurements taken perpendicular from the wound edge through the epidermis to the basement membrane zone using the OlyVIA 2.7 viewer software (Olympus). For immunohistochemistry, tissue sections (6 μm) were deparaffinized, and blocked with 100% horse serum (Invitrogen, Grand Island, NY) at room temperature for 2 h. Sections were then incubated overnight at 4 °C with a mouse monoclonal antibody against 8-oxo-2'-deoxyguanosine (8-oxo-2’-dG, 1:200, Abcam, Cambridge, MA), or control mouse IgG (ProSci, Atlanta, GA). After washing, sections were incubated at room temperature for 30 min with biotinylated horse anti-mouse secondary antibody (Vector Labs, Burlingame, CA). Antibody binding was visualized using a DAB Peroxidase Substrate Kit (Vector Labs).
Studies on skin barrier function
The integrity of mouse skin was assessed using a gpskin Barrier® analyzer (GPOWER Inc, Seoul, South Korea). Skin barrier strength (SBS), moisture level (ML), stratum corneum hydration (SCH), temperature (T), humidity (H), and trans-epidermal water loss (TEWL) were measured. Overall barrier score (OBS), a measure of the strength of the skin barrier, was determined by gpskin Barrier® software.
Western blot analysis
Protein expression in mouse epidermal lysates was analyzed by Western blotting using 4-15% Tris-HCl polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA). Protein concentrations were determined by a bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL) using a bovine serum albumin standard. Proteins from gels were transferred to nitrocellulose membranes and blocked with 5% nonfat dry milk in 0.1% Tris phosphate buffered saline for 30 min at 37 °C. Blots were then incubated with primary antibodies against p53 and phosphorylated p53 (1:1,000, Cell Signaling Technologies, Danvers, MA), histone H2A.X and phosphorylated histone H2A.X (1:1000, Cell Signaling Technologies), heme oxygenase-1 (HO-1, 1:1000, Enzo Life Science Inc., Burlington, NC), thioredoxin reductase (TrxR, 1:1,000, Santa Cruz Biotechnology, Dallas, TX), glutathione reductase (GR, 1:1,000, Abcam), heat shock protein 27 (HSP27, 1:1,000, Abcam), cyclooxygenase-2 (COX-2, 1:1,000, Cell Signaling Technologies), 4-hydroxynonenal (4-HNE, 1:1,000, R&D Systems, Minneapolis, MN) or superoxide dismutase (SOD, 1:1,000, Cell Signaling Technologies). β-Actin (1:1,000, Cell Signaling Technologies) was used as the protein loading control. Proteins were visualized using HRP-linked secondary antibodies (anti-rabbit, or anti-mouse, 1:5,000, Cell Signaling Technologies) and Luminata Forte Western HRP substrate (Millipore, Billerica, MA). A Fluorchem Imager (ProteinSimple, Santa Clara, CA) was used to visual the substrate, which forms chemiluminescent protein-antibody complexes. ImageJ Software (version 1.5) was used for semi-quantitative analysis of protein bands on blots.
Statistical analyses
Data was expressed as the mean ± SD. Multiple treatment groups were compared using one-way ANOVA; p-values ≤ 0.05 were considered statistically significant.
RESULTS
Effects of NM on mouse epidermis
In initial experiments, the effects of NM on epidermal cell proliferation and inflammation were analyzed. Control skin contained a 1-2 cell layer thick epidermis with a contiguous stratum corneum (Fig. 1). Changes in the epidermis were observed at 12 h post NM exposure; at this time, an inflammatory cell infiltrate was noted at the dermal/epidermal junction. By 24 h epidermal hyperplasia and dermal edema were evident (Fig. 1 and not shown). A 60-80% increase in punch biopsy weight, a marker of edema, was observed at 12 and 24 h post-NM exposure, when compared to control (Fig. 2; upper panel). In addition, a two-fold increase in epidermal thickness was evident in the skin 24 h post NM exposure (Fig. 2, lower panel).
Fig. 2. Effects of NM on edema and epidermal thickness.
Punch biopsies from control mouse skin (CTL) and mouse skin collected 1-24 h post-NM were weighed and analyzed for changes in epidermal thickness. Wound weights (upper panel) and epidermal thickness (lower panel) were assessed as described in the Materials and Methods section. Each bar represents the mean ± SD (n = 4). *Significantly different from CTL (p ≤ 0.05).
We next measured mast cell degranulation as a marker of dermal inflammation. While the total number of mast cells at the dermal epidermal junction was unchanged following NM, the percentage of degranulated mast cells was increased 3-24 h post exposure (Fig. 3 upper graphs). In control skin, mast cells were evident in the dermis, adjacent to the dermal appendages and at the dermal/epidermal junction; over 60% of the mast cells were granulated as measured by metachromatic granules surrounded by a well-defined cell membrane (Fig. 3 lower left panel). Twenty fours post-NM, 75% of the mast cells at the dermal/epidermal junction were degranulated; isolated metachromatic granules were also observed in the dermis, as well as non-degranulated mast cells (Fig. 3, lower right panel).
Fig. 3. Effects of NM on mast cell degranulation.
Panel A. Histological sections, prepared from control mouse skin (CTL) and mouse skin collected 1-24 hours post-NM, were stained for mast cells using toluidine blue (upper panels, original magnification, x400). Insets show intact and degranulated mast cells in CTL skin and NM treated skin. ND, No degranulation; MD, major degranulation. Panel B. The number of mast cells at the dermal/epidermal (D/E) junction in CTL and NM treated skin and the percentage of mast cells that were degranulated (right graph). Each bar is the mean + SD (n = 4) of 10 fields at 40x magnification. *Significant from CTL (p < 0.05).
