Abstract
Contact lenses represent a widely utilized form of vision correction with more than 140 million wearers worldwide. Although generally well-tolerated, contact lenses can cause corneal infection (microbial keratitis), with an approximate annualized incidence ranging from ~2 to ~20 cases per 10,000 wearers, and sometimes resulting in permanent vision loss. Research suggests that the pathogenesis of contact lens-associated microbial keratitis is complex and multifactorial, likely requiring multiple conspiring factors that compromise the intrinsic resistance of a healthy cornea to infection. Here, we outline our perspective of the mechanisms by which contact lens wear sometimes renders the cornea susceptible to infection, focusing primarily on our own research efforts during the past three decades. This has included studies of host factors underlying the constitutive barrier function of the healthy cornea, its response to bacterial challenge when intrinsic resistance is not compromised, pathogen virulence mechanisms, and the effects of contact lens wear that alter the outcome of host-microbe interactions. For almost all of this work, we have utilized the bacterium Pseudomonas aeruginosa because it is the leading cause of lens-related microbial keratitis. While not yet common among corneal isolates, clinical isolates of P. aeruginosa have emerged that are resistant to virtually all currently available antibiotics, leading the United States CDC (Centers for Disease Control) to add P. aeruginosa to its list of most serious threats. Compounding this concern, the development of advanced contact lenses for biosensing and augmented reality, together with the escalating incidence of myopia, could portent an epidemic of vision-threatening corneal infections in the future. Thankfully, technological advances in genomics, proteomics, metabolomics and imaging combined with emerging models of contact lens-associated P. aeruginosa infection hold promise for solving the problem - and possibly life-threatening infections impacting other tissues.
Keywords: Corneal infection, Contact lens, Pseudomonas aeruginosa, Epithelial barrier function, Para-inflammation, Innate defenses
1. Introduction
The contact lens was first conceptualized by Leonardo DaVinci in 1508, and the first glass contact lenses brought into use for vision correction, albeit for very brief (hours) periods of wear, in the 19th century. Soft contact lens wear, as we know it today, was initiated by the pivotal invention of biocompatible and transparent hydrophilic hydrogel polymers (Wichterle and Lím, 1960). However, it soon became evident to clinicians that while contact lenses could be of therapeutic value by promoting corneal epithelial healing (Lawrence et al., 1969; Leibowitz and Rosenthal, 1971), contact lens wear had also become an important risk factor for microbial infection of the cornea (microbial keratitis or infectious keratitis) with significant potential for permanent vision loss (Dixon et al., 1966; Galentine et al., 1984; Golden et al., 1971). Importantly, Pseudomonas spp. (invariably Pseudomonas aeruginosa) were at that time, and continue to be, identified as a leading cause of contact lens-associated microbial keratitis: e.g. 23% of isolates in one study (Galentine et al., 1984), and more than 50% in another (Green et al., 2008) (see also Cheng et al., 1999; Cho and Lee, 2018; Golden et al., 1971; Lim et al., 2016; Schein et al., 1989a, 1989b; Stapleton et al., 2008; Stapleton and Carnt, 2012). Multiple epidemiological studies have consistently shown that the annualized incidence of contact lens-related microbial keratitis significantly increases with overnight and/or extended wear versus daily wear (e.g. ~5 to 10-fold or more), that other risk factors can also participate (e.g. patient compliance and hand hygiene, type of lens care solution used, microbial contamination of the lens storage case), and that the introduction of silicone hydrogel lenses with greatly increased oxygen transmissibility has not reduced disease incidence (Cheng et al., 1999; Dart et al., 2008; Robertson, 2013; Schein et al., 1989a; Stapleton et al., 2013, 2008). These patient-based studies have provided important clues as to how lens wear leads to infection pathogenesis, and suggest it is complex and multifactorial.
For almost three decades, this laboratory has focused exclusively on understanding why contact lens wear predisposes the cornea to infection by P. aeruginosa, from the perspective of both host defense and bacterial virulence. This effort has necessitated delving into an array of topics and disciplines, some not previously studied in the same context, and has led to development of models and methods not previously available.
Our general approach has been to ask the following inter-related questions: 1) how does the intact healthy cornea intrinsically resist infection in vivo? 2) how are key components of this resistance impacted by contact lens wear to trigger infection risk? 3) how do bacteria take advantage of these conditions to cause infection? After this brief introduction (Section 1), this perspective paper provides a comprehensive review of our own work, including only relevant research done by colleagues in the field. We begin with studies aimed at understanding the constitutive defenses of the healthy cornea that usually prevent microbes from traversing through the surface epithelium and associated basement membrane (basal lamina) to reach the vulnerable underlying stroma (Section 2). This is followed by examination of bacterial virulence factors and mechanisms involved in P. aeruginosa interactions with non-lens wearing corneas (Section 3), and studies of how contact lens wear compromises those defenses and/or renders microbes able to overcome them (Section 4). After a summative discussion (Section 5), we have outlined future directions that we believe will eventually help solve the problem of contact lens-associated P. aeruginosa keratitis (Section 6), which is followed by a short conclusion (Section 7).
It is our hope that this line of research will lead to strategies for completely avoiding infection and therefore infection-related pathology. There may also be applications beyond the lens-wearing eye, given the numerous sites that this versatile and life-threatening human pathogen can infect.
Before moving forward, we would like to emphasize that our interest in corneal defense relates only to the barriers that usually prevent microbes from accessing the vulnerable corneal stroma. We believe this knowledge is foundational to understanding how lens wear compromises those barriers. Our research focus has generally not extended to host immune responses occurring after bacteria have already arrived at corneal stroma, a separate important topic elegantly investigated and reviewed by others (e.g. Foldenauer et al., 2013; Hazlett, 2004; Sun et al., 2010; Sun et al., 2012; Thanabalasuriar et al., 2019; Willcox, 2007). Also important to note, our studies have almost exclusively utilized P. aeruginosa, the most common cause of contact lens-related infection.
We are cognizant that there are many other important topics that relate to infection and contact lens wear, and that there are other lens-related adverse events. These include: the efficacy of contact lens disinfection, the role of storage case contamination, patient compliance and hygiene, the impact of lens wear on corneal and tear film physiology, and the epidemiology of multiple contact lens-related phenomena including infection. Further, infections can involve microbes other than P. aeruginosa, they can be associated with therapeutic contact lenses used for other corneal epithelial pathologies, and microbes can instead cause inflammatory events such as CLARE (Contact Lens-induced Acute Red Eye). While some of these topics are discussed in this review, many excellent articles have thoroughly reviewed those topics, to which we direct the reader (Carnt et al., 2007; Carnt and Stapleton, 2016; Dartt and Willcox, 2013; Efron et al., 2013; Foulks, 2006; Jones and Powell, 2013; Muntz et al., 2015; Stapleton et al., 2007; Stapleton and Carnt, 2012).
2. How do intact healthy corneas intrinsically resist infection?
Knowing how lens wear alters corneal resistance to infection necessitates first knowing how infection resistance is maintained without lens wear. Indeed, the cornea is constantly exposed to the outside environment and is barraged with particulate matter and allergens, not only microbes and their antigens. At the same time, the cornea needs to maintain clarity critical for vision, which depends on barrier function and proper regulation of ion and fluid transport by cells in both the endothelium and epithelium not just the highly specialized arrangement of collagen fibrils that confers the appropriate optical properties. This incredible biological achievement also relies on the immune-privileged nature of the cornea to minimize potentially damaging inflammation from unwanted immune responses to environmental antigens (microbial or otherwise) (Hamrah and Dana, 2007; Niederkorn, 2011; Streilein, 2003). Much has been learned about how each of these elements contribute to corneal homeostasis and transparency.
However, significant gaps in our knowledge remain. For example, how the cornea resists the assortment of potential pathogens encountered on a daily basis makes little sense based on our current understanding of the biology. In a healthy cornea, the corneal epithelium withstands challenge with even enormous inocula of potentially pathogenic bacteria. This includes P. aeruginosa and S. aureus, the two leading causes of bacterial keratitis. Indeed, our research has shown that topical inoculation of mouse eyes with ~109 Colony-Forming Units [CFU] of bacterial cells of either of these pathogens contained in a ~5 μL drop (translating to a ~1011 CFU/mL thick suspension) results in very few bacteria adhering to the corneal surface and none penetrating into it (Alarcon et al., 2011; Augustin et al., 2011; Mun et al., 2009; Wan et al., 2018). Even more remarkable, the outcome is the same if the eye is first excised and submerged ex vivo into the bacterial suspension for 6 h (Metruccio et al., 2017; Tam et al., 2011).
This fact that the cornea continues to resist bacterial adhesion even after the eye is removed shows that unbound components of tear fluid are not required at the time of bacterial inoculation. However, the surface corneal epithelial cells do not act alone to accomplish this amazing feat. When the same corneal epithelial cells are raised in vitro in tissue culture (i.e. without other in vivo components) they become exquisitely sensitive to virulence strategies of both P. aeruginosa and S. aureus even when 5-log fewer bacteria are used (Fleiszig et al., 1995, 1996b; Fleiszig et al., 1997a; Jett and Gilmore, 2002). In other words, the state of the corneal epithelium in terms of its capacity to resist bacteria is vastly different between in vivo/ex vivo and in vitro conditions. Thus, performing only in vitro experiments using cultured cells grown in tissue culture media can be potentially misleading with results that are not applicable in vivo. Beyond corneal infection research, it is disconcerting that much of our knowledge of cell biology in general has been derived from experiments using cultured cells. On a more positive note, comparing results of in vivo/ex vivo and in vitro experiments can be used as a strategy to better understand how epithelial cells resist bacteria within the intact cornea.
Also important to consider, in the absence of contact lens wear, P. aeruginosa (and other) infections do not generally occur unless there is sufficient injury (trauma) to the cornea to expose the stroma, (Keay et al., 2006; Schein et al., 1989b). As will be discussed later, this is because the overlying epithelium and epithelial basement membrane both function as barriers to microbe penetration (Alarcon et al., 2009b). For this reason, researchers intending to study events occurring during corneal infection have used either scarification or stromal injection to introduce microbes directly into the stroma of animals. These models have been of great value as they reproduce a common cause of corneal infection world-wide, i.e. trauma (Keay et al., 2006; Schein et al., 1989b).
Electron microscopy has shown that lens wear causes little change to corneal surface ultrastructure (Forte et al., 2010). Thus, in predisposing to microbial keratitis, contact lens wear is instead thought to impact the ocular environment in more subtle ways, e.g. reducing tear exchange and adsorbing tear fluid components (Luensmann and Jones, 2012; McNamara et al., 1999a; Muntz et al., 2015).
While contact lenses can sometimes cause clinically evident mechanical epithelial injury, e.g. Superior Epithelial Arcuate Lesions (SEALs) or corneal erosions (reviewed by Lin and Yeh, 2013), these are not generally associated with microbial keratitis. Similarly, lens-induced corneal infiltrative events do not usually result in infection even when associated with bacteria-contaminated lenses (Willcox, 2013b). That infections do not follow suggests that these adverse responses do not compromise the epithelium/basement membrane barriers sufficiently to expose the stroma, the stroma has adopted a higher level of resistance, or that the microbes present were not in a pathogenic state.
Foundational to our understanding of how infections occur during lens wear is knowing how each of the anatomical elements overlying the corneal stroma contribute to preventing microbial penetration when a lens is not worn. Next, is determining how microbes sometimes avoid, or overcome, those defenses when a lens is worn and under what circumstances. Since neither can be studied using scarification or injection models that deliberately by-pass these anatomical elements, much of our knowledge about epithelial barrier function and epithelialmicrobe interactions (for the eye and elsewhere) has arisen from work done exclusively in vitro using cultured cells.
To address this knowledge gap, our research efforts in the past decade have focused largely on developing new animal models to allow corneal epithelial barrier function to be studied in vivo, in addition to strategies for quantifying outcomes in the absence of overt infectious pathology. Importantly, we have been using these methods to delineate the roles of various players in protecting the cornea against microbes during health.
2.1. Role of the ocular surface microbiome?
Microbes are ubiquitous in our environment, and the past few decades have brought an immense amount of research demonstrating the importance of the microbial world to human health (Sharma and Gilbert, 2018; Turnbaugh and Stintzi, 2011). It is clear that microbes on and in the human body play a vital role in maintaining health, and that disease is often associated with a disturbance in the abundance or diversity of these microbial communities, or microbiomes (Marchesi et al., 2016).
Research to date has primarily focused on the role of the intestinal (gut) microflora, often using animal studies, with less known regarding microbiomes at other sites. For example, segmented filamentous bacteria, common inhabitants of the murine intestinal microbiome, facilitate and modulate local and remote protective mucosal immunity (Gauguet et al., 2015; Ivanov et al., 2009; Ivanov and Littman, 2010; Wu et al., 2010a). However, in recent years, it has become clear that microbes at the murine ocular surface, or elsewhere, can also modulate immunity in the cornea, including the host response to an ongoing infection (Kugadas et al., 2016; St. Leger et al., 2017). Given the obvious importance to our research goals, which include understanding the corneal barrier, we were also interested in whether a live microbiome exists on the healthy murine corneal surface, versus the conjunctiva and tear fluid.
Historically, the most common approach used to investigate microbiomes was to culture microorganisms from samples. Swabs of various bodily sites or samples of bodily substances can be taken and plated on media containing nutrients to facilitate microbial growth. Several studies (including our own work in the early 1990’s) showed that culturing bacteria from the human conjunctiva is possible. However, there is much variability from person to person, with some eyes being culture negative. When bacteria are isolated, there are often only a few, and results have varied from study to study (Willcox, 2013a). The most commonly isolated include coagulase-negative Staphylococcus spp., Corynebacterium spp. and Propionibacterium spp. (Doan et al., 2016; Fleiszig and Efron, 1992b; Willcox, 2013a). These are also the most common constituents of the skin, raising the possibility that at least some of them are contaminants from hands or eyelids.
On the other hand, it is well known that standard laboratory culture techniques do not allow for growth of all bacteria. In fact, it has even been argued that over 99% of the bacterial kingdom cannot be cultured using these standard methods (Epstein, 2013). With the advent of molecular technologies, high-throughput culture-independent techniques have been developed to overcome this obstacle. The technique of 16S ribosomal RNA (rRNA) gene sequencing became the gold standard for analyzing microbial communities (Turnbaugh et al., 2007), since this gene is highly conserved in the bacterial kingdom. Additionally, the presence of variable regions allows for the identification of bacteria down to a species level. Several studies have employed this technique to further characterize the microbial community of the human conjunctiva, and have revealed a more diverse set of bacterial constituents, although still much fewer compared to other body sites (0.06 bacteria/conjunctival cell versus 12–16 bacteria/skin or oral cavity cell) (Doan et al., 2016; Dong et al., 2011; Graham et al., 2007). It is also important to note that a microbiome includes all microbial species, not just bacteria. Taking this into consideration, one study specifically looked at the virome of the human conjunctiva, and found that a majority of participants (65% of conjunctival samples) also harbored the Torque teno virus (TTV), previously associated with post-operative endophthalmitis and other intraocular inflammatory conditions (Doan et al., 2016). However, the significance of conjunctival TTV to ocular health (or disease) is unknown.
Nucleic acid contamination is a significant problem with 16S rRNA gene sequencing as it can be very difficult to separate out the true signal from noise, particularly when working with samples of low biomass such as in the eye. Additionally, sequencing errors and difficulties in assessing operational taxonomic units (OTUs) provide further limitations. One study that explored the accuracy and reliability of 16S rRNA genesequencing found that a sample in which only four known species were intentionally mixed resulted in identification of 13 species (Salter et al., 2014). Last, this method detects bacterial nucleic acids, and so there is no information about whether detected DNA comes from a live microbe. As such, a degree of caution is needed in the interpretation of conjunctival microbiome data obtained purely from genesequencing. Nevertheless, human conjunctiva microbiome inhabitants most commonly identified from 16S rRNA gene sequencing consistently mirror traditional culture studies, indicating the presence of a resident conjunctival microbiome that influences ocular homeostasis, with its composition influenced by ocular surface diseases (Ozkan et al., 2017; Ozkan and Willcox, 2019).
To ask if live bacteria were actually associated with the conjunctival tissue (as opposed to being in the overlying tear fluid), and to overcome limitations of culture (e.g. lack of cultivability) and sequencing (footprint, not necessarily live bacteria), we employed imaging techniques that label only live bacteria. This involved the use of click chemistry involving an alkyne-functionalized D-alanine (alkDala) probe, specific to only metabolically-active bacteria undertaking peptidoglycan cell wall synthesis (Shieh et al., 2014; Siegrist et al., 2013). Mice were used for these experiments, which enabled us to enucleate the eyes and perform the experiments ex vivo thereby excluding the presence of tear fluid. The results showed numerous very long filamentous structures on the surface of the conjunctiva (Fig. 1). Further experiments identified these structures as filamentous bacteria belonging to the Corynebacterineae (Wan et al., 2018). This result aligned with studies done by us and others that identified Corynebacterium spp. as relatively common inhabitants of the human conjunctiva (Fleiszig and Efron, 1992b; Willcox, 2013a). Moreover, C. mastitidis, present on the conjunctiva of some mice, can drive a protective response against inflammation involving an interleukin-17 (IL-17) response from gamma-delta T cells in the ocular mucosa, reducing damaging pathology from P. aeruginosa infection in a scratch injury model (St. Leger et al., 2017). Other studies have also shown roles for both conjunctival and intestinal commensal bacteria in supporting IL-1β driven mucosal immunity at the murine ocular surface including involvement of coagulase-negative Staphylococcus spp. (Kugadas et al., 2016). Whether conjunctival commensals also play roles in maintaining the normal murine corneal barrier against microbial penetration during health is to be determined.
Fig. 1.
Images of bacterial filamentous forms associated with the healthy murine conjunctiva. A) Control showing that eyes incubated in D-alanine without an alkyne did not cause fluorescent labeling. B) Using wild-type transgenic mice with fluorescent red cell membranes [mT/mG knock-in mice (Muzumdar et al., 2007) in conjunction with alkDala labeling showed that filamentous structures on the conjunctiva (upper panels) did not colocalize with host cell membranes (Mem-dtom). Conversely, when filament-like structures were present in host tissue, alkDala labeling was not present (lower panels). Images shown on the left and right are of the same fields of view with different emission filters. (C) Imaging of murine conjunctival epithelial tissue shows that DMN-Tre, a probe specific for Corynebacterineae (red), labeled most of the same conjunctival forms as alkDala (green). All images are from the same field of view with different emission filters (Reproduced from Wan et al., 2018).
Contrasting with the conjunctiva, the human cornea is not as straightforward to sample. Thus, ocular surface microbiome studies have largely ignored the cornea, or simply assumed that the cornea and conjunctiva would be similar given that they are neighboring tissues.
To search for bacteria on the cornea, we utilized the same probe and experimental protocol that revealed bacteria on the conjunctiva (alkDala). Again we used mice, as the reagents cannot be used in humans. Results showed that very few bacteria were present (~60 per mouse cornea) and that none were filamentous. For confirmation, we also performed fluorescent in situ hybridization (FISH) using a universal 16S ribosomal RNA gene probe that detects metabolically-active bacteria even if they lack peptidoglycan cell wall synthesis - including viable but non-culturable bacteria (see Section 4.6.2). Results revealed ~100 bacteria per mouse cornea, not significantly different from the quantity detected using alkDala metabolic labeling. Control experiments involving deliberate inoculation of mouse eyes with P. aeruginosa (i.e. a bacterial suspension dropped onto the ocular surface as described previously) showed that both methods can detect bacteria in the context of the cornea when they are present (Wan et al., 2018).
Importantly, we found that the absence of viable bacteria on the murine cornea depended on both IL-1R [Interleukin-1 receptor] and MyD88 [Myeloid differentiation primary response 88], as knockouts lacking either harbored commensal-type bacteria on their corneas (Wan et al., 2018). In both cases, this correlated with a loss of antimicrobial activity in corneal homogenates (Sullivan et al., 2015; Wan et al., 2018). In addition to providing important information about the mechanisms by which the healthy cornea remains microbe free (IL-1R and MyD88 regulation-dependent, possibly via regulated antimicrobials), this set of results validated the methods and our conclusion that the cornea does not harbor a live bacterial microbiome.
Thus, the mouse cornea differs from the mouse conjunctiva in being devoid of a resident viable bacterial microbiome (Wan et al., 2018). Whether the same is true for human corneas remains an open question.
The lack of a microbiome on the cornea is perhaps not surprising given what is known about the microbiome in the gut, which harbors the most significant microbiome in both bacterial number and importance to health. Even in that location, there is a 50 μm clear zone between the epithelial cell layer and the mass of microbes (Vaishnava et al., 2011). This region, described as a “demilitarized zone,” is maintained by antimicrobial peptides expressed by the epithelial cells lining the lumen of the gut. Since the tear film overlying the human cornea is only ~7 μm thick, even if corneal epithelial cells were no more capable of repelling microbes than gut epithelial cells (unlikely), we would not expect a microbiome worth noting at the surface of the cornea. A further contributor would be conjunctival epithelial cells lining the inside of the eyelid, which presumably also create a “demilitarized zone” on the other side of the thin tear fluid layer during blinking and eye closure. The likelihood will further be reduced by blinking itself, which exerts a significant shear force against the smooth corneal surface several times every minute.
This begs the question of why does the conjunctiva harbor a microbiome? A major difference between the conjunctiva and cornea is that the smoothness of the cornea renders the overlying tear film to spread as a relatively thin layer. In contrast, the conjunctiva contains many folds, which could trap microbes and prevent them from being swept away, while at the same time allowing some of them to potentially avoid close contact with epithelial cells. Further, the sweeping action of blinking is less relevant to the conjunctiva, which derives less benefit from the lid-wiper edge. Even though there are some bacteria present (on both the mouse and human conjunctiva), they are few in number, and those associated with the tissue itself (actually visible) appear to be mostly Corynebacterium spp. and are filamentous. Corynebacterium spp. (and Propionibacterium spp.) are similar to Mycobacterium spp, and possess a fatty acid cell wall enabling them to resist a large array of antimicrobials (Brennan and Nikaido, 1995; Gebhardt et al., 2007), providing them with an advantage for colonizing an antimicrobial surface. Filamentation, a survival strategy that can be performed by many bacteria (including P. aeruginosa), also renders microbes more resistant to killing by antimicrobials (Barrett et al., 2019; Bos et al., 2014). Filamentation additionally interferes with phagocytosis in part because the microbes become too big to be engulfed (Horvath et al., 2011; Prashar et al., 2013), and filamentation also promotes surface colonization (Möller et al., 2013). Thus, the filamentous nature of bacterial forms on the murine conjunctiva is likely to contribute to their resistance to killing by host antimicrobials at the conjunctiva, while also helping them avoid physical removal by blinking or phagocytosis. Indeed, we have observed microbial filaments in the mouse conjunctiva to be tangled around folds in the tissue and around each other (Wan et al., 2018).
It would be remiss if we were not to mention possible caveats to our studies, and acknowledge work done by others that does not necessarily align with our own findings or theoretical models. The methods we have used to study the murine cornea cannot be used on humans. One difference between experimental animals (such as mice) and humans is that humans blink much more often. Thus, the ocular surface of animals is likely to differ from humans to compensate for functions otherwise provided by more regular blinking. Whether this translates to differences in the presence of a microbiome on the cornea is unknown at present. Similar limitations exist for all basic research involving animal and cell models. A study by Ozkan and colleagues suggested that bacteria (with pathogenic potential) might reside within human ocular surface tissues below the surface (Ozkan et al., 2018). Conjunctiva (bulbar, fornix and limbal areas) and eyelid tissue were studied, but not cornea. Since the tissue revealing a potentially pathogenic microbe was derived from patients undergoing surgery for preexisting ocular surface diseases, it is unclear whether such microbes reside deep in ocular surface tissues in a healthy human eye, even within the conjunctiva. Clearly, ethical considerations often prevent the performance of otherwise ‘ideal’ experiments on healthy human subjects, and this is especially true for cornea research. In healthy mice, we did not detect live microbes below the tissue surface in either the cornea or conjunctiva (Wan et al., 2018).
As previously alluded to, in addition to resisting colonization by environmental bacteria in vivo, the healthy murine cornea also rapidly clears large inocula of P. aeruginosa and other potentially pathogenic bacteria, e.g. S. aureus, without any signs of infection (Augustin et al., 2011; Mun et al., 2009; Wan et al., 2018). This again depends on MyD88 and IL-1R. Mechanisms by which the protective system modulated by these two innate response modulators operates to keep the cornea free from both commensals and pathogens, and how it is impacted by contact lens wear, is currently being investigated in our laboratory.
While the lack of a microbiome on the murine cornea rules out the possibility that bacteria at the corneal surface in some way directly restrict access of other bacteria to the corneal epithelial surface (as hinted at in scientific and lay-press articles), it is important to think about whether the microbiome on the adjacent conjunctiva might participate from a distance. As discussed above, murine conjunctival Corynebacterium spp. modulate the immune response to P. aeruginosa after disease is initiated via the scratch injury infection model (St. Leger et al., 2017). Whether murine conjunctival Corynebacterium spp. play roles in intrinsic barrier function of the healthy, uninjured cornea (i.e. preventing bacterial penetration into the stroma) is a different question that has not yet been answered. Suggesting this might not be the case, mouse eyes with their conjunctivae naturally lacking the specific Corynebacterium sp. studied (C. mastitidis) have corneas with normal barrier function against P. aeruginosa (St. Leger et al., 2017). However, it remains possible that other conjunctival commensals might take on this function in those mice. While there is little evidence from the clinic to suggest that antibiotics at the ocular surface increase the risk of P. aeruginosa corneal infection, antibiotics do not necessarily kill bacteria. Even when they do, microbial debris (ubiquitous) can activate responses via host cell pattern recognition receptors (see Section 2.3.7), some MyD88-dependent, and other triggered responses can depend on IL-1R. Additionally, the murine gut microbiome was shown to influence the biology of distant body sites, including the ocular surface (Kugadas et al., 2016). Thus, lack of a live microbiome at a specific site does not necessarily rule out a role for microbes and/or their components in regulating barrier function at that site.
2.2. Blinking and tear fluid
Blinking evenly distributes the tear fluid over the corneal surface (Holly, 1973), and in doing so flushes unwanted foreign particles including microbes. The tear fluid also plays a variety of other roles in protecting the ocular surface epithelium. This includes the antimicrobial activities of multiple molecular components, some that directly kill bacteria and others that prevent bacterial replication. Mechanisms of action used include targeting the bacterial cell wall, restricting iron availability, inhibiting protein synthesis, direct binding to cause aggregation, or otherwise inhibiting their ability to target host cells. Known antibacterial factors in tear fluid include; lysozyme, lactoferrin, antimicrobial peptides, lipocalin, soluble mucins, surfactant proteins, secretory IgA, and keratin-derived antimicrobial peptides (KAMPs) (Chan et al., 2018; Dartt and Willcox, 2013; Evans et al., 2007; Evans and Fleiszig, 2013; Flanagan and Willcox, 2009; Fluckinger et al., 2004; Masinick et al., 1997; McDermott, 2013; McNamara et al., 1999b; Ni et al., 2005; Tam et al., 2012).
Despite this array of antimicrobials, we have demonstrated that human tear fluid is not sufficient to kill or even inhibit the growth of many P. aeruginosa isolates (Fleiszig et al., 2003). This is partly explained by expression of LPS (as for most Gram-negative bacteria), which forms an outer layer that protects their cell wall. Beyond that, P. aeruginosa is unusually well equipped to survive in adverse environments compared to other Gram-negative bacteria. Nevertheless, we have found that human tear fluid, while unable to kill P. aeruginosa, can inhibit its virulence against cultured human corneal epithelial cells and mouse corneas in vivo (Fleiszig et al., 2003; Kwong et al., 2007). Likely involved in those effects, our data show that tear fluid has multiple impacts on bacterial morphology and function. For P. aeruginosa, tear fluid causes bacterial chain formation and clumping, can interfere with contact lens-associated biofilm formation, and can cause loss of two types of motility, swimming used for movement in fluid, and twitching motility used for traveling on surfaces (Fleiszig et al., 2003; Li et al., 2017; Wu et al., 2017). With respect to the latter, twitching motility is important for P. aeruginosa to traffic through corneal epithelial cell layers (Alarcon et al., 2009a), to exit host cells after internalization (Alarcon et al., 2009a), and for virulence in vivo (Zolfaghar et al., 2003) (). Recently, we identified glycoprotein DMBT1 (Deleted in Malignant Brain Tumors 1) as the ingredient in tear fluid responsible for inhibition of P. aeruginosa twitching motility function. Importantly, we found DMBT1 prevented P. aeruginosa from penetrating through multilayers of cultured human corneal epithelial cells, and could protect against infection in mice (Li et al., 2017).
Apart from its actions on bacteria, human tear fluid can act directly on corneal epithelial cells to enhance their resistance to bacteria (Mun et al., 2011). Tear fluid upregulates a plethora of genes in corneal epithelial cells, including global stress response factors NF- κB and AP-1 to resist P. aeruginosa, with part of this tear-mediated protection involving upregulation of host cell antimicrobial (RNase7) and immunomodulatory (ST2) factors (Mun et al., 2011), a process regulated by microRNAs (Mun et al., 2013). Thus, in addition to modulating bacterial virulence directly, tear fluid also primes defense mechanisms in corneal epithelial cells, and in doing so influences the outcome of bacterial exposure in two different ways.
Also present in the tear fluid are soluble mucins. In 1994, we published the first study showing that ocular mucins could inhibit bacterial adhesion to the corneal surface. This was shown using mucins that were collected from the ocular surface of multiple rat eyes ex vivo, pooled and purified, then tested for their ability to prevent adhesion of bacteria to other healthy or superficially-injured rat corneas ex vivo after tear fluid was first removed by rinsing (Fleiszig et al., 1994b)(). Showing that this defense can be regulated, other types of epithelial cells grown in vitro produce soluble mucins MUC2 and MUC5AC after inoculation with P. aeruginosa antigens (e.g. LPS or flagellin) (Dohrman et al., 1998; McNamara et al., 2001; Yu et al., 2012). We, and others, have since used more sophisticated methods, and other bacterial species, to confirm that ocular mucins can modulate adhesion of microbes to the cornea, and that regulation of their expression is complex, involving multiple cell types at the ocular surface (Dartt and Willcox, 2013; Gipson et al., 2014; Gipson and Argüeso, 2003; Jolly et al., 2017; Mantelli et al., 2013). In addition to preventing bacterial adhesion, soluble mucins can also cause dispersal of previously attached populations. For example, both MUC2 and MUC5AC were found to mediate disruption and dispersal of established P. aeruginosa biofilms (Co et al., 2018), suggesting an additional mechanism by which soluble mucins could contribute to host defense.
Surface-associated mucins of the corneal epithelial glycocalyx, which sits at the interface between the tear fluid and corneal epithelium, also help defend against bacterial adhesion, as discussed in detail below.
2.3. The corneal epithelium
Bacteria that survive the antimicrobial/virulence-altering potential of tear fluid and attempt to colonize the cornea next encounter the corneal epithelium, composed of a multilayer of stratified squamous epithelial cells. Research done by us, and others, has revealed a plethora of defenses associated with this cell multilayer, but there is good reason to believe that our current understanding remains far from complete.
2.3.1. History
When the first author of this review began working in this field (1986), little was known about how the corneal epithelium resists microbes. While it was known that tight-junctions between superficial cells formed a physical barrier between cells, and that regular desquamation (a.k.a. exfoliation, shedding or sloughing) helped rid the corneal surface of bound bacteria, it was assumed that these physical features fully explained resistance. Antimicrobial peptides, and the capacity of epithelial cells to actively respond to their environment to impact immunity (locally or at distant sites), had not yet been discovered in general. While the surface glycocalyx had been discovered a few years earlier, its composition was unknown beyond it “containing many highly charged polyanions” (Nichols et al., 1983), and its contributions to resisting microbes were not yet appreciated.
Even today, we know far less about how epithelial barrier function works in the cornea (or elsewhere) than might be expected given advances in related knowledge and technology. Likely, this is because the commonly used in vivo models appropriate for studying ongoing infection and immune responses (i.e. scarification, stromal injection), are less useful for studying normal barrier function of the intact cornea.
2.3.2. Studying corneal epithelial barrier function: method development and results
To address knowledge gaps in our understanding of epithelial barrier function against microbes, we have placed significant effort and resources into development of needed tools. Our goal was to develop experimental models allowing barrier function against microbes to be studied while it is not fully compromised, such that factors contributing to the lack of clinically evident infection could be studied. Thus, we turned to inoculating corneas completely healthy, or after introducing subtle manipulations that still did not result in infection susceptibility. When visible pathology is not an outcome, strategies other than disease scores are needed to evaluate results. Thus, we advanced intravital imaging methods to enable individual bacteria to be localized at subcellular resolution within the cornea, and developed a suite of computational methods for analysis of the data obtained. While continuing to improve upon these methodologies, we have been using them to systematically tease apart the mechanisms contributing to intrinsic resistance to bacteria during health. While some results have confirmed existing schools of thought, others have challenged them, and we have also discovered some novel players.
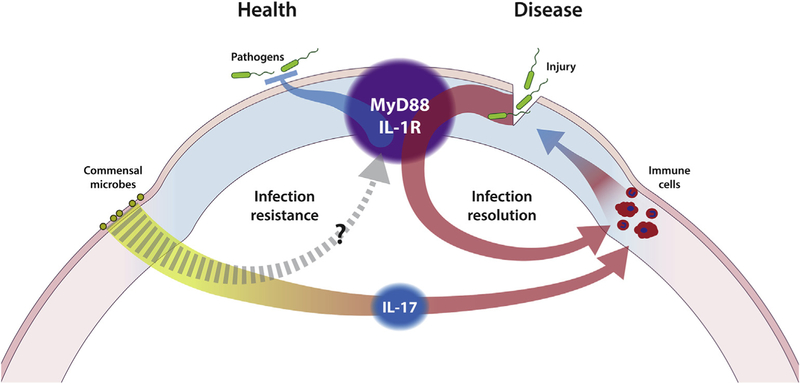
The schematic diagram shown in Fig. 2 illustrates the difference in research approach between studying ocular defenses during health versus defense responses to infections established after by-passing the epithelial barrier. Distinguishing these research approaches is important but can sometimes be confounded by involvement of the same factors, e.g. IL-1R, MyD88. That said, distinctions will likely be made as future studies determine the relative contributions of myeloid-derived versus non-myeloid-derived cells to defenses during health (see later discussion, Section 2.3.7).
