Abstract
As longevity has increased for people living with HIV (PLWH) in the United States and Europe, there has been a concomitant increase in the prevalence of cardiovascular disease (CVD) risk factors and morbidity in this population. Whereas the availability of HIV antiretroviral therapy has resulted in dramatic increases in life expectancy in sub-Saharan Africa (SSA), where over two thirds of PLWH reside, if and how these trends impact the epidemiology of CVD is less clear. In this review, we describe the current state of the science on how both HIV and its treatment impact CVD risk factors and outcomes among PLWH in sub-Saharan Africa, including regional factors (unique to SSA) likely to differentiate these relationships from the global North. We then outline how current regional guidelines address CVD prevention among PLWH and which clinical and structural interventions are best poised to confront the co-epidemics of HIV and CVD in the region. We conclude with a discussion of key research gaps that need to be addressed to optimally develop an actionable public health response.
Keywords: Cardiovascular risk factors, Cardiovascular disease, People living with HIV, Sub-Saharan Africa
Background
Approximately two thirds of the world’s population of people living with HIV (PLWH) reside in sub-Saharan Africa (SSA).1 After a lag in resource allocation and implementation of HIV control programs, the continent has seen a meteoric rise in access to HIV treatment over the past decade – with antiretroviral therapy (ART) coverage rates rising from virtually none in 1990s to 62% by 2018.1 This massive public health commitment and implementation has resulted in significantly increased longevity for PLWH. Due to this longevity, the leading causes of mortality among PLWH are now cardiovascular disease (CVD), non-AIDS malignancies, and liver disease.2 In the United States and Europe, there is evidence of heightened risk of myocardial infarction, stroke, heart failure,3,4 and sudden cardiac death5 among PLWH. Globally, the risk of CVD in PLWH is estimated to be 2.5-fold higher than HIV-uninfected persons, contributing to 2.6 million disability-associated life-years (DALYs) annually (a third of which are in SSA).6
In the wake of these epidemiologic shifts among PLWH, there is growing interest in measuring CVD risk factors and outcomes in the sub-Saharan African region has grown; however, most of these data have been limited to risk factor assessments, such as blood pressure (BP) and diabetes and lipids. Despite heterogeneity in unmeasured effects (genetics and environmental risk factors) and relationships between risk factors and outcomes, these data have been used to assess CVD risk, with risk prediction models derived from Western populations, such as the Framingham and Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Risk scores.7,8
Consequently, public health programs for HIV care are at a crucial crossroads, with parallel (or competing) priorities of ensuring all PLWH have access to care, maximizing HIV prevention efforts, and promoting health and quality of life for PLWH. Achieving these goals will require a thorough understanding of CVD epidemiology among PLWH. However, a number of critical questions remain unanswered related to CVD risk among PLWH in SSA:
What is the epidemiology of traditional (long-established) CVD risk factors among PLWH on chronic ART?
Which regionally relevant risk factors modify CVD risk among PLWH?
How do currently available data inform us about how HIV infection directly affects CVD risk in SSA?
How well do current regional guidelines account for CVD prevention through routine screening and management?
What CVD prevention interventions are most likely to reduce morbidity and mortality in the region?
What are the major research gaps that need to be addressed to ensure CVD risk is optimally minimized among PLWH in SSA?
In this systemic review, we aim to address these questions and lay the groundwork for future research and programmatic priorities for this field.
The epidemiology of traditional CVD risk factors among PLWH taking long-term ART
Hypertension (HTN)
As described globally, HTN ranks as the leading risk factor for CVD in PLWH in sub-Saharan Africa (SSA).9 According to a recent meta-analysis, the prevalence of HTN in PLWH on ART is 35% and rising.10 PLWH in SSA experience a high incidence of new-onset HTN, particularly in the first 1–2 years after ART initiation.11 Some evidence suggests that PLWH who have HTN may experience an even higher risk of CVD than HIV-negative adults with HTN.12 Though there were conflicting early reports on the burden of HTN in PLWH compared to uninfected people, recent studies from SSA demonstrate lower rates of HTN in PLWH compared to controls, and that women living with HIV have higher rates than men living with HIV.13–16 Interestingly, while women have a higher prevalence of HTN, they are more likely to be aware, and more likely to have BP control.17
As the population of PLWH in SSA is aging, HTN may provide a valuable and easy-to-measure indicator and target of escalating overall risk for CVD. Data from large cohorts in the United States and Europe have demonstrated that over half of PLWH have HTN and those with HTN usually have multiple CVD risk factors.18,19 A modeling-based study from Zimbabwe predicts that a similar trend will occur in the next 20 years in SSA.20 HTN is often the first harbinger of metabolic syndrome, which is a major CVD risk factor in PLWH.21 Even younger PLWH have high lifetime CVD risk scores, and this is largely driven by HTN.22
Notably, risk factors and treatment implications for HTN in PLWH in SSA may differ from other populations due to several factors. First, modest drug-drug interactions between ART and BP lowering drugs may affect the choice of medications for PLWH with HTN, although these drug interactions generally do not prevent titration to the highest doses.23 Second, growing evidence suggests that the potency of BP lowering drugs for people in SSA may differ from other populations with better response to combinations, including calcium channel blockers.24 Third, the best method for measuring BP and the optimal threshold for starting medications for HTN for PLWH has not yet been determined.25 Finally, the pathophysiology of HTN in PLWH in SSA may not be the same as the mechanisms in HIV-uninfected adults.26 The complex interplay between traditional risk factors, HIV viral tropism, ART, HIV-related chronic inflammation, rapid changes in adiposity and other factors leading to HTN in PLWH may require new strategies for HTN prevention and treatment in this population.27–29
Despite the growing knowledge of HTN among PLWH, the HIV health system in SSA is ill-equipped to handle the rising tide of HTN in PLWH with most HIV clinical care services lacking basic equipment such as BP cuffs and sphygmomanometers and front-line healthcare workers with limited basic knowledge about HTN.30 This is compounded by fragmented clinical care for HIV and for HTN, frequent stock outs of BP lowering drugs, as such PLWH with HTN often face severe physical and financial barriers to obtaining treatment for both HIV and HTN.31 Integration of HIV clinical care with HTN care provides a unique opportunity to improve awareness, treatment and control for HTN in PLWH.32,33
Diabetes mellitus (DM)
In SSA, DM is predicted to increase by 160% by 2030, partly owing to urbanization and unhealthy lifestyles.34 Abnormal glucose regulation associated with HIV per se35 and ART36 may lead to a rise in DM.37 Limited cross sectional evidence describe up to a four-fold higher risk of DM in PLWH taking ART compared to uninfected counterparts.38,39 In the context of the rollout of the HIV treat all campaign, and the increased availability of ART, the number of PLWH on ART with an increased risk of dysglycemia and DM will escalate.
However, in western countries the positive association between HIV and prevalent DM correlates with traditional risk factors, such as increasing age and obesity,40,41 suggesting that these factors, rather than ART play a major role in the development of DM among PLWH. Fortunately, the use of older generations of ART drugs such as indinavir, zidovudine, saquinavir, stavudine, and didanosine, which have been associated with a higher prevalence of DM,42,43 has been curtailed in SSA and thus a reduction in ART-induced DM.
Disparities by regional settings notwithstanding, health care providers should follow existing DM screening guidelines for PLWH before and after starting ART.44 In addition, the increased prevalence of DM among younger and non-obese PLWH, suggests modification of DM screening guidelines might help address this. Moreover, improved diagnostics for DM diagnosis and monitoring among PLWH are needed, based on studies demonstrating lower hemoglobin levels in PLWH raise limitations about HbA1c accuracy among PLWH.41,45,46 Similar reductions in HbA1c validity have been shown in those with sickle cell trait, which is highly prevalent in much of SSA.47 Outstanding gaps to identify optimal DM management strategies among PLWH abound. Traditional strategies to improve insulin sensitivity, such as weight loss and medical therapy have been shown to be less effective.48 For example, drug-drug interactions of metformin and dolutegravir, a cost-effective integrase-strand transfer inhibitor used in first-line ART regimens in most national HIV care programs in SSA, should be evaluated for optimal glycemic control in PLWH in the era of test and treat.49,50
Obesity
Overweight and obesity are the fifth leading risk factors for global deaths51 and the risk of mortality increases with morbid obesity (body mass index (BMI) > 40 kg/m2).52 In addition, 44% of the DM burden, 23% of the ischemic heart disease burden, and between 7% and 41% burdens of prostate, colon, and breast cancers are attributable to being overweight and obese.53,54
Obesity is increasingly common among PLWH in SSA with prevalence rates ranging from 5% in Nigeria55 to 23% in South Africa56 representing a remarkable transformation from the epoch when HIV was defined by “wasting” and “slimming”.57 In SSA, PLWH have low pre-ART BMI with subsequent weight gain following ART initiation.58 However, the ART associated weight gain is disproportionate resulting in central/visceral adiposity and eventually lipodystrophy that is believed to drive subsequent dysglycemia.59 Further still, newer first-line ART regimens that include dolutegravir have been documented to result in significant weight gain compared to previously favored, efavirenz-based ART regimens.60 These increases appear to be greatest in women, and appear to primarily affect the truncal areas of the body.60
Among PLWH, women have higher prevalence of obesity and overweight than men16,56,61,62 though associated factors do not differ from those for the general population.56 Modifiable factors associated with obesity include physical inactivity from increased urbanization, intake of energy dense diets that include high-fat, carbohydrate-rich foods, sugars and salt.53,54,63
Finally, additional data are needed to clarify optimal thresholds of BMI risk stratification among PLWH in SSA. Data from SSA, where traditionally-defined elevated BMI, particularly in women, is often seen in approximately 50% of the population, are needed to develop locally-relevant thresholds that predict downstream health outcomes.56
Dyslipidemia
To-date, few studies have examined the impact of dyslipidemia on cardiovascular health in SSA, and locally validated thresholds are lacking. Recently, an elevation of the ratio of total cholesterol to high density lipoprotein-cholesterol (HDL-c) - a validated measure of dyslipidemia -was shown to predict short-term all-cause mortality among PLWH in Malawi.64
Though dyslipidemia is common in the general adult population, PLWH taking ART have a higher burden compared to their HIV-uninfected counterparts.65 A meta-analysis by Noubiap et al.66 of 177 facility- and population-based studies of dyslipidemia across Africa reported a prevalence for elevated total cholesterol of 26.2% in PLWH compared to 25.5% in the general population, and more than two-fold higher prevalence for elevated low density lipoprotein (LDL)-cholesterol (45.6% vs 21.4%).66
A study from Malawi described population attributable fraction for stroke by hypercholesterolemia (total cholesterol ≥ 6.2 mmol/L) was 3% among adults younger than 45 years and 1% among those older than 45 years,67 demonstrating the comparatively minimal role of dyslipidemia in CVD morbidity and mortality compared to other risk factors. Nonetheless, hypercholesterolemia’s contribution to CVD burden among PLWH receiving ART in SSA might be considerably higher as demonstrated in USA and Europe.