Earlier studies showed that mustards damage the integrity of the skin causing increases in stratum corneum hydration and TEWL (Davoudi et al., 2009; Jansen van Rensburg et al., 2019; Reid et al., 2007). We found that NM caused an 88% decrease in skin barrier strength and a 90% increase in TEWL within 1 h of exposure (Fig. 4). Skin moisture level and stratum corneum hydration were also increased 24 h post-NM exposure, by 80% and 96%, respectively. No significant effects were evident in the overall barrier score, ambient temperature or relative humidity at the skin surface.
Fig. 4. Biophysical properties of the skin following treatment with NM.
A gpskin Barrier® analyzer (GPOWER Inc, Seoul, South Korea) was used to characterize the biophysical properties of control (CTL) and NM-treated mouse skin 1 h post NM (upper panel) and 24 h post NM (lower panel). OBS, overall barrier score; SBS, skin barrier strength; ML, moisture level; TEWL, trans epidermal water loss; SCH, stratum corneum hydration; T, temperature; H, humidity. Each point represents the mean ± SD (n = 3). *Significant from CTL (p ≤ 0.05).
NM-induced DNA damage and oxidative stress
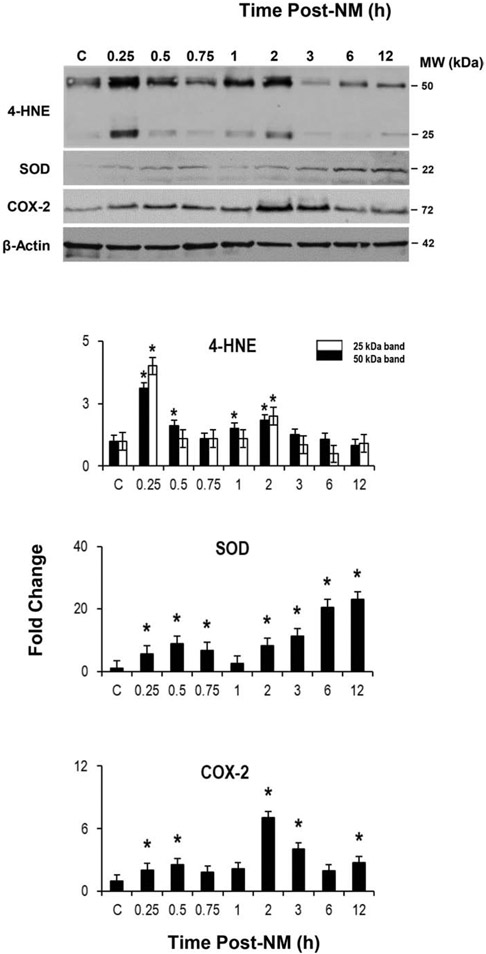
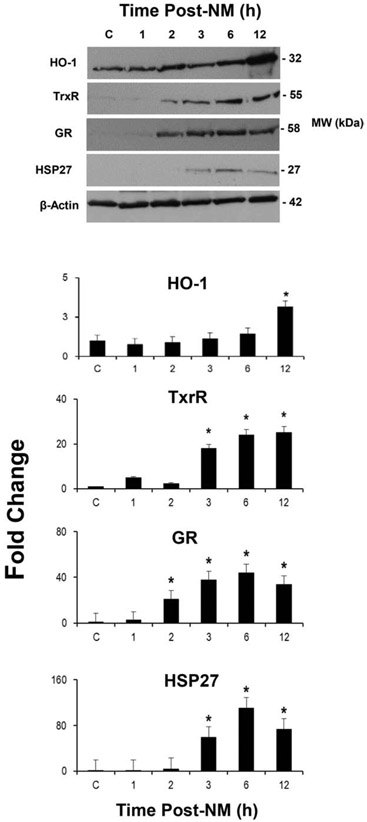
We next examined markers of the DNA damage response pathway, p53 and H2A.X, in the epidermis. NM caused an increase in phospho–p53, the activated form of p53, 3-12 h post NM exposure (Fig. 5). Total p53 protein also increased 1-3 h post-NM and then decreased. Expression of the DNA double strand break marker, phospho-H2A.X, was increased 3-12 h post NM; conversely, there were little or no changes in the expression of total H2A.X. Time related increases in oxidative stress markers were also noted in mouse epidermis after NM exposure. Within 15 min of NM, increases in the appearance of 4-HNE modified proteins were noted; this remained increased for 2 h, although at reduced levels (Fig. 6). Expression of SOD and COX-2 was also upregulated in the epidermis at all times post NM except at 0.75 and/or 1 h (Fig. 6). At 3-12 h post-NM, there were also marked increases in thioredoxin reductase, glutathione reductase and HSP-27; HO-1 was also upregulated at 12 h (Fig. 7). The oxidized DNA product, 8-oxo-2’-deoxyguanosine, was increased in basal keratinocytes within 1 h of NM, a response that increased with time up to 24 h post NM (Fig. 8).
Fig. 5. NM alters epidermal DNA damage response proteins.
Upper panel, Epidermis isolated from control mice (CTL) and mice treated with NM was lysed and analyzed by Western blotting using antibodies to phospho p53 (pp53), p53, phospho H2A.X (pH2A.X) and H2A.X. Lower panels, Protein expression was quantified using densitometry of western blots from 3 independent experiments. Data are presented as mean ± SD (n = 3). *Significant from CTL, p ≤ 0.05.