Fig. 2.
Schematic diagram illustrating differences in research approach towards investigating the pathogenesis of P. aeruginosa keratitis. By-passing the epithelial barrier by injury allows acute infection of the cornea (disease) and has been invaluable for detailed mechanistic study of host responses. Understanding contact lens-related P. aeruginosa keratitis, however, requires first understanding defenses during health, followed by lens effects on epithelial barrier function in the absence of overt injury. IL-1R and MyD88 participate in ocular defense in both health and disease. The same may also be true for ocular commensals for which an IL-17 mediated role in defense responses to acute infection (disease) have already been shown.
2.3.2.1. The scratch and heal model.
Our first attempt to study epithelial barrier function in vivo led to development of the “scratch and heal” model. This was a modification of the traditional scratch (scarification) model in which we allowed time for healing re-epithelialization (e.g. 6, 9, or 12 h) prior to challenging the cornea with bacteria (Lee et al., 2003b). It was based on results showing time points during re-epithelialization when the epithelium had already regained its multilayered morphology yet remained susceptible to infection (e.g. 6 h).
Results using the scratch and heal model showed that fluorescein staining was still apparent at 12 h when the epithelium had regained its resistance to infection. This showed a disconnect between infection resistance and lack of fluorescein staining, interesting considering that fluorescein is commonly used in the clinic to assess “barrier function”. These findings suggested that the mechanisms contributing to barrier function can depend upon the nature of the factor(s) being excluded.
The scratch and heal model has also proven useful for studying host-microbe interactions between corneal epithelium and bacteria in vivo. For example, we used it to decipher virulence factors used by P. aeruginosa to traverse a susceptible corneal epithelium (discussed below). We also used it to show that human tear fluid can protect the corneal epithelium against P. aeruginosa colonization and keratitis, as was discussed in the previous section (Kwong et al., 2007). However, the usefulness of this scratch and heal model for understanding contact lens-related infection is unclear, given that epithelial susceptibility could occur by different mechanisms during lens wear. Similarly, a healthy cornea’s infection resistance might not equate to that of a healing cornea.
2.3.2.2. Development of imaging methods to study epithelial barrier function.
To detect subtle outcomes using models in which overt disease does not occur, we have advanced and customized a suite of imaging methods that enable us to perform 4D imaging in high resolution using live samples. This allows visualization of events not detected at lower resolution or without temporal information, and it avoids fixation/sectioning and associated artifacts. Use of high resolution additionally allows detection and quantification of the number and location of bacteria relative to subcellular structures. Foregoing fixation and sectioning allows the imaging of changes in the z-plane over time, i.e. to image in 4D. By performing imaging in vivo, or imaging freshly excised eyes after in vivo experiments are performed, we can also avoid in vitro-introduced artifacts. Exposure time is also limited to reduce potentially damaging impacts, and to minimize photobleaching of fluorophores. While we have utilized label-free autofluorescence or reflection methods as needed, we have also employed both mice and bacteria that express fluorescent reporters to gain more detailed information (Metruccio et al., 2016, 2017; Sullivan et al., 2015; Tam et al., 2011). Quantitative metrics we have developed/used include dimensions of corneal features (Sullivan et al., 2015), individual cell status via NAD(P)H autofluorescence (Tam et al., 2011), bacterial position mapping (Sullivan et al., 2015), and live responses of resident and infiltrative immune cells (Metruccio et al., 2017, 2019).
2.3.2.3. “Null-infection” model.
It has long been appreciated that inoculation of P. aeruginosa onto a naïve healthy ocular surface yields no disease (Gerke and Magliocco, 1971). With the exception of immature corneas, e.g. age P5 in mice (Singh et al., 1991), it does not even result in bacterial adherence (Ramphal et al., 1981). Based on this principle, we developed a simple model that we called the “null infection” model (Mun et al., 2009). This involves inoculating healthy eyes of normal mice and asking questions about why their corneas do not become infected. Strategies that can be used to explore outcomes include tracking bacterial clearance from the ocular surface over time quantitatively, and a variety of imaging tools as described above to study the tissue response and microbe location (Augustin et al., 2011; Mun et al., 2009).
Results using this method have shown that both Gram-negative (P. aeruginosa) and Gram-positive (S. aureus) bacteria inoculated onto a healthy cornea are completely cleared within hours, irrespective of the size of the inoculum (Mun et al., 2009; Wan et al., 2018). Despite lack of disease and no microbial colonization, we found a detectable host response consisting of an increase in the number of CD11c-positive cells (most likely dendritic cells [DCs]) and changes to their morphology (Metruccio et al., 2017). While this result raised the possibility that corneal barriers to infection were not passive even in the completely healthy cornea, subsequent experiments showed that while corneas were still able to resist bacterial adhesion when the eye was excised, they were unable to mount the CD11c-positive cell response to bacterial inoculation. Thus, the CD11c-positive cell response is not required for resistance to microbial colonization when the cornea is healthy. More likely, it represents a “stand-by” response in anticipation of further insult.
There are many interesting unanswered questions about this CD11c-positive cell response, including what attracts these cells into the healthy cornea. The absence of a response ex vivo (freshly excised eyeball), suggests a requirement of factors outside of the eyeball, e.g. lymphatic vessels/lymph nodes, tear fluid, and/or cell bodies of corneal sensory nerves.
2.3.3. Surface-associated mucins
The apical face of the superficial cells within the corneal epithelium display an array of membrane-bound mucins (e.g. MUC1, MUC 4, MUC16 and MUC20). This forms a dense glycocalyx, difficult for pathogens to pass through and gain access to host membranes (Gipson and Argüeso, 2003; Mantelli and Argüeso, 2008). The notion that the glycocalyx plays a role in corneal resistance to bacteria was first explored 25 years ago, when we used N-acetylcysteine to remove endogenous mucins from rat and rabbit corneas before inoculating with P. aeruginosa (Fleiszig et al., 1994b). Results showed that bacteria were able to adhere more readily after N-acetylcysteine treatment compared to PBS-treated control eyes. Even earlier studies done by others hinted at the now appreciated complexity of this topic by showing: 1) enhanced P. aeruginosa binding to unwounded mouse corneas after neuraminidase treatment to remove sialic acid residues (Singh et al., 1991), 2) that P. aeruginosa can actually interact with asialo-GM1 in scarification-injured corneas (Hazlett et al., 1993), and 3) that P. aeruginosa pilus adhesins can interact with corneal glycoproteins (Rudner et al., 1992). While mice deficient in MUC1, a transmembrane mucin of the corneal epithelium, showed no specific phenotypical change (Danjo et al., 2000), knockdown of a different transmembrane mucin (MUC16) enabled more bacteria binding, and also disrupted tight-junctions between epithelial cells (Gipson et al., 2014). Further, it has been demonstrated that barrier function of the corneal epithelial glycocalyx against the adherence of S. aureus is a result of its extensive O-glycosylation (Ricciuto et al., 2008).
Our recent studies also add to the complexity. Using a click-chemistry method that labels only newly synthesized mucins, we found that epithelial surface glycosylation in the mouse cornea in vivo requires IL-1R, but not MyD88 - despite the fact that IL-1R signaling is generally MyD88-dependent (Jolly et al., 2017). As will be discussed in more detail later, we also showed that IL-1R and MyD88 are both required for resistance to bacterial adhesion (Metruccio et al., 2017; Tam et al., 2011). Additionally, images captured using the click-chemistry method showed that the pattern of P. aeruginosa binding to individual cells was not predictable by glycosylation level. This again suggests that the relationship between glycosylation and susceptibility to bacterial adhesion is not straightforward (Jolly et al., 2017), with the caveat that the method we used only labeled glycosylation occurring during the time of the experiment, and pre-existing mucins might have been affected differently.
A potential explanation for the body of published data on this topic would be that the role of glycosylation in preventing bacterial adhesion is to sequester responsible factors rather than being directly involved. Candidates include antimicrobial peptides, the production of which can depend on both IL-1R and MyD88 (McDermott et al., 2003; Redfern et al., 2011), with a subset already known to bind mucins (Felgentreff et al., 2006). Other candidate possibilities would be clusterin or galectin-3 sequestered at the ocular surface, as both make known contributions to other aspects of corneal epithelial barrier function (AbuSamra and Argüeso, 2018; Fini et al., 2016).
2.3.4. The impact of superficial injury
Several years ago, we demonstrated that superficial epithelial in jury, induced by blotting the corneal surface with tissue paper (Kimwipe™), renders the otherwise healthy cornea more susceptible to P. aeruginosa binding without resulting in infection (Alarcon et al., 2011; see also Klotz et al., 1989; Metruccio et al., 2017; Tam et al., 2011). Closer inspection of what the tissue paper blotting process did to the corneal epithelium revealed that while it remained multilayered with relatively normal architecture (Alarcon et al., 2011), the epithelium stained extensively with fluorescein penetrating all the way into the stroma, suggesting that junctions normally excluding small molecules were disrupted (Alarcon et al., 2011; Tam et al., 2011). Blotting also reduced viability of surface corneal epithelial cells, and removed some glycocalyx expressing surface cells (Jolly et al., 2017). Yet, bacteria adhering to blotted corneas stayed localized to the surface of epithelium no matter how large the inoculum. Even more surprising, the result was the same when the inoculation step was performed on enucleated eyes (eyeball placed into the large inoculum) (Alarcon et al., 2011). These findings illustrate the incredible efficacy of defenses associated with the epithelium beyond its anti-adhesive properties, that they are independent of barriers preventing fluorescein penetration, and that they do not require the presence of tear fluid (at least at the time of inoculation) to be effective.
Perhaps not surprisingly, corneas superficially injured by blotting showed an even more robust CD11c-positive cell (DC) response than did healthy corneas. Furthermore, the dendrites of some CD11c-positive cells in blotted (only) corneas extended all the way to the corneal surface and colocalized with adherent bacteria. Showing this response actually contributes to defense in blotted corneas, CD11c-positive cell depletion rendered blotted corneas even more susceptible to P. aeruginosa adhesion (Metruccio et al., 2017), contrasting with unblotted corneas that continued to resist adhesion following CD11c-positive cell depletion. However, bacteria adhering to blotted corneas still did not penetrate beyond the surface despite the additional bacteria adhering. Thus, while CD11c-positive cells (DCs) contribute to defense against surface adhesion after superficial injury, they are not required for the corneal epithelium to resist bacterial penetration beyond the surface.
Taken together, these data show that there are three separate tiers to epithelial barrier function: 1) baseline defenses against surface adhesion that can be overcome by superficial injury/blotting, 2) backup defenses against surface adhesion dependent on a CD11c-positive cell response that can continue to operate after superficial injury, and 3) defenses preventing adherent bacteria from traversing the epithelium that operate independently of the CD11c-positive cell response.
2.3.5. Junctional complexes/cell polarity
Finding that tissue paper blotting and CD11c-positive cell depletion both promote bacterial binding without bacteria subsequently penetrating the corneal epithelium shows that defenses operating to prevent adherent bacteria from traversing the epithelium are separable from those that protect against bacterial adhesion. Hypothesizing that defenses against traversal might include cell-to-cell junctions, we explored the impact of treatment with EGTA [Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid] (100 mM), a Ca2+ chelator, used after superficial blotting. This allowed bacteria to readily traverse the epithelium (Alarcon et al., 2011; Sullivan et al., 2015). Supporting the possibility that junctions were involved, EGTA/calcium-chelation disrupted ZO-1 a junctional protein (Alarcon et al., 2011).
Junctional complexes could potentially contribute to preventing epithelial traversal by bacteria via one or both of two separate mechanisms. One would be if they form a physical barrier that escapes being damaged by blotting (e.g. if they are not at the surface). Such a barrier would need to be leaky/size-dependent given that fluorescein, but not bacteria, penetrates the blotted epithelium. Multiple types of junctional complexes are known to exist in the deeper epithelium, including desmosomes, adherens and gap junctions (Mantelli et al., 2013). Additionally, claudin-expressing suprabasal junctions function deep within the corneal epithelium to prevent access of some molecular factors (e.g. phalloidin) and leukocytes between adjacent corneal epithelial cells (Sosnová-Netuková et al., 2007).
A second potential role for junctions would be to maintain normal cell polarity. Epithelial cell membranes can be divided into apical and basolateral compartments kept separate by junctional complexes, and maintained by polarized sorting of membrane constituents (Drubin and Nelson, 1996). Correct localization of proteins and other constituents is essential for proper cell and tissue function (Martin-Belmonte and Mostov, 2008; St Johnston and Sanson, 2011). We have demonstrated the importance of correct cell polarity for corneal and other epithelial cells to protect themselves against P. aeruginosa. That data showed that corneal epithelial cells grown in vitro became more susceptible to invasion and killing by P. aeruginosa when they had lost their polarity as a result of EGTA-mediated calcium chelation (Fleiszig et al., 1997b). Tying this directly to loss of cell polarity, similar results were obtained when we used MDCK [Madin-Darby Canine Kidney] cell clones with and without polarity defects, and when corneal epithelial cells were treated with hepatocyte growth factor to disrupt polarity without disrupting tight-junctions (Fleiszig et al., 1998). We found the same result for lung epithelial cells (Lee et al., 1999), and later studies done by others have elegantly expanded upon the mechanisms underlying the differences between apical and basolateral surfaces of polarized epithelial cells in susceptibility to P. aeruginosa (Bucior et al., 2010, 2012; Eierhoff et al., 2014; Tran et al., 2014).
Possibly of relevance here, tear fluid treatment of multilayered corneal epithelial cells in vitro causes a significant elevation of transepithelial resistance (Kwong et al., 2007), a marker of tight-junction formation and cell polarity. Thus, the mechanisms by which tear fluid regulates corneal epithelial cell defenses against P. aeruginosa (discussed above, Mun et al., 2011), might involve an impact on epithelial cell polarity.
However, calcium chelation can potentially disrupt a plethora of other cellular functions beyond junctional integrity that might contribute to why calcium chelation reduces barrier function. For example, some antimicrobial peptides are calcium-dependent in either their production or their action. Other calcium-dependent factors expressed by host cells at the ocular surface include surfactant protein D, which we showed can limit both P. aeruginosa adherence and traversal in the corneal epithelium (Alarcon et al., 2011), and DMBT1 (Bikker et al., 2002), which as previously discussed inhibits P. aeruginosa twitching motility.
2.3.6. Antimicrobial peptides
An array of antimicrobial peptides (AMPs) are present at the ocular surface both in the tear fluid and the associated tissues (McDermott, 2009, 2013; Mohammed et al., 2017). These include defensins and cathelicidins (LL-37 in humans), their very presence suggesting key roles in defense against infection. These are generally considered broad-spectrum in activity, mostly cationic in nature, kill bacteria by membrane disruption, and are ubiquitous across species (Zasloff, 2002). First evidence of AMP presence in the eye was reported two decades ago when we and others discovered that β-defensins are expressed by human corneal and conjunctival epithelial cells (Haynes et al., 1999, 1998; McNamara et al., 1999b). In our study, we additionally showed that these could be regulated by pathogen associated molecular patterns (McNamara et al., 1999b). Others have since shown that both IL-1β and TLR-receptor signaling also regulate the expression of AMPs at the ocular surface (McDermott et al., 2003; Redfern et al., 2011), and that AMPs are involved in resolving P. aeruginosa corneal infections after they are initiated by scratching (Wu et al., 2009). Interestingly, AMP expression is not always upregulated in microbial keratitis, e.g. hBD-9 was downregulated in Gram-positive bacterial keratitis (Otri et al., 2012).
Our own studies of AMPs have remained focused on their role in infection resistance, i.e. their contributions to preventing bacterial adhesion to the corneal epithelium and subsequent bacterial penetration through the layer. We have found that several are involved. For example, small interfering RNA knockdown of hBD-1, −2, −3, and LL-37 in telomerase-immortalized multilayered human corneal epithelial (hTCEpi) cells, each reduced their barrier function against P. aeruginosa, and in combination they had additive effects (Augustin et al., 2011). We subsequently used mice deficient in the murine ortholog of hBD-2 (mBD-3) and imaging methods to prove this contribution to epithelial barrier function in vivo (Augustin et al., 2011).
In addition to confirming roles for known antimicrobial peptides, we have also discovered novel antimicrobial compounds expressed by corneal epithelial cells that contribute directly to barrier function against P. aeruginosa. Systematic fractionation of human corneal epithelial cells combined with mass spectrometry revealed a series of glycine-rich C-terminal peptides of human cytokeratin 6A (Tam et al., 2012). These keratin-derived antimicrobial peptides (KAMPs) were the first of their type to be discovered and they have bactericidal activity against a range of Gram-negative and Gram-positive bacteria, including P. aeruginosa, E. coli, S. aureus, and S. epidermidis. Showing that they contribute to constitutive barrier function against P. aeruginosa, we found that knockdown of their expression in mice resulted in a ~5-fold increase in bacterial adherence to otherwise healthy corneas (Tam et al., 2012). More recently, Dr. Connie Tam’s research group (now independent) has shown that KAMPs have a unique structure, are membrane-active and exert bactericidal activity in part by pore formation in the bacterial cell envelope. KAMPs are constitutively generated by corneal epithelial cells, and their levels can be upregulated after antigen-challenge via ubiquitin-proteasome processing (Chan et al., 2018; Lee et al., 2016).
Given the almost incomprehensible resistance of the corneal epithelium to infection in vivo compared to the in vitro susceptibility of cultured corneal epithelial cells, it is possible that these cells express other novel antimicrobial peptides when in situ. Epithelial cell lysates derived from MyD88 gene-knockout mouse corneas (susceptible to bacterial penetration) have significantly reduced antimicrobial activity compared to wild-type, at baseline and after antigen challenge, and proteomics results showed a plethora of potential candidates for involvement that differed between each condition (Sullivan et al., 2015). The same is likely true for lysates of IL-1R knockouts, which also show reduced antimicrobial activity (Wan et al., 2018). It is perhaps not surprising that knockout of either one of these factors could broadly influence antimicrobial activity of host cells given that both MyD88 and IL-1R profoundly influence multiple downstream signaling events after activation. Research in this area aimed at identifying key signaling pathways and effector molecules involved could lead to new therapeutics while advancing our understanding of corneal defenses against infection. As in our study above, it would be important to examine not only baseline (constitutive) epithelial cell antimicrobial activity, but that of lysates prepared after microbial antigen-challenge; the latter likely to contain a different spectrum of potential antimicrobial factors, including some not present at baseline.
2.3.7. Regulation of epithelial barrier function
Also important to understanding corneal epithelial barrier function against microbes is that it depends on regulators and effectors of innate immune responses (MyD88 and IL-1R), somewhat surprisingly even in the constitutive (non-inflamed) state (Sullivan et al., 2015; Tam et al., 2011).
Our published data showed that P. aeruginosa could readily traverse the otherwise healthy corneal epithelium of MyD88 knockout mice, i.e. even in the absence of any form of superficial injury. In this way, we demonstrated that MyD88 regulates defenses against bacterial adhesion and also their subsequent traversal through the layer (Tam et al., 2011).
The outcome was more complicated when we used IL-1R knockout mice (Metruccio et al., 2017). Those data showed that for healthy corneas (i.e. no blot), IL-1R regulated adhesion, but was not needed for defense against subsequent traversal. Intriguingly, chimera experiments showed that bone marrow-derived cells contributed ~50% to the role of IL-1R in protecting the healthy uninjured corneal surface against bacterial adhesion (Metruccio et al., 2017). Since CD11c-positive cells were not required (discussed previously), other bone marrow-derived cells appear to be important for constitutive barrier function in a healthy cornea. Even more surprising results were obtained when corneas were superficially injured by blotting, which unmasked a role for IL-1R (this time not involving bone marrow-derived cells) in defending the corneal epithelium against bacterial traversal. Together, these results suggest that IL-1R plays two separate roles in corneal epithelial barrier function against P. aeruginosa, neither requiring CD11c-positive cells: 1) defending a healthy corneal epithelium against bacterial adhesion involving both bone marrow-derived cells and other cell types, and 2) conditionally preventing adherent bacteria from traversing the epithelium (e.g. after superficial injury), accomplished independently of bone marrow-derived cells.
Two other MyD88-dependent receptors contributed even more selectively, each overlapping with one of the two IL-1R-dependent/CD11c-positive cell-independent defenses. TLR4 was found to protect against baseline adhesion, while TLR5 protected against traversal but only after blotting. TLRs 2, 7 and 9 played no role (Metruccio et al., 2017). TLR4 and TLR5 involvement suggests active detection of multiple microbial ligands (likely LPS and flagellin) mediates barrier function.
Our data showing separable roles for MyD88, IL-1R, TLR4, TLR5 and CD11c-positive cells/bone marrow-derived cells reveal mechanistic differences between healthy and superficially-injured corneal epithelium in how barrier function is maintained. They also suggest at least three sets of events can contribute, involving both overlapping and distinct players.
It is important to reflect upon involvement of the TLR/IL-1R family, and their signaling adaptor protein MyD88, in mediating resistance of a healthy cornea to bacterial colonization, and the correlation with their role in maintenance of corneal surface antimicrobial activity (and for IL-R, its role in ocular surface glycosylation). Over the past two decades, a vast amount of research has shown that these, and other, patternrecognition receptors (PRRs) participate in innate defense of multicellular organisms via the recognition of, and response to, pathogen-associated molecular patterns (PAMPS), i.e. common microbial ligands such as LPS, flagellin, components of cell wall peptidoglycan, DNA and RNA etc. (see Akira et al., 2001; Barton and Medzhitov, 2003; Beutler, 2004; Beutler, 2009; Garlanda et al., 2013). The role of innate defense PRRs at the ocular surface has been well demonstrated in terms of driving inflammation and disease pathology, and in influencing the balance between pro-inflammatory and anti-inflammatory responses that ultimately affect disease outcome (Foldenauer et al., 2013; Huang et al., 2006, 2005; Kumar et al., 2008; Kumar and Yu, 2006; Pearlman et al., 2008, 2013).
However, resisting infection during health represents an entirely different challenge for the host than responding to infection for multiple reasons. For the cornea this includes the need to remain transparent, a task the host abandons altogether when inflammation is triggered. The distinction between these topics has not always been well appreciated within the field, perhaps understandable given the data showing some cellular and molecular factors play roles in both, and variations in the use of terminology surrounding these events.
When we have referred to intrinsic resistance to infection (i.e. processes that prevent entry of bacteria into the corneal stroma when healthy), we have used terminology such as “constitutive” defense, “maintenance of ocular homeostasis,” or “resistance to infection” to indicate we are not studying responsive defense during active infection. In other settings, the term “resistance” is often used to refer to an infection that eventually resolves versus “susceptibility” when it does not (e.g. Hazlett, 2007; Hazlett, 2004). “Constitutive” and “homeostasis” have also been used to describe roles of MyD88 and other regulators in maintenance of corneal and conjunctival expression of cytokines, chemokines and metalloproteinases (e.g. Reins et al., 2017). To add to the complexity, such constitutive-homeostatic roles of PRRs may still involve microbial ligands either locally at the ocular surface, and/or at distant mucosal sites. For example, resident bacterial flora of the conjunctiva (St. Leger et al., 2017), and/or gut-associated bacteria (Kugadas et al., 2016) can help resolve (as opposed to prevent) P. aeruginosa keratitis in scarification models of infection. Perhaps they also play roles in preventing infection: for example, resident conjunctival flora and/or environmental exposure may provide a source of microbial ligands to the tear fluid and in that way might contribute to how tear fluid boosts baseline epithelial cell defenses against bacterial virulence (as described earlier, Mun et al., 2011). Also feasible, tear fluid may contain non-microbial “tear-associated molecular patterns” or “TAMPS” that accomplish this (Fig. 3A).
Fig. 3.
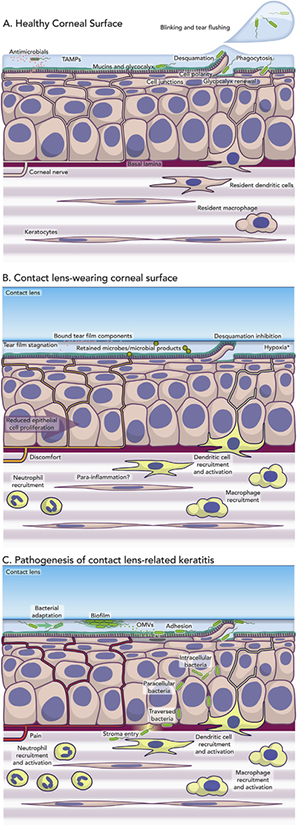
A) Schematic representation of known constitutive defenses of the healthy cornea. The tear fluid and epithelium combine to form a formidable barrier to microbial attack supported by the basal lamina and resident immune cells. Many of these defenses can also be upregulated in response to TAMPs (Tear-Associated Molecular Patterns) which are likely to include both microbial and non-microbial ligands. B) Schematic representation of known, and potential, effects of contact lens wear on constitutive defenses of the cornea that could help predispose to P. aeruginosa keratitis. Effects of lenses in binding tear components, reducing basal epithelial cell proliferation and surface cell desquamation (exfoliation, sloughing) are well established. However, effects of bound microbes (e.g. commensals), tear film stagnation, and lens-induced parainflammation (e.g. dendritic cell activation, “quiescent” neutrophil infiltration), and their consequences, remain to be determined. C) Schematic representation of potential events underlying the initiation of Pseudomonas aeruginosa keratitis during contact lens wear. Biofilm formation on contact lenses in vivo (or on lenses in storage cases before introduction onto the ocular surface) could promote phenotypic and genotypic changes that promote bacterial survival and virulence, as could adaptations to the ocular environment over time. Release of OMVs could prime the corneal epithelium for bacterial adhesion, the latter also favoring expression of the T3SS (see Section 3.3). Lens inhibition of epithelial sloughing could also help retain bound and internalized bacteria at the ocular surface. Pathology of P. aeruginosa keratitis requires bacterial entry into the corneal stroma with activation of inflammatory and immune cells. However, stromal entry in the presence of a contact lens requires bacteria to traverse the multilayered epithelium via intracellular or paracellular pathways (or both). Further understanding is needed of the dynamics and timing of the known bacterial and host events depicted, along with an ongoing appreciation of the potential for unknown factors to participate. * Hypoxia was thought to be responsible for the initiation of P. aeruginosa keratitis, but incidence of infection did not change after introduction of silicone hydrogel lenses with high oxygen transmissibility. However, those lenses do show significantly reduced risk of severe keratitis after extended wear (see Section 4.2).
Thus, further work on the role of resident mucosal microbiomes (conjunctival, or gut-associated) or other factors in maintaining constitutive defenses or driving responsive processes, from the perspective of their impact on microbial colonization or on the host itself, will likely involve multiple areas of investigation. Moreover, some factors could be involved in multiple phenomena, and overlapping mechanisms might contribute. To this end, further studies are warranted to identify the key PRR-regulated factors that enable the in vivo cornea to be naturally resistant to infection. In this effort, it would be of value to consider the cell types with access to the environment at the surface of the cornea when intact and healthy. These include corneal CD11c-positive cells (Hamrah and Dana, 2007), which we have shown undergo morphological changes consistent with activation when the healthy cornea is challenged with P. aeruginosa in vivo; including extension of their processes to the epithelial surface after superficial injury to interact with, and clear, adherent bacteria (Metruccio et al., 2017).
There are some obvious candidates for downstream-regulated effectors of barrier function that have already been discussed, e.g. cell-cell junctions and antimicrobial peptides produced by cells in the cornea, and some of these factors are known to be calcium-dependent (see sections 2.3.5 and 2.3.6). While we have demonstrated contributions of tight junctions and antimicrobial factors (Alarcon et al., 2011; Augustin et al., 2011; Mun et al., 2009; Tam et al., 2012; Sullivan et al., 2015; Wan et al., 2018), there is likely much more to discover since the incredible efficiency of the corneal epithelial barrier is not fully explained by our current knowledge. Similar epithelial barriers line our other body surfaces and protect us against life-threatening infections. Thus, research in this area has the potential to be of broader significance beyond contact lens-related corneal infection.
2.4. The basal lamina
Disrupting only the epithelial barrier (e.g. via blotting and EGTA, or using IL-1R, MyD88 knockouts) does not actually result in infection. Explaining why not, our imaging data show that bacteria penetrating all the way through susceptible corneal epithelium accumulate against the underlying basement membrane (known in the cornea as the basal lamina) rather than entering the vulnerable stroma (Alarcon et al., 2011; Tam et al., 2011). Accordingly, animal models have shown that in the absence of contact lens wear, infection occurs in adult mice only if injury penetrates into the stroma (past the basal lamina), e.g. via scratch injury or intrastromal injection. This is true for virtually all microbes studied to date. The physical barrier role played by the basal lamina is even visibly apparent in scratch injury-related infections, as bacteria can be seen entering the corneal stroma in areas where the basal lamina is visibly disrupted, and are found trapped against it where it remains intact (Alarcon et al., 2009b).
The basal lamina is composed of extracellular matrix proteins including collagen and laminin, and it provides the foundation for overlying epithelial architecture and function in addition to forming a physical barrier (Torricelli et al., 2013). Relevant to its filtering function, it is topographically organized as a mesh with pores smaller than the size of most bacteria (< 0.2 μm versus ~1 μm, respectively). (Abrams et al., 2000). We have shown that the filtering effect of the basal lamina can be modeled in vitro using Matrigel™. Experiments utilizing multilayers of human corneal epithelial cells grown on Matrigel™-coated Transwell™ filters showed ~100-fold less bacteria accessing the basolateral compartment below the filter. Matrigel alone also provided a physical barrier to bacterial movement (Alarcon et al., 2009b).
In addition to its physical filtering function, the basal lamina appears to play a second role in barrier function. Cross-sectional imaging of P. aeruginosa-challenged cells grown on Matrigel™ showed that while many of the bacteria accumulated against the Matrigel™ layer, others accumulated at the epithelial surface (Alarcon et al., 2009b). Possibly, this relates to the role that basement membrane proteins play in modulating epithelial cell polarity and junctional integrity, which as discussed above could contribute to epithelial barrier function.
Thus, the basal lamina appears to be a key component of corneal epithelial defense against P. aeruginosa. Since its mechanism is based on size exclusion, it should function independently of antimicrobial resistance profile. Therefore, it is likely to protect broadly against multiple bacterial species. This “safety net” role likely explains why injury must be deep and penetrating to render a cornea susceptible to infection with virtually all types of bacteria in both experimental animal models and also in the human eye.
2.5. Conclusion
Research shows that the combination of blinking, tear fluid/flow, the corneal epithelium, and the basal lamina in the healthy eye work together with regulatory elements to form a formidable barrier protecting the vulnerable corneal stroma against microbial penetration (Fig. 3A). The data also suggest a significant amount of redundancy among these various defense layers and their function. The transparency of the corneal stroma depends on a highly organized arrangement of cells, collagen fibrils, glycosaminoglycans and carefully maintained levels of hydration. The provision of multiple “safety nets” to protect it against microbes aligns with the importance of stromal transparency in vision, and therefore survival.
3. How does P. aeruginosa interact with the non-lens wearing cornea?
Another topic foundational to knowing how contact lens wear enables corneal infection is understanding bacterial virulence capacity in the context of the tear film, corneal epithelium, and basal lamina. Our efforts in this area have focused almost exclusively on P. aeruginosa, because it continues to be the most common cause of contact lens-related corneal infections after five decades of soft contact lens wear. This focus has also enabled us to delve more deeply into mechanisms.
As discussed earlier, injury models of infection have been invaluable for deciphering host responses to infection and bacterial virulence factors contributing to bacterial persistence after infection is initiated. However, it is intuitive that models bypassing the epithelial and basal lamina barriers to deposit bacteria directly into the stroma have limited value for studying bacterial-host interactions that operate in the context of barrier components. While bacterial interactions with whatever remains of the epithelium might influence disease outcome, they are not necessarily relevant to how bacteria gained access to the stroma to initiate disease. Importantly, cells in an injured and infected epithelium might differ in their responses to bacteria compared to cells not injured in that manner and in a cornea not yet infected.
A separate problem complicating studies aimed at understanding bacterial virulence is that their physical presence (dead or alive) and associated ligands (e.g. LPS) can trigger host inflammatory responses typical of the disease process irrespective of what other virulence factors they possess (Liang et al., 2007; Schultz et al., 2000). Thus, injury models can make the role of some known bacterial virulence factors at other sites appear completely redundant (Preston et al., 1995). Accordingly, the use of injury models has led to the conclusion that surface-expressed bacterial components (LPS, flagellin, pili etc.) are the most important, whereas this may relate to their roles as PRR ligands rather than virulence factors per se. Elucidating bacterial virulence factors contributing to traversal of the epithelial/basal lamina barriers when susceptible (e.g. during pathogenesis of contact lens-related infection) requires use of different in vivo models and other experimental approaches. Clearly, the development and use of contact lens-wearing animal models (discussed below) advances tools to elucidate the relevant mechanisms involved. Using a mouse model, we recently showed that contact lens wear can enable intact-appearing epithelium in vivo to become susceptible to bacterial traversal (Metruccio et al., 2019).
3.1. In vitro cell culture models
As previously discussed, while the epithelial cells on the healthy corneal surface resist adhesion of even large inocula, the same cells become exquisitely vulnerable to P. aeruginosa virulence strategies if cultured in vitro (Fleiszig et al., 1995, 1996b, 1997a,1997b). Despite their greater vulnerability to bacteria, such cultured cells can be utilized, with carefully designed experiments, to understand bacterial capabilities when they encounter susceptible epithelial cells in vivo as occurs during contact lens wear (Metruccio et al., 2019).
Primary corneal epithelial cells can be cultured from animal and human donor corneas and subsequently passaged using well-established protocols (Cubitt et al., 1993; Gipson and Grill, 1982; O’Brien et al., 2006). Historically, preparing primary cell cultures from mice has been challenging, and used sparingly by comparison (Hazlett et al., 1996). However, updated protocols for maintaining mouse corneal epithelial cells may be useful for experiments to bridge findings between transgenic mouse lines and in vitro experiments (Kobayashi et al., 2009). An important advantage of primary cultured cells is that they survive longer in the presence of P. aeruginosa and its secreted exotoxins than immortalized cell lines (Goldufsky et al., 2015). Further, cultured primary corneal epithelial cells benefit from recent exposure to in vivo factors such as tear fluid with associated benefits described previously (Section 2.2). However, disadvantages of primary culture are that it is time-consuming, and more expensive and more difficult to control than other forms of cell culture. Exfoliating (sloughing) primary cells can also be collected from human subjects by irrigation (Fullard and Wilson, 1986). We and others have used these exfoliated cells to study P. aeruginosa adherence (Fleiszig et al., 1995; Ladage et al., 2002).