As with obesity and diabetes mellitus discussed above, newer ART regimens with fewer dyslipidemic effects are increasingly available; however, continued research is needed to delineate the metabolic effects of newer ART, and particularly so for dolutegravir, which is now a first-line agent in much of the region and which preliminary studies implicate in significant weight gain.68,69
Smoking
Smoking is the leading behavioral cause of CVD worldwide.70 Though smoking rates are decreasing worldwide in the wake of tightened regulations such as the World Health Organization’s Framework Convention on Tobacco Control, progress has lagged across much of SSA,71–73 likely driven by differences in tobacco taxation and policies regarding advertising and public smoking.73
Similar to trends in high-income settings, PLWH in SSA are more likely to smoke than their HIV-uninfected counterparts. In a meta-analysis of nationally-representative studies from 28 low and middle income countries, Mdege et al.74 found that 24% of men with HIV and 1% of women with HIV in SSA smoke tobacco products. Compared to the HIV-uninfected population, this translates to a 47% higher risk of smoking among men and 87% higher risk of smoking among women with HIV. Reasons why PLWH may smoke more than the general population include self-treatment of the comorbid anxiety and depression that is prevalent among PLWH, risky behaviors driven by the perception that life expectancy is too short to experience tobacco-related health effects, and self-management of HIV-associated symptoms.75,76
In high income settings, smoking decreases life expectancy more than HIV infection itself for PLWH taking ART,77 though it is unknown whether this relationship holds for PLWH in SSA. Though smoking and HIV infection each independently increase the risk for CVD, whether PLWH have increased susceptibility to smoking-associated CVD compared to HIV-uninfected smokers remains unknown. Furthermore, the types and forms of tobacco use in SSA differ from that found in other settings, but little is known regarding whether the unique sources of tobacco have different influences on cardiovascular health. PLWH require concerted smoking cessation efforts, but further work is needed to characterize the most effective cessation strategies in this population.
Regionally relevant risk factors modify CVD risk among PLWH in sub-Saharan Africa
Sex
Women living with HIV (WLWH) in North America and Europe are estimated to have 2 to 4 times higher risk of myocardial infarction, stroke and heart failure as compared to women without HIV infection and approximately equivalent risk compared to men living with HIV (MLWH).78–81 The reasons behind this elevated risk are multifactorial, including 1) increased levels of systemic inflammation and interaction with sex-specific hormones,82 2) sub-optimal implementation of guideline-directed preventive therapy for traditional cardiometabolic risk factors,83 and 3) heightened levels of behavioral and psychosocial risk factors as compared to the general population.84
Whether relationships between sex and CVD risk are similar in SSA as elsewhere is unknown. Large-scale comparative data assessing the links between sex and subsequent development of CVD are lacking in the region. As discussed above, cross-sectional studies have demonstrated increased prevalence of a variety of CVD risk factors (obesity, dyslipidemia and HTN) among women in SSA compared to men, with significant variation depending on the population that is studied.85,86 One potential contributor to sex-determined CVD risk is pregnancy-related physiologic changes and its’ downstream effects on chronic health. Hypertensive disorders of pregnancy (HDP), are a major cause of mortality and morbidity worldwide, complicating 5%–10% of all pregnancies.87 The impact of HIV and its treatment on HDP has yet to be fully elucidated. In several studies, WLWH have been found to carry similar risks for HDP compared to uninfected counterparts; however, the influence of ART on this risk remains unclear.88–91 In some studies, pregnant women living with HIV taking ART have been shown to have higher rates of HDP compared to those not taking ART or uninfected.92–95 However, the role ART and or HIV in increasing the risk of HDP and gestational DM are yet to be fully elucidated.
As of yet, no large-scale, prospective cohort studies have assessed the comparative risk of developing coronary artery disease or stroke by sex with use of end-organ diagnostics, such as coronary angiogram or brain magnetic resonance imaging. Several studies have shown an increased prevalence of secondary markers of CVD in women, such as ischemic findings on ECG in Uganda, and angina symptoms as reported on the Rose Angina Questionnaire in South Africa.85,96 Numerous studies have looked at carotid intimal-medial thickness (cIMT) as a surrogate for the prevalence of CVD, with mixed results. Ssinabuulya et al. found no difference in mean cIMT in WLWH as compared to MLWH in Uganda, whereas Schoffelen et al. found a slightly lower mean cIMT among WLWH in South Africa,97,98 and Nonterah et al. demonstrated lower cIMT values among PLWH as compared to the HIV-negative population, with no significant difference by sex.99 Larger-scale, prospective studies are needed for a comprehensive understanding of the relative risk of CVD in women versus men and to quantify the contribution of ART and HIV to that risk.
Further work building upon these findings is needed to elucidate if and how sex and traditional risk factors combine to alter CVD risk among WLWH in SSA. If the preliminary relationships are confirmed, entirely new frameworks for considering female sex as a potential risk factor for CVD, an inverse of the standard conceptualization in western settings, would be needed.
Environmental factors including air pollution
Globally, air pollution is responsible for at least one out of every four deaths from both CVD and cerebrovascular disease.100 Air pollution results from a variety of human-related activities and natural events that include emissions from vehicles, factories and power plants; burning of biomass fuels (e.g., charcoal, firewood, animal dung, crop residues) for home cooking and heating; dust storms; forest fires; and volcanic eruptions. The pollutants released from these sources include particulate matter (PM), volatile organic compounds, and gases. These affect cardiovascular health by inciting systemic inflammation, oxidative stress, coagulation, and endothelial dysfunction through both local pulmonary parenchymal damage and by crossing the lung alveolar endothelium to directly enter the bloodstream.101,102 Air pollution exposure in SSA is among the highest in the world103 (Fig. 1) as a result of both rapid urbanization and industrialization as well as lack of air quality regulations.104
Fig. 1.