Fig. 6. NM alters epidermal oxidative stress response proteins.
Upper panel, Epidermis isolated from control mice (CTL) and mice treated with NM was analyzed by western blotting using antibodies to 4-HNE-modified proteins, SOD and COX-2. β-Actin served as a representative protein loading control. Lower panel, Protein expression was quantified using densitometry. Data are presented as mean ± SD (n = 3). For the 4-HNE modified proteins, the black bars represent the high MW band and the white bars represent the low MW band). *Significant from CTL, p ≤ 0.05.
Fig. 7. NM alters epidermal oxidative stress response proteins.
Upper panel, Epidermis isolated from control mice (CTL) and mice treated with NM was lysed analyzed by western blotting using antibodies to heme oxygenase-1 (HO-1), thioredoxin reductase (TrxR), glutathione reductase (GR) and heat shock protein 27 (HSP27). β-Actin served as a representative protein loading control. Lower panels, Protein expression was quantified using densitometry. Data are presented as mean ± SD (n = 2-3). *Significant from CTL, p ≤ 0.05.
Fig. 8. Effects of NM on the formation of 8-oxo-2’-deoxyguanosine in mouse epidermis.
Upper panels, Punch biopsies from control mouse skin (CTL) and mouse skin collected 1-24 h post-NM were stained with antibodies to 8-oxo-2’-deoxyguanosine. Antibody binding was visualized using a Vectastain Elite ABC kit. Black arrows show 8-oxo-2’-deoxyguanosine positive cells. One representative section from 3 mice/treatment group is shown (original magnification, x400). Lower panel, 8-oxo-2’-deoxyguanosine positive nuclei were counted in 10 randomly selected fields at 40x magnification. Each bar is the mean ± SD (n = 3).
Discussion
Earlier studies in mice demonstrated that SM causes significant damage to the skin; this was associated with a rapid inflammatory response characterized by edema and an inflammatory cell infiltration into the dermis, degranulation of mast cells, degradation of collagen, degeneration of the basement membrane and alterations in the structure of both basal and suprabasal keratinocytes including evidence of apoptosis and necrosis (Brown and Rice, 1997; Jain et al., 2014a; Joseph et al., 2018). Consistent with these reports, we found that NM causes edema, infiltration of inflammatory cells, mast cell degranulation and increases in epidermal thickness (see Fig. 9 for a summary)(Wohlman et al., 2016). These data demonstrate that our murine skin patch model can be utilized to effectively investigate mechanisms of action of vesicants, and to assess the efficacy of potential therapeutics. This is important, as currently there are no approved therapeutics to treat skin injury induced by mustards.
Fig. 9. Summary of the effects of NM on mouse skin.
Mouse skin was exposed to NM using a modified patch-test model. At various times post NM exposure, skin tissue was analyzed for histopathological changes, DNA damage and markers of oxidative stress.
The present studies also demonstrate that NM caused marked changes in skin hydration and transepidermal water loss, an indication that skin barrier function is impaired. Similar findings have been described after dermal exposure to SM in swine, guinea pig, mouse and human skin (Barillo et al., 2019; Chilcott et al., 2000; Clery-Barraud et al., 2013; Davoudi et al., 2009). In addition to disruption of the stratum corneum, changes in blood perfusion, metabolism and temperature are thought to contribute to increases in TEWL and skin hydration (Fujimura et al., 2017; Rodrigues et al., 2004; Singh and Maibach, 2013). It remains to be determined which of these factors contributes to alterations in the biophysical properties of the skin following NM exposure.
Techniques in immunohistochemistry have been used to identify changes in expression of markers of mustard-induced epidermal injury (Chang et al., 2018; Joseph et al., 2016; Tewari-Singh et al., 2009; Wohlman et al., 2016). Tissue biopsies of whole skin prepared as homogenates have also been used to study biochemical and molecular mechanisms underlying mustard-induced injury (Chang et al., 2014; Jain et al., 2014b). These latter techniques are limited as they are not specific for epidermal damage and often include changes in dermal and subcutaneous structures. Using our modified patch model, we were able to assess mustard-induced damage and oxidative stress in isolated epidermis. These studies showed that NM rapidly induced oxidative stress in the epidermis, as indicated by the appearance of 4-HNE-modified proteins. These data are consistent with our immunochemical findings of increased 8-8-oxo-2’-deoxyguanosine in the nuclei of epidermal cells post NM. Treatment of mouse skin with mustards has been reported to cause the formation of epidermal DNA oxidation products and an accumulation of lipid peroxidation end products in the tissue (Kumar et al., 2015; Sawale et al., 2013; Zhang et al., 2017). Mechanisms causing oxidative stress in the epidermis following exposure to mustards are multifactorial. They include inflammatory reactions from infiltrating neutrophils and macrophages releasing ROS and nitric oxide, disruption of dissipative structures such as mitochondria, lipid peroxidation, depletion of intracellular antioxidants, and inhibition of antioxidant enzymes (Jan et al., 2015; Laskin et al., 2010; Naghii, 2002).