Other options for in vitro experiments include human corneal epithelial cells immortalized with either SV40 or telomerase for continued passaging (Araki-Sasaki et al., 1995; Kahn et al., 1993; Robertson et al., 2005). Development of a telomerase-immortalized human corneal epithelial cell line (hTCEpi) represented a considerable advance for in vitro cell culture models (Robertson et al., 2005). These cells can be grown on Transwell™ tissue culture inserts to a confluent monolayer, and after air-lifting for 7 days, differentiate into multilayered cultures with morphology resembling basal, wing, and squamous layers. Indeed, these cells were later used to reconstitute a denuded mouse cornea (Robertson et al., 2011a). We and others have used these, and other cell lines, to study P. aeruginosa virulence in vitro (see below), and to elucidate some of the corneal host defenses described above (Section 2.3).
3.2. Bacterial invasion of corneal epithelial cells
P. aeruginosa is able to bind to a wide range of biotic and abiotic surfaces (e.g. extracellular matrix proteins, glass, plastic, contact lenses etc.) owing to a large array of encoded adhesins (Alarcon et al., 2009b; Fletcher et al., 1993a, 1993b; Hazlett et al., 1993; Tran et al., 2011a; Willcox et al., 2001; Willcox, 2013b). However, it is less effective at adhering to the apical side of corneal epithelial cells, either in vitro or in vivo due to cell polarity-associated defenses (see Section 2.3.5). Time-lapse imaging of P. aeruginosa interacting with cultured cells shows that even when it does gain access to the basal surface of an epithelial cell in culture, most bacteria travel along the substratum on which the cell is grown rather than adhering to the overlying cell membrane (Fleiszig, 2006). Of those that do bind to corneal epithelial cells, some become intracellular.
It has been 25 years since we first demonstrated that P. aeruginosa can invade epithelial cells in vivo; first in a murine corneal infection model (Fleiszig et al., 1994a)() and subsequently in a lung infection model (Fleiszig et al., 1997a). While sometimes still referred to as an “extracellular” pathogen, we and others have since published a large body of work related to the mechanisms and significance of intracellular P. aeruginosa using corneal and other epithelial cell types (Angus et al., 2008, 2010; Heimer et al., 2013; Kroken et al., 2018; Sana et al., 2015; Zaidi et al., 1996, 1999). This includes a comprehensive study showing internalization and rapid intracellular replication rates for both clinical and laboratory isolates (Fleiszig et al., 1995). Importantly, we also showed that human corneal epithelial cells rinsed from the corneal surface internalized P. aeruginosa and supported its intracellular survival. This suggested that internalization by sloughing cells (which have lost their polarity) might be an intended defense mechanism to enclose bacteria, and in combination with tear fluid and blinking, clear them from the eye. As discussed in detail later, this could represent a problem if intracellular bacteria prevail in the eye within a stagnated epithelial cell.
Gentamicin protection (exclusion) assays can be used to quantify intracellular bacterial numbers over time. The basis for these assays is that intracellular bacteria are protected from the bactericidal action of aminoglycoside antibiotics such as gentamicin (or amikacin) which do not readily cross host cell plasma membranes. Using these assays, we and our collaborators found that P. aeruginosa internalization was mediated by the outer core of LPS, while host cells with defects in the CFTR (Cystic Fibrosis Transmembrane-conductance Regulator), as found in Cystic Fibrosis (CF), have a reduced capacity to internalize bacteria (Pier et al., 1996a,b; Zaidi et al., 1996). Defects in internalization were postulated as a mechanism for P. aeruginosa colonization in the CF airways (Pier et al., 1996a,b) where bacteria later persist extracellularly in thick biofilms (Høiby et al., 2010; Lyczak et al., 2002). However, in other circumstances, internalization into host cells can actually contribute to P. aeruginosa pathogenesis by providing bacteria a protective niche against extracellular antimicrobials and immune factors. Indeed, CFTR-mediated bacterial internalization contributes to P. aeruginosa persistence and disease in the injured murine cornea (Zaidi et al., 1999).
Since epithelial cell invasion provides a potential mechanism for P. aeruginosa to traverse the epithelium of mucosal barriers and persist in vivo, we have placed considerable effort into understanding mechanisms of entry and subsequent intracellular survival. For example, we have shown that P. aeruginosa internalization occurs preferentially through basolateral surfaces of epithelial cells, with apical surfaces of polarized cells being relatively resistant (Fleiszig et al., 1997b). Using host cell inhibitors, we also showed the involvement of protein tyrosine kinase activity, MEK-ERK signaling, calcium (Ca2+)-calmodulin signaling, and the actin cytoskeleton in P. aeruginosa internalization by corneal and other epithelial cells (Evans et al., 1998, 2002b, 2002c).
Furthering our understanding of bacterial factors involved, we have found roles for flagellar genes flhA (flagellar assembly), fliC (flagellin protein, ligand for TLR5) (Fleiszig et al., 2001), and fleQ, a regulator of flagellar gene expression as discussed below (Kroken et al., 2018).
Others have shown that P. aeruginosa internalization can involve phosphatidyl-inositol 3 kinase (PI3K)/Akt (Kierbel et al., 2005), with this pathway helping disrupt epithelial cell polarity to promote internalization (Kierbel et al., 2007). Lipid rafts on host cell membranes have been shown to be involved (Yamamoto et al., 2005), the mechanism for involvement associated with CFTR (Zaidi et al., 2008). Furthermore, apical N-glycans or basolateral heparan-sulfate proteoglycans can serve as receptors for P. aeruginosa internalization (Bucior et al., 2010), with pili or flagella acting as their respective ligands (Bucior et al., 2012). Others have also shown P. aeruginosa internalization involves a host surface glycolipid Gb3 (Eierhoff et al., 2014), and microtubule stimulation by a Type 6 Secretion System (H2-T6SS) effector (VgrG2b) (Sana et al., 2015). Thus, P. aeruginosa internalization is a complex event involving multiple bacterial ligands and host cell surface receptors.
Shortly after discovering that P. aeruginosa invaded corneal epithelial cells, we examined a collection of 10 clinical isolates for this capacity. Strikingly, the results showed that the strains varied widely in their capacity to invade, and this inversely correlated with their capacity to be acutely cytotoxic (Fleiszig et al., 1996b). This observation using corneal infection isolates initially caused a good deal of intrigue and debate. However, it was to make a significant contribution to the discovery of the P. aeruginosa Type 3 Secretion System (T3SS) and its currently known effector functions. In collaboration with several groups in Pseudomonas research, it became evident that P. aeruginosa strains could be classified as invasive or cytotoxic towards host cells depending on which toxins were expressed by the T3SS (Fleiszig et al., 1997a). Today, the T3SS is known to be the most significant virulence determinant of P. aeruginosa as a pathogen in general. Thus, the history behind these discoveries, and the role of the T3SS in virulence, are explained in detail in the next section.
3.3. The type three secretion system (T3SS)
Type 3 Secretion Systems are used broadly by Gram-negative bacteria to deliver effector toxins with anti-host enzymatic activity. The T3SS apparatus consists of a molecular syringe to enable export of the T3SS effector toxins across both the inner and outer membranes of a Gram-negative bacterium. These effectors are then either secreted extracellularly or they can be injected across a host cell membrane utilizing the pore forming capacity of T3SS associated translocon proteins.
The earliest work related to the T3SS in P. aeruginosa was identification of a second source of ADP ribosyltransferase (ADPr) activity distinct from the previously discovered Exotoxin A (Iglewski et al., 1978). That toxin was named ExoS, and it turned out to be a bifunctional enzyme with a N-terminal RhoGTPase Activating Protein (RhoGAP) domain and a “promiscuous” C-terminal ADPr domain that targets a number of different host proteins (see review by Barbieri and Sun, 2004). That ExoS secretion was via a T3SS was discovered much later by random transposon mutation, with research showing that mutants deficient in ExoS had insertions into genes with high homology to the then newly discovered Yersinia spp. T3SS apparatus proteins () (Yahr et al., 1996b). A discovery made around the same time showed that most strains of P. aeruginosa also secrete ExoT, an immunologically-related effector 75% identical to ExoS with a biochemically identical RhoGAP domain, but divergent ADPr domain (Yahr et al., 1996a) [Note: ExoT was originally thought to be a higher molecular weight form of ExoS due to the challenge in generating specific antibodies to each exoenzyme (Nicas and Iglewski, 1984)]. At this point in time, others had already shown that P. aeruginosa could be acutely cytotoxic towards cultured epithelial (MDCK) cells (Apodaca et al., 1995). The inverse correlation between P. aeruginosa internalization and acute cytotoxicity among clinical isolates (Fleiszig et al., 1996b), and a fruitful collaborative effort utilizing our bank of corneal isolates, lead to discovery of a third effector ExoU, a potent cytotoxin (Finck-Barbançon et al., 1997). ExoU was later shown to have patatin-like phospholipase activity (Sato et al., 2003). Our collaboration also provided first insights into functional roles of ExoT, showing contribution to cytotoxic activity and inhibition of internalization, each similar functions to ExoS (see review by Barbieri and Sun, 2004). Importantly, we also showed that invasive P. aeruginosa isolates express ExoS, but do not encode ExoU, with the opposite generally true for cytotoxic P. aeruginosa (Fleiszig et al., 1997a). Later, a fourth T3SS effector (ExoY) was discovered, a nucleotidyl cyclase that can elevate intracellular cyclic AMP (Yahr et al., 1998). All four known T3SS effectors require mammalian cytosolic cofactor proteins for activity: 14–3-3 proteins for ExoS and ExoT, actin for ExoY, and ubiquitin for ExoU (Anderson et al., 2011; Belyy et al., 2016; Fu et al., 1993).
Work done by others using isolates from a wide range of infection sites have since confirmed our results with corneal isolates, showing that nearly all P. aeruginosa strains encode ExoT, around 90% encode ExoY, and that ExoS and ExoU are usually mutually exclusive with only rare isolates containing both (Feltman et al., 2001).
On the other hand, P. aeruginosa isolates from corneal infections do show some differences in T3SS effector distribution versus those from other sites, with keratitis isolates more often favoring epidemic clones of cytotoxic strains that encode ExoU (Lomholt et al., 2001), which also express different protease profiles (discussed further below). This was also shown in another collection of corneal isolates with ExoU encoded in > 50% of the strains (Stewart et al., 2011; Winstanley et al., 2005). Fewer ExoU encoding isolates were found among 101 P. aeruginosa strains isolated from the Steroids for Corneal Ulcers Trial (SCUT), with only 18 encoding ExoU, 56 encoding ExoS, and 27 encoding both exoenzymes or neither (Borkar et al., 2013). The fewer ExoU-bearing keratitis isolates found in the SCUT study may reflect the minimal number of contact lens-related cases, the latter known to favor ExoU-bearing isolates (Choy et al., 2008; Shen et al., 2012). Selection of ExoU-encoding isolates in contact lens-related corneal infections may relate to the use of contact lens disinfection solutions for which ExoU-encoding isolates show higher levels of resistance (Lakkis and Fleiszig, 2001), or it might relate to the fact that they are generally more resistant to certain antimicrobials, e.g. fluoroquinolones (Agnello and Wong-Beringer, 2012; Borkar et al., 2014; Sawa et al., 2014; Zhu et al., 2006).
Discovery of ExsA as the transcriptional activator of the T3SS (Frank et al., 1994; Yahr and Frank, 1994), and generation of mutants in exsA and/or one or more effectors, allowed the various roles of the T3SS in P. aeruginosa pathogenesis to be elucidated. Many in vitro and in vivo studies have since demonstrated the biological activities of the T3SS and their impact on the host. All four effectors, and the machinery (needle and translocon pore proteins) required for intracellular effector delivery, exert an effect on P. aeruginosa virulence at some level. These studies have been summarized and synthesized in excellent reviews to which we refer the reader (Engel and Balachandran, 2009; Hauser, 2009). Here, we will focus only on T3SS effects in the context of the cornea.
We performed the first studies to explore the role of the T3SS in bacterial uptake by cells. Those experiments utilized exsA mutants lacking the entire T3SS system, before the system had been discovered. The results showed that these mutants were better able to invade epithelial cells than wild-type P. aeruginosa, showing that ExsA regulated antiphagocytic activity (Evans et al., 1998). Once the T3SS had been identified, we used mutants in ExsA-regulated factors to show that expression of either ExoT or ExoS alone (without other T3SS effectors) inhibited the capacity of P. aeruginosa to invade corneal epithelial cells (Cowell et al., 2000). The latter study also suggested that the RhoGAP domain was required since anti-internalization activity was retained by an ADPr activity mutant of ExoS. Subsequently, others showed that the RhoGAP domains of ExoT and ExoS were required for this activity (see Barbieri and Sun, 2004; Garrity-Ryan et al., 2000). For ExoS, the ADPr activity has additionally been shown to block receptor-mediated endocytosis in other cell types (Deng and Barbieri, 2008). When ExoY was later discovered as a T3SS effector encoded by most strains, we showed that its adenylate cyclase activity also had anti-phagocytic impact for (corneal) epithelial cells (Cowell et al., 2005).
We also published the first study to show the importance of T3SS effectors in an in vivo system with results revealing that either ExoU or ExoT were sufficient to promote P. aeruginosa virulence in the murine scarification model (Lee et al., 2003a). We additionally used the scratch-heal model (described above) to show that the T3SS regulator ExsA was required for a cytotoxic strain (ExoU encoding), but not an invasive strain (ExoS encoding) to traverse the healing corneal epithelium (Lee et al., 2003b). With the invasive strain, increased healing time actually allowed more bacterial colonization of the epithelium. Later, others confirmed the importance of the T3SS in the lung and other tissues (Lee et al., 2005; Vance et al., 2005), and we showed that the phospholipase activity of ExoU was required for cytotoxic strain virulence in the scarification model (Tam et al., 2007).
More recently, we have used the blotting/EGTA treatment method (described previously) to study the role of the T3SS in epithelial traversal by an invasive strain and found that the results differed from the scratch-heal model, illustrating the importance of using the right model to study specific mechanisms. The data showed that, even for an invasive strain (ExoS encoding), ExsA was required for bacteria to traverse the corneal epithelium (Sullivan et al., 2015). Also showing that virulence can depend on how one enables susceptibility, ExsA was again dispensable when we used MyD88 gene-knockout mice, as it was for the scratch-heal model (Sullivan et al., 2015).
We have also explored the contributions of individual T3SS effectors in epithelial traversal. Results showed that, for cytotoxic strains, ExoU and its phospholipase activity contribute to corneal epithelial traversal in vitro (Ramirez et al., 2012), while ExoU and ExoT both promote P. aeruginosa survival in the murine scarification model by modulating either phagocyte viability, infiltration, or other functions (Zolfaghar et al., 2006). Later studies done by others using the murine scarification model of keratitis showed P. aeruginosa survival was favored by promotion of neutrophil apoptosis via ExoS and ExoT ADPr activity (Y. Sun et al., 2012), and that ExoS-mediated ADP-ribosylation of Ras promoted bacterial survival in the cornea by blocking neutrophil oxidative burst (Vareechon et al., 2017). Data we have yet to publish suggest that multiple T3SS effectors contribute specifically to corneal epithelial traversal in vitro and in vivo.
Our in vitro cell culture models have also shed light on why invasive strains encode ExoS. In demonstrating that P. aeruginosa can invade epithelial cells, we showed it could survive and replicate inside the cells for extended time periods (Fleiszig et al., 1995) dependent on intact LPS (Evans et al., 2002a). Subsequently, we showed that intracellular P. aeruginosa form membrane blebs in the cells which they then occupy (bleb-niches), and swim inside using flagellar-mediated motility (Angus et al., 2008), a phenomenon that also occurs in vivo in mouse corneal infection models (Tam et al., 2011) (Fig. 4). The generation of membrane blebs by P. aeruginosa, and its intracellular survival, depend on the T3SS largely as a result of ExoS (Angus et al., 2008, 2010). Importantly, typical cytotoxic strains adopt the same phenotype if engineered to express ExoS instead of ExoU (Angus et al., 2008, 2010), showing the requirement of ExoS versus ExoU for enabling this virulence strategy.
Fig. 4.
Images of a P. aeruginosa-induced membrane bleb in the murine corneal epithelium ex vivo obtained using transgenic mice expressing CFP-labeled membranes (cyan) (Melichar et al., 2011). A) Bacterial-induced membrane bleb shown as a spherical membrane projection (arrow) extending away from the epithelial cells. Representative view shown in the xz plane. B) Higher magnification image revealing a bleb-confined, GFP-labeled, P. aeruginosa bacterium (green) (Reproduced from Tam et al., 2011).
The profound impact of the T3SS in vivo during infection is clinically evident. Some of the effectors can influence the infiltration of immune cells to cause a visible phenomenon called ring infiltration in mouse corneas (Zolfaghar et al., 2006), a feature often occurring during infections in people. Different T3SS genotypes have also been shown to correlate with differences in visual outcome in infected people. For example, our involvement in the SCUT study allowed us to illustrate that ulcers involving cytotoxic strains initially presented with worse visual acuity, but that those involving invasive strains showed less improvement after three months (Borkar et al., 2013). This correlated with differences in visible pathology reflecting known impacts of their encoded T3SS effectors. Similarly, responses to therapeutics (antibiotics and corticosteroids) in these people were predictable based upon known roles of encoded effectors. Another study also showed that keratitis caused by invasive strains was associated with poorer prognosis with regard to best corrected visual acuity (Shen et al., 2014).
Thus, we have gone from bedside (collecting corneal isolates) to the bench (dissecting apart the T3SS and its specific activities) and back again (comparing isolates to disease outcome and therapeutic responses). This journey into discovery related to the P. aeruginosa T3SS provides an example of how basic mechanistic research can eventually translate to a better understanding and management of human disease, even without developing new therapeutic strategies. It also shows that corneal research with its unique advantages (e.g. clarity and accessibility) can turn out to be relevant to diseases beyond the eye. There are now a number of potential therapies aimed at the T3SS in clinical trials for treating infections caused by this life-threatening pathogen that is rapidly developing resistance to all currently available antibiotics. Even if these become available for use in the cornea, we will need to remain cognizant that the role of the T3SS is complex and depends on how the cornea becomes susceptible to infection.
3.4. Intracellular survival and replication of P. aeruginosa
Compared to traditionally recognized intracellular pathogens (e.g., Listeria spp., Salmonella spp., and Shigella spp.), the intracellular lifestyle of P. aeruginosa has received much less attention largely because of the persistent dogma that it is exclusively an extracellular pathogen. This dogma exists despite a growing body of literature published by us and others demonstrating its intracellular capacities that takes the evidence far beyond what has been shown for other facultative intracellular pathogens (discussed above). This includes movies showing bacteria localized, replicating and motile inside cells using methods that conclusively demonstrate that they are not extracellular, and also illustrating that the host cell can remain alive during the process (Kroken et al., 2018; Nieto et al., 2019). Other publications show that this also occurs in vivo during infection (Tam et al., 2011).
An unfortunate byproduct of this persistent dogma is that little is known about what transpires intracellularly apart from our own research efforts in this area. What we do know is that the T3SS (and importantly the ADPr activity of ExoS) is used for both intracellular survival/replication, and for the formation of the membrane bleb-niches in both corneal and other epithelial cells (Angus et al., 2010, 2008). We have also shown that these blebs can disconnect from the cell while continuing to exclude gentamicin, showing that the membrane remains intact (and bacteria alive) throughout that process. This suggests that membrane bleb formation is a strategy by which P. aeruginosa might disseminate within tissues whilst inside a protected niche.
ExoS ADPr-mediated intracellular survival/replication can also occur without bleb-niche formation. We showed this using translocon (PopB) mutants that cannot inject T3SS effectors across host membranes, the results showing that the bacteria remain within intracellular vacuoles (Hritonenko et al., 2012). Illustrating continued importance in intracellular survival, additional mutation of ExoS renders PopB mutants unable to thrive inside cells. ExsA mutants, or the sub-population of wild-type bacteria that do not/cannot activate the T3SS, remain trapped within acidified membrane-bound vacuoles that limit their intracellular replication (Heimer et al., 2013). We have also established that: 1) the adenylate cyclase activity of ExoY can also induce membrane bleb formation in corneal epithelial cells, but without promoting intracellular survival (Hritonenko et al., 2011), and 2) ExoS-induced bleb formation is driven by osmotic forces that are usually opposed by functional CFTR, i.e. inhibition or mutation of CFTR (as occurs in CF) promotes bleb formation and size (Jolly et al., 2015).
Recently, we compared the internalization and intracellular survival/replication of four commonly used P. aeruginosa strains in relation to T3SS (and ExoS) expression, and the results reconcile differences of opinion regarding P. aeruginosa internalization, and the apparent paradox of invasive isolates of P. aeruginosa expressing effectors (ExoS, ExoT) with RhoGAP-mediated anti-internalization activity (see above) (Kroken et al., 2018). Briefly, our findings showed that invasive P. aeruginosa isolates are internalized into epithelial cells despite their ability to encode effectors with anti-internalization activity (ExoS, ExoT, ExoY) due to differences in the bistability of the T3SS, i.e. different levels of T3SS expression within a given population as they encounter an epithelial cell. Bacteria with high levels of T3SS expression remain extracellular, while low level T3SS expression allows internalization, with subsequent intracellular expression driving survival/replication and bleb-niche formation (Kroken et al., 2018). The results, moreover, suggest that the dogma P. aeruginosa is strictly an extracellular pathogen has been fed by the extensive use of strain PA103 for in vitro studies of P. aeruginosa-host cell interactions, particularly those focused on the T3SS. Strain PA103 has a mutation in fleQ that prevents flagellum expression, which we showed reduces bacterial internalization. We further showed that it has a T3SS bistability set-point (unrelated to fleQ mutation) that drives expression towards very high levels in a given population compared to other P. aeruginosa isolates. When combined with use of a double effector null (exoUexoT) mutant of PA103 to deliver ExoS into epithelial cells (as is commonly done to study ExoS function), the result is a nearly total absence of intracellular bacteria (Kroken et al., 2018). This is not generally the case for P. aeruginosa isolates. Clearly, these data do not preclude significant T3SS-mediated effects on host cells mediated by extracellular bacteria, but they do support a further role(s) for the T3SS in the intracellular pathogenesis of P. aeruginosa in the cornea, and at other tissue sites, and help explain the diverse viewpoints (reconcile apparently contradictory information) about P. aeruginosa internalization.
It is also worth mentioning here that much of the above information regarding internalization and fate of intracellular P. aeruginosa resulted from a commitment to imaging, particularly the use of live cells, time-lapse, and novel strategies for quantitative analysis of those results. Although expensive and relatively time-consuming, live imaging has allowed us to obtain invaluable insights into individual host cell/bacterial invasion events over an extended time. It also enabled us to discover phenomena that do not survive washing and fixation steps, such as membrane bleb-niches (Jolly et al., 2015; Kroken et al., 2018). A remaining challenge that will need to be overcome is that the use of fluorescent proteins for labeling bacteria and monitoring gene expression requires bacteria to make fluorescent proteins, which draws energy and resources away from other functions and can potentially modify bacterial virulence. Further efforts to improve upon methods that monitor host-microbe interactions will help unravel the biology of infection and might eventually result in strategies for visualizing infection in detail in the context of human patients.
3.5. Bacterial motility
P. aeruginosa can utilize multiple strategies to be motile including twitching, swimming, swarming, and sliding. Twitching motility is a form of surface-associated movement facilitated by extension and retraction of type IV pili (T4P) (Burrows, 2012; Mattick, 2002), a process reminiscent of walking. In contrast, swimming occurs in solution dependent on rotation of this bacterium’s single flagellum (Blair, 2003; Dasgupta et al., 2003). Swarming and sliding are less well-studied phenomena that occur if bacteria are grown on a semisolid surface, with swarming requiring both pili and flagella, and believed to be a hybrid of swimming and twitching (Köhler et al., 2000). In contrast, sliding motility occurs in the absence of both pili and flagella under conditions that promote swarming (Murray and Kazmierczak, 2008). For obvious reasons, motility directly impacts P. aeruginosa interactions with corneal components in vitro and in vivo.
The type IV pili that enable twitching motility are composed of pilin protein (PilA), with extension requiring PilB and retraction dependent on PilT and PilU, ATPases that antagonistically polymerize and depolymerize PilA, respectively (Mattick, 2002). In vitro, we have found that twitching is used for P. aeruginosa to spread out under a cell layer (Fleiszig, 2006), for penetrating corneal epithelial cell multilayers, and for exiting invaded corneal epithelial cells (Alarcon et al., 2009a). More recently, we have discovered that P. aeruginosa can use twitching motility to move intracellularly (Nieto et al., 2019), with mutants lacking twitching capacity accumulating as sessile aggregates in the host cell cytoplasm, reminiscent of biofilm formation. In vivo, twitching is also used to spread along collagen fibrils (Robertson et al., 2017; Tam et al., 2011). Accordingly, twitching contributes to disease severity in vivo, with mutants defective in twitching but still expressing pili causing disease that is more discretely localized within the cornea (Zolfaghar et al., 2003)(). Mutants in retS, a global virulence regulator discovered by our group, also showed loss of twitching motility, with reduced pilin production, and attenuated association with, and invasion of, corneal epithelial cells (Zolfaghar et al., 2005). In vitro experiments by others have additionally shown that type IV pili and twitching function can play roles in adhesion to cells and in cytotoxic activity (Comolli et al., 2002), the latter involving complex cross-regulation of T3SS expression (Burrows, 2012; Diaz et al., 2011; Persat et al., 2015). Type IV pili can also promote adhesion to inanimate surfaces, including contact lenses (Fletcher et al., 1993b; Rudner et al., 1992). To add further complexity to this topic, bacterial surface structures (including both pili and flagella) are recognized by host PRR’s, and thereby can drive immune responses to either decrease or increase disease severity depending on circumstances. Fortunately, the availability of mutants lacking twitching, but possessing pili, have enabled separation of function (virulence factor versus ligand for host responses) to some extent, while also allowing roles of motility to be separated from structural properties. Given the importance of twitching motility to P. aeruginosa virulence in the cornea, it is perhaps not surprising that human tear fluid can alter the expression of a multitude of genes known to regulate motility (unpublished data courtesy of Dr. Melinda Grosser, Fleiszig laboratory). We recently identified the abundant tear fluid glycoprotein DMBT1 as an inhibitor of P. aeruginosa twitching motility that can bind to pili, and reduce bacterial virulence in vivo (Li et al., 2017). Interestingly, we found that twitching motility inhibition by DMBT1 is independent of its binding to pili and instead involves its N-glycosylation. It is also independent of the DMBT1 Scavenger Receptor Cysteine-Rich (SRCR) peptide domain that binds other bacteria, including Streptococcus spp. (Li et al., 2019).
Swimming motility in aqueous environments is accomplished by rotation of a single polar flagellum, composed of flagellin (FliC), as a means for propelling the bacteria towards nutrients or away from hazards. Similar to twitching, there is highly complex regulation of swimming function, with chemical sensors (chemotaxis machinery) enabling rotation of the flagellum to influence trajectory changes by altering forward running (counter-clockwise rotation) to reverse running (clockwise rotation) (Meliani and Bensoltane, 2017; Sampedro et al., 2014; Vater et al., 2014). The roles of the flagellum and its swimming motility function in driving virulence have been difficult to discern given that it is a ligand for the PRR TLR5. During P. aeruginosa infection, TLR5 plays major role in regulating both host defense against infection (Metruccio et al., 2017), and also responses to infection (Kumar et al., 2008; Zhang et al., 2003), confounding the results of experiments using flagellin and flagellin mutants. In this regard, while we have found that flagellin (FliC), and its associated regulators, are involved in bacterial-epithelial cell interactions in vitro, impacting adhesion to and invasion of these cells (Fleiszig et al., 2001), it remains unclear if the mechanisms go beyond host cell recognition of flagellin to swimming motility, or structural function, because in vitro grown corneal epithelial cells also express TLR5 that can respond to flagellin.
3.6. Other P. aeruginosa virulence determinants
While the T3SS plays major roles in P. aeruginosa virulence, results using T3SS-negative P. aeruginosa isolates have shown that other factors additionally contribute to virulence, even in the scratch-injury model (Toska et al., 2014; Zolfaghar et al., 2005). Furthermore, factors that are not required in injury models might contribute to infection under other conditions of susceptibility, e.g. when it is contact lens-induced.
3.6.1. Lipopolysaccharide
Few would argue the importance of lipopolysaccharide (LPS) to the virulence of P. aeruginosa, or other Gram-negative bacteria. As a key component of the outer membrane of Gram-negative bacteria, LPS plays a crucial role in maintaining the integrity of the bacterial cell envelope, and the permeability barrier function of the outer membrane, e.g. against antimicrobials (Nikaido, 2003). The endotoxin component of LPS, lipid A, is also notorious for its potent role in Gram-negative sepsis (Morrison and Ryan, 1987). For P. aeruginosa, each major component of LPS (lipid A, core oligosaccharides, and O antigens) play important roles in virulence and the induction of inflammation in host tissues (Pier, 2007), the latter primarily via TLR4 interactions as previously discussed (Section 2.3). In addition to its contribution as a PAMP, however, LPS also contributes to pathogenesis of P. aeruginosa keratitis. Mechanisms include: mediating adherence to contact lenses and corneal epithelium (Fletcher et al., 1993a), binding to CFTR to promote bacterial internalization into corneal epithelial cells (Zaidi et al., 1996, 1999), and promoting survival of intracellular P. aeruginosa (Evans et al., 2002a). As such, LPS has been, and remains, a potential target for therapeutic or preventive intervention (Pier, 2007; Welsh et al., 1984).
3.6.2. Exotoxin A
While our research group has not studied exotoxin A, it is a well-established P. aeruginosa virulence factor with known impacts on host cells, and therefore could potentially be relevant to the pathogenesis of contact lens-related infection. Exotoxin A is an AB-type toxin with ADPr activity that targets host elongation factor 2 to disrupt protein synthesis (Allured et al., 1986; Iglewski et al., 1977b). Mechanistically similar to diphtheria toxin (albeit using a different cell surface receptor), it is similarly produced under iron-limiting conditions (Frank and Storey, 1994). In the cornea, direct injection of purified exotoxin A into the corneal stroma in rabbits caused dose-dependent pathology attributed to widespread cell death (Iglewski et al., 1977a). While exotoxin A deficient mutants adhered to injured corneas and induced keratitis just as efficiently as wild-type bacteria, they were more readily cleared suggesting exotoxin A helps P. aeruginosa persist in the cornea once infection is already established (Pillar and Hobden, 2002). However, like all virulence factors, the role of exotoxin A can be complicated to study in vivo, which needs consideration when designing experiments and interpreting results. For example, in whole rabbit corneas, exotoxin A inhibits synthesis of many proteins including host Matrix Metallo-Proteinase 9 (MMP9), while activating other host MMPs (Twining et al., 1993). It is also degraded by P. aeruginosa proteases, which has driven some to only use strains producing less protease than typical isolates (Liu, 1973). In vitro, exotoxin A diminished barrier function of epithelial cell monolayers, and exhibited a complementary role with P. aeruginosa proteases (discussed below) in facilitating P. aeruginosa traversal (Azghani, 1996). As such, further studies under different conditions of host susceptibility could be useful to determine the significance of exotoxin A in the establishment of P. aeruginosa keratitis.
3.6.3. Bacterial proteases
P. aeruginosa encodes numerous protease enzymes that are obvious candidates for how it might overcome epithelial barrier function when given the opportunity. Analysis of P. aeruginosa strain PAO1 predicted 155 proteases: ~2.8% of its genome (Hoge et al., 2010). Of these, elastase B (or LasB) is among the best studied. A zinc metalloprotease with structural similarity and 28% sequence identity with thermolysin (Thayer et al., 1991), LasB can degrade host elastin (Wretlind and Pavlovskis, 1983), cell-cell junctions (Golovkine et al., 2014), immunoglobulin G (Holder and Wheeler, 1984), SP-A and SP-D (Mariencheck et al., 2003; Mun et al., 2009), cytokines (Parmely et al., 1990), and complement components (Hong and Ghebrehiwet, 1992). Bacterial proteases have direct effects on tissue, but most have weak activity against mammalian collagen, a major component of the corneal stroma (Okamoto et al., 1997). Elastase B can degrade collagen III and IV, but has limited activity on collagen I, and none versus collagens II and V (Heck et al., 1986b). In the human cornea, Bowman’s membrane is comprised of collagen IV, and the stroma comprised predominantly of collagen I and ~10% collagen V (Bailey, 1987).
As previously discussed, the corneal epithelial basal lamina acts as a “filter” with pores too small for bacteria to cross (Abrams et al., 2000; Alarcon et al., 2009b). Our studies using in vitro grown cells cultured on Matrigel™ (artificial basement membrane) have shown that wild-type P. aeruginosa does have a “low level” capacity to penetrate a basement membrane (at least in vitro) dependent on LasB, with LasB mutants lacking this ability altogether (Alarcon et al., 2009b). The reason why wild-type does not generally penetrate the corneal basal lamina in vivo utilizing LasB might relate to the regulation of its expression by quorum sensing (Pearson et al., 1997), with the latter thought to require a relatively large number of bacteria to be in close enough proximity to be able to detect each other’s chemical signals. As such, quorum sensing might be restricted in the context of the tightly-packed intact corneal epithelium in vivo. Relevant here, it has been shown that very low numbers of bacteria can activate quorum sensing in a microfluidic environment, albeit with considerable variability (Boedicker et al., 2009), but the significance of that finding in epithelia, particularly in vivo, is unknown. When it is able to gain access into the corneal stroma, a location less densely packed with cells, P. aeruginosa spreads laterally, oriented to collagen lamellae (Robertson et al., 2017; Tam et al., 2011). Since LasB has limited activity against collagen types found in the corneal stroma, stromal necrosis during keratitis likely involves the activation of other bacterial proteases or host matrix metalloproteinases (MMPs) (Okamoto et al., 1997).