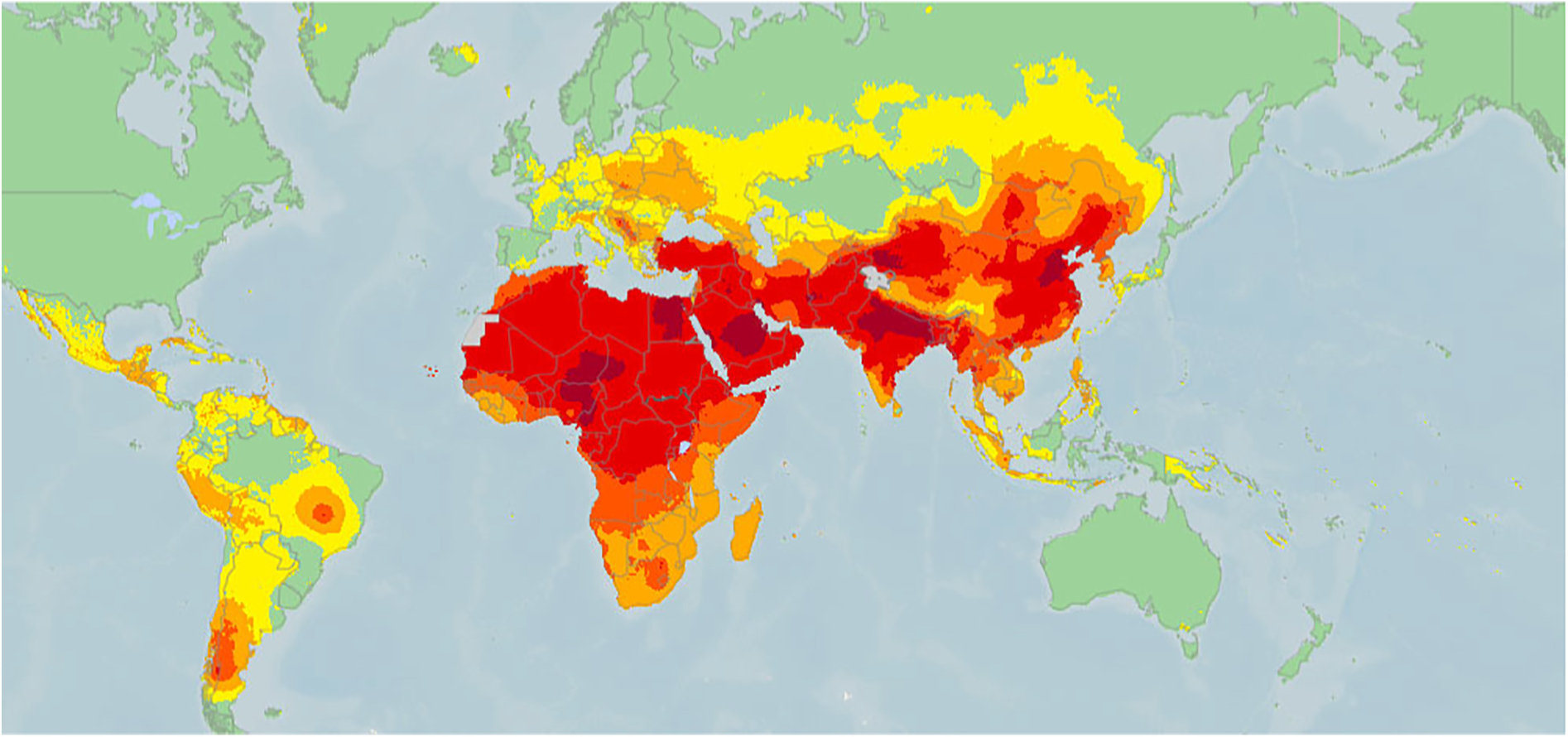
Global mean concentrations (μg/m3) of ambient particulate matter (PM) of diameter of <2.5 μm in 2016. Reprinted with permission of the American Thoracic Society. Copyright © 2019 American Thoracic Society.105
Ambient air pollution
There are few studies of the cardiovascular effects of ambient pollution in SSA that directly measure pollutant concentrations, and none that focus specifically on PLWH. The Prospective Urban Rural Epidemiology (PURE) Study which included only one African country (Tanzania) estimated that increased ambient pollution exposure was associated with 3 to 8% increased risk of CVD-related death, incident CVD events, myocardial infarction, or stroke.106 Based upon the higher ambient pollutant levels in SSA, authors suggested that ambient pollution exposure may account for the higher CVD burden. A multi-country cross-sectional study of 45,625 adults that included Ghana and South Africa showed that each 10 μg/m3 increase in average PM2.5 exposure over the preceding three years was associated with 13% higher odds of self-reported stroke.107 Wichmann and Voyi demonstrated that interquartile range increases in particulate matter, nitrogen dioxide and sulfur dioxide were each associated with increased daily CVD and/or cerebrovascular mortality, particularly in the warm season, among 149,667 residents of Cape Town, South Africa from 2001 to 2006.108 In non-African settings, ambient pollution exposure has also been associated with angina, acute myocardial infarction, heart failure hospitalizations, arrhythmias, cardiac arrest, carotid intimal thickening, aortic atherosclerotic plaques, coronary calcium progression, and adverse cardiac remodeling.109 None of this work has evaluated the potential effect modification of HIV or ART.
Household air pollution (HAP)
A leading source of air pollution in SSA is HAP, which results from indoor biomass burning and disproportionately influences the health of women and children.110 Agarwal et al. measured HAP exposure and cardiac function in 44 Kenyan women and found that higher HAP exposure was associated with lower left ventricular ejection fraction and higher pulmonary artery diastolic pressure. In this same cohort, decreasing HAP exposure through a clean cookstove intervention led to reductions in both systolic and diastolic BP over 6 months.111 Similarly, HAP was associated with higher systolic and diastolic blood pressure among 44 Ghanaian women,112 and both diastolic pressure and hypertension prevalence decreased among 324 pregnant Nigerian women randomized to cook with cleaner as compared to traditional fuel sources.113 In non-African settings, HAP exposure has been associated with ST segment changes, cIMT, right ventricular systolic pressure, and decreased right and left ventricular function.109 There are no studies of the influence of HAP on CVD among PLWH.
Early and mechanistic data suggest that the impact of air pollution on CVD might be particularly problematic in SSA and among PLWH.114 Yet, comprehensive exposure metrics exclusive of household-only studies are largely lacking, and those that do exist focus predominantly on urban settings. Additionally, the effects of air pollution are influenced by pollutant source.115–118 Much of the global knowledge regarding the health effects of air pollution originates from high income settings, where traffic and industry-related activities drive air pollution, while air pollution in SSA is driven largely by biomass fuel burning.119 Based upon similarities in the systemic inflammatory influences of both air pollution and chronic HIV infection, the potential for interactive effects on cardiovascular health is high. However, it is not yet known whether PLWH are exposed to higher levels of air pollution or whether they are more susceptible to air pollution-related cardiovascular effects.
How do currently available data inform us about how HIV infection directly affects CVD risk in SSA?
Much of the data from western settings strongly support relationships between HIV infection and risks of both pre-clinical CVD120–127 and CVD events.78,81,128–130 As discussed above, this relationship is primarily driven by a combination of traditional CVD risk factor profiles in PLWH81,127,131 and an inflammatory profile, which persists despite suppressive ART.132,133 However, critical differences exist between western and SSA populations relating to traditional risk factor profiles, regional risk factors, and clinical infrastructures for addressing CVD risk. Thus, investigation of relationships between HIV and risk of pre-clinical, clinical, and fatal CVD events in SSA remains a crucial priority.
Arteriosclerosis and pre-clinical atherosclerosis
Like in the United States, early studies assessing relationships between HIV and atherosclerosis largely focused on use of non-invasive, surrogate markers of disease, namely ankle brachial index, pulse wave velocity and cIMT. Data on arteriosclerosis, which is predictive of future CVD events, but not a marker itself, have generally demonstrated increased arterial stiffness in PLWH than HIV-uninfected comparators. For example, cohort studies from Cameroon and South Africa demonstrated increased pulse-wave velocity among ART-naïve PLWH compared to HIV-uninfected comparators, which was most pronounced in people older than 50 years.134,135 Similarly, a study in Uganda of people over 45 years old found significantly increased arterial stiffness as measured by ankle-brachial index in PLWH on long-term ART therapy compared to community-based HIV-uninfected comparators, which remained significant after adjustment for CVD risk factors.136
However, there is conflicting evidence for a similar, if not decreased burden of pre-clinical carotid atherosclerosis among PLWH compared to HIV-uninfected counterparts in SSA. For example, early studies in South Africa and Uganda, found no difference in cIMT between ART-naïve, ART-treated, and HIV-uninfected comparators, before or after adjustment of CVD risk factors.98,137,138 Interestingly, these findings were despite PLWH having higher inflammatory markers, including intracellular adhesion molecule and vascular cell adhesion molecule in South Africa, and soluble CD14, high sensitivity C-reactive protein, and fatty acid binding protein-2 in Uganda.139 Similar results suggesting no difference in cIMT between PLWH taking ART and uninfected comparators, have since been replicated in urban populations in South Africa.140 On the contrary, a large meta-analysis across sub-Saharan Africa including nearly 9000 individuals from four countries (Burkina Faso, Kenya, Ghana, and South Africa),99 showed that although traditional CVD risk factors, such as BP, LDL, and age were associated with increased cIMT, HIV serostatus was notably associated with a decreased cIMT (−8.86 μm, 95% confidence interval: −15.7, −2.0). The combined sample appeared strongly representative of older individuals in the region (median age 50 years), with nearly 85% of PLWH on ART. The first study in the region to move beyond cIMT to assess more strongly predictive measures of CVD outcomes also found similar rates of coronary artery calcium (CAC) between PLWH on ART and HIV-uninfected comparators in an urban Kampala cohort.141 That study, which compared CAC between individuals in Uganda with age and sex-matched individuals in the United States found remarkably lower prevalence of any CAC in Uganda (9% versus 48%, P < 0.01). These data are all limited by a number of factors, including cross-sectional designs, the possibility of residual confounding, and perhaps most importantly, limited correlation with downstream CVD events.
Coronary artery disease (CAD) and acute coronary syndromes (ACS)
Although pre-clinical data do not suggest increased risk of CAD among PLWH in SSA, a multitude of factors have been associated with increased risk of CAD among PLWH in the USA and Europe, including: HIV-vascular dysfunction due to immune activation caused by HIV itself, metabolic derangements, including hyperglycemia and dyslipidemia related to ART, and age-related atherosclerotic CVD disease due to risk factors present in the general population.142,143 Similarly, progression of atherosclerotic CVD into clinical coronary artery disease and acute coronary syndrome among PLWH has been observed to occur faster than that observed in the general population.144 Finally, PLWH who present with ACS tend to be younger than their HIV-uninfected counterparts.144 Based on these relationships, modeling studies that account for HIV prevalence and CVD risk factors have suggested an outsized effect of HIV on CAD in SSA.6
Unfortunately, due largely to the lack of CAD diagnostic resources in the region, primary data on CAD epidemiology outcomes in SSA are rare. Until such data become available, it will be premature to assume that predictive models for CVD risks and outcomes, which are largely based on relationships in Western populations between risk factors and outcomes, predict CVD events in the SSA region.