As bifunctional alkylating agents, NM and SM readily alkylate DNA forming both monoalkylation products and interstrand and intrastrand DNA cross-links (Malaviya et al., 2019; Osborne et al., 1995; Shakarjian et al., 2010). Single and double stranded DNA breaks are generated in target cells as a consequence of repair of these DNA lesions (Scully and Xie, 2013). As a result, mustards can suppress DNA replication and transcription causing mutations and cytotoxicity (Jowsey et al., 2009; Kehe et al., 2009; Panahi et al., 2018). Activation of DNA damage response pathways is critical for DNA repair (Inturi et al., 2014; Kitagawa and Kastan, 2005; Meador et al., 2008). Previous studies have shown that NM has the capacity to activate DNA damage repair pathways mediated by ATR (ataxia telangiectasia mutated- and Rad3-related), ATM (ataxia telangiectasia mutated) and DNA-PKcs (DNA-dependent protein kinase catalytic subunit) (Jan et al., 2019). As a replication stress kinase, ATR is recruited by DNA damage to stalled replication forks that interfere with replication; ATR participates in homologous recombination repair and nucleotide excision repair. DNA-PKcs mediate nonhomologous end joining repair of double strand breaks while ATM mediates homologous recombination repair of DNA double strand breaks (Blackford and Jackson, 2017; Jette and Lees-Miller, 2015). ATM/ATR downstream targets important in DNA repair include the tumor suppressor p53, and the histone variant H2A.X (Inturi et al., 2013; Jowsey et al., 2012). The present studies demonstrate that within 3 h, NM readily activates epidermal phospho-p53 and pH2A.X; an initial increase followed by a decrease in total p53 protein was evident 6-12 h post NM treatment. p53 and H2A.X have been reported to be activated in intact mouse skin 12-24 h post NM exposure (Kumar et al., 2015). Activation of H2A.X has also been observed in mouse epidermis treated with SM (Joseph et al., 2016). These data indicate that epidermal cells readily sense NM-induced DNA damage and recruit proteins important in DNA repair. Our findings of an increase and then a decrease in total p53 protein with time is likely due to changes in turnover of the protein during DNA repair (Joerger and Fersht, 2016). Conversely, phospho-p53 remains elevated suggesting that DNA repair processes are prolonged. Decreases in overall p53 protein might also be due to apoptosis of cells whose DNA is damaged beyond repair. The remaining cells suffered less damage and therefore, are undergoing repair.
Consistent with NM-induced oxidative stress, epidermal expression of a number of antioxidant enzymes/stress proteins including Cu, Zn-SOD, HO-1, thioredoxin reductase, glutathione reductase, HSP27 and COX-2 were upregulated. Cu, Zn-SOD protects against the formation of ROS via detoxification of superoxide anion, while HO-1, the rate limiting enzyme in the metabolism of the prooxidant heme, acts as an antiinflammatory and an antioxidant protein (Araujo et al., 2012; Choi and Alam, 1996; Nourani et al., 2015). The NADPH thioredoxin reductase and glutathione-glutaredoxin systems consists of thioredoxin and thioredoxin reductase, and glutathione reductase, glutathione and glutaredoxin, respectively, which control cellular redox balance (Jan et al., 2015). HSP27, a heat shock protein, is a class of molecular chaperones important in the folding, assembly and degradation of proteins that are upregulated in response to cellular stress and contribute to the wound repair process (Black et al., 2011; Hirano et al., 2002; Suarez et al., 2014; Vidal Magalhaes et al., 2012). COX-2 was also upregulated in the epidermis at early times (within 0.25-0.5 h) after NM treatment, peaking after 2-3 h. These data are in accord with earlier studies showing increased COX-2 expression in full thickness hairless mouse skin treated with NM, SM or the half mustard 2-chloroethyl ethyl sulfide (CEES) as well as in mouse keratinocytes and in a human 3-D skin construct model (Black et al., 2010; Jain et al., 2015; Joseph et al., 2011; Tewari-Singh et al., 2012). That COX-2 is important in mustard-induced toxicity is indicated by fact that inhibitors of this enzyme suppress mustard-induced skin inflammation and injury; SM toxicity is blunted in COX-2 deficient mice (Casillas et al., 2000; Dachir et al., 2004; Wormser et al., 2004; Young et al., 2012). The fact that all of these antioxidant/stress enzymes were upregulated following NM exposure likely reflects an adaptive cytoprotective response to oxidative stress important in limiting tissue injury and promoting wound repair (Gozzelino et al., 2010; Hanselmann et al., 2001; Scandalios, 2005).
Mechanisms by which mustards induce antioxidant/stress proteins are not well understood. An important pathway known to mediate expression of many antioxidant/cytoprotective genes is via Keap1/Nrf2 signaling (Cheng et al., 2016; Tonelli et al., 2018). As a negative regulator of Nrf2, Keap-1 maintains Nrf2 in the cytoplasm where it promotes its ubiquitination and proteasomal degradation. However, electrophiles modify Keap1; resulting in structural changes in the protein that allow Nrf2 to translocate into the nucleus and bind antioxidant response elements controlling expression of antioxidant/stress proteins (Kaspar et al., 2009; Ma, 2013). It is possible that NM activates the Keap1/Nrf2 pathway in the epidermis; earlier studies have shown that SM can activate this pathway in lung tissues (Meng et al., 2017). In this regard, treatment of mice with sulforaphane, a cancer chemopreventive agent that activates Nrf2 signaling, has been reported to suppress CEES-induced mutation frequency in mouse skin (Abel et al., 2013; Fahey and Talalay, 1999; Kwak and Kensler, 2010). Sulforaphane and methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate (CDDO-Me), another chemopreventive agents that activates Nrf2 signaling, also protects mouse and human keratinocytes from mustard-induced cytotoxicity (Abel et al., 2011; Gross et al., 2006; Udasin et al., 2016). Additional transcription factors such as NF-κB and AP1 are also known to regulate expression of antioxidants/stress proteins and these may also be important in controlling their expression in the epidermis (Hellweg et al., 2016; Kurutas, 2016).