The protease profiles of individual P. aeruginosa strains are diverse, making this another difficult topic to study. Prior to widely-available sequencing methodology, most proteases were characterized by their molecular weight on zymogram or other enzymatic assay, so our knowledge about specific proteases correlates with those more readily detected in that way. Other secreted proteases identified in P. aeruginosa that have been studied include; Elastase A (LasA), Alkaline Protease (AprA), Protease IV, P. aeruginosa small protease (PASP), and LepA. LasA acts synergistically with LasB (Peters et al., 1992), but must be activated by LasB (or AprA). AprA can degrade laminin, a key component of the basal lamina (Heck et al., 1986a), and can also degrade complement, similarly to LasB (Hong and Ghebrehiwet, 1992). LasA (and LasB) are secreted by a Type Two Secretion System, and AprA by a Type One Secretion System (Hoge et al., 2010). Protease IV, a serine protease, was initially discovered in strain PA103, which does not appreciably produce LasA, LasB or AprA. The lack of these three proteases (and flagellin) in a strain that remains fully virulent in the cornea highlights redundancies in function among P. aeruginosa proteases. For PA103, protease IV contributes substantially to P. aeruginosa keratitis in rabbit intrastromal injection and mouse scratch-injury models (Engel et al., 1997; O’Callaghan et al., 1996; Twining et al., 1993), is capable of degrading host immunological proteins (Engel et al., 1998), and is conserved across many P. aeruginosa strains (Caballero et al., 2004), appearing to be universally found in clinical isolates. Interestingly, sequence variations of up to 2.5% segregate into two groups correlating with expression of ExoS or ExoU (Conibear et al., 2012).
Another protease discovered in strain PA103 is PASP, capable of cleaving collagen I, and when purified, causing the destruction of rabbit corneas after intrastromal injection (Marquart et al., 2005). It is widely encoded by both cytotoxic and invasive P. aeruginosa strains (Tang et al., 2009). LepA is a relatively newly identified protease, not yet studied in a keratitis model. It activates protease activated receptor 2 (PAR-2) on host cells (Kida et al., 2008), and appears to co-operate with hemolytic phospholipase C (PlcH) in contributing to P. aeruginosa growth and virulence in a murine model of systemic infection (Kida et al., 2011).
Liquefactive necrosis of the corneal stroma is a hallmark of P. aeruginosa keratitis. As outlined above, it has long been known that purified proteases injected into the corneal stroma can cause tissue necrosis and keratitis (Kreger and Gray, 1978). However, results from rabbit and mouse in vivo models using different protease mutants have shown variable to no effects on virulence (Hobden, 2002; Preston et al., 1997). This is likely due to redundancy among bacterial virulence factors (e.g. 155 proteases). Supporting this in the cornea, exogenous expression of LasB or AprA from P. aeruginosa into P. putida (normally not virulent in the cornea) enabled disease in a rabbit intrastromal injection model (Thibodeaux et al., 2007). Another consideration is that production of virulence factors is usually regulated by complex systems to be expressed only when needed, and it has been demonstrated that some strains encode proteases that are not expressed during corneal infection (Kernacki et al., 1995).
Review of the published literature reveals that most studies on the topic of P. aeruginosa proteases in the context of the cornea relate to their impact within the stroma. An exception is one of our studies that showed a role for P. aeruginosa proteases in compromising ocular surface clearance mechanisms in healthy mouse eyes, likely via SP-D degradation (Mun et al., 2009). Given their potential to degrade host factors, it would be of interest to examine if they play other roles, given sufficient time, in overcoming other protective functions of the tear fluid, and enable the bacteria to traverse corneal epithelial or basal lamina barriers in vivo. Indeed, in vitro experimentation has shown roles for P. aeruginosa proteases in compromising both epithelial and basement membrane barrier function (Alarcon et al., 2009b; Azghani, 1996; Azghani et al., 1993). Further, multiple tear fluid protective effects are lost after prolonged exposure to P. aeruginosa in vitro (Fleiszig et al., 2003), with protease degradation being a likely potential mechanism. Also important to consider is our published data showing that P. aeruginosa-derived proteases (e.g. LasB) impact the production of T3SS effectors (Cowell et al., 2003), so potentially might impact virulence indirectly in addition to directly compromising host defenses.
3.6.4. Biofilm formation
It has long been known that P. aeruginosa (and many other bacteria) can utilize biofilm formation to promote their survival in a diverse array of environments. Biofilms are surface-associated bacterial microcolonies, surrounded by a matrix containing exopolysaccharides, proteins, extracellular DNA, and other bacterial-derived factors (Hall-Stoodley et al., 2004; López et al., 2010; Whiteley et al., 2001). In the course of human infections, P. aeruginosa biofilms are associated with persistent colonization of (and dispersal from) indwelling medical devices, and other biomaterials, and the chronic colonization of CF airways (Cole et al., 2014; Høiby et al., 2010; Mulcahy et al., 2014; Wagner and Iglewski, 2008). Biofilm growth allows both phenotypic and genotypic changes that promote bacterial resistance to antimicrobials, host immune defenses, and other forms of potential adversity (Drenkard and Ausubel, 2002; Evans et al., 1991; Hoffman et al., 2005; Mah et al., 2003; Meluleni et al., 1995; Thanabalasuriar et al., 2019; Whiteley et al., 2001; Winstanley et al., 2016). Since the relationship between biofilms and P. aeruginosa virulence is a very active and extensive research field, the reader is referred to references cited, and many others in the literature, for further general information.
Given the importance of biofilms in other systems, it is perhaps not surprising that biofilms have been of considerable interest to researchers studying the pathogenesis of contact lens-related P. aeruginosa keratitis. Results show that P. aeruginosa, and many other environmental and host-derived bacteria, readily form biofilms in contact lens storage cases, from which they can colonize contact lenses and enter the eye (Willcox et al., 2001; Willcox, 2013b; Wu et al., 2015b). Moreover, in contact lens-wearing animal models of corneal infection, bacterial lens colonization and biofilm formation can readily occur in vivo (Tam et al., 2010; Metruccio et al., 2019). The potential significance of biofilm formation in contact lens-related P. aeruginosa keratitis is discussed in other sections below (see Sections 4.5 and 4.6).
3.6.5. Second messenger signaling
Bacterial second messengers control expression of genes involved in lifestyle transitions of bacteria, e.g. by promoting biofilm production (Hickman et al., 2005), modulating motility (Paul et al., 2010), and influencing virulence factor expression (Persat et al., 2015). Cyclic di-GMP (c-di-GMP) is an intracellular signaling molecule that coordinates the switch from planktonic to sessile bacteria as the bacterium encounters changes to its environment, specifically, contact with a surface. Levels of c-di-GMP are maintained inside bacterial cells via synthesis and degradation by diguanylate cyclases (DGCs) and phosphodiesterases (PDEs) respectively (Ryjenkov et al., 2005; Schmidt et al., 2005). After a bacterial population has attached to a surface, an increase of c-di-GMP by DGCs decreases bacterial metabolism, and increases the production of extracellular matrix components (exopolysaccharides, proteins, extracellular DNA) to form the biofilm (Allesen-Holm et al., 2006; Fong and Yildiz, 2015). P. aeruginosa has multiple DGCs that promote surface-associated growth: SiaD, SadC, RoeA, WspR, and YfiN/TpbB; while biofilm dispersal is facilitated by DGCs: GcbA and NicD, or PDEs: DipA, NbdA, and RbdA (Valentini and Filloux, 2016). C-di-GMP can bind effector proteins, such as the transcriptional regulator, FleQ in P. aeruginosa, to directly act on specific targets. For example, at high levels of c-di-GMP, FleQ stimulates the expression of cdr, pel, and psl genes that control production of adhesins and exopolysaccharides, critical components for biofilm formation (Hickman and Harwood, 2008). Interestingly, at low levels of c-di-GMP, it is also FleQ that functions as the master activator of flagellar gene expression (Hickman and Harwood, 2008). Importantly, FleQ also regulates P. aeruginosa invasion of corneal epithelial cells, with contributions additional to that of flagellin (Kroken et al., 2018).
Cyclic AMP (cAMP) also serves as a bacterial second messenger that impacts virulence factors, including the type II secretion system (T2SS, which secretes LasB and other proteases discussed above) and the type III secretion system (T3SS), via the activation of the virulence factor regulator (Vfr) (Whitchurch et al., 2005; Wolfgang et al., 2003). Cyclic AMP has also recently been implicated in regulating twitching motility in P. aeruginosa in vitro (Persat et al., 2015). Cyclic AMP levels are increased by an adenylate cyclase, CyaB, which is activated by the Chp chemosensory system, a two-component signal transduction system that is similar to the flagellar chemotaxis system (Fulcher et al., 2010). It has been suggested that the Chp system is activated by surface contact made by pili during twitching motility, which in turn positively regulates pili assembly and twitching motility in vitro. Unpublished data from our laboratory (courtesy of Dr. Vincent Nieto) indicates that cyaB mutants of P. aeruginosa are defective in their capacity to exit invaded human corneal epithelial cells, similar to twitching mutants. Whether this occurs via reduced twitching function or reduced expression of virulence factors (e.g. Vfr-regulated), remains to be determined.
3.7. Co-infection
Sometimes a microbe does not act alone in the pathogenesis of infection. Indeed, biofilms are typically polymicrobial, and infections outside of the eye often involve multiple pathogens. This includes infections involving P. aeruginosa, which commonly partners with S. aureus in wound infections (DeLeon et al., 2014; Pastar et al., 2013), and with S. aureus or Burkholderia cenocepacia in the lungs of CF patients (Bragonzi et al., 2012; Maliniak et al., 2016). While it is often the only microbe cultured from contact lens-related infections, P. aeruginosa is sometimes present during Acanthamoeba keratitis, as a potential endosymbiont within the amoeba (Iovieno et al., 2010). A recent study that combined these two significant causative organisms of keratitis (P. aeruginosa and Acanthamoeba castellanii) suggested that they work synergistically, in that the presence of P. aeruginosa was required for the development of Acanthamoeba keratitis in a rabbit stromal injection model (Nakagawa et al., 2017). Also interesting to ponder is a recent paper showing that some P. aeruginosa strains encode bacteriophages released during cell infection that manipulate host cell responses towards combatting viruses (Sweere et al., 2019). Thus, in addition to taking a trojan horse approach, P. aeruginosa can use other microbes as decoys to distract and occupy the host that it is infecting - in both cases using organisms belonging to other kingdoms. Interestingly, we recently found that the bacteriophage shown to sway host immune responses is a hotspot for the development of mutations during contact lens-related infection in mice (unpublished data courtesy of Dr. Matteo Metruccio, Fleiszig laboratory), suggesting a possible role for such mutations in pathogenesis.
4. Impact of contact lens wear on corneal intrinsic resistance and microbial virulence
4.1. Impact of lens wear on the human cornea and tear film
Over the past several decades, research done using human subjects has provided a wealth of information about how lens wear impacts the human ocular surface. Some of these studies have focused on changes visible using equipment readily available in the clinic; e.g. a slit-lamp biomicroscope to detect epithelial microcysts, mucin balls, endothelial blebs, edema, corneal staining etc. Others have developed and used more specialized equipment to study temperature, pH, osmolarity, biochemistry/rheology of the tear fluid, tear exchange, epithelial permeability, vascular responses and cellular changes. Much important information has arisen from such studies, and we refer the reader to a sample of numerous papers/reviews on these topics (Craig et al., 2013; Efron, 2017; Efron et al., 2010; Holden et al., 1985; Hori et al., 2006; Maldonado-Codina et al., 2004; McNamara et al., 1998, 1999a; Purslow et al., 2005; Rohit et al., 2013; Stapleton et al., 2006). However, it has been difficult to determine which, if any, of these phenomena observed in people relate to infection pathogenesis. This is because proving causation usually requires introducing perturbations that would be unethical in people; such as inoculation with microbes, interfering with candidate defenses, and/or use of invasive techniques to assess outcome. Thus, we and others have developed various alternative model systems.
4.2. Contact lens infection models and implications of results
One option for experimentation not suited to human subjects, is use of human corneal cells in vitro. In one study, we used an irrigation method developed by Fullard and Wilson in the mid-1980’s (Fullard and Wilson, 1986) to collect exfoliating corneal epithelial cells from people wearing lenses and control non-lens wearers. We then inoculated the collected cells in vitro with P. aeruginosa. Results showed that extended lens wear enhanced P. aeruginosa binding to the exfoliated cells (Fleiszig et al., 1992). In another study, we placed contact lenses over human corneal epithelial cells grown in culture for 72 h prior to inoculating the cells with P. aeruginosa. Results showed that the capacity of the cells to upregulate hBD-2 in response to P. aeruginosa antigen challenge was compromised (Maltseva et al., 2007). As previously discussed, hBD-2 is a crucial antimicrobial peptide in the corneal epithelium that contributes to preventing P. aeruginosa binding. How accurately results using these in vitro methods predict events occurring in vivo is not certain. A subsequent study done by others adopting our exfoliated cell method showed hypoxia was important for enhanced bacterial binding (Ladage et al., 2002), yet contact lenses that overcome hypoxia have not reduced the infection risk (Stapleton et al., 2008, 2013). As mentioned earlier, there can be major differences between cells grown in culture in vitro and cells in vivo in how they respond to bacteria, and exfoliated cells will have lost normal polarity that we now know is involved in defense against microbes (Section 2.3.5).
Other studies have been done using animal models, which enable invasive manipulation in vivo. Most have utilized rabbits as they can be fitted with human lenses. A limitation is that the nictitating membrane (absent in humans) is often surgically removed to allow wear of human contact lenses when eyes are sutured closed (Ichijima et al., 1993). Without suturing, it is difficult to keep a lens in the rabbit eye, and the lens dehydrates. Nevertheless, the results have been informative.
For example, a rabbit model was used to study the impact of hypoxia induced by soft lenses, the results showing higher levels of P. aeruginosa adhesion (Imayasu et al., 1994), bacterial internalization into surface epithelial cells via lipid-raft colocalization (Yamamoto et al., 2005, 2006) and also risk of infection (Solomon et al., 1994). However, a more recent study utilizing rigid lenses showed no differences in infection rates between low- and high-oxygen transmissible materials (Wei et al., 2014). The results also showed that while both lens types caused infection, only the low oxygen transmissible lenses (visibly) damaged the rabbit corneal epithelium. Thus, in addition to showing that hypoxia is not necessary for infection to occur, this study showed a disconnect between visible epithelial injury and infection risk. This also aligns with what we know for human soft lens wearers, i.e. that neither hypoxia nor injury necessarily lead to infection during contact lens wear nor are they required. A more recent study using a lens-wearing rabbit model (without removing the nictitating membrane and without epithelial injury) showed that microbial keratitis required the presence of viable P. aeruginosa on the contact lens in vivo, i.e. bacterial antigens (e.g. LPS) did not mimic the disease (Dutta et al., 2016). That study also showed the potential for a lens-bound antimicrobial peptide, melimine, to reduce the risk of microbial keratitis, in addition to showing the value of lens-wearing in vivo models to find key factors important for disease.
That epithelial injury is neither necessary nor sufficient for corneal infection risk is also supported by our own research using the tissue paper blotting method in mice and rats (discussed in detail earlier, Section 2.3.4). That data showed no disease after superficial injury no matter how large the inoculum added, despite the induction of bacterial adhesion to the corneal surface, significant fluorescein staining, and the loss of surface cells and their associated glycosylation (glycocalyx). Even more compelling, blotting had no impact on either the timing or the severity of infection induced by lens wear in rats (Tam et al., 2010). That there was still a delay in disease onset of several days when the corneas were superficially-injured before being fitted with P. aeruginosa contaminated lenses, is interesting to ponder. Indeed, it provides important clues as to what is (and what is not) involved in the pathogenesis of contact lens-related infection.
Other studies using rabbits have shown that rigid gas permeable lens wear alone (without bacteria) reduces epithelial cell turnover in the central cornea (Ladage et al., 2001, 2003). Similarly, another study using both human subjects and a rabbit model of contact lens wear showed that both rigid gas permeable and soft contact lenses reduced corneal epithelial cell proliferation rates, and suppressed apoptosis and exfoliation (desquamation) of surface cells (Ladage et al., 2002). In that study, contact lens effects on cell proliferation were attributed to oxygen transmissibility and the lens itself. Reduced cell turnover is potentially very important for multiple reasons. For example, surface corneal epithelial cells (including sloughing cells) can engulf P. aeruginosa (Fleiszig et al., 1995; Fleiszig et al., 1992). Once corneal epithelial cells internalize bacteria, they can traffic them intracellularly to acidified vacuoles (most likely lysosomes) where they are killed by the cell (Heimer et al., 2013). This is likely to be an important defense strategy of the immune-privileged corneal surface. However, as described previously, P. aeruginosa can evade being trafficked to lysosomes and subsequently can “set up shop” inside the corneal epithelial cell (Heimer et al., 2013) (Section 3.4). This combination of P. aeruginosa internalization by cells, and reduced cell turnover, could potentially contribute to infection risk during contact lens wear. A related important question is whether contact lens wear interferes with the inherent capacity of these cells to engulf bacteria, or to eradicate them intracellularly, irrespective of the type of microbe involved.
Rats have also been used to study impacts of contact lens wear. The first of these studies explored immunological features, showing no significant change in the expression of a panel of cytokines and chemokines when silicone hydrogel lenses were worn by rats (Szliter et al., 2002). However, repeated P. aeruginosa inoculation of lens and eye caused significant corneal inflammation involving upregulation of IL-1β and IL-6, changes in Langerhans (dendritic) cells and neutrophil infiltration without development of severe microbial keratitis (Szliter et al., 2006). Later use of this model also revealed effects of hypoxia, e.g. low-Dk lenses were associated with increased numbers of conjunctival Langerhans cells versus high-Dk lenses, and some eyes wearing low-Dk lenses showed infection after P. aeruginosa challenge, not seen with high-Dk lenses (Zhang et al., 2008). Guinea pig models of soft (hydrogel) contact lens wear have also shown P. aeruginosa-induced inflammatory events without microbial keratitis (Vijay et al., 2009).
Our own studies using rat lenses focused on the pathogenesis of infection during inoculated lens wear rather than inflammatory impacts. We used extended wear of hydrogel (low-Dk, hypoxia inducing) lenses and only a single inoculum of P. aeruginosa (introduced with the lens). The results showed this consistently caused microbial keratitis after an onset delay (median ~8 days) independently of inoculum size (Tam et al., 2010). Introduction of superficial injury had no impact of time of disease onset or disease severity (as previously mentioned). Importantly, disease was associated with significant bacterial biofilm formation on the lens surface facing the cornea (posterior lens surface), but the anterior surface was free of adherent microbes.
More recently, we developed a mouse contact lens wear model (Metruccio et al., 2019). This has the added advantage of allowing utilization of the many reagents available only for use in mice, including mice engineered to lack specific factors or to express fluorescently labeled cells/proteins. Data we have collected using custom-made, silicone hydrogel (high-Dk) lenses show they can be used to induce infection with P. aeruginosa.
While uninoculated mouse lens wear caused no obvious changes to corneal health (similar to human lens wear), high resolution imaging using mice expressing fluorescent membranes revealed some interesting phenomena. For example, small round vesicles were identified within cells in the superficial layers of the epithelium, and some keratocytes in the stroma appeared jagged. Most striking was the presence of small, motile cells trafficking along and over keratocytes within the stroma (Metruccio et al., 2019). In that study, using mice with fluorescent myeloid-derived (immune) cells, we showed that lens wear caused an influx of immune cells not seen in contralateral control eyes. Further experiments revealed Ly6G+ (neutrophil) infiltration of the corneal stroma after 5 days of continuous lens wear requiring both MyD88 and IL-1R. This was a surprising result considering neutrophil infiltration into the stoma is normally associated with outright inflammation/corneal swelling, and neither were observed. Thus, the (likely) neutrophils infiltrating were in a quiescent state. Throughout this study, both male and female mice showed similar responses to contact lens wear. The phenotype of the Ly6G + infiltrate during lens wear, and the other lens-associated corneal changes, are under investigation.
It is not yet known if lens wear in humans also routinely causes infiltration of quiescent neutrophils, and that would be more difficult to ascertain. However, we did observe other phenomena also known to occur during human lens wear. This included a dendritic (CD11c-positive) cell response observable by 24 h of lens wear (Metruccio et al., 2019), which occurs in people in a similar time frame (Alzahrani et al., 2016). Also reminiscent of human lens wear (Szczotka-Flynn et al., 2010b; Willcox, 2013b), lenses worn by mice accumulated commensal-type bacteria on their posterior surfaces in vivo. Thus, the mouse model appears to faithfully replicate at least some events occurring during human lens wear. In both humans and mice, accumulation of microbes on the lens might relate to the dendritic cell response, which appears very similar to the dendritic cell response occurring when mouse corneas are inoculated with P. aeruginosa (Metruccio et al., 2017).
Parainflammation is a term used to describe an intermediate tissue-adaptive response between basal homeostasis and classical inflammation (Medzhitov, 2008), thought to help restore tissue homeostasis under conditions of cell and tissue stress. With persistent or increased stress, this can then lead to induction of outright inflammation. The notion that contact lens wear causes parainflammation was first proposed by researchers studying human lens wearers (Efron, 2017), and our results with mice support this idea. Thus, we have adopted this terminology to refer to the CD11c-positive cell (DC) and quiescent neutrophil cell responses occurring during mouse lens wear (see Fig. 3B). The trigger for, and consequences of, contact lens-induced parainflammation remain to be established. As eluded to above, microbes or their antigens are prime suspects given that the normal cornea does not host a viable bacterial microbiome (Wan et al., 2018), that worn lenses readily become contaminated with several types of environmental microbes or commensals from the skin or conjunctiva (Metruccio et al., 2019), and that microbes alone can trigger similar responses (see Section 2.3.7).
Important to consider, is the significance of the parainflammatory response. A possibility is that it functions to protect the cornea against bacterial colonization. FISH with a universal 16S rRNA gene probe to examine mouse corneas after extended contact lens wear revealed very few bacteria colonizing the cornea after 11 days despite the lenses being colonized (Metruccio et al., 2019). Whatever the case, it is likely that the parainflammatory response is involved in priming the cornea to prepare for the possibility of further trouble. For example, it might help mount the more potentially damaging immune responses that occur during an actual infection. In that way, it might be a key contributor to the pathogenesis of contact lens-related infection pathology.
When P. aeruginosa was introduced with mouse lens wear it did result in infection (Metruccio et al., 2019). Further, the pathogenesis involved bacterial penetration of intact appearing corneal epithelium (see Fig. 3C), explaining why outright epithelial injury is not required for infection to occur during lens wear. This was followed by bacterial entry into the stroma and induction of an acute immune response similar to that occurring in other in vivo infection models (Metruccio et al., 2019). Thus, the mouse contact lens wear model will be useful for further studies of how and why P. aeruginosa reaches the corneal stroma despite epithelial and basal lamina barriers that remain visibly intact during lens wear in the absence of P. aeruginosa inoculation. Fig. 3B illustrates some of the known and potential effects of lens wear on corneal defenses against P. aeruginosa that might ultimately predispose to microbial keratitis.
As always, further research on this and other areas will need to bear in mind that there are differences between humans and animals, and between in vitro and in vivo experiments. Lessons have been learned from the role of hypoxia, which was apparent in multiple animal models and sloughed human corneal cells, but not in the live human population. A related example was a rabbit study showing that lens wear impacts the glycocalyx by increasing wheat germ agglutinin (WGA) receptors more significantly with wear of low-Dk versus high-Dk lenses, possibly implicating hypoxia (Latkovic and Nilsson, 1997), while a more recent study with human subjects using fluorescein-conjugated WGA showed the opposite result with soft lens wear significantly reducing WGA binding (Fukui et al., 2016).
Sometimes, however, animal and human data might appear not to align because of study design details or how the data are interpreted. Indeed, one study showed that the severity of infection can be influenced by hypoxia in human lens wearers even though it does not alter overall incidence (Morgan et al., 2005). Thus, the results of animal studies need to be interpreted carefully, bearing in mind complexities in the system, and cross-validation of observations should be made with human subjects wherever possible. Indeed, the above discussion illustrates the value of animal studies when guided by results of research using human subjects.
4.3. Lens effects on tear fluid
A potential mechanism by which contact lens-wear could promote P. aeruginosa persistence at the ocular surface, and also reduce epithelial defenses against it, is by disrupting the composition and function of tear fluid at the corneal surface, and/or reducing tear exchange. As discussed previously (Section 2.2), tear fluid exerts many direct antimicrobial effects on bacteria, including the inhibition of motility, induction of clumping or chain formation, and strain-dependent bacteriostatic effects. It also protects epithelial cells against bacterial invasion and cytotoxicity. Our data show that tear fluid loses these capacities if incubated with bacteria for 8 h, suggesting a relatively short active lifespan of static tear fluid (Fleiszig et al., 2003). Notably, fresh tear fluid applied to the same bacteria was fully effective, implying that bacteria had not adapted to tear fluid, but rather a breakdown of tear components had occurred, possibly mediated by the bacteria (Fleiszig et al., 2003). Further, the protective effects of tear fluid were lost with dilution, showing the importance of maintaining the correct concentration of active factors (Fleiszig et al., 2003). When bacteria are present on the posterior surface of a contact lens between the lens and cornea in vivo, they reside in a space where reduced tear exchange is likely (McNamara et al., 1999a; Muntz et al., 2015) which might contribute to increased bacterial survival at posterior lens surfaces in vivo (Tam et al., 2010).
Even without the prolonged presence of pathogenic bacteria, the antimicrobial longevity of tear fluid is compromised by lens wear (Wu et al., 2015a). Specifically, tear fluid collected from non-lens-wearing volunteers retained full antimicrobial activity for up to 8 h, whereas antimicrobial activity of tears collected on the posterior side of worn contact lenses reduced with the length of time the lenses were worn (Wu et al., 2015a). Loss of tear antimicrobial activity in the post-lens tear fluid in the presence of a contact lens may occur through several mechanisms. In addition to potential decay over time, or destruction by microbes or their products, tear components can bind to the lens, on which their activities can be altered. Proteins shown to bind to lenses include lysozyme and lactoferrin, which could clearly compromise antimicrobial efficacy (see review by Luensmann and Jones, 2012). Lens binding of other tear components, e.g. proteinase inhibitors, could also compromise the protective function of tears by creating excessive proteinase activity that injures the ocular surface. Protein binding may also remove tear factors that normally influence epithelial defensive functions that were discussed previously. Thus, reduced tear exchange and/or lens binding of tear proteins could provide mechanisms for contact lens-mediated compromise to tear fluid defenses. The latter, in turn, could result in a reduced capacity of the post-lens tear film to protect against bacterial persistence and potential corneal attachment, compared to an intact tear film with normal tear exchange (Fig. 3B). These mechanisms are likely to contribute to the reasons why daily disposable lenses (replaced each day) have been found safer than conventional daily wear during which the same lens is “cleaned” and worn again the next day (Dart et al., 2008).
Here, it is important to mention the work done by others that shows contact lens wear can alter cytokine and chemokine levels in the tear fluid. For example, significant differences were observed between nonadapted and adapted contact lens wearers, and between these groups and non-lens-wearing controls, for certain tear fluid proinflammatory, chemoattractant mediators (e.g. IL-8 and LTB4) and PMNs (e.g. neutrophils) (Thakur and Willcox, 2000). A more recent study of established contact lens wearers revealed significantly higher cytokine levels (e.g. IL-1β, IL-12, IL-17A) in daily wear (reusable) versus daily-disposable wear (Chao et al., 2017). However, another study of established contact lens wearers found no significant changes in tear cytokines after ~24 h versus non-lens wearing controls (Duong et al., 2017).
Further studies are needed to more clearly define the influence of contact lens wear on tear biochemistry, including cytokines and phagocytes. This will be of particular importance for the post-lens tear film between the cornea and contact lens since this is of obvious significance to understanding infection, not only non-infectious inflammation during lens wear.
4.4. Lens wear in the closed eye
The risk of infection increases with both overnight and extended wear (Cheng et al., 1999; Dart et al., 2008; Schein et al., 1989a; Stapleton et al., 2008). In addition to increasing length of wear time before removal, overnight and extended wear both involve wear during sleep when there is a normal physiological response to eye closure that resembles an inflammatory state. This involves upregulation of pro-inflammatory mediators, complement (C3) activation, increased levels of secretory IgA and serum proteins, and phagocyte (neutrophil) infiltration with associated proteinases (Sack et al., 1992, 2000, 2009). Damage to the ocular surface from the closed eye inflammatory state is limited by several factors including regulators of complement activation and anti-proteinase expression that minimize ocular surface damage (Sack et al., 2000, 2009). More recently, it has been shown that tear neutrophils present in the closed eye environment exhibit a relatively non-inflammatory phenotype in that they are capable of oxidative burst, but do not respond to traditional stimuli, e.g. LPS (Gorbet et al., 2015). These differ considerably from blood-derived neutrophils, although the factors driving the tear neutrophil phenotype have yet to be determined. Exposure of blood-derived neutrophils to hypoxia, corneal epithelial cells, or artificial tear fluid does not recapitulate the tear-derived phenotype, so it remains unclear what programs these cells differently from other neutrophil types (Postnikoff and Gorbet, 2018). Nevertheless, the observation of distinct neutrophil phenotypes between tear fluid and blood is consistent with the extensive, and growing, field of investigation in neutrophil biology, regarding neutrophil phenotypic plasticity, licensing, and consequences for inflammation and host defense (Deguine et al., 2017; Hong, 2017; Kolaczkowska and Kubes, 2013).
How contact lens wear impacts the normal events occurring at the corneal surface during eye closure when a lens is worn on an overnight or extended wear basis remains an open question. Given that tear exchange is even more reduced than in an open blinking eye, likely this will be significant. Also not known, is the extent to which lens wear in an open eye recapitulates the subclinical inflammation of closed eye tear fluid, given coverage by the lens and the various pro-inflammatory components that might bind to it (Szczotka-Flynn et al., 2010b). There are intriguing parallels between the subclinical inflammation of closed-eye tear fluid observed in humans, and corneal parainflammation observed in lens-wearing corneas in vivo (Metruccio et al., 2019).
4.5. Lens wear impact on microbes at the ocular surface
Another important topic to consider when thinking about the pathogenesis of contact lens-related infection is how the lens wear environment might influence microbes and their virulence capacity. Considering what we know about microbes in general, and P. aeruginosa specifically, this could be at least as important as any impact of the lens on the ocular surface. In this section, we discuss the known, and potential, impacts of contact lens wear on bacteria and their virulence mechanisms at the ocular surface (schematically illustrated in Fig. 3C).
4.5.1. How does lens wear influence the microbial environment?
Contact lens care introduces numerous factors that can potentially increase the opportunity for microbes to interact with the cornea. Microbes could enter the eye via the eyelids, fingers when inserting/removing the lens, contamination from the lens itself, disinfectant solutions used, and the contact lens storage case. It has been reported that only 32% of patients are compliant in all aspects of the lens care regimen (Bui et al., 2010). In some instances, not following proper lens care instructions has been linked to infection, but clearly that is not always the case given the relatively low incidence. Cases of severe microbial keratitis in planned replacement lens users correlated with the presence of pathogenic bacteria in lens cases (Dart et al., 2008). Additionally, 30% of daily disposable lens users have reported wearing them for occasional or regular overnight wear which increases the risk of infection (Dart et al., 2008). Many studies have indicated that poor hygiene with lens care, extended use of disinfectant solutions (> 3 months), and lens case contamination with bacteria in biofilms are associated with increased risk of microbial keratitis and other lens-related complications (Lim et al., 2016; Stapleton et al., 2008, 2013; Wu et al., 2015b). While lens-associated bacteria have not been shown to be directly causative of corneal infection, studies have shown that bacteria isolated from a corneal ulcer are commonly the same as those isolated from a patient’s worn lenses or lens cases (Konda et al., 2014; Stapleton et al., 1995).
At this stage it is important to consider whether this high incidence of lens and case contamination and lack of compliance actually changes the microbial community in the eyes of lens wearers. Over the years, many studies have addressed this question - too many to summarize comprehensively here. Our own studies on this topic were done more than three decades ago using standard culture methods, which was all that was available at the time. One was a longitudinal study examining the effect of rigid gas permeable lens wear on conjunctival flora. Interestingly, this revealed an increased number of potentially pathogenic bacteria, even though these lenses do not generally predispose to infection (Fleiszig and Efron, 1992a). The other study explored the effects of soft-contact lens wear, which is more often associated with infections. Ironically we found no differences in the conjunctival flora in lens wearers compared to non-lens wearers (Fleiszig and Efron, 1992b). A surprising result was that former lens wearers were culture-positive for commensal-type bacteria more often than current lens wearers, possibly explaining their discontinuation as lens wearers.
A much more recent study used 16S rRNA gene-sequencing techniques and showed that contact lens wear altered the conjunctival bacteria flora to be closer to those of the skin (Shin et al., 2016). Given the methods used, it is very likely that the samples were contaminated by debris arising from the skin during lens handling. Nevertheless, studies have shown that bacteria do accumulate on the contact lens when worn by people (Retuerto et al., 2019), and as discussed above, this also happens when mice wear lenses.
Use of the mouse model has also allowed us to look for microbes directly on the corneal surface with FISH labeling (using a 16S rRNA gene probe) which cannot be done using people. Those results revealed very few viable microbes on the mouse cornea after lens wear (similar to no lens wear) despite microbes being present on the lens (Metruccio et al., 2019). Taken together, these results suggest (at least in mice) that under normal circumstances, contact lens wear does not compromise epithelial defenses against the type of bacteria colonizing contact lenses (commensals, not P. aeruginosa).
4.5.2. Does contact lens wear impact microbial virulence capacity?
Studies defining the microbial environment of the eye and what influences it, such as those discussed above, have helped understand the origin of microbes that ultimately cause infections. However, there needs to be more to the pathogenesis of lens-related infection, because the microbes involved (including P. aeruginosa) are normally not virulent to the cornea. The notion that corneal defenses against these microbes might be compromised was discussed previously. Here, we will consider the possibility that lens wear also, or instead, sets up conditions for microbes to be more virulent, either by enabling them to avoid being subjected to normal defenses, or by upregulating their virulence capacity.
4.5.2.1. Bacterial adhesion to lenses and biofilm formation.
As already discussed, contact lenses can provide a surface for microbes to adhere to (Willcox, 2013b), even though they do not typically persist on the corneal surface. This might confer protection from physical removal, such as by blinking shear forces, particular if they are attached to the posterior lens surface where such forces would be greatly reduced, and where the tear fluid trapped between the cornea and lens can become less bacteriostatic with time of wear (Wu et al., 2015a).