Stroke
In the United States, PLWH appear to have approximately 40–60% increased risk of stroke, which appears greatest in those with advanced HIV disease.78,129,145 Studies from SSA consistently demonstrate that HIV infection is an independent risk factor for stroke.146–148 This was best demonstrated in a large (n = 775) case-control study in Malawi, that noted a tripling of the odds of stroke among PLWH.67 The same study described HIV infection as accounting for 42% of total strokes among adults younger than 45 years, 6% strokes among those older than 45 years, demonstrating that stroke in PLWH in SSA disproportionately affects the young and is associated with poor outcomes.149
Putative mechanisms associated with stroke in PLWH can be classified into either traditional or HIV-specific risk factors. Traditional risk factors, such as HTN, DM, dyslipidemia, CVD and physical inactivity have been consistently shown in SSA to be associated with stroke.148,150 In addition, the burden of traditional stroke risk factors in PLWH is sharply on the rise with reports suggesting that up to 50% of PLWH in SSA have metabolic syndrome.7,13 The role of HIV-specific risk factors in stroke is supported by the fact that stroke in PLWH is almost always due to ischemic stroke indicating an underlying propensity towards hypercoagulability and thrombosis. More than 90% of strokes in PLWH are due to ischemic stroke149 as opposed to a reported figure of 50–60% in HIV-uninfected individuals in SSA.151,152
A commonly proposed HIV-specific risk factor is HIV-induced coagulopathy, which is thought to arise from deficiency of protein C and S, hyper homocysteinemia, antiphospholipid syndromes and elevated d-dimer among others.153–155 Other HIV-specific factors include HIV-induced vasculopathy and opportunistic infections of the central nervous system. The exact mechanisms by which these factors cause stroke are yet to be elucidated; however, HIV induced vasculopathy, which is a heterogeneous term denoting HIV vasculitis, accelerated carotid atherosclerosis and small vessel disease occurring in the absence of atherosclerosis or infectious vasculitis, is the leading cause of ischemic stroke in PLWH in SSA.156,157 Crucially, these mechanisms of HIV-specific risk are at least partially due to untreated and advanced disease. This was most notable in the Malawi case-control study, which suggested that the risk of stroke among PLWH drastically decreased after 6 months of ART and with CD4 counts over 500 cells/μL.67
How well do current regional guidelines account for CVD prevention through routine screening and management?
CVD risk prediction scores in SSA populations
One major strategy to promoting CVD prevention is use of risk prediction scores to identify those at high risk of CVD outcomes for cost saving interventions. Multiple studies have reported high CVD risk distributions among PLWH in SSA with a moderate to high risk among older-age groups.159–161 However, questions exist as to whether currently available risk stratification tools, such as the Framingham and Pooled Cohort equations, have been appropriately calibrated to reflect true risk both among PLWH in general, as well as in SSA.85 For example, studies in the United States have under-predicted CVD risk for both CAD and stroke events for PLWH,162 more pronounced in African Americans.163 Studies in both Botswana and Uganda have shown only modest correlations between the Framingham Risk Score, the Atherosclerotic Cardiovascular Disease (ASCVD) Risk Score, and the Reynolds Risk Score and presence of pre-clinical atherosclerosis measured with cIMT165 Consequently, as efforts are made to improve CVD event data collection, an important parallel effort is needed to develop CVD risk prediction models that are better suited to populations of PLWH in SSA.
Health systems and guidelines
Standardized guidelines for the primary prevention of CAD in PLWH have been suggested by a number of international and national bodies. Of the international guidelines, the European AIDS Clinical Society (2008) was among the first to provide guidelines for the prevention and treatment of CVD in PLWH.166 Recommendations were mostly extrapolated from current general medical guidelines which included assessing CVD risk with the Framingham risk score, measuring fasting lipids regularly, and performing an electrocardiogram annually. Risk categorization into low, moderate and high-risk groups was done identically to the general population. The HIV Medicine Association of the Infectious Diseases Society of America Primary Care Guideline (2013) also addressed coronary artery disease risk specifically for PLWH.44 In addition to applying the current CAD risk assessment as in the general population (i.e., Framingham risk score), the authors alluded to HIV as an independent risk factor for CVD, leaving the option open for “more aggressive management of lipids”. Smoking cessation was strongly encouraged regardless of level of CAD risk, as was annual BP checks, screening for DM, blood lipid measurement after initiation of ART, and avoidance of harmful drug-drug interactions. In 2015, the National Lipid Association conducted a thorough review and addition of HIV-specific recommendations for prevention of CVD and management of dyslipidemia. The review largely concluded that there was insufficient evidence for a unique management profile for this population, aside from ensuring risk screening for all PLWH, and potentially consideration of HIV as an independent CVD risk factor.167
More recently, the 2018 EACS Guidelines delved further into the topic of prevention of CVD and risk assessment by specifying targets and treatment algorithms for BP, smoking, coagulation, glucose and lipid management.168 EACS continues to recommend the Framingham risk score or regional corollary using a >10% 10-year risk threshold for initiation of primary prevention drug therapy, largely keeping with the idea of applying assessments and therapies as is the case in the general population. Similarly, the International Association of Providers of AIDS Care (2018) provided protocols for the management of CVD risk, specifying treatment algorithms for HTN, but did not address primary prevention of ASCVD.169 Finally, in an effort to promote public health practice, the British HIV Association introduced auditable targets, considered to be important areas of patient care, among which includes the proportion of PLWH aged 40 or older with measured 10-year CVD risk using the QRISK2 score, a smoking history and blood pressure measurement.170,171 In addition, annual screening for HTN, DM and dyslipidemia are recommended for those with >10%, 10-year CVD risk.
Less attention has been paid to primary prevention of CVD risk among PLWH in SSA. The World Health Organization CVD risk charts (2019) were adapted to the circumstances of 21 global regions including four WHO regions in SSA.172 However, HIV infection was not included in the modeling approach. Most of the national CVD prevention and management guidelines that discuss CVD prevention in SSA do not deviate from the general guidance of other societies, and many countries explicitly follow recommendations from European or American societies. Of guidelines and national strategic plans that were available to review from Ethiopia, Sudan and Kenya,173,174,175 HIV was not considered as an atherosclerotic CVD risk factor nor were CVD prevention guidelines specific to PLWH.
HIV-specific guidelines for CVD risk factor screening and management vary widely. As an example, in Kenya, guidelines for CVD screening among PLWH are thorough, and mirror those of western settings. These include BP and weight screening at each visit, lipid and fasting glucose assessment prior to ART initiation and annually, and recommendations to stop smoking.176 In Uganda, risk factor assessment is recommended at baseline, and then at every clinic visit, though DM and HTN are the only CVD risk factors explicitly targeted.177 By contrast, in South Africa, home to the largest population of PLWH globally, primary care guidelines lack specific recommendations for measurement of these indicators among PLWH, aside from lipid monitoring once every three months after initiation of protease inhibitors.178 Similarly, the 2018 update to the South African dyslipidemia guideline consensus statement specifically addressed the contribution of HIV to CVD risk. Considering the relatively young age of most PLWH and the paucity of other CVD risk factors, the experts considered South African PLWH to be at low risk for CVD events and recommended lipid management and indications for lipid lowering drugs to be the same as in the general population.178
What CVD prevention interventions are most likely to reduce morbidity and mortality in the region?
Multi-level barriers to CVD risk prevention among PLWH in SSA
Studies on primary prevention interventions in PLWH in the region are lacking, but early efforts will first depend on developing the infrastructure necessary to implement interventions as they are shown to be effective. Currently, there are significant barriers to CVD screening and management across the patient, provider, and health systems levels. Early evidence suggests awareness alone presents a major challenge, with only about 50% of PLWH in some settings having adequate knowledge and perception of these conditions.179 In addition to low awareness, low motivation for management of asymptomatic conditions and poor self-efficacy have been reported.180,181 There is poor education of health care providers with gaps in initiation and intensification of CVD management.182–184 Staffing shortages and siloed health care systems also contribute to sub-optimal health systems responses.185,186 Moreover, complex environmental and societal factors such as transportation and urban planning, as well as food processing, distribution, and marketing impact CVD risk factors.53 Finally, these patient, provider, and health system factors often interact to thwart CVD care in the region.187,188 As such, it is unsurprising that large-scale assessments of health system readiness for CVD management in Tanzania and Uganda have shown significant shortages in resources.190
As better data on CVD risk among PLWH in SSA accrue, public health efforts to reduce CVD risk in PLWH in SSA should accentuate accomplishing three major public health priorities: 1) early identification of HIV infection and prompt initiation of ART; 2) strengthening health system-based approaches to CVD risk factor measurement at appropriate and routine intervals; and 3) empowerment of patients and health care providers to respond to those with identified risk factors.
Early ART initiation
The increased CVD risk among PLWH decreases as both CD4 nadir and population of CD4+ T cells rise.129,191–193 This data strongly suggests that at least a proportion of the CVD risk attributable to HIV infection can be mitigated with early ART initiation. Notably, the World Health Organization guidelines and most national guidelines now recommend immediate ART initiation for all PLWH. Efforts to execute these guidelines are paramount. Nonetheless, low CD4 T cell nadirs have proved stubbornly persistent in SSA, despite public health prioritization to provide ART for all PLWH.194–197
Routine CVD risk screening
To promote routine screening for CVD risk factors, increased attention to CVD by national health ministries will be required. The diversity of national guidelines for CVD risk assessments described above presents a clear and actionable priority for the region to prioritize and standardize CVD risk assessment and management. Because HIV care is implemented through a public health approach in the region, which is largely determined by national guidelines, such an effort could have far-reaching effects. Moreover, because PLWH in SSA have high rates of contact with the healthcare system (typically seen once every 1–6 months), there is a unique opportunity to integrate CVD disease screening within HIV care. Early evidence both from community-based programs and in HIV-CVD care co-implementation have demonstrated promising results198,199, and appears to be cost effective,200 but will require additional validation outside of academic and research-based partnerships.
Empowering patients and providers to respond to CVD risk
In addition to strengthening CVD guidelines and their implementation, efforts are clearly needed to empower patients and providers to translate such guidelines into health benefits. For such interventions to be sustainable and effective on a population scale, they will likely need to address barriers across the cascade of care. In high-resource settings, such interventions tend to be multi-component, including training and capacity building for health care providers, as well as measures to improve patient adherence and self-efficacy, such as home-based disease monitoring.201 Unfortunately, early data on interventions that focus on only one strategy, such as nurse training and support, have not shown clinical benefits in the region.202
What are the major research gaps that need to be addressed to ensure CVD risk is optimized among PLWH in SSA?