In summary, the present studies demonstrate that NM alters the biophysical properties of the skin at early times which may contribute to toxicity. Our findings that the epidermal antioxidant/stress is upregulated after NM exposure suggests that they may be important in protecting the skin from injury. Further studies are needed to determine if agents that augment expression of antioxidant/stress genes in the epidermis can protect against NM- and SM-induced injury as this may lead to the development of therapeutic interventions targeting these pathways.
Acknowledgements
Supported by NIH grants AR055073, ES005022 and T32ES007148
Footnotes
Conflict of Interest
The authors have declared that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abel EL, Boulware S, Fields T, McIvor E, Powell KL, DiGiovanni J, Vasquez KM, MacLeod MC, 2013. Sulforaphane induces phase II detoxication enzymes in mouse skin and prevents mutagenesis induced by a mustard gas analog. Toxicol. Appl. Pharmacol 266, 439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel EL, Bubel JD, Simper MS, Powell L, McClellan SA, Andreeff M, MacLeod MC, DiGiovanni J, 2011. Protection against 2-chloroethyl ethyl sulfide (CEES)-induced cytotoxicity in human keratinocytes by an inducer of the glutathione detoxification pathway. Toxicol. Appl. Pharmacol 255, 176–183. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Zhang M, Yin F, 2012. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol 3, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A, Munoz MF, Arguelles S, 2014. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev 2014, 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillo DJ, Croutch CR, Barillo AR, Thompson CK, Roseman J, Reid F, 2019. Debridement of sulfur-mustard skin burns: A comparison of three methods. J. Burn. Care Res 2019, in press. [DOI] [PubMed] [Google Scholar]
- Beigi Harchegani A, Khor A, Tahmasbpour E, Ghatrehsamani M, Bakhtiari Kaboutaraki H, Shahriary A, 2019. Role of oxidative stress and antioxidant therapy in acute and chronic phases of sulfur mustard injuries: A review. Cutan. Ocul. Toxicol 38, 9–17. [DOI] [PubMed] [Google Scholar]
- Black AT, Hayden PJ, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD, 2011. Regulation of Hsp27 and Hsp70 expression in human and mouse skin construct models by caveolae following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol. Appl. Pharmacol 253, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AT, Joseph LB, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD, 2010. Role of MAP kinases in regulating expression of antioxidants and inflammatory mediators in mouse keratinocytes following exposure to the half mustard, 2-chloroethyl ethyl sulfide. Toxicol. Appl. Pharmacol 245, 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford AN, Jackson SP, 2017. ATM, ATR, and DNA-PK: The trinity at the heart of the DNA damage response. Mol. Cell 66, 801–817. [DOI] [PubMed] [Google Scholar]
- Brown RF, Rice P, 1997. Histopathological changes in Yucatan minipig skin following challenge with sulphur mustard. A sequential study of the first 24 hours following challenge. Int. J. Exp. Pathol 78, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cals-Grierson MM, Ormerod AD, 2004. Nitric oxide function in the skin. Nitric Oxide. 10, 179–913. [DOI] [PubMed] [Google Scholar]
- Casillas RP, Kiser RC, Truxall JA, Singer AW, Shumaker SM, Niemuth NA, Ricketts KM, Mitcheltree LW, Castrejon LR, Blank JA, 2000. Therapeutic approaches to dermatotoxicity by sulfur mustard. I. Modulaton of sulfur mustard-induced cutaneous injury in the mouse ear vesicant model. J. Appl. Toxicol 20 Suppl 1, S145–151. [DOI] [PubMed] [Google Scholar]
- Chang YC, Soriano M, Hahn RA, Casillas RP, Gordon MK, Laskin JD, Gerecke DR, 2018. Expression of cytokines and chemokines in mouse skin treated with sulfur mustard. Toxicol. Appl. Pharmacol 355, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Wang JD, Hahn RA, Gordon MK, Joseph LB, Heck DE, Heindel ND, Young SC, Sinko PJ, Casillas RP, Laskin JD, Laskin DL, Gerecke DR, 2014. Therapeutic potential of a non-steroidal bifunctional anti-inflammatory and anticholinergic agent against skin injury induced by sulfur mustard. Toxicol. Appl. Pharmacol 280, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Wu R, Guo Y, Kong AN, 2016. Regulation of Keap1-Nrf2 signaling: The role of epigenetics. Curr. Opin. Toxicol 1, 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcott RP, Brown RF, Rice P, 2000. Non-invasive quantification of skin injury resulting from exposure to sulphur mustard and Lewisite vapours. Burns. 26, 245–250. [DOI] [PubMed] [Google Scholar]
- Choi AM, Alam J, 1996. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol 15, 9–19. [DOI] [PubMed] [Google Scholar]
- Clery-Barraud C, Nguon N, Vallet V, Sentenac C, Four E, Arlaud C, Coulon D, Boudry I, 2013. Sulfur mustard cutaneous injury characterization based on SKH-1 mouse model: relevance of non-invasive methods in terms of wound healing process analyses. Skin Res. Technol 19, e146–156. [DOI] [PubMed] [Google Scholar]