Also previously mentioned (Section 3.6.4) bacteria can form biofilms on surfaces to which they adhere. P. aeruginosa is especially adept at this, with its biofilms generally consisting of complex structures containing bacterial communities residing in self-produced polymeric matrix. While our published data show that adding tear fluid to a P. aeruginosa biofilm already existing on a contact lens reduces bacterial cultivability, biofilms can still form on a lens when tear fluid is present -even if the experiment is done in vitro (Wu et al., 2017). Accordingly, P. aeruginosa can readily form biofilm on the posterior surfaces of lenses worn in vivo as we showed in a rat model (Tam et al., 2010).
The survival advantage that biofilm growth enables in diverse potentially hostile environments includes elevated resistance to antimicrobials, immune effectors, and shear forces, making them very difficult to eradicate (Section 3.6.4). As such, P. aeruginosa biofilm formation on contact lenses, lens cases, and in vivo represents a significant threat to the cornea from several different perspectives, additional to increasing resistance to antimicrobial factors such as those at the ocular surface. From this vantage-point, bacteria could gradually disperse to continuously reseed the environment. Bacteria within the protected niche of the biofilm could also contribute to disease pathogenesis through the release of a multitude of factors that trigger inflammation (e.g. LPS, flagellin, and other ligands for PRRs), or virulence factors with potential to compromise barriers against infection e.g. proteases, toxins (and OMVs, to be discussed later). A further major threat is that lens-associated biofilms in vivo could provide a niche for bacterial cell adaptations, e.g. changes to gene expression or development of mutations, that favor bacterial virulence in the ocular environment. This possibility is discussed in more detail below. Also important to consider, is that when bacteria adhere to the lens surface (in a biofilm or otherwise) they can remain at a slight distance from the epithelial surface potentially protected from some of its more direct defenses. This is a notion supported by the fact that commensals (and P. aeruginosa) can colonize the posterior surface of worn lenses whilst the corneal surface remains microbe free.
The capacity of lenses to adhere bacteria has been studied extensively, mostly in vitro. Results show that bacterial adhesion varies by storage conditions, bacterial strain (not just type), temperature, medium used, timing, how extensively the lenses are washed, and methods used to quantify outcomes (e.g. Fleiszig et al., 1996a; Vijay et al., 2012; Willcox et al., 2001; Willcox, 2013b). Contact lens material can also influence bacterial attachment with P. aeruginosa and S. aureus, for example in some studies they have been shown to adhere more effectively to silicone hydrogel lenses compared to other materials (Kodjikian et al., 2008; Willcox et al., 2001). Adding to the complexity, in vivo factors can have a profound influence. For example, bacterial adherence to silicone hydrogel lenses was greater for worn versus unworn lenses (Willcox et al., 2001), likely due to the impact of residual tear components, cell debris, inflammatory mediators etc. In another study, addition of neutrophils enhanced P. aeruginosa biofilm formation on hydrogel lenses more than 30-fold (Robertson et al., 2011b). Epithelial cell debris even enhanced P. aeruginosa adhesion to 96-well plates in vitro, with clear implications for biofilm contamination of lenses and lens storage cases, where cell debris can accumulate (Burnham et al., 2012).
We contributed to this research topic many years ago, then again more recently using more sophisticated methods. We showed that numerous bacterial surface ligands including LPS, flagella, and pili, can be utilized by P. aeruginosa to modulate its association with contact lenses (Fletcher et al., 1993a, 1993b; Tran et al., 2011a, 2011b). The two more recent of these studies showed that individual bacteria within a population can behave differently, using a range of surface components, even when they are all of the same genotype and are all in the same environment. The type of attachment that these individuals exhibit includes combinations of end-on or side-on, and fixed or temporary, while they can also display a range of surface-associated mobility strategies. While this data suggests a good deal of complexity, carefully designed studies aimed at answering key questions will likely continue to be of value given that bioburden is associated with a greater risk of corneal infiltrative events (Szczotka-Flynn et al., 2010a), and that biofilm formation on contact lenses and lens cases has been associated with increased risk of microbial keratitis in people (McLaughlin-Borlace et al., 1998; Stapleton and Dart, 1995).
4.5.2.2. Bacterial adaptations favoring virulence.
The genome of P. aeruginosa is particularly large and plastic, as evidenced by the extensive intraspecies diversity found between P. aeruginosa strains (an estimated ~11% global variability between strains), underscoring its potential to evolve rapidly under a variety of selective pressures (Shen et al., 2006). During infection at other body sites, P. aeruginosa has been shown to undergo adaptations that include alterations to gene expression and development of mutations that benefit their capacity to prevail. These phenotypic (gene expression) and genotypic (mutational) changes have been studied in great detail in some instances. From clinical isolates (Jeukens et al., 2014; Mahenthiralingam et al., 1994; Workentine et al., 2013) and isolates from an artificial sputum model (Davies et al., 2016, 2017), genetic sequencing analysis has identified common mutations related to antibiotic resistance, biofilm formation, type III secretion, quorum sensing, and motility (both swimming and twitching). Some of these variants have been shown to out-compete their parental strain (as observed through fitness assays), suggesting that population diversity is advantageous to the fitness of the overall population during infection (Davies et al., 2016, 2017; Flynn et al., 2016; Winstanley et al., 2016). Genetic diversification is believed to be facilitated by several factors, including changes to niche environment and density of the bacterial population.
An example where P. aeruginosa experiences changes in both environment and density is in the lung of patients with cystic fibrosis (CF), where it migrates from the sinuses into the lower airways of the lung. As the disease progresses, it experiences a reduction in nutrient availability, O2 levels, and is exposed to antibiotics, culminating with increased mucus (i.e., sputum) association (Winstanley et al., 2016). Bacterial adaptation has been studied in CF patients (Folkesson et al., 2012; Winstanley et al., 2016) and related models (Davies et al., 2016, 2017; Fothergill et al., 2014). Interestingly, nasopharyngeal colonization was shown to serve as a silent reservoir for evolutionary adaptation and chronic reseeding of lung infections following intermittent clearance from the lungs (Fothergill et al., 2014). Also apparent are frequent mutations in the quorum-sensing regulator lasR associated with poor clinical prognosis (Hoffman et al., 2009). Interestingly, similar mutations have been found evident in P. aeruginosa isolates from microbial keratitis, again associated with worse clinical outcome (Hammond et al., 2016). Another result suggesting P. aeruginosa adaptations occurring in the eye and airways can be similar, is the finding that sputum induces a loss of motility in P. aeruginosa infection isolates (Davies et al., 2017), as we showed previously using human tear fluid (Fleiszig et al., 2003; Li et al., 2017).
Results obtained using our rat model support the notion that P. aeruginosa can undergo important adaptations during the pathogenesis of contact lens-related infection, underpinning an ~8 day delay in infection onset after the bacteria-contaminated lenses were placed on rat eyes. This was shown by fitting naïve rats with lenses removed from infected rats that already harbored eye-adapted P. aeruginosa biofilms, which reduced the ~8 day disease onset delay to only ~2 days (Tam et al., 2010). These findings illustrated that bacteria present on a transferred lens can be primed for virulence, with appropriate controls done to rule other possibilities. Thus, in addition to protecting bacteria from ocular clearance by shear forces and host antimicrobials, lens-associated biofilms may contribute to virulence of P. aeruginosa by allowing bacteria to prevail for long enough to enable critical adaptations. Possibly of relevance, we have shown increased expression of a key virulence factor (the T3SS, discussed in Section 3.3) in contact lens-associated P. aeruginosa biofilms, which is further enhanced by incubation of the lens-related biofilm in human tear fluid (Wu et al., 2017).
4.5.3. Bacterial breakdown of barrier function
As discussed above, bacteria causing contact lens-related infection might change in some way to enhance their virulence when in the eye with a lens in place. Another possibility is that they use “garden-variety” mechanisms usually accessible to them to gradually “chip away” at host defenses, simply because they can prevail at the ocular surface longer than is normally possible when on or under the lens. P. aeruginosa and other bacteria causing infections can produce many factors that could potentially compromise cell-cell junctions, antimicrobial peptides, basement membrane proteins, and other defense factors directly, or through impacts on factors that regulate them (see Section 3). Two specific examples are provided below, but there are other possible contributors yet to be explored.
4.5.3.1. Outer membrane vesicles.
A strategy P. aeruginosa and other Gram-negative bacteria can use to attack host cells from a distance is to release outer membrane vesicles (OMVs) containing virulence factors, some of which can alter host cell function (Bomberger et al., 2009; Schwechheimer and Kuehn, 2015). OMVs can fuse with a target host cell to transfer their contents or lyse to release their contents to the extracellular space. An extracellular vesicle (EV) equivalent has been shown for Gram-positive bacteria, including the corneal pathogen S. aureus (Gurung et al., 2011; Lee et al., 2009). There are several advantages to using OMVs/EVs for the bacterium. One is that it can remain at a safe distance from the cell it is targeting. As previously discussed, host cell surfaces can be hostile for multiple reasons, including the presence of antimicrobial peptides and the glycocalyx (see Section 2.3), and the threat of being phagocytosed. OMVs also allow bacterial factors to be delivered to sites not accessible to the larger bacterium, or that are otherwise undesirable. P. aeruginosa is particularly adept at generating OMVs when in a biofilm, with OMVs actually thought to contribute to construction of the biofilm matrix (Schooling and Beveridge, 2006).
A variety of factors can be packaged into OMVs, which for P. aeruginosa depends on the strain and how the bacteria are grown (Choi et al., 2011). One such factor is the toxin Cif (CFTR inhibitory factor) that can hijack the host ubiquitin system (Bomberger et al., 2011). Recent evidence shows Salmonella spp. can package T3SS effectors into OMVs to deliver them into host cells without the T3SS apparatus being involved (Kim et al., 2018), a possibility not yet explored for P. aeruginosa.
Relevant to this discussion, our published data show that human tear fluid induces OMV production by P. aeruginosa (Metruccio et al., 2016). The tear ingredient lysozyme is involved in this triggering, and the released OMVs are toxic towards human corneal epithelial cells in vitro. Importantly, OMVs could prime the in vivo corneal surface for subsequent adhesion of P. aeruginosa bacteria that otherwise did not adhere (Metruccio et al., 2016). Sonicated OMVs were unable to prime the cornea in this way, suggesting intact OMVs bind to cells to deliver the responsible factor(s). Given that OMVs can contain hundreds of proteins (Choi et al., 2011), and are a major component of bacterial biofilms (Schooling and Beveridge, 2006), more work will be required to understand the mechanisms for this phenomenon. It would also be of value to determine if OMVs contribute to the pathogenesis of contact lens-related infection. This could feasibly involve release of OMVs from biofilms on the posterior lens surface triggered by tear fluid in vivo, or via their transfer from contaminated contact lens care solutions or cases wherein biofilms are also known to occur.
4.5.3.2. Chipping away at ocular surface mucins.
Given the role of mucin in host defense at the epithelial surface, another strategy that might help bacteria infect would be disruption of the ocular surface glycocalyx mucins. Indeed, S. pneumoniae secretes a metalloproteinase ZmpC, that removes the ectodomain of MUC16 from ocular surface epithelia to promote bacteria-host cell interactions (Govindarajan et al., 2012). As another example, P. aeruginosa utilizes host sialidase NEU1 to desialylate MUC1 increasing its adherence to airway epithelia, to which host cells respond by shedding the MUC1 ectodomain that then serves as a decoy receptor (Lillehoj et al., 2015). While lens wear does not appear to alter mucin gene expression in the conjunctiva of established lens wearers, nor levels of tear fluid MUC5AC, lenses can bind ocular mucins in vivo (Hori et al., 2006). In another study utilizing human subjects, lens wear reduced tear fluid levels of Neu5Ac (Yasueda et al., 2005). Thus, it is plausible that contact lens wear, or adherent or trapped microbes, might impact ocular surface mucins in a way that contributes to infection pathogenesis.
4.6. Impact of lens care
4.6.1. Microbe interactions with lens care solutions and lens cases
Lens care solutions are an integral part of reusable contact lens care, and their intended purpose is to reduce microbial contamination of the eye. However, they can unintentionally provide a source of bacterial inoculation if there is microbial resistance or improper use. Sub-par efficacy could subsequently create stress conditions for bacteria, thereby encouraging further adaptations to enhance their capacity to survive, including biofilm formation, production of potentially toxic OMVs, and release of components that trigger host inflammatory responses (PAMPs). Bacterial virulence factors and PAMPs could then be transferred into the eye to cause inflammation or challenge intrinsic barrier function even in the absence of the microbe they originated from.
Accordingly, multiple studies have shown an association between poor compliance to lens care hygiene recommendations and risk of bacterial keratitis (Lim et al., 2016; Stapleton and Carnt, 2012). Failure of lens care solutions has also been connected to significant outbreaks of fungal and protozoan keratitis (Chang et al., 2006; Khor et al., 2006; Verani et al., 2009).
There are multiple reasons for disinfection failure. Lens care solutions can vary in antimicrobial efficacy in situ depending on their composition (Mohammadinia et al., 2012; Wu et al., 2010b). Additionally, there can be inherent differences among individual bacteria, even of the same species. This can, in turn, select the most virulent among them. For example, research in our laboratory showed variable inherent resistance to lens care disinfectants among P. aeruginosa strains correlating with their relative cytotoxicity activity towards corneal epithelial cells (Lakkis and Fleiszig, 2001). This was directly related to the expression of ExoU, the most potent cytotoxin encoded by P. aeruginosa (Lakkis and Fleiszig, 2001). In this way, inefficient lens care disinfection might contribute to the skewed high prevalence of cytotoxic/ExoU-encoding P. aeruginosa strains among contact-lens related keratitis isolates (discussed previously under the T3SS, Section 3.3).
As discussed earlier (section 4.5.2), bacteria can become more resistant to killing when grown within a biofilm. Directly relevant here, strains of P. aeruginosa, S. aureus, and S. marcescens normally susceptible to killing by biguanide-preserved multipurpose lens care solutions (> 6-log viability reduction) became almost completely resistant when grown as biofilms on the surface of lotrafilcon A silicone hydrogel lenses (Szczotka-Flynn et al., 2009). Similar resistance has been shown to occur when biofilms form within a contact lens case (Szczotka-Flynn and Chalmers, 2013; Wilson et al., 1991). Importantly, manufacturer guidelines for cleaning lens cases that typically recommend a rinse with a multipurpose disinfection solution followed by an air-dry, are often ineffectual at removing bacterial biofilms (,Wu et al., 2010b 2011). Inclusion of a simple rubbing step in lens case disinfection routines can significantly reduce accumulated biofilm, and is thus a valuable addition to manufacturer recommendations (,Wu et al., 2010b 2011).
In Section 4.5.2, the potential for evolutionary adaptations to occur when bacteria colonize a lens in vivo was discussed. It is quite possible that long-term exposure to lens care disinfectant solutions within storage cases may also lead to heritable evolutionary adaptations. In this way, extended use of a lens case could serve as a silent reservoir for continual adaptation and reseeding of increasingly more virulent/more resistant P. aeruginosa onto the ocular surface, a topic that has received little attention to date.
Also worth considering here, lens care solutions might also influence infection susceptibility independently of their direct interactions with microbes if they compromise intrinsic resistance (Carnt et al., 2007; Willcox, 2013c). Indeed, lens care solutions containing boric acid can alter expression of MUC1 and MUC16 in human corneal epithelial cells in vitro and rat corneas in vivo (Tchedre et al., 2013). As discussed earlier, such cell surface associated mucins are known to contribute to defense against infection. Thus, in some instances lens care solutions might work synergistically with microbes to compromise defense.
4.6.2. Generation of viable but non-culturable bacteria and other persistent forms
Multiple events surrounding lens care raise concerns about the emergence of viable but non-culturable bacteria (VBNCs) and bacterial persister cells. These include; biofilm formation, failed disinfection regimens, and bacterial starvation in lens storage solutions. Importantly, there are always persisters when an antimicrobial is used to kill bacteria, and these can recover to become normal (fully culturable, virulent, and antibiotic susceptible) given sufficient time without the antimicrobial agent. This is an entirely different phenomenon from antimicrobial resistance, which is much less common and more permanently changes the microbe to resist the antimicrobial.
Persister cells represent subpopulations of bacteria with reduced or absent growth and/or metabolic activity (Fisher et al., 2017; Wood et al., 2013). Their reduced growth rates confer transient antibiotic tolerance, and they are thought to occur among nearly all bacterial species. VBNCs are again a different subpopulation of bacterial cells with antibiotic tolerance, although evidence suggests they are metabolically active (Ramamurthy et al., 2014). Both populations may overlap to some degree, part of a “dormancy continuum” (Ayrapetyan et al., 2015), but for which there could also be distinct, and important, phenotypic differences beyond metabolic activity. Nevertheless, the fact that antimicrobial treatment targeting a mostly susceptible population does not eradicate persisters/VBNCs (that can later resume growth) makes it difficult to completely prevent recurrent contamination. Clearly, the nature of persister and VBNC bacteria may make them difficult to identify via typical culture methods resulting in a potential underestimation of their presence.
Another related phenomenon likely to occur within biofilms in contact lens solutions (or in the eye during lens wear) is development of phenotypic resistance or tolerance. Similar to persisters, phenotypically tolerant bacteria display nonheritable resistance to antimicrobials; however, unlike persisters, this resistance is not due to metabolic inactivity, but rather to altered gene expression or protein production affecting drug extrusion, degradation, inactivation, or target modification (Drenkard and Ausubel, 2002; Fisher et al., 2017; Wiuff et al., 2005).
Metabolically-active subpopulations of P. aeruginosa that have acquired non-heritable tolerance to antimicrobial peptides (AMPs) emerge in P. aeruginosa biofilms in vitro (Pamp et al., 2008). Thus, preexisting biofilms on lenses, and corresponding phenotypic tolerance, could increase the risk of keratitis due to the prominent role of AMPs in protecting against corneal infection (see Section 2.3.6).
It is important to mention two other strategies bacteria can use to escape being killed by antibiotics: filamentation and generation of L-forms. As already discussed, the Corynebacterium spp. on the conjunctiva use the filamentation strategy, probably to help them remain in that location on the conjunctiva despite the expression of antimicrobial peptides by the tissue (see Section 2.1). By definition, bacteria that maintain a filamentous state would be difficult to culture, given that filamentation is elongation without the cell actually dividing. Concerningly, we have observed that throughout the process of filamentation, P. aeruginosa can still actively express its T3SS that delivers toxins into host cells (Movie S1).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.preteyeres.2019.100804
Contrasting with other bacterial forms, L-forms completely lack the bacterial cell wall. In addition to being non-culturable, they cannot be visualized using standard methods. L-forms can be generated after environmental stress, e.g. extreme temperatures, antibiotics, disinfectants, or ultraviolet light (Wright et al., 1988). L-form bacteria have been described primarily in Bacillus subtilis as antibiotic-resistant bacteria that generate excess amounts of cell membrane, leading to cell shape alteration (Kawai et al., 2015). This cell-wall-deficient L-form state is stabilized by an additional mutation in ipsA, a gene that prevents bacterial lysis in B. subtilis by affecting the polyprenoid synthetic pathway (Julsing et al., 2007). There is little published work regarding P. aeruginosa L-forms, but they are known to exist, and thus are likely to be a part of P. aeruginosa persistence.
An example of how extremely resilient P. aeruginosa can become was provided to our research group when we performed an in vivo experiment using our rat model of extended contact lens wear. A suction pen, previously used only to handle P. aeruginosa-inoculated rat lenses, had been ethanol-disinfected, air dried, and then stored dry for 6 months, before being used to place control (uninoculated) lenses onto rat eyes. All of the “control” lenses that were not deliberately inoculated developed P. aeruginosa keratitis caused by the same strain that had not been grown in the laboratory since disinfecting the suction pen. Culture of the infected eyes and suction pen revealed the original bacteria remaining on the suction pen showing that they had retained viability, and infectious capability (Tam et al., 2010). The mechanism(s) behind this finding require further study, but could relate to the ability of some bacterial cells within the original population to enter a state of persistence.
4.6.3. Summary
While designed to reduce microbial contamination of the eye, lens care solutions and lens storage systems might sometimes have considerable impact on the risk of P. aeruginosa keratitis by providing a ready source of bacterial inoculation of the ocular surface, and a conduit for bacterial plasticity that fosters survival via phenotypic and genotypic adaptation and which could favor even greater virulence. This might relate to why P. aeruginosa keratitis sometimes occurs despite good patient compliance with lens care hygiene and the use of disinfection systems. Nevertheless, lens care systems are not the only factor modulating susceptibility to P. aeruginosa keratitis, given that infections can still occur during wear of daily-disposable contact lenses that do not require the use of solutions or lens cases (Dart et al., 2008; Stapleton et al., 2017).
5. Summary and discussion
5.1. Why are certain microbes involved?
Both injury and contact lens-related infections most often involve opportunistic pathogens. By definition, these are the microbes we consider harmless under normal circumstances that can “take advantage” when our defenses are down. While some opportunists have minimal disease-causing capacity, others possess an impressive array of virulence factors. These differ from outright pathogens by being ubiquitous in our environment as a result of their capacity to be adaptive. This has forced us to evolve protective defenses against them. As logic predicts, it is these opportunists that are the most likely to infect us when our defenses are compromised. A good example is P. aeruginosa, which encodes more virulence factors (and strategies for adaptation and resistance to antimicrobials), than most of the most worrisome outright human pathogens, assisted by an unusually large genome encoding a remarkable number of systems for gene regulation. P. aeruginosa can cause disease in virtually any species of animal or plant. It can even quickly and effectively eradicate almost all other bacteria, a talent now being considered for its potential to combat infections caused by other pathogenic bacteria. The virulence potential of P. aeruginosa, its wide environmental distribution, and its unusual capacity to adapt and resist killing, likely explains why it has held the title as the most common cause of contact lens-related infection ever since soft lenses were first introduced in the 1960’s. This naturally leads to the next question, which much of this article has focused on.
5.2. Why is the cornea normally resistant to infection?
Knowing how lens wear predisposes to infection, requires first considering how it maintains its exquisite resistance to infection when a lens is not worn. The fact that corneal epithelial cells become highly susceptible to P. aeruginosa when cultured in vitro shows that they depend upon other in vivo factors to function as a protective barrier against microbes.
In previous sections, we reviewed our current understanding of how the corneal epithelium collaborates with other ocular surface components to prevent microbes from gaining access into the vulnerable corneal stroma, wherein damaging infection-related pathology might be triggered. We have learned that epithelial-associated defenses are multifactorial, including an array of antimicrobial peptides (e.g. defensins, cathelicidin, keratin-derived peptides, RNase7), surfactant proteins, surface-expressed mucins, cell polarity, cell-to-cell junctions, phagocytosis/lysosomal killing of bacteria, and cell desquamation (Alarcon et al., 2011; Augustin et al., 2011; Fleiszig et al., 1995, 1997b; Gipson et al., 2014; Heimer et al., 2013; Ladage et al., 2002; McDermott et al., 2003; McNamara et al., 1999b; Mun et al., 2011; Ni et al., 2005; Tam et al., 2012). We have also learned that corneal staining with fluorescein (used in the clinic to assess surface epithelial integrity) does not necessarily predict infection risk because cells in deeper layers of the epithelium provide a backup. Further, the epithelium does not act alone to protect the corneal stroma, with direct contributions made by the tear fluid overlying the epithelium, and the basal lamina below it.
While tear fluid does not reliably kill P. aeruginosa, or even inhibit its growth, it can modulate bacterial virulence. It can also directly act on corneal epithelial cells to render them less susceptible to microbes. These protective effects of tear fluid involve regulation of gene expression in both the microbe and the corneal epithelial cell. If bacteria overcome the defenses provided by tear fluid and the epithelium, and successfully traverse the corneal epithelium, they next encounter the underlying basal lamina which functions as a size-exclusion filter. This elegant non-specific barrier protects the underlying stroma against bacteria that are larger than its pores, most bacteria being ~1–2 μm versus a pore-size of < 0.2 μm. In principle, this size-exclusion filtering strategy should work irrespective of bacterial Gram-type or antimicrobial susceptibility profile. Basal lamina components can also act indirectly by modulating barrier function of the overlying corneal epithelial cells.
Other players that can contribute to constitutive barrier function include resident CD11c-positive cells (e.g. DCs) that can respond to microbes even when the epithelium is completely healthy. If super-ficial-injury occurs, these cells actually play a role in limiting bacterial adhesion to the corneal epithelium - even if the extent of injury is not sufficient enough to allow adherent bacteria to penetrate beyond the most superficial epithelial cells. Indeed, in this case, CD11c-positive cells can be seen extending processes all the way to the epithelial surface to localize under surface-bound bacteria. The role of these cells in the non-susceptible, subtle-injured state, might be regulatory of other constitutive defenses (such as those mentioned in the previous paragraph), but other mechanisms might be at play possibly involving factors with defensive roles during inflammation.
Since superficial epithelial injury and tear fluid disruption are a common occurrence in daily life, it is fortuitous that there is redundancy among the various contributors to barrier function, and that multiple layers - not just multiple mechanisms - are involved. This sheds light on why researchers studying a wide variety of microbes have had to resort to extreme measures (such as scarification or stromal injection) to enable infection in their animal models.
5.3. How does contact lens wear predispose to infection without directly exposing the corneal stroma?
There is little evidence to suggest that contact lens infection in people arises because of overt or even superficial injury, although superficial injury can occur during lens wear just as it can in daily life when a lens is not worn. Soft lenses cause less mechanical trauma than hard lenses, yet cause more infections. We and others have shown using animal models (mice, rats, and rabbits) and P. aeruginosa that it is not necessary to introduce any form of injury for contact lens-related infection to occur. Introducing deliberate superficial injury had no impact on disease severity or timing (delayed 8 days) in contact lens-wearing rats (Tam et al., 2010). Showing more directly that an epithelial breach is not required for P. aeruginosa to cross the corneal epithelium during lens wear, we have observed it penetrating through intact corneal epithelium in our mouse contact lens model (Metruccio et al., 2019).
How lens wear compromises epithelial defenses against P. aeruginosa to allow it to traverse is likely to be multifactorial given the redundancy among protective mechanisms discussed above. There are various potential mechanisms by which tear fluid biochemistry could be altered. Associated with greatest risk, soft lenses have a bigger impact on reducing tear exchange under the lens during blinking. The resultant stagnation could potentially degrade protective components, enable accumulation of factors arising from the corneal/limbal area surface, and/or exclude factors arising from elsewhere around the ocular surface. Additionally, contact lens surfaces can adsorb tear components, including tear defense factors, potentially reducing tear concentration and altering function. Production of tear components might also be altered if relevant cells are impacted, potentially causing an imbalance of tear factors that could compromise protective mechanisms. Such tear fluid alterations could in turn cause a “chain-reaction” affecting epithelial and basal lamina defenses. While some information exists about how lens wear impacts tear biochemistry, little has been done to study the critical post-lens tear compartment between the lens and corneal surface. However, we have shown that the posterior surface of contact lenses becomes less bacteriostatic against P. aeruginosa after 8 h of wear.
Given the limitations of human experimentation, even less is known about how lens wear directly compromises epithelial or basal lamina barriers to microbes. While sloughed corneal epithelial cells bind more bacteria after lens wear (Fleiszig et al., 1992), given the various in vivo modifiers discussed above (and loss of cell polarity), sloughed cells might not actually predict adherence in situ. Further, susceptibility to bacterial adherence does not necessarily translate to infection susceptibility, as illustrated by our in vivo blotting (superficial injury) model. As another example, bacterial adhesion to sloughed human cells predicted a role for hypoxia, now known not to contribute to infection incidence in people, even though it might impact infection severity.
For bacteria to cross the epithelium without an epithelial breach, as we observed in the mouse model, it follows that epithelial defenses against bacterial adhesion and subsequent traversal must be compromised. As discussed at length above, this likely involves a combination of lens effects on the cornea, lens impacts on the microbe, and microbial impacts on normal defense. Defenses impacted also include some yet to be discovered. Compromise could occur directly or via regulatory elements such as IL-1R, MyD88, TLR4, TLR5, or CD11c-positive cells, which each differentially regulate overlapping aspects of the epithelial barrier to microbes. Since contact lens wear reduces epithelial cell desquamation, as shown in humans and rabbits, increased exposure to microbes adhering to or internalized by stagnated superficial cells might also contribute.
How the basal lamina barrier is compromised to allow bacterial access to the stroma is another open question. This could involve alterations to the epithelial cells that produce and maintain the layer. Alternatively, it might be related to the dendritic cell (DC) and/or neutrophil responses occurring during lens wear, since both cell types can produce enzymes capable of degrading basement membrane proteins. Such cells can cross tissue barriers, and in doing so could create spaces large enough for bacteria to follow. In some infections, bacteria can even “hitch a ride” inside these cell types to cross tissue barriers. Possibly relevant, our imaging experiments (unpublished) after 7 days of uninoculated contact lens wear in mice have shown myeloid-derived cells, possibly DCs, in the corneal epithelium appearing to extend processes across the basal lamina into the stroma (Movie S2).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.preteyeres.2019.100804
Another potential role for dendritic cell/neutrophil responses during contact lens wear might be to prime the cornea for more robust immune responses to pathogen exposure. Studying the impacts of lens wear on innate immune cell distribution and function might provide links between the pathogenesis of inflammation and infection. However, the fact that infection rates are similar for hydrogel and silicone-hydrogel lenses, yet inflammation occurs less often with hydro-gels, suggest that any such relationship will be complex.
5.4. What factors associated with contact lens wear trigger infection susceptibility?
The previous lack of a mouse model amenable to a wide range of modern biological tools meant that most research in this field has utilized human subjects or rabbits. Limitations to what can be done to people or rabbits have made it difficult to prove some important hypotheses, delaying answers to key questions formulated decades ago, spinning off heated debates among researchers in the field, and leading to tangential pursuits. An example was development of silicone hydrogel (high-Dk) lenses as an attempt to try to solve the infection problem. This was based mostly on circumstantial correlative evidence, and that hypoxia was known to cause some other more common and easy to study complications of lens wear. While silicone hydrogel lenses did solve some less serious problems associated with soft lens wear, they did not reduce the infection rate, and introduced some new problems while also increasing inflammatory complications.
Research utilizing human subjects has shown that the ocular surface environment changes in various other ways when a lens is worn. In addition to stagnating the tear film (as discussed above) and exposing the eye to a different array of microbes (via lens, hand, and solution contamination), there can be changes to temperature, osmolarity and pH. Moreover, mechanical forces exerted on the corneal surface are likely to differ from those exerted when a lens is not worn, during both daytime open-eye blinking and nighttime eyelid closure/REM sleep. While the relative contributions of each of these environmental alterations remain to be determined, we now know that multiple cell types are resident at the ocular surface that can respond to virtually all of these potential triggers. This includes epithelial cells, goblet cells, sensory nerve endings, dendritic cells, and neutrophils. Given what is already known about how these cell types respond in other circumstances, there are feasible hypotheses that could be tested.
The pathogen itself might contribute to corneal defense compromise during lens wear, in addition to taking advantage of lens-mediated effects. While P. aeruginosa does not normally cause corneal infection (or even bind to the cornea), this might be a question of timing. When a lens is not worn, P. aeruginosa is quickly cleared from the ocular surface. However, if it is trapped for sufficient time under or on a lens, it will almost certainly deploy adaptations to enhance its capacity for persistence and counter defenses. This master of adaptation possesses more than 70 two-component regulatory systems used to sense the environment, and in response it can alter the regulation of a plethora of factors involved in survival, antimicrobial resistance, and virulence.
Our studies using a rat lens-wearing model support the notion that bacterial adaptations do contribute to contact lens-related infection. While we found a long delay in disease initiation (~8 days) that was not shortened by introducing superficial injury, lenses removed from already-infected rats that harbored in vivo formed biofilm caused infection more rapidly (2 days), suggesting improved virulence with practice. Moreover, our yet to be published RNA-seq studies have revealed a large number of genes altered in regulation when P. aeruginosa is exposed to human tear fluid, or to human corneal epithelial cells grown in culture. As expected, these include many genes involved in virulence, adaptation, and resistance to killing.
Another strategy by which P. aeruginosa could prime the cornea given sufficient time would be via OMV release. As discussed earlier, P. aeruginosa releases OMVs when exposed to human tear fluid, that when added to mouse eyes in vivo, can kill superficial corneal epithelial cells, and prime the cornea for adhesion by subsequently added P. aeruginosa. Given their small size, OMVs should (in principle) be able to penetrate through the pores of the basal lamina.
P. aeruginosa itself might also cross the basal lamina under some circumstances, given that it produces many proteases, including some able to break down basement membrane components. Because their production can be regulated by quorum sensing (regulated by bacterial density), the key to enabling this might be a critical mass of bacteria reaching the basal lamina.
Whether P. aeruginosa can adopt alternate survival states (e.g. L-forms, VBNCs, persisters, filaments) in lens care solutions, on or under worn lenses, in tear fluid, or in the cornea is not yet known. If so, this would render them difficult to detect using standard methods. Meanwhile, they might be even more resistant to killing by antimicrobials, including possibly those derived from host cells and disinfectants used in lens care solutions.
Other clues to the pathogenesis of contact lens-related infection come from results of meticulous epidemiological research using human subjects that has been done by other investigators. Those efforts, showing that infections occur more commonly with extended wear, suggest that induction of susceptibility takes time. The lower severity of corneal infection with daily disposable lenses versus conventional daily wear lenses (in which lenses are cleaned and reused) strongly implicates a role for use of lens care solutions or factors depositing on the lens (possibly microbes or their debris). Greater incidence during soft versus rigid lens wear points to lack of tear exchange, coverage of the limbus, or the very close apposition associated with soft lenses. The latter possibility is further supported by the increased infection risk with reverse-geometry rigid lenses that sit closer to the cornea than regular rigid lenses, and the extremely low risk associated with scleral lenses that vault far away from corneal and limbal surfaces, yet completely stagnate tear fluid and cover the limbus. The similar incidence of infection with silicone hydrogels and conventional hydrogel lenses, but different risk of inflammation, suggests any relationship between infection and inflammation is likely to be complex. The demonstrated increased risk for males suggests hormonal influences possibly via known different healing-inflammatory mediator responses. Association with youth suggests a capacity for a more robust immune response might increase risk, as is the case for other diseases targeting this age group. Increased risk for smokers combined with the relatively common occurrence of P. aeruginosa corneal infection in the high air pollution environs of India (even in the absence of lens wear or overt injury) suggests the contribution of an “irritant” effect. Associations with non-compliance, solution contamination, showering and swimming all point towards an increased exposure to microbes. There appears to be little evidence that lens material, or type of disinfectant used, influences the risk of infection for P. aeruginosa, but has been related to occasional outbreaks with other opportunistic microbes. These many fascinating correlations and associations have resulted from painstaking research done by multiple individuals in the field. They form a solid foundation for further research aimed at proving causation and establishing mechanisms. That next step will be necessary if we are to eventually solve the problem of lens-related corneal infections.