Primary research priorities include development of observational prospective cohort studies that include collection of CVD events to enable determination of CVD epidemiology. These include which events are most common, which traditional, regional, and HIV-specific risk factors have the greatest contributions to them, and identification of intervention points. At least two active studies are specifically designed to address these needs. The Ndlovu Study is enrolling 2000 individuals in the rural Limpopo State of South Africa, mixed by HIV serostatus, into a perspective longitudinal cohort study with 6-monthly CVD risk factor, surrogate marker (cIMT, pulse-wave velocity) and clinical outcome data.203 The UGANDAC study in rural Uganda is enrolling 600 individuals from the Mbarara region204 mixed by HIV serostatus, and conducting cardiovascular disease risk profiling, local risk factor sampling (e.g. personal air pollution monitoring), and conducting computed tomography coronary artery angiography in an attempt to definitively assess the presence of CAD.137 Ultimately, large, population-based cohorts similar in design, scope, and duration as the Framingham Cohort and inclusive of CVD events will be needed to truly discern the impact of HIV on CVD risk and identify preventative interventions to reduce it.
Interventional studies, including both traditional clinical randomized clinical trials and implementation science designs for health systems evaluations are needed to translate data into public health knowledge. The REPREIVE study is an ongoing randomized clinical trial of statins for primary prevention of CVD in PLWH with low CVD risk. The study includes six sites in SSA across four countries (Uganda, Zimbabwe, Botswana, and South Africa) and has the potential to provide region-specific data on the value of a low-cost and scalable primary prevention of CVD in PLWH in SSA. To translate such interventions, health systems implementation studies are also needed. Pilot studies of such interventions have been conducted, but the implementation science literature on integration of CVD services into HIV care remains under-developed and is an untapped opportunity.205 The US National Institute of Health; National Heart, Lung, and Blood Institute recent funding opportunity announcement; Heart, Lung, and Blood Co-morbiditieS Implementation Models in People Living with HIV (HLB SIMPLe)” (UG3/UH3), is one of such opportunities to implement strategies to deliver proven-effective prevention and treatment interventions for heart, lung, blood, and sleep comorbid diseases and disorders in PLWH.
Conclusions
Despite decades of accrued data on increased CVD risk among PLWH in the United States and Europe, similarly compelling data in SSA remain lacking. Data largely derived from cross-sectional studies suggest the following key points: 1) PLWH in SSA are living longer with ART, and are thus increasingly susceptible to CVD risk factors; 2) regional risk factors, such as low nadir CD4 counts, and exposure to air pollution are likely to differentiate risk factors for CVD in the SSA population; 3) PLWH have similar prevalence of CVD risk factors as people without HIV in the region, some evidence of decreased pre-clinical atherosclerosis as measured by cIMT, but appear to be at increased risk of stroke, particularly prior to ART initiation; 4) traditional CVD risk scores do not perform well (using surrogate markers as outcomes) in PLWH in SSA; and 5) there are a multitude of barriers to CVD risk screening and prevention in SSA across the patient, provider and health system levels that require urgent attention. A variety of study designs are needed to respond to the known gaps in the literature and barriers to care, including large prospective cohort studies with outcome estimation, clinical trials, and implementation science evaluations of health systems interventions. These studies must be adequately powered to demonstrate clinical benefits in SSA and are urgently needed to ensure health systems are designed accounting for regional epidemiology and health system capacities. Until the field takes such strides, optimal methods of CVD risk prevention among PLWH in SSA will remain poorly understood and largely unaddressed.
Acknowledgements
All individuals who contributed to this publication have been included as authors.
Abbreviations and acronyms:
- ACS
acute coronary syndromes
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- BP
blood pressure
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CD
cluster of differentiation
- cIMT
carotid intimal-medial thickness
- CVD
cardiovascular disease
- DALYs
disability-associated life-years
- DM
diabetes mellitus
- EACS
European AIDS clinical society
- HAP
household air pollution
- HbA1c
glycated hemoglobin
- HDL-c
high density lipoprotein-cholesterol
- HDP
hypertensive disorders of pregnancy
- HIV
human immunodeficiency virus
- HTN
hypertension
- LDL-c
low density lipoprotein cholesterol
- MLWH
men living with HIV
- PLWH
people living with HIV
- PM
particulate matter
- SSA
sub-Saharan Africa
- WLWH
women living with HIV
Footnotes
Declaration of competing interest
SO and RP are supported by a career development grant from the Fogarty International Institute of the National Institutes of Health (K43 TW010715 and K01 TW01281 respectively). MJS receives research support from the National Institutes of Health (R01 AI124718, R01 HL141053, R01 AG059504).
References
- 1.UNAIDS. Fact sheet—World AIDS Day. 2019.
- 2.Farahani M, Mulinder H, Farahani A, Marlink R. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS 2017;28: 636–650. [DOI] [PubMed] [Google Scholar]
- 3.Feinstein MJ, Steverson AB, Ning H, et al. Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc 2018;7, e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang C-CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012;59:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah AS, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018;138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muyanja D, Muzoora C, Muyingo A, Muyindike W, Siedner MJ. High prevalence of metabolic syndrome and cardiovascular disease risk among people with HIV on stable ART in southwestern Uganda. AIDS Patient Care STDS 2016;30:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muiru AN, Bibangambah P, Hemphill L, et al. Distribution and performance of cardiovascular risk scores in a mixed population of HIV-infected and community-based HIV-uninfected individuals in Uganda. JAIDS Journal of Acquired Immune Deficiency Syndromes 2018;78:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(WHO) WHO. Cardiovascular diseases (CVDs) fact sheet. Vol 20202017.
- 10.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens 2017;11:530–540. [DOI] [PubMed] [Google Scholar]
- 11.Okello S, Kanyesigye M, Muyindike WR, et al. Incidence and predictors of hypertension in adults with HIV initiating antiretroviral therapy in Southwestern Uganda. J Hypertens 2015;33:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armah KA, Chang C-CH, Baker JV, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and-uninfected veterans. Clin Infect Dis 2013;58:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Heerden A, Barnabas RV, Norris SA, Micklesfield LK, van Rooyen H, Celum C. High prevalence of HIV and non-communicable disease (NCD) risk factors in rural KwaZulu-Natal, South Africa. J Int AIDS Soc 2017;20, e25012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okello S, Ueda P, Kanyesigye M, et al. Association between HIV and blood pressure in adults and role of body weight as a mediator: cross-sectional study in Uganda. The Journal of Clinical Hypertension 2017;19:1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwarisiima D, Balzer L, Heller D, et al. Population-based assessment of hypertension epidemiology and risk factors among HIV-positive and general populations in rural Uganda. PLoS One 2016;11, e0156309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloomfield GS, Hogan JW, Keter A, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One 2011;6, e22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jardim TV, Reiger S, Abrahams-Gessel S, et al. Hypertension management in a population of older adults in rural South Africa. J Hypertens 2017;35:1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong C, Gange SJ, Moore RD, et al. Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis 2017;66: 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelchen-Matthews A, Ryom L, Borges Á, et al. Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. Aids 2018;32:2405–2416. [DOI] [PubMed] [Google Scholar]
- 20.Smit M, Olney J, Ford NP, et al. The growing burden of noncommunicable disease among persons living with HIV in Zimbabwe. AIDS (London, England) 2018;32: 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Iguacel R, Negredo E, Peck R, Friis-Møller N. Hypertension is a key feature of the metabolic syndrome in subjects aging with HIV. Curr Hypertens Rep 2016;18:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kingery JR, Alfred Y, Smart LR, et al. Short-term and long-term cardiovascular risk, metabolic syndrome and HIV in Tanzania. Heart 2016;102:1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanidas E, Papadopoulos DP, Velliou M, Tsioufis K, Barbetseas J, Papademetriou V. Human immunodeficiency virus infection and hypertension. Is there a connection? Am J Hypertens 2017;31:389–393. [DOI] [PubMed] [Google Scholar]
- 24.Ojji DB, Mayosi B, Francis V, et al. Comparison of dual therapies for lowering blood pressure in black Africans. N Engl J Med 2019;380:2429–2439. 10.1056/NEJMoa1901113. [DOI] [PubMed] [Google Scholar]
- 25.Kent ST, Bromfield SG, Burkholder GA, et al. Ambulatory blood pressure monitoring in individuals with HIV: a systematic review and meta-analysis. PLoS One 2016;11, e0148920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV-infected adults: novel path-ophysiologic mechanisms. Hypertension 2018;72:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maffongelli G, Alteri C, Gentilotti E, et al. Impact of HIV-1 tropism on the emergence of non-AIDS events in HIV-infected patients receiving fully suppressive antiretroviral therapy. AIDS (London, England) 2016;30:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okello S, Asiimwe SB, Kanyesigye M, Muyindike WR, Boum Y. D-dimer levels and traditional risk factors are associated with incident hypertension among HIV-infected individuals initiating antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr 2016;73:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker JV, Sharma S, Achhra AC, et al. Changes in cardiovascular disease risk factors with immediate versus deferred antiretroviral therapy initiation among HIV-positive participants in the START (Strategic Timing of Antiretroviral Treatment) Trial. J Am Heart Assoc 2017;6, e004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peck R, Mghamba J, Vanobberghen F, et al. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: a cross-sectional survey. Lancet Glob Health 2014;2:e285–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight L, Schatz E, Mukumbang FC. “I attend at Vanguard and I attend here as well”: barriers to accessing healthcare services among older South Africans with HIV and non-communicable diseases. Int J Equity Health 2018;17:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manne-Goehler J, Montana L, Gómez-Olivé FX, et al. The ART advantage: healthcare utilization for diabetes and hypertension in rural South Africa. J Acquir Immune Defic Syndr 2017;75:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muddu M, Tusubira AK, Sharma SK, Akiteng AR, Ssinabulya I, Schwartz JI. Integrated hypertension and HIV care cascades in an HIV treatment program in eastern Uganda: a retrospective cohort study. J Acquir Immune Defic Syndr 2019;81:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 35.Dooko CBA, De Wit S, Neuhaus J, et al. Interleukin-6, high sensitivity C-reactive protein, and the development of type 2 diabetes among HIV positive patients taking antiretroviral therapy. J Acquir Immune Defic Syndr 2014;67:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes 2009;50:499–505. [DOI] [PubMed] [Google Scholar]