- Composto GM, Laskin JD, Laskin DL, Gerecke DR, Casillas RP, Heindel ND, Joseph LB, Heck DE, 2016. Mitigation of nitrogen mustard mediated skin injury by a novel indomethacin bifunctional prodrug. Exp. Mol. Pathol 100, 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachir S, Cohen M, Kamus-Elimeleh D, Fishbine E, Sahar R, Gez R, Brandeis R, Horwitz V, Kadar T, 2012. Characterization of acute and long-term pathologies of superficial and deep dermal sulfur mustard skin lesions in the hairless guinea pig model. Wound Repair Regen. 20, 852–861. [DOI] [PubMed] [Google Scholar]
- Dachir S, Fishbeine E, Meshulam Y, Sahar R, Chapman S, Amir A, Kadar T, 2004. Amelioration of sulfur mustard skin injury following a topical treatment with a mixture of a steroid and a NSAID. J. Appl. Toxicol 24, 107–113. [DOI] [PubMed] [Google Scholar]
- Davoudi SM, Keshavarz S, Sadr B, Shohrati M, Naghizadeh MM, Farsinejad K, Rashighi-Firouzabadi M, Zartab H, Firooz A, 2009. Skin hydration and transepidermal water loss in patients with a history of sulfur mustard contact: A case-control study. J. Eur. Acad. Dermatol. Venereol 23, 940–944. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Talalay P, 1999. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol 37, 973–979. [DOI] [PubMed] [Google Scholar]
- Frank JD, Manson JM, Cartwright ME, 1995. Separation of epidermis from dermis in the rhesus monkey. Exp. Dermatol 4, 89–92. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Shimotoyodome Y, Nishijima T, Sugata K, Taguchi H, Moriwaki S, 2017. Changes in hydration of the stratum corneum are the most suitable indicator to evaluate the irritation of surfactants on the skin. Skin Res. Technol 23, 97–103. [DOI] [PubMed] [Google Scholar]
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Shoja MM, 2010. Mustard gas toxicity: the acute and chronic pathological effects. J. Appl. Toxicol 30, 627–643. [DOI] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, Soares MP, 2010. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol 50, 323–354. [DOI] [PubMed] [Google Scholar]
- Graham JS, Schoneboom BA, 2013. Historical perspective on effects and treatment of sulfur mustard injuries. Chem. Biol. Interact 206, 512–522. [DOI] [PubMed] [Google Scholar]
- Gross CL, Nealley EW, Nipwoda MT, Smith WJ, 2006. Pretreatment of human epidermal keratinocytes with D,L-sulforaphane protects against sulfur mustard cytotoxicity. Cutan. Ocul. Toxicol 25, 155–163. [DOI] [PubMed] [Google Scholar]
- Hanselmann C, Mauch C, Werner S, 2001. Haem oxygenase-1: A novel player in cutaneous wound repair and psoriasis? Biochem. J 353, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellweg CE, Spitta LF, Henschenmacher B, Diegeler S, Baumstark-Khan C, 2016. Transcription factors in the cellular response to charged particle exposure. Front. Oncol 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Rees RS, Gilmont RR, 2002. MAP kinase pathways involving hsp27 regulate fibroblast-mediated wound contraction. J. Surg. Res 102, 77–84. [DOI] [PubMed] [Google Scholar]
- Inturi S, Tewari-Singh N, Agarwal C, White CW, Agarwal R, 2014. Activation of DNA damage repair pathways in response to nitrogen mustard-induced DNA damage and toxicity in skin keratinocytes. Mutat. Res 763-764, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturi S, Tewari-Singh N, Jain AK, Roy S, White CW, Agarwal R, 2013. Absence of a p53 allele delays nitrogen mustard-induced early apoptosis and inflammation of murine skin. Toxicology. 311, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Kumar D, Orlicky DJ, Agarwal C, White CW, Agarwal R, 2015. Flavanone silibinin treatment attenuates nitrogen mustard-induced toxic effects in mouse skin. Toxicol. Appl. Pharmacol 285, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Orlicky DJ, White CW, Agarwal R, 2014a. Histopathological and immunohistochemical evaluation of nitrogen mustard-induced cutaneous effects in SKH-1 hairless and C57BL/6 mice. Exp. Toxicol. Pathol 66, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Orlicky DJ, White CW, Agarwal R, 2014b. Myeloperoxidase deficiency attenuates nitrogen mustard-induced skin injuries. Toxicology. 320, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YH, Heck DE, Casillas RP, Laskin DL, Laskin JD, 2015. Thioredoxin cross-linking by nitrogen mustard in lung epithelial cells: Formation of multimeric thioredoxin/thioredoxin reductase complexes and inhibition of disulfide reduction. Chem. Res. Toxicol 28, 2091–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YH, Heck DE, Laskin DL, Laskin JD, 2019. Sulfur mustard analog mechlorethamine (bis(2-chloroethyl)methylamine) modulates cell cycle progression via the DNA damage response in human lung epithelial A549 cells. Chem. Res. Toxicol 32, 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YH, Heck DE, Malaviya R, Casillas RP, Laskin DL, Laskin JD, 2014. Crosslinking of thioredoxin reductase by the sulfur mustard analogue mechlorethamine (methylbis(2-chloroethyl)amine) in human lung epithelial cells and rat lung: Selective inhibition of disulfide reduction but not redox cycling. Chem. Res. Toxicol 27, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen van Rensburg S, Franken A, Du Plessis JL, 2019. Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: A review. Skin Res. Technol 25, 595–605. [DOI] [PubMed] [Google Scholar]