6. Future research
If we are to understand why contact lens wear predisposes to infection, research will need to continue in three inter-related topics: 1) mechanisms by which the cornea resists infection when healthy, 2) how lens wear compromises key components of that resistance, and 3) how microbes take advantage of the situation to cause disease. Specific questions that require further investigation include:
Which epithelial-associated defenses are compromised by contact lens wear and how? Understanding this will require studying known defenses (e.g. antimicrobial peptides, mucins, cell polarity/junctions, phagocytosis and intracellular killing of bacteria), while continuing to uncover other contributors yet to be discovered. Ideally this would be done using in vivo contact lens wear models given the complexities and redundancies discussed previously. It will be of utmost importance to circle back and determine which of the impacted defenses actually contribute to enabling infection.
Does lens wear compromise regulators of epithelial defense against traversal, i.e. IL-1R, MyD88, TLR4, and TLR5 signaling, or the dendritic cell response to microbes? Each of these is important for maintaining epithelial barrier function against P. aeruginosa, but there are likely to be others also involved.
What is the significance of reduced cell sloughing during lens wear? Superficial epithelial cells engulf bacteria, and P. aeruginosa can exploit this to establish an intracellular niche. It would be important to know if contact lens wear stagnates sloughed cells containing intracellular bacteria either on the cornea or under the lens, and if so, the contribution to initiation of infection.
How does lens wear impact the biochemistry of the post-lens tear film? Only the conjunctival sac tear film has been extensively studied with and without lens wear. Important here will be to determine if changes found actually contribute to compromising functional effects of tears, such as their ability to kill bacteria, suppress virulence, or to reprogram epithelial cells to be more resistant.
Does lens wear impact polymodal nociceptors to impact defense? These can respond to changes in temperature, osmolarity, pH, microbes, mechanical forces, and are sensitized by inflammatory mediators. In turn, they can release modulators of inflammation.
Does parainflammation induced by lens wear play a role in defense against microbes or in mediating pathology?
Does lens wear impact the overnight tear fluid neutrophil response to affect defense or susceptibility to infection? Even without lens-wear, the ocular surface contains a significant number of neutrophils during eye closure. While these have been shown to be in a relatively quiescent state, their function has not yet been determined, and it is not known if they can contribute to disease. We have observed neutrophils containing intracellular bacteria trapped between the lens and cornea in a mouse lens infection model (Metruccio et al., 2019). Does trapping (or exclusion) of tear neutrophils influence susceptibility to infection during lens wear in either direction? What about other impacts of eye closure during lens wear on pathogenesis? The closed eye has been shown to have different tear fluid biochemistry, but how lens wear impacts that in the post-lens tear film remains unexplored.
What happens to the basal lamina during lens wear? Does lens wear alter its structure or function? Do epithelial cells or traversing bacteria produce proteases or other factors that break it down?
Do specific bacterial adaptations to the ocular surface during lens wear contribute to driving infection? Is there a role for alternate bacterial forms such as filamentation, L-forms, persisters, or VBNCs. What host factors drive these adaptations?
What is the role of bacterial OMVs? Tear fluid triggers their production and they prime the cornea for P. aeruginosa adhesion. Bacteria trapped under or on the lens are likely to release them. Is this the key to breaking down the epithelial barrier during lens wear? Are they delivered across the basal lamina to drive pathology in the stroma? If so, that pathology might subsequently feedback to disrupt the basal lamina and thereby also allow access of the larger microbe.
What is the role of the adjacent conjunctiva in the corneal epithelial barrier during health, and in driving lens-related parainflammation? Moreover, does the palpebral conjunctiva (under the eyelids) normal apposition/direct contact with the cornea play roles in why the healthy cornea is resistant to bacteria? If so, what happens when these tissues are physically separated by a contact lens?
What is the role of genetic factors in contact lens-related P. aeruginosa keratitis? Studies to date have shown some associations between single nucleotide polymorphisms (SNPs) in genes encoding cytokines (e.g. IL-6) with severity of microbial keratitis (Carnt et al., 2012; Stapleton and Carnt, 2012). Given the multifactorial nature of corneal defenses discussed in this article, and the increasing ease and availability of genetic analysis, pursuit of genetic risk factors is an important avenue for investigation.
7. Conclusion
This manuscript illustrates that the pursuit of a seemingly simple question can sometimes occupy an entire career. The quest by us and others to determine how contact lens wear causes infection has driven forays into multiple topics in biology, leading to important information about ocular surface biology in general. It has also provided novel insights about an important human pathogen with relevance beyond the eye.
Things have moved on since the first author of this paper wrote the concluding sentence of her PhD thesis (circa 1991) with a similar title to this paper. It stated; “Thus, contact lens-related infectious keratitis remains a serious health issue for which there is no obvious solution”. Much has been learned since that time about the biology of the eye, the microbes that cause lens-related infection, and about biological processes in general. There are now modern biological tools that continue to evolve, including methods for screening and manipulating genomes and proteomes, quantitative high-resolution in vivo imaging, computational methods that can handle large data sets, and animal models for lens wear amenable to all of these tools. This leads to more confidence that contact lens-related infection can be understood and solved despite its complexities, and that the significance of doing so will extend beyond the topic of contact lens wear.
Supplementary Material
Acknowledgements
Drs. Fleiszig and Evans gratefully acknowledge funding of this research over several decades by the National Eye Institute (currently active EY011221, EY024060, EY030350), and the National Institute of Allergy and Infectious Diseases (AI079192). Dr. Kroken was supported by the National Eye Institute (EY025069) as is Dr. Nieto (EY029152). Dr. Wan was supported in part by the UC Berkeley Vision Science Training Grant from the National Eye Institute (EY007043). Dr. Grosser was supported by a fellowship from the American Heart Association. Generous support for this research has also been provided by Alcon Laboratories Inc., Allergan Inc., and CooperVision Inc. We would like to express our sincere thanks and appreciation to the many undergraduate and graduate students, post-doctoral fellows, staff research associates, and junior faculty researchers of the Fleiszig-Evans laboratory for their invaluable contributions to this research over several decades (see cited publications). We would also like to express our gratitude to our numerous colleagues, collaborators and mentors for their ongoing collegiality, support, and friendship. A special thank you to Dr. Nathan Efron, who sparked the first author’s interest in research, continues to serve as a thoughtful and generous mentor, and who has an uncanny knack of remaining on the same wavelength despite our divergent approaches.
Footnotes
Ethics statement
All procedures involving animals described in this manuscript, and our other published work, were carried out in accordance with standards established by the Association for Research in Vision and Ophthalmology, under a protocol approved by the Animal Care and Use Committee, University of California Berkeley, an AAALAC accredited institution. All of our studies involving human subjects were conducted under protocols approved by the Committee for the Protection of Human Subjects, University of California, Berkeley. Our research has followed the tenets of the Declaration of Helsinki.
Declaration of competing interest
Drs. Fleiszig and Evans gratefully acknowledge funding of this research over several decades by the National Eye Institute (currently active EY011221, EY024060, EY030350), and the National Institute for Allergy and Infectious Diseases. Dr. Kroken was supported by the National Eye Institute (EY025069) as is Dr. Nieto (EY029152). Dr. Wan was supported in part by the UC Berkeley Vision Science Training Grant from the National Eye Institute (EY007043). Dr. Grosser was supported by a fellowship from the American Heart Association. Generous support for this research has also been provided by Alcon Laboratories Inc., Allergan Inc., and CooperVision Inc. The funding agencies had no role in study design, collection, analysis and interpretation of data, writing the manuscript, or decision to publish. Drs. Fleiszig and Evans are identified as co-inventors on two US patents; US 9,545,461 B2 (therapeutic use of keratin-derived antimicrobial peptides) and US 7,332,470 B2 (therapeutic use of collectins). Dr. Fleiszig is also identified as a co-inventor on one US patent US 6,984,622 B2 (upregulation of antimicrobial peptides for therapeutic purposes). The authors have no other competing interests.
References
- Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ, 2000. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 299, 39–46. 10.1007/s004410050004. [DOI] [PubMed] [Google Scholar]
- AbuSamra DB, Argüeso P, 2018. Lectin-glycan interactions in corneal infection and inflammation. Front. Immunol 9, 2338 10.3389/fimmu.2018.02338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnello M, Wong-Beringer A, 2012. Differentiation in quinolone resistance by virulence genotype in Pseudomonas aeruginosa. PLoS One 7, e42973 10.1371/journal.pone.0042973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T, 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol 2, 675–680. 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Alarcon I, Evans DJ, Fleiszig SMJ, 2009a. The role of twitching motility in Pseudomonas aeruginosa exit from and translocation of corneal epithelial cells. Investig. Ophthalmol. Vis. Sci 50, 2237–2244. 10.1167/iovs.08-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon I, Kwan L, Yu C, Evans DJ, Fleiszig SMJ, 2009b. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect. Immun 77, 3264–3271. 10.1128/IAI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon I, Tam C, Mun JJ, LeDue J, Evans DJ, Fleiszig SMJ, 2011. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Investig. Ophthalmol. Vis. Sci 52, 1368–1377. 10.1167/iovs.10-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T, 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol 59, 1114–1128. 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- Allured VS, Collier RJ, Carroll SF, McKay DB, 1986. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc. Natl. Acad. Sci. U. S. A 83, 1320–1324. 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani Y, Pritchard N, Efron N, 2016. Changes in corneal Langerhans cell density during the first few hours of contact lens wear. Contact Lens Anterior Eye 39, 307–310. 10.1016/j.clae.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Schmalzer KM, Sato H, Casey M, Terhune SS, Haas AL, Feix JB,Frank DW, 2011. Ubiquitin and ubiquitin-modified proteins activate the Pseudomonas aeruginosa T3SS cytotoxin, ExoU. Mol. Microbiol 82, 1454–1467. 10.1111/j.1365-2958.2011.07904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus AA, Evans DJ, Barbieri JT, Fleiszig SMJ, 2010. The ADP-ribosylation domain of Pseudomonas aeruginosa ExoS is required for membrane bleb niche formation and bacterial survival within epithelial cells. Infect. Immun 78, 4500–4510. 10.1128/IAI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus AA, Lee AA, Augustin DK, Lee EJ, Evans DJ, Fleiszig SMJ, 2008Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect. Immun 76, 1992–2001. 10.1128/IAI.01221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Bomsel M, Lindstedt R, Engel J, Frank D, Mostov K, Wiener-Kronish J, 1995. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: Glycosylation-defective host cells are resistant to bacterial killing. Infection and Immunity 63, 1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H, 1995. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci 36, 614–621. [PubMed] [Google Scholar]
- Augustin DK, Heimer SR, Tam C, Li WY, Le Due JM, Evans DJ, Fleiszig SMJ, 2011. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect. Immun 79, 595–605. 10.1128/IAI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayrapetyan M, Williams TC, Oliver JD, 2015. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol. 23, 7–13. 10.1016/J.TIM.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Azghani AO, 1996. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am. J. Respir. Cell Mol. Biol 15, 132–140. 10.1165/ajrcmb.15.1.8679217. [DOI] [PubMed] [Google Scholar]
- Azghani AO, Gray LD, Johnson AR, 1993. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect. Immun 61, 2681–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AJ, 1987. Structure, function and ageing of the collagens of the eye. Eye 1,175–183. 10.1038/eye.1987.34. [DOI] [PubMed] [Google Scholar]
- Barbieri JT, Sun J, 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol 152, 79–92. 10.1007/s10254-004-0031-7. [DOI] [PubMed] [Google Scholar]
- Barrett TC, Mok WWK, Murawski AM, Brynildsen MP, 2019. Enhanced antibiotic resistance development from fluoroquinolone persisters after a single exposure to antibiotic. Nat. Commun 10, 1177 10.1038/s41467-019-09058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R, 2003. Toll-like receptor signaling pathways. Science 300, 1524–1525. 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- Belyy A, Raoux-Barbot D, Saveanu C, Namane A, Ogryzko V, Worpenberg L,David V, Henriot V, Fellous S, Merrifield C, Assayag E, Ladant D, Renault L, Mechold U, 2016. Actin activates Pseudomonas aeruginosa ExoY nucleotidyl cyclase toxin and ExoY-like effector domains from MARTX toxins. Nat. Commun 7, 13582 10.1038/ncomms13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler BA, 2004. Inferences, questions and possibilities in Toll-like receptor signalling.Nature 430, 257–263. 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- Beutler BA, 2009. TLRs and innate immunity. Blood 113, 1399–1407. 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikker FJ, Ligtenberg AJM, Nazmi K, Veerman ECI, Hof WVNT, Bolscher JGM, Poustka A, Nieuw Amerongen AV, Mollenhauer J, 2002. Identification of the bacteria-binding peptide domain on salivary agglutinin (gp-340/DMBT1), a member of the scavenger receptor cysteine-rich superfamily. J. Biol. Chem 277, 32109–32115. 10.1074/jbc.M203788200. [DOI] [PubMed] [Google Scholar]
- Blair DF, 2003. Flagellar movement driven by proton translocation. FEBS Lett. 545,86–95. 10.1016/S0014-5793(03)00397-1. [DOI] [PubMed] [Google Scholar]
- Boedicker JQ, Vincent ME, Ismagilov RF, 2009. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angew. Chem. Int. Ed 48, 5908–5911. 10.1002/anie.200901550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomberger JM, MacEachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA, 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5, e1000382 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomberger JM, Ye S, MacEachran DP, Koeppen K, Barnaby RL, O’Toole GA, Stanton BA, 2011. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 7, e1001325 10.1371/journal.ppat.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkar DS, Acharya NR, Leong C, Lalitha P, Srinivasan M, Oldenburg CE,Cevallos V, Lietman TM, Evans DJ, Fleiszig SMJ, 2014. Cytotoxic clinical isolates of Pseudomonas aeruginosa identified during the Steroids for Corneal Ulcers Trial show elevated resistance to fluoroquinolones. BMC Ophthalmol. 14, 54 10.1186/1471-2415-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkar DS, Fleiszig SMJ, Leong C, Lalitha P, Srinivasan M, Ghanekar AA, Tam C, Li WY, Zegans ME, McLeod SD, Lietman TM, Acharya NR, 2013. Association between cytotoxic and invasive Pseudomonas aeruginosa and clinical outcomes in bacterial keratitis. JAMA Ophthalmol. 131, 147–153. 10.1001/jamaophthalmol.2013.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J, Zhang Q, Vyawahare S, Rogers E, Rosenberg SM, Austin RH, 2014. Emergence of antibiotic resistance from multinucleated bacterial filaments. Proc. Natl. Acad. Sci 112, 178–183. 10.1073/pnas.1420702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragonzi A, Farulla I, Paroni M, Twomey KB, Pirone L, Lorè NI, Bianconi I, Dalmastri C, Ryan RP, Bevivino A, 2012. Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS One 7, e52330 10.1371/journal.pone.0052330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Nikaido H, 1995. The envelope of mycobacteria. Annu. Rev. Biochem 64,29–63. 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- Bucior I, Mostov K, Engel JN, 2010. Pseudomonas aeruginosa-mediated damage requires distinct receptors at the apical and basolateral surfaces of the polarized epithelium. Infect. Immun 78, 939–953. 10.1128/IAI.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucior I, Pielage JF, Engel JN, 2012. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 8, e1002616 10.1371/journal.ppat.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TH, Cavanagh HD, Robertson DM, 2010. Patient compliance during contact lens wear: perceptions, awareness, and behavior. Eye Contact Lens 36, 334–339. 10.1097/ICL.0b013e3181f579f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham GW, Cavanagh HD, Robertson DM, 2012. The impact of cellular debris on Pseudomonas aeruginosa adherence to silicone hydrogel contact lenses and contact lens storage cases. Eye Contact Lens 38, 7–15. 10.1097/icl.0b013e31823bad0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows LL, 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol 66, 493–520. 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- Caballero A, Thibodeaux B, Marquart M, Traidej M, O’Callaghan R, 2004Pseudomonas keratitis: protease IV gene conservation, distribution, and production relative to virulence and other Pseudomonas proteases. Investig. Ophthalmol. Vis. Sci 45, 522–530. 10.1167/iovs.03-1050. [DOI] [PubMed] [Google Scholar]
- Carnt N, Jalbert I, Stretton S, Naduvilath T, Papas E, 2007. Solution toxicity in soft contact lens daily wear is associated with corneal inflammation. Optom. Vis. Sci 84, 309–315. 10.1097/OPX.0b013e318046551b. [DOI] [PubMed] [Google Scholar]
- Carnt N, Stapleton F, 2016. Strategies for the prevention of contact lens-related Acanthamoeba keratitis: a review. Ophthalmic Physiol. Opt 36, 77–92. 10.1111/opo.12271. [DOI] [PubMed] [Google Scholar]
- Carnt NA, Willcox MDP, Hau S, Garthwaite LL, Evans VE, Radford CF, Dart JKG, Chakrabarti S, Stapleton F, 2012. Association of single nucleotide polymorphisms of interleukins-1β, −6, and −12B with contact lens keratitis susceptibility and severity. Ophthalmology 119, 1320–1327. 10.1016/j.ophtha.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Chan JKL, Yuen D, Too PHM, Sun Y, Willard B, Man D, Tam C, 2018. Keratin 6a reorganization for ubiquitin-proteasomal processing is a direct antimicrobial response. J. Cell Biol 217, 731–744. 10.1083/jcb.201704186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DC, Grant GB, O’Donnell K, Wannemuehler KA, Noble-Wang J, Rao CY, Jacobson LM, Crowell CS, Sneed RS, Lewis FMT, Schaffzin JK, Kainer MA, Genese CA, Alfonso EC, Jones DB, Srinivasan A, Fridkin SK, Park BJ, 2006. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. J. Am. Med. Assoc 296, 953–963. 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- Chao C, Stapleton F, Willcox MDP, Golebiowski B, Richdale K, 2017Preinflammatory signs in established reusable and disposable contact lens wearers. Optom. Vis. Sci 94, 1003–1008. 10.1097/OPX.0000000000001129. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PGH, Geerards AJM, Kijlstra A, 1999. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 354, 181–185. 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- Cho CH, Lee S-B, 2018. Comparison of clinical characteristics and antibiotic susceptibility between Pseudomonas aeruginosa and P. putida keratitis at a tertiary referral center: a retrospective study. BMC Ophthalmol. 18, 204 10.1186/s12886-018-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Kim DK, Choi SJ, Lee J, Choi JP, Rho S, Park SH, Kim YK, Hwang D, Gho YS, 2011. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 11, 3424–3429. 10.1002/pmic.201000212. [DOI] [PubMed] [Google Scholar]
- Choy MH, Stapleton F, Willcox MDP, Zhu H, 2008. Comparison of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens- and non-contact lens-related keratitis. J. Med. Microbiol 57, 1539–1546. 10.1099/jmm.0.2008/003723-0. [DOI] [PubMed] [Google Scholar]
- Co JY, Cárcamo-Oyarce G, Billings N, Wheeler KM, Grindy SC, Holten-Andersen N, Ribbeck K, 2018. Mucins trigger dispersal of Pseudomonas aeruginosa biofilms. NPJ Biofilms Microbiomes 4, 23 10.1038/s41522-018-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SJ, Records AR, Orr MW, Linden SB, Lee VT, 2014. Catheter-associated urinary tract infection by Pseudomonas aeruginosa is mediated by exopolysaccharide-independent biofilms. Infect. Immun 82, 2048–2058. 10.1128/iai.01652-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comolli JC, Hauser AR, Waite L, Whitchurch CB, Mattick JS, Engel JN, 2002. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun 70, 3625–3630. 10.1128/IAI.70.1.1-4.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear TCR, Willcox MDP, Flanagan JL, Zhu H, 2012. Characterization of protease IV expression in Pseudomonas aeruginosa clinical isolates. J. Med. Microbiol 61, 180–190. 10.1099/jmm.0.034561-0. [DOI] [PubMed] [Google Scholar]
- Cowell BA, Chen DY, Frank DW, Vallis AJ, Fleiszig SMJ, 2000. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect. Immun 68, 403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell BA, Evans DJ, Fleiszig SMJ, 2005. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol. Lett 250, 71–76. 10.1016/j.femsle.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Cowell BA, Twining SS, Hobden JA, Kwong MSF, Fleiszig SMJ, 2003. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology 149, 2291–2299. 10.1099/mic.0.26280-0. [DOI] [PubMed] [Google Scholar]
- Craig JP, Willcox MDP, Argüeso P, Maissa C, Stahl U, Tomlinson A, Wang J, Yokoi N, Stapleton F, 2013. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Investig. Ophthalmol. Vis. Sci 54, 123–156. 10.1167/iovs.13-13235.TFOS. [DOI] [PubMed] [Google Scholar]
- Cubitt CL, Tang Q, Monteiro C. a, Lausch RN, Oakes JE, 1993. IL-8 gene expression in cultures of human corneal epithelial cells and keratocytes. Investig. Ophthalmol. Vis. Sci 34, 3199–3206. [PubMed] [Google Scholar]
- Danjo Y, Hazlett LD, Gipson IK, 2000. C57BL/6 mice lacking Muc1 show no ocular surface phenotype. Investig. Ophthalmol. Vis. Sci 41, 4080–4084. [PubMed] [Google Scholar]
- Dart JKG, Radford CF, Minassian D, Verma S, Stapleton F, 2008. Risk factors for microbial keratitis with contemporary contact lenses. A case-control Study. Ophthalmology 115, 1647–1654. 10.1016/j.ophtha.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Dartt DA, Willcox MDP, 2013. Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp. Eye Res 117, 1–3. 10.1016/j.exer.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Wolfgang MC, Goodman AL, Arora SK, Jyot J, Lory S, Ramphal R, 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol 50, 809–824. 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- Davies EV, James CE, Brockhurst MA, Winstanley C, 2017. Evolutionary diversification of Pseudomonas aeruginosa in an artificial sputum model. BMC Microbiol. 17,3 10.1186/s12866-016-0916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies EV, James CE, Williams D, O’Brien S, Fothergill JL, Haldenby S, Paterson S, Winstanley C, Brockhurst MA, 2016. Temperate phages both mediate and drive adaptive evolution in pathogen biofilms. Proc. Natl. Acad. Sci 113, 8266–8271. 10.1073/pnas.1520056113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguine J, Wei J, Barbalat R, Gronert K, Barton GM, 2017. Local TNFR1 signaling licenses murine neutrophils for increased TLR-dependent cytokine and eicosanoid production. J. Immunol 198, 2865–2875. 10.4049/jimmunol.1601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon S, Clinton A, Fowler H, Everett J, Horswill AR, Rumbaugh KP, 2014. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect. Immun 82, 4718–4728. 10.1128/IAI.02198-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Barbieri JT, 2008. Modulation of host cell endocytosis by the type III cytotoxin, Pseudomonas ExoS. Traffic 9, 1948–1957. 10.1111/j.1600-0854.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, King JM, Yahr TL, 2011. Intrinsic and extrinsic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Front. Microbiol 2, 89 10.3389/fmicb.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JM, Young CA, Baldone JA, Halberg GP, Sampson W, Stone W, 1966. Complications associated with the wearing of contact lenses. J. Am. Med. Assoc 195, 901–903. 10.1001/jama.1966.03100110069017. [DOI] [PubMed] [Google Scholar]
- Doan T, Akileswaran L, Andersen D, Johnson B, Ko N, Shrestha A, Shestopalov V, Lee CS, Lee AY, Van Gelder RN, 2016. Paucibacterial microbiome and resident DNA virome of the healthy conjunctiva. Investig. Ophthalmol. Vis. Sci 57, 5116–5126. 10.1167/iovs.16-19803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C, 1998. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta 1406, 251–259. 10.1016/S0925-4439(98)00010-6. [DOI] [PubMed] [Google Scholar]
- Dong Q, Brulc JM, Iovieno A, Bates B, Garoutte A, Miller D, Revanna KV, Gao X, Antonopoulos DA, Slepak VZ, Shestopalov VI, 2011. Diversity of bacteria at healthy human conjunctiva. Investig. Ophthalmol. Vis. Sci 52, 5408–5413. 10.1167/iovs.10-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenkard E, Ausubel FM, 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416, 740–743. 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ, 1996. Origins of cell polarity. Cell 84, 335–344. 10.1016/S0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Duong K, Chao C, Willcox M, Richdale K, 2017. Changes in tear cytokines following a short period of daily and overnight silicone hydrogel lens wear. J. Contact Lens Res. Sci 1, 3–11. 10.22374/jclrs.v1i1.10. [DOI] [Google Scholar]
- Dutta D, Vijay AK, Kumar N, Willcox MDP, 2016. Melimine-coated antimicrobial contact lenses reduce microbial keratitis in an animal model. Investig. Ophthalmol. Vis. Sci 57, 5616–5624. 10.1167/iovs.16-19882. [DOI] [PubMed] [Google Scholar]
- Efron N, 2017. Contact lens wear is intrinsically inflammatory. Clin. Exp. Optom 100, 3–19. 10.1111/cxo.12487. [DOI] [PubMed] [Google Scholar]
- Efron N, Al-Dossari M, Pritchard N, 2010. Confocal microscopy of the bulbar conjunctiva in contact lens wear. Cornea 29, 43–52. 10.1097/ICO.0b013e3181acf82a. [DOI] [PubMed] [Google Scholar]
- Efron N, Brennan NA, Bright FV, Glasgow BJ, Jones LW, Sullivan DA,Tomlinson A, Zhang J, 2013. 2. Contact lens care and ocular surface homeostasis. Contact Lens Anterior Eye 36 (Suppl. 1). 10.1016/S1367-0484(13)60004-1.S9-13. [DOI] [PubMed] [Google Scholar]
- Eierhoff T, Bastian B, Thuenauer R, Madl J, Audfray A, Aigal S, Juillot S, Rydell GE, Muller S, De Bentzmann S, Imberty A, Fleck C, Romer W, 2014. A lipid zipper triggers bacterial invasion. Proc. Natl. Acad. Sci 111, 12895–12900. 10.1073/pnas.1402637111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Balachandran P, 2009. Role of Pseudomonas aeruginosa type III effectors in disease. Curr. Opin. Microbiol 12, 61–66. 10.1016/j.mib.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Engel LS, Hill JM, Caballero AR, Green LC, O’Callaghan RJ, 1998. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J. Biol. Chem 273, 16792–16797. 10.1074/jbc.273.27.16792. [DOI] [PubMed] [Google Scholar]
- Engel LS, Hobden JA, Moreau JM, Callegan MC, Hill JM, O’Callaghan RJ, 1997. Pseudomonas deficient in protease IV has significantly reduced corneal virulence. Investig. Ophthalmol. Vis. Sci 38, 1535–1542. [PubMed] [Google Scholar]
- Epstein SS, 2013. The phenomenon of microbial uncultivability. Curr. Opin. Microbiol 16, 636–642. 10.1016/j.mib.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Allison DG, Brown MRW, Gilbert P, 1991. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: effect of specific growth rate. J. Antimicrob. Chemother 27, 177–184. 10.1093/jac/27.2.177. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Fleiszig SMJ, 2013. Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am. J. Ophthalmol 155, 961–970. 10.1016/j.ajo.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DJ, Frank DW, Finck-Barbançon V, Wu C, Fleiszig SMJ, 1998. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect. Immun 66, 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DJ, Kuo T, Kwong M, Van R, Fleiszig SMJ, 2002a. Pseudomonas aeruginosa strains with lipopolysaccharide defects exhibit reduced intracellular viability after invasion of corneal epithelial cells. Exp. Eye Res 75, 635–643. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Kuo TC, Kwong M, Van R, Fleiszig SMJ, 2002b. Mutation of csk, encoding the C-terminal Src kinase, reduces Pseudomonas aeruginosa internalization by mammalian cells and enhances bacterial cytotoxicity. Microb. Pathog 33, 135–143. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Maltseva IA, Wu J, Fleiszig SMJ, 2002c. Pseudomonas aeruginosa internalization by corneal epithelial cells involves MEK and ERK signal transduction proteins. FEMS Microbiol. Lett 213, 73–79. [DOI] [PubMed] [Google Scholar]
- Evans DJ, McNamara NA, Fleiszig SMJ, 2007. Life at the front: dissecting bacterial-host interactions at the ocular surface. Ocul. Surf 5, 213–227. [DOI] [PubMed] [Google Scholar]
- Felgentreff K, Beisswenger C, Griese M, Gulder T, Bringmann G, Bals R, 2006. The antimicrobial peptide cathelicidin interacts with airway mucus. Peptides 27, 3100–3106. 10.1016/j.peptides.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR, 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147, 2659–2669. 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SMJ, Wu C, Mende-Mueller L, Frank DW, 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol 25, 547–557. [DOI] [PubMed] [Google Scholar]
- Fini ME, Bauskar A, Jeong S, Wilson MR, 2016. Clusterin in the eye: an old dog with new tricks at the ocular surface. Exp. Eye Res 147, 57–71. 10.1016/j.exer.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Fisher RA, Gollan B, Helaine S, 2017. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol 15, 453–464. 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- Flanagan JL, Willcox MDP, 2009. Role of lactoferrin in the tear film. Biochimie 91,35–43. 10.1016/j.biochi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Fleiszig SMJ, Efron N, 1992a. Conjunctival flora in extended wear of rigid gas permeable contact lenses. Optom. Vis. Sci 69, 354–357. [DOI] [PubMed] [Google Scholar]
- Fleiszig SMJ, Efron N, 1992b. Microbial flora in eyes of current and former contact lens wearers. J. Clin. Microbiol 30, 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Evans DJ, Mowrey-McKee MF, Payor R, Zaidi TS, Vallas V,Muller E, Pier GB, 1996a. Factors affecting Staphylococcus epidermidis adhesion to contact lenses. Optom. Vis. Sci 73, 590–594. [DOI] [PubMed] [Google Scholar]
- Fleiszig SMJ, Zaidi TS, Preston MJ, Grout M, Evans DJ, Pier GB, 1996b. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun 64, 2288–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Vallas V, Jun CH, Mok L, Balkovetz DF, Roth MG, Mostov KE, 1998. Susceptibility of epithelial cells to Pseudomonas aeruginosa invasion and cytotoxicity is upregulated by hepatocyte growth factor. Infect. Immun 66, 3443–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW, 1997a. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun 65, 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Zaidi TS, Fletcher EL, Preston MJ, Pier GB, 1994a. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect. Immun 62, 3485–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Zaidi TS, Pier GB, 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun 63, 4072–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, 2006. The Glenn A. Fry award lecture 2005. The pathogenesis of contact lens-related keratitis. Optom. Vis. Sci 83, 866–873. 10.1097/01.opx.0000250045.85499.55. [DOI] [PubMed] [Google Scholar]
- Fleiszig SMJ, Arora SK, Van R, Ramphal R, 2001. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect. Immun 69, 4931–4937. 10.1128/IAI.69.8.4931-4937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Efron N, Pier GB, 1992. Extended contact lens wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Investig. Ophthalmol. Vis. Sci 33, 2908–2916. [PubMed] [Google Scholar]
- Fleiszig SMJ, Evans DJ, Do N, Vallas V, Shin S, Mostov KE, 1997b. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun 65, 2861–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Zaidi TS, Ramphal R, Pier GB, 1994b. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect. Immun 62, 1799–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Kwong MSF, Evans DJ, 2003. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect. Immun 71, 3866–3874. 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EL, Fleiszig SM, Brennan NA, 1993a. Lipopolysaccharide in adherence of Pseudomonas aeruginosa to the cornea and contact lenses. Investig. Ophthalmol. Vis. Sci 34, 1930–1936. [PubMed] [Google Scholar]
- Fletcher EL, Weissman BA, Efron N, Fleiszig SMJ, Curcio AJ, Brennan NA, 1993b. The role of pili in the attachment of Pseudomonas aeruginosa to unworn hydrogel contact lenses. Curr. Eye Res 12, 1067–1071. [DOI] [PubMed] [Google Scholar]
- Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B, 2004. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob. Agents Chemother 48, 3367–3372. 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn KM, Dowell G, Johnson TM, Koestler BJ, Waters CM, Cooper VS, 2016. Evolution of ecological diversity in biofilms of Pseudomonas aeruginosa by altered cyclic diguanylate signaling. J. Bacteriol 198, 2608–2618. 10.1128/JB.00048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldenauer MEB, McClellan SA, Berger EA, Hazlett LD, 2013. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J. Immunol 190, 5649–5658. 10.4049/jimmunol.1203094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Hoiby N, Molin S, 2012. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat. Rev. Microbiol 10, 841–851. 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- Fong JNC, Yildiz FH, 2015. Biofilm matrix proteins. Microbiol. Spectr 3 10.1128/microbiolspec.MB-0004-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte R, Cennamo G, Del Prete S, Cesarano I, Del Prete A, 2010. Scanning electron microscopy of corneal epithelium in soft contact lens wearers. Cornea 29, 732–736. 10.1097/ICO.0b013e3181c32f1a. [DOI] [PubMed] [Google Scholar]