- 37.Althoff KN, McGinnis KA, Wyatt CM, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2014;60:627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omech B, Sempa J, Castelnuovo B, et al. Prevalence of HIV-associated metabolic abnormalities among patients taking first-line antiretroviral therapy in Uganda. ISRN AIDS 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maganga E, Smart LR, Kalluvya S, et al. Glucose metabolism disorders, HIV and antiretroviral therapy among Tanzanian adults. PLoS One 2015;10, e0134410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripathi A, Liese A, Jerrell J, et al. Incidence of diabetes mellitus in a population-based cohort of HIV-infected and non-HIV-infected persons: the impact of clinical and therapeutic factors over time. Diabet Med 2014;31:1185–1193. [DOI] [PubMed] [Google Scholar]
- 41.Tien PC, Schneider MF, Cox C, et al. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr 2012;61:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen LD, Mathiesen ER, Kronborg G, Gerstoft J, Obel N. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PLoS One 2012;7, e44575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care 2017;5, e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2013;58:e1–e34. [DOI] [PubMed] [Google Scholar]
- 45.Slama L, Palella FJ Jr, Abraham AG, et al. Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother 2014;69:3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briker SM, Aduwo JY, Mugeni R, et al. A1C underperforms as a diagnostic test in Africans even in the absence of nutritional deficiencies, anemia and hemoglobinopathies: insight from the Africans in America study. Front Endocrinol (Lausanne) 2019;10:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skinner S, Diaw M, Ndour Mbaye M, et al. Evaluation of agreement between hemoglobin A1c, fasting glucose, and fructosamine in Senegalese individuals with and without sickle-cell trait. PLoS One 2019;14, e0212552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han JH, Crane HM, Bellamy SL, Frank I, Cardillo S, Bisson GP. HIV infection and glycemic response to newly initiated diabetic medical therapy. AIDS (London, England) 2012;26:2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gervasoni C, Minisci D, Clementi E, Rizzardini G, Cattaneo D. How relevant is the interaction between dolutegravir and metformin in real life? JAIDS Journal of Acquired Immune Deficiency Syndromes 2017;75:e24–e26. [DOI] [PubMed] [Google Scholar]
- 50.Cattaneo D, Resnati C, Rizzardini G, Gervasoni C. Dolutegravir and metformin: a clinically relevant or just a pharmacokinetic interaction? Aids 2018;32:532–533. [DOI] [PubMed] [Google Scholar]
- 51.Vangile Mkhatshwa B, Ogunbanjo GA, Mabuza LH. Knowledge, attitudes and management skills of medical practitioners regarding weight management. Afr J Prm Health Care Fam Med 2016;8 [a1187–1189pages]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma Anjali, Hoover Donald R, et al. Relationship between body mass index and mortality in HIV-infected HAART users in the women’s interagency HIV study. PLoS One 2015;10, e0143740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellulu Mohammed, Yehia Abed, Asmah Rahmat, Yazan Ranneh, Ali aF. Epidemiology of obesity in developing countries: challenges and prevention. Global epidemic Obes 2014;2:2–5. [Google Scholar]
- 54.Rynal D, Esterhuizen TM, Govender RD. Overweight and obesity amongst Black women in Durban, KwaZulu-Natal: a “disease” of perception in an an area of high HIV prevalence. Afr J Prm Health Care Fam Med 2013;5 [7 pages]. [Google Scholar]
- 55.Ogunmola Olarinde Jeffrey, Yusuf Oladosu Olatunji, Olamoyegun Michael Adeyemi. Association of hypertension and obesity with HIV and antiretroviral therapy in a rural tertiary health center in nigeria: a cross-sectional cohort study. Vasc Health Risk Manag 2014;10:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malaza Mossong J, Barnighausen T, N M-L. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One 2012;7(10), e47761 [7: e47761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serwadda D, Sewankambo NK, Carswell J, et al. Slim disease: a new disease in Uganda and its association with HTLV-III infection. The Lancet 1985;326:849–852. [DOI] [PubMed] [Google Scholar]
- 58.Koethe JR, Jenkins CA, Lau B, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016;32:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price J, Hoy J, Ridley E, Nyulasi I, Paul E, Woolley I. Changes in the prevalence of lipodystrophy, metabolic syndrome and cardiovascular disease risk in HIV-infected men. Sex Health 2015;12:240–248. [DOI] [PubMed] [Google Scholar]
- 60.Hill WFV A, Delaporte E, Sokhela S, et al. Progressive rises in weight and clinical obesity for TAF/FTC/DTG and TDF/FTC/DTG versus TDF/FTC/EFV: ADVANCE and NAMSAL trials. 10th IAS conference on HIV science. Vol 22. Mexico City, Mexico J Int AIDS Soc 2019;22(S5):e25327 10.1002/jia2.25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirigo AT, Tesfaye DY. Influences of gender in metabolic syndrome and its components among people living with HIV virus using antiretroviral treatment in Hawassa, southern Ethiopia. BMC Res Notes 2016;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asiki Gershim a b M SF, et al. Sociodemographic and behavioural factors associated with body mass index among men and women in Nairobi slums: AWI-Gen Project. Glob Health Action 2018;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siyabonga Kunene H, Taukobong NP. Dietary habits among health professionals working in a district hospital in KwaZulu-Natal, South Africa. Afr J Prm Health Care Fam Med 2017;9 [5 pages]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amberbir A, Banda V, Singano V, et al. Effect of cardio-metabolic risk factors on all-cause mortality among HIV patients on antiretroviral therapy in Malawi: a prospective cohort study. PLoS One 2019;14, e0210629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol 2013;42:1754–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noubiap JJ, Bigna JJ, Nansseu JR, et al. Prevalence of dyslipidaemia among adults in Africa: a systematic review and meta-analysis. Lancet Glob Health 2018;6:e998–e1007. [DOI] [PubMed] [Google Scholar]
- 67.Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology 2015;4(86): 324–333. 10.1212/WNL.0000000000002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagathu C, Bereziat V, Gorwood J, et al. Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin Drug Saf 2019;18:829–840. [DOI] [PubMed] [Google Scholar]
- 69.Menard A, Meddeb L, Tissot-Dupont H, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS 2017;31:1499–1500. [DOI] [PubMed] [Google Scholar]
- 70.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. The Lancet 2019;19 (S0140–6736):32008 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reitsma MB, Fullman N, Ng M, et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. The Lancet 2017;389:1885–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson C, Becher H, Winkler V. Tobacco control progress in low and middle income countries in comparison to high income countries. Int J Environ Res Public Health 2016;13:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brathwaite R, Addo J, Smeeth L, Lock K. A systematic review of tobacco smoking prevalence and description of tobacco control strategies in Sub-Saharan African countries; 2007 to 2014. PLoS One 2015;10, e0132401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K. Tobacco use among people living with HIV: analysis of data from demographic and health surveys from 28 low-income and middle-income countries. Lancet Glob Health 2017;5:e578–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reynolds NR, Neidig JL, Wewers ME. Illness representation and smoking behavior: a focus group study of HIV-positive men. Journal of the Association of Nurses in AIDS Care 2004;15:37–47. [DOI] [PubMed] [Google Scholar]
- 76.Humfleet GL, Delucchi K, Kelley K, Hall SM, Dilley J, Harrison G. Characteristics of HIV-positive cigarette smokers: a sample of smokers facing multiple challenges. AIDS Educ Prev 2009;21:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reddy KP, Parker RA, Losina E, et al. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: a US-based modeling study. J Infect Dis 2016;214:1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012;60:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janjua SA, Triant VA, Addison D, et al. HIV infection and heart failure outcomes in women. J Am Coll Cardiol 2017;69:107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stone L, Looby SE, Zanni MV. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr Opin HIV AIDS 2017;12:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zanni MV, Awadalla M, Toribio M, et al. Immune correlates of diffuse myocardial fibrosis and diastolic dysfunction among aging women with human immunodeficiency virus. J Infect Dis 2019. 10.1093/infdis/jiz184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hatleberg CI, Ryom L, El-Sadr W, et al. Gender differences in HIV-positive persons in use of cardiovascular disease-related interventions: D:A:D study. J Int AIDS Soc 2014;17:19516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015;162:335–344. [DOI] [PubMed] [Google Scholar]
- 85.Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, et al. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural south africa: the HAALSI (Health and Aging in Africa: longitudinal studies of INDEPTH communities) study. BMC Public Health 2017;17:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magodoro IM, Feng M, North CM, et al. Female sex and cardiovascular disease risk in rural Uganda: a cross-sectional, population-based study. BMC Cardiovasc Disord 2019;19:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol 2013;25:124–132. [DOI] [PubMed] [Google Scholar]
- 88.Adams JW, Watts DH, Phelps BR. A systematic review of the effect of HIV infection and antiretroviral therapy on the risk of pre-eclampsia. Int J Gynecol Obstet 2016;133:17–21. [DOI] [PubMed] [Google Scholar]
- 89.Boyajian T, Shah PS, Murphy KE. Risk of preeclampsia in HIV-positive pregnant women receiving HAART: a matched cohort study. J Obstet Gynaecol Can 2012;34:136–141. [DOI] [PubMed] [Google Scholar]
- 90.Browne JL, Schrier VJ, Grobbee DE, Peters SA, Klipstein-Grobusch K. HIV, antiretroviral therapy, and hypertensive disorders in pregnancy: a systematic review and meta-analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes 2015;70: 91–98. [DOI] [PubMed] [Google Scholar]
- 91.Maharaj NR, Moodley J, Chuturgoon A. Association of HIV and highly active antiretroviral therapy with clinical and biochemical indices among women with pre-eclampsia. Int J Gynecol Obstet 2016;134:304–308. [DOI] [PubMed] [Google Scholar]
- 92.Phoswa WN, Naicker T, Ramsuran V, Moodley J. Pre-eclampsia: the role of highly active antiretroviral therapy and immune markers. Inflamm Res 2019;68:47–57. [DOI] [PubMed] [Google Scholar]
- 93.Sansone M, Sarno L, Saccone G, et al. Risk of preeclampsia in human immunodeficiency virus–infected pregnant women. Obstet Gynecol 2016;127:1027–1032. [DOI] [PubMed] [Google Scholar]
- 94.Sebitloane HM, Moodley J, Sartorius B. Associations between HIV, highly active antiretroviral therapy, and hypertensive disorders of pregnancy among maternal deaths in South Africa 2011–2013. Int J Gynecol Obstet 2017;136:195–199. [DOI] [PubMed] [Google Scholar]
- 95.Tooke L, Riemer L, Matjila M, Harrison M. Antiretrovirals causing severe pre-eclampsia. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health 2016;6:266–268. [DOI] [PubMed] [Google Scholar]
- 96.Kentoffio K, Albano A, Koplan B, et al. Electrocardiographic evidence of cardiac disease by sex and HIV serostatus in Mbarara, Uganda. Glob Heart 2019;14:395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schoffelen AF, de Groot E, Tempelman HA, Visseren FL, Hoepelman AI, Barth RE. Carotid intima media thickness in mainly female HIV-infected subjects in rural South Africa: association with cardiovascular but not HIV-related factors. Clin Infect Dis 2015;61:1606–1614. [DOI] [PubMed] [Google Scholar]
- 98.Ssinabulya I, Kayima J, Longenecker C, et al. Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS One 2014;9, e89537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nonterah EA, Boua PR, Klipstein-Grobusch K, et al. Classical cardiovascular risk factors and HIV are associated with carotid intima-media thickness in adults from sub-Saharan Africa: findings from H3Africa AWI-Gen Study. J Am Heart Assoc 2019;8, e011506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Organization WH. Air Pollution. Vol 2019.