- Jette N, Lees-Miller SP, 2015. The DNA-dependent protein kinase: A multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog. Biophys. Mol. Biol 117, 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger AC, Fersht AR, 2016. The p53 pathway: Origins, inactivation in cancer, and emerging therapeutic approaches. Annu. Rev. Biochem 85, 375–404. [DOI] [PubMed] [Google Scholar]
- Joseph LB, Composto GM, Heck DE, 2016. Tissue injury and repair following cutaneous exposure of mice to sulfur mustard. Ann. N. Y. Acad Sci 1378, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph LB, Composto GM, Perez RM, Kim HD, Casillas RP, Heindel ND, Young SC, Lacey CJ, Saxena J, Guillon CD, Croutch CR, Laskin JD, Heck DE, 2018. Sulfur mustard induced mast cell degranulation in mouse skin is inhibited by a novel anti-inflammatory and anticholinergic bifunctional prodrug. Toxicol. Lett 293, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph LB, Gerecke DR, Heck DE, Black AT, Sinko PJ, Cervelli JA, Casillas RP, Babin MC, Laskin DL, Laskin JD, 2011. Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage. Exp. Mol. Pathol 91, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourd'heuil D, Jourd'heuil FL, Kutchukian PS, Musah RA, Wink DA, Grisham MB, 2001. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J. Biol. Chem 276, 28799–28805. [DOI] [PubMed] [Google Scholar]
- Jowsey PA, Williams FM, Blain PG, 2009. DNA damage, signalling and repair after exposure of cells to the sulphur mustard analogue 2-chloroethyl ethyl sulphide. Toxicology. 257, 105–112. [DOI] [PubMed] [Google Scholar]
- Jowsey PA, Williams FM, Blain PG, 2012. DNA damage responses in cells exposed to sulphur mustard. Toxicol. Lett 209, 1–10. [DOI] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK, 2009. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med 47, 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Steinritz D, Thiermann H, 2009. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 263, 12–19. [DOI] [PubMed] [Google Scholar]
- Kehe K, Szinicz L, 2005. Medical aspects of sulphur mustard poisoning. Toxicology. 214, 198–209. [DOI] [PubMed] [Google Scholar]
- Kitagawa R, Kastan MB, 2005. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb. Symp. Quant. Biol 70, 99–109. [DOI] [PubMed] [Google Scholar]
- Kumar D, Tewari-Singh N, Agarwal C, Jain AK, Inturi S, Kant R, White CW, Agarwal R, 2015. Nitrogen mustard exposure of murine skin induces DNA damage, oxidative stress and activation of MAPK/Akt-AP1 pathway leading to induction of inflammatory and proteolytic mediators. Toxicol. Lett 235, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurutas EB, 2016. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J 15, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Kensler TW, 2010. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol 244, 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin JD, Black AT, Jan YH, Sinko PJ, Heindel ND, Sunil V, Heck DE, Laskin DL, 2010. Oxidants and antioxidants in sulfur mustard-induced injury. Ann. N. Y. Acad. Sci 1203, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, 2013. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol 53, 401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Heck D, Casillas R, Laskin J, Laskin D, 2019. Mustard Vesicants in: Luckey BJ, Romano JA, Salem H (Eds.), Chemical Warfare Agents: Biomedical and Psychological Effects, Medical Countermeasures, and Emergency Response. CRC Press, Boca Raton, FL, pp. 131–144. [Google Scholar]
- Meador JA, Zhao M, Su Y, Narayan G, Geard CR, Balajee AS, 2008. Histone H2AX is a critical factor for cellular protection against DNA alkylating agents. Oncogene. 27, 5662–5671. [DOI] [PubMed] [Google Scholar]
- Meng W, Pei Z, Feng Y, Zhao J, Chen Y, Shi W, Xu Q, Lin F, Sun M, Xiao K, 2017. Neglected role of hydrogen sulfide in sulfur mustard poisoning: Keap1 S-sulfydration and subsequent Nrf2 pathway activation. Nature.com/Scientific Reports, Nature.com, pp. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghii MR, 2002. Sulfur mustard intoxication, oxidative stress, and antioxidants. Mil. Med 167, 573–575. [PubMed] [Google Scholar]
- Nourani MR, Mahmoodzadeh Hosseini H, Imani Fooladi AA, 2015. Comparative transcriptional and translational analysis of heme oxygenase expression in response to sulfur mustard. J. Recept. Signal Transduct. Res 35, 479–484. [DOI] [PubMed] [Google Scholar]
- Osborne MR, Wilman DE, Lawley PD, 1995. Alkylation of DNA by the nitrogen mustard bis(2-chloroethyl)methylamine. Chem. Res. Toxicol 8, 316–20. [DOI] [PubMed] [Google Scholar]
- Otter J, D'Orazio JL, 2019. Blister Agents (Mustard, Vesicants, Hd, Hn1-3, H) Toxicity StatPearls [Internet], Treasure Island (FL). [Google Scholar]
- Panahi Y, Fattahi A, Nejabati HR, Abroon S, Latifi Z, Akbarzadeh A, Ghasemnejad T, 2018. DNA repair mechanisms in response to genotoxicity of warfare agent sulfur mustard. Environ. Toxicol. Pharmacol 58, 230–236. [DOI] [PubMed] [Google Scholar]
- Paromov V, Suntres Z, Smith M, Stone WL, 2007. Sulfur mustard toxicity following dermal exposure: role of oxidative stress, and antioxidant therapy. J. Burns Wounds 7, e7. [PMC free article] [PubMed] [Google Scholar]