- Fothergill JL, Neill DR, Loman N, Winstanley C, Kadioglu A, 2014. Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat. Commun 5, 4780 10.1038/ncomms5780. [DOI] [PubMed] [Google Scholar]
- Foulks GN, 2006. Prolonging contact lens wear and making contact lens wear safer. Am. J. Ophthalmol 141, 369–373. 10.1016/j.ajo.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Frank DW, Nair G, Schweizer HP, 1994. Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect. Immun 62, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Storey DG, 1994. Regulation of expression of Pseudomonas exotoxin A by iron. Methods Enzymol. 235, 502–517. [DOI] [PubMed] [Google Scholar]
- Fu H, Coburn J, Collier RJ, 1993. The eukaryotic host factor that activates exoenzyme S of Pseudomonas aeruginosa is a member of the 14–3-3 protein family. Proc. Natl. Acad. Sci. U. S. A 90, 2320–2324. 10.1073/pnas.90.6.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Yamada M, Akune Y, Shigeyasu C, Tsubota K, 2016. Fluorophotometric analysis of the ocular surface glycocalyx in soft contact lens wearers. Curr. Eye Res 41, 9–14. 10.3109/02713683.2014.999948. [DOI] [PubMed] [Google Scholar]
- Fulcher NB, Holliday PM, Klem E, Cann MJ, Wolfgang MC, 2010. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Mol. Microbiol 76, 889–904. 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullard RJ, Wilson GS, 1986. Investigation of sloughed corneal epithelial cells collected by non-invasive irrigation of the corneal surface. Curr. Eye Res 5, 847–856. 10.3109/02713688609029236. [DOI] [PubMed] [Google Scholar]
- Galentine PG, Cohen EJ, Laibson PR, Adams CP, Michaud R, Arentsen JJ, 1984. Corneal ulcers associated with contact lens wear. Arch. Ophthalmol 102, 891–894. 10.1001/archopht.1984.01040030711025. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A, 2013. The interleukin-1 family: back to the future. Immunity 39, 1003–1018. 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity-Ryan L, Kazmierczak BI, Kowal R, Comolli JC, Hauser AR, Engel JN, 2000. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun 68, 7100–7113. 10.1128/IAI.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauguet S, D’Ortona S, Ahnger-Pier K, Duan B, Surana NK, Lu R, Cywes-Bentley C, Gadjeva M, Shan Q, Priebe GP, Pier GB, 2015. Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infect. Immun 83, 4003–4014. 10.1128/IAI.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt H, Meniche X, Tropis M, Krämer R, Daffé M, Morbach S, 2007. The key role of the mycolic acid content in the functionality of the cell wall permeability barrier in Corynebacterineae. Microbiology 153, 1424–1434. 10.1099/mic.0.2006/003541-0. [DOI] [PubMed] [Google Scholar]
- Gerke JR, Magliocco MV, 1971. Experimental Pseudomonas aeruginosa infection of the mouse cornea. Infect. Immun 3, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Argüeso P, 2003. Role of mucins in the function of the corneal and conjunctival epithelia. Int. Rev. Cytol 231, 1–49. 10.1016/S0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Grill SM, 1982. A technique for obtaining sheets of intact rabbit corneal epithelium. Investig. Ophthalmol. Vis. Sci 23, 269–273. [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB, 2014. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One 9, e100393 10.1371/journal.pone.0100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden B, Fingerman LH, Allen HF, 1971. Pseudomonas corneal ulcers in contact lens wearers. Epidemiology and treatment. Arch. Ophthalmol 85, 543–547. 10.1001/archopht.1971.00990050545004. [DOI] [PubMed] [Google Scholar]
- Goldufsky J, Wood S, Hajihossainlou B, Rehman T, Majdobeh O, Kaufman HL, Ruby CE, Shafikhani SH, 2015. Pseudomonas aeruginosa exotoxin T induces potent cytotoxicity against a variety of murine and human cancer cell lines. J. Med. Microbiol 64, 164–173. 10.1099/jmm.0.000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkine G, Faudry E, Bouillot S, Voulhoux R, Attrée I, Huber P, 2014. VE-cadherin cleavage by LasB protease from Pseudomonas aeruginosa facilitates type III secretion system toxicity in endothelial cells. PLoS Pathog. 10, 1–17. 10.1371/journal.ppat.1003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbet M, Postnikoff C, Williams S, 2015. The noninflammatory phenotype of neutrophils from the closed-eye environment: a flow cytometry analysis of receptor expression. Investig. Ophthalmol. Vis. Sci 56, 4582–4591. 10.1167/iovs.14-15750. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Menon BB, Spurr-Michaud S, Rastogi K, Gilmore MS, Argüeso P, Gipson IK, 2012. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PLoS One 7, e32418 10.1371/journal.pone.0032418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Moore Jonathan E., Jiru X, Moore John E., Goodall EA, Dooley JSG, Hayes VEA, Dartt DA, Downes CS, Moore TCB, 2007. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Investig. Ophthalmol. Vis. Sci 48, 5616–5623. 10.1167/iovs.07-0588. [DOI] [PubMed] [Google Scholar]
- Green M, Apel A, Stapleton F, 2008. Risk factors and causative organisms in microbial keratitis. Cornea 27, 22–27. 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, Lee YC, Seol SY, Cho DT, Kim SI, Lee JC, 2011. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS One 6, e27958 10.1371/journal.pone.0027958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P, 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol 2, 95–108. 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Hammond JH, Hebert WP, Naimie A, Ray K, Van Gelder RD, DiGiandomenico A, Lalitha P, Srinivasan M, Acharya NR, Lietman T, Hogan DA, Zegans ME, 2016. Environmentally endemic Pseudomonas aeruginosa strains with mutations in lasR are associated with increased disease severity in corneal ulcers. mSphere 1, e00140 10.1128/mSphere.00140-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Dana MR, 2007. Corneal antigen-presenting cells. Chem. Immunol. Allergy 92, 58–70. 10.2147/OTT.S85383. [DOI] [PubMed] [Google Scholar]
- Hauser AR, 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol 7, 654–665. 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RJ, Tighe PJ, Dua HS, 1999. Antimicrobial defensin peptides of the human ocular surface. Br. J. Ophthalmol 83, 737–741. 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RJ, Tighe PJ, Dua HS, 1998. Innate defence of the eye by antimicrobial defensin peptides. Lancet 352, 451–452. 10.1016/S0140-6736(05)79185-6. [DOI] [PubMed] [Google Scholar]
- Hazlett L, Masinick S, Mezger B, Barrett R, Kurpakus M, Garrett M, 1996Ultrastructural, immunohistological and biochemical characterization of cultured mouse corneal epithelial cells. Ophthalmic Res. 28, 50–56. 10.1159/000267873. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, 2007. Bacterial infections of the cornea (Pseudomonas aeruginosa). Chem. Immunol. Allergy 92, 185–194. 10.1159/000099269. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, 2004. Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res 23, 1–30. 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, Masinick S, Barrett R, Rosol K, 1993. Evidence for asialo GM1 as a corneal glycolipid receptor for Pseudomonas aeruginosa adhesion. Infect. Immun 61, 5164–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck LW, Morihara K, Abrahamson DR, 1986a. Degradation of soluble laminin and depletion of tissue-associated basement membrane laminin by Pseudomonas aeruginosa elastase and alkaline protease. Infect. Immun 54, 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck LW, Morihara K, McRae WB, Miller EJ, 1986b. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect. Immun 51, 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer SR, Evans DJ, Stern ME, Barbieri JT, Yahr T, Fleiszig SMJ, 2013. Pseudomonas aeruginosa utilizes the type III secreted toxin ExoS to avoid acidified compartments within epithelial cells. PLoS One 8, e73111 10.1371/journal.pone.0073111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman JW, Harwood CS, 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol 69, 376–389. 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman JW, Tifrea DF, Harwood CS, 2005. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci 102, 14422–14427. 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobden JA, 2002. Pseudomonas aeruginosa proteases and corneal virulence. DNA CellBiol. 21, 391–396. 10.1089/10445490260099674. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, D’Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI, 2005Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436, 1171–1175. 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, Kulasekara HD, Emerson J, Houston LS, Burns JL, Ramsey BW, Miller SI, 2009. Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J. Cyst. Fibros 8, 66–70. 10.1016/j.jcf.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge R, Pelzer A, Rosenau F, Wilhelm S, 2010. Weapons of a pathogen: proteases and their role in virulence of Pseudomonas aeruginosa In: Mendez-Vilas (Ed.), Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Formatex Research Center, Badajoz, Spain, pp. 383–395. [Google Scholar]
- Høiby N, Ciofu O, Bjarnsholt T, 2010. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 5, 1663–1674. 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- Holden BA, Sweeney DF, Vannas A, Nilsson KT, Efron N, 1985. Effects of long-term extended contact lens wear on the human cornea. Investig. Ophthalmol. Vis. Sci 26, 1489–1501. [PubMed] [Google Scholar]
- Holder IA, Wheeler R, 1984. Experimental studies of the pathogenesis of infections owing to Pseudomonas aeruginosa: elastase, an IgG protease. Can. J. Microbiol 30, 1118–1124. 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Holly FJ, 1973. Formation and rupture of the tear film. Exp. Eye Res 15, 515–525. 10.1016/0014-4835(73)90064-X. [DOI] [PubMed] [Google Scholar]
- Hong C-W, 2017. Current understanding in neutrophil differentiation and heterogeneity. Immune Netw. 17, 298–306. 10.4110/in.2017.17.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Ghebrehiwet B, 1992. Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin. Immunol. Immunopathol 62, 133–138. 10.1016/0090-1229(92)90065-V. [DOI] [PubMed] [Google Scholar]
- Hori Y, Argüeso P, Spurr-Michaud S, Gipson IK, 2006. Mucins and contact lens wear. Cornea 25, 176–181. 10.1097/01.ico.0000177838.38873.2f. [DOI] [PubMed] [Google Scholar]
- Horvath DJ, Li B, Casper T, Partida-Sanchez S, Hunstad DA, Hultgren SJ,Justice SS, 2011. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microb. Infect 13, 426–437. 10.1016/j.micinf.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hritonenko V, Evans DJ, Fleiszig SMJ, 2012. Translocon-independent intracellular replication by Pseudomonas aeruginosa requires the ADP-ribosylation domain of ExoS. Microb. Infect 14, 1366–1373. 10.1016/j.micinf.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hritonenko V, Mun JJ, Tam C, Simon NC, Barbieri JT, Evans DJ, Fleiszig SMJ, 2011. Adenylate cyclase activity of Pseudomonas aeruginosa ExoY can mediate bleb-niche formation in epithelial cells and contributes to virulence. Microb. Pathog 51, 305–312. 10.1016/j.micpath.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Barrett RP, McClellan SA, Hazlett LD, 2005. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci 46, 4209–4216. 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- Huang X, Du W, McClellan SA, Barrett RP, Hazlett LD, 2006. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci 47, 4910–4916. 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- Ichijima H, Ohashi J, Petroll WM, Cavanagh HD, 1993. Morphological and biochemical evaluation for rigid gas permeable contact lens extended wear on rabbit corneal epithelium. CLAO J. 19, 121–128. [PubMed] [Google Scholar]
- Iglewski BH, Burns RP, Gipson IK, 1977a. Pathogenesis of corneal damage from Pseudomonas exotoxin A. Investig. Ophthalmol. Vis. Sci 16, 73–76. [PubMed] [Google Scholar]
- Iglewski BH, Liu PV, Kabat D, 1977b. Mechanism of action of Pseudomonas aeruginosa exotoxin A: adenosine diphosphate ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect. Immun 15, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski BH, Sadoff J, Bjorn MJ, Maxwell ES, 1978. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc. Natl. Acad. Sci. U. S. A 75, 3211–3215. 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayasu M, Petroll WM, Jester JV, Patel SK, Ohashi J, Cavanagh HD, 1994. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology 101, 371–388. 10.1016/S0161-6420(94)31326-1. [DOI] [PubMed] [Google Scholar]
- Iovieno A, Ledee DR, Miller D, Alfonso EC, 2010. Detection of bacterial endosymbionts in clinical Acanthamoeba isolates. Ophthalmology 117, 445–452. 10.1016/j.ophtha.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR, 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498. 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Littman DR, 2010. Segmented filamentous bacteria take the stage. MucosalImmunol. 3, 209–212. 10.1038/mi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett BD, Gilmore MS, 2002. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect. Immun 70, 4697–4700. 10.1128/IAI.70.8.4697-4700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeukens J, Boyle B, Kukavica-Ibrulj I, Ouellet MM, Aaron SD, Charette SJ,Fothergill JL, Tucker NP, Winstanley C, Levesque RC, 2014. Comparative genomics of isolates of a Pseudomonas aeruginosa epidemic strain associated with chronic lung infections of cystic fibrosis patients. PLoS One 9, 1–15. 10.1371/journal.pone.0087611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly AL, Agarwal P, Metruccio MME, Spiciarich DR, Evans DJ, Bertozzi CR, Fleiszig SMJ, 2017. Corneal surface glycosylation is modulated by IL-1R and Pseudomonas aeruginosa challenge but is insufficient for inhibiting bacterial binding. FASEB J. 31, 2393–2404. 10.1096/fj.201601198R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly AL, Takawira D, Oke OO, Whiteside SA, Chang SW, Wen ER, Quach K, Evans DJ, Fleiszig SMJ, 2015. Pseudomonas aeruginosa-induced bleb-niche formation in epithelial cells is independent of actinomyosin contraction and enhanced by loss of cystic fibrosis transmembrane-conductance regulator osmoregulatory function. mBio 6, e02533 10.1128/mBio.02533-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Powell CH, 2013. Uptake and release phenomena in contact lens care by silicone hydrogel lenses. Eye Contact Lens 39, 29–36. 10.1097/ICL.0b013e31827d4f25. [DOI] [PubMed] [Google Scholar]
- Julsing MK, Rijpkema M, Woerdenbag HJ, Quax WJ, Kayser O, 2007. Functional analysis of genes involved in the biosynthesis of isoprene in Bacillus subtilis. Appl. Microbiol. Biotechnol 75, 1377–1384. 10.1007/s00253-007-0953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn CR, Young E, Ihn Hwan Lee, Rhim JS, 1993. Human corneal epithelial primary cultures and cell lines with extended life span: In vitro model for ocular studies. Investig. Ophthalmol. Vis. Sci 34, 3429–3441. 10.1186/1471-244X-14-29. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Mercier R, Wu LJ, Domínguez-Cuevas P, Oshima T, Errington J, 2015. Cell growth of wall-free L-form bacteria is limited by oxidative damage. Curr. Biol 25, 1613–1618. 10.1016/j.cub.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay L, Edwards K, Naduvilath T, Taylor HR, Snibson GR, Forde K, Stapleton F, 2006. Microbial keratitis: predisposing factors and morbidity. Ophthalmology 113, 109–116. 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Kernacki KA, Hobden JA, Hazlett LD, Fridman R, Berk RS, 1995. In vivo bacterial protease production during Pseudomonas aeruginosa corneal infection. Investig. Ophthalmol. Vis. Sci 36, 1371–1378. [PubMed] [Google Scholar]
- Khor WB, Aung T, Saw SM, Wong TY, Tambyah PA, Tan AL, Beuerman R, Lim L, Chan WK, Heng WJ, Lim J, Loh RSK, Lee SB, Tan DTH, 2006. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. J. Am. Med. Assoc 295, 2867–2873. 10.1001/jama.295.24.2867. [DOI] [PubMed] [Google Scholar]
- Kida Y, Higashimoto Y, Inoue H, Shimizu T, Kuwano K, 2008. A novel secreted protease from Pseudomonas aeruginosa activates NF-KB through protease-activated receptors. Cell Microbiol. 10, 1491–1504. 10.1111/j.1462-5822.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- Kida Y, Shimizu T, Kuwano K, 2011. Cooperation between LepA and PlcH contributes to the in vivo virulence and growth of Pseudomonas aeruginosa in mice. Infect. Immun 79, 211–219. 10.1128/IAI.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierbel A, Gassama-Diagne A, Mostov K, Engel JN, 2005. The phosphoinositol-3-kinase–protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell 16, 2577–2585. 10.1091/mbc.E04-08-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierbel A, Gassama-Diagne A, Rocha C, Radoshevich L, Olson J, Mostov K, Engel J, 2007. Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane. J. Cell Biol 177, 21–27. 10.1083/jcb.200605142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SI, Kim S, Kim E, Hwang SY, Yoon H, 2018. Secretion of Salmonella pathogenicity island 1-encoded type III secretion system effectors by outer membrane vesicles in Salmonella enterica serovar typhimurium. Front. Microbiol 9, 2810 10.3389/fmicb.2018.02810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz SA, Au YK, Misra RP, 1989. A partial-thickness epithelial defect increases the adherence of Pseudomonas aeruginosa to the cornea. Investig. Ophthalmol. Vis. Sci 30, 1069–1074. [PubMed] [Google Scholar]
- Kobayashi T, Yoshioka R, Shiraishi A, Ohashi Y, 2009. New technique for culturing corneal epithelial cells of normal mice. Mol. Vis 15, 1589–1593. [PMC free article] [PubMed] [Google Scholar]
- Kodjikian L, Casoli-Bergeron E, Malet F, Janin-Manificat H, Freney J, Burillon C, Colin J, Steghens J-P, 2008. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefe’s Arch. Clin. Exp. Ophthalmol 246, 267–273. 10.1007/s00417-007-0703-5. [DOI] [PubMed] [Google Scholar]
- Köhler T, Curty LK, Barja F, van Delden C, Pechère JC, 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol 182, 5990–5996. 10.1128/JB.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P, 2013. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol 13, 159–175. 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Konda N, Motukupally SR, Garg P, Sharma S, Ali MH, Willcox MDP, 2014. Microbial analyses of contact lens–associated microbial keratitis. Optom. Vis. Sci 91, 47–53. 10.1097/OPX.0000000000000082. [DOI] [PubMed] [Google Scholar]
- Kreger AS, Gray LD, 1978. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of Pseudomonal protease-induced rabbit corneal damage. Infect. Immun 19, 630–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroken Abby R., Chen CK, Evans DJ, Yahr TL, Fleiszig SMJ, 2018. The impact of ExoS on Pseudomonas aeruginosa internalization by epithelial cells is independent of fleQ and correlates with bistability of type three secretion system gene expression. mBio 9, e00668 10.1128/mBio.00668-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugadas A, Christiansen SH, Sankaranarayanan S, Surana NK, Gauguet S, Kunz R, Fichorova R, Vorup-Jensen T, Gadjeva M, 2016. Impact of microbiota on resistance to ocular Pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 12, e1005855 10.1371/journal.ppat.1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Hazlett LD, Yu FS, 2008. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect. Immun 76, 89–96. 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yu FS, 2006. Toll-like receptors and corneal innate immunity. Curr. Mol. Med 6, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong MSFF, Evans DJ, Ni M, Cowell BA, Fleiszig SMJ, 2007. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect. Immun 75, 2325–2332. 10.1128/IAI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladage PM, Yamamoto K, Li L, Ren DH, Petroll WM, Jester JV, Cavanagh HD, 2002. Corneal epithelial homeostasis following daily and overnight contact lens wear. Contact Lens Anterior Eye 25, 11–21. 10.1016/S1367-0484(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Ladage PM, Yamamoto K, Ren DH, Jester JV, Petroll WM, Bergmanson JPG, Cavanagh HD, 2003. Recovery time of corneal epithelial proliferation in the rabbit following rigid gas-permeable extended contact-lens wear. Eye Contact Lens 29, 61–64. 10.1097/01.ICL.0000060781.84166.80. [DOI] [PubMed] [Google Scholar]
- Ladage PM, Yamamoto K, Ren DH, Li L, Jester JV, Matthew Petroll W,Bergmanson JPG, Dwight Cavanagh H, 2001. Proliferation rate of rabbit corneal epithelium during overnight rigid contact lens wear. Investig. Ophthalmol. Vis. Sci 42, 2804–2812. [PubMed] [Google Scholar]
- Lakkis C, Fleiszig SMJ, 2001. Resistance of Pseudomonas aeruginosa isolates to hydrogel contact lens disinfection correlates with cytotoxic activity. J. Clin. Microbiol 39, 1477–1486. 10.1128/JCM.39.4.1477-1486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkovic S, Nilsson SE, 1997. The effect of high and low Dk/L soft contact lenses on the glycocalyx layer of the corneal epithelium and on the membrane associated receptors for lectins. CLAO J. 23, 185–191. [PubMed] [Google Scholar]
- Lawrence HM, Dyer JA, Karlson AG, 1969. Effect of contact lenses on Pseudomonas ulcers in rabbit corneas. Arch. Ophthalmol 81, 843–848. 10.1001/archopht.1969.00990010845017. [DOI] [PubMed] [Google Scholar]
- Lee A, Chow D, Haus B, Tseng W, Evans D, Fleiszig S, Chandy G, Machen T, 1999. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am. J. Physiol 277, L204–L217. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Cowell BA, Evans DJ, Fleiszig SMJ, 2003a. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Investig. Ophthalmol. Vis. Sci 44, 3892–3898. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Evans DJ, Fleiszig SMJ, 2003b. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Investig. Ophthalmol. Vis. Sci 44, 5220–5227. [DOI] [PubMed] [Google Scholar]
- Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS, 2009. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9, 5425–5436. 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- Lee JTY, Wang G, Tam YT, Tam C, 2016. Membrane-active epithelial keratin 6A fragments (KAMPs) are unique human antimicrobial peptides with a non-αβ structure. Front. Microbiol 7, 1799 10.3389/fmicb.2016.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VT, Smith RS, Tümmler B, Lory S, 2005. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect. Immun 73, 1695–1705. 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz HM, Rosenthal P, 1971. Hydrophilic contact lenses in corneal disease: I. Superficial, sterile, indolent ulcers. Arch. Ophthalmol 85, 163–166. 10.1001/archopht.1971.00990050165008. [DOI] [PubMed] [Google Scholar]
- Li J, Metruccio MME, Smith BE, Evans DJ, Fleiszig SMJ, Smith BE, Evans DJ, Fleiszig SMJ, 2017. Mucosal fluid glycoprotein DMBT1 suppresses twitching motility and virulence of the opportunistic pathogen Pseudomonas aeruginosa. PLoS Pathog. 13, e1006392 10.1371/journal.ppat.1006392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wan SJ, Metruccio MME, Ma S, Nazmi K, Bikker FJ, Evans DJ, Fleiszig SMJ, 2019. DMBT1 inhibition of Pseudomonas aeruginosa twitching motility involves its N-glycosylation and cannot be conferred by the Scavenger Receptor Cysteine-Rich bacteria-binding peptide domain. Sci. Rep 9, 13146 10.1038/s41598-019-49543-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Brignole-Baudouin F, Labbé A, Pauly A, Warnet J-M, Baudouin C, 2007. LPS-stimulated inflammation and apoptosis in corneal injury models. Mol. Vis 13, 1169–1180. [PubMed] [Google Scholar]
- Lillehoj EP, Won Hyun S, Liu A, Guang W, Verceles AC, Luzina IG, Atamas SP, Chul Kim K, Goldblum SE, 2015. NEU1 sialidase regulates membrane-tethered mucin (MUC1) ectodomain adhesiveness for Pseudomonas aeruginosa and decoy receptor release. J. Biol. Chem 290, 18316–18331. 10.1074/jbc.M115.657114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CHL, Carnt NA, Farook M, Lam J, Tan DT, Mehta JS, Stapleton F, 2016Risk factors for contact lens-related microbial keratitis in Singapore. Eye 30, 447–455. 10.1038/eye.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MC, Yeh TN, 2013. Mechanical complications induced by silicone hydrogel contact lenses. Eye Contact Lens 39, 115–124. 10.1097/ICL.0b013e31827c77fd. [DOI] [PubMed] [Google Scholar]
- Liu PV, 1973. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J. Infect. Dis 128, 506–513. 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Lomholt JA, Poulsen K, Kilian M, 2001. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect. Immun 69, 6284–6295. 10.1128/IAI.69.10.6284-6295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López D, Vlamakis H, Kolter R, 2010. Biofilms. Cold Spring Harb. Perspect. Biol 2, a398 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luensmann D, Jones L, 2012. Protein deposition on contact lenses: the past, the present, and the future. Contact Lens Anterior Eye 35, 53–64. 10.1016/j.clae.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB, 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev 15, 194–222. 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA, 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426, 306–310. 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- Mahenthiralingam E, Campbell ME, Speert DP, 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun 62, 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Codina C, Morgan PB, Schnider CM, Efron N, 2004. Short-term physiologic response in neophyte subjects fitted with hydrogel and silicone hydrogel contact lenses. Optom. Vis. Sci 81, 911–921. [PubMed] [Google Scholar]
- Maliniak ML, Stecenko AA, McCarty NA, 2016. A longitudinal analysis of chronic MRSA and Pseudomonas aeruginosa co-infection in cystic fibrosis: a single-center study. J. Cyst. Fibros 15, 350–356. 10.1016/j.jcf.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Maltseva IA, Fleiszig SMJ, Evans DJ, Kerr S, Sidhu SS, McNamara NA,Basbaum C, 2007. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp. Eye Res 85, 142–153. 10.1016/j.exer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Mantelli F, Argüeso P, 2008. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol 8, 477–483. 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Mauris J, Argüeso P, 2013. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr. Opin. Allergy Clin. Immunol 13, 563–568. 10.1097/ACI.0b013e3283645899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G,Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A, 2016. The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339. 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariencheck WI, Alcorn JF, Palmer SM, Wright JR, 2003. Pseudomonas aeruginosa elastase degrades surfactant proteins A and D. Am. J. Respir. Cell Mol. Biol 28, 528–537. 10.1165/rcmb.2002-0141OC. [DOI] [PubMed] [Google Scholar]
- Marquart ME, Caballero AR, Chomnawang M, Thibodeaux BA, Twining SS,O’Callaghan RJ, 2005. Identification of a novel secreted protease from Pseudomonas aeruginosa that causes corneal erosions. Investig. Ophthalmol. Vis. Sci 46, 3761–3768. 10.1167/iovs.04-1483. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Mostov K, 2008. Regulation of cell polarity during epithelial morphogenesis. Curr. Opin. Cell Biol 20, 227–234. 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Masinick SA, Montgomery CP, Montgomery PC, Hazlett LD, 1997. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Investig. Ophthalmol. Vis. Sci 38, 910–918. [PubMed] [Google Scholar]
- Mattick JS, 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol 56,289–314. 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- McDermott AM, 2013. Antimicrobial compounds in tears. Exp. Eye Res 117, 53–61. 10.1016/j.exer.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM, 2009. The role of antimicrobial peptides at the ocular surface.Ophthalmic Res. 41, 60–75. 10.1159/000187622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM, Redfern RL, Zhang B, Pei Y, Huang L, Proske RJ, 2003. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci 44, 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Borlace L, Stapleton F, Matheson M, Dart JK, 1998. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J. Appl. Microbiol 84, 827–838. 10.1046/j.1365-2672.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- McNamara N, Khong A, McKemy D, Caterina M, Boyer J, Julius D, Basbaum C, 2001. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc. Natl. Acad. Sci. U. S. A 98, 9086–9091. 10.1073/pnas.161290898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara NA, Polse KA, Brand RJ, Graham AD, Chan JS, McKenney CD, 1999a. Tear mixing under a soft contact lens: effects of lens diameter. Am. J. Ophthalmol 127, 659–665. 10.1016/S0002-9394(99)00051-3. [DOI] [PubMed] [Google Scholar]
- McNamara NA, Polse KA, Fukunaga SA, Maebori JS, Suzuki RM, 1998. Soft lens extended wear affects epithelial barrier function. Ophthalmology 105, 2330–2335. 10.1016/S0161-6420(98)91237-4. [DOI] [PubMed] [Google Scholar]
- McNamara NA, Van R, Tuchin OS, Fleiszig SMJ, 1999b. Ocular surface epithelia express mRNA for human beta defensin-2. Exp. Eye Res 69, 483–490. 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, 2008. Origin and physiological roles of inflammation. Nature 454,428–435. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Meliani A, Bensoltane A, 2017. Pseudomonas chemotaxis, motility and host-pathogen interactions. MOJ Immunol. 5, 1–6. 10.15406/moji.2017.05.00167. [DOI] [Google Scholar]
- Melichar HJ, Li O, Herzmark P, Padmanabhan RK, Oliaro J, Ludford-Menting MJ, Bousso P, Russell SM, Roysam B, Robey EA, 2011. Quantifying subcellular distribution of fluorescent fusion proteins in cells migrating within tissues. Immunol. Cell Biol 89, 549–557. 10.1038/icb.2010.122. [DOI] [PubMed] [Google Scholar]
- Meluleni GJ, Grout M, Evans DJ, Pier GB, 1995. Mucoid Pseudomonas aeruginosa growing in a biofilm in vitro are killed by opsonic antibodies to the mucoid exopolysaccharide capsule but not by antibodies produced during chronic lung infection in cystic fibrosis patients. J. Immunol 155, 2029–2038. [PubMed] [Google Scholar]
- Metruccio MME, Evans DJ, Gabriel MM, Kadurugamuwa JL, Fleiszig SMJ, 2016. Pseudomonas aeruginosa outer membrane vesicles triggered by human mucosal fluid and lysozyme can prime host tissue surfaces for bacterial adhesion. Front. Microbiol 7, 871 10.3389/fmicb.2016.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metruccio MME, Tam C, Evans DJ, Xie AL, Stern ME, Fleiszig SMJ, 2017. Contributions of MyD88-dependent receptors and CD11c-positive cells to corneal epithelial barrier function against Pseudomonas aeruginosa. Sci. Rep 7, 13829 10.1038/s41598-017-14243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metruccio MME, Wan SJ, Horneman H, Kroken AR, Sullivan AB, Truong TN, Mun JJ, Tam CKP, Frith R, Welsh L, George MD, Morris CA, Evans DJ, Fleiszig SMJ, 2019. A novel murine model for contact lens wear reveals clandestine IL-1R dependent corneal parainflammation and susceptibility to microbial keratitis upon inoculation with Pseudomonas aeruginosa. Ocul. Surf 17, 119–133. 10.1016/J.JTOS.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadinia M, Rahmani S, Eslami G, Ghassemi-Broumand M, Aghazadh Amiri M, Aghaie G, Tabatabaee SM, Taheri S, Behgozin A, 2012. Contact lens disinfecting solutions antibacterial efficacy: comparison between clinical isolates and the standard ISO ATCC strains of Pseudomonas aeruginosa and Staphylococcus aureus. Eye 26, 327–330. 10.1038/eye.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed I, Said DG, Dua HS, 2017. Human antimicrobial peptides in ocular surface defense. Prog. Retin. Eye Res 61, 1–22. 10.1016/j.preteyeres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Möller J, Emge P, Vizcarra IA, Kollmannsberger P, Vogel V, 2013. Bacterial filamentation accelerates colonization of adhesive spots embedded in biopassive surfaces. New J. Phys 15, 125016 10.1088/1367-2630/15/12/125016. [DOI] [Google Scholar]
- Morgan PB, Efron N, Hill EA, Raynor MK, Whiting MA, Tullo AB, 2005. Incidence of keratitis of varying severity among contact lens wearers. Br. J. Ophthalmol 89, 430–436. 10.1136/bjo.2004.052688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DC, Ryan JL, 1987. Endotoxins and disease mechanisms. Annu. Rev. Med 38, 417–432. 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Mulcahy LR, Isabella VM, Lewis K, 2014. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol 68, 1–12. 10.1007/s00248-013-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun J, Tam C, Chan G, Kim JH, Evans D, Fleiszig S, 2013. MicroRNA-762 is upregulated in human corneal epithelial cells in response to tear fluid and Pseudomonas aeruginosa antigens and negatively regulates the expression of host defense genes encoding RNase7 and ST2. PLoS One 8, e57850 10.1371/journal.pone.0057850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun JJ, Tam C, Evans DJ, Fleiszig SMJ, 2011. Modulation of epithelial immunity by mucosal fluid. Sci. Rep 1, 8 10.1038/srep00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun James J., Tam C, Kowbel D, Hawgood S, Barnett MJ, Evans DJ, Fleiszig SMJ, 2009. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect. Immun 77, 2392–2398. 10.1128/IAI.00173-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntz A, Subbaraman LN, Sorbara L, Jones L, 2015. Tear exchange and contact lenses: a review. J. Opt 8, 2–11. 10.1016/j.optom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TS, Kazmierczak BI, 2008. Pseudomonas aeruginosa exhibits sliding motility in the absence of type IV pili and flagella. J. Bacteriol 190, 2700–2708. 10.1128/JB.01620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L, 2007. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605. 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Hattori T, Koike N, Ehara T, Narimatsu A, Kumakura S, Matsumoto T, Goto H, 2017. Number of bacteria and time of coincubation with bacteria required for the development of Acanthamoeba keratitis. Cornea 36, 353–357. 10.1097/ICO.0000000000001129. [DOI] [PubMed] [Google Scholar]
- Ni M, Evans DJ, Hawgood S, Anders EM, Sack RA, Fleiszig SMJ, 2005Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect. Immun 73, 2147–2156. 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas TI, Iglewski BH, 1984. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect. Immun 45, 470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B, Dawson CR, Togni B, 1983. Surface features of the conjunctiva and cornea. Investig. Ophthalmol. Vis. Sci 24, 570–576. [PubMed] [Google Scholar]
- Niederkorn JY, 2011. Cornea: window to ocular immunology. Curr. Immunol. Rev 7, 328–335. 10.1210/jc.2013-39028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto V, Kroken AR, Grosser MR, Smith BE, Metruccio MME, Hagan P, Hallsten ME, Evans DJ, Fleiszig SMJ, 2019. Type IV pili can mediate bacterial motility within epithelial cells. mBio 10, e02880 10.1128/mBio.02880-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H, 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev 67, 593–656. 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WJ, Krema C, Heimann T, Zhao H, 2006. Expression of NADPH oxidase in rabbit corneal epithelial and stromal cells in culture. Investig. Ophthalmol. Vis. Sci 47, 853–863. 10.1167/iovs.05-1063. [DOI] [PubMed] [Google Scholar]