- 101.Nemmar A, Hoet PM, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation 2002;105:411–414. [DOI] [PubMed] [Google Scholar]
- 102.Brook RD, Rajagopalan S, Pope III CA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 103.Institute HE. State of Global Air 2019 2019.
- 104.Amegah AK, Agyei-Mensah S. Urban air pollution in Sub-Saharan Africa: time for action. Environ Pollut 2017;220:738–743. [DOI] [PubMed] [Google Scholar]
- 105.(WHO) WHO. Global Ambient Air Pollution. Vol 20192019.
- 106.Corsi DJ, Subramanian SV, Chow CK, et al. Prospective Urban Rural Epidemiology (PURE) study: baseline characteristics of the household sample and comparative analyses with national data in 17 countries. Am Heart J 2013;166:636–646 e634. [DOI] [PubMed] [Google Scholar]
- 107.Lin H, Guo Y, Di Q, et al. Ambient PM2.5 and stroke: effect modifiers and population attributable risk in six low-and middle-income countries. Stroke 2017;48:1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wichmann J, Voyi K. Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001–2006. Int J Environ Res Public Health 2012;9:3978–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pena MSB, Rollins A. Environmental exposures and cardiovascular disease: a challenge for health and development in low-and middle-income countries. Cardiol Clin 2017;35:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The lancet 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bloomfield GS, Kirwa K, Agarwal A, et al. Effects of a cookstove intervention on cardiac structure, cardiac function, and blood pressure in western Kenya. J Am Soc Echocardiogr 2019;32:427–430. [DOI] [PubMed] [Google Scholar]
- 112.Quinn AK, Ayuurebobi K, Kinney PL, et al. Ambulatory monitoring demonstrates an acute association between cookstove-related carbon monoxide and blood pressure in a Ghanaian cohort. Environ Health 2017;16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alexander D, Northcross A, Wilson N, et al. Randomized controlled ethanol cookstove intervention and blood pressure in pregnant Nigerian women. Am J Respir Crit Care Med 2017;195:1629–1639. [DOI] [PubMed] [Google Scholar]
- 114.Mocumbi AO, Stewart S, Patel S, Al-Delaimy WK. Cardiovascular effects of indoor air pollution from solid fuel: relevance to sub-Saharan Africa. Curr Environ Health Rep 2019;6:116–126. [DOI] [PubMed] [Google Scholar]
- 115.Owili P, Lien W-H, Muga M, Lin T-H. The associations between types of ambient PM2.5 and under-five and maternal mortality in Africa. Int J Environ Res Public Health 2017;14:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thurston GD, Burnett RT, Turner MC, et al. Ischemic heart disease mortality and long-term exposure to source-related components of US fine particle air pollution. Environ Health Perspect 2015;124:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hampel R, Peters A, Beelen R, et al. Long-term effects of elemental composition of particulate matter on inflammatory blood markers in European cohorts. Environ Int 2015;82:76–84. [DOI] [PubMed] [Google Scholar]
- 118.Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six US cities. Environ Health Perspect 2000;108:941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karagulian F, Belis CA, Dora CFC, et al. Contributions to cities’ ambient particulate matter (PM): a systematic review of local source contributions at global level. Atmos Environ 2015;120:475–483. [Google Scholar]
- 120.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006;20:2275–2283. [DOI] [PubMed] [Google Scholar]
- 121.Jerico C, Knobel H, Calvo N, et al. Subclinical carotid atherosclerosis in HIV-infected patients: role of combination antiretroviral therapy. Stroke 2006;37:812–817. [DOI] [PubMed] [Google Scholar]
- 122.Currier JS, Kendall MA, Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS 2007;21:1137–1145. [DOI] [PubMed] [Google Scholar]
- 123.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS 2009;23:1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Volpe GE, Tang AM, Polak JF, Mangili A, Skinner SC, Wanke CA. Progression of carotid intima-media thickness and coronary artery calcium over 6 years in an HIV-infected cohort. J Acquir Immune Defic Syndr 2013;64:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012;26:2409–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fitch KV, Lo J, Abbara S, et al. Increased coronary artery calcium score and noncalcified plaque among HIV-infected men: relationship to metabolic syndrome and cardiac risk parameters. J Acquir Immune Defic Syndr 2010;55:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010;24:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr 2010;55:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marcus JL, Leyden WA, Chao CR, et al. HIV infection and incidence of ischemic stroke. AIDS 2014;28:1911–1919. [DOI] [PubMed] [Google Scholar]
- 130.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr 2014;65:160–166. [DOI] [PubMed] [Google Scholar]
- 131.Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015;61:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009;200:1212–1215. [DOI] [PubMed] [Google Scholar]
- 133.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003;187:1534–1543. [DOI] [PubMed] [Google Scholar]
- 134.Fourie C, van Rooyen J, Pieters M, Conradie K, Hoekstra T, Schutte A. Is HIV-1 infection associated with endothelial dysfunction in a population of African ancestry in South Africa? Cardiovasc J Afr 2011;22:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ngatchou W, Lemogoum D, Ndobo P, et al. Increased burden and severity of metabolic syndrome and arterial stiffness in treatment-naive HIV+ patients from Cameroon. Vasc Health Risk Manag 2013;9:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Okello S, Millard A, Owori R, et al. Prevalence of lower extremity peripheral artery disease among adult diabetes patients in southwestern Uganda. BMC Cardiovasc Disord 2014;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feinstein MJ, Kim JH, Bibangambah P, et al. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV-infected and uninfected Ugandans. AIDS Res Hum Retroviruses 2017;33:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fourie CM, Schutte AE, Smith W, Kruger A, van Rooyen JM. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis 2015;240:154–160. [DOI] [PubMed] [Google Scholar]
- 139.Siedner MJ, Zanni M, Tracy RP, et al. Increased systemic inflammation and gut permeability among women with treated HIV infection in rural Uganda. J Infect Dis 2018;218(6):922–926. 10.1093/infdis/jiy244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vos AG, Hoeve K, Barth RE, et al. Cardiovascular disease risk in an urban African population: a cross-sectional analysis on the role of HIV and antiretroviral treatment. Retrovirology 2019;16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alencherry B, Erem G, Mirembe G, et al. Coronary artery calcium, HIV and inflammation in Uganda compared with the USA. Open Heart 2019;6, e001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nordell AD, McKenna M, Borges AH, et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014;3, e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Severino P, Calcagno S, Sardella G, et al. Pathophysiology of acute coronary syndrome in HIV positive patients: insight from virtual histology analysis. J Am Coll Cardiol 2015;65:A43. [Google Scholar]
- 144.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009;23:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chow FC, He W, Bacchetti P, et al. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology 2014;83:1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Namale G, Kamacooko O, Kinengyere A, et al. Risk factors for hemorrhagic and ischemic stroke in Sub-Saharan Africa. J Trop Med 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology 2016;86:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Walker RW, Jusabani A, Aris E, et al. Stroke risk factors in an incident population in urban and rural Tanzania: a prospective, community-based, case-control study. Lancet Glob Health 2013;1:e282–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abdallah A, Chang JL, O’Carroll CB, et al. Stroke in human immunodeficiency virus-infected individuals in Sub-Saharan Africa (SSA): a systematic review. J Stroke Cerebrovasc Dis 2018;27:1828–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. The lancet 2016;388:761–775. [DOI] [PubMed] [Google Scholar]
- 151.Kaduka L, Muniu E, Oduor C, et al. Stroke mortality in Kenya’s public tertiary hospitals: a prospective facility-based study. Cerebrovascular diseases extra 2018;8:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fekadu G, Adola B, Mosisa G, Shibiru T, Chelkeba L. Clinical characteristics and treatment outcomes among stroke patients hospitalized to Nekemte referral hospital, western Ethiopia. J Clin Neurosci 2019;71(2020):170–176. 10.1016/j.jocn.2019.08.075. [DOI] [PubMed] [Google Scholar]