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K, 2014. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int 2014, 761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid FM, Niemuth NA, Shumaker SM, Waugh JD, Graham JS, 2007. Biomechanical monitoring of cutaneous sulfur mustard-induced lesions in the weanling pig model for depth of injury. Skin Res. Technol 13, 217–225. [DOI] [PubMed] [Google Scholar]
- Rodrigues LM, Pinto PC, Magro JM, Fernandes M, Alves J, 2004. Exploring the influence of skin perfusion on transepidermal water loss. Skin Res. Technol 10, 257–262. [DOI] [PubMed] [Google Scholar]
- Rothmiller S, Schroder S, Strobelt R, Wolf M, Wang J, Jiang X, Worek F, Steinritz D, Thiermann H, Schmidt A, 2018. Sulfur mustard resistant keratinocytes obtained elevated glutathione levels and other changes in the antioxidative defense mechanism. Toxicol. Lett 293, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawale SD, Ambhore PD, Pawar PP, Pathak U, Deb U, Satpute RM, 2013. Ameliorating effect of S-2(omega-aminoalkylamino) alkylaryl sulfide (DRDE-07) on sulfur mustard analogue, 2-chloroethyl ethyl sulfide-induced oxidative stress and inflammation. Toxicol. Mech. Methods 23, 702–710. [DOI] [PubMed] [Google Scholar]
- Scandalios JG, 2005. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res 38, 995–1014. [DOI] [PubMed] [Google Scholar]
- Scully R, Xie A, 2013. Double strand break repair functions of histone H2AX. Mutat. Res 750, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL, Laskin JD, 2010. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 114, 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Maibach H, 2013. Climate and skin function: An overview. Skin Res. Technol 19, 207–212. [DOI] [PubMed] [Google Scholar]
- Suarez E, Syed F, Rasgado TA, Walmsley A, Mandal P, Bayat A, 2014. Skin equivalent tensional force alters keloid fibroblast behavior and phenotype. Wound Repair Regen. 22, 557–568. [DOI] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, Agarwal C, White CW, Agarwal R, 2012. Silibinin attenuates sulfur mustard analog-induced skin injury by targeting multiple pathways connecting oxidative stress and inflammation. PLoS One. 7, e46149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Rana S, Gu M, Pal A, Orlicky DJ, White CW, Agarwal R, 2009. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicol. Sci 108, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe GW, Fong CS, Alic N, Higgins VJ, Dawes IW, 2004. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc. Natl. Acad. Sci. U. S. A 101, 6564–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli C, Chio IIC, Tuveson DA, 2018. Transcriptional Regulation by Nrf2. Antioxid. Redox. Signal 29, 1727–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udasin RG, Wen X, Bircsak KM, Aleksunes LM, Shakarjian MP, Kong AN, Heck DE, Laskin DL, Laskin JD, 2016. Nrf2 regulates the sensitivity of mouse keratinocytes to nitrogen mustard via multidrug resistance-associated protein 1 (Mrp1). Toxicol. Sci 149, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal Magalhaes W, Gouveia Nogueira MF, Kaneko TM, 2012. Heat shock proteins (HSP): Dermatological implications and perspectives. Eur. J. Dermatol 22, 8–13. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Laskin JD, Sunil VR, Sinko PJ, Heck DE, Laskin DL, 2011. Sulfur mustard-induced pulmonary injury: Therapeutic approaches to mitigating toxicity. Pulm. Pharmacol. Ther 24, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlman IM, Composto GM, Heck DE, Heindel ND, Lacey CJ, Guillon CD, Casillas RP, Croutch CR, Gerecke DR, Laskin DL, Joseph LB, Laskin JD, 2016. Mustard vesicants alter expression of the endocannabinoid system in mouse skin. Toxicol. Appl. Pharmacol 303, 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser U, Langenbach R, Peddada S, Sintov A, Brodsky B, Nyska A, 2004. Reduced sulfur mustard-induced skin toxicity in cyclooxygenase-2 knockout and celecoxib-treated mice. Toxicol. Appl. Pharmacol 200, 40–47. [DOI] [PubMed] [Google Scholar]
- Young SC, Fabio KM, Huang MT, Saxena J, Harman MP, Guillon CD, Vetrano AM, Heck DE, Flowers RA 2nd, Heindel ND, Laskin JD, 2012. Investigation of anticholinergic and non-steroidal anti-inflammatory prodrugs which reduce chemically induced skin inflammation. J. Appl. Toxicol 32, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mei Y, Wang T, Liu F, Jiang N, Zhou W, Zhang Y, 2017. Early oxidative stress, DNA damage and inflammation resulting from subcutaneous injection of sulfur mustard into mice. Environ. Toxicol. Pharmacol 55, 68–73. [DOI] [PubMed] [Google Scholar]
- Zheng H, Ma HP, Wang J, Ma M, 2013. Preoperative HO-1 levels as prognostic factor for adverse cardiac events in elder patients undergoing non-cardiac surgery. PLoS One. 8, e58567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Heck DE, Mishin V, Black AT, Shakarjian MP, Kong AN, Laskin DL, Laskin JD, 2014. Modulation of keratinocyte expression of antioxidants by 4-hydroxynonenal, a lipid peroxidation end product. Toxicol. Appl. Pharmacol 275, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]