- O’Callaghan RJ, Engel LS, Hobden JA, Callegan MC, Green LC, Hill JM, 1996. Pseudomonas keratitis. The role of an uncharacterized exoprotein, protease IV, in corneal virulence. Investig. Ophthalmol. Vis. Sci 37, 534–543. [PubMed] [Google Scholar]
- Okamoto T, Akaike T, Suga M, Tanase S, Horie H, Miyajima S, Ando M, Ichinose Y, Maeda H, 1997. Activation of human matrix metalloproteinases by various bacterial proteinases. J. Biol. Chem 272, 6059–6066. 10.1074/jbc.272.9.6059. [DOI] [PubMed] [Google Scholar]
- Otri AM, Mohammed I, Al-Aqaba MA, Fares U, Peng C, Hopkinson A, Dua HS, 2012. Variable expression of human beta defensins 3 and 9 at the human ocular surface in infectious keratitis. Investig. Ophthalmol. Vis. Sci 53, 757–761. 10.1167/iovs.11-8467. [DOI] [PubMed] [Google Scholar]
- Ozkan J, Coroneo M, Willcox MDP, Wemheuer B, Thomas T, 2018. Identification and visualization of a distinct microbiome in ocular surface conjunctival tissue. Investig. Opthalmol. Vis. Sci 59, 4268–4276. 10.1167/iovs.18-24651. [DOI] [PubMed] [Google Scholar]
- Ozkan J, Nielsen S, Diez-Vives C, Coroneo M, Thomas T, Willcox MDP, 2017. Temporal stability and composition of the ocular surface microbiome. Sci. Rep 7, 9880 10.1038/s41598-017-10494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan J, Willcox MDP, 2019. The ocular microbiome: molecular characterisation of a unique and low microbial environment. Curr. Eye Res 44, 685–694. 10.1080/02713683.2019.1570526. [DOI] [PubMed] [Google Scholar]
- Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T, 2008. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol. Microbiol 68, 223–240. 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- Parmely M, Gale A, Clabaugh M, Horvat R, Zhou WW, 1990. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect. Immun 58, 3009–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I, Nusbaum AG, Gil J, Patel SB, Chen J, Valdes J, Stojadinovic O, Plano LR, Tomic-Canic M, Davis SC, 2013. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One 8, e56846 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM, 2010. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol. Cell 38, 128–139. 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E, Johnson A, Adhikary G, Sun Y, Chinnery HR, Fox T, Kester M, McMenamin PG, 2008. Toll-like receptors at the ocular surface. Ocul. Surf 6, 108–116. 10.1016/S1542-0124(12)70279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E, Sun Y, Roy S, Karmakar M, Hise AG, Szczotka-Flynn L, Ghannoum M, Chinnery HR, McMenamin PG, Rietsch A, 2013. Host defense at the ocular surface. Int. Rev. Immunol 32, 4–18. 10.3109/08830185.2012.749400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JP, Pesci EC, Iglewski BH, 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol 179, 5756–5767. 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z, 2015. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci 112, 7563–7568. 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JE, Park SJ, Darzins A, Freck LC, Saulnier JM, Wallach JM, Galloway DR, 1992. Further studies on Pseudomonas aeruginosa LasA: analysis of specificity. Mol. Microbiol 6, 1155–1162. 10.1111/j.1365-2958.1992.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Pier GB, 2007. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int. J. Med. Microbiol 297, 277–295. 10.1016/j.ijmm.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Grout M, Zaidi TS, Goldberg JB, 1996a. How mutant CFTR may contribute to Pseudomonas aeruginosa infection in cystic fibrosis. In: Am. J. Respir. Crit. Care Med, vol. 154 pp. S175–S182. 10.1164/ajrccm/154.4_Pt_2.S175. [DOI] [PubMed] [Google Scholar]
- Pier GB, Grout M, Zaidi TS, Olsen JC, Johnson LG, Yankaskas JR, Goldberg JB, 1996b. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 271, 64–67. 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillar CM, Hobden JA, 2002. Pseudomonas aeruginosa exotoxin A and keratitis in mice. Investig. Ophthalmol. Vis. Sci 43, 1437–1444. 10.1016/j.ajo.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Postnikoff C, Gorbet M, 2018. The effect of closed-eye tear film conditions on blood-isolated neutrophils, in vitro. Ocul. Immunol. Inflamm 26, 706–716. 10.1080/09273948.2017.1281423. [DOI] [PubMed] [Google Scholar]
- Prashar A, Bhatia S, Gigliozzi D, Martin T, Duncan C, Guyard C, Terebiznik MR, 2013. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. J. Cell Biol 203, 1081–1097. 10.1083/jcb.201304095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston MJ, Fleiszig SM, Zaidi TS, Goldberg JB, Shortridge VD, Vasil ML, Pier GB, 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun 63, 3497–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston MJ, Seed PC, Toder DS, Iglewski BH, Ohman DE, Gustin JK, Goldberg JB, Pier GB, 1997. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect. Immun 65, 3086–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purslow C, Wolffsohn JS, Santodomingo-Rubido J, 2005. The effect of contact lens wear on dynamic ocular surface temperature. Contact Lens Anterior Eye 28, 29–36. 10.1016/j.clae.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S, 2014. Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front. Public Health 2, 103 10.3389/fpubh.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JC, Fleiszig SMJ, Sullivan AB, Tam C, Borazjani R, Evans DJ, 2012. Traversal of multilayered corneal epithelia by cytotoxic Pseudomonas aeruginosa requires the phospholipase domain of ExoU. Investig. Ophthalmol. Vis. Sci 53, 448–453. 10.1167/iovs.11-8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R, McNiece MT, Polack FM, 1981. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Ann. Ophthalmol 13, 421–425. [PubMed] [Google Scholar]
- Redfern RL, Reins RY, McDermott AM, 2011. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp. Eye Res 92, 209–220. 10.1016/j.exer.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reins RY, Courson J, Lema C, Redfern RL, 2017. MyD88 contribution to ocular surface homeostasis. PLoS One 12, e0182153 10.1371/journal.pone.0182153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retuerto MA, Szczotka-Flynn L, Mukherjee PK, Debanne S, Iyengar SK,Richardson B, Cameron M, Ghannoum MA, 2019. Diversity of ocular surface bacterial microbiome adherent to worn contact lenses and bacterial communities associated with care solution use. Eye Contact Lens 45, 331–339. 10.1097/ICL.0000000000000578. [DOI] [PubMed] [Google Scholar]
- Ricciuto J, Heimer SR, Gilmore MS, Argueso P, 2008. Cell surface O-glycans limit Staphylococcus aureus adherence to corneal epithelial cells. Infect. Immun 76, 5215–5220. 10.1128/IAI.00708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, 2013. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. In: Eye and Contact Lens, vol. 39 pp. 67–72. 10.1097/ICL.0b013e31827c5b73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Kalangara JP, Baucom RB, Petroll WM, Cavanagh HD, 2011a. A reconstituted telomerase-immortalized human corneal epithelium in vivo: a pilot study. Curr. Eye Res 36, 706–712. 10.3109/02713683.2011.582662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV, 2005. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investig. Ophthalmol. Vis. Sci 46, 470–478. 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Parks QM, Young RL, Kret J, Poch KR, Malcolm KC, Nichols DP, Nichols M, Zhu M, Cavanagh HD, Nick JA, 2011b. Disruption of contact lens-associated Pseudomonas aeruginosa biofilms formed in the presence of neutrophils. Investig. Ophthalmol. Vis. Sci 52, 2844–2850. 10.1167/iovs.10-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Rogers NA, Petroll WM, Zhu M, 2017. Second harmonic generation imaging of corneal stroma after infection by Pseudomonas aeruginosa. Sci. Rep 7, 1–10. 10.1038/srep46116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohit A, Willcox M, Stapleton F, 2013. Tear lipid layer and contact lens comfort: a review. Eye Contact Lens 39, 247–253. 10.1097/ICL.0b013e31828af164. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Zheng Z, Berk RS, Irvin RT, Hazlett LD, 1992. Corneal epithelial glycoproteins exhibit Pseudomonas aeruginosa pilus binding activity. Investig. Ophthalmol. Vis. Sci 33, 2185–2193. [PubMed] [Google Scholar]
- Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M, 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol 187, 1792–1798. 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R, Sathe S, Beaton AR, McNamara N, Fleiszig S, Ni M, 2009. Protein array characterization of bioactive proteins secreted by immortalized human corneal epithelium in response to Pseudomonas constituents. Curr. Eye Res 34, 92–98. 10.1080/02713680802539869. [DOI] [PubMed] [Google Scholar]
- Sack RA, Beaton A, Sathe S, Morris C, Willcox M, Bogart B, 2000. Towards a closed eye model of the pre-ocular tear layer. Prog. Retin. Eye Res 19, 649–668. 10.1016/S1350-9462(00)00006-9. [DOI] [PubMed] [Google Scholar]
- Sack RA, Tan KO, Tan A, 1992. Diurnal tear cycle: evidence for a nocturnal inflammatory constitutive tear fluid. Investig. Ophthalmol. Vis. Sci 33, 626–640. [PubMed] [Google Scholar]
- Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW, 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 12, 87 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro I, Parales RE, Krell T, Hill JE, 2014. Pseudomonas chemotaxis. FEMSMicrobiol. Rev 39, 17–46. 10.1111/1574-6976.12081. [DOI] [PubMed] [Google Scholar]
- Sana TG, Baumann C, Merdes A, Soscia C, Rattei T, Hachani A, Jones C, Bennett KL, Filloux A, Superti-Furga G, Voulhoux R, Bleves S, 2015. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. mBio 6, e00712 10.1128/mBio.00712-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, Finck-Barbançon V, Buchaklian A, Lei M, Long RM, Wiener-Kronish J, Sawa T, 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J 22, 2959–2969. 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa T, Shimizu M, Moriyama K, Wiener-Kronish JP, 2014. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit. Care 18, 668 10.1186/s13054-014-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR, 1989a. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. N. Engl. J. Med 321, 773–778. 10.1056/NEJM198909213211201. [DOI] [PubMed] [Google Scholar]
- Schein OD, Ormerod LD, Barraquer E, Alfonso E, Egan KM, Paton BG, Kenyon KR, 1989b. Microbiology of contact lens-related keratitis. Cornea 8, 281–285. 10.1097/00003226-198912000-00011. [DOI] [PubMed] [Google Scholar]
- Schmidt AJ, Ryjenkov DA, Gomelsky M, 2005. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J. Bacteriol 187, 4774–4781. 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooling SR, Beveridge TJ, 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol 188, 5945–5957. 10.1128/JB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CL, Buret AG, Olson ME, Ceri H, Read RR, Morck DW, 2000Lipopolysaccharide entry in the damaged cornea and specific uptake by polymorphonuclear neutrophils. Infect. Immun 68, 1731–1734. 10.1128/IAI.68.3.1731-1734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Kuehn MJ, 2015. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol 13, 605–619. 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Gilbert JA, 2018. Microbial exposure and human health. Curr. Opin. Microbiol 44, 79–87. 10.1016/J.MIB.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Shen EP, Hsieh YT, Chu HS, Chang SC, Hu FR, 2014. Correlation of Pseudomonas aeruginosa genotype with antibiotic susceptibility and clinical features of induced central keratitis. Investig. Ophthalmol. Vis. Sci 56, 365–371. 10.1167/iovs.14-15241. [DOI] [PubMed] [Google Scholar]
- Shen EP, Tsay RY, Chia JS, Wu S, Lee JW, Hu FR, 2012. The role of type III secretion system and lens material on adhesion of Pseudomonas aeruginosa to contact lenses. Investig. Ophthalmol. Vis. Sci 53, 6416–6426. 10.1167/iovs.11-8184. [DOI] [PubMed] [Google Scholar]
- Shen K, Sayeed S, Antalis P, Gladitz J, Ahmed A, Dice B, Janto B, Dopico R, Keefe R, Hayes J, Johnson S, Yu S, Ehrlich N, Jocz J, Kropp L, Wong R, Wadowsky RM, Slifkin M, Preston RA, Erdos G, Post JC, Ehrlich GD, Hu FZ, 2006. Extensive genomic plasticity in Pseudomonas aeruginosa revealed by identification and distribution studies of novel genes among clinical isolates. Infect. Immun 74, 5272–5283. 10.1128/IAI.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh P, Siegrist MS, Cullen AJ, Bertozzi CR, 2014. Imaging bacterial peptidoglycan with near-infrared fluorogenic azide probes. Proc. Natl. Acad. Sci 111, 5456–5461. 10.1073/pnas.1322727111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Price K, Albert L, Dodick J, Park L, Dominguez-Belloa MG, 2016. Changes in the eye microbiota associated with contact lens wearing. mBio 7, e00198 10.1128/mBio.00198-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist MS, Whiteside S, Jewett JC, Aditham A, Cava F, Bertozzi CR, 2013. D-amino acid chemical reporters reveal peptidoglycan dynamics of an intracellular pathogen. ACS Chem. Biol 8, 500–505. 10.1021/cb3004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Hazlett L, Berk RS, 1991. Characterization of Pseudomonal adherence to unwounded cornea. Investig. Ophthalmol. Vis. Sci 32, 2096–2104. [PubMed] [Google Scholar]
- Solomon OD, Loff H, Perla B, Kellis A, Belkin J, Roth AS, Zucker J, 1994Testing hypotheses for risk factors for contact lens-associated infectious keratitis in an animal model. CLAO J. 20, 109–113. [PubMed] [Google Scholar]
- Sosnová-Netuková M, Kuchynka P, Forrester JV, 2007. The suprabasal layer of corneal epithelial cells represents the major barrier site to the passive movement of small molecules and trafficking leukocytes. Br. J. Ophthalmol 91, 372–378. 10.1136/bjo.2006.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Leger AJ, Desai JV, Drummond RA, Kugadas A, Almaghrabi F, Silver P,Raychaudhuri K, Gadjeva M, Iwakura Y, Lionakis MS, Caspi RR, 2017. An Ocular Commensal protects against corneal infection by driving an interleukin-17 response from mucosal γδ T cells. Immunity 47, 148–158. 10.1016/j.immuni.2017.06.014.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Sanson B, 2011. Epithelial polarity and morphogenesis. Curr. Opin. CellBiol 23, 540–546. 10.1016/j.ceb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Carnt N, 2012. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye 26, 185–193. 10.1038/eye.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Dart J, 1995. Pseudomonas keratitis associated with biofilm formation on a disposable soft contact lens. Br. J. Ophthalmol 79, 864–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Dart JKG, Seal DV, Matheson M, 1995. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol. Infect 114, 395–402. 10.1017/S0950268800052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Keay L, Edwards K, Holden B, 2013. The epidemiology of microbial keratitis with silicone hydrogel contact lenses. Eye Contact Lens 39, 79–85. 10.1097/ICL.0b013e3182713919. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Keay L, Edwards K, Naduvilath T, Dart JKG, Brian G, Holden BA, 2008. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 115, 1655–1662. 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Keay L, Jalbert I, Cole N, 2007. The epidemiology of contact lens related infiltrates. Optom. Vis. Sci 84, 257–272. 10.1097/OPX.0b013e3180485d5f. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Naduvilath T, Keay L, Radford C, Dart J, Edwards K, Carnt N,Minassian D, Holden B, 2017. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS One 12, e0181343 10.1371/journal.pone.0181343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Stretton S, Papas E, Skotnitsky C, Sweeney DF, 2006. Silicone hydrogel contact lenses and the ocular surface. Ocul. Surf 4, 24–43. 10.1016/S1542-0124(12)70262-8. [DOI] [PubMed] [Google Scholar]
- Stewart RMK, Wiehlmann L, Ashelford KE, Preston SJ, Frimmersdorf E,Campbell BJ, Neal TJ, Hall N, Tuft S, Kaye SB, Winstanley C, 2011. Genetic characterization indicates that a specific subpopulation of Pseudomonas aeruginosa is associated with keratitis infections. J. Clin. Microbiol 49, 993–1003. 10.1128/JCM.02036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, 2003. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol 3, 879–889. 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Sullivan AB, Tam KPC, Metruccio MME, Evans DJ, Fleiszig SMJ, 2015. The importance of the Pseudomonas aeruginosa type III secretion system in epithelium traversal depends upon conditions of host susceptibility. Infect. Immun 83, 1629–1640. 10.1128/IAI.02329-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, Pearlman E, 2010. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J. Immunol 185, 4272–4283. 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Karmakar M, Taylor PR, Rietsch A, Pearlman E, 2012. ExoS and ExoT ADP ribosyltransferase activities mediate Pseudomonas aeruginosa keratitis by promoting neutrophil apoptosis and bacterial survival. J. Immunol 188, 1884–1895. 10.4049/jimmunol.1102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere JM, Van Belleghem JD, Ishak H, Bach MS, Popescu M, Sunkari V, Kaber G, Manasherob R, Suh GA, Cao X, de Vries CR, Lam DN, Marshall PL, Birukova M, Katznelson E, Lazzareschi DV, Balaji S, Keswani SG, Hawn TR, Secor PR, Bollyky PL, 2019. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 363, eaat9691 10.1126/science.aat9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczotka-Flynn L, Chalmers R, 2013. Incidence and epidemiologic associations of corneal infiltrates with silicone hydrogel contact lenses. In: Eye and Contact Lens, vol.39 pp. 49–52. 10.1097/ICL.0b013e318271d3dc. [DOI] [PubMed] [Google Scholar]
- Szczotka-Flynn L, Lass JH, Sethi A, Debanne S, Benetz BA, Albright M, Gillespie B, Kuo J, Jacobs MR, Rimm A, 2010a. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Investig. Ophthalmol. Vis. Sci 51, 5421–5430. 10.1167/iovs.10-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczotka-Flynn LB, Imamura Y, Chandra J, Yu C, Mukherjee PK, Pearlman E, Ghannoum MA, 2009. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea 28, 918–926. 10.1097/ICO.0b013e3181a81835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczotka-Flynn LB, Pearlman E, Ghannoum M, 2010b. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens 36, 116–129. 10.1097/ICL.0b013e3181d20cae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szliter EA, Barrett RP, Gabriel MM, Zhang Y, Hazlett LD, 2006. Pseudomonas aeruginosa-induced inflammation in the rat extended-wear contact lens model. Eye Contact Lens 32, 12–18. 10.1097/01.icl.0000167611.03883.58. [DOI] [PubMed] [Google Scholar]
- Szliter EA, Morris CA, Carney F, Gabriel MM, Hazlett LD, 2002. Development of a new extended-wear contact lens model in the rat. CLAO J. 28, 119–123. 10.1097/01.ICL.0000018044.92196.3D. [DOI] [PubMed] [Google Scholar]
- Tam C, LeDue J, Mun JJ, Herzmark P, Robey EA, Evans DJ, Fleiszig SMJ, 2011. 3D quantitative imaging of unprocessed live tissue reveals epithelial defense against bacterial adhesion and subsequent traversal requires MyD88. PLoS One 6, e24008 10.1371/journal.pone.0024008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C, Lewis SE, Li WY, Lee E, Evans DJ, Fleiszig SMJ, 2007. Mutation of the phospholipase catalytic domain of the Pseudomonas aeruginosa cytotoxin ExoU abolishes colonization promoting activity and reduces corneal disease severity. Exp. Eye Res 85, 799–805. 10.1016/j.exer.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C, Mun JJ, Evans DJ, Fleiszig SMJ, 2012. Cytokeratins mediate epithelial innate defense through their antimicrobial properties. J. Clin. Investig 122, 3665–3677. 10.1172/JCI64416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C, Mun JJ, Evans DJ, Fleiszig SMJ, 2010. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Investig. Ophthalmol. Vis. Sci 51, 3100–3106. 10.1167/iovs.09-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Marquart ME, Fratkin JD, McCormick CC, Caballero AR, Gatlin HP, O’Callaghan RJ, 2009. Properties of PASP: a Pseudomonas protease capable of mediating corneal erosions. Investig. Ophthalmol. Vis. Sci 50, 3794–3801. 10.1167/iovs.08-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchedre K, Imayasu M, Hori Y, Cavanagh HD, 2013. Contact lens care solutions downregulate membrane-associated mucins 1 and 16 in cultured human corneal epithelial cells and at the rat corneal surface in vivo. Eye Contact Lens Sci. Clin. Pract 39, 394–399. 10.1097/ICL.0b013e3182a2f8d9. [DOI] [PubMed] [Google Scholar]
- Thakur A, Willcox MDP, 2000. Contact lens wear alters the production of certain inflammatory mediators in tears. Exp. Eye Res 70, 255–259. 10.1006/EXER.1999.0767. [DOI] [PubMed] [Google Scholar]
- Thanabalasuriar A, Scott BNV, Peiseler M, Willson ME, Zeng Z, Warrener P, Keller AE, Surewaard BGJ, Dozier EA, Korhonen JT, Cheng L.I. tin, Gadjeva M, Stover CK, DiGiandomenico A, Kubes P, 2019. Neutrophil extracellular traps confine Pseudomonas aeruginosa ocular biofilms and restrict brain invasion. Cell Host Microbe 25, 526–536. 10.1016/j.chom.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer MM, Flaherty KM, McKay DB, 1991. Three-dimensional structure of the elastase of Pseudomonas aeruginosa at 1.5-Å resolution. J. Biol. Chem 266, 2864–2871. [DOI] [PubMed] [Google Scholar]
- Thibodeaux BA, Caballero AR, Marquart ME, Tommassen J, O’Callaghan RJ, 2007. Corneal virulence of Pseudomonas aeruginosa elastase B and alkaline protease produced by Pseudomonas putida. Curr. Eye Res 32, 373–386. 10.1080/02713680701244181. [DOI] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Santhiago MR, Wilson SE, 2013. The corneal epithelial basement membrane: structure, function, and disease. Investig. Ophthalmol. Vis. Sci 54, 6390–6400. 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toska J, Sun Y, Carbonell DA, Foster ANS, Jacobs MR, Pearlman E, Rietsch A, 2014. Diversity of virulence phenotypes among type III secretion negative Pseudomonas aeruginosa clinical isolates. PLoS One 9, e86829 10.1371/journal.pone.0086829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CS, Eran Y, Ruch TR, Bryant DM, Datta A, Brakeman P, Kierbel A,Wittmann T, Metzger RJ, Mostov KE, Engel JN, 2014. Host cell polarity proteins participate in innate immunity to Pseudomonas aeruginosa infection. Cell Host Microbe 15, 636–643. 10.1016/j.chom.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran VB, Fleiszig SMJ, Evans DJ, Radke CJ, 2011a. Dynamics of flagellum- and pilus-mediated association of Pseudomonas aeruginosa with contact lens surfaces. Appl. Environ. Microbiol 77, 3644–3652. 10.1128/AEM.02656-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran VB, Sung YS, Fleiszig SMJ, Evans DJ, Radke CJ, 2011b. Dynamics of Pseudomonas aeruginosa association with anionic hydrogel surfaces in the presence of aqueous divalent-cation salts. J. Colloid Interface Sci 362, 58–66. 10.1016/j.jcis.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI, 2007. The human microbiome project. Nature 449, 804–810. 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Stintzi A, 2011. Human health and disease in a microbial world. Front. Microbiol 2, 190 10.3389/fmicb.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining Sally S., Kirschner SE, Mahnke LA, Frank DW, 1993. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Investig. Ophthalmol. Vis. Sci 34, 2699–2712. [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV, 2011. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258. 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini M, Filloux A, 2016. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem 291, 12547–12555. 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Rietsch A, Mekalanos JJ, 2005. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect. Immun 73, 1706–1713. 10.1128/IAI.73.3.1706-1713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareechon C, Zmina SE, Karmakar M, Pearlman E, Rietsch A, 2017. Pseudomonas aeruginosa effector ExoS inhibits ROS production in human neutrophils. Cell Host Microbe 21, 611–618. 10.1016/j.chom.2017.04.001.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater SM, Weiße S, Maleschlijski S, Lotz C, Koschitzki F, Schwartz T, Obst U, Rosenhahn A, 2014. Swimming behavior of Pseudomonas aeruginosa studied by holographic 3D tracking. PLoS One 9, e87765 10.1371/journal.pone.0087765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani JR, Lorick SA, Yoder JS, Beach MJ, Braden CR, Roberts JM, Conover CS, Chen S, McConnell KA, Chang DC, Park BJ, Jones DB, Visvesvara GS, Roy SL, 2009. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg. Infect. Dis 15, 1236–1242. 10.3201/eid1508.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay AK, Sankaridurg P, Zhu H, Willcox MDP, 2009. Guinea pig models of acute keratitis responses. Cornea 28, 1153–1159. 10.1097/ICO.0b013e3181a87a0b. [DOI] [PubMed] [Google Scholar]
- Vijay AK, Zhu H, Ozkan J, Wu D, Masoudi S, Bandara R, Borazjani RN, Willcox MDP, 2012. Bacterial adhesion to unworn and worn silicone hydrogel lenses. Optom. Vis. Sci 89, 1095–1106. 10.1097/opx.0b013e318264f4dc. [DOI] [PubMed] [Google Scholar]
- Wagner VE, Iglewski BH, 2008. P. aeruginosa biofilms in CF infection. Clin. Rev. Allergy Immunol 35, 124–134. 10.1007/s12016-008-8079-9. [DOI] [PubMed] [Google Scholar]
- Wan SJ, Sullivan AB, Shieh P, Metruccio MME, Evans DJ, Bertozzi CR, Fleiszig SMJ, 2018. IL-1R and MyD88 contribute to the absence of a bacterial microbiome on the healthy murine cornea. Front. Microbiol 9, 1117 10.3389/fmicb.2018.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Zhu M, Matthew Petroll W, Robertson DM, 2014. Pseudomonas aeruginosa infectious keratitis in a high oxygen transmissible rigid contact lens rabbit model. Investig. Ophthalmol. Vis. Sci 55, 5890–5899. 10.1167/iovs.14-14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh NH, Rauch AJ, Gaffin SL, 1984. Topical immunotherapy for Pseudomonas keratitis in rabbits: use of antilipopolysaccharide plasma. Br. J. Ophthalmol 68, 828–832. 10.1136/bjo.68.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch CB, Beatson SA, Comolli JC, Jakobsen T, Sargent JL, Bertrand JJ, West J, Klausen M, Waite LL, Kang PJ, Tolker-Nielsen T, Mattick JS, Engel JN, 2005. Pseudomonas aeruginosa fimL regulates multiple virulence functions by intersecting with Vfr-modulated pathways. Mol. Microbiol 55, 1357–1378. 10.1111/j.1365-2958.2005.04479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S,Greenberg EP, 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413, 860–864. 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- Wichterle O, Lím D, 1960. Hydrophilic gels for biological use. Nature 185, 117–118. 10.1038/185117a0. [DOI] [Google Scholar]
- Willcox MDP, Harmis N, Cowell B, Williams T, Holden B, 2001. Bacterial interactions with contact lenses; effects of lens material, lens wear and microbial physiology. Biomaterials 22, 3235–3247. 10.1016/S0142-9612(01)00161-2. [DOI] [PubMed] [Google Scholar]
- Willcox MDP, 2013a. Characterization of the normal microbiota of the ocular surface.Exp. Eye Res 117, 99–105. 10.1016/j.exer.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Willcox MDP, 2013b. Microbial adhesion to silicone hydrogel lenses. Eye Contact Lens Sci. Clin. Pract 39, 60–65. 10.1097/ICL.0b013e318275e284. [DOI] [PubMed] [Google Scholar]
- Willcox MDP, 2013c. Solutions for care of silicone hydrogel lenses. In: Eye and Contact Lens, vol. 39 pp. 24–28. 10.1097/ICL.0b013e318275e0d9. [DOI] [PubMed] [Google Scholar]
- Willcox MDP, 2007. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom. Vis. Sci 84, 273–278. 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- Wilson LA, Sawant AD, Ahearn DG, 1991. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch. Ophthalmol 109, 1155–1157. 10.1001/archopht.1991.01080080115043. [DOI] [PubMed] [Google Scholar]
- Winstanley C, Kaye SB, Neal TJ, Chilton HJ, Miksch S, Hart CA, 2005Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J. Med. Microbiol 54, 519–526. 10.1099/jmm.0.46005-0. [DOI] [PubMed] [Google Scholar]
- Winstanley C, O’Brien S, Brockhurst MA, 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 24, 327–337. 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiuff C, Zappala RM, Regoes RR, Garner KN, Baquero F, Levin BR, 2005. Phenotypic tolerance: antibiotic enrichment of noninherited resistance in bacterial populations. Antimicrob. Agents Chemother 49, 1483–1494. 10.1128/AAC.49.4.1483-1494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang MC, Lee VT, Gilmore ME, Lory S, 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4, 253–263. 10.1016/S1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Wood TK, Knabel SJ, Kwan BW, 2013. Bacterial persister cell formation and dormancy. Appl. Environ. Microbiol 79, 7116–7121. 10.1128/AEM.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workentine ML, Sibley CD, Glezerson B, Purighalla S, Norgaard-Gron JC, Parkins MD, Rabin HR, Surette MG, 2013. Phenotypic heterogeneity of Pseudomonas aeruginosa populations in a cystic fibrosis patient. PLoS One 8, e60225 10.1371/journal.pone.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretlind B, Pavlovskis OR, 1983. Pseudomonas aeruginosa elastase and its role in Pseudomonas infections. Rev. Infect. Dis 5 (Suppl. 5), S998–S1004. 10.1093/clinids/5.Supplement_5.S998. [DOI] [PubMed] [Google Scholar]
- Wright JB, Costerton JW, McCoy WF, 1988. Filamentous growth of Pseudomonas aeruginosa. J. Ind. Microbiol 3, 139–146. 10.1007/BF01569520. [DOI] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D, 2010a. Gut-residing segmented filamentous bacteria drive auto-immune arthritis via T helper 17 cells. Immunity 32, 815–827. 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, McClellan SA, Barrett RP, Zhang Y, Hazlett LD, 2009. Beta-defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa keratitis. J. Immunol 183, 8054–8060. 10.4049/jimmunol.0902140. [DOI] [PubMed] [Google Scholar]
- Wu YT, Tam C, Zhu LS, Evans DJ, Fleiszig SMJ, 2017. Human tear fluid reduces culturability of contact lens-associated Pseudomonas aeruginosa biofilms but induces expression of the virulence-associated type III secretion system. Ocul. Surf 15, 88–96. 10.1016/j.jtos.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Zhu H, Willcox M, Stapleton F, 2011. The effectiveness of various cleaning regimens and current guidelines in contact lens case biofilm removal. Investig. Ophthalmol. Vis. Sci 52, 5287–5292. 10.1167/iovs.10-6785. [DOI] [PubMed] [Google Scholar]
- Wu YT, Zhu H, Willcox M, Stapleton F, 2010b. Removal of biofilm from contact lens storage cases. Investig. Ophthalmol. Vis. Sci 51, 6329–6333. 10.1167/iovs.10-5796. [DOI] [PubMed] [Google Scholar]
- Wu YT, Zhu LS, Tam KPC, Evans DJ, Fleiszig SMJ, 2015a. Pseudomonas aeruginosa survival at posterior contact lens surfaces after daily wear. Optom. Vis. Sci 92, 659–664. 10.1097/OPX.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Willcox M, Zhu H, Stapleton F, 2015b. Contact lens hygiene compliance and lens case contamination: a review. Contact Lens Anterior Eye 38, 307–316. 10.1016/j.clae.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Yahr TL, Barbieri JT, Frank DW, 1996a. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J. Bacteriol 178, 1412–1419. 10.1128/jb.178.5.1412-1419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Frank DW, 1994. Transcriptional organization of the trans-regulatory locus which controls exoenzyme S synthesis in Pseudomonas aeruginosa. J. Bacteriol 176, 3832–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Goranson J, Frank DW, 1996b. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol 22, 991–1003. 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW, 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. U. S. A 95, 13899–13904. 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Naoka, Yamamoto Nobutaka, Petroll MW, Cavanagh HD, Jester JV, 2005. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci 46, 1348–1355. 10.1167/iovs.04-0542. [DOI] [PubMed] [Google Scholar]
- Yamamoto Nobutaka, Yamamoto Naoka, Jester JV, Petroll WM, Cavanagh HD, 2006. Prolonged hypoxia induces lipid raft formation and increases Pseudomonas internalization in vivo after contact lens wear and lid closure. Eye Contact Lens 32, 114–120. 10.1097/01.icl.0000177384.27778.4c. [DOI] [PubMed] [Google Scholar]
- Yasueda SI, Yamakawa K, Nakanishi Y, Kinoshita M, Kakehi K, 2005. Decreased mucin concentrations in tear fluids of contact lens wearers. J. Pharm. Biomed. Anal 39, 187–195. 10.1016/j.jpba.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhou X, Wen S, Xiao Q, 2012. Flagellin/TLR5 responses induce mucus hypersecretion by activating EGFR via an epithelial cell signaling cascades. Exp. Cell Res 318, 723–731. 10.1016/j.yexcr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Zaidi TS, Fleiszig SM, Preston MJ, Goldberg JB, Pier GB, 1996Lipopolysaccharide outer core is a ligand for corneal cell binding and ingestion of Pseudomonas aeruginosa. Investig. Ophthalmol. Vis. Sci 37, 976–986. [PubMed] [Google Scholar]
- Zaidi TS, Lyczak J, Preston M, Pier GB, 1999. Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect. Immun 67, 1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi Tanweer, Bajmoczi M, Zaidi Tauqeer, Golan DE, Pier GB, 2008. Disruption of CFTR-dependent lipid rafts reduces bacterial levels and corneal disease in a murine model of Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci 49, 1001–1009. 10.1167/iovs.07-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M, 2002. Antimicrobial peptides of multicellular organisms. Nature 415,389–395. 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu K, Ambati B, Yu FS, 2003. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Investig. Ophthalmol. Vis 44, 4247–4254. 10.1167/iovs.03-0219.Sci. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gabriel MM, Mowrey-McKee MF, Barrett RP, McClellan S, Hazlett LD, 2008. Rat silicone hydrogel contact lens model: effects of high- versus low-Dk lens wear. Eye Contact Lens Sci. Clin. Pract 34, 306–311. 10.1097/ICL.0b013e3181891421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Conibear TCR, Bandara R, Aliwarga Y, Stapleton F, Willcox MDP, 2006. Type III secretion system-associated toxins, proteases, serotypes, and antibiotic resistance of Pseudomonas aeruginosa isolates associated with keratitis. Curr. Eye Res 31, 297–306. 10.1080/02713680500536746. [DOI] [PubMed] [Google Scholar]
- Zolfaghar I, Angus AA, Kang PJ, To A, Evans DJ, Fleiszig SMJ, 2005. Mutation of retS, encoding a putative hybrid two-component regulatory protein in Pseudomonas aeruginosa, attenuates multiple virulence mechanisms. Microb. Infect 7, 1305–1316. 10.1016/j.micinf.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Zolfaghar I, Evans DJ, Fleiszig SMJ, 2003. Twitching motility contributes to the role of pili in corneal infection caused by Pseudomonas aeruginosa. Infect. Immun 71, 5389–5393. 10.1128/IAI.71.9.5389-5393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SMJ, 2006. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect. Immun 74, 3880–3889. 10.1128/IAI.01891-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.