- 153.Mochan A, Modi M, Modi G. Protein S deficiency in HIV associated ischaemic stroke: an epiphenomenon of HIV infection. J Neurol Neurosurg Psychiatry 2005;76:1455–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Longo-Mbenza B, Longokolo Mashi M, Lelo Tshikwela M, et al. Relationship between younger age, autoimmunity, cardiometabolic risk, oxidative stress, HAART, and ischemic stroke in Africans with HIV/AIDS. ISRN cardiology 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zimba S, Ntanda PM, Lakhi S, Atadzhanov M. HIV infection, hypercoagulability and ischaemic stroke in adults at the University Teaching Hospital in Zambia: a case control study. BMC Infect Dis 2017;17:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Benjamin LA, Allain TJ, Mzinganjira H, et al. The role of human immunodeficiency virus–associated vasculopathy in the etiology of stroke. J Infect Dis 2017;216:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Benjamin LA, Bryer A, Lucas S, et al. Arterial ischemic stroke in HIV: defining and classifying etiology for research studies. Neurol Neuroimmunol Neuroinflamm 2016;3, e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mashinya F, Alberts M, Van Geertruyden JP, Colebunders R. Assessment of cardiovascular risk factors in people with HIV infection treated with ART in rural South Africa: a cross sectional study. AIDS Res Ther 2015;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mateen FJ, Kanters S, Kalyesubula R, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens 2013;31:1372–1378 discussion 1378. [DOI] [PubMed] [Google Scholar]
- 160.Kavishe B, Vanobberghen F, Katende D, et al. Dyslipidemias and cardiovascular risk scores in urban and rural populations in north-western Tanzania and southern Uganda. PLoS One 2019;14, e0223189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mateen FJ, Post WS, Sacktor N, et al. Long-term predictive value of the Framingham risk score for stroke in HIV-positive vs HIV-negative men. Neurology 2013;81:2094–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Feinstein MJ, Nance RM, Drozd DR, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol 2017;2:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mosepele M, Hemphill LC, Palai T, et al. Cardiovascular disease risk prediction by the American College of Cardiology (ACC)/American Heart Association (AHA) Atherosclerotic Cardiovascular Disease (ASCVD) risk score among HIV-infected patients in sub-Saharan Africa. PLoS One 2017;12, e0172897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lundgren JD, Battegay M, Behrens G, et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med 2008;9:72–81. [DOI] [PubMed] [Google Scholar]
- 165.Jacobson TA, Maki KC, Orringer CE, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol 2015;9 [S1–S122. e121]. [DOI] [PubMed] [Google Scholar]
- 166.(EACS) EACS. EACS guidelines 2018. 2018.
- 167.(IAPAC) IAoPoAC. IAPAC protocols for the integrated management of HIV and noncommunicable diseases. 2018.
- 168.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. Bmj 2008;336:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Angus B, Brook G, Awosusi F, et al. BHIVA guidelines for the routine investigation and monitoring of adult HIV-1-positive individuals. 2016.
- 170.Kaptoge S, Pennells L, De Bacquer D, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ministry of Health FDRoE. Guidelines on clinical and programmatic management of major non communicable diseases. Addis Ababa, Ethiopia: Ministry of Health; 2016. [Google Scholar]
- 172.Directorate General of Public Health and Emergency NMoH, Republic of Sudan. Non-communicable disease national strategic plan 2010–2015. Khartoum, Sudan: 2010. [Google Scholar]
- 173.Division of Non-communicable Diseases MoH, Republic of Kenya. Kenya national guidelines for cardiovascular diseases management. 2018.
- 174.National AIDS & STI Control Program MoHK. Guidelines on use of antiretroviral drugs for treating and preventing HIV Infection in Kenya. Vol 2018 edition. Nairobi, Kenya: National AIDS and STI Control Program; 2018. [Google Scholar]
- 175.(MOH) UMoH. Consolidated guidelines for prevention and treatment of HIV in Uganda. Kampala, Uganda: 2016:71. [Google Scholar]
- 176.Klug E, Raal F, Marais A, et al. South African dyslipidaemia guideline consensus statement: 2018 update a joint statement from the South African Heart Association (SA Heart) and the Lipid and Atherosclerosis Society of Southern Africa (LASSA). S Afr Med J 2018;108:973–1000. [PubMed] [Google Scholar]
- 177.Kagaruki GB, Mayige MT, Ngadaya ES, et al. Knowledge and perception on type2 diabetes and hypertension among HIV clients utilizing care and treatment services: a cross sectional study from Mbeya and Dar es Salaam regions in Tanzania. BMC Public Health 2018;18:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Murphy K, Chuma T, Mathews C, Steyn K, Levitt N. A qualitative study of the experiences of care and motivation for effective self-management among diabetic and hypertensive patients attending public sector primary health care services in South Africa. BMC Health Serv Res 2015;15:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dube L, Rendall-Mkosi K, Van den Broucke S, Bergh A-M, Mafutha NG. Self-management support needs of patients with chronic diseases in a South African township: a qualitative study. J Community Health Nurs 2017;34:21–31. [DOI] [PubMed] [Google Scholar]
- 180.Das J, Hammer J. Quality of primary care in low-income countries: facts and economics. Annual Review of Economics 2014;6:525–553. [Google Scholar]
- 181.Das J, Woskie L, Rajbhandari R, Abbasi K, Jha A. Rethinking assumptions about delivery of healthcare: implications for universal health coverage. BMJ 2018;361:k1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.National Academies of Sciences Engineering and Medicine. Crossing the global quality chasm: improving health care worldwide. Washington, DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- 183.Maimela E, Van Geertruyden J-P, Alberts M, et al. The perceptions and perspectives of patients and health care providers on chronic diseases management in rural South Africa: a qualitative study. BMC Health Serv Res 2015;15:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rabkin M, Palma A, McNairy ML, et al. Integrating cardiovascular disease risk factor screening into HIV services in Swaziland: lessons from an implementation science study. AIDS 2018;32(suppl 1):S43–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004;27:1535–1540. [DOI] [PubMed] [Google Scholar]
- 186.Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med 2008;23:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Katende D, Mutungi G, Baisley K, et al. Readiness of Ugandan health services for the management of outpatients with chronic diseases. Trop Med Int Health 2015;20: 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis 2015;60:1278–1280. [DOI] [PubMed] [Google Scholar]
- 189.Siedner MJ. START or SMART? Timing of antiretroviral therapy initiation and cardiovascular risk for people with human immunodeficiency virus infection. Open Forum Infect Dis 2016;3, ofw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV 2017;5(3):e116–e125. 10.1016/S2352-3018(17)30205-9. [DOI] [PubMed] [Google Scholar]
- 192.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis 2015;60:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.IeDEA and Cohere Cohort Collaborations. Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis 2018;66:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Auld AF, Shiraishi RW, Oboho I, et al. Trends in prevalence of advanced HIV disease at antiretroviral therapy enrollment - 10 countries, 2004–2015. MMWR Morb Mortal Wkly Rep 2017;66:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chamie G, Kwarisiima D, Clark TD, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One 2012;7, e43400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Walsh KF, Lee MH, Martelly S, et al. Integrating hypertension services at an HIV clinic in Port-au-Prince, Haiti: a report from the field. J Clin Hypertens (Greenwich) 2018;20:1485–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rawat A, Uebel K, Moore D, Yassi A. Integrated HIV-care into primary health care clinics and the influence on diabetes and hypertension care: an interrupted time series analysis in Free State, South Africa over 4 years. JAIDS Journal of Acquired Immune Deficiency Syndromes 2018;77:476–483. [DOI] [PubMed] [Google Scholar]
- 198.Mills J, Duffy M. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index. Am Fam Physician 2018;98:754–755. [PubMed] [Google Scholar]
- 199.Fairall LR, Folb N, Timmerman V, et al. Educational outreach with an integrated clinical tool for nurse-led non-communicable chronic disease management in primary care in South Africa: a pragmatic cluster randomised controlled trial. PLoS Med 2016;13, e1002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vos A, Tempelman H, Deville W, et al. HIV and risk of cardiovascular disease in sub-Saharan Africa: rationale and design of the Ndlovu Cohort Study. Eur J Prev Cardiol 2017;24:1043–1050. [DOI] [PubMed] [Google Scholar]
- 201.Siedner MJ. Epidemiology of coronary artery disease among people with HIV in rural sub-Saharan Africa. Vol 2019 Grantome. [Google Scholar]
- 202.Kemp CG, Weiner BJ, Sherr KH, et al. Implementation science for integration of HIV and non-communicable disease services in sub-Saharan Africa: a systematic review. AIDS 2018;32(suppl 1):S93–S105. [DOI] [PubMed] [Google Scholar]


